Abstract
In this work there was investigated the synergistic effect of the nanomaterials-the Montmorillonite (MMT) and the vanadium pentoxide (V2O5) on the polyvinyl alcohol (PVA)/starch composite. The composite films were prepared by the solvent casting method. The characterization of the composites showed that the addition of the MMT and the V2O5 to PVA/starch composite decreased the water solubility and water absorption capacity of the film. Both of the reinforcement materials enriched values of thermal conductivity and thermal stability of the composite. The TG/DTA and universal testing machine (UTM) analysis exhibited that MMT and V2O5 augmented the thermal robustness and tensile strength of composites and decreased the strain to break. It was also observed that greater MMT concentration accelerates mechanical strength deterioration of the film owing to agglomeration. The scanning electron microscopy (SEM) analysis reflected great change in the surface morphology of the films in the presence and absence of MMT and V2O5. This was due to the interaction amid constituents of the composite. The chemical interaction between the PVA, Starch, MMT and the V2O5 was also established via Fourier-transform infrared spectroscopy (FTIR) analysis, which revealed fluctuations in the absorbance position and intensity of the PVA/Starch. Antimicrobial activities against seven different cultures of bacteria (both-gram positive and -negative) and one fungus (Candida albicans), exposed that antimicrobial performance of the PVA amplified upon addition of the starch, MMT and V2O5, making these composites prospective candidates for the biodegradable packaging materials.
Keywords: Montmorillonite, Vanadium pentoxide, Polyvinyl alcohol, Thermal conductivity, TG/DTA, UTM, FTIR, Antimicrobial, Biodegradable
1. Introduction
Plastic products made from petroleum are used as packing materials in food and other industries because of their low cost and excellent mechanical properties. However, plastic obtained from petroleum products is non-biodegradable in environment and cause waste disposal problems. Moreover, increased consumption of petroleum in different forms causes a decrease in petroleum resources throughout the world [1]. Due to limited resources of petroleum, increased awareness and knowledge about its environmental pollution potential, there is a need for the production of environment-friendly packing materials alternatives to petroleum products. Natural polymer composites derived from renewable resources are considered as the best alternative for petroleum products because of their low cost and easy preparation. Polymeric matrix composite is a material made up of polymer shared with fortifying dispersed phase. Starch being a natural polymer is second in abundance after cellulosic polymer and can be used in natural polymer composites formation. There are various starch sources, such as potato, wheat, lentils, corn, maize, rice, tapioca and peas etc. [2]. Starch is a low-cost biodegradable material and has some excellent properties due to which it is widely engaged in the preparation of packaging materials. Moreover, starch is hydrophilic in nature as it has many hydroxyl groups due to which it has a strong affinity towards water, hence making it unsatisfactory option without nano filler addition when considering its use as a packaging material [3]. The development of environment-friendly packaging materials is an area of continuing challenge for packaging technologists. The incorporation of nano-fillers is crucial for the green composite film to achieve high mechanical strength, antimicrobial efficacy, and air absorptivity [4]. Montmorillonite (MMT) is a naturally occurring clay, which is hydrophilic in nature and forms a stable suspension in water. The strong hydrophilic nature makes it easy to disperse in water-soluble polymer mixture, namely polyvinyl alcohol (PVA). Films containing polyvinyl alcohol as dispersion medium and the MMT as a dispersed phase are widely used as packaging materials [5]. Owing to the increased costs of the PVA, it is uneconomical to use it alone as a packaging material. Fabrication of composites of starch and PVA not only increases the properties of the material, but makes it also cost-effective method. However, both- PVA and starch have a large number of hydroxyl groups and thus, introduce a strong hydrophilic behavior, thus the PVA/starch composite has poor water barrier properties and mechanical performance [6]. To improve the water barrier properties, resistance to moisture, flame redundancy and mechanical properties of polymers, nano polymeric composites are prepared by combining nano clay, carbon nanotubes, graphene oxide and metal oxide, namely the TiO2 or the V2O5 [7]. The synthesis of inorganic polymer nanocomposites has gained profound attention recently. When applied in combination, these materials display exceptional properties, comprising electrical, mechanical, and optical features, which individual components cannot achieve on their own.
Based upon aforementioned features, this paper endeavors to prepare biodegradable nanocomposites based on PVA/Starch/MMT with the addition of V2O5. A robust solvent casting technique is employed to attain superior biodegradability, high mechanical strength, improved water barrier properties, and antimicrobial attributes. These attributes enrichments render them particularly suitable for applications in the food packaging industry. Our study primarily focuses on the physical and antimicrobial properties of the composites, and further work is needed to assess their performance in real-world food industry applications. Understanding the long-term durability and stability of these composites in food packaging or other relevant applications is an important area for future research.
