Abstract
Traditional optical waveguides or mediums are often silica-based materials, but their applications in biomedicine and healthcare are limited due to the poor biocompatibility and unsuitable mechanical properties. In term of the applications in human body, a biocompatible hydrogel system with excellent optical transparency and mechanical flexibility could be beneficial. In this review, we explore the different designs of hydrogel-based optical waveguides derived from natural and synthetic sources. We highlighted key developments such as light emitting contact lenses, implantable optical fibres, biosensing systems, luminating and fluorescent materials. Finally, we expand further on the challenges and perspectives for hydrogel waveguides to achieve clinical applications.
Keywords: Optical fibres, Transparent hydrogels, Flexibility, Biocompatibility, Photodynamic therapy
Graphical abstract
Highlights
-
•
Design requirements, optimization, and properties of implantable waveguides are summarized.
-
•
Precursor polymers for hydrogel fabrication and design are discussed.
-
•
Current applications of transparent light delivery hydrogels are explored.
-
•
Challenges and outlook of hydrogel-based waveguides in biomedical applications are discussed.
1. Introduction
Optical techniques have long been established in the diagnosis and treatment of patients. A simple torch light to the tiniest of scopes is vastly utilized to evaluate the human body. However, the ability of light is heavily reliant on its waveguiding medium. The development of an ideal waveguide or light responsive platforms for biomedical applications requires extensive considerations and challenges to overcome. The observation of light propagation in different mediums can be defined with an optical parameter called Refractive Index (RI) and it is a fundamental step to determine in the development of light guiding techniques [1].
Traditionally, the optical waveguides or mediums are silica-based materials (glass) that possess excellent optical transparency with very minimal propagation loss. These silica optical fibres (SOF) can only be a temporary insertion into the body for treatment or diagnostics as it is limited by other aspects such as compatibility, brittleness, and rigidity [2]. A typical endoscope generally consists of a bundle of optical fibres, and it is the simplest but effective form of visualization in the body. In the ablation of kidney stones, SOF are a common medium for laser delivery in the body [3]. However, its mechanical fragility increases the chances of injury in a dynamic body environment and the hardness of such material conflicts with the soft tissue environment which may lead to rejection and damage [4]. Undoubtedly, biocompatibility and surface morphology of the implantable system plays a significant role in its suitability. Polymeric Optic Fibres (POF) are advantageous in this aspect due to its functionalization abilities with surface chemistry techniques, whereas in SOF, coatings are able to play significant role in improving biocompatibility [5].
Subsequently, polymeric-based materials have been developed to address these shortcomings by achieving similar optical transparency with better biocompatibility and mechanical properties. An example would be polymethyl methacrylate (PMMA), which is widely used to fabricate these POFs for connectivity and telecommunications, but also in medicine as health monitoring and treatment. Unlike the traditional SOF waveguides, POFs are able to expand into other applications in medicine due to its low cost and inert properties. However, POFs still exhibit extremely high Young's Modulus (1 MPa–10 GPa) as compared to soft human tissues that are only in the 10 kPa range [6,7]. The mechanical properties of these waveguides are critical when it comes implantation in the human body. The need for implantation brings about additional considerations to the material such as biocompatibility, adverse interactions, biodegradation, as well as mechanical compliance [8,9].
In recent years, soft biocompatible optical guides to be worn or implanted in the body have become an interest in research. Hydrogels are considered a promising candidate for biomedical waveguides mediums to achieve the requirements of biocompatibility, elasticity, and optical transparency. Hydrogels are a class of polymeric materials with three-dimensional crosslinked network, that has the ability to uptake aqueous solutions and can be functionalized to respond to pH, temperature, UV light, or other stimuli [10]. It's tunability can potentially generate hydrogels with mechanical properties similar to body tissues, favourable for implantation and remain sufficiently transparent for light guiding applications [11]. In comparison to hydrogels, the Young's Modulus, E of SOF and POF is much larger and deviates further from human tissues (Fig. 1a). Looking into hydrogels specifically the Ashby plot of Fig. 1b as well as Table 1 demonstrates certain overlaps of characteristics between different hydrogel-based materials and the human tissue. In the rapidly growing field of photomedicine, hydrogels are an ideal alternative material to incorporate biocompatibility and biodegradability to the system.
Fig. 1.
a) An overview of the optical materials across the mechanical properties and refractive index, b) An Ashby Plot of the different hydrogels [9,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]].
Table 1.
| Human Tissues | Refractive Index, n | Hydrogels | Refractive Index, n |
|---|---|---|---|
| Epidermis | 1.37–1.56 | PEG-based | 1.33–1.47 |
| Skin | 1.32–1.52 | PAM-based | 1.46–1.50 |
| Adipose Tissues | 1.44–1.46 | Alginate-based | 1.34–1.54 |
| Muscles | 1.37–1.39 | Silk-based | 1.50–1.70 |
| Neck | 1.40 | Chitosan-based | 1.40–1.50 |
| Myocardium | 1.51 | Cellulose | 1.48 |
| Stomach | 1.45 | ||
| Liver | 1.51 | ||
| Intestine | 1.33 | ||
| Blood | 1.42 | ||
| Malignant tumour | 1.43 | ||
While the interest of this remains relatively niche, it has gained more attention of late. Yang's group dives deep into a wider perspective of biopolymers for optical techniques [25]. The review covers living biomaterials to a combination of natural and synthetic polymers for applications such as optical waveguides and arrays. Albeit brief, Zhao's group discussed the various systems of hydrogels and its key components for its ability to be tuned for the desired outcome [26]. Yun's group narrows down into optical technologies for medical applications, exploring nature's own waveguides, to different types of polymeric wave guides, not limited to hydrogels [9]. Recently, Demirci's and Kim's group had published a comprehensive review on hydrogel-based biomedical photonics [27,28]. del Campo expanded into light delivery and responsive strategies for photoactivation, and highlights the challenges and promising outlook [29]. While there are existing summaries in their respective field, there are no comprehensive summary of hydrogels as waveguides or light manipulating materials in biomedical applications. In the perspective of material development, we summarize the recent work on hydrogel waveguides and light manipulation systems in biomedical applications. The review was structured to highlight firstly, the requirements of an ideal hydrogel waveguide, the challenges of light delivery in the human body, and then the appeal of hydrogels as a promising medium. Subsequently, we discuss the different reported approaches of light guiding, categorized as natural-, synthetic-, or hybrid-based hydrogels. We summarized the work through the perspective of light guiding in the human body such as light transmittance, biocompatibility, and mechanical friendliness. Moreover, we discussed the applications of hydrogels and their competitive edge in biomedical fields such as the future of contact lenses, in vivo light delivery for therapy and diagnostics, and biosensing. The niche area of light-guided hydrogel plays an important role in paving the pathway for unconventional but necessary materials in biomedicine.
2. Light propagation in hydrogels
To achieve good light propagation, light needs travel through the medium with minimal loss of energy (high optical transparency) and in between two mediums of a similar RI. The RI of a material indicates the bending and speed of light when travelling across, thus giving an idea on the loss of energy if the mediums are drastically different. Snell's law can be used to describe the relation of the light travel between two mediums expressed as follows,
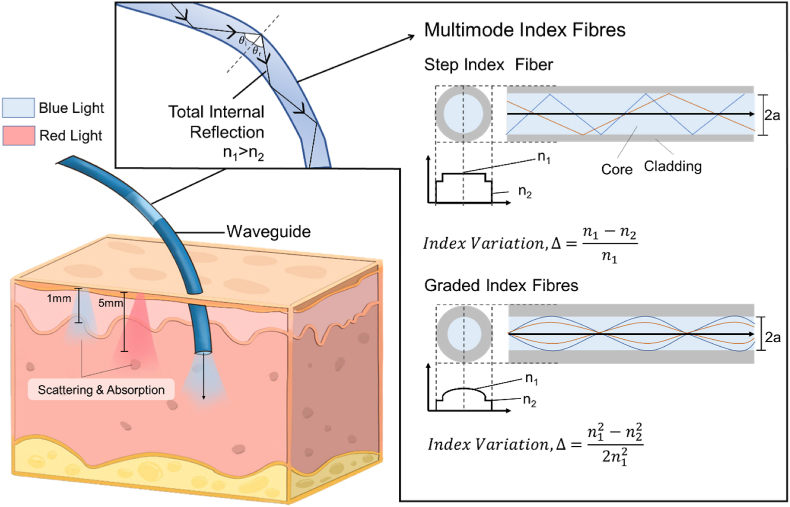
whereby, n1 and n2 are the refractive indices of the medium and θ1 is the incident angle and θ2 is the refracted angle [30]. This concept is suitable for light bending and delivery through transparent mediums, applicable for ocular vitreous tamponades, contact lenses, and wound dressings. On another hand, for waveguides to achieve excellent efficiency, matching the RI of the waveguide materials is not ideal, but instead, manipulating light such that Total Internal Reflection (TIR) occurs. This is a fundamental concept of waveguiding to trap and facilitate light delivery. For TIR to happen within the body, the RI of a single material system must be higher than the surrounding tissues (1.38–1.41) [31]. Whereas, for a multi-material system, the core must exhibit higher RI than the cladding (Fig. 2). The conventional design of a waveguide involves a single direction guide, typically cylindrical and is guided by the principles of step-index or graded-index (Fig. 2), Single mode fibres are generally step-index and multimode can be both step-index and graded-index fibres [32,33]. For TIR to occur, the incident angle must be larger than the critical angle and light must travel from a higher RI medium to a lower RI medium. With incident angles less than the critical angle between the mediums, light will be split into refraction and reflection, resulting in less effective reflection. The critical angle, θc, can be determined from the aforementioned Snell's law,
Fig. 2.
An overview of the light propagation in human tissues without waveguides, that leads to scattering and absorption of light. The longer wavelength of red light (625–750 nm) is able to penetrate deeper into the skin as compared to shorter wavelengths of blue light (450–485 nm). The use of a waveguide enables direct and greater depth access in the delivery of light through the concept of total internal reflection with two conventional designs, step index fibre and graded index fibre.
To confine the light propagation, the RI and incident angle θ1 needs to be carefully designed to achieve the ideal guided mode, as opposed to the radiation and substrate radiation mode [33].
Biomedical waveguides require not only good light guiding properties, but also flexibility and biocompatibility if implantable. The dynamic nature of the human body and the softness of the internal tissues makes traditional silica-based waveguides difficult to be implanted or utilized for long. The difference in stiffness of the internal tissues and the waveguides can lead to inflammation and incompatibility.
The inherent nature of human tissues is an unfavourable environment for light delivery as optical signals are often interfered and influenced by tissue scattering and absorption [34]. This is a major obstacle of light delivery in the body in biomedical applications. The heterogeneous properties of living tissues contribute to the scattering of light which can be indicated with the scattering coefficient, μs (cm−1), defined the probability of single scattering in the cross section, ρs and the density of the particles present in the volume, σs:
This is the probability of scatter to happen during a unit length of photon travel, describing the light distribution profile [35]. In simulation models of light scattering studies, Mie scattering is a common theory to apply as it indicates the scattering of particles larger than the wavelength of light as oppose to Rayleigh [36]. However, both Mie and Rayleigh theories are vastly applied in biomedical optics to evaluate the scattering properties in the body. Human tissues consist of both strong and weak scattering, whereby the in blood dominant regions such as the skin, brain, and vascular systems exhibit absorption and multiple scattering model. Whereas, for the cornea and lens, the scattering model can be described as low-step scattering [34,37]. Aside from scattering, human tissues also absorb light, denoted by the absorption coefficient, μa, and is particularly prominent in the circulatory system. In an assumed homogenous medium of particles, the is defined by the probability of the absorption in the cross section, ρa and the density of the particles present in the volume, σa:
The coefficient indicates the probability of photon absorption in the medium per unit photon travel [36]. The scattering coefficient of tissues decreases as the incident wavelength increases, whereas the absorption coefficient varies across the different tissues and light spectrum [38]. The presences of chromophores (haemoglobin and melanin) contribute heavily to the absorption of light internally. Therefore, the path of light delivery in the human body is a significant challenge, and perhaps we can seek guidance from other organisms.
Over the years of evolution, some organisms have adapted to maximize the capture of light to generate chemical energy, to scatter light for camouflage, and to emit light for defence or signalling. For example, Diphylleia grayi flowers will appear white when dry and transparent when wet due to the loose structural arrangement of cells. The flower petals appear transparent as they achieve the same refractive index between (water-cytolymph), allowing unobstructed light to pass through to maximize energy acquisition at the leaves or signalling pollination [39]. This reversible transparency kindled various strategies in material developments for optical applications [[40], [41], [42]]. On another hand, Leachia sp. and many other deep sea creatures exhibit translucent body void of pigment contents, allowing light to bend through the body for camouflage [43]. Midwater squid, Galiteuthis phyllura, emit bioluminescent light due to the production of photophores that aids in counterillumination and communication. The structural cross section of photophores function as leaky light guides that counterilluminate to match downwelling sunlight for camouflage [44]. Deep-sea creatures are an excellent source of inspiration for light manipulating strategies such as the leaky waveguides in Galiteuthis phyllura [45], transparent teeth from Idiacanthus atlanticus [46,47], and the body of jellyfishes [48,49]. By studying these natural wonders, the capabilities in light guiding and manipulation can be applied to innovative materials not only in biomedical fields but others as well.
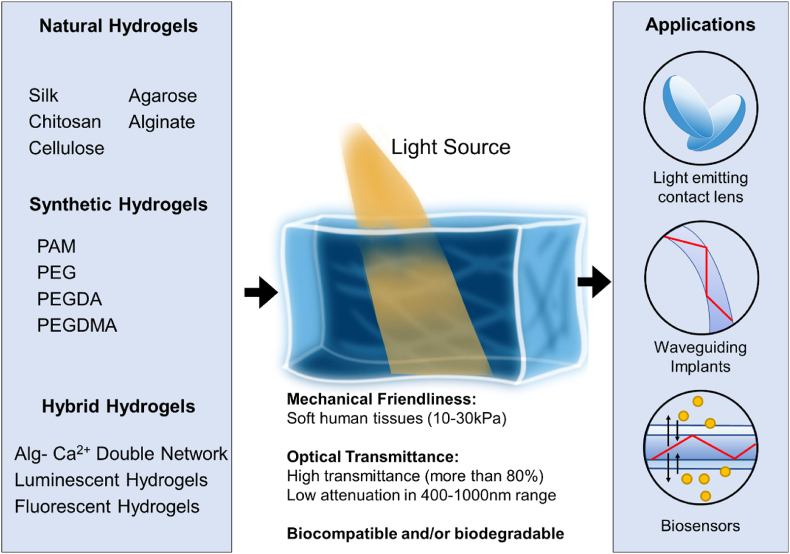
Hydrogels are three dimensional crosslinked polymeric networks that is able to maintain its soft 3D structure by the stability of its swell state in aqueous environment [50]. These polymeric chains are generally held together by hydrogen bonding, van der Waals interactions, or covalent bonding, through either chemical or physical crosslinks [51,52]. In recent years, the use of hydrogels to facilitate light exposure has gained traction, as a promising non-invasive approach to monitor body conditions [53,54], triggering the release of therapeutic drugs [55,56], and tumour ablation capabilities [57,58]. Due to its tailorability, hydrogels can be classified by many different categories depending on its size, precursors, bonding characteristics, physical nature, or synthesis route. Fig. 3 is a schematic on the classification, requirements, and application of hydrogels in light guiding and manipulating. Mahinroosta et al. Thakur et al., and Bashir et al. had summarized the different ways of classifying a hydrogel in the aforementioned ways comprehensively [10,51,52].
Fig. 3.
Illustrates one of the classification methods of hydrogel-based light guides in terms of its polymers and the biomedical applications.
The versatility of hydrogels holds promise to enhance various applications, including optics, tissue engineering scaffolds, drug delivery systems, and tamponade systems for wound healing. A notable example is the development of soft contact lens (CL) in the early 1970s, denoting the first era of hydrogel development [51]. The breakthrough of soft CLs led to a surge of hydrogel research, resulting with critical understanding of how hydrogels can facilitate light delivery and manipulation. Peng et al. wrote a comprehensive overview of hydrogelations via light and hydrogel responses after light exposure. Light-responsive mechanisms such as bond cleaving and link formation, photoisomerization, and photothermy were discussed. Despite progress in this area, Peng et al. acknowledged that gaps are still wide and more needs to be done to match application needs [59]. Nonetheless, the dynamic nature of hydrogels is considerably a significant advantage in biomedical applications. Li et al. explored designs of light responsive hydrogels and the emerging applications such as controlled drug delivery, smart biointerfaces and self-healing properties [60]. Tomatsu et al. describes the use of light to control the properties of hydrogels through photoresponsive groups, suitable for drug release and cell culturing applications [61]. Since inception, the field of hydrogels experienced exponential growth, resulting in the development hydrogels in biomedical applications.
3. Optical hydrogels
Among the recent developments, hydrogel-based waveguides or light manipulators are one of the emerging technologies aim to better photo-based theragnostic. However, the realization of this ideation has been proven difficult due to the surrounding environment under the skin that hinders the optical intensity. Hydrogels from natural sources such as silk and alginate have been exploited for optical waveguides and optogenetic modulations. Hydrogels from synthetic sources such as polyethylene glycol (PEG) and polyacrylamide (PAM) have been studied for its flexible, non-degradable waveguide properties. Here, we explore the hydrogels capabilities for the use of light delivery and stimulation in biomedical applications.
3.1. Optical hydrogels from natural polymers
Naturally occurring polymers are prized for its biocompatibility and biodegradation, making them suitable for medical applications. These polymers are commonly derived from plants, animals, or bacterial sources. A well explored strategy of utilising natural polymers is to create composites, be it all natural or hybrid. Herein, we explore the design and properties of these naturally occurring polymers and shortcomings as a light guiding hydrogel (Table 2).
Table 2.
A summary of transparent hydrogels derived from natural polymers.
| Polymer | Resource | Gelation | Hydrogel | Applications | Reference |
|---|---|---|---|---|---|
Silk 
|
Silkworm cocoon, spider silk | Binary solvent-exchange-induced self-assembly |  |
Light waveguide for photodynamic therapy, lenses for light emitting diode (LED), birefringence | [62] |
| Gel with horseradish peroxidase and hydrogen peroxide |  |
[63] | |||
 |
[64] | ||||
Chitosan 
|
Crusteacean shells, Mushrooms | Crosslink with glutaraldehyde prepared in HEPES buffer | – | Biosensors, leaky waveguides | [65] |
Cellulose 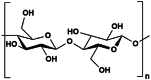
|
Trees, plants | Crosslink with Al3+ ions |  |
biosensor and signal transmission | [66] |
Agarose 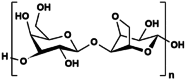
|
Brown seaweed | Gel upon cooling | – | in vivo imaging, light delivery, biosensor | [15] |
Alginate 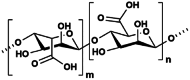
|
Green seaweed | Crosslink with calcium sulphate dihydrate solution |  |
tamponading agent for vitreous substitute | [67] |
3.1.1. Silk
The naturally occurring silk fibre obtained from silkworms is mainly divided into two fractions of silk fibroin (SF) (almost 75%) and silk sericin. The degumming process solubilizes silk sericin, leaving zig-zag polypeptide chains of SF. The SF structure comprises of heavy and light molecular chains linked with a disulphide bond. In the typical Bombyx mori SF, silk I (α-helix) and silk II (β-sheets) are two components of the crystalline structure [68]. The flexibility of SF is attributed to the interaction and formation of β-sheets, allowing hydrogen bond and van der Waals interactions to occur inter- and intra-chains [69,70]. The mechanical properties of SF can be tailored through hydrogel formation to accommodate to natural tissues. Furthermore, SF hydrogels possesses various key properties for biomedical applications, such as its inherent biocompatibility, biodegradability and ease of manipulation [[71], [72], [73]]. Optical losses of SF exhibit as low as 0.25 dB/cm at 846 nm and 0.25 at 1549 nm, suggesting a strong potential for waveguiding mediums [74]. Through the different extraction methods of SF such as physically [75], chemically [76], enzymatic [77] or a combination of methods [78], waveguiding SF hydrogels were developed over the years.
Sahoo et al. for example had synthesized a range of biocompatible SF hydrogels by using the enzyme, horseradish peroxidase, coupled with hydrogen peroxide [77]. By increasing the degumming (extraction) time (DT), the content of β-sheet (crystals) drops and better optical transparency can be achieved. The mean transmittance at 700 nm was reported to be 91% for the 120 DT as compared to 70% at 1 DT. The shorter DT hydrogels have longer β-sheet chains that would likely contribute to larger clusters of hetero-structures which increases light scattering in the silk structure. However, SF hydrogels with the longest DT exhibited the largest hysteresis loss in compressive loading cycles of 30%. The heterogenous networks of 1DT contributed to quick gelation and plastic deformation. In terms of its cytocompatibility, all SF hydrogels exhibit at least 90% survival rates, however after 8 days, the 30DT exhibited the best metabolic activity, alluding to an optimal stiffness for cellular activity compared to both end extremities of 1DT and 120DT [77]. The hydrogels can be implemented in diverse applications such as tissue engineering and vitreous substitutions, to name a few. The appeal of transparency and tunable mechanical properties of SF hydrogels makes it an ideal material for biological optical components in diagnostics and sensing applications. The fabrication of SF hydrogels is straightforward and results in a flexible gel suitable for lens applications (Fig. 4ai,ii,iii) [79].
Fig. 4.
Silk hydrogel for optic applications. a) i) Schematic illustration of the SF hydrogel fabrication from treating silk cocoons in 0.02 M sodium carbonate, dissolving the SF in lithium bromide to purifying through dialysis and forming hydrogel by mixing the purified SF solution with acetone. ii) Transparent hydrogel formed from SF solution mixed with acetone. Scale bar = 7.5 mm. iii) SF hydrogel prepared in the shape of meniscus lens. Scale bar = 5 mm, adapted with permission from © 2015 American Chemical Society [79], b)i) Illustration of using SF ink to attain both straight and curvy silk waveguides via direct-write assembly. ii) Schematic representation of the set up for capturing and analyzing the transverse face of silk waveguides and the optimization could be attained by controlling the position of the optical condenser. These images were adapted with permission from © 2009 Advanced Materials [81]. c) i) Schematic representation of printed SF with straight and curved designs. ii) Absolute irradiance measured for the different wavelengths using cool white LED and warm white LED. iii) Influence of the concentration of silk hydrogels on the transmittance, adapted © 2017 Springer Nature [64].
In another approach, Shu et al. developed birefringent SF hydrogels with the use of binary solvent-exchange-induced self-assembly (BSEISA). These SF hydrogels exhibit mechanical properties similar to cartilage, skin and eye crystalline lens [62]. In general, the modulus of SF hydrogels can be tailored in between 0.07 and 6.5 MPa, similar to soft tissues [80]. The BSEISA derived SF hydrogels transparent and mostly in the visible spectrum. Soft birefringent materials are highly desired in photomedicine, diagnostics and tissue engineering, as it is able to accommodate irregular geometries. The development of such materials is difficult, but SF can provide a potential solution.
As a waveguide, Applegate et al. reported the use of SF to fabricate a functioning prototype for in vivo photomedicine. The design incorporated two different silk components – silk film and silk hydrogel in a standard fibre optic design. The silk film acts as a core and the silk gel is the cladding to guide light within the core and minimise propagation loss [63]. The SF waveguide exhibited refractive index of 1.33–1.36 collectively and the optical loss at 540 nm was reported to be 2.0 dB/cm, within the range of conventional POF [63]. Although it was not reported, the work summarizes that the waveguide would eventually be consumed by cells due to the biocompatibility and biodegradation nature of silk. Melikov et al. explored the use of SF hydrogels as an alternative green lens to optoelectronics such as light-emitting diodes (LEDs). As opposed to the waveguide shape, here the SF hydrogels were shaped into a spherical dome shape and its light extraction efficiency for 3 wt% was reported as the most favourable (Fig. 4ai). Similarly Parker et al. printed optical SF waveguides (Fig. 4bi,ii) with propagation losses of 0.25 dB/cm and 0.81 dB/cm for straight and curved light guides respectively at 633 nm, indicating good guiding abilities of innate SF ink [64,81]. The average transmittance of (3-18 wt%) SF hydrogels exhibit a drop of only 0.41 dB/com in the visible spectrum and the irradiation of cool to warm light was measured to be efficient (Fig. 4cii,iii).
3.1.2. Chitosan
Chitosan is a derivative of chitin and is the second most abundant natural polymer after cellulose. Chitin is most commonly found in shells of crustaceans, exoskeletons of insects and cell walls of fungi [82]. The structure of chitosan is positively charged due to the amino groups. Similarly to silk, chitosan is capable of forming a transparent gel with good compatibility and degradation, all of which are ideal characteristics for hydrogel waveguides [83]. Therefore, the addition of chitosan in hydrogels provides an excellent platform to explore light guiding and sensing materials to be integrated with soft tissues.
Alamrani et al. developed a mesoporous chitosan-based leaky waveguide sensor to detect thrombin presence in blood [84]. The porosity of the waveguide allows species to diffuse in an interact with immobilized aptamers. The interaction between the chitosan free amine groups, the aptamers and the concentration of thrombin will alter the resonance angle in the waveguide. Following up, the group evolved diffraction-based leaky waveguide to have the self-referencing ability that minimised environmental and non-specific effect during the analyte measurement. This was achieved through the covalent attachment of biotinylated linker to chitosan, photocleavable at 365 nm. Through selective exposure, regions of high and low loading of proteins were established to serve as sensor and reference respectively [65]. A follow up study revealed that the chitosan diffraction-based leaky waveguide could differentiate the non-specific adsorption of bovine serum albumin (BSA) from the antibody binding even when the BSA concentration was more than 30 000 times of the maximum antibody concentration [18].
The transparency and biocompatibility of chitosan makes it a suitable precursor to hydrogels for tissue engineering scaffolds, particularly for cornea regeneration. Feng et al. developed a thermogel scaffold derived from oligoethylene glycol (G1) (OEG)-based dendronized chitosan (DCs), with transition temperature of 37 °C (Fig. 5ai,ii). The resulting hydrogel exhibit transmittance of over 90% at visible light range from 25 °C to 40 °C, and the sol-gel transition point decreases as the concentration increases (Fig. 5aiii) [85]. The naked chitosan chain is populated with hydrophilic amino groups and hydroxyl groups that contributes to the self-assembly and formation of supramolecular fibres. Similarly, Ding et al. demonstrated a tough chitosan hydrogel for stem cell and tissue engineering (Fig. 5b). The chitosan hydrogel exhibit a 94% optical transmittance compared to 81% of chitin hydrogel at 800 nm, with Young's Modulus, E ranging from 7.9 MPa to 92.0 MPa (increasing with the degree of deacetylation) [86]. Isobe et al. developed a highly transparent and hydrogel network comprising of highly crystalline α-chitin. The hydrogel exhibits similar light transmission to regular contact lenses with robust mechanical properties [87].
Fig. 5.
Chitosan hydrogels. a) i) Schematic illustration of the fabrication of dendronized chitosan hydrogel via self-assembly and heating ii) Chemical structure representation of the dendronized chitosan hydrogel synthesis iii) Optical image of the thermoresponsive dendronized chitosan hydrogel. Adapted with permission from © 2021 American Chemical Society [85]. b) Optical image of transparent chitosan hydrogels after different rounds of deacetylation. Adapted with permission from © 2016 American Chemical Society [86]. c) i) Optical image of N-acetylated chitosan hydrogel ii) Benchmark of the light transmittance capability of the N-acetylated chitosan hydrogel based on high degree of polymerization against commercial contact lens iii) Photo of the N-acetylated chitosan hydrogel fabricated into the shape of contact lens. Adapted with permission from © 2020 American Chemical Society [87].
3.1.3. Cellulose
Cellulose is the most abundant polymer, commonly found in cell walls of vascular plants, marine organisms and produced organically by microbial biosynthesis [88]. The structure of cellulose is highly crystalline and extensively networked with a large degree of polymerization. Due to the complex structure and interactions between the chains, the solubility of cellulose is highly restricted [13]. Therefore, further pre-treatments are necessary to break and depolymerize these chains to better exploit the polymer. Organic light guiding systems can benefit from the characteristics of cellulose, such as biocompatibility, degradation, and the ease of functionalization [89]. In terms of light manipulation strategies, the form of cellulose can vary widely from facile top-down approaches of transparent wood to the addition of nanocellulose (NC) in hydrogels [[90], [91], [92]].
Orelma et al. developed an optical fibre from regenerated cellulose as the core and cellulose acetate as the cladding through a dry-jet wet spinning method. The cellulose fibre guides light from 500 to 1400 nm range while absorbing UV range. The reported attenuation constant stands at 6.3 dB/cm (1300 nm), higher than conventional silica fibres, while the maximum tensile strength was found to be 120 MPa [93]. While the focal point of the study is towards water sensing applications, the inherent biocompatibility properties warrant additional investigations for potential photomedicine uses.
In a study conducted by Li et al. carboxymethyl cellulose (CMC) was incorporated with carbon nanotubes (CNT) to generate hydrogel composite that was subsequently used to build optical fibre sensor [94]. The advantages of such sensors as compared to traditional electrical sensors lie in overcoming the issues of corrosion and interference from complex electromagnetic environments, with the addition of better response speed. Kuddushi et al. fabricated a transparent hydrogel film (PVA-CMC-Na-Alg) that exhibit light transmittance at 85% between 400 and 800 nm [95]. This facile mixture of different gelation components exhibits excellent mechanical properties due to their hydrogen bonding, resulting in maximum breakage at 100 MPa. The transparency of the film enables the user to visually check on the area without removing the film, enhancing the convenience of wound healing. Light guiding can be a potential application from this material as it already exhibit good transmittance and mechanical properties. Similarly, Agate et al. developed a method of fabricating unconventional hydrogels such as CMC/riboflavin-based hydrogels by photoablation to crease the lens-shape of contact lens [96].
On another hand, Zhou et al. demonstrated state-switchable cellulose hydrogels with the use of Ca2+ and Zn2+ ion exchange. The hydrogels are able to shift from rigid, fluid, and brittle characteristics depending on the concentration of ZnCl2 and CaCl2. The addition of Ca2+ ions greatly improve the cross-linking stability and the recovery area ratio of Gel-L-Ca2+ is 75%, indicating good recoverability [97]. These transparent gels exhibit good pressure sensing properties and can be further explored for multi-responsive sensor or light guiding.
3.1.4. Agarose
Agarose is a seaweed-derived linear polysaccharide that has been explored for hydrogel waveguiding application. In addition to being biocompatible [98], agarose is often low cost and food grade which are ultimately appealing for biomedical usage [15]. As a waveguiding material, agarose exhibit high transparency in the visible range, with adjustable refractive index and thermo-reversible gelation property [15]. The change in agarose concentration by 0.5% (w/v) can alter the refractive index of its hydrogel by approximately 0.001 [99].
To develop a light guide, Fujiwara et al. demonstrated a simple method to utilize agarose to craft structured optical fibre. This involves pouring the agarose solution over stacked rods arranged in a mould, and upon cooling, the rods and the mould were removed freeing the solidified waveguide. The waveguides exhibit relatively higher optical losses at 3.2 dB/cm (633 nm) compared to silica waveguides but the transmission can be improved to (0.8 dB/cm losses) by eliminating noise and incorporating glycerol [100]. Through the control of agarose composition and mould design, the refractive index and the fibre geometry of the optical fibre could be altered accordingly to the needs respectively.
Gupta and Goddard demonstrated the use of agarose hydrogel and reactive blue 4 dye (RB4) to form leaky waveguide. The success was exhibited in the reduction of the response time and the detection limit of bovine serum albumin. The resonance of the waveguide can be visualised due to the addition of the dye and the RI can be measured real time at any location [101].
3.1.5. Alginate
Alginate is an anionic charged block copolymer that is found in structures of algae and capsular polysaccharides in bacteria such as Pseudomonas sp. and Azotobacter sp. [102]. The structure of alginate is derived from randomized polymerization of 2 units; β-1,4-d-mannuronic acid (M block) and α-1,4-l-guluronic acid (G block). The rich hydroxyl and carboxyl groups in its chains proves to be useful in gelation, biodegradation, and compatibility [103]. The use of alginate is often coupled other synthetic polymers, such as polyethylene glycol (PEG) and polyacrylamide to create hydrogel waveguides [17,19,22].
A notable study revolves around the potential alginate in the development of vitreous substitute. The high molecular weight alginate gels with the presence of calcium sulphate resulting in a hydrogel exhibiting a RI value of 1.33 in both sol and gel states. The hydrogel achieved 90% transmittance in the visible light range, suitable for light manipulation, not only for vitreous substitution but potentially for delivery [67]. Wang et al. fabricated an alginate-polyacrylamide (alg-PAAm) hydrogel optical fibre for in vivo optogenetics applications. The optical fibre exhibits a propagation loss 0.25 dB/cm and Young's modulus of 48–91 kPa, similar to certain human tissues. The biocompatible fibre provides an alternative to silica fibres and alleviate tissues responses [55].
3.2. Optical hydrogels from synthetic materials
The demands on biomaterials for optical purposes are numerous, calling for characteristics such as biocompatibility and appropriate chemical and mechanical properties, while necessitating optimal material-light interactions. While there are several natural materials have met these criteria as described in the previous section, there is also an expansive range of synthetic materials that offer the added benefits such as better mechanical properties, extensive functional techniques, and reproducibility [[104], [105], [106]]. As such, various synthetic polymers have found their places as important candidates in this area (Fig. 6) [9,27].
Fig. 6.
Structures, gelation approaches, optical properties, mechanical properties, and applications of synthetic hydrogels in waveguide-related applications [4,[107], [108], [109], [110], [111], [112]]. The images were adapted with permission © 2018, Springer Nature [109]. © 2019 Sensors [112], © 2019 Optical Publishing Group [113], © 2021 Wiley‐VCH GmbH [4].
3.2.1. PEG
PEG has been known for its functionalities in optical fibre cores, and have been proposed to be a suitable material for optical fibres with soft mechanical properties [27,107]. This can be attributed to their high optical transparency and low light attenuation, as well as their low Young's modulus and high flexibility [108]. This combination of properties also allowed for the development of PEG-based hydrogels with reversible photo-induced stiffness switching, through the incorporation of Dronpa145 N, a photo switchable protein, as a crosslinker between 4-armed PEGs, which lends further tunability to its mechanical properties. The hydrogel could be spatially and temporally tuned to be softened by approximately 20 times within 14min of illumination by 505 nm light, serving to control cell migration through this change of substrate stiffness [109]. PEG's optimal optical properties have also seen used in being incorporated into other less optically desirable systems, such as elastin-like protein (ELP)-based biomaterial scaffolds, in order to improve their optical properties for cellular imaging purposes.
There are two main derivatives of modified PEG that are commonly used in the development of hydrogels optical fibres: PEG diacrylate (PEGDA) form, and PEG in its dimethacrylate (PEGDMA) form, and they can be used to develop hydrogels of varying properties based on the underlying polymer structures (Fig. 7) [27,107]. As a PEG derivative, PEGDMA gels also possess properties, such as elasticity, refractive index and optical transparency, which are affected by monomer length and concentration. This allows for tuning of the refractive index within the wide range of 1.344–1.437 to accomplish waveguiding within a core-clad structure while preventing total reflection at increased angles of incidence [110]. In particular, PEGDA-based hydrogels are thought highly of due to their ability to balance transparency and biocompatibility, achieving non-immunogenicity and resisting protein adsorption [114].
Fig. 7.
Fabrication of transparent hydrogels from synthetic polymers for biomedical purposes. a) (i) Printing setup for the printing of (ii) degradable and optically transparent 70% wt% PEGDA-DTT fibres. Adapted with permission from © 2020 Wiley‐VCH GmbH [115], b) PEGDA hydrogel (i) used in an optical setup in a microfluidic chip as an integrated waveguide (ii) exhibiting excellent transparency at 60% w/v concentration (iii) fabricated into a 1 × 4 waveguide splitter. Adapted from © 2019 Sensors [112]. c) PEGDMA hydrogel with fibre inserted, adapted with permission from © 2019 Optical Publishing Group [113].
Studies have shown that their transparency was improved upon increasing the molecular weight of these constituent polymers, as well as their concentrations [116]. At the same time, the large refractive index difference between the waveguide and its surrounding environment increases linearly with polymer content, rendering high polymeric concentrations more favourable in these circumstances. Therefore, a balance must be struck between the different parameters to optimize the properties. It is critical to understand as each polymer can interact differently, for example, low molar mass PEG is associated with improved dissolution but lowered optical transparency and undesirable stiffness and vice versa. Therefore, using higher molecular weight PEG is far suitable for this application. To explore other possible functionalities, the addition of methacrylic acid (MAA) to PEGDMA results in a copolymer with carboxylic groups that facilitates in cell attachment and proliferation. The dual function waveguide not only delivers light for optogenetics but also create a bioactive environment to the surroundings [111].
3.2.2. PAM
A common water-soluble polymer that easily forms hydrogels, [117], PAM is one of the most commonly used polymers in the fabrication of waveguides and optical fibres [107]. When considering its potential as an optical biomaterial, it has many desirable properties, such as flexibility and optical clarity, as well as non-toxicity, bio-inertness, non-absorbability and a long working lifetime [9].
Notably, PAM has been used in the detection of uric acid in aqueous samples through surface plasmon resonance in a fibre optic biosensor. The setup was achieved entrapping Uricase in a PAM gel and subsequently coating this onto silver and silicon layers with a small unclad length of silica optic fibre in the middle. During the increase of uric acid solution concentration, a reaction between oxygen, uric acid, and the uricase forms a complex that increases the refractive index of the PAM layer, resulting in a red shift in resonance wavelength that can be detected [118]. A similar sensor has also been created using SPR principles through the entrapment of tyrosinase in PAM for the detection of phenolic compounds [119].
PAM hydrogels have also been explored as coatings to improve optical fibres through the imparting of three different properties: mechanical similarities to native tissue, low-loss light transmission, the providing of a 3D network for additional functionalization. In the study, the PAM hydrogel was coated over PDMS as the core and was found to function with insignificant effects on the bulk mechanical properties on the optical fibre, while increasing its effectiveness in light propagation. Compared to the uncoated optical fibre which had a propagation loss of 0.52 dB/cm in air, the PAM hydrogel showed reduced light loss at the core-air interface, at only 0.39 dB/cm [4]. Preliminary studies have also attempted to use PAM as a substrate for opto-regulated drug release through an engineered self-replenishing living material. This study made use of PAM and agarose hydrogel matrices as a substrate for the sustenance of active endotoxin-free Escherichia coli that have been engineered to secrete deoxy-viocin, an antimicrobial and antitumoral drug, upon photo-stimulation, which could be spatially and temporally controlled with a focused light beam [120].
3.3. Hybrid systems
Both naturally derived and synthetic hydrogels possess their own set of advantages and limitations. While natural hydrogels excel in biocompatibility and biodegradation capabilities, their capabilities as waveguides on its own is challenging and minimally explored. In addition to the aforementioned strategies, natural and synthetic origins or even inorganic additives can interact to achieve synergistic and multi-functional properties for their applications [17,53,55,121,122].
A transparent and conductive hydrogel was derived from crosslinking carboxymethyl chitosan (CMCS) using N-methylol acrylamide (NMA), and the addition of CaCl2 introduces conductivity and mechanical enhancement additive through the carboxymethyl group in CMCS. The CMCS-Ca2+/PAAm/PNMA hydrogel with water content ∼74.29%. The hydrogel exhibited rapid response and recovery, excellent toughness and good fatigue resistance [123]. N-(Hydroxymethyl)acrylamide (NMA) forms the main skeleton and has strong hydrogen bonds with PAAm. The non-covalent bonds between CMCS, PAAm, and Ca2+ act as sacrificial bonds when subjected to external forces. In the hydrogel, hydrogen bonds between CMCS and PAAm molecules forms the transient non-covalent bonds cross-linking networks, as well as the metal-ligand coordination bonds between Ca2+ and CMCS. This improves the density and mechanical properties of the hydrogel crosslinked network. These transparent conductive hydrogels demonstrated applications in monitor of the movement of body joints, including the movements of wrists, elbows, and knee joints.
Similarly, calcium alginate-zwitterionic copolymers ADN double network also showed high transparent (transmittance 92.2%) (Fig. 8a) [124]. Ca2+ crosslinked alginate as dense network, and copolymer of anionic sodium p-styrene sulfonate (NAS) and cationic acryloxyethyl trimethyl ammonium chloride (DAC) as interpenetrating loose network. The hydrogel exhibits transparency, mechanically resilience with stretchability (1375%) and toughness (0.57 MJ/m3), as well as self-healing and high conductivity (0.25 S/m). All these properties together enable the hydrogel as a flexible wearable strain sensor for large joint flexion (such as finger, elbow, and knee), foot planter pressure measurement, and local muscle movement (such as eyebrow and mouth). ion-conductive alginate hydrogel network was developed in the present of Ca2+ with remarkable stretchability (∼975%) and high optical transmittance (∼96.2%) [126]. The hydrogels were physically cross-linked by Ca2+ and semi-interpenetrating copolymers consisting of zwitterionic [2-(methacryloyloxy) ethyl] dimethyl-(3-sulfopropyl) ammonium hydroxide (SBMA) and 2-hydroxyethyl methacrylate (HEMA) and is able to self-adhesive to diverse substrates (Fig. 8b). A prototype of sensor was created to assess bioactivities of the human body in real-time, by detecting force and temperature signals.
Fig. 8.
a) Schematic of the preparation process of the ADN hydrogel, adapted from © 2022 Frontiers in Bioengineering and Biotechnology [124], b) The fabrication process of the magneto-birefringence-based transparent hydrogel (MB-hydrogel), adapted from © 2022 Nature Communications [125] The MB-resin is a mixed suspension, comprising 2D Co-doped TiO2 (CTO) materials, water, monomer (PEGDA, molecular weight MW of 700 g/mol), and photo-initiator (Irgacure 2959, MW of 224.25 g/mol). c) The schematic illustration of the alginate/P(SBMA-co-HEMA) hydrogel (AHS) with a semi-interpenetrating network cross-linked by reversible noncovalent interactions, adapted with permission from © 2021 American Chemical Society [126].
On another hand, Ding et al. developed a transparent magneto-birefringence-based hydrogel system with synthetic precursor poly (ethylene glycol) diacrylate (PEGDA), and Irgacure 2959 with cobalt-doped titanium oxide (CTO) embedded in the system (Fig. 8c) [125]. The hydrogel exhibited sensitive magnetic responses, uniform optical capable of multiple transmitted interference colours. This magneto-birefringence hydrogel showed mechanochromic and thermochromic property as well. It shows promising optical applications such as the optical phase retarder, gradient optical attenuator, magnetic see-through colour imager, and mechano-chromic indicator.
In another form of light manipulation, hydrogels have been studied as a holding medium and strategy for luminating materials. The luminescent hydrogels can be fabricated by physical embedding of luminescent particles or covalently conjugating luminophors onto the hydrogel chains [127,128]. Typically, a stimuli response such as pH, temperature, or redox will activate the luminating hydrogels. Ning et al. developed an HRP/COD/luminol/Alg hydrogel-based chemiluminescent sensor to detect the levels of cholesterol. The addition of luminol exhibit a characteristic peak at 410 nm and the hydrogel system shows minimal absorbance of light at visible light range. The luminating light is stable in the presence of cholesterol and have a limit of detection of 7.2 μM [129]. Luminol is a chemiluminescent reagent that luminates blue light when oxidised, exhibiting a glow-type luminescence. As it does not require an external power source, it is a good alternative as a cold light or as a marker for biological interactions. Ye et al. developed a stable self-assembled guanosine-borate hydrogel with luminol and hemin embedded in the gel. The gel exhibited stable luminating intensity over time after 500s and excellent response to H2O2 [130].
Aside from chemiluminescence, luminescent additives such as lanthanide ions, carbon dots (CDs), quantum dots (QDs), and organic dyes contribute to the luminating properties of the hydrogel [127,131]. Lanthanide ions such as Eu3+ or Tb3+ complexes emits light when energy is transferred from the ligand to the core of the ions. Wei et al. developed an electrical stimulated multi coloured hydrogel as a biomimetic skin for robotic applications. The red- and green-luminescent hydrogels were prepared with lanthanide ions complexes as core, using potassium 6-acrylamidopicolinate (K6APA) as a ligand, thus forming, Eu–K6APA complex and Tb–K6APA complex. Whereas, the blue luminescent hydrogel was developed via radical polymerization of AIE-active 4-(dimethylamino)ethoxy-N-allyl-1,8-naphthalimide (DAEAN), and methylene-bis-acrylamide (MBAA) with N-isopropylacrylamide (NIPAM), DAEAN-PNIPAM [132]. Zhi et al. developed a luminous hydrogel by embedding Eu3+ ion complexes into poly (vinyl alcohol) (PVA) hydrogels and boric acid. The Eu-PVA gels exhibited stable luminescent property in dry and wetted state. In its natural state, it exists as a transparent gel but luminates under 385 nm excitation to display red intensity peaks [133].
Fluorescence is a phenomenon whereby longer wavelength of light (low energy) is emitted after absorbing light of a lower wavelength (higher energy) from a material. Here, that material is fluorescent hydrogels that incorporates various moieties such as organic dye protein molecules, lanthanide metal ions, QDs, and CDs [[134], [135], [136], [137], [138]]. Sophisticated strategies vary in terms of the assembly of moieties in the hydrogel and the mechanism of fluorescence. Hydrogels possess the ability to provide a transparent system that supports these fluorescent moieties in terms of physical and chemical stability. In return, the hydrogels are able to exhibit fluorescence and potentially boost the mechanical properties. Fluorescent materials are significantly important in the next generation methods of biosensing, therapeutics, and diagnostics. Guo et al. developed a tapered PEGDA hydrogel waveguide and coated with glutathione-coated QDs and thioglycolic acid (TGA)-coated QDs for metal ion sensing. Total internal reflection was achieved in the waveguide with a refractive index of 1.402. The fluorescence in the waveguide exhibited slight drop in light emission in water or tissue, but the relatively strong emission would still be suitable for in vivo applications. The fluorescence of QDs at 615 nm were significantly quenched by Pb2+ as compared to other metal ions, indicating the selectivity of glutathione-coated QDs [139].
In addition, fluorescent hydrogels can be incorporated into biomedical applications that most sensing devices and systems are limited by. Li et al. developed a multifunctional NIR-based fluorescent hydrogel to monitor and facilitate wound healing. Chitosan was grafted with dihydrocaffeic acid, an antioxidant drug, l-arginine, to facilitate angiogenesis and insulin secretion, and then metformin hydrochloride was grafted onto aldehyde groups of polyionic liquid. The resulting PIL-CS hydrogel absorb NIR at 820 nm and emit at increasing intensities as the pH at the microenvironment decreases [140].
4. Potential applications of optical hydrogels
In terms of optical applications, hydrogels have been studied on for vitreous tamponades [11], drug delivery contact lenses [141,142], delivering light into deep tissue for clinical applications including optogenetic stimulation [55], photodynamic therapy, [143], real-time in-vivo tissue imaging, [144], as well as biological sensing [145,146]. Hydrogels are able to break the limitations of traditional optical materials such as glass, crystals, rigid polymer, semi-conductors which are prone to injuring the soft tissues when implanted due to their stiffness and brittleness [147]. Moreover, hydrogels are easily designed to be biodegradable, [148], thus the implanted hydrogel based waveguides and optic fibres often do not require secondary removal procedures and this significantly improves patient comfort levels.
4.1. Contact lens devices
Ophthalmic lenses (or CLs) are the hallmark of hydrogels in commercial applications and has now taken a step further as potential drug delivery systems and smart CLs [149]. Hydrogels are especially suitable in ocular applications as they can be designed to possess high optical clarity with the correct toughness [150]. While most commercial CLs today are for the sole purpose of correcting visual acuity, [151], hydrogel-based CLs have been explored to enhance the drive the future of smart CLs [152]. The pores in the hydrogel matrix allow oxygen to permeate through it to the cornea and sufficient oxygenation is essential to the patient's comfort [153]. High water content in the hydrogels would allow the cornea to remain well hydrated, [154], which is a key factor that determines comfort level for the user. A well explored example would be Poly (2-hydroxyethyl methacrylate) (pHEMA)-based hydrogel [155] and silicone hydrogel [156] as medicated ophthalmic lenses. In addition to the previously mention characteristics, the polymeric matrix of the hydrogel can be employed to encapsulate drugs or therapeutic proteins and also act as the physical barrier to slow diffusion in order to achieve sustained and localised delivery of therapeutics [70]. As the medicated hydrogel lenses are better at resisting clearance due to frequent blinking, permanent tear production (0.5–2.2 μL min-1), [157], and nasolacrimal drainage than eye drops, [149], they allow drugs and biologics to penetrate the cornea more effectively and thus have been reported to enhance therapeutic effectiveness [158]. On another hand, smart CLs have been explored as electronic CLs devices [159], biosensing CLs [160,161], and light mediated ocular therapies [162].
Phototherapeutic approaches have attracted interests over the years as a non-invasive method of medicine. The tradition usage of CLs is no longer limited to visual correction but have been explored as a wearable ophthalmologic treatment modality. Through printing miniaturized inorganic light emitting diodes (ILED) pixel onto soft CLs, 645 nm can be emitted directly to the retina without disrupting visual clarity. The CLs device remained functional after a week of storage in the storing liquid and can be turned on wirelessly through a nanofiber antenna [162]. Similarly, Lee et al. developed a wireless light emitting diode (LED) CLs to combat diabetic retinopathy using red/NIR wavelength light (630–1000 nm) (Fig. 9a) [163]. Diabetic retinopathy can lead to blindness if not treated early. The typical treatment modalities for the condition are drug delivery, surgery, and laser photocoagulation [164]. The CLs exhibit 90% light transmittance in the 300–800 nm light range and minimal inflammatory responses were detected. However, in both of these approaches, heat generated is a large concern. The temperature rise is not acutely damaging but is restricted by the duration of exposure.
Fig. 9.
a) Two photographic images of smart CLs that emits red, blue, and green light for phototherapy in diabetes retinopathy. Images were adapted with permission from ©Advanced Science, 2022 [163], b) a photograph image of the pHEMA hydrogel substrate and functional device sensing IOP and the SEM image of the pyramid microstructures. Images were retrieved with permission from ©ACS Sensors, 2022 [161] c) the schematic diagram of glucose monitoring smart CLs. Images were adapted with permission from ©Advanced Materials, 2022 [160].
Intraocular pressure (IOP) is a critical aspect of eye health that indicates glaucoma diagnosis and progression. As IOP fluctuates considerably over time, it is difficult to accurately monitor and confirm the condition of the eye [165]. In a study conducted by Zhu et al. smart hydrogel CLs capable of wireless IOP monitoring based on inductor-capacitor-resistor (LCR) sensor was developed (Fig. 9b). The smart CLs consists of pyramid-shaped micro-structures that increases the sensitivity of LCR sensor. This coupled with an impedance-matching tuneable reader (IMTR) on the glass wear results in a sensitive IOP sensor to measure real-time with convenience [161]. CLs sensing devices not only detect ocular health but may potentially monitor glucose levels from tears in the eyes. Kim et al. fabricated nanoporous hydrogels to embed gold-platinum bimetallic nanocrystals (HA-Au@Pt BiNCs) (Fig. 9c). The diffused glucose and oxygen molecules interact with glucose oxidase in the hydrogel pores which results in hydrogen peroxide that quicky gets decomposed by the nanoparticles and emit two electrons for amperometry responses. The smart CLs was able to accurately detect glucose levels of 94.9% acceptance with no temperature increase for 30 min [160]. The rise of smart CLs is a promising indication of new horizons in biomedical applications. The development to clinical applications is limited as biocompatibility over usage has yet to be explored, the durability in wet environment, and safety of inorganic-based sensors would raise concerns. As discussed previously, heat generated can be of concern in prolonged usage. Furthermore, sophistication and miniaturization of other components are crucial to integrate on a thin and soft lens.
4.2. Light-guiding implants
While ophthalmic lenses are targeted for application to the eyes, hydrogel-based waveguides and optical fibres are also being developed rapidly with potential applications throughout the body. In the field of optogenetics, hydrogel waveguides have opened a new horizon in light delivery to excite cells as it can facilitate light delivery in deep tissues, expanding the potential of optogenetics. Hydrogel waveguides developed for optogenetics and photodynamic therapies are commonly implanted into tissue of significant depth and connected to light source at the external end [139]. Light coming in from the external thin optic fibre will spread out as it enters the waveguide before being extracted by the surrounding tissue [115,166]. This enables deep-tissue stimulation and overcomes the poor penetration of light via the skin (1–2 mm for visible light and ∼ 3 mm for near-infrared light) [167] (Fig. 10).
Fig. 10.
I) Comb-shaped waveguide connected to a light source via optical fibre with light being distributed via each protrusion, ii) difference int the delivery of light between waveguide and without, iii) the employment of waveguide significantly increased the depth of light penetration Adapted from, © 2016 Springer Nature [166], b) the blue laser light (492 nm) delivery of step-index hydrogel optic fibre in air, (ii) in tissues (ex vivo), (iii) the light propagation loss in different mediums. Adapted from © 2015 John Wiley and Sons [17] c) a diagram excerpt of i) the PDT activation in mouse model, ii) the H&E staining of muscle slices, iii) the temperature change during activation and an illustration of heat conductivity at the localised area. Adapted from © Nature Communications [168].
The waveguides generally have refractive indices in between that of the optic fibre and the extraction medium and the shape and surface of the waveguides are specially designed to control the intensity and spatial extraction of light [139,166,169]. There are various design and fabrication methods to achieve hydrogel-based waveguides. The common fabrication techniques involve small-scale assembly templates that utilizes moulding and coating methods [170], 3D printing [169] with nano or micro scale features fabricated via imprinting [171] or laser direct writing [172] methods. It is essential for these hydrogels to possess low absorbance and low scattering in order to reduce attenuation of optical power [173]. Hydrogel-based optical fibres are generally more challenging to fabricate compared to the traditional means. This is due to the swelling nature of the material and the softness that may impede its structural integrity. However, between traditional phosphate glass based optic fibres and hydrogel or polymeric based ones, the latter group generally have attenuations that are one order of magnitude higher than the former [174]. The advantages of hydrogel based optic fibres still lie in their flexibility and biocompatibility, but there are still significant challenges to overcome in terms of the mechanical strength and batch-to-batch variations of these hydrogel optic fibres [175].
The key design feature to minimise attenuation in optic fibres is to clad the fibre with a material of lower refractive index as compared to the fibre core [22]. For example, this step index profile allows for total internal reflection and thus entraps the light to within the optic fibre [176]. The fabrication of step-index hydrogel based optic fibre is usually done by first casting the inner core in a tube mould followed by dip-coating the core in another hydrogel precursor solution to form the cladding [17]. During the casting phase, the hydrogel core is commonly formed by thermal- or photo-polymerization of the monomers. Of the cladding material, alginate-based hydrogel is frequently employed as it can easily adhere to the core and also solidify by exposing to calcium ions [17]. Recently Zhu et al. fabricated a novel method of in situ growth via surface polymerization of a slippery cladding layer on PEGDA with poly (N-acryloylglycinamide) (PNAGA). Iron ions (Fe2+) were loaded onto PEGDA core fibres and subsequently submerged into NAGA monomers to form the PNAGA cladding over the core at different concentration and growth timing. The resulted fibre exhibits decreasing attenuation as the wavelength increase from 450 nm, 532 nm and 660 nm. Furthermore, the Young's modulus can be closely matched to soft tissues in the body. The PEGDA@PNAGA hydrogel optical fibre can potentially regulate the release of drug through light stimuli [177]. An alternative method involves creating a double network hydrogel system within a microfluidic device that enable the assembly of aligned capillaries to realise the formation of either one or two core structures. Covalently crosslinked acrylamide (AAm) and ionically crosslinked alginate (Alg) were combined to form the hydrogel system and dopamine methacrylamide (DMA) was incorporated to better microenvironment interactions with the biological tissues. The optical fibres exhibited relatively low attenuations of 0.10–0.12 dB/cm attributing to the uniformly fabricated core-cladding structure. Through the use of microfluidics, the geometry and structures of the fibre can be easily manipulated [121].
In the aspect of light delivery in optogenetics, Feng et al. proposed the incorporation of DL-dithiothreitol (DTT) as a bridge to form PEGDA-DTT hydrogels through the reaction between the acrylate groups of PEGDA and the thiol groups of DTT. The resulting optical waveguide facilitated sufficient light delivery under the tissue to activate optogenetic switches in the cells. The hydrogel demonstrated a reduction of scattering of approximately 15% compared to a pure PEGDA hydrogel at a polymer concentration of 70%, and a tuneable refractive index which increased with increased DTT concentration, or polymer content, producing more optically optimal hydrogels for the formation of waveguides. The addition of DTT also imparted greater biodegradability, and the resulting PEGDA-DTT solutions were found to be printable through the coupling of an extrusion-based printing and in-situ photopolymerization. The hydrogels to exhibit low attenuation of 0.1–0.4 dB/cm in air and 0.25–0.70 in tissue. Adjustments to the PEGDA-DTT molar mass can tailor the Young's modulus within the range of 100 kPa-8MPa [115] Johannsmeier et al. developed hydrogel PEGMA waveguides that enables optogenetic activation whilst enabling cell attachment on the surface via coating [110,111]. In another interesting aspect, Chen et al. proposed a versatile integrated light-triggered dynamic wet spinning (ILDWS) method to fabricate core-sheath hydrogel fibres. It demonstrated large-scale fabrication capabilities that current fabrication techniques are grossly limited by. The method is able to fabricate core-sheath type hydrogels from a variety of monomers for optogenetic stimulations, with the ability to fine tune the diameter, mechanical properties and optical transmission capabilities [178]. In the recent years, different fabrication methods have been reported in the development of hydrogel waveguides, one in particular is the use of 3D printing [179,180]. In a recent approach, a graded-index hydrogel optical fibre made from PAM was fabricated using a novel method of projection-suspended photocuring (PSP) 3D printing. The high resolution of the 3D printer enabled microscopic sizes to be printed, suitable for gradual gradient modulation for the graded index characteristics. The optical fibre exhibited the lowest transmission loss at 0.25 dB/cm at 635 cm with favourable bending performances [179].
On another hand, the optical properties of PEGDA have also seen use when coupled with upconversion nanoparticles (UCNPs) for photodynamic therapeutic applications. Through the use of a PEGDA hydrogel core, and a fluorinated ethylene propylene (FEP) cladding to tune the refractive index for light guiding purposes in tissue environments, two types of silica coated UCNPs could be encapsulated in the PEGDA hydrogel core in a 1:1 ratio. This wireless UCNPs implantable could be inserted into the brain of mice and secured using only surgical glue, and the applied photodynamic therapy was able to shrink tumour size 16 days just after the transplantation [57]. In the application of PDT, there are possible risks of during the light exposure. Although the adverse effects can be managed with controlled dosage and exposure, hydrogel waveguides have also been studied to dampen the thermal impact on the surrounding areas. A thermal regulated hydrogel waveguide (THFOW) was developed with PEGDA, N-isopropylacrylamide (NIPAM) and N, N-dimethylacrylamide (DMAAm) via an integrated homogeneous-dynamic-crosslinking-spinning method, exhibiting high transparency [168]. The resulting hydrogel waveguide minimises temperature rise in the surrounding areas as compared to the PMMA fibre for PDT (Fig. 10c).
The use of hydrogels and PDT extends further than cancer therapy with a recent studies of stimuli responsive gelation systems fabricated with hydrogels for on-demand theranostics against bacterial infections in wound management. The materials facilitate dual responses and are not necessarily exploiting light delivery capabilities. Wang et al. developed a thermos-responsive chitosan-based hydrogel that is pH-sensitive to indicate colour changes which facilitates fast diagnosis for PTT [181]. In another example, an antimicrobial peptide (ε-polylysine)-derived hydrogel system does not deliver light for activation but instead gets activated to undergo sol-gel transition to induce PDT. The hydrogel was also designed to be pH responsive with quick reversible colour changes for bacterial detection [182].
4.3. Biosensing systems
Biosensor can be defined as a device or a component of a machine, which uses biological molecules to detect the environment inputs. The use of optics taps on to the interactions of light and specific set of sensing molecules in the biological tissues, enabling minimally invasive alternatives. However, it is severely limited by the depth of which light can reach and real time monitoring. In this field of application, the use of hydrogels generally acts as a system to embed the critical molecules and allow light interactions or as a enhanced response system to the surroundings [183]. For example, lysophosphatidic acid (LPA) is an early-stage ovarian cancer biomarker that is challenging to detect on its own. A dually crosslinked supramolecular hydrogel based on the RAFT polymerization of N,N′-dimethylacrylamide (DMAAm) and 2-vinyl-4,4-dimethylazlactone (VDMA) was fabricated and has been explored to facilitate the detection via its swelling response to LPA which in return enables detection from surface plasmon resonance [184,185].
On another hand, hydrogel-based optical fibres have been intensely studied as a suitable material for diagnostic applications [22,53,54]. There is a need for a suitable implant that can achieve real-time monitoring of the internal body conditions via illumination and detection. This is prominent in the detection of glucose levels of the body for diabetic patients. Yetisen et al. developed a poly (acrylamide-co-poly (ethylene glycol) diacrylate) p (AMco-PEGDA) core guide cladded with calcium alginate for glucose sensing via 3-(acrylamido)-phenylboronic acid (3-APBA) molecules in the core. The glucose levels in the environment reversibly changes the fibre diameter of the hydrogel waveguide, thus altering the light propagation properties. This response enables the quantitate measurements of glucose in a set of range [19]. In addition, Chen et al. incorporated photonic crystals (PhC) and phenylboronic acid on to a hydrogel platform as an optical glucose sensor. The PhC converts the environment stimuli to optical signals that can be picked up easily with a detector. The fabricated gel can additionally detect saccharides and self-heal due to the boronate ester bond [186].
In recent years, research has not only focused on the application of hydrogel as a carrier but also to better the development and fabrication of the hydrogel system for in vivo applications. A novel method of fabricating hydrogel optical fibres through using multichannel microfluidic device had recently been reported that may path the way for facile fabrication techniques. The fabrication design was able to achieve dual-core optical fibre that is able to simultaneously deliver lights of two different wavelengths. The double network structure of acrylamide (Aam) and Alg, coupled with further functionalization with methacrylamide (DMA) developed a tough fibre with high tunability on the mechanical and optical properties suitable for spectroscopic measurements [121].
5. Perspective and conclusion
The current field of hydrogel research has been vastly developed, with potential applications in almost all industries, particularly prominent in the biomedical and agriculture applications. In this review, we explore a niche area of hydrogel waveguides for light delivery and manipulation. The appeal of hydrogels lies in the various amalgamation and modalities it can achieve. In many of the hydrogel waveguide research, it is not only of its own nature but also incorporates other disciplinary studies of nanomaterials, inorganic moieties, biological molecules, and printed electronics. The versatility of hydrogels, stimuli responsiveness, and tailorable properties of hydrogels give rise to multifaceted light manipulation and delivery platforms for devices, treatments, sensors, and monitoring in biomedical applications. In comparison to silica- and polymeric-based materials, hydrogels show great promise for in vivo applications.
Over the years, the pursuit of hydrogel waveguides and light manipulating systems for biomedical applications have continued to progress and innovate. However, there are challenges that prevents the implementation of these waveguides that needs to be further investigated. Poor mechanical property is a significant limitation in hydrogel development which would result in material damage during storage, handling, or dynamic body movement. Through the adjustments in the molecular weight of polymers, the crosslinking density, double network strategies, or the concentration of additives added, the mechanical strength of the hydrogel can be tailored. In the recent developments, double network strategies have been vastly explored to improve mechanical properties. Highly entangled double network hydrogels were developed by introducing an already densely entangled polymer network into a second network monomer solution resulting in excellent mechanical performance with high fracture energy under deformation [187]. This indicates promising potential to be adapted into hydrogel fabrication for light delivery.
Typically, hydrogels would swell or dehydrate depending on the water content, and water loss in the hydrogel system can lead to a stiff and brittle state. The swelling of hydrogels and swelling rate is dependent on the molecular weight or the crosslinking density of the polymer network. The increase in crosslinking density would cause a reduction in the swelling ratio, indicating lesser water uptake. The transparency of the hydrogels can be comprised if the swelling is not ideal. Furthermore, the swelling state of hydrogels can be utilized to better the mechanical properties of the hydrogel. A recent study indicated a swelling-stiffening property of 4D printed hydrogel scaffold, that may be beneficial in aqueous environments [188].
With the availability of natural and synthetic precursors, the development of hydrogel waveguides is still considerably challenging due the various mix of requirements, and most importantly, transparency. In the development of waveguides or light systems, additives to improve hydrogel characteristics or incorporate multifunctional capabilities can result in further light scattering and absorption that reduces the transmission of the hydrogels. As a result, majority of light-guiding hydrogel systems discussed are single functional materials. Notably, recent studies have developed dual function or switch-like waveguides through novel fabrication methods [168,177]. This indicates a promising direction towards multifunctional light guiding systems.
As we move forward, hydrogels are favourable candidates for light-guiding or photonic applications as the ideal refractive index can be achieve, and sophisticated fabrication methods shows a promising future. By understanding the intention of hydrogels, the fabrication methods can vary from a conventional highly transparent hydrogel to waveguiding strategies. In light manipulation of hydrogels, matching refractive index is ideal to ensure efficient light transfer between mediums. The shape of the hydrogel and the direction of light can also influence the direction of light propagation, for example a dome-like shape and cylindrical-shape fibres. In addition to the fibres, hydrogel waveguides commonly employ the concept of TIR. In some approaches, waveguides can be developed from a single core material without cladding and is dependent on the surrounding tissues to enclose the light propagation. These waveguides exhibit equally effective light guides in vivo applications. In most cases, hydrogel waveguide is made with at least two layers via mould injection and dip coating [19], co-axial extrusion [168], microfluidic channels [121], and most recently reported, surface polymerization of cladding [177]. In these approaches the refractive index of the cladding must be made lower than the core. Attributing to hydrogels low refractive index, many POF and SOF turn to hydrogels as a biocompatible cladding for in vivo applications.
In many research approaches, long-term stability of hydrogels in vivo has yet to be explored. There are multiple factors affecting the stability of waveguides, such as the exposure in the surrounding environment, erosion of the networks and the movement in the surroundings. Most research studies evaluate primarily on the biocompatibility of the hydrogels and not the biodegradation. The long-term stability is a critical aspect of hydrogels that can be estimated, possibly through erosion, repeated cycling, and simulation studies before moving forward into clinical applications.
Ethics approval and consent to participate
The manuscript is a comprehensive review on publicly reported research literatures, the informed consent and IRB approvals are not applicable.
CRediT authorship contribution statement
Pek Yin Michelle Yew: Writing – review & editing, Writing – original draft, Conceptualization. Pei Lin Chee: Writing – original draft. Qianyu Lin: Writing – original draft. Cally Owh: Writing – original draft. Jiayi Li: Writing – original draft. Qing Qing Dou: Writing – original draft. Xian Jun Loh: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. Kai Dan: Conceptualization, Resources, Supervision, Writing – review & editing. Yong Zhang: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is supported by the RIE2025 MTC Individual Research Grants (M22K2c0085) and Central Research Fund, administered by the Agency of Science, Technology and Research (A*STAR). This research is also supported by National Research Foundation (NRF) Singapore under its NRF Investigatorship (NRF-NRFI07–2021–0003). This work is also supported by the National Medical Research Council (NMRC), Singapore, under its Clinician Scientist-Individual Research Grant (CS-IRG) [MOH-001357-00]. The first author would like to acknowledge the Agency for Science, Technology and Research (A*STAR) for providing the sponsorship for her Eng. D programme in Biomedical Engineering at the National University of Singapore.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xian Jun Loh, Email: lohxj@imre.a-star.edu.sg.
Dan Kai, Email: kaid@imre.a-star.edu.sg.
Yong Zhang, Email: yozhang@cityu.edu.hk.
References
- 1.Khan R., Gul B., Khan S., Nisar H., Ahmad I. Refractive index of biological tissues: review, measurement techniques, and applications. Photodiagnosis Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2021.102192. [DOI] [PubMed] [Google Scholar]
- 2.Yuk H., Lu B., Zhao X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019;48:1642–1667. doi: 10.1039/c8cs00595h. [DOI] [PubMed] [Google Scholar]
- 3.Hardy L.A., Kennedy J.D., Wilson C.R., Irby P.B., Fried N.M. Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones. J. Biophot. 2017;10:1240–1249. doi: 10.1002/jbio.201600010. [DOI] [PubMed] [Google Scholar]
- 4.Liu B., Zhu H., Zhao D., Nian G., Qu S., Yang W. Hydrogel coating enabling mechanically friendly, step-index, functionalized optical fiber. Adv. Opt. Mater. 2021;9 [Google Scholar]
- 5.Biswas D. Optical fiber coatings for biomedical applications. Opt. Eng. 1992;31 [Google Scholar]
- 6.Egorov V., Tsyuryupa S., Kanilo S., Kogit M., Sarvazyan A. Soft tissue elastometer. Med. Eng. Phys. 2008;30:206–212. doi: 10.1016/j.medengphy.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacour S.P., Courtine G., Guck J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016;1 [Google Scholar]
- 8.Rezapour Sarabi M., Jiang N., Ozturk E., Yetisen A.K., Tasoglu S. Biomedical optical fibers. Lab Chip. 2021;21:627–640. doi: 10.1039/d0lc01155j. [DOI] [PubMed] [Google Scholar]
- 9.Shabahang S., Kim S., Yun S.-H. Light-guiding biomaterials for biomedical applications. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahinroosta M., Jomeh Farsangi Z., Allahverdi A., Shakoori Z. Hydrogels as intelligent materials: a brief review of synthesis, properties and applications. Mater. Today Chem. 2018;8:42–55. [Google Scholar]
- 11.Lin Q., Lim J.Y.C., Xue K., Su X., Loh X.J. Polymeric hydrogels as a vitreous replacement strategy in the eye. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120547. [DOI] [PubMed] [Google Scholar]
- 12.Chang H., Meng L., Shao C., Cui C., Yang J. Physically cross-linked silk hydrogels with high solid content and excellent mechanical properties via a reverse dialysis concentrated procedure. ACS Sustain. Chem. Eng. 2019;7:13324–13332. [Google Scholar]
- 13.Reimer M., Zollfrank C. Cellulose for light manipulation: methods, applications, and prospects. Adv. Energy Mater. 2021;11 [Google Scholar]
- 14.Zuluaga-Vélez A., Cómbita-Merchán D.F., Buitrago-Sierra R., Santa J.F., Aguilar-Fernández E., Sepúlveda-Arias J.C. Silk fibroin hydrogels from the Colombian silkworm Bombyx mori L: evaluation of physicochemical properties. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara E., Cabral T.D., Sato M., Oku H., Cordeiro C.M.B. Agarose-based structured optical fibre. Sci. Rep. 2020;10:7035. doi: 10.1038/s41598-020-64103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu L.-H., Qi C., Ma M.-G., Wan P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B. 2019;7:1541–1562. doi: 10.1039/c8tb02331j. [DOI] [PubMed] [Google Scholar]
- 17.Choi M., Humar M., Kim S., Yun S.H. Step‐index optical fiber made of biocompatible hydrogels. Adv. Mater. 2015;27:4081–4086. doi: 10.1002/adma.201501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R., Goddard N.J. A study of diffraction-based chitosan leaky waveguide (LW) biosensors. Analyst. 2021;146:4964–4971. doi: 10.1039/d1an00940k. [DOI] [PubMed] [Google Scholar]
- 19.Yetisen A.K., Jiang N., Fallahi A., Montelongo Y., Ruiz-Esparza G.U., Tamayol A., Zhang Y.S., Mahmood I., Yang S.-A., Kim K.S., Butt H., Khademhosseini A., Yun S.-H. Glucose-sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv. Mater. 2017;29 doi: 10.1002/adma.201606380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L., Ramezani H., Sun M., Xie M., Nie J., Lv S., Cai J., Fu J., He Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020;8:5020–5028. doi: 10.1039/d0bm00896f. [DOI] [PubMed] [Google Scholar]
- 21.Gaharwar A.K., Rivera C.P., Wu C.-J., Schmidt G. Transparent, elastomeric and tough hydrogels from poly(ethylene glycol) and silicate nanoparticles. Acta Biomater. 2011;7:4139–4148. doi: 10.1016/j.actbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Guo J., Liu X., Jiang N., Yetisen A.K., Yuk H., Yang C., Khademhosseini A., Zhao X., Yun S.H. Highly stretchable, strain sensing hydrogel optical fibers. Adv. Mater. 2016;28:10244–10249. doi: 10.1002/adma.201603160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J.-Y., Zhao X., Illeperuma W.R.K., Chaudhuri O., Oh K.H., Mooney D.J., Vlassak J.J., Suo Z. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding H., Lu J.Q., Wooden W.A., Kragel P.J., Hu X.H. Refractive indices of human skin tissues at eight wavelengths and estimated dispersion relations between 300 and 1600 nm. Phys. Med. Biol. 2006;51:1479–1489. doi: 10.1088/0031-9155/51/6/008. [DOI] [PubMed] [Google Scholar]
- 25.Shan D., Gerhard E., Zhang C., Tierney J.W., Xie D., Liu Z., Yang J. Polymeric biomaterials for biophotonic applications. Bioact. Mater. 2018;3:434–445. doi: 10.1016/j.bioactmat.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Liu J., Lin S., Zhao X. Hydrogel machines. Mater. Today. 2020;36:102–124. [Google Scholar]
- 27.Guimarães C.F., Ahmed R., Marques A.P., Reis R.L., Demirci U. Engineering hydrogel‐based biomedical photonics: design, fabrication, and applications. Adv. Mater. 2021;33 doi: 10.1002/adma.202006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umar M., Min K., Kim S. Advances in hydrogel photonics and their applications. APL Photonics. 2019;4 [Google Scholar]
- 29.Pearson S., Feng J., del Campo A. Lighting the path: light delivery strategies to activate photoresponsive biomaterials in vivo. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 30.Schechter R.J. Snell's Law: optimum pathway analysis. Surv. Ophthalmol. 1977;21:464–466. doi: 10.1016/s0039-6257(77)80002-7. [DOI] [PubMed] [Google Scholar]
- 31.Nazempour R., Zhang Q., Fu R., Sheng X. Biocompatible and implantable optical fibers and waveguides for biomedicine. Materials. 2018;11:1283. doi: 10.3390/ma11081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zubia J., Aldabaldetreku G., Durana G., Arrue J., Jiménez F. Light propagation in multi-step index optical fibres. Laser Photon. Rev. 2008;2:182–202. [Google Scholar]
- 33.Numai T. Springer Japan; Tokyo: 2015. Optical Waveguides, Fundamentals of Semiconductor Lasers; pp. 41–59. [Google Scholar]
- 34.Richards-Kortum R., Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- 35.Jacques S. Fractal nature of light scattering in tissues. J. Innov. Optical Health Sci. 2011;4 [Google Scholar]
- 36.Batool S., Nisar M., Mangini F., Frezza F., Fazio E. Scattering of light from the systemic circulatory system. Diagnostics. 2020;10:1026. doi: 10.3390/diagnostics10121026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuchin V.V. Light scattering study of tissues. Phys. Usp. 1997;40:495–515. [Google Scholar]
- 38.Welch A.J., Van Gemert M.J. 2011. Optical-thermal Response of Laser-Irradiated Tissue, Springer. [Google Scholar]
- 39.Yong J., Chen F., Yang Q., Du G., Shan C., Bian H., Farooq U., Hou X. Bioinspired transparent underwater superoleophobic and anti-oil surfaces. J. Mater. Chem. A. 2015;3:9379–9384. [Google Scholar]
- 40.Yoo G.Y., Lee S., Ko M., Kim H., Lee K.N., Kim W., Do Y.R. Diphylleia grayi-inspired intelligent hydrochromic adhesive film. ACS Appl. Mater. Interfaces. 2020;12:49982–49991. doi: 10.1021/acsami.0c13185. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C., Liu G., Lin Y., Li X., Meng N., Wang X., Fu S., Yu J., Ding B. Diphylleia grayi-inspired intelligent temperature-responsive transparent nanofiber membranes. Nano-Micro Lett. 2024;16:65. doi: 10.1007/s40820-023-01279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacucci G., Schertel L., Zhang Y., Yang H., Vignolini S. Light management with natural materials: from whiteness to transparency. Adv. Mater. 2021;33 doi: 10.1002/adma.202001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnsen S. Hidden in plain sight: the ecology and physiology of organismal transparency. Biol. Bull. 2001;201:301–318. doi: 10.2307/1543609. [DOI] [PubMed] [Google Scholar]
- 44.Holt A.L., Sweeney A.M. Open water camouflage via 'leaky' light guides in the midwater squid Galiteuthis. J. R. Soc. Interface. 2016;13 doi: 10.1098/rsif.2016.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J., Song C., Shan H., Jiang Y., Zhou L., Tao P., Wu J., Shang W., Deng T. Bioinspired color change through guided reflection. Adv. Opt. Mater. 2018;6 [Google Scholar]
- 46.Han S., Tang J., Qi X., Sun W., Jiang Z., Hou Y., Yang M., Feng S. Transparent hierarchical columnar nanocomposites. Chem. Eng. J. 2023;466 [Google Scholar]
- 47.Salameh C., Salviat F., Bessot E., Lama M., Chassot J.-M., Moulongui E., Wang Y., Robin M., Bardouil A., Selmane M., Artzner F., Marcellan A., Sanchez C., Giraud-Guille M.-M., Faustini M., Carminati R., Nassif N. Origin of transparency in scattering biomimetic collagen materials. Proc. Natl. Acad. Sci. USA. 2020;117:11947–11953. doi: 10.1073/pnas.2001178117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y Z.P., Huang H., Zhu J. Bio-Inspired transparent soft jellyfish robot. Soft Robot. 2023;10:590–600. doi: 10.1089/soro.2022.0027. [DOI] [PubMed] [Google Scholar]
- 49.Liu S., Tso C.Y., Du Y.W., Chao L.C., Lee H.H., Ho T.C., Leung M.K.H. Bioinspired thermochromic transparent hydrogel wood with advanced optical regulation abilities and mechanical properties for windows. Appl. Energy. 2021;297 [Google Scholar]
- 50.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakur S., Thakur V.K., Arotiba O.A. In: Hydrogels: Recent Advances. Thakur V.K., Thakur M.K., editors. Springer Singapore; Singapore: 2018. History, classification, properties and application of hydrogels: an overview; pp. 29–50. [Google Scholar]
- 52.Bashir S., Hina M., Iqbal J., Rajpar A., Mujtaba M., Alghamdi N., Wageh S., Ramesh K., Ramesh S. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. 2020;12:2702. doi: 10.3390/polym12112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elsherif M., Hassan M.U., Yetisen A.K., Butt H. Hydrogel optical fibers for continuous glucose monitoring. Biosens. Bioelectron. 2019;137:25–32. doi: 10.1016/j.bios.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Heo Y.J., Shibata H., Okitsu T., Kawanishi T., Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13399–13403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L., Zhong C., Ke D., Ye F., Tu J., Wang L., Lu Y. Ultrasoft and highly stretchable hydrogel optical fibers for in vivo optogenetic modulations. Adv. Opt. Mater. 2018;6 [Google Scholar]
- 56.Wang Y., Xie K., Yue H., Chen X., Luo X., Liao Q., Liu M., Wang F., Shi P. Flexible and fully implantable upconversion device for wireless optogenetic stimulation of the spinal cord in behaving animals. Nanoscale. 2020;12:2406–2414. doi: 10.1039/c9nr07583f. [DOI] [PubMed] [Google Scholar]
- 57.Teh D.B.L., Bansal A., Chai C., Toh T.B., Tucker R.A.J., Gammad G.G.L., Yeo Y., Lei Z., Zheng X., Yang F. A flexi‐PEGDA upconversion implant for wireless brain photodynamic therapy. Adv. Mater. 2020;32 doi: 10.1002/adma.202001459. [DOI] [PubMed] [Google Scholar]
- 58.Xing R., Liu K., Jiao T., Zhang N., Ma K., Zhang R., Zou Q., Ma G., Yan X. An injectable self-assembling collagen–gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016;28:3669–3676. doi: 10.1002/adma.201600284. [DOI] [PubMed] [Google Scholar]
- 59.Peng K., Zheng L., Zhou T., Zhang C., Li H. Light manipulation for fabrication of hydrogels and their biological applications. Acta Biomater. 2022;137:20–43. doi: 10.1016/j.actbio.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Li L., Scheiger J.M., Levkin P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019;31 doi: 10.1002/adma.201807333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomatsu I., Peng K., Kros A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011;63:1257–1266. doi: 10.1016/j.addr.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Shu T., Zheng K., Zhang Z., Ren J., Wang Z., Pei Y., Yeo J., Gu G.X., Ling S. Birefringent silk fibroin hydrogel constructed via binary solvent-exchange-induced self-assembly. Biomacromolecules. 2021;22:1955–1965. doi: 10.1021/acs.biomac.1c00065. [DOI] [PubMed] [Google Scholar]
- 63.Applegate M.B., Perotto G., Kaplan D.L., Omenetto F.G. Biocompatible silk step-index optical waveguides. Biomed. Opt Express. 2015;6:4221–4227. doi: 10.1364/BOE.6.004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melikov R., Press D.A., Kumar B.G., Dogru I.B., Sadeghi S., Chirea M., Yılgör İ., Nizamoglu S. Silk-hydrogel lenses for light-emitting diodes. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-07817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pal A.K., Goddard N.J., Dixon H.J., Gupta R. A self-referenced diffraction-based optical leaky waveguide biosensor using photofunctionalised hydrogels. Biosensors. 2020;10:134. doi: 10.3390/bios10100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaiswal A.K., Hokkanen A., Kapulainen M., KhakaloNonappa A., Ikkala O., Orelma H. Carboxymethyl cellulose (CMC) optical fibers for environment sensing and short-range optical signal transmission. ACS Appl. Mater. Interfaces. 2022;14:3315–3323. doi: 10.1021/acsami.1c22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulz A., Rickmann A., Wahl S., Germann A., Stanzel B.V., Januschowski K., Szurman P. Alginate- and hyaluronic acid–based hydrogels as vitreous substitutes: an in vitro evaluation. Transl. Vision Sci. & Technol. 2020;9:34. doi: 10.1167/tvst.9.13.34. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran H.A., Hoang T.T., Maraldo A., Do T.N., Kaplan D.L., Lim K.S., Rnjak-Kovacina J. Emerging silk fibroin materials and their applications: new functionality arising from innovations in silk crosslinking. Mater. Today. 2023;65:244–259. [Google Scholar]
- 69.Reizabal A., Costa C.M., Pérez‐Álvarez L., Vilas‐Vilela J.L., Lanceros‐Méndez S. Silk fibroin as sustainable advanced material: material properties and characteristics, processing, and applications. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 70.Galante R., Oliveira A.S., Topete A., Ghisleni D., Braga M., Pinto T.J., Colaco R., Serro A.P. Drug-eluting silicone hydrogel for therapeutic contact lenses: impact of sterilization methods on the system performance. Colloids Surf. B Biointerfaces. 2018;161:537–546. doi: 10.1016/j.colsurfb.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 71.Zheng H., Zuo B. Functional silk fibroin hydrogels: preparation, properties and applications. J. Mater. Chem. B. 2021;9:1238–1258. doi: 10.1039/d0tb02099k. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y., Zhu Z.S., Guan J., Wu S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021;125:57–71. doi: 10.1016/j.actbio.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Qiao X., Qian Z., Li J., Sun H., Han Y., Xia X., Zhou J., Wang C., Wang Y., Wang C. Synthetic engineering of spider silk fiber as implantable optical waveguides for low-loss light guiding. ACS Appl. Mater. Interfaces. 2017;9:14665–14676. doi: 10.1021/acsami.7b01752. [DOI] [PubMed] [Google Scholar]
- 74.Prajzler V., Arif S., Min K., Kim S., Nekvindova P. All-polymer silk-fibroin optical planar waveguides. Opt. Mater. 2021;114 [Google Scholar]
- 75.Yucel T., Cebe P., Kaplan D.L. Vortex-induced injectable silk fibroin hydrogels. Biophys. J. 2009;97:2044–2050. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umar M., Min K., Jo M., Kim S. Ultra-thin, conformal, and hydratable color-absorbers using silk protein hydrogel. Opt. Mater. 2018;80:241–246. [Google Scholar]
- 77.Sahoo J.K., Choi J., Hasturk O., Laubach I., Descoteaux M.L., Mosurkal S., Wang B., Zhang N., Kaplan D.L. Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties. Biomater. Sci. 2020;8:4176–4185. doi: 10.1039/d0bm00512f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu X., Yan L., Liu S., Tan Y., Xiao J., Cao Y., Chen K., Xiao W., Li B., Liao X. Preparation of silk fibroin/hyaluronic acid hydrogels with enhanced mechanical performance by a combination of physical and enzymatic crosslinking. J. Biomater. Sci. Polym. Ed. 2021;32:1635–1653. doi: 10.1080/09205063.2021.1932070. [DOI] [PubMed] [Google Scholar]
- 79.Mitropoulos A.N., Marelli B., Ghezzi C.E., Applegate M.B., Partlow B.P., Kaplan D.L., Omenetto F.G. Transparent, nanostructured silk fibroin hydrogels with tunable mechanical properties. ACS Biomater. Sci. Eng. 2015;1:964–970. doi: 10.1021/acsbiomaterials.5b00215. [DOI] [PubMed] [Google Scholar]
- 80.Galateanu B., Hudita A., Zaharia C., Bunea M.-C., Vasile E., Buga M.-R., Costache M. In: Cellulose-Based Superabsorbent Hydrogels. Mondal M.I.H., editor. Springer International Publishing; Cham: 2019. Silk-based hydrogels for biomedical applications; pp. 1791–1817. [Google Scholar]
- 81.Parker S.T., Domachuk P., Amsden J., Bressner J., Lewis J.A., Kaplan D.L., Omenetto F.G. Biocompatible silk printed optical waveguides. Adv. Mater. 2009;21:2411–2415. [Google Scholar]
- 82.Lv S., Zhang S., Zuo J., Liang S., Yang J., Wang J., Wei D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023;242 doi: 10.1016/j.ijbiomac.2023.124915. [DOI] [PubMed] [Google Scholar]
- 83.Tang W., Wang J., Hou H., Li Y., Wang J., Fu J., Lu L., Gao D., Liu Z., Zhao F., Gao X., Ling P., Wang F., Sun F., Tan H. Review: application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023;240 doi: 10.1016/j.ijbiomac.2023.124398. [DOI] [PubMed] [Google Scholar]
- 84.Alamrani N.A., Greenway G.M., Pamme N., Goddard N.J., Gupta R. A feasibility study of a leaky waveguide aptasensor for thrombin. Analyst. 2019;144:6048–6054. doi: 10.1039/c9an01421g. [DOI] [PubMed] [Google Scholar]
- 85.Feng L., Liu R., Zhang X., Li J., Zhu L., Li Z., Li W., Zhang A. Thermo-gelling dendronized chitosans as biomimetic scaffolds for corneal tissue engineering. ACS Appl. Mater. Interfaces. 2021;13:49369–49379. doi: 10.1021/acsami.1c16087. [DOI] [PubMed] [Google Scholar]
- 86.Ding B., Gao H., Song J., Li Y., Zhang L., Cao X., Xu M., Cai J. Tough and cell-compatible chitosan physical hydrogels for mouse bone mesenchymal stem cells in vitro. ACS Appl. Mater. Interfaces. 2016;8:19739–19746. doi: 10.1021/acsami.6b05302. [DOI] [PubMed] [Google Scholar]
- 87.Isobe N., Tsudome M., Kusumi R., Wada M., Uematsu K., Okada S., Deguchi S. Moldable crystalline α-chitin hydrogel with toughness and transparency toward ocular applications. ACS Appl. Polym. Mater. 2020;2:1656–1663. [Google Scholar]
- 88.Liu K., Du H., Zheng T., Liu H., Zhang M., Zhang R., Li H., Xie H., Zhang X., Ma M. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021;259 doi: 10.1016/j.carbpol.2021.117740. [DOI] [PubMed] [Google Scholar]
- 89.Zhao D., Zhu Y., Cheng W., Chen W., Wu Y., Yu H. Cellulose‐based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021;33 doi: 10.1002/adma.202000619. [DOI] [PubMed] [Google Scholar]
- 90.Zhu S., Kumar Biswas S., Qiu Z., Yue Y., Fu Q., Jiang F., Han J. Transparent wood-based functional materials via a top-down approach. Prog. Mater. Sci. 2023;132 [Google Scholar]
- 91.Surendran G., Sherje A.P. Cellulose nanofibers and composites: an insight into basics and biomedical applications. J. Drug Deliv. Sci. Technol. 2022;75 [Google Scholar]
- 92.Babaei-Ghazvini A., Vafakish B., Patel R., Falua K.J., Dunlop M.J., Acharya B. Cellulose nanocrystals in the development of biodegradable materials: a review on CNC resources, modification, and their hybridization. Int. J. Biol. Macromol. 2024;258 doi: 10.1016/j.ijbiomac.2023.128834. [DOI] [PubMed] [Google Scholar]
- 93.Orelma H., Hokkanen A., Leppänen I., Kammiovirta K., Kapulainen M., Harlin A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose. 2020;27:1543–1553. [Google Scholar]
- 94.Li J., Zhang J., Sun H., Yang Y., Ye Y., Cui J., He W., Yong X., Xie Y. An optical fiber sensor based on carboxymethyl cellulose/carbon nanotubes composite film for simultaneous measurement of relative humidity and temperature. Opt Commun. 2020;467 [Google Scholar]
- 95.Kuddushi M., Deng X., Nayak J., Zhu S., Xu B.B., Zhang X. A transparent, tough and self-healable biopolymeric composites hydrogel for open wound management. ACS Appl. Bio Mater. 2023;6:3810–3822. doi: 10.1021/acsabm.3c00455. [DOI] [PubMed] [Google Scholar]
- 96.Agate S., Argyropoulos D.S., Jameel H., Lucia L., Pal L. 3D photoinduced spatiotemporal resolution of cellulose-based hydrogels for fabrication of biomedical devices. ACS Appl. Bio Mater. 2020;3:5007–5019. doi: 10.1021/acsabm.0c00517. [DOI] [PubMed] [Google Scholar]
- 97.Zhou S., Guo K., Bukhvalov D., Zhang X.F., Zhu W., Yao J., He M. Cellulose hydrogels by reversible ion‐exchange as flexible pressure sensors. Adv. Mater. Technol. 2020;5 [Google Scholar]
- 98.Akashah M.H.N., Rani R.A., Saad N.H., Rahman M.K.A., Scully P.J., Makhsin S.R. 2021 IEEE Regional Symposium on Micro and Nanoelectronics (RSM) IEEE; 2021. Facile microwave-assisted synthesis of agarose hydrogel for fibre optic biosensors application; pp. 50–53. [Google Scholar]
- 99.Jain A., Yang A.H., Erickson D. Gel-based optical waveguides with live cell encapsulation and integrated microfluidics. Opt. Lett. 2012;37:1472–1474. doi: 10.1364/OL.37.001472. [DOI] [PubMed] [Google Scholar]
- 100.Fujiwara E., Cordeiro C.M., Oku H., Suzuki C.K. Sensing with agar-based optical waveguides. SPIE Future Sensing Technol. 2023:130–134. SPIE, 2023. [Google Scholar]
- 101.Goddard N.J., Gupta R. Speed and sensitivity–integration of electrokinetic preconcentration with a leaky waveguide biosensor. Sensor. Actuator. B Chem. 2019;301 [Google Scholar]
- 102.Ahmad Raus R., Wan Nawawi W.M.F., Nasaruddin R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021;16:280–306. doi: 10.1016/j.ajps.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Z. Chen, W. Wei, B.-J. Ni, Chapter 3 - algae-based alginate biomaterial: production and applications, in: H. Ngo, W. Guo, A. Pandey, J.-S. Chang, D.-J. Lee (Eds.) Biomass, Biofuels, and Biochemicals, Elsevier2022, pp. 37-66..
- 104.Sadeque M.S.B., Chowdhury H.K., Rafique M., Durmuş M.A., Ahmed M.K., Hasan M.M., Erbaş A., Sarpkaya İ., Inci F., Ordu M. Hydrogel-integrated optical fiber sensors and their applications: a comprehensive review. J. Mater. Chem. C. 2023;11:9383–9424. [Google Scholar]
- 105.Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madduma-Bandarage U.S.K., Madihally S.V. Synthetic hydrogels: synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021;138 [Google Scholar]
- 107.Vandekerckhove B., Missinne J., Vonck K., Bauwens P., Verplancke R., Boon P., Raedt R., Vanfleteren J. Technological challenges in the development of optogenetic closed-loop therapy approaches in epilepsy and related network disorders of the brain. Micromachines. 2020;12:38. doi: 10.3390/mi12010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y., Huang Y., Bai H., Wang G., Hu X., Kumar S., Min R. Biocompatible and biodegradable polymer optical fiber for biomedical application: a review. Biosensors. 2021;11:472. doi: 10.3390/bios11120472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu X., Huang W., Wu W.-H., Xue B., Xiang D., Li Y., Qin M., Sun F., Wang W., Zhang W.-B. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res. 2018;11:5556–5565. [Google Scholar]
- 110.Johannsmeier S., Torres-Mapa M.L., Ripken T., Heinemann D., Heisterkamp A. European Conference on Biomedical Optics. Optical Society of America; 2019. Hydrogels for light delivery in in vivo optogenetic applications. [Google Scholar]
- 111.Johannsmeier S., Nguyen M.T.T., Hohndorf R., Drager G., Heinemann D., Ripken T., Heisterkamp A. PEGDMA hydrogels for cell adhesion and optical waveguiding. ACS Appl. Bio Mater. 2020;3:7011–7020. doi: 10.1021/acsabm.0c00885. [DOI] [PubMed] [Google Scholar]
- 112.Torres-Mapa M.L., Singh M., Simon O., Mapa J.L., Machida M., Günther A., Roth B., Heinemann D., Terakawa M., Heisterkamp A. Fabrication of a monolithic lab-on-a-chip platform with integrated hydrogel waveguides for chemical sensing. Sensors. 2019;19:4333. doi: 10.3390/s19194333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johannsmeier S., Torres-Mapa M.L., Dipresa D., Ripken T., Heinemann D., Heisterkamp A. Hydrogels for targeted waveguiding and light diffusion. Opt. Mater. Express. 2019;9:3925–3940. [Google Scholar]
- 114.Zhang Z.F., Ma X., Wang H., Ye F. Influence of polymerization conditions on the refractive index of poly(ethylene glycol) diacrylate (PEGDA) hydrogels. Appl. Phys. A. 2018;124:283. [Google Scholar]
- 115.Feng J., Zheng Y., Bhusari S., Villiou M., Pearson S., del Campo A. Printed degradable optical waveguides for guiding light into tissue. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 116.Choi M., Choi J.W., Kim S., Nizamoglu S., Hahn S.K., Yun S.H. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics. 2013;7:987–994. doi: 10.1038/nphoton.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bae J., Park J., Kim S., Cho H., Kim H.J., Park S., Shin D.-S. Tailored hydrogels for biosensor applications. J. Ind. Eng. Chem. 2020;89:1–12. [Google Scholar]
- 118.Kant R., Tabassum R., Gupta B.D. Fiber optic SPR-based uric acid biosensor using uricase entrapped polyacrylamide gel. IEEE Photon. Technol. Lett. 2016;28:2050–2053. [Google Scholar]
- 119.Singh S., Mishra S.K., Gupta B.D. SPR based fibre optic biosensor for phenolic compounds using immobilization of tyrosinase in polyacrylamide gel. Sensor. Actuator. B Chem. 2013;186:388–395. [Google Scholar]
- 120.Sankaran S., Becker J., Wittmann C., Del Campo A. Optoregulated drug release from an engineered living material: self‐replenishing drug depots for long‐term, light‐regulated delivery. Small. 2019;15 doi: 10.1002/smll.201804717. [DOI] [PubMed] [Google Scholar]
- 121.Fitria G., Kwon M., Lee H., Singh A., Yoo K., Go Y., Kim J., Kim K.S., Yoon J. Microfluidic fabrication of highly efficient hydrogel optical fibers for in vivo fiber-optic applications. Adv. Opt. Mater. 2023;11 [Google Scholar]
- 122.Li Y., Luo S., Gui Y., Wang X., Tian Z., Yu H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors. 2023;13:287. doi: 10.3390/bios13020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu X., He C., Luo F., Wang H., Peng Z. Transparent, conductive hydrogels with high mechanical strength and toughness. Polymers. 2021;13 doi: 10.3390/polym13122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen K., Liu M., Wang F., Hu Y., Liu P., Li C., Du Q., Yu Y., Xiao X., Feng Q. nnHighly transparent, self-healing, and self-adhesive double network hydrogel for wearable sensors. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.846401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding B., Zeng P., Huang Z., Dai L., Lan T., Xu H., Pan Y., Luo Y., Yu Q., Cheng H.-M., Liu B. A 2D material–based transparent hydrogel with engineerable interference colours. Nat. Commun. 2022;13:1212. doi: 10.1038/s41467-021-26587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei H., Wang Z., Zhang H., Huang Y., Wang Z., Zhou Y., Xu B.B., Halila S., Chen J. Ultrastretchable, highly transparent, self-adhesive, and 3D-printable ionic hydrogels for multimode tactical sensing. Chem. Mater. 2021;33:6731–6742. [Google Scholar]
- 127.Cheng Q., Hao A., Xing P. Stimulus-responsive luminescent hydrogels: design and applications. Adv. Colloid Interface Sci. 2020;286 doi: 10.1016/j.cis.2020.102301. [DOI] [PubMed] [Google Scholar]
- 128.Liu S., Yuan H., Bai H., Zhang P., Lv F., Liu L., Dai Z., Bao J., Wang S. Electrochemiluminescence for electric-driven antibacterial therapeutics. J. Am. Chem. Soc. 2018;140:2284–2291. doi: 10.1021/jacs.7b12140. [DOI] [PubMed] [Google Scholar]
- 129.Ning Y., Lu F., Liu Y., Yang S., Wang F., Ji X., He Z. Glow-type chemiluminescent hydrogels for point-of-care testing (POCT) of cholesterol. Analyst. 2021;146:4775–4780. doi: 10.1039/d1an00676b. [DOI] [PubMed] [Google Scholar]
- 130.Ye J., Zhu L., Yan M., Xiao T., Fan L., Xue Y., Huang J., Yang X. An intensive and glow-type chemiluminescence of luminol-embedded, guanosine-derived hydrogel. Talanta. 2021;230 doi: 10.1016/j.talanta.2021.122351. [DOI] [PubMed] [Google Scholar]
- 131.Russell G.M., Inamori D., Masai H., Tamaki T., Terao J. Luminescent and mechanical enhancement of phosphorescent hydrogel through cyclic insulation of platinum-acetylide crosslinker. Polym. Chem. 2019;10:5280–5284. [Google Scholar]
- 132.Wei S., Qiu H., Shi H., Lu W., Liu H., Yan H., Zhang D., Zhang J., Theato P., Wei Y., Chen T. Promotion of color-changing luminescent hydrogels from thermo to electrical responsiveness toward biomimetic skin applications. ACS Nano. 2021;15:10415–10427. doi: 10.1021/acsnano.1c02720. [DOI] [PubMed] [Google Scholar]
- 133.Zhi H., Fei X., Tian J., Jing M., Xu L., Wang X., Liu D., Wang Y., Liu J. A novel transparent luminous hydrogel with self-healing property. J. Mater. Chem. B. 2017;5:5738–5744. doi: 10.1039/c7tb00975e. [DOI] [PubMed] [Google Scholar]
- 134.Li Y., Young D.J., Loh X.J. Fluorescent gels: a review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019;3:1489–1502. [Google Scholar]
- 135.Ganguly S., Margel S. Fluorescent quantum dots-based hydrogels: synthesis, fabrication and multimodal biosensing. Talanta Open. 2023;8 [Google Scholar]
- 136.Xue J., Xu X., Zhu Y., Yang D. Lanthanide based white-light-emitting hydrogel mediated by fluorescein and carbon dots with high quantum yield and multi-stimuli responsiveness. J. Mater. Chem. C. 2020;8:3380–3385. [Google Scholar]
- 137.Wang R., Zhang Y., Lu W., Wu B., Wei S., Wu S., Wang W., Chen T. Bio-inspired structure-editing fluorescent hydrogel actuators for environment-interactive information encryption. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202300417. [DOI] [PubMed] [Google Scholar]
- 138.Li S., Zeng Y., Hou W., Wan W., Zhang J., Wang Y., Du X., Gu Z. Photo-responsive photonic hydrogel: in situ manipulation and monitoring of cell scaffold stiffness. Mater. Horiz. 2020;7:2944–2950. [Google Scholar]
- 139.Guo J., Huang H., Zhou M., Yang C., Kong L. Quantum dots-doped tapered hydrogel waveguide for ratiometric sensing of metal ions. Anal. Chem. 2018;90:12292–12298. doi: 10.1021/acs.analchem.8b03787. [DOI] [PubMed] [Google Scholar]
- 140.Zong Y., Zong B., Zha R., Zhang Y., Li X., Wang Y., Fang H., Wong W.-L., Li C. An antibacterial and anti-oxidative hydrogel dressing for promoting diabetic wound healing and real-time monitoring wound pH conditions with a NIR fluorescent imaging system. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202300431. [DOI] [PubMed] [Google Scholar]
- 141.Li Z., Cheng H., Ke L., Liu M., Wang C.G., Jun Loh X., Li Z., Wu Y.L. Recent advances in new copolymer hydrogel‐formed contact lenses for ophthalmic drug delivery. ChemNanoMat. 2021;7:564–579. [Google Scholar]
- 142.Xiao W., Zhang X., Hao L., Zhao Y., Lin Q., Wang Z., Song W., Liang D. Modification of silicone hydrogel contact lenses for the selective adsorption of ofloxacin. J. Nanomater. 2022:2022. [Google Scholar]
- 143.Ji S., Zhou S., Zhang X., Chen W., Jiang X. Oxygen-sensitive probe and hydrogel for optical imaging and photodynamic antimicrobial chemotherapy of chronic wounds. Biomater. Sci. 2022;10:2054–2061. doi: 10.1039/d2bm00153e. [DOI] [PubMed] [Google Scholar]
- 144.Patrick P.S., Bear J.C., Fitzke H.E., Zaw-Thin M., Parkin I.P., Lythgoe M.F., Kalber T.L., Stuckey D.J. Radio-metal cross-linking of alginate hydrogels for non-invasive in vivo imaging. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gong J., Tanner M.G., Venkateswaran S., Stone J.M., Zhang Y., Bradley M. A hydrogel-based optical fibre fluorescent pH sensor for observing lung tumor tissue acidity. Anal. Chim. Acta. 2020;1134:136–143. doi: 10.1016/j.aca.2020.07.063. [DOI] [PubMed] [Google Scholar]
- 146.Wu C., Liu X., Ying Y. Soft and stretchable optical waveguide: light delivery and manipulation at complex biointerfaces creating unique windows for on-body sensing. ACS Sens. 2021;6:1446–1460. doi: 10.1021/acssensors.0c02566. [DOI] [PubMed] [Google Scholar]
- 147.Parsons A., Sharmin N., Shaharuddin S.I., Marshall M. Viscosity profiles of phosphate glasses through combined quasi-static and bob-in-cup methods. J. Non-Cryst. Solids. 2015;408:76–86. [Google Scholar]
- 148.Li Y., Yang H.Y., Lee D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Contr. Release. 2021;330:151–160. doi: 10.1016/j.jconrel.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Fang G., Yang X., Wang Q., Zhang A., Tang B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater. Sci. Eng. C. 2021;127 doi: 10.1016/j.msec.2021.112212. [DOI] [PubMed] [Google Scholar]
- 150.Hiroki A., Taguchi M. Development of environmentally friendly cellulose derivative-based hydrogels for contact lenses using a radiation crosslinking technique. Appl. Sci. 2021;11:9168. [Google Scholar]
- 151.Van Wyk H. Contact lens care. SA Pharmacist's Assistant. 2018;18:22–23. [Google Scholar]
- 152.Kim J., Cha E., Park J.-U. Recent advances in smart contact lenses. Adv. Mater. Technol. 2020;5 [Google Scholar]
- 153.Tran N.-P.-D., Yang M.-C. Synthesis and characterization of silicone contact lenses based on TRIS-DMA-NVP-HEMA hydrogels. Polymers. 2019;11:944. doi: 10.3390/polym11060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang M.-C., Tran-Nguyen P.L. Evaluation of silicone hydrogel contact lenses based on poly (dimethylsiloxane) dialkanol and hydrophilic polymers. Colloids Surf. B Biointerfaces. 2021;206 doi: 10.1016/j.colsurfb.2021.111957. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y., Zhang S., Cui Q., Ni J., Wang X., Cheng X., Alem H., Tebon P., Xu C., Guo C. Microengineered poly (HEMA) hydrogels for wearable contact lens biosensing. Lab Chip. 2020;20:4205–4214. doi: 10.1039/d0lc00446d. [DOI] [PubMed] [Google Scholar]
- 156.Lotfy N.M., Alasbali T., Alsharif A.M., Al-Gehedan S.M., Jastaneiah S., Al-Hazaimeh A., Ali H., Khandekar R. Comparison of the efficacy of lotrafilcon B and comfilcon A silicone hydrogel bandage contact lenses after transepithelial photorefractive keratectomy. Med. Hypothesis, Discov. Innovation (MEHDI) Ophthalmol. 2021;10:43–49. doi: 10.51329/mehdiophthal1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sánchez-López E., Espina M., Doktorovova S., Souto E., García M. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye–Part I–Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017;110:70–75. doi: 10.1016/j.ejpb.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 158.Desai D.T., Maulvi F.A., Desai A.R., Shukla M.R., Desai B.V., Khadela A.D., Shetty K.H., Shah D.O., Willcox M.D. In vitro and in vivo evaluation of cyclosporine-graphene oxide laden hydrogel contact lenses. Int. J. Pharm. 2022;613 doi: 10.1016/j.ijpharm.2021.121414. [DOI] [PubMed] [Google Scholar]
- 159.Vásquez Quintero A., Arai R., Yamazaki Y., Sato T., De Smet H. Near-field communication powered hydrogel-based smart contact lens. Adv. Mater. Technol. 2020;5 [Google Scholar]
- 160.Kim S.-K., Lee G.-H., Jeon C., Han H.H., Kim S.-J., Mok J.W., Joo C.-K., Shin S., Sim J.-Y., Myung D., Bao Z., Hahn S.K. Bimetallic nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens. Adv. Mater. 2022;34 doi: 10.1002/adma.202110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhu H., Yang H., Zhan L., Chen Y., Wang J., Xu F. Hydrogel-based smart contact lens for highly sensitive wireless intraocular pressure monitoring. ACS Sens. 2022;7:3014–3022. doi: 10.1021/acssensors.2c01299. [DOI] [PubMed] [Google Scholar]
- 162.Park Y.-G., Cha E., An H.S., Lee K.-P., Song M.H., Kim H.K., Park J.-U. Wireless phototherapeutic contact lenses and glasses with red light-emitting diodes. Nano Res. 2020;13:1347–1353. [Google Scholar]
- 163.Lee G.-H., Jeon C., Mok J.W., Shin S., Kim S.-K., Han H.H., Kim S.-J., Hong S.H., Kim H., Joo C.-K., Sim J.-Y., Hahn S.K. Smart wireless near-infrared light emitting contact lens for the treatment of diabetic retinopathy. Adv. Sci. 2022;9 doi: 10.1002/advs.202103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Current approaches in treatment of diabetic retinopathy and future perspectives. J. Ocul. Pharmacol. Therapeut. 2020;36:487–496. doi: 10.1089/jop.2019.0137. [DOI] [PubMed] [Google Scholar]
- 165.Kim K.H., Lee J.O., Du J., Sretavan D., Choo H. Real-time in vivo intraocular pressure monitoring using an optomechanical implant and an artificial neural network. IEEE Sensor. J. 2017;17:7394–7404. doi: 10.1109/JSEN.2017.2760140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nizamoglu S., Gather M.C., Humar M., Choi M., Kim S., Kim K.S., Hahn S.K., Scarcelli G., Randolph M., Redmond R.W. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 2016;7:1–7. doi: 10.1038/ncomms10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jacques S.L. Optical properties of biological tissues: a review. Phys. Med. Biol. 2013;58 doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 168.Chen G., Hou K., Yu N., Wei P., Chen T., Zhang C., Wang S., Liu H., Cao R., Zhu L., Hsiao B.S., Zhu M. Temperature-adaptive hydrogel optical waveguide with soft tissue-affinity for thermal regulated interventional photomedicine. Nat. Commun. 2022;13:7789. doi: 10.1038/s41467-022-35440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.H. Ramollari, P. Measor, A low-loss 3D printed ridge waveguide via stereolithography, Proc. SPIE Vol, pp. 120040S-120041..
- 170.Guo J., Yang C., Dai Q., Kong L. Soft and stretchable polymeric optical waveguide-based sensors for wearable and biomedical applications. Sensors. 2019;19:3771. doi: 10.3390/s19173771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elsherif M., Hassan M.U., Yetisen A.K., Butt H. Glucose sensing with phenylboronic acid functionalized hydrogel-based optical diffusers. ACS Nano. 2018;12:2283–2291. doi: 10.1021/acsnano.7b07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hou Z., Sun S., Zheng B., Yang R., Li A. Stimuli-responsive protein-based micro/nano-waveguides. RSC Adv. 2015;5:77847–77850. [Google Scholar]
- 173.Zhuo X., Shen H., Bian Y., Xu A., Zhu R. Projection-suspended stereolithography 3D printing for low-loss optical hydrogel fiber fabrication. APL Photonics. 2021;6 [Google Scholar]
- 174.Ceci-Ginistrelli E., Pugliese D., Boetti N.G., Novajra G., Ambrosone A., Lousteau J., Vitale-Brovarone C., Abrate S., Milanese D. Novel biocompatible and resorbable UV-transparent phosphate glass based optical fiber. Opt. Mater. Express. 2016;6:2040–2051. [Google Scholar]
- 175.Gierej A., Geernaert T., Van Vlierberghe S., Dubruel P., Thienpont H., Berghmans F. Challenges in the fabrication of biodegradable and implantable optical fibers for biomedical applications. Materials. 2021;14:1972. doi: 10.3390/ma14081972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song D., Yang R., Wang H., Fang S., Liu Y., Long F., Zhu A. Development of dual-color total internal reflection fluorescence biosensor for simultaneous quantitation of two small molecules and their affinity constants with antibodies. Biosens. Bioelectron. 2019;126:824–830. doi: 10.1016/j.bios.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 177.Zhu B., Liu D., Wu J., Meng C., Yang X., Wang Y., Jia X., Jiang P., Wang X. Slippery core-sheath hydrogel optical fiber built by catalytically triggered interface radical polymerization. Adv. Funct. Mater. 2024;a [Google Scholar]
- 178.Chen G., Wang G., Tan X., Hou K., Meng Q., Zhao P., Wang S., Zhang J., Zhou Z., Chen T., Cheng Y., Hsiao B.S., Reichmanis E., Zhu M. Integrated dynamic wet spinning of core-sheath hydrogel fibers for optical-to-brain/tissue communications. Natl. Sci. Rev. 2020;8 doi: 10.1093/nsr/nwaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhuo X., Han Y., Bian Y., Xu A., Shen H. A promising optical bio-interface: graded-index hydrogel fiber. Adv. Opt. Mater. 2024;12 [Google Scholar]
- 180.Guimarães C.F., Ahmed R., Mataji-Kojouri A., Soto F., Wang J., Liu S., Stoyanova T., Marques A.P., Reis R.L., Demirci U. Engineering polysaccharide-based hydrogel photonic constructs: from multiscale detection to the biofabrication of living optical fibers. Adv. Mater. 2021;33 doi: 10.1002/adma.202105361. [DOI] [PubMed] [Google Scholar]
- 181.Wang H., Zhou S., Guo L., Wang Y., Feng L. Intelligent hybrid hydrogels for rapid in situ detection and photothermal therapy of bacterial infection. ACS Appl. Mater. Interfaces. 2020;12:39685–39694. doi: 10.1021/acsami.0c12355. [DOI] [PubMed] [Google Scholar]
- 182.Ran P., Xia T., Zheng H., Lei F., Zhang Z., Wei J., Li X. Light-triggered theranostic hydrogels for real-time imaging and on-demand photodynamic therapy of skin abscesses. Acta Biomater. 2023;155:292–303. doi: 10.1016/j.actbio.2022.11.039. [DOI] [PubMed] [Google Scholar]
- 183.Alqurashi Y., Elsherif M., Hendi A., Essa K., Butt H. Optical hydrogel detector for pH measurements. Biosensors. 2022;12:40. doi: 10.3390/bios12010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li J., Ji C., Lu B., Rodin M., Paradies J., Yin M., Kuckling D. Dually crosslinked supramolecular hydrogel for cancer biomarker sensing. ACS Appl. Mater. Interfaces. 2020;12:36873–36881. doi: 10.1021/acsami.0c08722. [DOI] [PubMed] [Google Scholar]
- 185.Li J., Ji C., Yu X., Yin M., Kuckling D. Dually cross-linked supramolecular hydrogel as surface plasmon resonance sensor for small molecule detection. Macromol. Rapid Commun. 2019;40 doi: 10.1002/marc.201900189. [DOI] [PubMed] [Google Scholar]
- 186.Chen Q., Wei Z., Wang S., Zhou J., Wu Z. A self-healing smart photonic crystal hydrogel sensor for glucose and related saccharides. Mikrochim. Acta. 2021;188:210. doi: 10.1007/s00604-021-04849-3. [DOI] [PubMed] [Google Scholar]
- 187.Zhu R., Zhu D., Zheng Z., Wang X. Tough double network hydrogels with rapid self-reinforcement and low hysteresis based on highly entangled networks. Nat. Commun. 2024;15:1344. doi: 10.1038/s41467-024-45485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu B., Li H., Meng F., Xu Z., Hao L., Yao Y., Zhu H., Wang C., Wu J., Bian S., Lu W.W., Liu W., Pan H., Zhao X. 4D printed hydrogel scaffold with swelling-stiffening properties and programmable deformation for minimally invasive implantation. Nat. Commun. 2024;15:1587. doi: 10.1038/s41467-024-45938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]