Highlights
-
•
The basic principle, signal processing, and source localization of magnetoencephalography (MEG) are outlined, along with its strengths and limitations.
-
•
The primary clinical applications of MEG in cognitive assessment, diagnoses of neurological diseases and mental disorders, and preoperative evaluation are highlighted.
-
•
The potential neural mechanisms revealed by MEG-based strategies are addressed in the context of the brain-computer interface (BCI).
Keywords: Magnetoencephalography (MEG), Basic principle, Signal processing, Source localization, Clinical application
Abstract
Magnetoencephalography (MEG) is a non-invasive technique that can precisely capture the dynamic spatiotemporal patterns of the brain by measuring the magnetic fields arising from neuronal activity along the order of milliseconds. Observations of brain dynamics have been used in cognitive neuroscience, the diagnosis of neurological diseases, and the brain-computer interface (BCI). In this study, we outline the basic principle, signal processing, and source localization of MEG, and describe its clinical applications for cognitive assessment, the diagnoses of neurological diseases and mental disorders, preoperative evaluation, and the BCI. This review not only provides an overall perspective of MEG, ranging from practical techniques to clinical applications, but also enhances the prevalent understanding of neural mechanisms. The use of MEG is expected to lead to significant breakthroughs in neuroscience.
1. Introduction
Brain science has emerged as one of the most challenging subjects in cutting-edge global research in recent years. The cerebral architecture comprises billions of interlinked neurons, and exhibits profound and intricate mechanisms. This complexity encompasses a multi-layered hierarchy of organizational strata, spanning from the microcosmic domain of neurons to the mesoscopic expanse of cerebral regions, and each tier plays a pivotal functional role. A variety of neuroimaging techniques have been used to obtain information on the structure and function of the nervous system in vivo, ranging from macroscopic to microscopic and static to dynamic, and have yielded unprecedented insights on the processing of information and coordination of complex tasks by the brain.
Researchers have used the modalities of neuroimaging based on a variety of principles to decode the mechanisms of the brain and to understand brain disorders at different scales (Poo et al., 2016). Magnetoencephalography (MEG) is the most sensitive of the modalities used to capture the transient and rapid electrical activity of the entire brain. It is used to measure the magnetic fields generated by neuronal activity, and provides a unique perspective to better understand the intricate workflow of the human brain (Gross, 2019). While it has the advantages of non-invasiveness and real-time processing, the main limitation of MEG is that its signal-to-noise ratio (SNR) tends to decrease significantly when probing deeper regions of the brain. This is because the weaker magnetic fields generated by deeper brain tissues are easily affected by background noise (Zhu et al., 2020, Ott et al., 2021).
This review summarizes the basic principle, signal processing, source localization, and clinical applications of MEG. Here, the source localization can be categorized as forward and inverse modeling. Since MEG has become a valuable tool not only in neurocognitive processes, but also in clinical settings (Salmelin and Hari, 1994, D. Van 't Ent et al., 2003). The clinical applications of MEG have been discussed in the context of cognitive assessment, neurological diseases and mental disorders, preoperative evaluation, and the brain-computer interface (BCI).
2. Search strategy
This article is a narrative review that aims to comprehensively present our current understanding of MEG in the context of brain science. To this end, we considered studies on the principle of MEG, the analysis of MEG signals, the inverse problem, and clinical applications of this technique that were published from January 2013 to February 2024. This involved querying several electronic databases, including the Web of Science, PubMed, and Google Scholar, by using a combination of the following keywords relevant to research on MEG: “magnetoencephalography,” “artifact removal,” “optically pumped magnetometers,” “magnetic source localization,” “forward problem,” “inverse problem,” “epilepsy,” and “multi-modal fusion.” After removing duplicate records of the retrieved studies, we subjected the remainder to a preliminary screening by reading the title and abstract of each. A total of 154 articles were finally considered for this review.
3. Basic principle of MEG
3.1. MEG signals
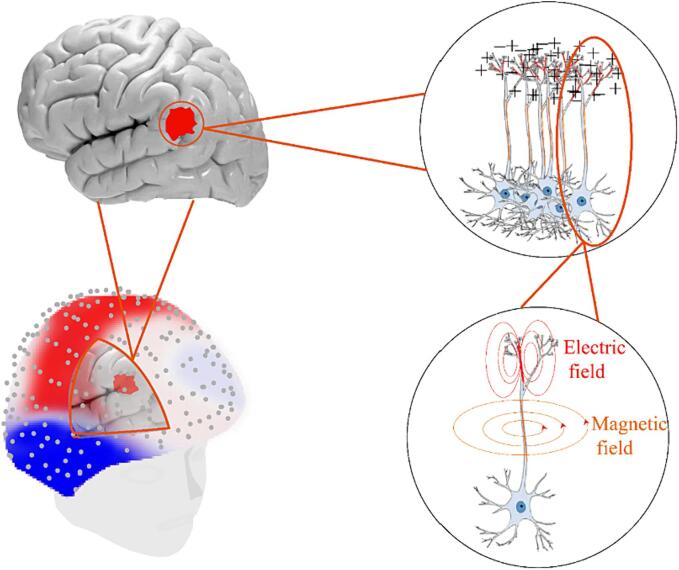
David Cohen used induction coils combined with signal stacking and superconducting technology to record MEG signals in a magnetically shielded environment in 1968, and this paved the way for the development of MEG technology (Cohen, 1968). The neuronal activity captured by MEG is not, as is perhaps expected, produced by the briefly lasting axonal action potentials of pyramidal cells, but rather by the net contributions of excitatory and inhibitory dendritic post-synaptic potentials (Malcolm et al., 2014). The current flowing through the apical dendrites (represented as a “dipole”) generates an extended radial magnetic field that causes MEG signals to be highly sensitive to dipoles arranged tangentially with respect to the skull (da Silva, 2013). The extensively folded human cortex promotes the orientation of a majority of cortical microcolumns (Hansen, 2010, Hämäläinen et al., 2020).
According to Lorente de Nó, cortical pyramidal neurons are the main source of MEG and electroencephalography (EEG) signals (Lorente De Nó, 1947). These neurons are organized in a manner whereby their apical dendrites are aligned perpendicularly to the cortical plane, and thus form “open fields” (Hämäläinen et al., 1993). Consequently, longitudinal intracellular current flows along the dendrites or axons, and can be described by the right-hand rule of electromagnetism. Characterized by their elongated apical dendrites, the cortical pyramidal neurons are regarded as “current dipoles” with synchronously activated coherent magnetic fields. The electrophysiological principle of the current dipole is shown in Fig. 1.
Fig. 1.
Electrophysiological principle of current dipoles.
The activity of the dipoles can be detected by sensors positioned close to the skull. MEG primarily captures neuronal currents flowing tangentially with respect to the surface of the scalp that originate from pyramidal neurons oriented in parallel within the sulci of the neocortex. Unlike EEG signals, MEG signals are insensitive to the radial currents that are perpendicular to the surface of the scalp, and typically emanate from the apical dendrites of pyramidal neurons located in the gyri. The gyrification of the cortex, which creates gyri and sulci, leads to certain neuronal populations with apical dendrites being perpendicular to the adjacent surface of the skull (atop the gyrus), while other populations are parallel to it (along the sulcal walls). This neuronal orientation with respect to the skull significantly influences the capture of MEG signals. The neuronal currents that are oriented radially with respect to the skull fail to produce a detectable external magnetic field (da Silva, 2013).
3.2. Characteristics of MEG
3.2.1. Benefits and limitations of MEG
Compared with EEG, functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (fNIRS), MEG has the advantages of a high temporal resolution, the capability of directly measuring neuronal activity, real-time results, and dynamic and accurate positioning. However, the application of MEG is constrained by its high expenses, low SNR, and vulnerability to variations in head structure (Hillebrand and Barnes, 2003, Singh, 2014), as shown in Table 1.
Table 1.
Comparison of non-invasive neuroimaging techniques.
| Modality | Characteristic | Temporal resolution | Spatial resolution | Strengths | Weaknesses | Clinical application |
|---|---|---|---|---|---|---|
| EEG | Monitoring and locating brain activity | 0.001 s-0.005 s (Gevins and B., 1996) |
11 mm (Kalogianni et al., 2018) |
High temporal resolution Applicable for long time recording |
Lower spatial resolution Limited signal penetration Finite localized information |
Epilepsy (Rosenow et al., 2015) Dementia disorders (Adebisi and Veluvolu, 2023) Parkinson’s Disease (Chu et al., 2022) Migraine (Zhang et al., 2023) |
| fMRI (Yoo et al., 2018) |
Simultaneously observing of brain anatomy and neural activity |
0.5 s-2 s | 1.5 mm-3.5 mm | High spatial resolution Robust signal fidelity Directly obtaining BOLD signals from the cerebral cortex |
Low temporal resolution Inefficient for the explanation of cognitive mechanisms Insensitive to high frequency oscillations |
Depressive disorders (Pitsik et al., 2023) Epilepsy (Sadjadi et al., 2021) |
| fNIRS (Peng and Wang, 2023) | Monitoring the activity of different brain regions | > 0.1 s | About 10 mm |
High degree of mobility and portability Suitable for children’s studies |
Low spatial resolution Detecting deep brain regions weakly |
Cognitive dysfunction (Sui et al., 2022) Depressive disorders (Wang et al., 2023) |
| MEG (Hämäläinen et al., 2020) |
Accurately locating brain functional activity | <0.001 s | 2 mm- 4 mm |
High temporal resolution High signal quality Direct measurement of neuronal activity High positioning accuracy Security and real time dynamic |
High use cost Low SNR Susceptible to the structure of head Unable to directly reflect the patterns of brain activity on the cortex |
Epilepsy (Pedersen et al., 2022) Autism spectrum disorder (Roberts et al., 2021) Alzheimer’s Disease (López-Sanz et al., 2018) Parkinson disease (Boon et al., 2019) |
MEG signals are used as complementary to EEG signals in clinical practice (Baillet, 2017). Hans Berger first recorded the rhythmic electrical activity of the human scalp in 1929, and published the first paper on EEG (Coenen and Zayachkivska, 2013). By placing electrodes on the scalp, EEG can be used to monitor electrical signals from the brain. In contrast to MEG signals that originate from tangential currents, EEG signals are generated by radial currents. The blood oxygen level is used to implement fMRI to non-invasively observe brain activity without applying any radiation. fNIRS can be used to calculate temporal changes in cerebral blood flow, and its distinct advantages are its portability and potential for long-term monitoring.
3.2.2. MEG fusion
MEG fusion, called “MEG+,” refers to the combination of MEG with other neuroimaging technologies. Such multi-modal fusion can represent the complementarity of the mechanisms of the brain by integrating the advantages of multiple techniques while overcoming the limitations of each (Hall et al., 2014).
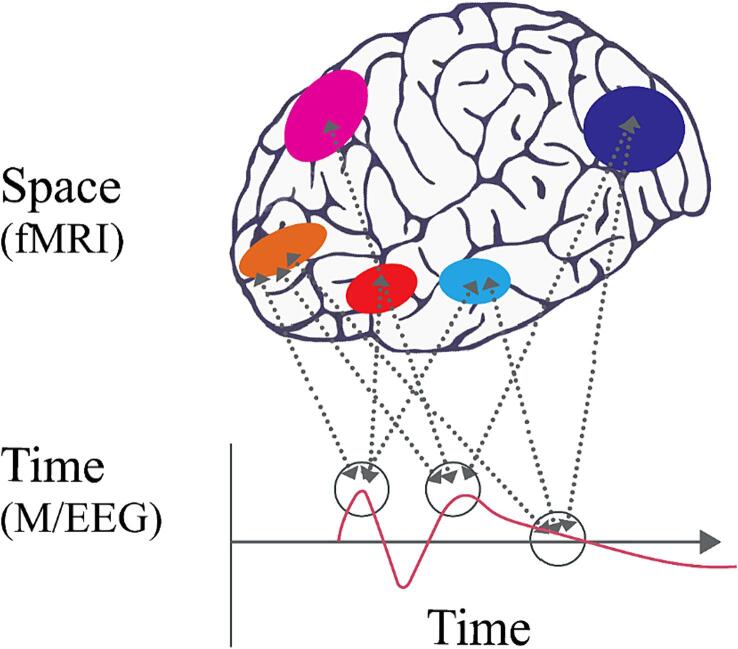
MEG and EEG signals have an excellent temporal resolution for dynamic neural activities, while fMRI can resolve brain activity-related changes in the properties of blood in regions of the brain below the order of millimeters. If considered in isolation, each technique involves many-to-many mappings between regions of the brain and underspecified time points (Cichy and Oliva, 2020) (Fig. 2).
Fig. 2.
Many-to-many mapping between brain regions and time points (From Cichy et al. (Cichy and Oliva, 2020)).
The benefits of MEG + are as follows: (1) The combination of MEG and fMRI leverages the strengths of both modalities to overcome their individual limitations owing to the high temporal resolution of MEG and the high spatial resolution of fMRI (Cargnelutti and Tomasino, 2023). (2) The combination of MEG and EEG can provide comprehensive information on the electromagnetic activity of the brain by using the sensitivity of MEG to deep sources of this activity and the high temporal resolution of EEG (Heitmann et al., 2023). (3) MEG, when combined with structural MRI (sMRI), can provide information on the structure and function of the brain (Cetin et al., 2023).
Multi-modal fusion can be used to simultaneously obtain information on brain activity at different levels and in different periods. This can help us better understand the function and structure of the brain (Ru et al., 2022). Cichy et al. (Cichy and Oliva, 2020) proposed a method to link the response patterns of the brain obtained from fMRI and MEG/EEG via representational similarity. They discussed the rationale, applications, and pros and cons of this technique, called MEG/EEG–fMRI fusion. Their investigation revealed large-scale patterns of spatio-temporal dynamics across networks, which they interpreted as four distinct spatio-temporal components of attention. These studies exemplify how MEG + can be used to study complex cognitive phenomena, and can help establish causal relationships between the neural responses of spatio-temporal cognition and cognitive functions.
3.3. MEG measurement
The magnetic field strength measured by MEG, ranging from 10-12 to 10-15 Tesla, is significantly weaker than the Earth’s magnetic field strength of 10-4 to 10-5 Tesla, and is even lower than the strengths of the fields generated by the heart and muscles (Muthukumaraswamy, 2013). Therefore, the main challenge to MEG measurements lies in isolating weak brain signals from strong signals generated outside the brain. Highly sensitive sensors are needed in the magnetic shielding chamber to this end.
The superconducting quantum interference device (SQUID) based on the Josephson effect was developed in 1969. It has significantly improved the detection of weak magnetic fields of the brain owing to its high sensitivity, and is still among the most sensitive magnetometers in use (Krzyzewski et al., 2019, Walsh et al., 2021). A series of experimental studies based on SQUID were conducted with the establishment of several international SQUID laboratories in the 1970 s (Mauro et al., 1970). SQUID technology has made significant progress in the 21st century based on magnetic resonance imaging (MRI) and magnetic force microscopy (MFM) (Mößle et al., 2006). Its sensitivity and resolution have been further improved, and this has led to its use in a wider range of fields.
In 2002, researchers at Princeton University reported the first atomic spin exchange relaxation-free (SERF) state and verified its sensitivity. Many research teams around the world have since reported on SERF atomic magnetometers (Allred et al., 2002, Kominis et al., 2003). The use of the SERF atomic magnetometer led to a significant breakthrough in biomedicine in 2006 owing to its highly sensitive measurements of signals of the magnetic field of the brain (Zotev et al., 2007), far surpassing those of the SQUID (Kim et al., 2014). Researchers at Sandia Laboratory in the United States focused on the measurement of weak magnetic fields in medicine, including brain magnetism and cardiac magnetism, in 2010, and developed an atomic magnetometer to detect signals of 5 fT/Hz1/2 under laboratory conditions. This is the first device used to measure the magnetic signals of the brain apart from SQUID atomic magnetometers (Dang et al., 2010, Xu et al., 2006). Research has shown that the SERF atomic magnetometer can be used as an alternative to SQUID, and can provide the foundation for next-generation wearable MEG systems owing to its small size and compactness.
The optically pumped magnetometer (OPM) has recently attracted attention due to its ability to operate in low-temperature environments, compactness, short detection distance, high signal sensitivity, and uniform coverage (Guo et al., 2023, Li et al., 2018, Brookes et al., 2022). One study used the OPM with the optical radio-frequency dual-resonance phenomenon to detect magnetic signals of the brain in 2017 (Boto et al., 2018). A probe was inserted into a 3D-printed helmet and located close to the surface of the head to accurately detect magnetic signals of the brain. In 2018, researchers at the University of Nottingham designed a wearable magnetometer, called OPM-MEG, that used a quantum spin magnetometer (Quspin)-based zero-field probe combined with MRI to obtain the magnetic activity of the brain and localize the magnetic source (Hassan and Wendling, 2018). This device has the advantages of a small weight and size, short detection distance, and flexibility.
Multiple sensors are placed in an array in commercial MEG devices to improve the accuracy of measurement and spatial resolution of MEG signals (Cao et al., 2023). In 2022, Brookes et al. (Brookes et al., 2022) designed a wearable OPM-MEG that yielded high-quality data (highly sensitive, with a high spatial resolution). It can be adapted according to the size and shape of the head (ranging from that of infants to adults), exhibits motor stability (the subject can freely move during scanning), and involves a simple imaging platform. Wang et al. (Wang et al., 2023) assessed the compatibility of movement of the wearable OPM-MEG, and highlighted its capabilities, the challenges to it, and its potential for future use.
The differences between the traditional SQUID-MEG and OPM-MEG are shown in Fig. 3. The traditional SQUID-MEG requires liquid helium at an ultra-low temperature of 4.2 K along with a detector located 3 cm from the scalp, while OPM-MEG can operate at a room temperature, with the detector closer to the cortex, and does not require a Dewar device. Table 2 lists the applicability, strengths, and weaknesses of the above instruments.
Fig. 3.
Comparison of traditional SQUID-MEG and OPM-MEG.
Table 2.
Comparison of MEG instruments.
| Instrument | Applicability | Strengths | Weaknesses |
|---|---|---|---|
| SQUID (Mößle et al., 2006) | Neuroscience research Medical imaging Geophysics |
High sensitivity Affordable Mature commercial market |
Depends on low temperature Shielding magnetic interference |
| SERF (Allred et al., 2002) | MRI enhancement: boosting MRI sensitivity, great for low-signal or high-contrast imaging. Nuclear Magnetic Resonance (NMR) improvement: improving sensitivity and SNR. Particle physics research |
Reduction other magnetic interference Sensitivity boost Theoretical maturity: benefiting fields like MRI and NMR, enhancing imaging resolution and analytical capabilities. |
Expensive equipment Environment-sensitive: be most sensitive in the range of extremely low-frequency magnetic fields. |
| OPM (Brookes et al., 2022) | Brain research Instant monitoring: responding rapidly and enabling real-time magnetic field tracking for immediate feedback. |
Precise measurement Quick response |
High cost Environmental sensitivity Technical complexity |
4. Signal processing
4.1. Preprocessing
As MEG has a high temporal resolution, its signals are easily contaminated by unpredictable noise that leads to two types of artifacts (Radüntz et al., 2017): those related to the instrument used for measurement, and physiological artifacts (Ge et al., 2017, Fatourechi et al., 2007, Burgess, 2020). The former are caused by faulty electrodes, line noise, and high electrode impedance, and can be avoided by using a precise recording system and strict measurement procedures. The latter are caused by eye movement and blinking, cardiac activity, and muscle activity, and can be eliminated by using regression analysis, wavelet transform, principal component analysis (PCA), independent component analysis (ICA), signal-space projection (SSP), signal-space separation (SSS), blind source separation (BSS) and etc (De Cheveigné and Simon, 2007, Jung et al., 2001, Susan Philip et al., 2023, Burgess, 2020, Vigario and Oja, 2008).
4.2. Feature selection and classification
Feature extraction and classification are crucial steps during the analysis and processing of magnetic signals of the brain. The analysis of magnetic signals of the brain can be divided into three types of time domain, frequency domain, and the network topology (Blankertz et al., 2007, Ba et al., 2014, Sanchez Bornot et al., 2018).
First, time domain algorithms are based on spectral decomposition-based analysis, which can be used to extract signals associated with specific stimuli or tasks. It is commonly used to analyze the event-related potential (ERP) of MEG signals. Time-frequency methods mainly include methods based on common spatial patterns (Blankertz et al., 2007) and discriminative spatial patterns, and those used to analyze the components of correlation (Liao et al., 2007, Tanaka and Miyakoshi, 2019, Zhang et al., 2018). These methods are relatively easy to implement, but may not be adequate for extracting important information regarding specific spectral features of complicated signals.
Second, frequency domain methods convert time domain signals into those in the frequency domain by using the Fourier transformation. In addition to the traditional Fourier transformation and wavelet analysis, new approaches have been developed for signals in the frequency domain, including spectral decomposition (Ba et al., 2014), state-space multi-dimensional time frequency analysis, higher-order spectral features, and topographic time frequency decomposition (Kim et al., 2018, Chella et al., 2017, König et al., 2001). These methods are suitable for identifying and quantifying the characteristics of the frequency of signals, including the frequency of oscillation, spectral peak, and spectral distribution. However, frequency-domain methods cannot be used for non-linear and non-stationary MEG signals.
Third, methods used to analyze the network topology explore the connections and interactions between regions of the brain by using connection matrices. This consists of network construction and analysis at the network level. The brain network is constructed by using coherence (Sanchez Bornot et al., 2018), phase synchronization, likelihood of synchronization, and Granger causality (Bruña et al., 2018, Aviyente et al., 2017, Li et al., 2019), and network analysis is performed by using such methods as time-varying brain network analysis, dynamic network analysis, and network restructuring (Rubinov and Sporns, 2010, Li et al., 2019, Duan et al., 2019, Li et al., 2019, Mandke et al., 2018, Harvy et al., 2019).
Time domain methods are suitable for event-related correlation and preliminary feature extraction, while frequency domain methods are suitable for extracting oscillations in the frequency and its characteristics during brain activity. Methods to analyze the network topology can help understand brain activities and the organization of the brain network for cognitive processes, disease-related mechanisms, and biomarkers of brain diseases. More advanced approaches need to be applied to comprehensively process MEG data.
5. Source localization
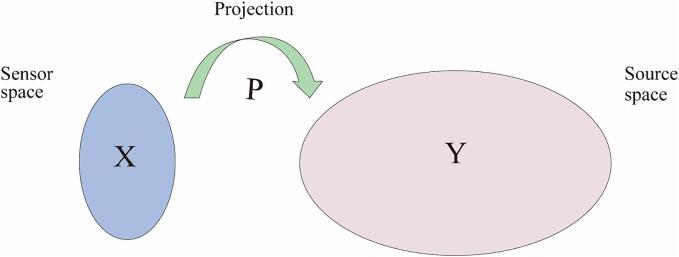
Source localization involves inferring changes in the position and intensity of brain activity based on the magnetic signals generated by it and recorded by sensors. Methods of source localization can be divided into those of forward and inverse modeling. Forward modeling estimates the magnetic field from known sources while inverse modeling localizes its sources from a known magnetic field that is deduced by using forward modeling. Magnetic source localization is also known as magnetic source imaging or magnetic source reconstruction, and operates on the principle of optimizing the projection operator P.
Fig 4.Shows the process of solving the inverse problem in MEG by optimizing the mapped projection of the sensor space to the source space, and is formulated as:
| (1) |
where P represents the projection operator, the sensor space X refers to the space in which magnetic signals of the brain are recorded on an array of magnetic induction sensors, and the source space Y is used to characterize the sources of brain activity in MEG. This represents a set of potential sources within the brain that can account for the magnetic or electric field data observed in the sensor space.
Fig. 4.
Spatial projection.
5.1. Forward modeling
Data from the intracellular tangential current in the brain can be obtained using MEG through forward modeling. It is critical to develop a comprehensive model of the head to this end that accurately represents the spatial distribution of tissue conductivity. The model of the head is not only related to the generator in the brain, but also depends on the surrounding tissues of the head. Such models can be divided into three types: spherical head model, standard head model and realistically shaped head model.
The forward problem is solved in applications by initially constructing a model of the head that elucidates the spatial patterns of tissue conductivities and then identifying a source current from the observed MEG signals. Cutting-edge and realistic models of the head can be established by using anatomical MRI. This process involves acquiring the anatomy of the head and transforming it into a digital model. The boundary element model is subsequently applied to simulate the volume of tissue of the entire head. This model typically replicates the distribution and characteristics of the surface tissues, including those of the scalp and skull (Piastra et al., 2018) (Fig. 5).
Fig. 5.
Relationship between the forward solution and the inverse problem.
5.2. Inverse modeling
The so-called problem of inverse modeling is ill posed because, in theory, any number of intracranial generators produce the same surface field (Mégevand and Seeck, 2018). The inverse problem in MEG involves estimating the source of current in the brain by measuring MEG signals on the scalp. The uniqueness of the solution to each inverse problem in MEG is essential for accurately determining the location of the original signals based on such factors such as the geometry of the conductor and the distribution of the current. Certain circumstances may give rise to “ill-posed” inverse problems during the analysis of MEG data. The challenges in this context are as follows: (1) There may be a number of mathematical solutions to the inverse problem. (2) The mathematical solution to the inverse problem may be highly sensitive to slight changes in the input parameters. Because of the non-unique properties of the inverse solution, it is necessary to apply finite constraints to it.
There are two approaches to solve the inverse problem for the models of distributed sources and dipole localization, as shown in Table 3 (He et al., 2018). The model of distributed sources assumes a uniform distribution of a large number of point sources on the gray matter grid in the brain, and estimates the intensity of activity of each source by solving a linear inverse problem. The model of dipole localization identifies certain dipoles that generate equivalent signals, and estimates the position and size of the dipoles through non-linear optimization and other methods (Fig. 6). The dynamic imaging of the underlying sources requires continuous MEG signals as well as the registration of the models of the source and the head.
Table 3.
MEG .
| Source Model | Method | Principle | Advantages | Limitations |
|---|---|---|---|---|
| Distributed source model | MNE (Hämäläinen et al., 1993) |
Estimating potential sources based on minimum norm reconstruction | Earliest method Suitable for distributed source model |
Beyond the scope of potential sources |
| LORETA (Iordanov et al., 2018) |
Estimating the three-dimensional distribution of brain activity sources | Better than MNE Improved superficial source bias |
Not suitable for local source estimation | |
| FOCUSS (Gorodnitsky and Rao, 1997) |
Emphasizing solutions for local underdetermined systems | Significantly improving positioning accuracy | Unable to estimate deeper source localization | |
| Bayesian estimation (Cai et al., 2018) | Combining spatial and temporal constraints within framework | Better than spatial regularization and more time-saving | Causing a single solution to the problem | |
| Beamformer (Zhang, 2022) | Weighted sources in specific directions | Robust Number of sources unknown |
Depend directly on the lead field and the physical size of the array (aperture) | |
| Dipole localization model | LSMN (Sánchez and Halliday, 2013) |
Minimize measured magnetic field data using nonlinear estimation algorithms | Simplify the complexity of function solving | Insufficient ability to resist overfitting |
| MUSIC (Mäkelä et al., 2018) |
Find source location as array popular vector | Improved localization | Inherent noise decrease accuracy |
source localization algorithms
Fig. 6.
Solution of MEG inverse problem.
Models of distributed sources include minimum norm estimation (MNE), low-resolution brain electromagnetic tomography (LORETA), focally underdetermined system solution (FOCUSS), Bayesian estimation, and the beamformer (Hämäläinen et al., 1993, Iordanov et al., 2018, Gorodnitsky and Rao, 1997, Cai et al., 2018, Zhang, 2022). The models of dipole localization include the least-squares minimum norm (LSMN), multiple signal classification algorithm (MUSIC), neural networks (NNs), and the genetic algorithm (Sánchez and Halliday, 2013, Mäkelä et al., 2018).
Research on distributed source estimation has led to many solutions based on LORETA and FOCUSS in conjunction with MNE (Tait et al., 2021). As a typical method of MNE, the beamformer enhances signals from the region of interest of the brain by weighting and combining sensor signals in the array. Bayesian estimation integrates different types of prior information in MNE to improve the accuracy of brain source localization. A combination of the beamformer and Bayesian estimation has been proposed to accurately identify the source of magnetic signals and reconstruct them by extracting the sources of brain activity from the beamformer, and then using the Bayesian network for inference and estimation (Zhang et al., 2021).
With the development of dipole positioning, MUSIC and LSMN have been used for signal processing and brain imaging. MUSIC is appropriate for locating the signal source, while the LSMN can be used to optimize the parameters and reconstruct the source of the magnetic signals. The choice of approach depends on the assumed number of signal sources and their distribution.
6. Clinical applications
6.1. Cognitive assessment
MEG is the ideal modality to determine the development of language, perception, execution of action, and multi-faceted cognitive abilities. Its high temporal and spatial resolutions enable researchers to track age-related changes in terms of both neural timing and location. Researchers have collected detailed spatial and temporal data from MEG signals, and have used them to examine the development of social, executive, and linguistic skills in normal children and those with autism spectrum disorders. The results have shown that variable and subtle changes in the brain function are related to age, sex, and clinical conditions (Taylor and Pang, 2014).
Ding et al. (Ding et al., 2016) used MEG to track whole-brain neural activities related to hierarchical language structures by designing a stimulus paradigm of a specific length and arrangement. Their results provided evidence of the ability of the human brain to rapidly construct multi-level linguistic structures. The large-scale neural activity measured by MEG was shown to concurrently follow the hierarchical linguistic structure of speech. The correlational experimental paradigm has been applied to evaluate the language processing capability of the brain and the level of consciousness of patients with consciousness disorders, such as those in a vegetative state. The experimental paradigm may involve testing the patient’s language processing ability as well as their perception and understanding of environments with specific stimuli or tasks. This helps doctors assess the state of consciousness of patients and their progress in rehabilitation (Gui et al., 2020).
6.2. Neurological diseases
With the advent of MEG, numerous studies have explored its potential in clinical applications of neurological diseases, such as epilepsy, Alzheimer’s disease, and Parkinson’s disease. The initial clinical application of MEG involved detecting and locating epileptic foci (Galanopoulou et al., 2012). This technology has played an important role in diagnosing and treating patients suffering from neurological diseases (Fred et al., 2022, Gross et al., 2013). Although the clinical applications of MEG are widely recognized, further research is needed to comprehensively understand the dynamics of the brain and widen the scope of applications of MEG, as shown in Table 4. The clinical applications of MEG are expected to broaden in scope as its cost decreased and innovative strategies for its use are developed.
Table 4.
MEG clinical applications (part).
| Diseases | Applications |
|---|---|
| Epilepsy | Accurate localization of spikes (Knowlton and Shih, 2004). Localizing electrophysiological activity in epilepsy using MEG is either based on interictal activity, usually in the form of interictal epileptic discharges (IEDs) and high frequency oscillations (HFOs), or ictal activity (Reuber, 2023). |
| Alzheimer’s disease (AD) |
Proficient in the early detection of dementia (Stam et al., 2006). Increase in count of dipoles in the delta and theta band (Osipova et al., 2006). Slow wave activity detection in the right temporal and parietal lobe of the brain (Fernández et al., 2002). |
| Schizophrenia | Resting state MEG are able to distinguish different subtypes of schizophrenia (Edgar et al., 2020). Detecting the brain oscillation that distinguishes between normal and schizophrenia subjects (Vandewouw et al., 2021). |
| Attention deficit hyperactivity disorder (ADHD) | Explored neural interactions using MEG between auditory cortices and the frontal cortices (Hare et al., 2022). |
| Chronic pain | Evidence on how resting-state MEG might serve as a diagnostic biomarker (Witjes et al., 2021, Zebhauser et al., 2022). |
| Stroke | Investigated the potential of perilesional and pathological activity to support language recovery in patients with poststroke aphasia (Piastra et al., 2022, Arheix-Parras et al., 2023). |
| Traumatic brain injury (TBI) | Analysis of brain networks computed from resting-state MEG with PAC and tensorial representation of connectivity profiles may provide a valuable biomarker for the diagnosis of TBI (Aaltonen et al., 2023, Itälinna et al., 2022). |
6.2.1. Epilepsy
A common neurodegenerative disease, epilepsy is caused by the abnormal discharge activity of neurons, and is manifested as transient and repetitive seizures (Vivekananda et al., 2020). Because MEG can record neuropsychological changes at a temporal resolution of the order of milliseconds and can localize the sources of the signals with a sub-centimeter accuracy, it plays an important role in locating epileptiform activity not only during seizures, but also for the non-invasive detection of interictal periods. This provides important insights into the underlying network dynamics of epilepsy (Geller et al., 2023, Knowlton, 2006, Alotaiby et al., 2017). Moreover, advanced methods of source localization have improved the efficacy of epileptic surgery by providing essential information for planning surgical interventions (Dorfer et al., 2020). A combination of MEG with models of the head can significantly improve the accuracy of identifying the sources of brain activity. This multi-disciplinary strategy can help improve the localization of intermittent epileptiform discharges, thereby improving the diagnosis and treatment of patients with epilepsy (Rampp et al., 2019, Hao et al., 2024).
6.2.2. Alzheimer’s disease
Alzheimer’s disease (AD) is a chronic neurological disease that is the most common cause of dementia, is among the leading causes of complications and death in the aging population, and constitutes 60 % to 70 % of the global population with dementia. Its causes are still unknown, and it is accompanied by a range of clinical symptoms that include memory loss, and the deterioration of such cognitive functions as attention, the capacity for thinking, skills of orientation, and language (Ballard et al., 2011, Zhao and Zhao, 2013). The identification of biomarkers can help detect AD at an early stage in clinical diagnosis. Because MEG can identify abnormal oscillatory characteristics of AD in the brain, it has the potential to be used for early detection (Mandal et al., 2018). By providing detailed information about the function and activities of the brain, MEG can detect abnormalities in neural activity early on, including changes in the patterns of functional connectivity in the brain of AD patients compared with those of healthy subjects (Sakkalis, 2011, Rodríguez-González et al., 2024).
6.2.3. Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disease that is characterized by dyskinesia and other symptoms (Bloem et al., 2021). The defining neuropathological feature of PD is the accumulation of Lewy bodies, which are predominantly composed of alpha-synuclein aggregates. As PD develops, lesions are no longer confined to the brain stem, and affect other regions of the cerebral cortex (Del Tredici et al., 2002, Ferrer, 2009). Owing to its high temporal resolution, MEG can be used to investigate brain activity and patterns of functional connectivity in case of PD. It has been used to assess PD-related neurophysiological characteristics both in the motor system and in the entire brain. MEG-based analyses of motor networks are spatially restricted to the motor cortex, and are usually performed in the source space. They can be combined with neurophysiological signals with different origins, including muscle activity recorded by using electromyography (EMG) (Boon et al., 2019). This underscores the importance of MEG as it can be used for the non-invasive diagnosis of PD based on neurophysiological foundations (Fred et al., 2022).
6.3. Mental disorders
Mental disorders involve significant disturbances in thinking, and emotion or behavior regulation. Examples include depression, schizophrenia, autism spectrum disorders, and attention deficit hyperactivity disorder (Reddy et al., 2023, Sanfratello et al., 2019, Edgar et al., 2014, Monge et al., 2015, Wang et al., 2019). MEG has exhibited significant potential for use in the early detection of mental disorders.
6.3.1. Depression
Depression is a mental disorder that is characterized by persistent feelings of sadness, loss of interest or pleasure, and a variety of physical and cognitive symptoms. Clinical treatment of depression mainly involves traditional psychological assessment and medication as well as psychotherapy (Alamian et al., 2017, Ye et al., 2019). An emerging treatment modality for depression involves transcranial magnetic stimulation (TMS), which is a procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of major depression (Leuchter et al., 2013). MEG has been used in research on biomarkers to determine the severity of depression in patients. This method is a preferable alternative to traditional pharmacotherapy and psychotherapy owing to its minimal side effects (Ho et al., 2020, Pettitt et al., 2022). Nonetheless, the use of MEG technology in this domain is still in its infancy, and it is not yet considered to be a routine diagnostic or treatment tool for depression.
6.3.2. Schizophrenia
Schizophrenia is a severe psychotic disorder that involves significant cognitive impairments. About 21 million people are estimated to suffer from schizophrenia worldwide (Weret and Mukherjee, 2014). It is characterized by significant impairments in perception and changes in behavior, including persistent delusions, hallucinations, highly disorganized behavior, and extreme agitation (Van Den Heuvel and Fornito, 2014). Regional and network-level power anomalies in the brain occur in case of schizophrenia, and research in the area has focused on brain regions associated with schizophrenic symptoms (Van Den Heuvel and Fornito, 2014). MEG can be used to identify the ERPs of schizophrenia patients based on specific stimuli or tasks, and to explore how the brain processes these stimuli. Changes in the oscillatory patterns of frequency waves can provide insights into the onset or symptomatic manifestations of the disease. One study on schizophrenia that used MEG identified correlations between the pathophysiology of the disorder and irregularities in synchronized oscillatory brain activities in the resting state (Nour et al., 2021).
6.3.3. Autism spectrum disorders
Autism spectrum disorders commonly occur in infancy or early childhood, and are identified by difficulties in social interactions, communication, and cognitive development (Edgar et al., 2014). Investigating the pathophysiological mechanisms underlying this condition in early infancy is vital for understanding its pathogeny and progression. Several researchers have used standard or child-sized MEG instruments to identify the neurophysiological foundations of various childhood neurodevelopmental disorders (Lord et al., 2020). Preschool- and school-aged children with ASD exhibit notable differences from healthy children in behavior, cognition, and social interactions (Picci et al., 2016). MEG can help better understand the heterogeneity of ASD, and can identify multi-variate biological markers to classify clinical sub-populations. MEG has been used to personalize the scanning experience of patients, and offers promise in the search for objective biological markers of brain dysfunction (Roberts et al., 2021).
6.4. Preoperative evaluation
Effective preoperative assessment relies on collaboration between experts in neurosurgery, neuroradiology, and neurophysiology. Non-invasive functional imaging techniques play an important role in assessing the feasibility, risks, and benefits of surgery (Xu et al., 2021). As an important technique for comprehensive preoperative evaluation, MEG can help locate crucial functional regions of the motor cortex, sensory cortex, and language centers to detect abnormal neural activity and neural dysfunction (Anderson et al., 2014, Nissen et al., 2016, Nissen et al., 2017, Hari et al., 2018). Clinicians can make detailed surgical plans, including surgical methods and resection criteria, based on insights provided by MEG (Maslarova et al., 2023).
6.5. Brain-computer interface
The brain-computer interface (BCI) has gradually attracted the attention of fundamental and clinical researchers in neuroscience, and enables the human brain to interactively communicate with computers or external devices. It can be used to create tools that support people with disabilities. MEG-based BCI has a wide range of real-time uses in technology, research, and healthcare. For people who cannot speak due to such problems as cerebral palsy and other neurological disorders, MEG-based BCI can be used to provide a means of communication. It is appropriate for neural speech decoding as well as prosthetic device control, and enables people who have lost a limb to regain part of the lost functionality.
MEG-based BCI can assist patients regain their motor function after a stroke or other neurological injury (Dash et al., 2020, Yanagisawa et al., 2019). BCI is also a treatment option for people suffering from brain injuries or neurological illnesses, and can help patients practice particular actions during rehabilitation (Roy et al., 2020, Susan Philip et al., 2023). Many researchers have investigated non-invasive BCI-based methods to make full use of the classification and decoding of MEG data (Li et al., 2023). Wearable MEG systems are still in the phase of laboratory research, and are expected to broaden the field of application of BCI.