2. Results and discussion
2.1. Water absorption capacity
The PVA film placed in 100 ml distilled water showed its water absorption of 100% after 24 h of the PVA film being dissolved in water. This was due to the existence of unattached hydroxyl groups in PVA. This result was in accordance with previous stated findings in literature [9]. The water absorption ability of PVA was reduced to 78% upon blending it with 30% of starch. This can be credited to the attraction of starch with hydroxyl moiety of the PVA to form hydrogen bond to prevent its interaction with water. This is in accordance with earlier findings [10]. Incorporating the MMT nano clay to the PVA/starch blend, the water absorption capacity was further reduced to 69%. This was due to the fact that the MMT also contains hydroxyl groups, which may establish hydrogen bonding between PVA and starch [11,12]. Doping of V2O5 to the PVA/Starch/MMT composite further improved water barrier by deteriorating the water absorption capacity of the composite to 58% owing to no solubility of V2O5 in water. This enhanced water barrier may be due to the formation of twisting path instead of direct owing to presence of V2O5 and high aspect ratio MMT. All these findings are shown in Fig. 1 as given below.
Fig. 1.
Water absorption capacities for fabricated composite materials.
2.2. Water solubility
Water solubility of the composite depends on the water sensitivity. Degradation in water or water solubility is greater when water absorption capacity is high. Water solubility of prepared films depicted the same trend as water absorption capacity: PVA/Starch had water solubility of 56% was achieved, PVA/Starch/Na-MMT had decreased water solubility of 43% whereas the lowest water solubility was achieved for PVA/Starch/Na-MMT/V2O5 with 36%. These results are displayed in Table 1 given below. Deteriorating of water solubility was witnessed by our findings. It can be deduced that inorganic fillers capable of forming hydrogen bonding with the polymeric matrix impede water solubility of the resulted polymeric films, thus making these films economic choice for enhancing shelf life with a number of edibles.
Table 1.
Water solubilities, Wo and Wd of used composites.
| Sample No | Type of composite | (Wo) in mg | (Wd) in mg | (Ws) |
|---|---|---|---|---|
| 1 | PVA | 67.9 | 0 | 100% |
| 2 | PVA/Starch | 234 | 102 | 56% |
| 3 | PVA/Starch/Na-MMT | 245 | 139 | 43% |
| 4 | PVA/Starch/Na-MMT/V2O5 | 216 | 138 | 36% |
2.3. Thermal conductivity analysis
The thermal conductivity of a material is a measure of its ability to conduct heat. The thermal conductivity of pure PVA was less amongst all the films. Blending PVA with starch had increased the thermal conductivity of the film as shown in Table 2, because of the formation of hydrogen bond between the PVA and starch, which caused the material to be more compact and hence caused an enormous increase in thermal conduction at a very low filler weight fraction. As in solid material, the conduction of heat occurs through vibration, thus the solid material with a more compact structure has a high thermal conductivity. The particle size of Starch and V2O5 are smaller than the particle size of PVA while Montmorillonite exhibits a unique two-dimensional arrangement characterized by stacked layers with a thickness of 0.96 nm responsible for more well-organized heat transfer pathways within the material. Decrease in the particle size and well-organized stacked layers has amplified thermal conductivity so the conductivity of the PVA was increased by blending it with starch, MMT and V2O5. This observation of particle size on the thermal conductivity of the composite is in agreement with previous published data [13]. Table 3 gives comparison of our findings with previous data on mechanical performance of polymeric composites. From thermal conductivity results it can be seen that all the constituents of the chosen composite hybrid materials are in nice compatibility at the chosen ratio, so making these films very good options for handling thermal materials.
Table 2.
Thermal conductivity of the composites.
| Sample No. | Type of composite | Thermal conductivity (W m−1 k−1) |
|---|---|---|
| 1 | PVA | 0.31 |
| 2 | PVA/Starch | 0.98 |
| 3 | PVA/Starch/MMT | 10.15 |
| 4 | PVA/Starch/MMT/V2O5 | 13.4 |
Table 3.
Comparative Thermal conductivities of polymeric composites.
| Sample No | Polymer Matrix | Comparative Thermal Conductivity of polymeric composites (W m−1 k−1) | Reference |
|---|---|---|---|
| 1 | PVA/GnPs with various weight fraction | 3.13–6.04 | [14] |
| 2 | PVA composite with hybrid particles (Polydopamin bridged ternary h-BN@PDA@TiO2 | 0.2–0.4 | [15] |
| 3 | PVA/TiO2 | 3.51 | [16] |
| 4 | PVA/CuCl2 | 0.26–0.33 | [17] |
| 5 | PVA/Starch/MMT/V2O5 | 13.4 | Current study |
2.4. FTIR analysis
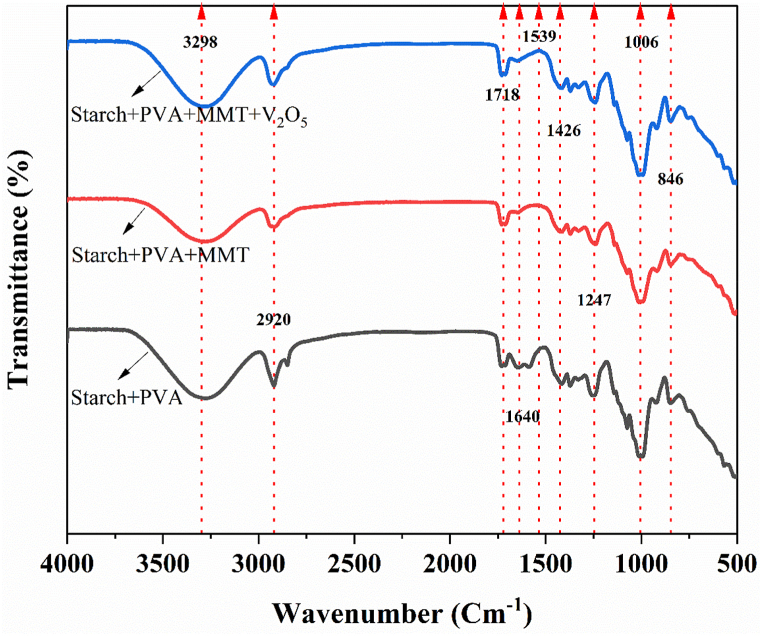
FTIR analyses shown in Fig. 2 of the prepared composite materials confirmed that the addition of starch and nanofillers to the PVA matrix changed chemical environment by fluctuating peak positions of the PVA. This has also been witnessed by documented literature [18]. Fig. 2 exposes characteristic OH stretching around 3277 cm−1, CH stretching at 2920 cm−1, C O stretching at 1712 cm−1, Intermolecular H-bond of the carboxyl group at 1640 cm−1, CH bending at 1426 cm−1, and C–O–C starching vibrations around (1000–800) cm−1. Fig. 2 clarifies that Peak positioning and intensities for OH stretching has altered in the composites. The shift of OH stretching from 3290 cm−1 for pure PVA to 3277 cm−1 reflects the possibility of intermolecular H bonding in PVA/Starch and PVA/Starch/MMT [19]. Similarly the peak corresponding to C–O–C stretching at 1012 cm−1 (PVA/Starch), 1006 cm−1 (PVA/Starch/MMT) has shifted to 1070 cm−1 upon incorporation of V2O5 to form PVA/Starch/MMT/V2O5 polymeric composite. Appearance of smaller peak at 763 cm−1 can be ascribed to the V–O–V asymmetric mode of stretching besides major differences observed around 1000 cm−1 attributing to the vibrational bond for terminal oxygen (V O) in the PVA/Starch/MMT/V2O5 polymeric composite. Our findings on FTIR are in close agreement with already documented literature [20].
Fig. 2.
FTIR spectrum of PVA/Starch, PVA/Starch/MMT and PVA/Starch/MMT/V2O5.
2.5. SEM analysis
The micrograph of a pure PVA film illustrated that the surface of the pure PVA is clear, smooth and has small cracks as shown in Fig. 3a. The morphology of the PVA/Starch composite exhibited rough and uniform compatible morphology without phase segregation due to starch H bonding with the PVA surface leading to increased amorphous region (as seen from Fig. 3b) and this was also in accordance with poor mechanical performance of the PVA/starch blend achieved (Table 4). The morphology of the PVA/Starch/MMT (Fig. 3c) showed some agglomeration. The reason of the agglomeration may be a poor distribution of the MMT at the given proportion. The agglomeration weakens the structure of material by forcing stress concentration spot. Thus, a high concentration of the MMT deteriorates the tensile strength. The trend is in consistency with previous findings [21]. Fig. 3d shows the surface morphology of the V2O5 reinforced PVA/Starch/MMT composite. Fig. 3d exposes that the V2O5 had altered the morphology of the composite and dispersion of the V2O5 was relatively good. There was very less aggregations of the observed material after addition of the V2O5 as witnessed from Fig. 3d. Morphological and structural tests confirmed the formation of orthorhombic structure of the V2O5. It can be perceived that there was good adhesion in the PVA/starch/MMT/V2O5, which has fortified the mechanical, and thermal properties along with enhanced water resistance due to the tortuous networking pattern as confirmed by Fig. 1. The SEM findings were fully supported by the FTIR analysis as exposed by Fig. 2.
Fig. 3.
SEM of: a) PVA b) PVA/starch. c) PVA/Starch/MMT d) PVA/Starch/MMT/V2O5.
Table 4.
Comparative Mechanical Performance of polymeric composites.
2.6. Mechanical analysis
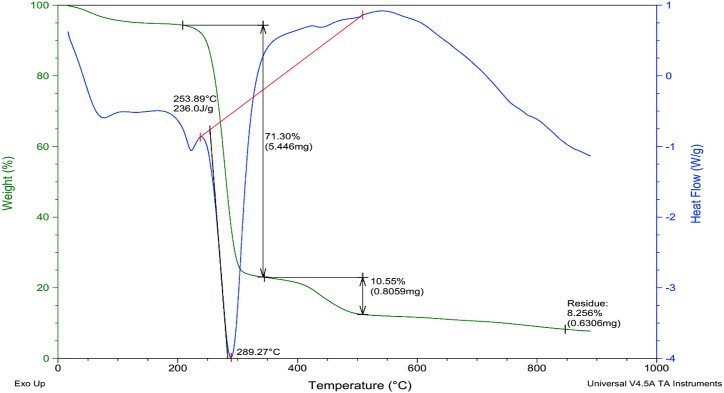
The mechanical performance of the composites was assessed and is depicted in Fig. 4. It is clear from Fig. 4, that pure PVA has the highest Young's modulus value and stress reflecting that PVA had the highest tensile strength. However, the elongation at the break was minimum in case of pure PVA. While blending PVA with starch had improved the stress to break and elongation at the break and decreased the Young's modulus of the composite. This decrease in the tensile strength of the PVA/Starch composite was due to the plastic nature of starch. Starch is plastic in nature and has very low tensile strength. Thus, its addition to the PVA decreases the tensile strength of the composite. Fig. 4 clarified that addition of small amount of the MMT declined elongation at the break and stress at the break. This decrease may be attributed to the fact that the MMT manifested as impurity enhancing stress concentration regions in the polymer and initiated rupture from these points [22]. MMT is a stiff reinforcement and thus, its addition to films decreased the strain to break. A glance on Table 4 exhibits that addition of V2O5 amplified Young's modulus, stress at the break and tensile strength of the PVA/Starch/MMT composite. This increase may be due to the fact that the V2O5 is inorganic in nature and has a high tensile strength itself. This trend is supported by the previous literature [23] and has also indorsed by the TG/DTA thermograms of PVA/Starch/MMT/V2O5 (5a) and PVA/Starch/MMT (5b) showing increased maximum decomposition temperature (289.27 οC, 71.30%) and complete degradation temperature around (350–550 οC, 10.55%) and enhanced char residue (8.256%) contrary to MMT loading to the PVA/Starch blend as shown below in Fig. 5a and b respectively. Table 4 displays comparison of our mechanical performance outcomes with the previously undertaken similar work, which clarified higher Young's modulus values achieved by our tests compared to earlier results available in literature [[24], [25], [26], [27]]. All our results from mechanical characterization were in full agreement with the trend shown by thermal performance analysis as exposed by Table 3 and Fig. 5 (a&b).
Fig. 4.
Mechanical Performance of polymeric composites.
Fig. 5.
a. TG/DTA thermogram of PVA/Starch/MMT/V2O5 Composite. b. TG/DTA thermogram of PVA/Starch/MMT composite.
2.7. Antimicrobial activity
The antimicrobial activity of the prepared composites was tested against different microbes, such as Eschercichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella Typhi, Proteus mirabilis as gram negative bacteria; Staphylococcus aureus, Bacillus subtilis as gram positive bacteria, Candida albicans as a diploid gram-positive fungus. For this purpose, various tests were carried out for seven different bacterial cultures and one fungus. Table 5 demonstrates the findings with the pure PVA film. It is clear from Table 5 that the PVA in pure form did not show any resistance against various microbes, it was detected that there was zero inhibitory zone surrounding the films, which explained that the non-loaded pure PVA didn't show any antibacterial or antifungal activity. This was in harmony with previous literature [28].
Table 5.
Antimicrobial activity of pure PVA thin films.
| Sample No. | Microbes | Disc 1 (mm) | Disc 2 (mm) |
|---|---|---|---|
| 1 | E. Coli (Escherichia coli) | 0 | 0 |
| 2 | P. Aeruginosa (Pseudomonas aeruginosa) | 0 | 0 |
| 3 | K. Pneumoniae (Klebsiella pneumoniae) | 0 | 0 |
| 4 | S. Typhi (Salmonella Typhi) | 0 | 0 |
| 5 | P. Mirabilis (Proteus mirabilis) | 0 | 0 |
| 6 | S. Aureus (Staphylococcus aureus) | 0 | 0 |
| 7 | B. Subtilis (Bacillus subtilis) | 0 | 0 |
| 8 | C. Albicans (Candida albicans) | 0 | 0 |
Fig. 6 illustrates antimicrobial effect of the PVA/Starch polymeric blend. It is obvious from Fig. 6 that addition of starch to the PVA had improved the antibacterial and antifungal effect of the pure PVA. The inhibitory role was endorsed from clear zone nearby circular film disc. As there was perfect inhibitory zone found when the PVA/Starch blend was treated with various microbes like bacteria and a fungus. So, the antibacterial experiments indicated that the PVA/Starch blends had a good activity against gram positive and gram-negative bacteria, being in accordance to previous literature [29].
Fig. 6.
Antimicrobial activity of PVA/Starch blend.
A glance at Fig. 7 reveals that antibacterial activity get augments with MMT incorporation to the PVA/Starch blend. The inhibitory zone is more pronouncedly distinguishable against the gram-negative bacteria contrary to the gram-positive bacteria (S. Aureus). The Candida albicans (yeast) also shows the presence of inhibitory zone. The combination of PVA, Starch, and MMT may create a synergistic effect, improving the overall biocidal performance. The explicit interaction amongst them and the microbial cells could pay to the experimental variances in inhibitory zones likewise the release kinetics of antimicrobial agents from the hybrid may favor a more sustained and effective release against gram-negative bacteria and yeast over time. This observation of antimicrobial resistivity of our fabricated hybrid composites was in harmony with biocidal activity manifested by the PVA/PSS packaging films [30,31]. Fig. 8 demonstrates that loading of V2O5 to the PVA/Starch/MMT composites improves the antimicrobial activity of the resulting composites, but the effect was almost similar to that observed with the addition of the MMT.
Fig. 7.
Antimicrobial activity of the PVA/starch/MMT composite.
Fig. 8.
Antimicrobial activity of the PVA/starch/MMT/V2O5 composite.
3. Conclusions
From the current study it was concluded that the addition of MMT and V2O5 had a significant effect on the solubility and water absorption capacity of the PVA/starch blends. The water absorption capacity and water solubility both declined by doping the PVA/starch blend with the MMT and the V2O5. This decrease in moisture absorption caused simultaneous increase in the tensile strength making these films very suitable options for boosting shelf life for numerous food items without compromising mechanical performance. The thermal conductivity analysis suggested these polymeric hybrid film (the PVA/starch blended with the MMT and the V2O5) as a viable choice for handling thermal materials as the addition of nanoparticle (MMT and the V2O5) boosted thermal conduction of the fabricated polymeric nanocomposite that may aid in degradation of thin films. The SEM analysis revealed that the surface morphology was highly affected by the presence of the nanofillers pointing towards the chemical interaction between the constituents of the composite. The FTIR results confirmed this behavior. Antimicrobial activities confirmed that PVA blending with the starch, MMT and the V2O5 demonstrates pronounced resistance towards microbes making these composites ideal to be used for food packing material in industries as well as for tissue scions and wounds curing purposes.
4. Experimental
4.1. Chemicals
PVA with a degree of hydrolysis 99 % was obtained from Sigma-Aldrich (Germany). To remove the moisture from the PVA, it was kept in an oven for 3 h at 70 οC. The starch used in the formation of films was also obtained from Sigma-Aldrich (Germany). Before use, starch was also kept in an oven at 60 οC to remove the moisture. Montmorillonite clay and vanadium pentoxide were also obtained from Sigma-Aldrich (Germany) and were used directly without any pretreatment.
4.2. Preparation of polymeric nanocomposites
First of all, PVA thin films were fabricated through simple solvent casting method by dissolving 3.5 g of pure PVA in 95 ml deionized water. The solution was kept on magnetic hot plate and heated at 45 οC while stirring continuously for 3 h until all the PVA was dissolved in water and a clear solution was obtained. The solution was poured into a Petri dish and was kept in oven to evaporate half of water from the Petri dish and the remaining water was evaporated at room temperature. When all the water was evaporated, thin film of the pure PVA remained in the Petri dish, which was removed carefully and was used for reference purpose. For the preparation of the starch blended PVA, solvent proportion was kept constant and 1.5 g starch was blended by adopting the same procedure while for the biodegradable nanocomposite thin films, the PVA/starch in mentioned proportion was mixed with 0.05 wt % (0.0025 g) of he MMT and the V2O5 separately by following the same protocol to estimate the effect of MMT and the V2O5 individually on the performance of the prepared polymer blends.
4.3. Characterization of biodegradable nanocomposites thin films
4.3.1. Water absorption capacity
Films were cut into 2 by 2 cm pieces and was dried in vacuum oven for 2 h. After drying, prepared films were cooled in desiccator. Then, initial weight of the films denoted as WO (Initial weight of film before absorption) was recorded. These prepared films were then kept in beakers containing 100 ml water for 24 h. After 24 h, the samples were removed from water and their surfaces were purified with clean tissue to remove the attached water molecule from the surface. Their weights were found again as W1 (Final weight of film after absorption). The Wa (water absorption capacity) was estimated by engaging equation (1) given below:
| (1) |
4.3.2. Water solubility test
Film samples after water absorption capacity test were employed to estimate water solubility (Ws) of the samples by using equation (2). After water immersion, samples were dried again in vacuum oven at 60 οC for 24 h. Then, the films were cooled up to room temperature in desiccator for 30 min. The samples were weighed again to measure dry mass after immersion (Wd).
| (2) |
4.3.3. Fourier-transform infrared spectroscopy (FTIR) analysis
The effect of MMT and V2O5 on the chemical nature of composites was studied through the FTIR analysis. FTIR analysis was done by Shimadzu IR Tracer 100 (Shimadzu, Japan) in the wavelength range of (4000–400) cm−1 and resolution of 4 cm−1.
4.3.4. Scanning electron microscopy (SEM) analysis
SEM studies of the samples were carried out by using Scanning Electron Microscope Quanta 250 FEG manufactured by FEI (Hillsboro, Oregon, USA). Carbon sputtering by using carbon rod was done before analyzing sample by SEM.
4.3.5. Universal testing machine (UTM) analysis
Mechanical performance of the materials was judged by tensile analysis via conventional tensile tester (Testometric Inc. 100-500 KN, UK). Samples were subjected at ambient conditions for tensile and compression testing at the specific tensile and compression speed of 1 mm/min. As per Standard Test Method for Tensile Properties (ASTM D-638), typical type IV sample geometry with a gauge length of 30 mm was used. Tensile test results were interpreted as percent elongation at break, tensile strength and the Young's modulus.
4.3.6. Thermo-gravimetric analysis
The thermal robustness of all samples was examined via Thermo-gravimetric/Differential Thermal Analyzer i.e. TG/DTA (SDT Q600) and thermal constant analyzer (New Castle, USA). Under nitrogen atmosphere, samples were heated from (25–600) °C on heating speed of 10 °C/min.
4.3.7. Antimicrobial activity
Antimicrobial activities were examined at Laboratories Complex of Pakistan Council of Scientific and Industrial Research (PCSIR Peshawar, Pakistan). The procedure documented earlier was adopted to inspect the biocidal activity of the nanocomposite [8]. The nutrient medium, cotton swab, and petri dishes were sterilized for culture production tests. Moisture was eliminated from applied Petri dishes in oven and further testing was done under laminar flow hood. At 37οC both-nutrient agar and broth were held in an incubator for one day to inspect its sterility while media was emptied in the petri dishes. On the next day, a hoop was immersed in nutrient broth containing bacteria, thereafter it was adulterated on fresh broth. 1 ml of broth culture was supplemented to a nutrient agar dish having uniform bacterial colonies present from field or mat of bacteria. Different composite materials were placed on bacterial test plate while zones of inhibitions were computed after 24 h in millimeters (mm) by Vernier caliper.
CRediT authorship contribution statement
Sabiha Sultana: Supervision, Methodology, Funding acquisition, Conceptualization. Sohail Imran: Methodology, Formal analysis, Data curation. Amir Naveed: Software, Resources, Project administration, Formal analysis. Sardar Hussain: Writing – original draft, Validation, Methodology. Rozina Khattak: Software, Resources. Luqman Ali Shah: Writing – review & editing, Formal analysis. Kamran Rehan: Writing – original draft, Data curation. Imran Rehan: Writing – review & editing, Validation. Mujeeb Ur Rehman: Writing – review & editing, Software, Resources. Uzma Hashmat: Writing – review & editing. Farzana Haider: Validation, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The corresponding author is highly grateful to the Higher Education Endowment Fund (HEREF), Project Management Unit (PMU), Khyber Pakhtunkhwa, Pakistan project No.118 and Higher Education Commission of Pakistan, HEC project No. 10164 to support this research work.
References
- 1.Tang J., Yi W., Yan J., Chen Z., Fan H., Zaldivar-Silva D., Wang S. Highly absorbent bio-sponge based on carboxymethyl chitosan/poly-γ-glutamic acid/platelet-rich plasma for hemostasis and wound healing. Int. J. Biol. Macromol. 2023;247 S doi: 10.1016/j.ijbiomac.2023.125754. [DOI] [PubMed] [Google Scholar]
- 2.Ramaraj B. Crosslinked poly (vinyl alcohol) and starch composite films. II. Physicomechanical, thermal properties and swelling studies. J. Appl. Polym. Sci. 2007;103:909–916. doi: 10.1002/app.25237. [DOI] [Google Scholar]
- 3.Popescu M.C., Dogaru B.I., Goanta M., Timpu D. Structural and morphological evaluation of CNC reinforced PVA/Starch biodegradable films. Int. J. Biol. Macromol. 2018;116:385–393. doi: 10.1016/j.ijbiomac.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Youssef H.F., El-Naggar E.M., Fouda F.K., Ahmed M. Youssef, Antimicrobial packaging film based on biodegradable CMC/PVA-zeolite doped with noble metal cations. Food Packag. Shelf Life. 2019;22 doi: 10.1016/j.fpsl.2019.100378. [DOI] [Google Scholar]
- 5.Strawhecker K.E., Manias E. Structure and properties of poly (vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000;12:2943–2949. doi: 10.1021/cm000506g. [DOI] [Google Scholar]
- 6.Tang X., Alavi S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydrate polymers. 2011;85:7–16. doi: 10.1016/j.carbpol.2011.01.030. [DOI] [Google Scholar]
- 7.Zamanian M., Sadrnia H., Khojastehpour M., Hosseini F., Kruczek B., Thibault J. Barrier properties of PVA/TiO2/MMT mixed-matrix membranes for food packaging. J. Polym. Environ. 2021;29:1396–1411. doi: 10.1007/s10924-020-01965-8. [DOI] [Google Scholar]
- 8.Rout Y., Behera S., Ojha A.K., Nayak P.L. Green synthesis of silver nanoparticles using Ocimum sanctum (Tulashi) and study of their antibacterial and antifungal activities. J. Microbiol. Antimicrob. 2012;4:103–109. doi: 10.5897/JMA11.060. [DOI] [Google Scholar]
- 9.Jain N., Singh V.K., Chauhan S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017;26:213–222. doi: 10.1515/jmbm-2017-0027. [DOI] [Google Scholar]
- 10.Asem M., Nawawi W.M.F.W., Jimat D.N. IOP Conference Series: Materials Science and Engineering. vol. 368. 2018. Evaluation of water absorption of polyvinyl alcohol-starch biocomposite reinforced with sugarcane bagasse nanofibre: Optimization using Two-Level Factorial Design. [DOI] [Google Scholar]
- 11.Jalalvandi E., Majid R.A., Ghanbari T. Processing, morphological, thermal and absorption behavior of PLA/thermoplastic starch/montmorillonite nanocomposites. International Journal of Materials and Metallurgical Engineering. 2012;6:1128–1132. [Google Scholar]
- 12.Ramesh P., Prasad B.D., Narayana K.L. Effect of MMT Clay on mechanical, thermal and barrier properties of treated aloe vera fiber/PLA-hybrid biocomposites. Silicon. 2020;12:1751–1760. doi: 10.1007/s10570-020-03268-6. [DOI] [Google Scholar]
- 13.Raheem Z., Tamara N. Thermal conductivity of PVA/rice starch and PVA/pistachia (biodegradable) blends. Indian Journal of Natural Sciences. 2017;8:12925–12930. ISSN: 0976 – 0997. [Google Scholar]
- 14.Liu Y., Wu K., Luo F., Lu M., Xiao F., Du X., Lu M. Significantly enhanced thermal conductivity in polyvinyl alcohol composites enabled by dopamine modified graphene nanoplatelets. Compos. Appl. Sci. Manuf. 2019;117:134–143. doi: 10.1016/j.compositesa.2018.11.015. [DOI] [Google Scholar]
- 15.Wang X., Hu W., Hu Y. Polydopamine-bridged synthesis of ternary h-BN@ PDA@ TiO2 as nanoenhancers for thermal conductivity and flame retardant of polyvinyl alcohol. Front. Chem. 2020;893 doi: 10.3389/fchem.2020.587474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Ye K., Liu Z., Wang M., Chee K.W., Lin C.T., Yu J. Effective thermal transport highway construction within dielectric polymer composites via a vacuum-assisted infiltration method. J. Mater. Chem. C. 2018;6:6494–6501. doi: 10.1039/C8TC01464G. [DOI] [Google Scholar]
- 17.Bama G.K., Devi P.I., Ramachandran K. Structural and thermal properties of PVA and its composite with CuCl2. AIP Conf. Proc. 2011;1349:537–538. doi: 10.1063/1.3605970. [DOI] [Google Scholar]
- 18.Alsaad A., Ahmad A., Al Dairy A.R., Qattan I.A., Al Fawares S., Al-Bataineh Q. Characterization of as-prepared (PMMA-PVA)/CuO-NPs hybrid nanocomposite thin films. Preprints. 2021 doi: 10.20944/preprints202101.0607.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil M.B., Mathad S.N., Patil A.Y., Khan A., Hussein M.A., Alosaimi A.M., Khan M.M.A., A M.M. Functional properties of grapefruit seed extract embedded blend membranes of poly (vinyl alcohol)/starch: potential application for antiviral activity in food safety to fight against COVID-19. J. Polym. Environ. 2023;31:2519–2533. doi: 10.1007/s10924-022-02742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajpai A.K., Goswami A., Bajpai J., Sinha B.K. Study of mechanical, optical, and electrical behaviors of calcium alginate/poly (vinyl alcohol)–vanadium pentoxide bio nanocomposite films. Macromol. Res. 2018;26:305–316. doi: 10.1007/s13233-018-6040-0. [DOI] [Google Scholar]
- 21.Tee T.T., Sin L.T., Gobinath R., Bee S.T., Hui D., Rahmat A.R., Fang Q. Investigation of nano-size montmorillonite on enhancing polyvinyl alcohol–starch blends prepared via solution cast approach. Compos. B Eng. 2013;47:238–247. doi: 10.1016/j.compositesb.2012.10.033. [DOI] [Google Scholar]
- 22.Majdzadeh-Ardakani K., Nazari B. Improving the mechanical properties of thermoplastic starch/poly (vinyl alcohol)/clay nanocomposites. Compos. Sci. Technol. 2010;70:1557–1563. doi: 10.1016/j.compscitech.2010.05.022. [DOI] [Google Scholar]
- 23.Wacharawichanant S., Wutanasiri N., Srifong P., Meesangpan U., Thongyai S. Effect of vanadium pentoxide on the mechanical, thermal, and electrical properties of poly (vinyl alcohol)/vanadium pentoxide nanocomposites. J. Appl. Polym. Sci. 2011;121:2870–2876. doi: 10.1002/app.33850. [DOI] [Google Scholar]
- 24.Shang S., Gan L., Yuen C.W.M. Water-dispersible graphene stabilized by PVA grafted graphene and its poly (vinyl alcohol) nanocomposites. Nano Science and Technology Institute. 2014:388–391. [Google Scholar]
- 25.Escobar-Sierra D.M., Perea-Mesa Y.P. Manufacturing and evaluation of Chitosan, PVA and Aloe Vera hydrogels for skin applications. Dyna. 2017;84:134–142. doi: 10.15446/dyna.v84n203.62742. [DOI] [Google Scholar]
- 26.Musa B.H., Hameed N.J. Study of the mechanical properties of polyvinyl alcohol/starch blends. Mater. Today Proc. 2020;20:439–442. doi: 10.1016/j.matpr.2019.09.161. [DOI] [Google Scholar]
- 27.Soundararajah Q.Y., Karunaratne B.S.B., Rajapaksa R.M.G. Mechanical properties of poly (vinyl alcohol) montmorillonite nanocomposites. J. Compos. Mater. 2010;44:303–311. doi: 10.1177/0021998309347. [DOI] [Google Scholar]
- 28.Abd El-Mohdy H.L., Ghanem S. Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J. Polym. Res. 2009;16:1–10. doi: 10.1007/s10965-008-9196-0. [DOI] [Google Scholar]
- 29.Wu Z., Wu J., Peng T., Li Y., Lin D., Xing B., Han G. Preparation and application of starch/polyvinyl alcohol/citric acid ternary blend antimicrobial functional food packaging films. Polymers. 2017;9:102. doi: 10.3390/polym9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahima S., Solimana O., Sultana M. Synergistic antimicrobial effect of xylitol with curcumin: water vapor barrier, mechanical and thermal properties of PSS/PVA packaging films. Int. J. Appl. Eng. Res. 2017;12:10360–10366. [Google Scholar]
- 31.Hajeeassa K.S., Hussein M.A., Anwar Y., Tashkandi N.Y., