Abstract
The biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in liquid cultures with municipal anaerobic sludge showed that at least two degradation routes were involved in the disappearance of the cyclic nitramine. In one route, RDX was reduced to give the familiar nitroso derivatives hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) and hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine (DNX). In the second route, two novel metabolites, methylenedinitramine [(O2NNH)2CH2] and bis(hydroxymethyl)nitramine [(HOCH2)2NNO2], formed and were presumed to be ring cleavage products produced by enzymatic hydrolysis of the inner C—N bonds of RDX. None of the above metabolites accumulated in the system, and they disappeared to produce nitrous oxide (N2O) as a nitrogen-containing end product and formaldehyde (HCHO), methanol (MeOH), and formic acid (HCOOH) that in turn disappeared to produce CH4 and CO2 as carbon-containing end products.
RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) (Royal Demolition Explosive) is a powerful, highly energetic chemical whose wide use in various military and civilian applications has resulted in severe soil and groundwater contamination (11, 28). It has been estimated that, during RDX manufacturing, up to 12 mg/liter may be discharged into the environment in process wastewaters (15). In general, cyclic nitramine explosives have been proven to be toxic (31, 35). The toxicity of cyclic nitramines necessitates that contaminated soil and groundwater be remediated, preferably biologically.
There is little existing information regarding biodegradation of cyclic nitramines such as RDX, particularly in regard to ring cleavage products and the enzymes and metabolic pathways that lead to their formation (4, 8, 17, 39, 40). RDX and HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazine) are reportedly degraded by anaerobic sludge (25); under nitrate-reducing, sulfidogenic, and/or methanogenic conditions (5, 9, 17); by specific isolates (17, 37, 39, 40); or by consortia (10). Despite these efforts, no further definition of the degradation pathway of RDX was obtained since the early work of McCormick et al. (25). A pathway based on the sequential reduction of RDX to hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX), hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine (DNX), and hexahydro-1,3,5-trinitroso-1,3,5-triazine (TNX) was suggested. The nitroso compounds were proposed to undergo further transformation to unstable hydroxylamino-RDX derivatives, HOHN-RDX, which subsequently undergo ring cleavage to eventually yield HCHO, CH3OH, NH2NH2, and (H3C)2NNH2. The pathway proposed by McCormick et al. (25) is speculative, since the authors did not identify any of the intermediate ring cleavage products. One objective from the present study is thus to apply a combination of analytical techniques (liquid chromatography-mass spectrometry [LC-MS], SPME-gas chromatography [GC]-MS, capillary electrophoresis [CE]-UV, and other GC techniques) using 15N- and 14C-labeled RDX to confirm the identity of certain intermediates during biodegradation of RDX with a municipal anaerobic sludge.
Commercial-grade RDX (with a purity of >99%) was provided by the Defence Research Establishment Valcartier, Val Bélair, Quebec, Canada. Uniformly 14C-labeled RDX, [U-14C]RDX, was synthesized and recrystallized to a chemical and radiochemical purity reaching 99 and 97%, respectively, by Ampleman et al. (1). The specific activity of the radioactive compound was 28.7 mCi/mmol. Ring-labeled [15N]RDX (purity, >98%) was also synthesized as described by Ampleman et al. (2). MNX and TNX were synthesized according to the method described by Brockman et al. (6). All other chemicals were of reagent grade. The municipal sludge, which in the past proved to be an excellent source of microorganisms, particularly methanogens (32), was obtained from a food factory (Cornwall, Ontario, Canada) and was used as the exogenous source of microorganisms. The sludge was always obtained fresh and stored at 4°C when not in use. The viability of the sludge was measured using the glucose activity test. On average, the biomass concentration of the sludge was 8 g of volatile suspended solid per liter with a O-mV reduction potential (Eh) before incubation that dropped down to a range of −250 to −300 mV during the biodegradation of RDX. A BBL dry anaerobic indicator (VWR; Canlab) was used to detect air leaks and to ensure anaerobic conditions.
Microcosms for the degradation of RDX were prepared as described earlier (13). Briefly, serum bottles (100 ml) were each charged with fresh municipal anaerobic sludge (5 ml), a phosphate-buffered (pH 7) mineral salt medium (10 ml), glucose (2.1 g/liter) to serve as a C source, and RDX (200 mg/liter) to serve as an N source. RDX was added in excess of its water solubility (ca. 60 mg/liter) (35, 38) to generate sufficient amounts of metabolites. The final working volume for the liquid medium in the microcosm was 50 ml. Some serum bottles (microcosms) were supplemented with [U-14C]RDX (100,000 dpm) and then fitted with a small test tube containing 1.0 ml of 0.5 M KOH to trap liberated carbon dioxide (14CO2) for subsequent measurement using a Packard Tri-Carb 4530 liquid scintillation counter (Model 2100 TR; Packard Instrument Company, Meriden, Conn.). The headspace in each microcosm was flushed with nitrogen gas to maintain anaerobic conditions and then sealed with butyl rubber septa and aluminum crimp seals to prevent the loss of CO2 and other volatile metabolites. For the analysis of N2 and N2O, the headspace was flushed with nitrogen-free argon gas prior to the onset of biodegradation. Two control microcosms were prepared: one contained the sludge without RDX, and the second contained RDX and an autoclaved sludge. Each microcosm was wrapped with aluminum foil to protect the mixture against photolysis.
The nitroso products MNX and DNX and the ring cleavage products were analyzed using a Micromass Platform bench top single-quadropole mass detector fronted by a Hewlett-Packard 1100 Series high-performance LC (HPLC) system. Analyte ionization was done in a negative electrospray (ES) ionization mode producing mainly the deprotonated mass ions, [M − H]− (13). Formaldehyde (HCHO) was analyzed as its oxime derivative using SPME fiber coated with poly(dimethylsiloxane)-divinylbenzene (Supelco) and O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine as a derivatizing agent as described by Martos and Pawliszyn (22). The SPME fiber, coated with the derivatizing agent, was placed inside the headspace of a fixed volume (1 ml) of the RDX-spiked culture medium at 55°C with stirring for 20 min. The SPME assembly was then allowed to undergo desorption (250°C) inside the injector port of a GC (HP 6890)-MSD (HP 5973) system. Formic acid (HCOOH) was analyzed by CE and UV detection using a Hewlett-Packard HP3D CE system consisting of a photodiode array detector and an HP fused-silica bubble capillary (inside diameter, 50 μm; length, 56 cm) as described by Chen et al. (7). [14C]RDX was added to the microcosms to help identify RDX metabolites (CH4, MeOH, and HCOOH), since most of them are expected to be generated from the sludge.
The presence of nitrous oxide was confirmed using GC-MS analysis and by monitoring the mass ion at m/z 45 Da (15N14NO). An SRI 8610 GC (INSUS Systems Inc.) connected to a Supelco Porapack Q column (2 m) was coupled with either an electron capture detector (330°C) for the detection of N2O or a GC-RAM radioactivity detector for the detection of 14CH4.
The attempted analysis of hydrazine and dimethyl hydrazines was carried out using a Dionex Model DX-500 ion chromatograph system consisting of a GP40 gradient pump and an ED40 electrochemical detector as described by Larson and Strong (21). A more sensitive analytical technique (SPME-GC-MS, with a picogram detection limit) was also employed to confirm the absence of hydrazines as RDX metabolites. Ammonium cation was analyzed for the aqueous phase of the culture medium using an SP 8100 HPLC system equipped with a Waters 431 conductivity detector and a Hamilton PRP-X200 (250 mm by 4.1 mm by 10 μm) analytical cation-exchange column using 30% methanol in 4 mM nitric acid at a flow rate of 0.75 ml/min. The NO2− was analyzed using CE as described by Okemgbo et al. (29).
For the time course study, new microcosms were prepared as described above, but each was sacrificed in the course of analysis to monitor the concentration of RDX and its metabolites. The disappearance of RDX was monitored by extracting the remaining fraction of RDX with acetonitrile (50 ml) and then measuring RDX concentrations with a Waters HPLC system (Waters Chromatography Division) composed of a model 600 pump, an autoinjector (Model 710 Plus), and a Model 996 photodiode array detector (254 nm).
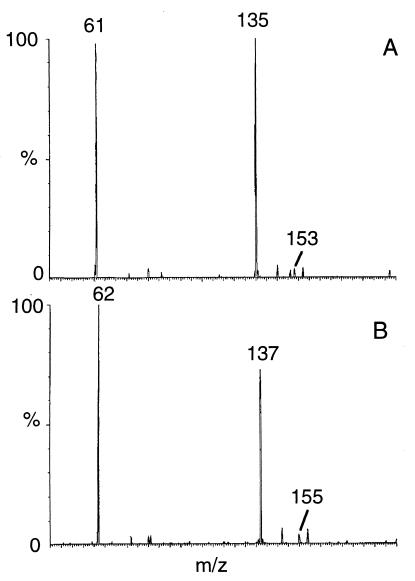
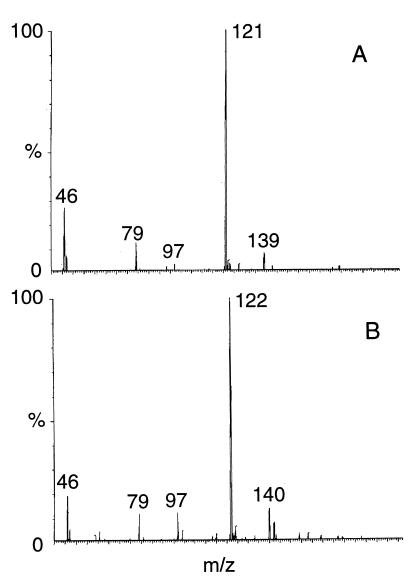
LC-MS (negative ES) analysis of liquid cultures obtained during biodegradation of RDX in the anaerobic sludge at pH 7 shows the presence of the nitroso products (MNX and DNX). Both were identified by their mass data using their deprotonated molecular mass ions and retention times ([M − H], daltons; retention time, minutes) as follows: (205, 16.3) and (189, 15.3), respectively. No TNX was detected. Two other LC-MS (negative-ES) peaks were detected and considered RDX ring cleavage products. For instance, one peak showed a deprotonated molecular mass ion [M − H] at 135 Da (m/z 136 Da), matching a molecular mass formula of CH4N4O4 (Fig. 1A). Using [U-15N]RDX, the [M − H] of peak 1 was observed at 137 Da (m/z 138 Da), indicating that two of the nitrogen atoms in the product were from the ring (Fig. 1B). Other relevant mass ions included those at m/z 153, 61, and 46 Da (traces) representing the solvent adduct ([M − H] + H2O), the fragment -NHNO2 and group -NO2, respectively. When the ring-labeled [15N]RDX was used, the mass ions occurring at m/z 153 and 61 Da appeared at 155 (two N atoms from the ring) and 62 (one N atom from the ring) Da, respectively. The metabolite was tentatively identified as methylenedinitramine [(O2NNH)2CH2]. On the other hand, the mass spectrum of the second LC-MS peak showed a [M − H] at 121 Da (m/z 122 Da), matching a molecular mass formula of C2H6N2O4 (Fig. 2A). Other relevant mass ions from the above LC-MS peak included one at m/z 139 Da representing the adduct ([M − H] + H2O). Another characteristic mass ion was detected at m/z 46 Da, representing the fragment mass ion -NO2. Once again, using the ring-labeled [15N]RDX, the deprotonated molecular mass ion [M − H] and its adduct with water were detected with 1 mass unit higher than those observed without labeling (122 and 140 Da instead of 121 and 139 Da, respectively), indicating that one N atom in the metabolite was originating from the ring (Fig. 2B). The metabolite was tentatively identified as bis(hydroxymethyl)nitramine [(HOCH2)2NNO2]. Both metabolites accounted for the six N atoms in RDX including the three -NO2 groups (two -NO2 groups in the first product and one in the second), supporting the views that the two metabolites originated from the cleavage of RDX. We did not study the fraction of RDX that was reduced to MNX and DNX and the fraction that led to ring cleavage in the present report.
FIG. 1.
Mass spectrum of methylenedinitramine, (O2NNH)2CH2, obtained during degradation of RDX. (A) Unlabeled RDX; (B) ring-labeled [15N]RDX.
FIG. 2.
Mass spectrum of bis(hydroxymethyl)nitramine, (HOCH2)2NNO2, obtained during degradation of RDX. (A) Unlabeled RDX; (B) ring-labeled [15N]RDX. Mass ions 79 and 97 were from the mobile phase.
When a control containing RDX and buffer (pH 7) was studied in the absence of sludge, negligible reductions in the concentration of RDX were observed. In addition, MNX, DNX, and the two ring cleavage products were not detected. The alkaline (pH > 8) hydrolysis of RDX has been reported to produce pentahydro-3,5-dinitro-1,3,5-triazacyclohex-1-ene (12, 14). Interestingly, this last intermediate was not formed during RDX treatment with the sludge, confirming the biotic nature of methylenedinitramine and bis(hydroxymethyl)nitramine formation. Furthermore, the treatment of RDX with autoclaved sludge did not produce any of the above products.
In an earlier study, Binks et al. (4) reported two intermediate products from the treatment of RDX with the aerobic isolate Stenotrophomonas maltophilia PB1. Using GC-MS (ES ionization), Binks et al. (4) identified the first of the two products as methylene-N-nitroamino-N′-acetoxyammonium chloride (m/z 171 Da) and the second as methylene-N-(hydroxymethyl)-hydroxylamine-N′-(hydroxymethyl)nitroamine (m/z 167 Da). The first product was suggested to originate from the impurity 1-acetylhexahydro-3,5-dinitro-1,3,5-triazine known to be present with RDX. The reaction of methylenedinitramine with HCl (the acid used by Binks et al. [4]) would give a salt with the same molecular mass ion (m/z at 171 Da) observed by Binks et al. (4). Interestingly, the mass spectrum by Binks et al. (4) contained a strong mass ion with m/z at 136 Da characteristic of the molecular mass ion of methylenedinitramine. More recently, Bier et al. (3) reported on the formation of methylenedinitramine (m/z 136 Da) while photolyzing RDX with Fenton reagent. The intermediate was detected as the biphosphate salt, supporting the views that the product observed by Binks et al. (4) at m/z 171 Da was more likely the chloride salt of methylenedinitramine. When we attempted to degrade TNX with the sludge, neither of the two ring cleavage products was observed. In contrast, TNX was found to be recalcitrant compared to RDX since its rate of disappearance was lower than that of RDX by at least a factor of 10. Based on the above observation, we proposed that the trinitroso derivative TNX (or its HOHN-RDX) did not play a critical role during RDX biodegradation with the municipal sludge used in the present study. In contrast, McCormick et al. (25, 26) postulated the presence of TNX as an RDX metabolite that was biotransformed further to HONH-RDX prior to ring cleavage.
Both metabolites [methylenedinitramine, (O2NNH)2CH2, and bis(hydroxymethyl)nitramine, (HOCH2)2NNO2] disappeared, and their disappearance was accompanied by the formation of a new set of products including N2O, HCHO, HCOOH, CH4, and CO2. The presence of nitrous oxide as an RDX metabolite was confirmed by using the ring-labeled [15N]RDX and monitoring the mass ion appearing at m/z 45 Da, matching a molecular formula of 15N14NO. At the end of the experiment, which lasted 50 days, relatively high yields of nitrous oxide (22.6%) and carbon dioxide (60%) were detected following the complete disappearance of RDX. In addition to the above products, 16.3% of the total C content and 5.5% of the total N content of RDX remained in the liquid phase as a soluble nonextractable fraction. The formation of soluble nonextractable products following RDX biodegradation has been observed previously (17, 25, 39, 40).
On the other hand, no hydrazine was detected during biodegradation of RDX with the sludge. In contrast, McCormick et al. (25) reported the formation of hydrazine, 1,1′-dimethylhydrazine, and 1,2-dimethylhydrazine without observing CH4 or N2O. Interestingly, the same group of authors did not observe any hydrazine from HMX and added that hydrazines are unstable in water, particularly under aerobic and anaerobic conditions (26). Reportedly, hydrazine reacts with formaldehyde to form the condensation product formaldazine, which has the tendency to polymerize (16, 23).
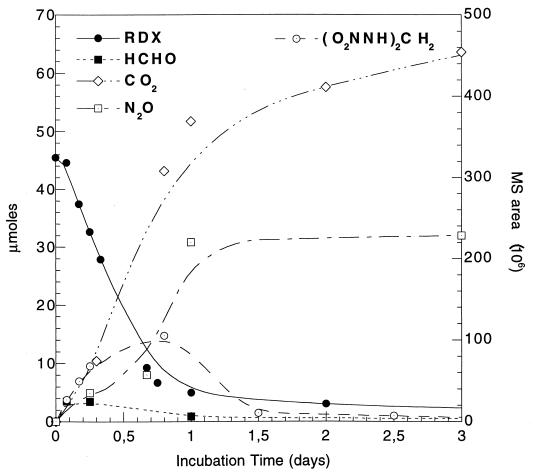
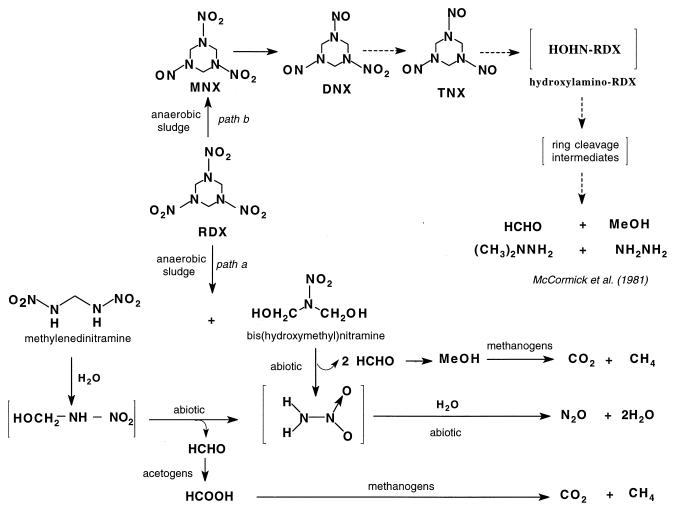
A time course study for the disappearance of RDX and the appearance and disappearance of its metabolites including the ring cleavage product methylenedinitramine is shown in Fig. 3. Figure 3 also shows the appearance and disappearance of formaldehyde and the accumulation of the end products nitrous oxide and carbon dioxide. The time course study for biodegradation of RDX shown in Fig. 3 is best explained by the degradation pathway constructed in Fig. 4. The mechanisms through which RDX disappeared and its intermediates MNX, DNX, and the ring cleavage products formed are not yet clear, and further investigation is required. The types of microorganisms and enzymes involved in the biodegradation process have not been identified. Nonetheless, the sludge used in the present study is expected to contain several microbial strains including the methanogenic and acetogenic bacteria. Earlier, Rocheleau et al. (32) used in situ hybridization and confocal scanning laser microscopy to confirm the presence of Methanosaeta concilii and Methanosarcina barkeri in the sludge.
FIG. 3.
A time course study for the disappearance of RDX (200 mg/liter) and the appearance and disappearance of methylenedinitramine [(O2NNH)2CH2], formaldehyde (HCHO), nitrous oxide (N2O), and carbon dioxide (14CO2) during treatment with municipal anaerobic sludge. The mass area is used for the quantification of methylenedinitramine [(O2NNH)2CH2] because no reference is available. Bis(hydroxymethyl)nitramine [(HOCH2)2NNO2] was not included in the study because its decomposition rate is high and only traces can be detected.
FIG. 4.
A constructed pathway for the biodegradation of RDX (200 mg/liter) with municipal anaerobic sludge (pH 7.0). Path a, postulated pathway via direct ring cleavage of RDX; path b, degradation via reduction to the nitroso derivatives followed by ring cleavage (modified McCormick et al. pathway [25]). Dashed arrows represent steps that have not been observed in the present study, and square brackets signify that no quantitation was done.
Initial enzymatic cleavage of the inner N—C—N bonds in RDX, possibly by a hydrolase, produced the two metabolites (O2NNH)2CH2 and (HOCH2)2NNO2, because we observed them only in the presence of the sludge (Fig. 4, path a). As far as we are aware, no specific enzymes have ever been reported to cause the inner C—N bonds in cyclic nitramine explosives such as RDX to cleave. Alkylnitramines and hydroxyalkylnitramine, however, are unstable in water and hydrolyze (enzymatically or abiotically) to produce nitramine, NH2NO2, and formaldehyde, HCHO (19, 20, 36). For example, hydroxymethylnitramine is known to exist as an equilibrated mixture with its dissociated products HCHO and NH2NO2 in water. Nitramines themselves undergo spontaneous decomposition to give N2O in water (14, 19, 20, 36) (Fig. 4, path a). It has also been reported that N2O can be generated from nitrite reduction under anaerobic conditions by first forming the hydroxylamine which in turn is transformed to N2O (34). In the present study, only traces of nitrite at the beginning of the experiment which did not accumulate were seen. When RDX was treated with the sludge under a blanket of argon, we were able to detect traces of nitrogen gas confirmed by the detection of 15N14N by MS (m/z 29 Da). We are not sure of the mechanisms that produced nitrogen from RDX, although it has been reported previously that facultatively and obligately anaerobic bacteria can reduce N2O to nitrogen (24).
Also, as the time course study in Fig. 3 reveals, formaldehyde (HCHO) did not accumulate during biodegradation of RDX with the sludge. We also observed formic acid, methanol, and methane, whose presence was confirmed using [U-14C]RDX. Apparently, acetogenic bacteria expected to be present in the sludge biotransformed HCHO to methanol and formic acid, whereas methanogens biotransformed methanol and formic acid into methane and carbon dioxide (Fig. 4, path a). We have not identified any microorganism in the sludge yet, and this should be the subject of future research.
Additionally, RDX could be reduced by a two-electron transfer process (type I nitroreductase?) to give MNX and DNX (Fig. 4, path b). Several other researchers (17, 39, 40) observed MNX, DNX, and TNX as RDX biotransformation products, and yet no reports have described hydroxylamino derivatives (HOHN-RDX) as RDX metabolites.
Alternatively, single-electron reduction could occur via type II nitroreductase to produce the free anion radical RDX·−, which should undergo denitration (-NO2−) to produce the unstable denitrated free radical, RDX·, which might also lead to a rapid ring cleavage. None of the above nitroreductases have been identified in the present study. Peterson et al. (30) reported that Escherichia coli can reduce nitrofurazone via the two types of nitroreductases (type I and type II) and confirmed the presence of free radicals (type II) by electron spin resonance spectroscopy. A nitroreductase enzyme capable of reducing nitrobenzene has been isolated and characterized from Pseudomonas pseudoalkaligenes JS45 (33). Kitts et al. (18) recently reported the degradation of RDX by the enteric bacterium Morganella morganii by the oxygen-insensitive type I nitroreductase (a two-electron transfer process). No intermediate products were described.
We assume that, following any initial enzymatic attack on RDX leading to the cleavage of any of the bonds in RDX (inner N—C or external N—NO2 and C—H bond), the resulting intermediate becomes very unstable. Melius (27) reported that, once an external bond in RDX is cleaved, some of the inner C—N bonds would be <2 kcal/mol and spontaneously decompose to produce N2, N2O, HCHO, and HCOOH.
The tentative conclusion is thus made that, once the RDX ring is cleaved, the fate of the resulting intermediates will be determined by competitive microbial and chemical processes. For instance, most of the products discussed in this article are thermally unstable and undergo fast hydrolytic cleavage in water. Due to the complexity of the anaerobic sludge, none of the microorganisms or the enzymes responsible for this hydrolysis were investigated. Nonetheless, the present study should open the way for future research to further enhance our understanding of the metabolic pathways, microbial communities, and molecular biology of biodegradation of cyclic nitramines. The present study revealed the presence of some RDX ring cleavage metabolites including methylene dinitramine [(O2NNH)2CH2] and bis(hydroxymethyl)nitramine [(HOCH2)2NNO2] and the end product nitrous oxide (N2O) that have never been reported before during biodegradation of cyclic nitramine explosives. Finally, the formation of N2O as an end product is seemingly a universal observation since preliminary data revealed that N2O can be generated during biodegradation of both cyclic nitramines RDX and HMX under various aerobic (with the fungus Phanerochaete chrysosporium and the soil isolate Rhodococcus sp.) and anaerobic (municipal sludge) conditions.
Acknowledgments
We thank the Department of National Defence, Canada, and the National Research Council Canada for supporting the work of A. Halasz and the Natural Science and Engineering Research Council Canada (NSERC) for supporting the work of T. Sheremata.
We also thank C. Beaulieu, A. Corriveau, and S. Deschamps for their analytical support.
Footnotes
NRCC publication number 43310.
REFERENCES
- 1.Ampleman G, Thiboutot S, Lavigne J, Marois A, Hawari J, Jones A M, Rho D. Synthesis of 14C-labelled hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), 2,4,6-trinitrotoluene (TNT), nitrocellulose (NC) and glycidylazide polymer (GAP) for use in assessing the biodegradation potential of these energetic compounds. J Label Compd Radiopharm. 1995;36:559–577. [Google Scholar]
- 2.Ampleman G, Marois A, Thiboutot S, Hawari J, Greer C W, Godbout J, Sunahara G I, Shen C F, Guiot S. Synthesis of 14C-labelled octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) and 15N-isotopically labelled hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) for use in microcosm experiments. DREV-TR-1999-99. Quebec, Canada: Defence Research Establishment Valcartier, Val Bélair; 1999. [Google Scholar]
- 3.Bier E L, Singh J, Li Z, Comfort S D, Shea P. Remediating hexahydro-1,3,5-trinitro-1,3,5-triazine-contaminated water and soil by Fenton oxidation. Environ Toxicol Chem. 1999;18:1078–1084. [Google Scholar]
- 4.Binks P R, Nicklin S, Bruce N C. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl Environ Microbiol. 1995;61:1318–1322. doi: 10.1128/aem.61.4.1318-1322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boopathy R, Gurgas M, Ullian J, Manning J F. Metabolism of explosive compounds by sulfate-reducing bacteria. Curr Microbiol. 1998;37:127–131. doi: 10.1007/s002849900350. [DOI] [PubMed] [Google Scholar]
- 6.Brockman F J, Downing D C, Wright G F. Nitrolysis of hexamethylenetetramine. Can J Res. 1949;27B:469–474. doi: 10.1139/cjr49b-049. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Preston B P, Zimmerman M J. Analysis of organic acids in industrial samples: comparison of capillary electrophoresis and ion chromatography. J Chromatogr A. 1997;781:205–213. [Google Scholar]
- 8.Coleman N V, Nelson D R, Duxbury T. Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol Biochem. 1998;30:1159–1167. [Google Scholar]
- 9.Freedman D L, Sutherland K W. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) under nitrate-reducing conditions. Water Sci Technol. 1998;38:33–40. [Google Scholar]
- 10.Funk S B, Roberts D J, Crawford D L, Crawford R L. Initial-phase optimization for bioremediation of munition compound-contaminated soils. Appl Environ Microbiol. 1993;59:2171–2177. doi: 10.1128/aem.59.7.2171-2177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas R, Schreiber I, von Löw E, Stork G. Conception for the investigation of contaminated munitions plants. 2. Investigation of former RDX-plants and filling stations. Fresenius J Anal Chem. 1990;338:41–45. [Google Scholar]
- 12.Hawari J, Paquet L, Zhou E, Halasz A, Zilber B. Enhanced recovery of the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) from soil: cyclodextrin versus anionic surfactants. Chemosphere. 1996;32:1929–1936. [Google Scholar]
- 13.Hawari J, Halasz A, Paquet L, Zhou E, Spencer B, Ampleman G, Thiboutot S. Characterization of metabolites in the biotransformation of 2,4,6-trinitrotoluene with anaerobic sludge: role of triaminotoluene. Appl Environ Microbiol. 1998;64:2200–2206. doi: 10.1128/aem.64.6.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffsommer J C, Kubose D A, Glover D J. Kinetic isotope effects and intermediate formation for the aqueous alkaline homogenous hydrolysis of 1,3,5-triaza-1,3,5-trinitrocyclohexane (RDX) J Phys Chem. 1977;81:380–385. [Google Scholar]
- 15.Jackson M, Green J M, Hash R L, Lindsten D C, Tatyrek A F. Nitramine (RDX and HMX) wastewater treatment at the Holston Army Ammunition Plant. Report ARLCD77013. Dover, N.J: U.S. Army Armament Research and Development Command; 1978. [Google Scholar]
- 16.Kamachi M, Murahashi S. The polymerization of formaldazine. I. The nature of formaldazine. Polymer J. 1974;6:295–301. [Google Scholar]
- 17.Kitts C L, Cunningham D P, Unkefer P J. Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Appl Environ Microbiol. 1994;60:4608–4611. doi: 10.1128/aem.60.12.4608-4611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitts C L, Green C E, Otley R A, Alvarez M A, Unkefer P J. Type I nitroreductases in soil enterobacteria reduce TNT (2,4,6-trinitrotoluene) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) Can J Microbiol. 2000;46:278–282. doi: 10.1139/w99-134. [DOI] [PubMed] [Google Scholar]
- 19.Lamberton A H, Lindley C, Owston P G, Speakman J C. Studies of nitroamines. Part V. Some properties of hydroxymethyl- and aminomethyl-nitroamines. J Chem Soc. 1949;355:1641–1646. [Google Scholar]
- 20.Lamberton A H, Lindley C, Speakman J C. Studies of nitroamines. Part VII. The decomposition of methylenedinitroamine in aqueous solutions. J Chem Soc. 1949;357:1650–1656. [Google Scholar]
- 21.Larson S L, Strong A B. Ion chromatography with electrochemical detection for hydrazine quantification in environmental samples. Technical report IRRP-96-3. Vicksburg, Miss: U.S. Army Corps of Engineers, Waterways Experiment Station; 1996. [Google Scholar]
- 22.Martos P A, Pawliszyn J. Sampling and determination of formaldehyde using solid-phase microextraction with on-fiber derivatization. Anal Chem. 1998;70:2311–2320. doi: 10.1021/ac9711394. [DOI] [PubMed] [Google Scholar]
- 23.Mashima M. The infra red absorption spectra of the condensation products with hydrazines. Bull Chem Soc Jpn. 1966;39:504–506. [Google Scholar]
- 24.Matsubara T, Mori T. Studies on the denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem (Tokyo) 1968;64:863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- 25.McCormick N G, Cornell J H, Kaplan A M. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Appl Environ Microbiol. 1981;42:817–823. doi: 10.1128/aem.42.5.817-823.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick N G, Cornell J H, Kaplan A M. The anaerobic biotransformation of RDX, HMX and their acetylated derivatives. Technical report AD report A149464 (TR85-008). Natick, Mass: U.S. Army Natick Research and Development Center; 1985. [Google Scholar]
- 27.Melius C F. Thermochemical modeling. I. Application to decomposition of energetic materials. In: Bulusu S N, editor. Chemistry and physics of energetic materials. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 21–49. [Google Scholar]
- 28.Myler C A, Sisk W. Bioremediation of explosives contaminated soils (scientific questions engineering realities) In: Sayler G S, Fox R, Blackburn J W, editors. Environmental biotechnology for waste treatment. New York, N.Y: Plenum Press; 1991. pp. 137–146. [Google Scholar]
- 29.Okemgbo A A, Hill H H, Metcalf S G, Bachelor M A. Determination of nitrate and nitrite in Hanford defense waste by reverse-polarity capillary zone electrophoresis. J Chromatogr A. 1999;844:387–394. [Google Scholar]
- 30.Peterson F G, Mason R P, Hovesepian J, Holtzman J L. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microcosms. J Biol Chem. 1979;254:4009–4014. [PubMed] [Google Scholar]
- 31.Robidoux, P. Y., J. Hawari, S. Thiboutot, G. Ampleman, and G. Sunahara. Chronic toxicity of energetic compounds in soil using the earthworm (Eisenia andrei) reproduction test. Environ. Toxicol. Chem., in press. [DOI] [PubMed]
- 32.Rocheleau S, Cimpoia R, Paquet L, van Koppen I, Guiot S R, Hawari J, Thiboutot S, Ampleman G, Sunahara G I. Ecotoxicological evaluation of a bioslurry process treating TNT and RDX contaminated soil. Bioremediation J. 1999;3:233–245. [Google Scholar]
- 33.Somerville C, Nishino S F, Spain J C. Purification and characterization of nitrobenzene from Pseudomonas pseudoalkaligenes JS45. J Bacteriol. 1995;177:3837–3842. doi: 10.1128/jb.177.13.3837-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. Jones R T, Hollocher T C. Nitrogen—15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977;252:212–218. [PubMed] [Google Scholar]
- 35.Talmage S S, Opresko D M, Maxwel C J, Welsh C J E, Cretella F M, Reno P H, Daniel F B. Nitroaromatic munition compounds: environmental effects and screening values. Rev Environ Contam Toxicol. 1999;161:1–156. doi: 10.1007/978-1-4757-6427-7_1. [DOI] [PubMed] [Google Scholar]
- 36.Urbanski T. Aliphatic nitramines and nitramides. In: Urbanski T, editor. Chemistry and technology of explosives. Vol. 3. Oxford, United Kingdom: PWN-Polish Scientific Publishers, Pergamon Press; 1967. pp. 15–47. [Google Scholar]
- 37.Yang Y X, Wang X, Yin P, Li W H, Zhou P J. Studies on three strains of Corynebacterium degrading cyclotrimethylene-triamine (RDX) Acta Microbiol Sin. 1983;23:251–256. [Google Scholar]
- 38.Yinon J. Toxicity and metabolism of explosives. Boca Raton, Fla: CRC Press, Inc.; 1990. [Google Scholar]
- 39.Young D M, Unkefer P J, Ogden K L. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a prospective consortium and its most effective isolate Serratia marcescens. Biotechnol Bioeng. 1997;53:515–522. doi: 10.1002/(SICI)1097-0290(19970305)53:5<515::AID-BIT9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Young D M, Kitts C L, Unkefer P J, Ogden K L. Biological breakdown of RDX in slurry reactors proceeds with multiple kinetically distinguishable paths. Biotechnol Bioeng. 1997;56:258–267. doi: 10.1002/(SICI)1097-0290(19971105)56:3<258::AID-BIT3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]