7. Conclusion
In contrast to other techniques of neuroimaging, MEG provides a comprehensive representation of brain activity at a high temporal resolution along the order of milliseconds. It is expected to revolutionize our understanding of the functions of the brain as well as the mechanisms of diseases, and clinical applications. When integrated with computer-aided algorithms, “MEG+” offers significant promise for the diagnosis and prognosis of neurological illnesses. Moreover, MEG-based BCI systems are expected to provide major breakthroughs in rehabilitation from neurological illnesses. The next generation of MEG technology will be highly sensitive, miniaturized, customized, wearable, and flexible in terms of deployment, and is expected to usher in a new era of brain-related measurements.
8. Financial support
This study was funded by the National Natural Science Foundation of China (Nos. 61971275, 81830052, and 82072228), the grants of the National Key Research and Development Program of China (2020YFC2008700) and Shanghai Municipal Commission of Science and Technology for Capacity Building for Local Universities (23010502700).
CRediT authorship contribution statement
Yanling Yang: Writing – original draft, Visualization, Resources, Formal analysis, Conceptualization. Shichang Luo: Writing – review & editing, Supervision, Methodology, Formal analysis. Wenjie Wang: Writing – review & editing, Formal analysis. Xiumin Gao: Supervision, Investigation. Xufeng Yao: Supervision, Funding acquisition, Conceptualization. Tao Wu: Supervision.
Data availability
No data was used for the research described in the article.
References
- Aaltonen J., Heikkinen V., Kaltiainen H., Salmelin R., Renvall H. Sensor-level MEG combined with machine learning yields robust classification of mild traumatic brain injury patients. Clinical Neurophysiology. 2023;153:79–87. doi: 10.1016/j.clinph.2023.06.010. [DOI] [PubMed] [Google Scholar]
- Adebisi A.T., Veluvolu K.C. Brain network analysis for the discrimination of dementia disorders using electrophysiology signals: A systematic review. Frontiers in Aging Neuroscience. 2023;15:1039496. doi: 10.3389/fnagi.2023.1039496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamian G., Hincapié A.-S., Pascarella A., Thiery T., Combrisson E., Saive A.-L., Martel V., Althukov D., Haesebaert F., Jerbi K. Measuring alterations in oscillatory brain networks in schizophrenia with resting-state MEG: State-of-the-art and methodological challenges. Clinical Neurophysiology. 2017;128:1719–1736. doi: 10.1016/j.clinph.2017.06.246. [DOI] [PubMed] [Google Scholar]
- Allred J., Lyman R., Kornack T., Romalis M.V.l. High-sensitivity atomic magnetometer unaffected by spin-exchange relaxation. Physical Review. 2002;89 doi: 10.1103/PhysRevLett.89.130801. [DOI] [PubMed] [Google Scholar]
- Alotaiby T.N., Alrshoud S.R., Alshebeili S.A., Alhumaid M.H., Alsabhan W.M. Epileptic MEG spike detection using statistical features and genetic programming with KNN. Journal of Healthcare Engineering. 2017;2017 doi: 10.1155/2017/3035606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.T., Carlson C.E., Li Z., Raghavan M. Magnetoencephalography in the preoperative evaluation for epilepsy surgery. Current Neurology Neuroscience Reports. 2014;14:1–8. doi: 10.1007/s11910-014-0446-8. [DOI] [PubMed] [Google Scholar]
- Arheix-Parras S., Glize B., Guehl D., Python G. Electrophysiological Changes in Patients with Post-stroke Aphasia: A Systematic Review. Brain Topography. 2023;36:135–171. doi: 10.1007/s10548-023-00941-4. [DOI] [PubMed] [Google Scholar]
- Aviyente S., Tootell A., Bernat E.M. Time-frequency phase-synchrony approaches with ERPs. International Journal of Psychophysiology. 2017;111:88–97. doi: 10.1016/j.ijpsycho.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Ba D., Babadi B., Purdon P.L., Brown E.N. Robust spectrotemporal decomposition by iteratively reweighted least squares. Proceedings of the National Academy of Sciences. 2014;111:E5336–E5345. doi: 10.1073/pnas.1320637111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nature Neuroscience. 2017;20:327–339. doi: 10.1038/nn.4504. [DOI] [PubMed] [Google Scholar]
- C. Ballard, S. Gauthier, A. Corbett, C. Brayne, D. Aarsland, E. Jones, Alzheimer's disease, the Lancet, 377 (2011) 1019-1031. [DOI] [PubMed]
- Blankertz B., Tomioka R., Lemm S., Kawanabe M., Muller K.-R. Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Processing Magazine. 2007;25:41–56. [Google Scholar]
- Bloem B.R., Okun M.S., Klein C. Parkinson's disease. The Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- Boon L.I., Geraedts V.J., Hillebrand A., Tannemaat M.R., Contarino M.F., Stam C.J., Berendse H.W. A systematic review of MEG-based studies in Parkinson's disease: The motor system and beyond. Human Brain Mapping. 2019;40:2827–2848. doi: 10.1002/hbm.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto E., Holmes N., Leggett J., Roberts G., Shah V., Meyer S.S., Muñoz L.D., Mullinger K.J., Tierney T.M., Bestmann S. Moving magnetoencephalography towards real-world applications with a wearable system. Nature. 2018;555:657–661. doi: 10.1038/nature26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes M.J., Leggett J., Rea M., Hill R.M., Holmes N., Boto E., Bowtell R. Magnetoencephalography with optically pumped magnetometers (OPM-MEG): the next generation of functional neuroimaging. Trends in Neurosciences. 2022 doi: 10.1016/j.tins.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruña R., Maestú F., Pereda E. Phase locking value revisited: teaching new tricks to an old dog. Journal of Neural Engineering. 2018;15 doi: 10.1088/1741-2552/aacfe4. [DOI] [PubMed] [Google Scholar]
- Burgess R.C. Recognizing and correcting MEG artifacts. Journal of Clinical Neurophysiology. 2020;37:508–517. doi: 10.1097/WNP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- Cai C., Sekihara K., Nagarajan S.S. Hierarchical multiscale Bayesian algorithm for robust MEG/EEG source reconstruction. NeuroImage. 2018;183:698–715. doi: 10.1016/j.neuroimage.2018.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Gao Z., Qi S., Chen K., Xiang M., An N., Ning X. Realistic three-layer head phantom for optically pumped magnetometer-based magnetoencephalography. Computers in Biology Medicine. 2023;164 doi: 10.1016/j.compbiomed.2023.107318. [DOI] [PubMed] [Google Scholar]
- Cargnelutti E., Tomasino B. Pre-Operative Functional Mapping in Patients with Brain Tumors by fMRI and MEG: Advantages and Disadvantages in the Use of One Technique over the Other. Life. 2023;13:609. doi: 10.3390/life13030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin E.B., Bilgici M.N.C., Tuncer G.O., Karabag I.S., Aydin S. Syndrome of megalencephaly, mega corpus callosum, and complete lack of motor development: an unusual case and a literature review. Child's Nervous System. 2023:1–7. doi: 10.1007/s00381-023-06150-5. [DOI] [PubMed] [Google Scholar]
- Chella F., D'Andrea A., Basti A., Pizzella V., Marzetti L. Non-linear analysis of scalp EEG by using bispectra: the effect of the reference choice. Frontiers in Neuroscience. 2017;11:262. doi: 10.3389/fnins.2017.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Zhang Z., Song Z., Xu Z., Wang J., Wang F., Liu W., Lu L., Liu C., Zhu X. An enhanced EEG microstate recognition framework based on deep neural networks: an application to Parkinson's disease. IEEE Journal of Biomedical Health Informatics. 2022;27:1307–1318. doi: 10.1109/JBHI.2022.3232811. [DOI] [PubMed] [Google Scholar]
- Cichy R.M., Oliva A. A M/EEG-fMRI fusion primer: resolving human brain responses in space and time. Neuron. 2020;107:772–781. doi: 10.1016/j.neuron.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen A., Zayachkivska O. Adolf Beck: A pioneer in electroencephalography in between Richard Caton and Hans Berger. Advances in Cognitive Psychology. 2013;9:216. doi: 10.2478/v10053-008-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968;161:784–786. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- da Silva F.L. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Dang H., Maloof A.C., Romalis M.V. Ultrahigh sensitivity magnetic field and magnetization measurements with an atomic magnetometer. Applied Physics Letters. 2010;97 [Google Scholar]
- Dash D., Ferrari P., Wang J. Decoding imagined and spoken phrases from non-invasive neural (MEG) signals. Frontiers in Neuroscience. 2020;14:290. doi: 10.3389/fnins.2020.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cheveigné A., Simon J.Z. Denoising based on time-shift PCA. Journal of Neuroscience Methods. 2007;165:297–305. doi: 10.1016/j.jneumeth.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K., Rüb U., De Vos R.A., Bohl J.R., Braak H. Where does parkinson disease pathology begin in the brain?, Journal of Neuropathology. Experimental Neurology. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Ding N., Melloni L., Zhang H., Tian X., Poeppel D. Cortical tracking of hierarchical linguistic structures in connected speech. Nature Neuroscience. 2016;19:158–164. doi: 10.1038/nn.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfer C., Rydenhag B., Baltuch G., Buch V., Blount J., Bollo R., Gerrard J., Nilsson D., Roessler K., Rutka J. How technology is driving the landscape of epilepsy surgery. Epilepsia. 2020;61:841–855. doi: 10.1111/epi.16489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Xia M., Womer F.Y., Chang M., Yin Z., Zhou Q., Zhu Y., Liu Z., Jiang X., Wei S. Dynamic changes of functional segregation and integration in vulnerability and resilience to schizophrenia. Human Brain Mapping. 2019;40:2200–2211. doi: 10.1002/hbm.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Lanza M.R., Daina A.B., Monroe J.F., Khan S.Y., Blaskey L., Cannon K.M., Jenkins J., III, Qasmieh S., Levy S.E. Missing and delayed auditory responses in young and older children with autism spectrum disorders. Frontiers in Human Neuroscience. 2014;8:417. doi: 10.3389/fnhum.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Guha A., Miller G.A. Magnetoencephalography for schizophrenia, Neuroimaging. Clinics. 2020;30:205–216. doi: 10.1016/j.nic.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatourechi M., Bashashati A., Ward R.K., Birch G.E. EMG and EOG artifacts in brain computer interface systems: A survey. Clinical Neurophysiology. 2007;118:480–494. doi: 10.1016/j.clinph.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Fernández A., Maestú F., Amo C., Gil P., Fehr T., Wienbruch C., Rockstroh B., Elbert T., Ortiz T. Focal temporoparietal slow activity in Alzheimer’s disease revealed by magnetoencephalography. Biological Psychiatry. 2002;52:764–770. doi: 10.1016/s0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Early involvement of the cerebral cortex in Parkinson's disease: convergence of multiple metabolic defects. Progress in Neurobiology. 2009;88:89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Fred A.L., Kumar S.N., Kumar Haridhas A., Ghosh S., Purushothaman Bhuvana H., Sim W.K.J., Vimalan V., Givo F.A.S., Jousmäki V., Padmanabhan P. A Brief introduction to magnetoencephalography (MEG) and its clinical applications. Brain Sciences. 2022;12:788. doi: 10.3390/brainsci12060788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A.S., Buckmaster P.S., Staley K.J., Moshé S.L., Perucca E., Engel J., Jr, Löscher W., Noebels J.L., Pitkänen A., Stables J. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53:571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S., Yang Q., Wang R., Lin P., Gao J., Leng Y., Yang Y., Wang H. A brain-computer interface based on a few-channel EEG-fNIRS bimodal system. IEEE Access. 2017;5:208–218. [Google Scholar]
- Geller A.S., Teale P., Kronberg E., Ebersole J.S. Magnetoencephalography for Epilepsy Presurgical Evaluation. Current Neurology Neuroscience Reports. 2023:1–12. doi: 10.1007/s11910-023-01328-5. [DOI] [PubMed] [Google Scholar]
- Gevins A.J.M., B. Modern High Resolution Electroencephalography. Medical and Biological Engineering and Computing. 1996;34:1–4. [Google Scholar]
- Gorodnitsky I.F., Rao B.D. Sparse signal reconstruction from limited data using FOCUSS: A re-weighted minimum norm algorithm. IEEE Transactions on Signal Processing. 1997;45:600–616. [Google Scholar]
- Gross J. Magnetoencephalography in cognitive neuroscience: a primer. Neuron. 2019;104:189–204. doi: 10.1016/j.neuron.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Gross J., Baillet S., Barnes G.R., Henson R.N., Hillebrand A., Jensen O., Jerbi K., Litvak V., Maess B., Oostenveld R. Good practice for conducting and reporting MEG research. Neuroimage. 2013;65 doi: 10.1016/j.neuroimage.2012.10.001. 349–363.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui P., Jiang Y., Zang D., Qi Z., Tan J., Tanigawa H., Jiang J., Wen Y., Xu L., Zhao J. Assessing the depth of language processing in patients with disorders of consciousness. Nature Neuroscience. 2020;23:761–770. doi: 10.1038/s41593-020-0639-1. [DOI] [PubMed] [Google Scholar]
- Guo Q.-Q., Hu T., Feng X.-Y., Zhang M.-K., Chen C.-Q., Zhang X., Yao Z.-K., Xu J.-Y., Wang Q., Fu F.-Y. A compact and closed-loop spin-exchange relaxation-free atomic magnetometer for wearable magnetoencephalography. Chinese Physics B. 2023;32 [Google Scholar]
- Hall E.L., Robson S.E., Morris P.G., Brookes M.J. The relationship between MEG and fMRI. Neuroimage. 2014;102:80–91. doi: 10.1016/j.neuroimage.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M., Hari R., Ilmoniemi R.J., Knuutila J., Lounasmaa O.V. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413. [Google Scholar]
- Hämäläinen M., Huang M., Bowyer S.M. Magnetoencephalography signal processing, forward modeling, inverse source imaging, and coherence analysis, Neuroimaging. Clinics. 2020;30:125–143. doi: 10.1016/j.nic.2020.02.001. [DOI] [PubMed] [Google Scholar]
- P. Hansen, M. Kringelbach, R. Salmelin, MEG: An introduction to methods, Oxford university press2010.
- Hao G., Yan H., Wang X., Gao R., Xue Y., Zhang X., Ni D., Shu W., Qiao L., He L. The role of magnetoencephalography in preoperative localization and postoperative outcome prediction in patients with posterior cortical epilepsy. CNS Neuroscience Therapeutics. 2024;30:e14602. doi: 10.1111/cns.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare M.M., Dick A.S., Graziano P.A. Adverse childhood experiences predict neurite density differences in young children with and without attention deficit hyperactivity disorder. Developmental Psychobiology. 2022;64:e22234. doi: 10.1002/dev.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Baillet S., Barnes G., Burgess R., Forss N., Gross J., Hämäläinen M., Jensen O., Kakigi R., Mauguière F. IFCN-endorsed practical guidelines for clinical magnetoencephalography. MEGClinical Neurophysiology. 2018;129:1720–1747. doi: 10.1016/j.clinph.2018.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvy J., Thakor N., Bezerianos A., Li J. Between-frequency topographical and dynamic high-order functional connectivity for driving drowsiness assessment. IEEE Transactions on Neural Systems Rehabilitation Engineering. 2019;27:358–367. doi: 10.1109/TNSRE.2019.2893949. [DOI] [PubMed] [Google Scholar]
- Hassan M., Wendling F. Electroencephalography source connectivity: aiming for high resolution of brain networks in time and space. IEEE Signal Processing Magazine. 2018;35:81–96. [Google Scholar]
- He B., Sohrabpour A., Brown E., Liu Z. Electrophysiological source imaging: a noninvasive window to brain dynamics. Annual Review of Biomedical Engineering. 2018;20:171–196. doi: 10.1146/annurev-bioeng-062117-120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann H., Zebhauser P.T., Hohn V.D., Henningsen P., Ploner M. Clinical; NeuroImage: 2023. Resting-state EEG and MEG biomarkers of pathological fatigue–A transdiagnostic systematic review; p. 103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R. The use of anatomical constraints with MEG beamformers. Neuroimage. 2003;20:2302–2313. doi: 10.1016/j.neuroimage.2003.07.031. [DOI] [PubMed] [Google Scholar]
- Ho C.S., Lim L.J., Lim A., Chan N.H., Tan R., Lee S., Ho R.C. Diagnostic and predictive applications of functional near-infrared spectroscopy for major depressive disorder: a systematic review. Frontiers in Psychiatry. 2020;11:378. doi: 10.3389/fpsyt.2020.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov T., Bornfleth H., Wolters C.H., Pasheva V., Venkov G., Lanfer B., Scherg M., Scherg T. LORETA with cortical constraint: choosing an adequate surface laplacian operator. Frontiers in Neuroscience. 2018;12:746. doi: 10.3389/fnins.2018.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V. Itälinna, H. Kaltiainen, N. Forss, M. Liljeström, L. Parkkonen, Detecting mild traumatic brain injury with MEG, normative modelling and machine learning, medRxiv, (2022) 2022.2009. 2029.22280521. [DOI] [PMC free article] [PubMed]
- Jung T.-P., Makeig S., McKeown M.J., Bell A.J., Lee T.-W., Sejnowski T.J. Imaging brain dynamics using independent component analysis. Proceedings of the IEEE. 2001;89:1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogianni K., de Munck J.C., Nolte G., Vardy A.N., van der Helm F.C., Daffertshofer A. Spatial resolution for EEG source reconstruction—A simulation study on SEPs. Journal of Neuroscience Methods. 2018;301:9–17. doi: 10.1016/j.jneumeth.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Kim K., Begus S., Xia H., Lee S.-K., Jazbinsek V., Trontelj Z., Romalis M.V. Multi-channel atomic magnetometer for magnetoencephalography: A configuration study. NeuroImage. 2014;89:143–151. doi: 10.1016/j.neuroimage.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Kim S.-E., Behr M.K., Ba D., Brown E.N. State-space multitaper time-frequency analysis. Proceedings of the National Academy of Sciences. 2018;115:E5–E14. doi: 10.1073/pnas.1702877115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton R.C. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behavior. 2006;8:91–101. doi: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Shih J. Magnetoencephalography in epilepsy. Epilepsia. 2004;45:61–71. doi: 10.1111/j.0013-9580.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- Kominis I., Kornack T., Allred J., Romalis M.V. A subfemtotesla multichannel atomic magnetometer. Nature. 2003;422:596–599. doi: 10.1038/nature01484. [DOI] [PubMed] [Google Scholar]
- König T., Marti-Lopez F., Valdes-Sosa P. Topographic time-frequency decomposition of the EEG. NeuroImage. 2001;14:383–390. doi: 10.1006/nimg.2001.0825. [DOI] [PubMed] [Google Scholar]
- Krzyzewski S., Perry A., Gerginov V., Knappe S. Characterization of noise sources in a microfabricated single-beam zero-field optically-pumped magnetometer. Journal of Applied Physics. 2019;126 doi: 10.1063/1.5098088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.F. Leuchter, I.A. Cook, Y. Jin, B. Phillips, The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder, Frontiers in human neuroscience, (2013) 37. [DOI] [PMC free article] [PubMed]
- Li X., Chen J., Shi N., Yang C., Gao P., Chen X., Wang Y., Gao S., Gao X. A hybrid steady-state visual evoked response-based brain-computer interface with MEG and EEG. Expert Systems with Applications. 2023;223 [Google Scholar]
- Li Y., Lei M., Cui W., Guo Y., Wei H.-L. A parametric time-frequency conditional granger causality method using ultra-regularized orthogonal least squares and multiwavelets for dynamic connectivity analysis in EEGs. IEEE Transactions on Biomedical Engineering. 2019;66:3509–3525. doi: 10.1109/TBME.2019.2906688. [DOI] [PubMed] [Google Scholar]
- Li F., Peng W., Jiang Y., Song L., Liao Y., Yi C., Zhang L., Si Y., Zhang T., Wang F. The dynamic brain networks of motor imagery: time-varying causality analysis of scalp EEG. International Journal of Neural Systems. 2019;29:1850016. doi: 10.1142/S0129065718500168. [DOI] [PubMed] [Google Scholar]
- Li J., Quan W., Zhou B., Wang Z., Lu J., Hu Z., Liu G., Fang J. SERF atomic magnetometer–recent advances and applications: A review. IEEE Sensors Journal. 2018;18:8198–8207. [Google Scholar]
- Li F., Yi C., Song L., Jiang Y., Peng W., Si Y., Zhang T., Zhang R., Yao D., Zhang Y. Brain network reconfiguration during motor imagery revealed by a large-scale network analysis of scalp EEG. Brain Topography. 2019;32:304–314. doi: 10.1007/s10548-018-0688-x. [DOI] [PubMed] [Google Scholar]
- Liao X., Yao D., Wu D., Li C. Combining spatial filters for the classification of single-trial EEG in a finger movement task. IEEE Transactions on Biomedical Engineering. 2007;54:821–831. doi: 10.1109/TBME.2006.889206. [DOI] [PubMed] [Google Scholar]
- López-Sanz D., Serrano N., Maestú F. The role of magnetoencephalography in the early stages of Alzheimer’s disease. Frontiers in Neuroscience. 2018;12:572. doi: 10.3389/fnins.2018.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Brugha T.S., Charman T., Cusack J., Dumas G., Frazier T., Jones E.J., Jones R.M., Pickles A., State M.W. Autism spectrum disorder. Nature Reviews Disease Primers. 2020;6:1–23. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De Nó R. Action potential of the motoneurons of the hypoglossus nucleus. Journal of Cellular and Comparative Physiology. 1947;29:207–287. doi: 10.1002/jcp.1030290303. [DOI] [PubMed] [Google Scholar]
- Mäkelä N., Stenroos M., Sarvas J., Ilmoniemi R.J. Truncated rap-music (trap-music) for MEG and EEG source localization. NeuroImage. 2018;167:73–83. doi: 10.1016/j.neuroimage.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Malcolm P., Mark W.W., Anna C.N., Martin R.T. Magnetoencephalography. Practical Neurology. 2014;14:336. doi: 10.1136/practneurol-2013-000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P.K., Banerjee A., Tripathi M., Sharma A. A comprehensive review of magnetoencephalography (MEG) studies for brain functionality in healthy aging and Alzheimer's disease (AD) Frontiers in Computational Neuroscience. 2018;12:60. doi: 10.3389/fncom.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandke K., Meier J., Brookes M.J., O'Dea R.D., Van Mieghem P., Stam C.J., Hillebrand A., Tewarie P. Comparing multilayer brain networks between groups: Introducing graph metrics and recommendations. NeuroImage. 2018;166:371–384. doi: 10.1016/j.neuroimage.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Maslarova A., Zhao Y., Rösch J., Dörfler A., Coras R., Blümcke I., Lang J., Schmidt M., Hamer H.M., Reindl C., Welte T.M., Rampp S., Rössler K., Buchfelder M., Brandner S. Surgical planning, histopathology findings and postoperative outcome in MR-negative extra-temporal epilepsy using intracranial EEG, functional imaging, magnetoencephalography, neuronavigation and intraoperative MRI. Clinical Neurology and Neurosurgery. 2023;226 doi: 10.1016/j.clineuro.2023.107603. [DOI] [PubMed] [Google Scholar]
- Mauro A., Conti F., Dodge F., Schor R. Subthreshold behavior and phenomenological impedance of the squid giant axon. The Journal of General Physiology. 1970;55:497–523. doi: 10.1085/jgp.55.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégevand P., Seeck M. Electroencephalography, magnetoencephalography and source localization: their value in epilepsy. Current Opinion in Neurology. 2018;31:176–183. doi: 10.1097/WCO.0000000000000545. [DOI] [PubMed] [Google Scholar]
- Monge J., Gómez C., Poza J., Fernández A., Quintero J., Hornero R. MEG analysis of neural dynamics in attention-deficit/hyperactivity disorder with fuzzy entropy. Medical Engineering Physics in Medicine Biology. 2015;37:416–423. doi: 10.1016/j.medengphy.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Mößle M., Han S.-I., Myers W.R., Lee S.-K., Kelso N., Hatridge M., Pines A., Clarke J. SQUID-detected microtesla MRI in the presence of metal. Journal of Magnetic Resonance. 2006;179:146–151. doi: 10.1016/j.jmr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Frontiers in Human Neuroscience. 2013;7:138. doi: 10.3389/fnhum.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen I., Stam C., Citroen J., Reijneveld J., Hillebrand A. Preoperative evaluation using magnetoencephalography: experience in 382 epilepsy patients. Epilepsy Research. 2016;124:23–33. doi: 10.1016/j.eplepsyres.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Nissen I.A., Stam C.J., Reijneveld J.C., van Straaten I.E., Hendriks E.J., Baayen J.C., De Witt Hamer P.C., Idema S., Hillebrand A. Identifying the epileptogenic zone in interictal resting-state MEG source-space networks. Epilepsia. 2017;58:137–148. doi: 10.1111/epi.13622. [DOI] [PubMed] [Google Scholar]
- Nour M.M., Liu Y., Arumuham A., Kurth-Nelson Z., Dolan R.J. Impaired neural replay of inferred relationships in schizophrenia. Cell. 2021;184:4315–4328. doi: 10.1016/j.cell.2021.06.012. e4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D., Rantanen K., Ahveninen J., Ylikoski R., Häppölä O., Strandberg T., Pekkonen E. Source estimation of spontaneous MEG oscillations in mild cognitive impairment. Neuroscience Letters. 2006;405:57–61. doi: 10.1016/j.neulet.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Ott L.R., Penhale S.H., Taylor B.K., Lew B.J., Wang Y.-P., Calhoun V.D., Stephen J.M., Wilson T.W. Spontaneous cortical MEG activity undergoes unique age-and sex-related changes during the transition to adolescence. NeuroImage. 2021;244 doi: 10.1016/j.neuroimage.2021.118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M., Abbott D.F., Jackson G.D. Wearable OPM-MEG: A changing landscape for epilepsy. Epilepsia. 2022;63:2745–2753. doi: 10.1111/epi.17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Wang Z. Diagnosis of motor function injury based on near-infrared spectroscopy brain imaging (fNIRS) technology. Preventive Medicine. 2023;174 doi: 10.1016/j.ypmed.2023.107641. [DOI] [PubMed] [Google Scholar]
- Pettitt R.M., Brown E.A., Delashmitt J.C., Pizzo M.N., Pettitt R., Delashmitt J., Pizzo M. The Management of anxiety and depression in pediatrics. Cureus. 2022;14 doi: 10.7759/cureus.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piastra M.C., Nüßing A., Vorwerk J., Bornfleth H., Oostenveld R., Engwer C., Wolters C.H. The discontinuous Galerkin finite element method for solving the MEG and the combined MEG/EEG forward problem. Frontiers in Neuroscience. 2018;12:30. doi: 10.3389/fnins.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piastra M.C., Oostenveld R., Schoffelen J.M., Piai V. Estimating the influence of stroke lesions on MEG source reconstruction. NeuroImage. 2022;260 doi: 10.1016/j.neuroimage.2022.119422. [DOI] [PubMed] [Google Scholar]
- Picci G., Gotts S.J., Scherf K.S. A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Developmental Science. 2016;19:524–549. doi: 10.1111/desc.12467. [DOI] [PubMed] [Google Scholar]
- Pitsik E.N., Maximenko V.A., Kurkin S.A., Sergeev A.P., Stoyanov D., Paunova R., Kandilarova S., Simeonova D., Hramov A.E. The topology of fMRI-based networks defines the performance of a graph neural network for the classification of patients with major depressive disorder. Chaos, Solitons Fractals. 2023;167 [Google Scholar]
- Poo M.-M., Du J.-L., Ip N.Y., Xiong Z.-Q., Xu B., Tan T. China brain project: basic neuroscience, brain diseases, and brain-inspired computing. Neuron. 2016;92:591–596. doi: 10.1016/j.neuron.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Radüntz T., Scouten J., Hochmuth O., Meffert B. Automated EEG artifact elimination by applying machine learning algorithms to ICA-based features. Journal of Neural Engineering. 2017;14 doi: 10.1088/1741-2552/aa69d1. [DOI] [PubMed] [Google Scholar]
- Rampp S., Stefan H., Wu X., Kaltenhäuser M., Maess B., Schmitt F.C., Wolters C.H., Hamer H., Kasper B.S., Schwab S. Magnetoencephalography for epileptic focus localization in a series of 1000 cases. Brain. 2019;142:3059–3071. doi: 10.1093/brain/awz231. [DOI] [PubMed] [Google Scholar]
- S.D. Reddy, S. Goyal, T.K. Reddy, Riemannian Approach Based Depression classification using Transfer Learning for MEG signals, 2023 IEEE 4th Annual Flagship India Council International Subsections Conference (INDISCON), IEEE, 2023, pp. 1-4.
- Reuber M. Seizure: European Journal of Epilepsy Star Reviewers 2022, Seizure-European. Journal of Epilepsy. 2023;105:52–55. [Google Scholar]
- Roberts T.P., Kuschner E.S., Edgar J.C. Biomarkers for autism spectrum disorder: opportunities for magnetoencephalography. MEGJournal of Neurodevelopmental Disorders. 2021;13:1–9. doi: 10.1186/s11689-021-09385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-González V., Núñez P., Gómez C., Hoshi H., Shigihara Y., Hornero R., Poza J. Unveiling the alterations in the frequency-dependent connectivity structure of MEG signals in mild cognitive impairment and Alzheimer’s disease. Biomedical Signal Processing and Control. 2024;87 [Google Scholar]
- Rosenow F., Klein K.M., Hamer H.M. Non-invasive EEG evaluation in epilepsy diagnosis. Expert Review of Neurotherapeutics. 2015;15:425–444. doi: 10.1586/14737175.2015.1025382. [DOI] [PubMed] [Google Scholar]
- Roy S., Rathee D., Chowdhury A., McCreadie K., Prasad G. Assessing impact of channel selection on decoding of motor and cognitive imagery from MEG data. Journal of Neural Engineering. 2020;17 doi: 10.1088/1741-2552/abbd21. [DOI] [PubMed] [Google Scholar]
- Ru X., He K., Lyu B., Li D., Xu W., Gu W., Ma X., Liu J., Li C., Li T. Multimodal neuroimaging with optically pumped magnetometers: A simultaneous MEG-EEG-fNIRS acquisition system. Neuroimage. 2022;259 doi: 10.1016/j.neuroimage.2022.119420. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sadjadi S.M., Ebrahimzadeh E., Shams M., Seraji M., Soltanian-Zadeh H. Localization of epileptic foci based on simultaneous EEG–fMRI data. Frontiers in Neurology. 2021;12 doi: 10.3389/fneur.2021.645594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkalis V. Applied strategies towards EEG/MEG biomarker identification in clinical and cognitive research. Biomarkers in Medicine. 2011;5:93–105. doi: 10.2217/bmm.10.121. [DOI] [PubMed] [Google Scholar]
- Salmelin R., Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology. 1994;91:237–248. doi: 10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sanchez Bornot J.M., Wong-Lin K., Ahmad A.L., Prasad G. Robust EEG/MEG based functional connectivity with the envelope of the imaginary coherence: sensor space analysis. Brain Topography. 2018;31:895–916. doi: 10.1007/s10548-018-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez J., Halliday D.M. 2013 Asilomar Conference on Signals, Systems and Computers. 2013. Reducing the effect of correlated brain sources in MEG using a linearly constrained spatial filter based on Minimum Norm; pp. 1828–1832. [Google Scholar]
- Sanfratello L., Houck J.M., Calhoun V.D. Relationship between MEG global dynamic functional network connectivity measures and symptoms in schizophrenia. Schizophrenia Research. 2019;209:129–134. doi: 10.1016/j.schres.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.P. Magnetoencephalography: basic principles. Annals of Indian Academy of Neurology. 2014;17:S107. doi: 10.4103/0972-2327.128676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., Jones B., Manshanden I., Van Walsum A.v.C., Montez T., Verbunt J.P., de Munck J.C., van Dijk B.W., Berendse H.W., Scheltens P. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer's disease. Neuroimage. 2006;32:1335–1344. doi: 10.1016/j.neuroimage.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Sui S.X., Hendy A.M., Teo W.-P., Moran J.T., Nuzum N.D., Pasco J.A. A Review of the Measurement of the Neurology of Gait in Cognitive Dysfunction or Dementia. Focusing on the Application of fNIRS during Dual-Task Gait Assessment, Brain Sciences. 2022;12:968. doi: 10.3390/brainsci12080968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susan Philip B., Prasad G., Hemanth D.J. A systematic review on artifact removal and classification techniques for enhanced MEG-based BCI systems, Brain-Computer. Interfaces. 2023:1–15. [Google Scholar]
- Tait L., Özkan A., Szul M.J., Zhang J. A systematic evaluation of source reconstruction of resting MEG of the human brain with a new high-resolution atlas: Performance, precision, and parcellation. Human Brain Mapping. 2021;42:4685–4707. doi: 10.1002/hbm.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Miyakoshi M. Cross-correlation task-related component analysis (xTRCA) for enhancing evoked and induced responses of event-related potentials. NeuroImage. 2019;197:177–190. doi: 10.1016/j.neuroimage.2019.04.049. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Pang E.W. From Signals to Dynamic Cortical Networks; Magnetoencephalography: 2014. MEG and cognitive developmental studies; pp. 557–577. [Google Scholar]
- Van Den Heuvel M.P., Fornito A. Brain networks in schizophrenia. Neuropsychology Review. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- Van 't Ent D., Manshanden I., Ossenblok P., Velis D.N., de Munck J.C., Verbunt J.P.A., Lopes da Silva F.H. Spike cluster analysis in neocortical localization related epilepsy yields clinically significant equivalent source localization results in magnetoencephalogram (MEG) Clinical Neurophysiology. 2003;114:1948–1962. doi: 10.1016/s1388-2457(03)00156-1. [DOI] [PubMed] [Google Scholar]
- Vandewouw M.M., Dunkley B.T., Lerch J.P., Anagnostou E., Taylor M.J. Characterizing Inscapes and resting-state in MEG: Effects in typical and atypical development. Neuroimage. 2021;225 doi: 10.1016/j.neuroimage.2020.117524. [DOI] [PubMed] [Google Scholar]
- Vigario R., Oja E. BSS and ICA in neuroinformatics: from current practices to open challenges. IEEE Reviews in Biomedical Engineering. 2008;1:50–61. doi: 10.1109/RBME.2008.2008244. [DOI] [PubMed] [Google Scholar]
- Vivekananda U., Mellor S., Tierney T.M., Holmes N., Boto E., Leggett J., Roberts G., Hill R.M., Litvak V., Brookes M.J. Optically pumped magnetoencephalography in epilepsy. Annals of Clinical Translational Neurology. 2020;7:397–401. doi: 10.1002/acn3.50995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.D., Jung W., Lee G.-H., Efetov D.K., Wu B.-I., Huang K.-F., Ohki T.A., Taniguchi T., Watanabe K., Kim P. Josephson junction infrared single-photon detector. Science. 2021;372:409–412. doi: 10.1126/science.abf5539. [DOI] [PubMed] [Google Scholar]
- Wang C., Cao F., Wang W., Xu W., Li W., Gao Z., An N. Methods for Improving Movement Compatibility of Wearable OPM-MEG: A Review. IEEE Sensors Journal. 2023 [Google Scholar]
- Wang Q., Tian S., Tang H., Liu X., Yan R., Hua L., Shi J., Chen Y., Zhu R., Lu Q. Identification of major depressive disorder and prediction of treatment response using functional connectivity between the prefrontal cortices and subgenual anterior cingulate: a real-world study. Journal of Affective Disorders. 2019;252:365–372. doi: 10.1016/j.jad.2019.04.046. [DOI] [PubMed] [Google Scholar]
- Wang G., Wu N., Tao Y., Lee W.H., Cao Z., Yan X., Wang G. The Diagnosis of Major Depressive Disorder through Wearable fNIRS by Using Wavelet Transform and Parallel-CNN Feature Fusion, IEEE Transactions on Instrumentation. Measurement. 2023 [Google Scholar]
- Weret Z.S., Mukherjee R. Prevalence of relapse and associated factors in patient with schizophrenia at Amanuel Mental Specialized Hospital. Addis Ababa, Ethiopia: Institution Based Cross Sectional Study, International Journal of Interdisciplinary Multidisciplinary Studies. 2014;2:184–192. [Google Scholar]
- Witjes B., Baillet S., Roy M., Oostenveld R., Huygen F.J., de Vos C.C. Magnetoencephalography reveals increased slow-to-fast alpha power ratios in patients with chronic pain. Pain Reports. 2021;6 doi: 10.1097/PR9.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Shan W., Qi J., Wu J., Wang Q. Presurgical evaluation of epilepsy using resting-state MEG functional connectivity. Frontiers in Human Neuroscience. 2021;15 doi: 10.3389/fnhum.2021.649074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Yashchuk V.V., Donaldson M.H., Rochester S.M., Budker D., Pines A. Magnetic resonance imaging with an optical atomic magnetometer. Proceedings of the National Academy of Sciences. 2006;103:12668–12671. doi: 10.1073/pnas.0605396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Fukuma R., Seymour B., Hosomi K., Kishima H., Shimizu T., Yokoi H., Hirata M., Yoshimine T., Kamitani Y. Using a BCI prosthetic hand to control phantom limb pain. Brain-Computer Interface Research: A State-of-the-Art Summary. 2019;7:43–52. [Google Scholar]
- Ye Q., Yan D., Yao M., Lou W., Peng W. Hyperexcitability of cortical oscillations in patients with somatoform pain disorder: a resting-state EEG study. Neural Plasticity. 2019;2019 doi: 10.1155/2019/2687150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo P.E., John S.E., Farquharson S., Cleary J.O., Wong Y.T., Ng A., Mulcahy C.B., Grayden D.B., Ordidge R.J., Opie N.L.J.N. 7T-fMRI: Faster Temporal Resolution Yields Optimal BOLD Sensitivity for Functional Network Imaging Specifically at High Spatial Resolution. 2018;164:214–229. doi: 10.1016/j.neuroimage.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Zebhauser P.T., Hohn V.D., Ploner M. Resting state EEG and MEG as biomarkers of chronic pain: A systematic review. Pain. 2022 10.1097. [Google Scholar]
- Zhang J. Depth-invariant beamforming for functional connectivity with MEG data, Statistics Its. Interface. 2022;15:359–371. [Google Scholar]
- Zhang N., Pan Y., Chen Q., Zhai Q., Liu N., Huang Y., Sun T., Lin Y., He L., Hou Y. Application of EEG in migraine. Frontiers in Human Neuroscience. 2023;17:1082317. doi: 10.3389/fnhum.2023.1082317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Sun J., Sun Y., Niu K., Wang P., Wu C., Chen Q., Wang X. Pretreatment source location and functional connectivity network correlated with therapy response in childhood absence epilepsy: A magnetoencephalography study. Frontiers in Neurology. 2021;12 doi: 10.3389/fneur.2021.692126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yin E., Li F., Zhang Y., Tanaka T., Zhao Q., Cui Y., Xu P., Yao D., Guo D. Two-stage frequency recognition method based on correlated component analysis for SSVEP-based BCI. IEEE Transactions on Neural Systems. 2018;26:1314–1323. doi: 10.1109/TNSRE.2018.2848222. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxidative Medicine Cellular Longevity. 2013;2013 doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Liu J., Ye C., Mathiak K., Astikainen P., Ristaniemi T., Cong F. Discovering dynamic task-modulated functional networks with specific spectral modes using MEG. NeuroImage. 2020;218 doi: 10.1016/j.neuroimage.2020.116924. [DOI] [PubMed] [Google Scholar]
- Zotev V.S., Matlachov A.N., Volegov P.L., Sandin H.J., Espy M.A., Mosher J.C., Urbaitis A.V., Newman S.G., Kraus R.H. Multi-channel SQUID system for MEG and ultra-low-field MRI. IEEE Transactions on Applied Superconductivity. 2007;17:839–842. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.