Abstract
Disulfiram (DSF) is a second-line drug for the clinical treatment of alcoholism and has long been proven to be safe for use in clinical practice. In recent years, researchers have discovered the cancer-killing activity of DSF, which is highly dependent on the presence of metal ions, particularly copper ions. Additionally, free DSF is highly unstable and easily degraded within few minutes in blood circulation. Therefore, an ideal DSF formulation should facilitate the co-delivery of metal ions and safeguard the DSF throughout its biological journey before reaching the targeted site. Extensive research have proved that nanotechnology based formulations can effectively realize this goal by strategic encapsulation therapeutic agents within nanoparticle. To be more specific, this is accomplished through precise delivery, coordinated release of metal ions at the tumor site, thereby amplifying its cytotoxic potential. Beyond traditional co-loading techniques, innovative approaches such as DSF-metal complex and metal nanomaterials, have also demonstrated promising results at the animal model stage. This review aims to elucidate the anticancer mechanism associated with DSF and its reliance on metal ions, as well as to provide a comprehensive overview of recent advances in the arena of nanomedicine based co-delivery strategies for DSF and metal ion in the context of cancer therapy.
Keywords: Disulfiram, Metal ion, Nanomedicine, Co-delivery, cancer treatment
Graphical abstract
Highlights
-
•
Co-delivery of disulfiram and metal ions has potential in cancer treatment.
-
•
Traditional formulation strategies face challenges in co-delivering disulfiram and metal ions.
-
•
Nanomedicine-based drug delivery enabled efficient co-delivery of disulfiram and metal ions, improving therapeutic outcomes.
-
•
More studies are needed to explore the precise synchronous release of metal ions for disulfiram action at the tumor site.
1. Introduction
Cancer is a malignant tumor originating in epithelial tissue due to abnormal cell differentiation and uncontrolled proliferation. Although cancer cells have share similar structural and functional characteristics with normal cells, they are capable of unlimited cell divisions and exhibit new properties, which contribute to their generally reduced sensitivity to existing chemotherapeutic agents (Yin et al., 2021). The creation of new drugs is costly in terms of both time and money. Currently, there is no suitable therapeutic option that can adequately address this problem. In recent years, some researchers have proposed the strategy of repurposing old drugs for new therapeutic effect, which greatly shortens the drug research and development cycle and reduces the risk of failure(McMahon et al., 2020).
Disulfiram (DSF) is a FDA approved drug for alcohol dependence. It has been used in clinic for over 60 years, with the advantages of limited toxicity and controllable adverse effect(Li et al., 2020a). Recently, DSF has been shown to perform as a cytotoxic drug and to have anticancer activity. As reported, DSF can inhibit acetaldehyde dehydrogenase (ALDH) activity, promote reactive oxygen species (ROS) production, and downregulate the NF-κB and MAPK signaling pathways(Nie et al., 2022). Several studies have substantiated the therapeutic potential of DSF in various cancer types, including gastric, breast, liver, and pancreatic cancers(Lu et al., 2021a). However, the anti-cancer effect of DSF alone is suboptimal, and the precise mechanism remains to be fully elucidated.
Concurrently, as research on the interplay between metal ions and cancer progresses, numerous metal ions have been implicated in tumor growth and development. For example, copper ions are involved in angiogenesis within tumor tissue and regulate the level of ROS in tumor cells(Ekinci et al., 2019; Kamiya, 2022). Obviously, the concentration of metal ions could affect the tumor cells progression and induce tumor cell apoptosis. Studies have employed metal-based compounds, such as oxaliplatin, to regulate metal ion homeostasis; oxaliplatin is widely utilized as an anticancer drug in clinical settings (Xu et al., 2021a). Chen et al. reported that DSF‑copper complex treated MDA-MB-231 underwent proteasome inhibition and cancer cell shrinkage, rounding, and a large number of vacuoles in cytoplasm, indicative of cellular autophagy and apoptosis(Chen et al., 2006). Additionally, researchers found that metal ions, such as iron and calcium, can also induce apoptosis in cancer cells through specific pathways(Gleitze et al., 2021; Sukumaran et al., 2021), that may overlap with the cell used by DSF. Thus, combining DSF with metal ions presents a promising therapeutic strategy for cancer treatment and has become a research focus, attracting the attention of many researchers. Among these metal ions, copper ion has been reported as the optimal partner for DSF in anticancer application. It has been demonstrated that when copper ions are present, DSF can induce massive ROS generation(Allensworth et al., 2015), contributing to the occurrence of oxidative stress and the subsequent death of tumor cells. With the copper ion, DSF can also exert anticancer effects much more efficiently by targeting ALDH(Viola-Rhenals et al., 2018) and ALDH-positive cancer stem cells(Qian et al., 2018), producing cytotoxicity through ALDH accumulation, decreasing cancer stem cell stemness, and inhibiting ubiquitinated protein degradation. In addition, it has also been shown that the combination of DSF and copper ion can also inhibit cancer cells migration, which can reduce the rate of cancer recurrence. Clearly, DSF could be used as a promising anticancer agent, but its metal-dependent potency should not be underestimated.
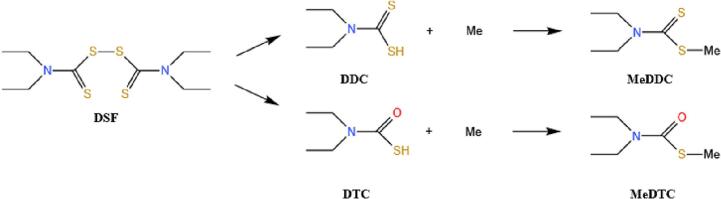
The DSF‑copper treatment is simple to apply at the cellular level; however, it can present challenges when administrated in vivo. In the absence of external copper supplementation, the efficacy of DFS‑copper combination is highly dependent on the intracellular copper ions level, and often cause uncontrollable outcomes due to individual differences in practical use. Consequently, co-delivery of DSF and metal ions holds considerable promise in cancer treatment, although many efforts have been made in regarding finding suitable carrier or formulations. DSF's physicochemical property also make it difficult to formulate(Farooq et al., 2019). As is known, DSF has low solubility and gastrointestinal stability, and it is rapidly metabolized in the body, so it often requires a very high dose when orally administrated and a simple injection formulation also could not solve its instability issue. Clinical trials have used DSF monotherapy(McMahon et al., 2020), where DSF is metabolized and converted into diethyldithiocarbamate (DDC) or diethylthiocarbamate (DTC), then chelates with metal ion and form a metal-DDC (DTC) complex to exert anti-cancer effect (Fig. 1).
Fig. 1.
Chemical structure of Disulfiram (DSF), S-methyl-N, N-diethylthiocarbamate (DDC), S-methyl-N, N-diethylthiocarbamate (DTC), and their related metal complexes. Metal is abbreviated as Me.
However, the amount of metal ions in the human body is limited and varies among individuals. Copper supplement has been used in clinic trials to provide an external source of copper ions and increase their concentration in the tumor microenvironment. However, the copper supplement has its limitations, as the increase in the copper ion level of tumor is redistricted, and large dose administration also raise the health concerns(Peng et al., 2020). The conventional combination of DSF and metal ion with separate formulation does not meet clinic need for cancer treatment. To address these issues, researchers have explored the potential of nanotechnology-based formulation carrier for the co-delivery DSF and metal ion. Nanomedicine could encapsulate DSF, providing protection during circulation and enchaining targeted delivery efficiency by minimizing undesired drug accumulation. For metal ion-co-delivery, scientists leverage nanotechnology through physical co-loading, polymer-chelation, and metal-based nanomaterials. While physical co-loading offers protective encapsulation for DSF, ensuring the desired concentration of metal ion loading is changeling, which can limit the tumor kill efficacy. The metal-DSF chelation strategy ensures synchronous loading and release, but the drugability of this complex requires further improvement. Metal-based nanomaterials have also been explored for DSF delivery, with the potential to release required metal ion under specific condition. However, the immature release of metal ions is an issue that needs further research. Here, we will discuss several common anticancer mechanism of DSF, including oxidative stress balance, related enzyme and protein activity, and the metal ion dependence of these mechanisms. Additionally, we will summarize the latest progress in nanomaterial strategies for DSF and metal ion co-delivery, including physical co loading of metal ion and DSF, DSF metabolite-metal chelation complex (CuET), and metal-based nanomaterials.
2. Metal ion dependent anti-cancer effect
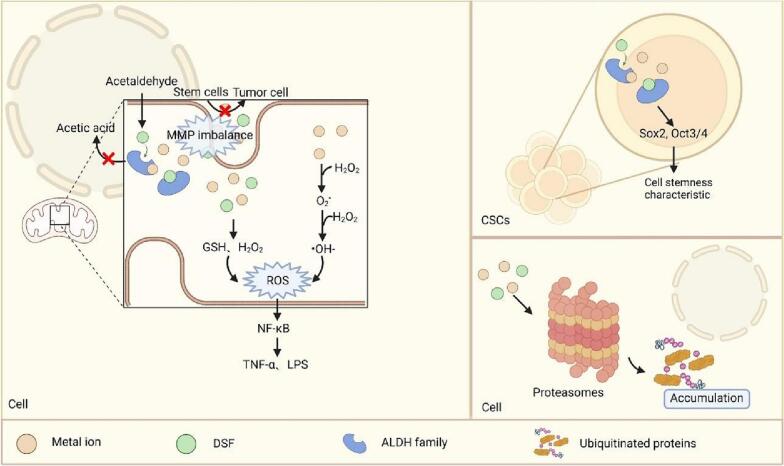
Numerous researchers have worked to find the underlying anti-cancer mechanisms of disulfiram and its dependence on metal ion. In this section, we will discuss the regulation of metal homeostasis within the tumor microenvironment and its impact on the drug action of disulfiram. Current research focuses on the cancer-killing mechanisms of disulfiram, as presented in Fig. 2. This includes its impact on ALDH, ROS levels, cancer stem cells, and the ubiquitin-proteasome system as potential therapeutic targets.
Fig. 2.
The anti-cancer mechanism of DSF in the presence of metal ions. DSF synergizes with metal ions in different ways to produce cytotoxic effects and reduce cancer progression.
2.1. Metal homeostasis and cancer development
Metal ions exert a profound influence on biological systems, and many can readily adopt various oxidation states through the loss or gain of electrons to undergo or mediate chemical reactions. The ability mentioned above plays a crucial role in various physiological processes, like the maintenance of cellular homeostasis, regulation of metabolic pathways, synthesis of substances, signaling mechanisms, and energy conversion(Chen et al., 2022b; Dow, 2017). By investigating the biological behavior of metal ions, researchers have discovered that an abnormal distribution of these ions can affect a variety of physiological functions within cells, thereby eliciting deleterious consequences or ever fatality. For instance, the introduction of copper ions into the drinking water of rats with chemically induced mammary tumors or pancreatic islet cell carcinomas resulted in an expedited growth rate of tumors in the experimental rat models(Skrajnowska et al., 2013). Meanwhile, many metal ions have been reported to induce cell death by perturbing osmolality, eliciting biocatalytic reactions, activating immune pathways, and instigating pro-oxidative effects. For example, in biology, iron plays a crucial role in the functionality of proteins containing iron‑sulfur clusters. Within mitochondria, certain iron‑sulfur cluster-containing proteins actively participate in the mitochondrial electron transport chain (ETC) to establish a proton gradient across the inner mitochondrial membrane (IMM), thereby facilitating oxidative phosphorylation reactions that are essential for adenosine triphosphate (ATP) production and energy storage. Moreover, iron is indispensable for the proper functioning of various other protein types involved in DNA replication, DNA repair, telomere maintenance, and ribosome assembly(Lal, 2020). Consequently, due to the pressing need for novel anticancer strategies, many researchers have directed their attention towards comprehending the pivotal role played by metal ions in maintaining tumor cell homeostasis (Chen et al., 2022b).
The impact of altered metal ion levels on oxidative stress, a key factor in the development and progression of various diseases including cancer, is widely recognized. ROS refers to a group of oxygen derived radicals and non-radicals that includes superoxide anions (), hydrogen peroxide (H2O2), hydroxyl radicals (•)(Srinivas et al., 2019). Elevated levels of ROS serve as indicators for the presence of oxidative stress.Previous studies have demonstrated that specific metals can directly or indirectly modulate ROS production and metabolic reactions, thereby perturbing the redox balance and inducing detrimental effects on human body. The production of is typically attributed to the NADPH oxidase in mitochondria, while its removal is achieved through a disproportionation reaction to maintain a stable level within the body. However, under conditions of metal ion overload, these ions can engage in a Fenton reaction with H2O2, resulting in the generation of . Subsequently, free radicals undergo a Haber-Weiss reaction, leading to the conversion of into highly active •. This cascade ultimately disrupts ROS homeostasis and prevents its return to steady-state levels. As we know, the excessive ROS levels are closely associated with cellular redox imbalance. These persistent free radicals exhibit high reactivity and readily engage in interaction with surrounding reactive components, such as DNA (via base double bonds), proteins (via sulfhydryl groups), and enzymes (via clusters), leading to their fragmentation or inactivation.Additionally, excessive ROS can activate the NF-κB signaling pathway, while active inducers of these pathways such as TNFα and LPS can reciprocally generate elevated levels of ROS(Qian et al., 2018). It has been shown that the NF-κB signaling pathway regulates multiple genes involved in cell transformation, proliferation, angiogenesis, and inflammatory response mediated by the nuclear transcription factor NF-κB. Moreover, due to its sensitivity to oxidative stress, the MAPK pathway can also be activated by ROS. The MAPK signaling pathway encompasses activation of nuclear transcription factors associated with DNA repair and apoptosis. Generally speaking, iron and copper ions directly participate in Fenton and Haber-Weiss reactions to facilitate ROS formation(Bahar et al., 2023). Although cadmium cannot directly engage in these reactions as a metal ion itself, it indirectly promotes tumor development through assisting in the production of ROS, RNS (reactive nitrogen species), and non-radical hydrogen peroxide (Wang et al., 2018).
Furthermore, the mitochondrial membrane potential is intricately linked to cellular differentiation, tumorigenicity, and malignancy of tumors. Notably, tumor cells generally exhibit elevated levels of mitochondrial membrane potential compared to normal cells. For instance, lung cancer cells, colon cancer cells, and melanoma cells demonstrate a minimum difference of 60 mV in this regard (Zhang et al., 2014). This phenomenon arises due to the propensity of high mitochondrial membrane potential-bearing cells for continued proliferation and increased tumorigenicity while low mitochondrial membrane potential-bearing cells tend to differentiate into other cell types, thereby diminishing their ability to proliferate into tumors. It has been observed that lower levels of ROS are associated with higher levels of mitochondrial membrane potential. In a recent study, an excess amount of copper was found to decrease the mitochondria's membrane potential and induce apoptosis through a cysteine-dependent pathway mediated by mitochondria(Yang et al., 2019a). Collectively, these studies indicate that metal ion concentrations can significantly influence the homeostasis of mitochondrial membrane potential via diverse pathways.
2.2. Acetaldehyde dehydrogenase (ALDH)
ALDH, a type of aldehyde dehydrogenase, catalyzes the oxidation of acetaldehyde to acetic acid. Upon administration into the body, DSF undergoes rapid conversion and metabolism to methyl diethyl dithiocarbamate (DTC), leading to the formation of diethylthiomethylcarbamate and other metabolites. These metabolites irreversibly inhibit the activity of all known cytoplasmic and mitochondrial ALDH isoforms by catalyzing Cys302 residue modification(Li et al., 2020a; Viola-Rhenals et al., 2018).Consequently, uncomfortable symptoms such as fever, flushing of the face or upper parts of the neck, chest and arms, scleral vasodilation, palpitations, nausea, and vomiting may occur due to acetaldehyde accumulation when alcohol is consumed (Lu et al., 2021b). This mechanism underlies DSF's efficacy in treating alcohol dependence since its approval as an oral medication for alcoholism treatment by FDA in 1951.
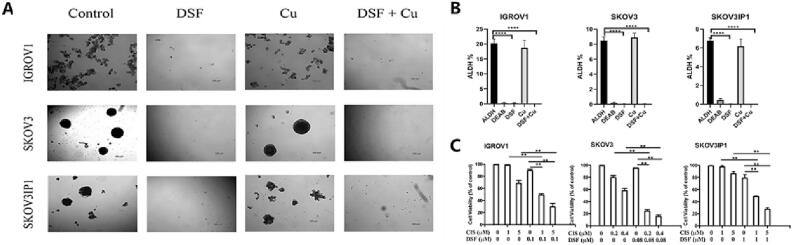
In recent years, research has revealed that the activity of ALDH and its family members, such as ALDH1A1, in certain tumor stem cells (CSCs) is generally higher than in normal tissues (Yang et al., 2018). Transcriptional activation of ALDH1 expression has been associated with drug resistance in tumors (Koppaka et al., 2012). CSCs are considered a subset of cancer cells. Similar to normal stem cells, these ALDH-positive cells possess the capacity for rapid proliferation and transformation into tumor cells. Even a small number of CSCs can rapidly initiate tumorigenesis (Lopez et al., 2019). Therefore, ALDH has been widely recognized as a biomarker for CSCs. Consequently, numerous researchers have utilized DSF either as a primary drug or in combination with other classic chemotherapeutics to selectively target and inhibit ALDH-positive CSCs (Qian et al., 2018). A previous study by Guo et al. (Fig. 3) demonstrated that the combination of DSF and cisplatin not only significantly reduced the survival rate of ovarian cancer stem cells from 21.7%, 8.4%, and 6.88% to approximately 0%, but also reversed the resistance of ALDH-positive cells to cisplatin while substantially decreasing overall cancer cell survival rates (Guo et al., 2019). Furthermore, this combination sensitized the cisplatin-resistant population resembling stem-like characteristics expressing high levels of ALDH to cisplatin treatment.
Fig. 3.
An example of co-administration of DSF and metal ions to reverse the resistance of ALDH positive cells to cisplatin. A) The ability of ovarian cancer cells to form spheroids when exposed to DSF (0.1 μM) and Cu2+ (0.1 μM). B) Analysis of ALDH activity in ovarian cancer cells exposed to DSF (10 μM) and Cu2+ (1 μM). Cells treated with diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor, were used as controls. C) Cell viability of ovarian cancer cell lines treated with different concentrations of DSF and cisplatin for 72 h. Reproduced from ref. (Guo et al., 2019) with permission from Elsevier, copyright 2019.
However, the efficacy of DSF alone did not meet expectations, possibly due to the disruption of sulfhydryl groups essential for DSF-induced cytotoxicity in the gastrointestinal or hepatic environment (Butcher et al., 2018). Evidence has demonstrated that combining DSF with copper ions can effectively target both ALDH1A1 and ALDH2, profoundly impeding B lymphocyte development, enhancing cancer cell sensitivity to chemotherapy, and addressing drug resistance issues(Li et al., 2020b). Furthermore, Song et al. (Song et al., 2016c) found that the combination of cisplatin and DSF reduced cell survival in the cisplatin-resistant lung cancer cell line A549DDP. This effect may be attributed to DSF's ability to inhibit CSC activity and stemness, reverse drug resistance, and sensitize ALDH-positive stem-like cells to cisplatin treatment. Although the precise mechanism underlying tumor stemness reduction by DSF/Cu co-treatment remains unvalidated, numerous clinical studies have confirmed its efficacy (Lu et al., 2021b; Lu et al., 2022).
Recently, Qian et al. discovered low levels of H2O2 and elevated levels of intracellular antioxidant enzymes, such as Cu/Zn-SOD-1 and manganese superoxide dismutase, in the CSC population of nasopharyngeal carcinoma(Qian et al., 2018). This finding suggests a potential involvement of metal ions in the aberrant activation of ROS in CSCs. Notably, ALDH enzymes have been identified as ROS scavengers(Guo et al., 2019). Consequently, incorporating metal ions into other chemotherapeutic agents has emerged as an effective strategy to mitigate drug resistance in tumor cells (Ekinci et al., 2019). In line with this notion, previous studies have demonstrated that combined treatment with DSF and copper ions can enhance intracellular ROS levels, thereby inducing apoptosis specifically in ALDH-positive stem cell-like cells (Kannappan et al., 2021). Simultaneously, co-administration of DSF and copper ions inhibits ALDH enzyme activity and consequently abolishes the protective effect mediated by ALDH against oxidative stress-induced damage (Guo et al., 2019).
2.3. ROS level and cancer resistance
Low levels of ROS promote cell proliferation and differentiation in normal cells, whereas high concentrations of ROS induce apoptosis. Therefore, elevated intracellular ROS levels are currently considered to be one of the mechanisms underlying the action of chemotherapeutic drugs associated with oxidative stress. During cancer treatment with chemotherapy drugs, cellular accumulation of ROS leads to oxidative stress. This disrupts the redox balance in cells and reduces their antioxidant capacity, resulting in damage to cell structure and triggering apoptosis (Li et al., 2020a). However, tumor cells can develop drug resistance by modulating intracellular ROS levels. In some cancer patients undergoing radiotherapy, a high ratio of oxidized/reduced glutathione (GSSG/GSH) is observed in tumor tissue, indicating persistent oxidation levels and suggesting poor local control rate for tumors under radiotherapy conditions.
Previous studies have demonstrated that DSF treatment leads to a reduction in the levels of GSH and H2O2 in tumor endothelial cells, indicating its potential as an inhibitor of tumor growth by suppressing antioxidant enzymes and elevating cellular ROS levels (Fong, and To, K. K. W, 2019). It is worth mentioning that DSF-induced ROS generation occurs through synergistic interactions with metal ions, followed by the reduction of oxidized metal ions by radical anion superoxide(Jomova and Valko, 2011). This crucial reaction facilitates metal regeneration, resulting in continuous production of free radicals and promoting sustained accumulation of ROS within the cell(Lu et al., 2022; Viola-Rhenals et al., 2018). Furthermore, copper has been identified as a vital factor influencing Cu/Zn-SOD enzyme activity, while also playing a significant role in maintaining mitochondrial membrane potential homeostasis. In serum-free medium, DSF fails to exert cytotoxicity without the addition of supplementary copper ions (Yang et al., 2019c).
As previously mentioned, copper plays a crucial role in maintaining mitochondrial membrane potential, ensuring normal mitochondrial oxidative phosphorylation reactions, and balancing cellular ROS content. DSF inhibits the NF-κB signaling pathway and reduces mitochondrial membrane potential, leading to the accumulation of ROS in cells and inducing cell apoptosis(Zhu et al., 2022). Importantly, as shown in Fig. 4, DSF/Cu also enhances ROS levels in tumor cells, continuously activates JNK and p38MAPK pathways, while simultaneously blocking NF-κB activation to promote tumor cell apoptosis or increase ubiquitination protein expression levels (Liu et al., 2022b). Similarly, copper also plays an indispensable role in this signaling pathway. Li et al. Reported that copper chaperone for superoxide dismutase could regulate ROS-mediated MAPK/ERK signaling pathways to promote cancer cell proliferation and metastases(Li et al., 2019).
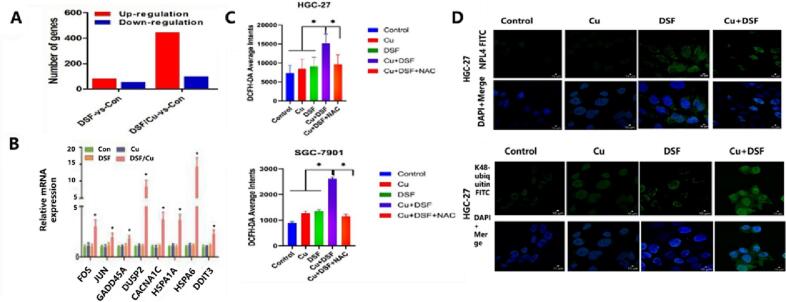
Fig. 4.
The co-administration of DSF and copper ions enhances ROS generation and cytotoxicity by activating MAPK signaling pathway. A) The effect of metal ions (copper ions) on gene expression levels in human undifferentiated gastric cancer (HGC-27) cells. B) The mRNA expression levels of eight MAPK related genes (FOS, JUN, GADD45A, CACANA1, DUSP2, HSPA1A, HSPA6, DDIT3) when DSF was co delivered with Cu2+ (0.2 μM). C) flow cytometric results of ROS generation in HGC-27 cells and human gastric adenocarcinoma (SGC-7901) cells after drug treatment. Drug dose, Cu2+ concentration: 0.2 μM, DSF concentration: 0.24 μM in HGC-27 cells, 0.30 μM in SGC7901 cells. N-acetylcysteine (NAC) was used as a ROS scavenger. D) Expression level of K48 ubiquitination protein in HGC-27 and SGC-7901 cells exposed to various concentrations of Cu2+ (0.2 μM) and DSF (0.24 μM in HGC-27 cells, 0.30 μM in SGC7901 cells) for 24 h. Reproduced from ref. (Liu et al., 2022b) with permission from Talor & Francis Group, copyright 2022.
It is well-established that hypoxia promotes the secretion of ROS and vascular endothelial growth factor (VEGF) by cells, thereby stimulating blood vessel growth and facilitating tumor angiogenesis and progression. Copper serves as a cofactor for pro-angiogenic molecules, promoting angiogenesis through various mechanisms such as oxygen and nutrient supply, upregulation of angiogenic mediators like VEGF, interleukin-1 (IL-1), and ROS release (Kannappan et al., 2021). DSF induces cell apoptosis, indirectly inhibits angiogenesis, and suppresses tumor cell growth. In a melanoma study using immunodeficient mice, it was observed that DSF effectively inhibits tumor angiogenesis in a zinc-dependent manner(Viola-Rhenals et al., 2018). Furthermore, co-administration of DSF/Cu has been shown to inhibit angiogenesis via the EGFR/SRC/VEGF pathway as well as the MMP-2 or MMP-9 pathway, leading to significant suppression of tumor growth(Li et al., 2015).
2.4. Cancer stem cells
Recently, researchers have initiated investigations into the dependence of cancer stem cells (CSC) and mesenchymal state cancer cells on iron ions (Rodriguez et al., 2022). Iron ions can permeate cells via pathways such as TFR1/TF to regulate cellular iron metabolism and maintain iron homeostasis, while pharmacological modulation of systemic iron levels can impede cell proliferation and metastatic dissemination. Previous studies have demonstrated that the transport of iron through the TFR1/TF pathway is augmented in CSC (Schonberg et al., 2015), and both total cellular iron content and labile iron pool are elevated in CD44high, a tumorigenic breast cancer cell model that recapitulates CSC characteristics, compared to normal epithelial cells (Mai et al., 2017). Excessive intracellular accumulation of iron may foster tumor development since malignant cells necessitate substantial amounts of this element for sustaining rapid proliferation upon transformation. Consequently, numerous investigators have shifted their focus towards exploring the anticancer potential of iron chelators, with DSF emerging as an ideal ligand capable of sequestering intracellular iron ions and reducing their concentration within cancerous cells. In addition to the aforementioned chelating effect, DSF also exhibits inhibitory effects on the expression of stemness transcription factors such as Sox2 and Oct3/4, leading to a reduction in the stemness characteristics of CSCs (Li et al., 2020a). Consequently, this dual action can impede cancer progression.
2.5. Ubiquitin-proteasome system
The ubiquitin-proteasome system (UPS) serves as the primary mechanism for endogenous protein degradation. As previously mentioned, an intact sulfhydryl group in DSF is essential for its cytotoxicity due to the high affinity of sulfhydryl groups towards thiol-containing proteins. Inactivation of sulfhydryl groups results in protein destruction. DSF acts as an inhibitor of both the 19S and 20S proteasomes(Hasinoff and Patel, 2017), hindering the degradation of ubiquitinated proteins by binding to the 26S proteasome. However, this inhibition differs from that exerted by true proteasome inhibitors which block the active site. DSF binds to other sites on the proteasome and exhibits action similar to certain non-competitive inhibitors (Yang et al., 2019c). Moreover, it has been demonstrated that cancer cells rely more heavily on UPS compared to normal cells (Damgaard, 2021). Nevertheless, DSF alone has limited inhibitory effects on the proteasome (Huang et al., 2016). This limitation arises from two factors: firstly, strong inhibitory effects of DSFs on tumor cells stem from their affinity towards proteins through sulfhydryl groups; secondly, their ability to chelate metals such as copper and zinc plays a crucial role in forming stable complexes.
Therefore, combining DSF with metal ions can achieve anticancer activity by inhibiting cancer proteasome activity. For instance, researchers have discovered that combining DSF with copper ions effectively inhibits protease activity in breast cancer cells without affecting normal cells. Dithiocarbamate pyrrolidine dithiocarbamate (an analog of DSF), when complexed with zinc and copper ions as a protease inhibitor, can induce tumor cell death by targeting caspase-3 and calpain, with IC50 values of 13.8 μM and 5.3 μM respectively(Viola-Rhenals et al., 2018).
Copper ions play a pivotal role in tumor development and progression, rendering them potential targets for therapeutic intervention. DSF exhibits potent anticancer activity in the presence of metal ions through synergistic mechanisms. Although the synergistic anticancer effect of DSF and metal ions has been extensively reported and demonstrated, effectively releasing these two components simultaneously to enable their concerted action remains a significant challenge.
3. Nanomedicine based strategies to co-deliver of DSF and metal ion
Numerous studies have demonstrated the inherent instability of DSF, characterized by a short half-life and limited water solubility. Moreover, DSF exhibits pH sensitivity and loses its efficacy within the gastrointestinal tract (Song et al., 2016a). One study revealed that DSF's solubility in acidic environments and blood circulation is merely 0.2 mg/mL, while its half-life in blood is as short as four minutes (Farooq et al., 2019). Consequently, when exposed to blood, DSF fails to attain adequate concentrations for stable and effective anti-cancer effects. In clinical practice, co-administration of DSF with metal ions typically involves sequential oral intake of metal supplements alongside DSF preparations. Metal supplements are clinically employed drugs used to address specific metal deficiencies and commonly exist as inorganic metal salts such as copper gluconate (McMahon et al., 2020) or copper chloride (Peng et al., 2020). Generally speaking, inorganic copper salts possess favorable water solubility at room temperature reaching up to 620 mg/mL for CuCl2 and 320 mg/mL for CuSO4 without exhibiting stability issues even when stored at low temperatures for several weeks (Wang et al., 2014). However, due to their heavy metal nature, excessive accumulation of these compounds in normal individuals can readily lead to heavy metal toxicity concerns.
Although the concentration of copper ions in the tumor microenvironment is slightly higher than that in general tissues (5–25 μM), it still fails to achieve a sufficient concentration difference (Lan et al., 2021; Zhao et al., 2022). Moreover, inorganic copper salts do not specifically target tumor cells (McMahon et al., 2020; Peng et al., 2020), which can significantly impact the therapeutic efficacy of DSF. Kalor et al. utilized parenteral copper ion injection (subcutaneous copper chloride-dithiramine) for Menkes disease treatment, but the injected copper ions were sequestered by the blood-brain barrier and did not exert a neurodegenerative effect (Kaler, 2014). Hoshina et al. conducted an intriguing study employing ^64Cu-labeled copper to visualize copper absorption and investigate the influence of DSF as a chelating agent on Cu absorption(Hoshina et al., 2018). In this study, DSF was administered prior to copper ion intake. The data revealed that compared to wild-type mice, DSF pretreatment significantly enhanced copper absorption in the upper small intestine at 6 h after administration and peaked at 6 and 8 h. Additionally, disulfiram pretreatment increased brain uptake of copper at 24 h in model mice while showing minimal uptake in wild-type mice throughout the entire 24-h period.
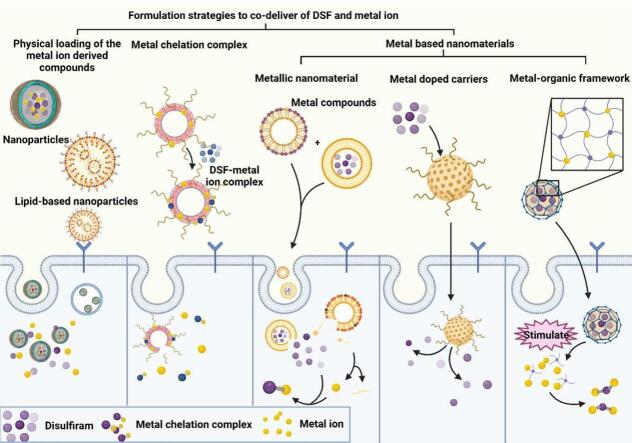
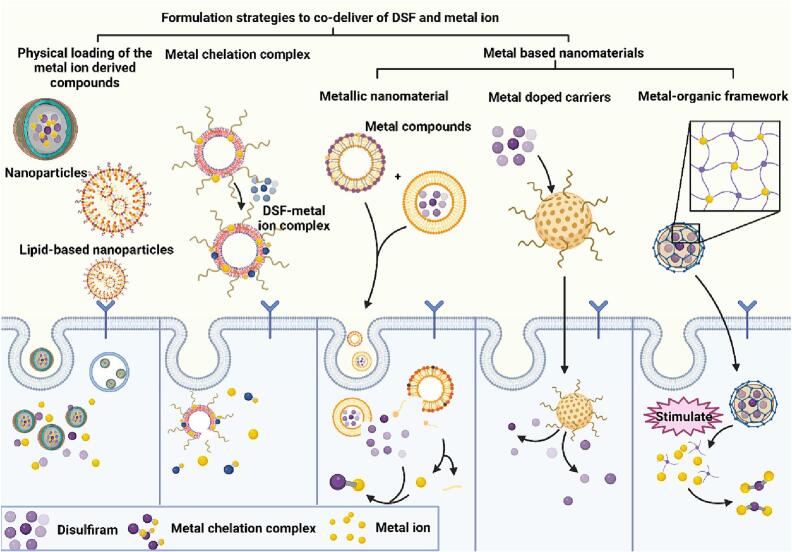
Ideally, the metal ion should be co-delivered with DSF in the same preparation and released simultaneously to create a metal ion-rich environment that maximizes the anti-cancer activity of DSF. In vitro cell studies can achieve nearly identical drug exposure by adding both drugs simultaneously to cultured cells. However, in vivo delivery requires innovative technologies to deliver DSF and metal ions together while targeting tumor sites for controlled release, thus preventing excessive accumulation of metal ions in the body. Nanotechnology-based nanomedicine offers a promising approach for co-loading these two components and achieving controllable release. Nanomedicine selectively accumulates at tumor sites, enhances drug stability through encapsulation, and reduces side effects. Moreover, nanoparticles enable loading and delivery of various therapeutic agents regardless of their physical properties (e.g., water solubility and molecular weight), thereby optimizing combination therapy efficacy. Furthermore, certain nanomaterials like gold nanoparticles not only serve as drug carriers but also function as imaging agents or directly exert anticancer activities themselves. Various strategies have been investigated for co-delivery of DSF and metal ions using nanomedicine-based approaches including physical co-loading of metal ion-derived compounds, formation of DSF-metal chelation complexes, and utilization of metal-based nanomaterials to provide sustained release of metal ions (Fig. 5). Additionally, we have compiled information on formulations characteristics of nanomedicine that combines DSF with metals from the past five years (Table 1).
Fig. 5.
Nanomedicine-based strategies for co-delivering disulfiram (DSF) and metal ions in cancer treatment. The physicochemical properties and delivery efficiency of drugs can be optimized by effectively incorporating DSF and metal ions into nanoparticles using various strategies. These strategies can be categorized into three groups based on different formulation methods, physical encapsulation of metal ion compounds, formation of metal chelates, and utilization of metal ion-based nanomaterials.
Table 1.
Formulation characteristics of example nanomedicine that combines DSF with metals from the past five years.
| Deliver strategy | Formulation design | Particle size (nm) | Zeta potential (mV) | Stability | Release characteristics | Major outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Physical co-loading | PEGylated calcium phosphate nanoparticles with Cu2+ in the internal calcium phosphate core, and DSF in the lipid layer. | 40 | – | Stable in neutral PBS buffer. | Show pH sensitivity. | 1) High drug loading rate and good tumor homing performance. 2) Degradation of CaP in tumor acidic microenvironment. |
(Li et al., 2022a) |
| A nanoparticles consist hollow mesoporous core for DSF loading and tannic acid shell for Cu2+ loading (by TA/Cu chelation). | 165.1 | −27.15 | The introduction of varying amounts of TA/Cu does not exert any influence on the dispersion of nanoparticles. |
|
Obtain high DSF drug loading rate (12.9%) and high release rate (>60%). | (Zhu et al., 2024) | |
| Mesoporous dopamine with tannic acid surfaced modification to achieve DSF loading and Cu2+ chelation. Then the surface was modified with tumor cell membrane camouflage. | <135 | – | The photothermal performance remains stable after five heating and cooling cycles. | The release of DSF is pH and photothermal dependent. | 1) Cell membrane coating enables immune escape and immunosuppression of nanoparticles. 2) Excellent photothermal conversion efficiency and photothermal stability. |
(Wang et al., 2024) | |
| A mesoporous polydopamine were co-loaded with DQ (a DSF prodrug), Cu2+, and GOx. Then the nanoparticle surface was further modified with folate receptors on nanoparticles. | 249.1 | – | The nanoparticles exhibit excellent dispersion in PBS, SBF, DMEM, and DMEM (FBS) solutions without significant aggregation after overnight. |
|
1) Loading of GOX can catalyze the reaction, leading to the production of H2O2 and facilitating the release of DTC. 2) The anticancer activity of Cu(DTC)2 is enhanced by its photothermal effect. |
(Hu et al., 2023) | |
| GSH-responsive nanoparticles with phospholipids and DSPE-PEG2000. DSF is encapsulated within a hydrophobic lipid layer for separate but simultaneous delivery. | ∼30 | – | Has good serum stability. | The release of DSF exhibits GSH responsiveness. | Achieves synchronous and separate delivery of DSF and Cu2+. | (Chen et al., 2022a) | |
| DSF metabolite-metal chelation complex | Metal organic nanoparticles are loaded with Cu(DDC)2 with the surface modified with BSA. | 63 | ∼ − 30 | The amino acid residues of BSA coordinate with transition metal ions, effectively adhering to the surface of Cu2+, forming a protective corona, thereby significantly improving the stability of the nanoparticles. | – |
|
(Chang et al., 2020) |
| Asymmetric bilayer solid nanoparticles with surface modified DSPE-PEG2000-SP94, loaded with Cu(DDC)2. | 188.2 | >9 | The particle size and PDI values remained unchanged during the 24-h serum co-treatment storage period. |
|
1) Directly encapsulating Cu(DDC)2 can prevent the outflow of copper ions. 2) The surface PEGlyation avoids the RES capture and improves the half-life and bioavailability. |
(Liu et al., 2022a) | |
| CuET self-assembles into nanoparticles. Surface modification with hyaluronic acid to achieve active targeting. | ∼125 | −23.2 | The size maintained unchanged during 48 days under both 4 °C and room temperature, indicating a super high stability. |
|
1) pH/GSH dual-responsive drug release was achieved through the triggering and cleavage of copper‑sulfur coordination bonds. 2) Even at low drug doses, a potent inhibitory effect on tumor growth is observed. |
(Peng et al., 2020) | |
| Phase-change materials (mainly lauric acid and stearic acid), DiR, and CuET were achieved by one-step self-assembly. | ∼123 | −20.8 | After three consecutive laser irradiations, the nanoparticles continue to demonstrate significant photothermal effects. | Drug release exhibits near-infrared response characteristics |
|
(Ren et al., 2020) | |
| Metal based nanomaterials | A copper doped silica frameworks loaded with DSF. Drug release is facilitated through near-infrared laser irradiation. | 195.38 | – | Maintain stable in DMEM containing 10% FBS. |
|
1) Obtain a high tumor inhibition rate (80.1%). 2) Exhibits an enhanced drug loading capacity (∼10%) due to its larger specific surface area, increased pore volume, and optimal pore size. |
(Zhou et al., 2023) |
| A copper doped zeolite imidazole framework 8 (ZIF-8) nanoparticle modified with PEG was prepared and loaded with DSF. | 208.3 | 12.13 | Exhibit good colloidal stability in various simulated physiological environments. | The drug release is pH dependent. | This nanomedicine show high sensitivity and specificity towards tumor microenvironment, offering a promising approach for low toxicity and effective anticancer therapy. | (Zhang et al., 2022a) | |
| Copper doped hollow zeolite imidazole ester skeleton nanoparticles were co-load with DSF and ICG. The surface of the nanoparticles was also modified with folic acid. | 505.03 ± 40.15 | 22.83 ± 0.6 | – | The drug release is pH dependent. | The Cu2+ doped hollow zeolite imidazole framework nanoparticle was prepared using a fast and low-cost method, serving as a carrier for DSF and ICG to achieve autonomous Cu2+ release. | (Zhao et al., 2022) | |
| Transferrin modified CuS porous nanoparticles for DSF loading. | 171.9 ± 1.9 | −6.0 ± 0.2 | – | DSF is released in a pH dependent sustained-release manner. | 1) Demonstrates excellent photothermal conversion performance. 2) The photothermal ability of the nano formulation remains unaffected by drug loading and surface modification. |
(Lan et al., 2021) | |
| Hollow mesoporous organosilica nanoparticles were loaded with DSF and further modifed with ultra small CuS nanoparticles with disulfide linker. CuS nanoparticles were utilized as Cu2+ donor. | 98.4 | – | – | The release of DSF and Cu2+ exhibits pH dependence. |
|
(Zhang et al., 2021) | |
| DSF loaded PEG functionalized hollow copper sulfide nanoparticle to achieve photothermal triggering of Cu2+ ions and in-situ generation of Cu(DTC)2 to enhance chemotherapy efficacy. | ∼263 | −1.67 ± 0.26 | The particle size remains stable within a 7-day period across various mediums. | The release of Cu2+ shows pH sensitivity. |
|
(Liu et al., 2021) |
3.1. Physical co-loading of metal ion and DSF
The idea of incorporating metal ion compounds into physical carriers originates from the in vivo instability of DSF for anticancer purposes and the inherent toxicity of metal ions themselves. Previous studies have demonstrated that transforming a drug into nanomedicine-based carriers can significantly enhance its stability and pharmacokinetics within the human body (Mohammad et al., 2018). Encapsulation of inorganic copper ions within carrier materials enables controlled release at specific regions, thereby increasing their local concentration in tumor tissues and facilitating binding and cellular uptake of DSF and metal ions at targeted sites(Farooq et al., 2019). The notion of preparing nanostructures composed of DSF with copper ions using delivery carriers has garnered considerable interest, leading to the development of various formulations such as self-assembled micelles, polymeric nanoparticles, and solid lipid nanoparticles. This formulation approach offers simplicity and flexibility in design, allowing customization not only regarding particle size to exploit the enhanced permeability and retention effect for tumor tissue entrapment, but also enabling modulation of surface properties to meet diverse in vivo requirements through different carrier materials. It is important to mention that most metal ions cannot be directly loaded into formulations; instead, they are often incorporated as salts. For example, copper ions are commonly formulated as chloride, sulfate or gluconate salts(Kelley et al., 2021; Li et al., 2020b; Zhang et al., 2022b). The choice of salt form may influence loading efficiency as well as drug efficacy when employing a metal ion co-loading strategy; thus it is crucial to specify both the type of metal ion and salt form.
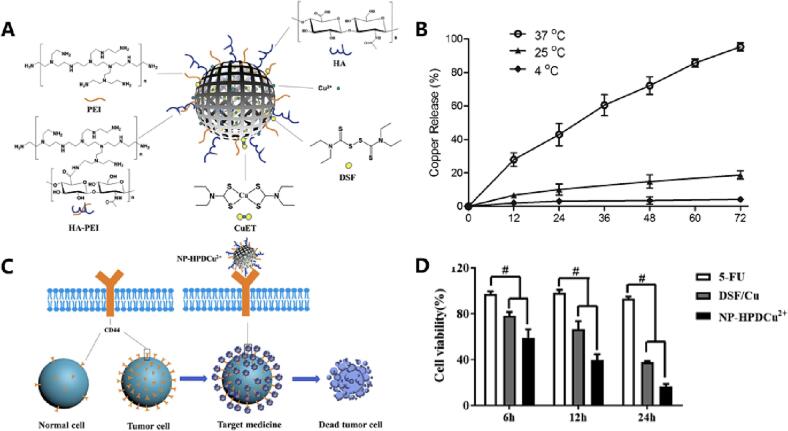
Polymeric nanoparticles are commonly used nanomedicine-based formulations for co-loading DSF and metal ions. Wang et al. developed PLGA-encapsulated DSF nanoparticles for therapeutic purposes(Wang et al., 2017). Following PLGA encapsulation, DSF-PLGA nanoparticles exhibited stable and sustained release over a period of seven days in a solution containing 0.5% Tween 80. Moreover, the half-life of DSF-PLGA in horse serum was up to seven hours - significantly higher than that observed for free DSF. This study demonstrated that polymeric nanoparticles enhance drug release characteristics and significantly extend drug stability upon contact with blood. In another study, Xu et al. utilized biologically well-characterized hyaluronic acid (HA) and polyethyleneimine (PEI) to generate nanostructures with a particle size of approximately 330 nm, enabling the co-loading of DSF and copper gluconate as depicted in Fig. 6(Xu et al., 2021b). Due to their size, these particles cannot directly enter cells through endocytosis; instead, they accumulate around the cells and target CD44 on the surface of esophageal squamous cell carcinoma cells (Eca109). This promotes Eca109 apoptosis while exhibiting no toxicity towards other normal organs. Other than HA, other biomaterials like PEG has also be used to enhance the hydrophilicity of the carrier material(Song et al., 2016b). This modification allows evasion of phagocytosis by the immune system, prevents direct contact between DSF and blood, prolongs in vivo circulation time while reducing toxicity associated with inorganic metal salts.
Fig. 6.
An example of polymer-modified nanoparticles for enhanced drug delivery efficiency. A) HA PEI nanoparticles (NP-HPD), loaded with DSF and copper ions, were synthesized by conjugating HA and PET through amide bonds. B) The release profiles of DSF and copper ions over a period of 72 h at temperatures of 4 °C, 25 °C, and 37 °C. C) The underlying mechanism involves the targeting of tumor sites via CD44 receptor binding facilitated by HA on the nanoparticles, leading to induction of tumor cell death. D) Cell viability.
Additionally, the nanoparticulate structure facilitates enhanced loading of DSF with metal ions, thereby improving the efficiency of targeted delivery. Wang et al. prepared PEGylated liposomes encapsulating DSF and CuCl2 through hydration extrusion(Wang et al., 2014). Owing to its exceptional water solubility, copper ions were uniformly distributed both inside and outside the liposomes, irrespective of the form or concentration of inorganic salts. Within a 72-h period at body temperature, copper ions exhibited nearly complete release in vivo; quantitative release curve analysis demonstrated a gradual release profile. This controlled release characteristic is one of the advantages offered by nanomedicine dosage forms.
Solid lipid nanoparticles (SLN), first proposed in 1991, are colloidal nanocarriers that range from 50 to 1000 nm and encapsulate drugs within lipid-like nuclei. These particles are composed of biodegradable lipids like lecithin and triacylglycerol, surfactants, and are free from organic solvents, avoiding residue issues. SLN's stability is enhanced by the solid lipid materials' stability at body temperature. Zhang et al. developed a pH-triggered TAT targeting PEG-lipid nanocarrier for DSF and copper co-encapsulation for cancer treatment that showed better cellular compared to DSF alone, indicating the enhanced stability and good targeting properties(Zhang et al., 2015). Manipulating the lipid type and solid/liquid ratio can further improve SLN stability by preventing drug leakage while increasing drug loading capacity, targeting ability, bioavailability and biocompatibility. Banerjee et al. prepared an optimized SLN system with D-α-tocopherol polyethylene glycol succinate modification using a modified emulsification sonication method for DSF loading(Banerjee et al., 2019). The authors found that adding too much TPGS surfactant could lead to excessive foam during preparation process which affects formation of SLNs. The particle size of SLN can be controlled through lyophilization, which limits the aggregation of nanoparticles in solid formulations (Safwat et al., 2017). Furthermore, inspired by the development of actively targeted agents, some studies have focused on the photothermal and pH-responsive properties of DSF nano-formulations. Wu et al. modified PVP/Cu-HMPB-encapsulated DSF and metal ion co-loading formulations to construct near-infrared light (NIR)-responsive nanodrugs(Wu et al., 2020). The PVP/Cu-HMPB formulation exhibited efficient photothermal conversion properties under NIR laser irradiation, thereby enhancing the therapeutic effect of this anticancer agent.
However, the incorporation of highly water-soluble inorganic copper ions into lipid carriers poses a potential risk of lipid carrier breakage, thereby compromising the integrity of drug encapsulation and hindering efficient delivery. To address this issue, the surface of nanoparticles can be coated with metal ions to mitigate drug leakage. Seo et al. proposed a method in their study where copper ions were incorporated into lipid membranes by coupling them with artificial lipids(Seo et al., 2008). Relevant experiments have demonstrated that the metal-containing liposomes prepared using this approach exhibit favorable pharmacokinetics and stability in both in vitro and in vivo settings. Therefore, embedding metal ions into the liposome membrane represents a promising choice. Another strategy involves transitioning from water-soluble salts to water-insoluble salts instead. Zhou et al. synthesized lipophilic copper salt, specifically copper oleate, as a source of copper supply and formulated it into the lipid bilayer of DSF-loaded liposomes (Zhou et al., 2018). Pharmacokinetic experiments revealed that copper oleate liposomes possess an extended circulation time and increased AUC compared to intravenous administration of inorganic copper ions, indicating that this strategy effectively prevents lipid membrane breakdown and enhances the circulation stability of liposomes. Furthermore, due to the metal coordination effect, these liposomes could exhibit different charge states at varying pH values. This property can be exploited for designing an electrostatic force-controlled “fixation effect” to enhance the stability of oleic acid molecules and facilitate the release of copper ions upon adjustment of pH value towards acidity.
However, the progress in co-loading both physical DSF and metal ions into a single nanocarrier appears to have stagnated. One significant obstacle is the limited drug loading efficiency (typically around 2%–5%) achieved through conventional preparation methods (Pan et al., 2019). This results in poor drug concentration within the formulation and potential metal toxicity due to premature release caused by strict regulation of copper systems in vivo. Therefore, achieving successful physical co-loading of metal ions and DSF with nanocarriers remains an ongoing challenge that requires further dedicated efforts.
3.2. DSF metabolite-metal chelation complex (CuET)
The concept of metal chelation is derived from metal chelation therapy (MCT), which was initially proposed over 50 years ago for the treatment of pathological disorders resulting from metal overload in the body. MCT employs a chelating agent (CA) as a scavenger, which binds to excessive metals at the lesion site and forms stable ligand complexes with target metal ions. This facilitates the elimination of bound chelated metals from the body (Šömen Joksić and Katz, 2015). In cancer treatment, tumor tissues exhibit slightly higher levels of copper compared to normal tissues, and diethyldithiocarbamate (DDC) or dithiocarbamate (or ester) (DTC) (Fig. 1) are rapidly metabolized from DSF upon entering the human body. These DSF metabolites, DDC and DTC, act as potent metal chelators that bind to various thiophilic metal ions(Peng et al., 2020). They also bind to excess copper ions at the tumor site, forming Cu(DDC)2 or Cu(DTC)2 metal chelates known as DSF metabolite-metal chelation complexes (CuET). Consequently, numerous studies have applied the concept of MCT in DSF-based tumor therapy by exploiting strong coordination between DSF's metabolic components in vivo and metal ions. Metal chelates offer an advantage by deviating from the conventional passive encapsulation approaches in nanomedicine. Moreover, the distinct spectral features of metal ion chelates facilitate visualization of the reaction between metal ions and ligands (Peng et al., 2020), leading to a significant increase in trapping efficiency (Wehbe et al., 2016).
However, Cu(DDC)2, a typical metal chelate derived from DSF, exhibits extremely low water solubility and limited solubility in organic solutions, rendering it unsuitable as a potential drug candidate. Conventional drug loading methods fail to achieve effective concentrations at the tumor site (Chang et al., 2020; Chen et al., 2018a), while general drug delivery approaches are inefficient and do not meet clinical requirements. Currently, two main delivery strategies are being explored for this treatment: direct administration of Cu(DDC)2 or Cu(DTC)2 encapsulated in formulations and co-delivery of DSF as a pre-drug along with copper ions (Pan et al., 2022). The preparation of CuET nanoparticles has been investigated (Chang et al., 2020), and compared to other nanoparticles, nanoscale coordination polymers possess unique properties such as high porosity and an oriented structure. Additionally, they can effectively encapsulate or absorb bioactive ligands for efficient drug delivery(Zhuang et al., 2014).
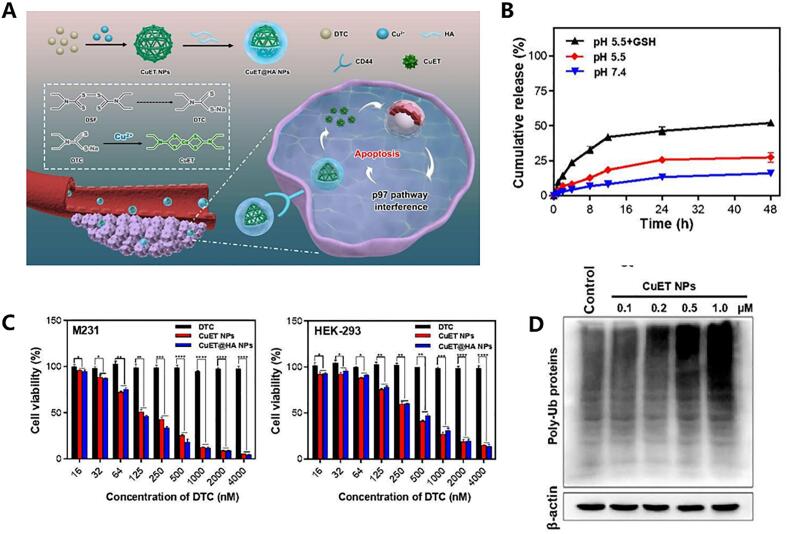
As mentioned, the formation of CuET exhibits low solubility and poses challenges in encapsulating it into nanoparticles. However, researchers have observed that CuET nanoparticles can be self-assembled through a strong coordination reaction between copper ions and DSF/DSF metabolites. Additionally, other biomaterials can be incorporated during the self-assembly process to confer additional functional properties onto the CuET nanoparticles. For instance, Peng et al. developed self-reactive HA-coated CuET nanoparticles (CuET@HA NPs) by leveraging the robust coordination reaction between Cu2+ and DTC (Peng et al., 2020) as shown in Fig. 7. The preparation process was spontaneous and resulted in well-dispersed particles in aqueous solution. Particle size tests conducted over 48 h and 48 days revealed no significant changes, indicating excellent stability of the self-assembled nanoparticles obtained through this method. Furthermore, release experiments demonstrated that within 48 h at different pH levels, <20% of the CuET@HA NPs were released, highlighting their slow-release characteristics conferred by metal-chelate formulation. Moreover, HA modification enhanced nanoparticle uptake by cancer cells while improving targeting capabilities of the formulation. A similar HA targeting PEI nanoparticle loaded with DSF and copper ions was reported and confirmed another group (Xu et al., 2021b).
Fig. 7.
An example of utilizing metal chelation complex for cancer treatment. A) Schematic graph of this design, including the self-assembly of CuET nanoparticles via strong coordination between DTC and Cu2+, as well as the mechanism by which these nanoparticles downregulate the p97-NPL4 pathway and promote cell apoptosis in vivo. B) In vitro drug release curves of CuET@HA NPs at pH 7.4, 5.5, and pH 5.5 with GSH addition. C) Cellular activity assays on two types of cancer cells using different concentrations of DTC, CuET NPs, and CuET@HA. D) Immunoblotting analysis of ubiquitinated proteins under different concentrations of CuET. Reproduced from ref. (Peng et al., 2020) with permission from ACS Publications, copyright 2020.
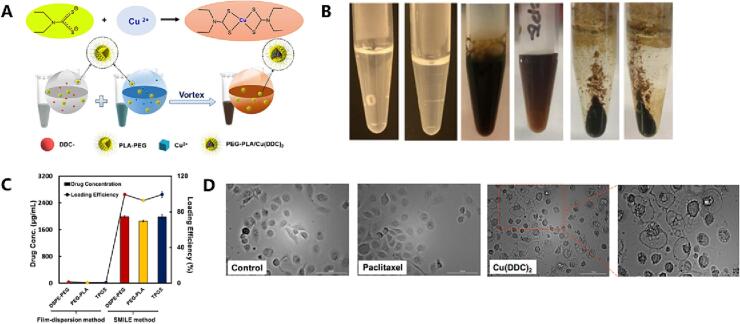
To address the instability of metal chelates when incorporated into nanoparticles, a novel technique called Stabilized Metal Ion Ligand Complex (SMILE) was develop (Chen et al., 2018b). Specifically, Cu(DDC)2 complex nanoparticles were prepared by combining DDC solution with copper chloride solution in the presence of appropriate stabilizers and adsorbing them onto the nanoparticle surface. This resulted in a drug loading capacity close to 100% for Cu(DDC)2 nanodrugs (Chen et al., 2018a; Wu et al., 2019). Importantly, this nanoparticle system induced cancer cell death through pyroptosis, as evidenced by extensive cytoplasmic vacuolation and no significant changes in caspase 3/7 activity under bright-field microscopy (Chen et al., 2018a)(Fig. 8). The SMILE method is characterized by its simple preparation process and high drug loading and release rates. Commonly used stabilizers in SMILE technology include Tween-20 and sodium dodecyl sulfate. It should be noted that particle size and nanoparticle stability are influenced by the choice of stabilizers, with suitable ones preventing uncontrolled aggregation.
Fig. 8.
An example of example of utilizing SMILE technology for the preparation of DSF metabolite-metal chelation complex (CuET) nanoparticles and their application in cancer treatment via the pyroptosis pathway. A) The preparation of CuET nanoparticles using SMILE technology. B) The impact of stabilizer selection on the formation of nanoparticle systems. C) Drug concentration and drug loading efficiency in nanoparticles prepared using SMILE technology. D) The morphology of analysis of DU145-TXR cells treated with CuET nanoparticles, paclitaxel, and blank PEG-PLA for 24 h. Reproduced from ref. (Chen et al., 2018a) with permission from ACS Publications, copyright 2018.
Prodrugs can also address the issue of poor stability associated with Cu(DDC)2 or Cu(DTC)2 and enhance drug bioavailability. The design of specific release prodrugs is based on the uptake of specific substances (Bakthavatsalam et al., 2018) or metabolic homeostasis in the tumor microenvironment (TME) (Pan et al., 2019; Zhou et al., 2018). For instance, solid tumors often exhibit elevated levels of ROS, leading to oxidative stress in cancer cells. Consequently, cancer cells upregulate their antioxidant system, such as glutathione, to adapt to this ROS-rich environment. In light of this, researchers utilized g-glutamic acid (gGlu) as a specific component for cancer cell identification and triggering DTC release by connecting gGlu and DTC through autolytic para-aminobenzyl (PAB) junctions within a prodrug called GGTDC (Bakthavatsalam et al., 2018). When the gGlu structure binds to tumor site-specific enzyme g-glutamyltransferase(GGT), GGTDC undergoes 1,6-benzyl elimination, thereby releasing the active component DTC. The liberated DTC then freely binds with nearby copper ions to generate Cu(DTC)2 chelates that promote Fenton reaction.
Furthermore, the combination of physicochemical techniques such as sound, light, and heat with DSF metal chelate preparations has demonstrated promising anti-cancer effects. Shi et al. utilized melanin dots (M-Dot) as carriers for Cu(DTC)2 to directly deliver it into tumor tissue(Shi et al., 2021). Flow cytometry results revealed that the cell necrosis rate achieved 51.0% when M-Dots-Cu(DTC)2 was combined with photothermal therapy. In vivo experiments demonstrated a remarkable tumor growth inhibition (TGI) value of 45.1% after administration of M-Dots-Cu(DTC)2, highlighting its excellent tumor inhibitory ability. Additionally, considering the poor solubility of Cu(DTC)2 itself, surface modification like HA modification on Sm-TCPP(Cu) nanoparticle has also been confirmed to water solubility and improve drug targeting and safety(Gao et al., 2020).
3.3. Metal based nanomaterials
3.3.1. Metallic nanomaterials
Metallic nanomaterials have been extensively investigated for their diverse antimicrobial properties, including the disruption of cell membranes, diffusion and degradation of internal cellular components (e.g., DNA, RNA, and enzymes) (Cheeseman et al., 2020). Additionally, they exhibit antimicrobial activity through the release of ions and leaching of metal ions (Zhong et al., 2022). Common metallic materials such as silver, gold, copper, zinc, and their corresponding oxides are synthesized into nanoparticles with various shapes and sizes (typically below 100 nm) s(Hoseinzadeh et al., 2017). One of our previous studies developed a glioma-targeted delivery system by designing a DSF/CuS nanocomplex with transferrin modification on the nanoparticle surface (Lan et al., 2021). DSF was loaded onto the nanoparticles via chelation reaction and released in an acidic tumor microenvironment. Western blot experiments demonstrated that introduction of these nanoparticles significantly increased apoptosis-associated protein caspase-3 levels and autophagy markers LC I/II in tumor cells. This suggests that the nanoparticles can induce apoptosis and promote autophagy in tumor cells.
Despite these advantages, metal-containing nanomaterials possess inherent drawbacks such as uncontrolled sustained release of metal ions (Zhong et al., 2022). Additionally, the diffusion properties and flexibility of nanomaterials, along with their interaction with cell membranes, may not favor a sustained and stable drug release. Consequently, metal nanomaterials are comparatively less effective than conventional counterparts. In this regard, next-generation nanomaterials can be activated by external stimuli like photocatalysis and photothermal technology (Chen et al., 2019), or magnetic response (Kumar and Mohammad, 2011) to generate ROS and localized temperature elevation in response to photothermal energy or magnetic fields for pathogen eradication. These advancements hold promise for further development of drug delivery systems.
3.3.2. Metal doped carriers
In addition to the method of metal ion injection into lipid membranes, researchers have recently developed environmentally and chemically inspired metal-doped porous materials (e.g., carbon, silicon, carbon-based nanomaterials) (Bibi et al., 2021; Sun et al., 2014). These materials possess non-toxicity, chemical stability, as well as photocatalytic properties and charge transfer capabilities. Moreover, the incorporation of abundant nanostructures in these metal-doped porous materials enables efficient loading of numerous signaling elements such as antibodies and enzymes while enhancing their electronic properties for generating detectable signals. This indirect detection approach leads to heightened sensitivity in immunoassays (Yang et al., 2019b). The inherent porosity of these materials provides a high surface area-to-volume ratio that facilitates increased drug delivery rates and improved controlled release efficiency (Parlak and Alver, 2017), allowing for free circulation in the bloodstream after intravenous administration while selectively accumulating within tumor tissues through either enhanced permeability and retention effect or carrier-modified components' affinity towards tumor cells (Wu et al., 2019).
The doping of metals enables targeted delivery of therapeutic ions and enhances the adsorption of encapsulated drugs to the carrier material (Ali et al., 2018). Zhao et al. developed a Cu2+-doped hollow zeolite imidazole backbone nanoparticle (D&I@HZIFCu-FA) that provides Cu2+ in a controlled manner, releases DSF at the tumor site, binds to Cu2+ as CuET, and triggers the Fenton reaction for ROS production (Zhao et al., 2022). Furthermore, Wu et al. fabricated hollow mesoporous silica nanoparticles doped with Cu2+ and loaded with DSF(Wu et al., 2019). The O-Cu2+ bond in the basic structure of copper-doped silicon nanoskeleton (Si-O(Cu2+)-Si) can break under acidic tumor microenvironment conditions, releasing Cu2+. Release experiments demonstrated over 85% release rate of Cu2+ within 48 h under weak acidic conditions simulating TME. This nanoparticle structure achieves targeted TME release while efficiently delivering DSF and Cu2+, thereby improving drug stability and bioavailability significantly. Currently, metal doping technology is widely used due to its rapid preparation and low cost advantages, offering a promising approach for simultaneous co-delivery of DSF and metal ions in cancer treatment.
3.3.3. Metal-organic framework
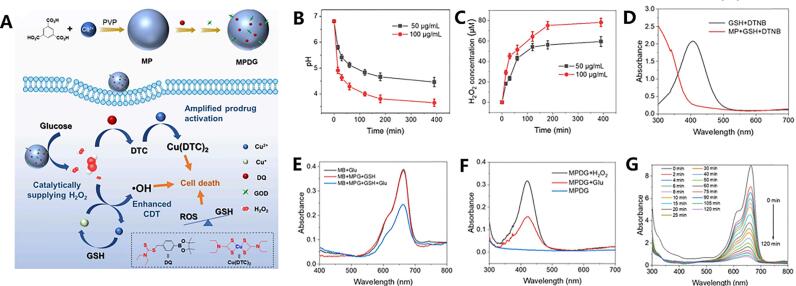
Metal organic frameworks (MOFs) are crystalline particles filled with molecular-sized pores formed by interconnected metals and organic linkers, exhibiting excellent biocompatibility(Bazzazzadeh et al., 2020). This material retains both the activity of the metal and the flexibility of the organic ligand, as well as the porous spatial structure formed by coordination between metal ions and organic ligands(Chen et al., 2020). Pan et al. employed a nanoscale Cu2+ metal-organic framework (MOF-199) as a nanocarrier and copper ion reservoir(Pan et al., 2022). Subsequently, they loaded the DSF prodrug DQ into this framework and modified it with glucose oxidase (GOD), enabling the MOF formulation to respond to high concentrations of glucose in tumors, thereby promoting the release of DQ along with Cu2+ ions and facilitating the formation of Cu(DTC)2 possessing anticancer activity (Fig. 9).
Fig. 9.
An example of the preparation of encapsulated DQ (a DSF prodrug) using nanoscale copper ion MOF to induce ROS generation through a Fenton-like reaction. A) The steps involved in preparing MOF nanoparticles and their mechanism of action, disrupting redox balance in tumor tissue for anti-cancer effects.B) The pH curve of the MPDG system (50 or 100 μg/mL) over time under high glucose concentration conditions. C) The concentration curve of H2O2 as the reaction progresses in the system. D) UV–Vis absorption spectra of GSH + DTNB and MP + GSH + DTNB. E) Time-dependent absorption profile of methyl bromide after treatment with methyl bromide and hydrogen peroxide. F) Degradation effect of Glu, MPG + GSH, and MPG + GSH + Glu treatments on MB demonstrated by UV–Vis absorption measurements. G) UV–Vis absorption spectra analysis of MPDG after 6-h treatment with hydrogen peroxide or glutamic acid. Reproduced from ref. (Pan et al., 2022) with permission from Elsevier, copyright 2022.
However, MOFs also possess certain limitations such as large gaps and modes of charge transfer between metals (LMCT), which pose challenges for reducing MOF development costs(Li et al., 2023). Chen et al. also developed a ROS-activated self-amplifying prodrug nano formulation comprising HA-coupled DTC prodrug (HA-DQ), modified photosensitizer Zn-TCPP, and Cu2+-coordinated MOF(Li et al., 2022b). This system can be specifically activated in tumor cells overexpressing ROS, leading to rapid release of DTC while remaining relatively stable in normal cells. The released DTC interacts with photosensitizer MOF capturing Cu2+, resulting in formation of active substance Cu(DTC)2. Simultaneously, MOF dissociates allowing recovery of photodynamic ability for Zn-TCPP which further promotes additional DTC release under near-infrared light at 630 nm wavelength forming a self-amplifying precursor/photosensitizer activated positive feedback circuit thus achieving cancer treatment.
Metals in metallic nanomaterials extend beyond copper ions. Zhao et al. developed a silk fibroin-modified DSF/ZnO nanocomposite to dissolve and stabilize DSF (Zhao et al., 2020). Similarly, acidic pH facilitates the release of zinc ions. Experimental evidence has demonstrated that DSF/ZnO nanoparticles can impede the migration of mouse cancer cells and enhance the production of anticancer agents. Furthermore, as previously indicated, metal ions not only promote effective utilization of DSF but also leverage their unique physicochemical properties to facilitate higher levels of tumor suppression, such as photothermal conversion effects and metal coordination properties exhibited by certain metal-based nanoparticles(Giliopoulos et al., 2020; Zhang et al., 2021). Therefore, researchers should further investigate the selection of metal ions and their interaction with organic or inorganic carriers.
4. Conclusion
DSF has been shown to possess a new and surprising therapeutic potential in cancer treatment, namely, metal ion-dependent cytotoxic effects. We review some anticancer mechanism of DSF and its correlation with metal ion. Among these, oxidative stress is an important cause of tumor development, and ROS can induce apoptosis in tumor cell. The reaction between metal-DSF complexes and local hydrogen peroxide cause excessive ROS generation (via Fenton and Haber-Weiss reaction), activation of MAPK signaling pathways, and affects on mitochondrial membrane potential, which promotes intracellular DNA damage. Previous studies have found that due to the high expression of ALDH in some CSCs, DSF inhibits the clearance of ROS in tumors by inhibiting ALDH activity. Therefore, targeting and killing these cells is also an important mechanism by which DSF inhibits tumor developments, and the presence of metal ions can help the agent to better target these cells and amplify the cytotoxic effects of DSF. The coordination of metal ion with DSF produces related compounds like Cu(DDC)2 or Cu(DTC)2, which also inhibit the development of tumor cells through regulation of the ALDH enzyme, ROS level, UPS and other pathways. Notably, the metals regenerated after Fenton and Haber-Weiss reaction can be reduced by the free radical anion superoxide to regenerate metal ions again, thereby sustaining the inhibitory effect on tumor growth.
However, the co-delivery of DSF and metal ion has long been regarded as a significant challenge, and the development of such system has not been straightforward. Conventional formulation method has a low drug loading capacity, and although inorganic copper salts has good aqueous solubility, their selectivity for tumor tissue is poor, and there is significant metal toxicity that cannot be ignored. Nanotechnology based nanomedicine has brought a change for this situation. In cancer treatment, nanomedicine has been widely used as drug carriers that help us target tumors more effectively with anti-cancer agents while leaving normal tissue alone. DSF could be encapsulated into various nanomedicine forms, such as micelles, nanoparticles, and liposomes. More importantly, nanocarrier could co-load different therapeutic agents, regardless of their physicochemical properties, making it possible to incorporate copper ions and DSF into the same nanostructure. Ideally, the DSF and metal ion could be released simultaneously and synergistically act upon cancer cells. A more common and feasible strategy is co-encapsulating DSF and soluble metal salt, such as copper gluconate, into the same nanocarriers. However, the premature release of drugs, especially metal salts, can make it difficult to ensure that sufficient metal ions are provided at the tumor site. Thus, achieving DSF delivery with proportionate metal ion remains challenging. To this end, scientists have proposed DSF-chelate complex to achieve proportional drug delivery, and more importantly, significantly reduce the transport loss of meal ion. One concern is the absence of direct interaction between DSF and metal, which could compromise the drug's action by affecting ROS generation. Although some scientists claim that DSF‑copper chelated complex exerted a higher cytotoxic effect as compared to DSF alone, there is no direct evidence that DSF-metal ion complex are superior than DSF in the presence of abundant metal ion. Further research could help to resolve this puzzle. Another promising strategy is utilizing metal nanoparticles to load DSF and provide metal ion in tumor microenvironment. Metal nanoparticle could play multiple roles in enhancing DSF's cancer killing activity by acting as a delivery carrier, releasing metal ion in a responsive manner, and even directly participating in cancer-suppression action. This method has significantly improved the bioavailability and drug loading efficiency of DSF, and demonstrated high targeted anticancer ability and excellent safety in in vitro and in vivo experiments. However, current data cannot provide precise information on metal ion level in vivo following DSF with metal nanoparticle, which is crucial for further research. Isotope labelling method could be used to confirm whether metal ion are locally generated and reach sufficient level for DSF activity. In the near future, we believe that the mechanism of cooperation between DSF and metals in anti-cancer will become more refined, and the co-delivery strategy for DSF and metal ions will become more sophisticated.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
This research was supported by the Zhejiang Provincial Natural Science Foundation (No.Y23H300012 and No. LZ23H040001), Zhejiang Medical and Health Science and Technology Program (No.2022KY881 and No.2023KY884), Wenzhou Municipal Science and Technology Bureau (No.Y20220908 and No. ZY2021019), and Excellent Young Scientist Training Program fund from Wenzhou Medical University.
CRediT authorship contribution statement
Xinyue Shen: Writing – original draft, Conceptualization. Huixiang Sheng: Writing – original draft, Methodology. Ying Zhang: Writing – review & editing, Validation. Xuan Dong: Writing – review & editing, Investigation. Longfa Kou: Writing – review & editing. Qing Yao: Writing – review & editing, Supervision. Xinyu Zhao: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank all of the colleagues and students who contributed to this study.
Contributor Information
Qing Yao, Email: yqpharm@163.com.
Xinyu Zhao, Email: zxy02pharm@163.com.
Data availability
Available from the corresponding author on reasonable request.
References
- Ali Shokuhi, Rad Sadegh, Mehdi, Society, A. J. C. a. p. t. o. j. o. t. K. P Potential of metal–fullerene hybrids as strong nanocarriers for cytosine and guanine nucleobases: A detailed DFT study. 2018;18:133–140. [Google Scholar]
- Allensworth J.L., et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol. 2015;9:1155–1168. doi: 10.1016/j.molonc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar M.E., Kim H.J., Kim D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct. Target. Ther. 2023;8:455. doi: 10.1038/s41392-023-01705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavatsalam S., Sleeper M.L., Dharani A., George D.J., Zhang T., Franz K.J. Leveraging gamma-Glutamyl Transferase to Direct Cytotoxicity of Copper Dithiocarbamates against Prostate Cancer Cells. Angew. Chem. Int. Ed. Eng. 2018;57:12780–12784. doi: 10.1002/anie.201807582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P., Geng T., Mahanty A., Li T., Zong L., Wang B. Integrating the drug, disulfiram into the vitamin E-TPGS-modified PEGylated nanostructured lipid carriers to synergize its repurposing for anti-cancer therapy of solid tumors. Int. J. Pharm. 2019;557:374–389. doi: 10.1016/j.ijpharm.2018.12.051. [DOI] [PubMed] [Google Scholar]
- Bazzazzadeh A., Dizaji B.F., Kianinejad N., Nouri A., Irani M. Fabrication of poly(acrylic acid) grafted-chitosan/polyurethane/magnetic MIL-53 metal organic framework composite core-shell nanofibers for co-delivery of temozolomide and paclitaxel against glioblastoma cancer cells. Int. J. Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119674. [DOI] [PubMed] [Google Scholar]
- Bibi S., Urrehman S., Khalid L., Yaseen M., Khan A.Q., Jia R. Metal doped fullerene complexes as promising drug delivery materials against COVID-19. Chem. Zvesti. 2021;75:6487–6497. doi: 10.1007/s11696-021-01815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K., et al. Investigation of the key chemical structures involved in the anticancer activity of disulfiram in A549 non-small cell lung cancer cell line. BMC Cancer. 2018;18:753. doi: 10.1186/s12885-018-4617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., et al. Biomimetic metal-organic nanoparticles prepared with a 3D-printed microfluidic device as a novel formulation for disulfiram-based therapy against breast cancer. Appl. Mater. Today. 2020;18 doi: 10.1016/j.apmt.2019.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman S., et al. Antimicrobial Metal Nanomaterials: from Passive to Stimuli-Activated applications. Adv. Sci. 2020;7 doi: 10.1002/advs.201902913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Cui Q.C., Yang H., Dou Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- Chen W., Yang W., Chen P., Huang Y., Li F. Disulfiram Copper Nanoparticles prepared with a Stabilized Metal Ion Ligand complex Method for Treating Drug-Resistant Prostate Cancers. ACS Appl. Mater. Interfaces. 2018;10:41118–41128. doi: 10.1021/acsami.8b14940. [DOI] [PubMed] [Google Scholar]
- Chen W., Yang W., Chen P., Huang Y., Li F. Disulfiram Copper Nanoparticles prepared with a Stabilized Metal Ion Ligand complex Method for Treating Drug-Resistant Prostate Cancers. ACS Appl. Mater. Interfaces. 2018;10:41118–41128. doi: 10.1021/acsami.8b14940. [DOI] [PubMed] [Google Scholar]
- Chen L., et al. Tumor-Targeted Drug and CpG delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple negative Breast Cancers. Adv. Mater. 2019;31 doi: 10.1002/adma.201904997. [DOI] [PubMed] [Google Scholar]
- Chen H., et al. Tumor-responsive copper-activated disulfiram for synergetic nanocatalytic tumor therapy. Nano Res. 2020;14:205–211. [Google Scholar]
- Chen J., et al. Reactive oxygen species-activated self-amplifying prodrug nanoagent for tumor-specific Cu-chelate chemotherapy and cascaded photodynamic therapy. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121513. [DOI] [PubMed] [Google Scholar]
- Chen L., Min J., Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Therap. 2022;7 doi: 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard R.B. The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021;28:423–426. doi: 10.1038/s41418-020-00703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow J.A. The essential roles of metal ions in insect homeostasis and physiology. Curr. Opin. Insect Sci. 2017;23:43–50. doi: 10.1016/j.cois.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Ekinci E., Rohondia S., Khan R., Dou Q.P. Repurposing Disulfiram as an Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Antican. Drug Discov. 2019;14:113–132. doi: 10.2174/1574892814666190514104035. [DOI] [PubMed] [Google Scholar]
- Farooq M.A., et al. Recent advances in the delivery of disulfiram: a critical analysis of promising approaches to improve its pharmacokinetic profile and anticancer efficacy. DARU J. Pharmaceut. Sci. 2019;27:853–862. doi: 10.1007/s40199-019-00308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W., To, K. K. W Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cell. Mol. Life Sci. 2019;76:3383–3406. doi: 10.1007/s00018-019-03134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., et al. A CD44-targeted Cu(ii) delivery 2D nanoplatform for sensitized disulfiram chemotherapy to triple-negative breast cancer. Nanoscale. 2020;12:8139–8146. doi: 10.1039/d0nr00434k. [DOI] [PubMed] [Google Scholar]
- Giliopoulos D., Zamboulis A., Giannakoudakis D., Bikiaris D., Triantafyllidis K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical applications. Molecules. 2020;25 doi: 10.3390/molecules25010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitze S., Paula-Lima A., Nunez M.T., Hidalgo C. The calcium-iron connection in ferroptosis-mediated neuronal death. Free Radic. Biol. Med. 2021;175:28–41. doi: 10.1016/j.freeradbiomed.2021.08.231. [DOI] [PubMed] [Google Scholar]
- Guo F., Yang Z., Kulbe H., Albers A.E., Sehouli J., Kaufmann A.M. Inhibitory effect on ovarian cancer ALDH+ stem-like cells by Disulfiram and copper treatment through ALDH and ROS modulation. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109371. [DOI] [PubMed] [Google Scholar]
- Hasinoff B.B., Patel D. Disulfiram is a slow-binding partial noncompetitive inhibitor of 20S proteasome activity. Arch. Biochem. Biophys. 2017;633:23–28. doi: 10.1016/j.abb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Hoseinzadeh E., et al. A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms. Curr. Drug Metab. 2017;18:120–128. doi: 10.2174/1389200217666161201111146. [DOI] [PubMed] [Google Scholar]
- Hoshina T., et al. Disulfiram enhanced delivery of orally administered copper into the central nervous system in Menkes disease mouse model. J. Inherit. Metab. Dis. 2018;41:1285–1291. doi: 10.1007/s10545-018-0239-3. [DOI] [PubMed] [Google Scholar]
- Hu X., et al. A cascade nanoplatform for the regulation of the tumor microenvironment and combined cancer therapy. Nanoscale. 2023;15:16314–16322. doi: 10.1039/d3nr03199c. [DOI] [PubMed] [Google Scholar]
- Huang J., et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neuro-Oncol. 2016;128:259–266. doi: 10.1007/s11060-016-2104-2. [DOI] [PubMed] [Google Scholar]
- Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kaler S.G. Neurodevelopment and brain growth in classic Menkes disease is influenced by age and symptomatology at initiation of copper treatment. J. Trace Elem. Med. Biol. 2014;28:427–430. doi: 10.1016/j.jtemb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T. Copper in the tumor microenvironment and tumor metastasis. J. Clin. Biochem. Nutr. 2022;71:22–28. doi: 10.3164/jcbn.22-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannappan V., et al. Recent advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.741316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K.C., et al. A phase 1 dose-escalation study of disulfiram and copper gluconate in patients with advanced solid tumors involving the liver using S-glutathionylation as a biomarker. BMC Cancer. 2021;21:510. doi: 10.1186/s12885-021-08242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppaka V., et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C.S., Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A. Iron in Health and Disease: an Update. Indian J. Pediatr. 2020;87:58–65. doi: 10.1007/s12098-019-03054-8. [DOI] [PubMed] [Google Scholar]
- Lan Q.H., et al. Disulfiram-loaded copper sulfide nanoparticles for potential anti-glioma therapy. Int. J. Pharm. 2021;607 doi: 10.1016/j.ijpharm.2021.120978. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett. 2015;369:86–96. doi: 10.1016/j.canlet.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. Copper Chaperone for Superoxide Dismutase Promotes Breast Cancer Cell Proliferation and Migration via ROS-Mediated MAPK/ERK Signaling. Front. Pharmacol. 2019;10:356. doi: 10.3389/fphar.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang J., Wu C., Wang L., Chen Z.-S., Cui W. The combination of disulfiram and copper for cancer treatment. Drug Discov. Today. 2020;25:1099–1108. doi: 10.1016/j.drudis.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Li H., Wang J., Wu C., Wang L., Chen Z.S., Cui W. The combination of disulfiram and copper for cancer treatment. Drug Discov. Today. 2020;25:1099–1108. doi: 10.1016/j.drudis.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Li Q., et al. Disulfiram loaded calcium phosphate nanoparticles for enhanced cancer immunotherapy. Biomaterials. 2022;291 doi: 10.1016/j.biomaterials.2022.121880. [DOI] [PubMed] [Google Scholar]
- Li X., et al. ROS-responsive hydrogel coating modified titanium promotes vascularization and osteointegration of bone defects by orchestrating immunomodulation. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121683. [DOI] [PubMed] [Google Scholar]
- Li R., et al. Metal-organic frameworks doped with metal ions for efficient sterilization: Enhanced photocatalytic activity and photothermal effect. Water Res. 2023;229 doi: 10.1016/j.watres.2022.119366. [DOI] [PubMed] [Google Scholar]
- Liu W., et al. Nanomedicine Enables Drug-Potency Activation with Tumor Sensitivity and Hyperthermia Synergy in the Second Near-infrared Biowindow. ACS Nano. 2021;15:6457–6470. doi: 10.1021/acsnano.0c08848. [DOI] [PubMed] [Google Scholar]
- Liu H., et al. The treatment of hepatocellular carcinoma with SP94 modified asymmetrical bilayer lipid-encapsulated Cu(DDC)2 nanoparticles facilitating Cu accumulation in the tumor. Expert Opin. Drug Deliv. 2022;20:145–158. doi: 10.1080/17425247.2023.2155631. [DOI] [PubMed] [Google Scholar]
- Liu Y., et al. Disulfiram/copper induces antitumor activity against gastric cancer via the ROS/MAPK and NPL4 pathways. Bioengineered. 2022;13:6579–6589. doi: 10.1080/21655979.2022.2038434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Ramchandani D., Vahdat L. Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic. 2019. 12. Copper Depletion as a Therapeutic Strategy in Cancer; pp. 303–330. [DOI] [PubMed] [Google Scholar]
- Lu C., Li X., Ren Y., Zhang X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother. Pharmacol. 2021;87:159–172. doi: 10.1007/s00280-020-04216-8. [DOI] [PubMed] [Google Scholar]
- Lu C., Li X., Ren Y., Zhang X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother. Pharmacol. 2021;87:159–172. doi: 10.1007/s00280-020-04216-8. [DOI] [PubMed] [Google Scholar]
- Lu Y., et al. Leveraging disulfiram to treat cancer: Mechanisms of action, delivery strategies, and treatment regimens. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2021.121335. [DOI] [PubMed] [Google Scholar]
- Mai T.T., et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017;9:1025–1033. doi: 10.1038/nchem.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A., Chen W., Li F. Old wine in new bottles: Advanced drug delivery systems for disulfiram-based cancer therapy. J. Control. Release. 2020;319:352–359. doi: 10.1016/j.jconrel.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Mohammad I.S., He W., Yin L. A Smart Paclitaxel-Disulfiram Nanococrystals for Efficient MDR Reversal and Enhanced Apoptosis. Pharm. Res. 2018;35:77. doi: 10.1007/s11095-018-2370-0. [DOI] [PubMed] [Google Scholar]
- Nie D., Chen C., Li Y., Zeng C. Disulfiram, an aldehyde dehydrogenase inhibitor, works as a potent drug against sepsis and cancer via NETosis, pyroptosis, apoptosis, ferroptosis, and cuproptosis. Blood Sci. 2022;4:152–154. doi: 10.1097/BS9.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., et al. A dithiocarbamate-based H(2)O(2)-responsive prodrug for combinational chemotherapy and oxidative stress amplification therapy. Chem. Commun. (Camb.) 2019;55:13896–13899. doi: 10.1039/c9cc05438c. [DOI] [PubMed] [Google Scholar]
- Pan Q., et al. Two birds with one stone: copper metal-organic framework as a carrier of disulfiram prodrug for cancer therapy. Int. J. Pharm. 2022;612 doi: 10.1016/j.ijpharm.2021.121351. [DOI] [PubMed] [Google Scholar]
- Parlak C., Alver Ö. A density functional theory investigation on amantadine drug interaction with pristine and B, Al, Si, Ga, Ge doped C60 fullerenes. Chem. Phys. Lett. 2017;678:85–90. [Google Scholar]
- Peng Y., Liu P., Meng Y., Hu S., Ding J., Zhou W. Nanoscale Copper(II)–Diethyldithiocarbamate Coordination Polymer as a Drug Self-delivery System for Highly Robust and specific Cancer Therapy. Mol. Pharm. 2020;17:2864–2873. doi: 10.1021/acs.molpharmaceut.0c00284. [DOI] [PubMed] [Google Scholar]
- Qian X., et al. Reactive oxygen species in cancer stem cells of head and neck squamous cancer. Semin. Cancer Biol. 2018;53:248–257. doi: 10.1016/j.semcancer.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Ren L., et al. Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting. Theranostics. 2020;10:6384–6398. doi: 10.7150/thno.45558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Schreiber S.L., Conrad M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell. 2022;82:728–740. doi: 10.1016/j.molcel.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat S., Ishak R.A.H., Hathout R.M., Mortada N.D. Nanostructured lipid carriers loaded with simvastatin: effect of PEG/glycerides on characterization, stability, cellular uptake efficiency and in vitro cytotoxicity. Drug Dev. Ind. Pharm. 2017;43:1112–1125. doi: 10.1080/03639045.2017.1293681. [DOI] [PubMed] [Google Scholar]
- Schonberg D.L., et al. Preferential Iron trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell. 2015;28:441–455. doi: 10.1016/j.ccell.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.W., Zhang H., Kukis D.L., Meares C.F., Ferrara K.W. A novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjug. Chem. 2008;19:2577–2584. doi: 10.1021/bc8002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Suo Y., Zhang Z., Liu R., Liu H., Cheng Z. Copper(II)-disulfiram loaded melanin-dots for cancer theranostics. Nanomedicine. 2021;32 doi: 10.1016/j.nano.2020.102340. [DOI] [PubMed] [Google Scholar]
- Skrajnowska D., Bobrowska-Korczak B., Tokarz A., Bialek S., Jezierska E., Makowska J. Copper and resveratrol attenuates serum catalase, glutathione peroxidase, and element values in rats with DMBA-induced mammary carcinogenesis. Biol. Trace Elem. Res. 2013;156:271–278. doi: 10.1007/s12011-013-9854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šömen Joksić A., Katz S.A. Chelation therapy for treatment of systemic intoxication with uranium: a review. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2015;50:1479–1488. doi: 10.1080/10934529.2015.1071154. [DOI] [PubMed] [Google Scholar]
- Song W., et al. Stable loading and delivery of disulfiram with mPEG-PLGA/PCL mixed nanoparticles for tumor therapy. Nanomedicine. 2016;12:377–386. doi: 10.1016/j.nano.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Song W., et al. Stable loading and delivery of disulfiram with mPEG-PLGA/PCL mixed nanoparticles for tumor therapy. Nanomedicine. 2016;12:377–386. doi: 10.1016/j.nano.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Song W., et al. Combining disulfiram and poly(l-glutamic acid)-cisplatin conjugates for combating cisplatin resistance. J. Control. Release. 2016;231:94–102. doi: 10.1016/j.jconrel.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Srinivas U.S., Tan B.W.Q., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P., et al. Calcium Signaling Regulates Autophagy and Apoptosis. Cells. 2021;10 doi: 10.3390/cells10082125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., et al. Ultrafast carrier trapping of a metal-doped titanium dioxide semiconductor revealed by femtosecond transient absorption spectroscopy. ACS Appl. Mater. Interfaces. 2014;6:10022–10027. doi: 10.1021/am5026159. [DOI] [PubMed] [Google Scholar]
- Viola-Rhenals M., Patel K.R., Jaimes-Santamaria L., Wu G., Liu J., Dou Q.P. Recent advances in Antabuse (Disulfiram): the Importance of its Metal-binding Ability to its Anticancer activity. Curr. Med. Chem. 2018;25:506–524. doi: 10.2174/0929867324666171023161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Evaluating the anticancer properties of liposomal copper in a nude xenograft mouse model of human prostate cancer: formulation, in vitro, in vivo, histology and tissue distribution studies. Pharm. Res. 2014;31:3106–3119. doi: 10.1007/s11095-014-1403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., et al. Poly lactic-co-glycolic acid controlled delivery of disulfiram to target liver cancer stem-like cells. Nanomedicine. 2017;13:641–657. doi: 10.1016/j.nano.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol. Appl. Pharmacol. 2018;353:23–30. doi: 10.1016/j.taap.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Orchestrating Precision within the Tumor Microenvironment by Biomimetic Nanoprodrugs for Effective Tumor Therapy. ACS Appl. Mater. Interfaces. 2024;16:8484–8498. doi: 10.1021/acsami.3c18239. [DOI] [PubMed] [Google Scholar]
- Wehbe M., Chernov L., Chen K., Bally M.B. PRCosomes: pretty reactive complexes formed in liposomes. J. Drug Target. 2016;24:787–796. doi: 10.1080/1061186X.2016.1186169. [DOI] [PubMed] [Google Scholar]
- Wu W., et al. Enhanced Tumor-specific Disulfiram Chemotherapy by in Situ Cu2+ Chelation-Initiated Nontoxicity-to-Toxicity transition. J. Am. Chem. Soc. 2019;141:11531–11539. doi: 10.1021/jacs.9b03503. [DOI] [PubMed] [Google Scholar]
- Wu W., Yu L., Pu Y., Yao H., Chen Y., Shi J. Copper-Enriched Prussian Blue Nanomedicine for in Situ Disulfiram Toxification and Photothermal Antitumor Amplification. Adv. Mater. 2020;32 doi: 10.1002/adma.202000542. [DOI] [PubMed] [Google Scholar]
- Xu J., et al. Metal-Coordinated Supramolecular Self-Assemblies for Cancer Theranostics. Adv. Sci. (Weinh) 2021;8 doi: 10.1002/advs.202101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Zhang K., Liang J., Gao F., Li J., Guan F. Hyaluronic acid/polyethyleneimine nanoparticles loaded with copper ion and disulfiram for esophageal cancer. Carbohydr. Polym. 2021;261 doi: 10.1016/j.carbpol.2021.117846. [DOI] [PubMed] [Google Scholar]
- Yang X., Yao R., Wang H. Update of ALDH as a potential Biomarker and Therapeutic Target for AML. Biomed. Res. Int. 2018;2018:1–5. doi: 10.1155/2018/9192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. in Vitro. 2019;54:310–316. doi: 10.1016/j.tiv.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Yang H., Xu W., Zhou Y. Signal amplification in immunoassays by using noble metal nanoparticles: a review. Microchim. Acta. 2019;186 doi: 10.1007/s00604-019-3904-9. [DOI] [PubMed] [Google Scholar]
- Yang Q., et al. An Updated Review of Disulfiram: Molecular Targets and strategies for Cancer Treatment. Curr. Pharm. Des. 2019;25:3248–3256. doi: 10.2174/1381612825666190816233755. [DOI] [PubMed] [Google Scholar]
- Yin W., Wang J., Jiang L., James Kang Y. Cancer and stem cells. Exp. Biol. Med. 2021;246:1791–1801. doi: 10.1177/15353702211005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.-B., Wang D.-G., Guo F.-F., Xuan C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer. 2014;14:19–23. doi: 10.1007/s10689-014-9757-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., et al. A Copper-Mediated Disulfiram-Loaded pH-triggered PEG-Shedding TAT Peptide-Modified Lipid Nanocapsules for use in Tumor Therapy. ACS Appl. Mater. Interfaces. 2015;7:25147–25161. doi: 10.1021/acsami.5b06488. [DOI] [PubMed] [Google Scholar]
- Zhang H., Song F., Dong C., Yu L., Chang C., Chen Y. Co-delivery of nanoparticle and molecular drug by hollow mesoporous organosilica for tumor-activated and photothermal-augmented chemotherapy of breast cancer. J. Nanobiotechnol. 2021;19:290. doi: 10.1186/s12951-021-01025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., et al. Disulfiram-loaded metal organic framework for precision cancer treatment via ultrasensitive tumor microenvironment-responsive copper chelation and radical generation. J. Colloid Interface Sci. 2022;615:517–526. doi: 10.1016/j.jcis.2022.01.187. [DOI] [PubMed] [Google Scholar]
- Zhang T., et al. Prospective clinical trial of disulfiram plus copper in men with metastatic castration-resistant prostate cancer. Prostate. 2022;82:858–866. doi: 10.1002/pros.24329. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-Z., et al. Silk Fibroin-Modified Disulfiram/Zinc Oxide Nanocomposites for pH Triggered Release of Zn2+ and Synergistic Antitumor Efficacy. Mol. Pharm. 2020;17:3857–3869. doi: 10.1021/acs.molpharmaceut.0c00604. [DOI] [PubMed] [Google Scholar]
- Zhao L., et al. Buffet-style Cu(II) for enhance disulfiram-based cancer therapy. J. Colloid Interface Sci. 2022;624:734–746. doi: 10.1016/j.jcis.2022.06.009. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Zheng X.T., Zhao S., Su X., Loh X.J. Stimuli-Activable Metal-Bearing Nanomaterials and Precise On-demand Antibacterial strategies. ACS Nano. 2022;16:19840–19872. doi: 10.1021/acsnano.2c08262. [DOI] [PubMed] [Google Scholar]
- Zhou L., et al. Membrane Loaded Copper Oleate PEGylated Liposome combined with Disulfiram for improving Synergistic Antitumor effect in Vivo. Pharm. Res. 2018;35 doi: 10.1007/s11095-018-2414-5. [DOI] [PubMed] [Google Scholar]
- Zhou J., et al. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202213922. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Lei C., Jiang Q., Yu Q., Qiu L. DSF/Cu induces antitumor effect against diffuse large B-cell lymphoma through suppressing NF-kappaB/BCL6 pathways. Cancer Cell Int. 2022;22:236. doi: 10.1186/s12935-022-02661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., et al. In situ generation of copper(II)/diethyldithiocarbamate complex through tannic acid/copper(II) network coated hollow mesoporous silica for enhanced cancer chemodynamic therapy. J. Colloid Interface Sci. 2024;660:637–646. doi: 10.1016/j.jcis.2024.01.121. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Kuo C.H., Chou L.Y., Liu D.Y., Weerapana E., Tsung C.K. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8:2812–2819. doi: 10.1021/nn406590q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available from the corresponding author on reasonable request.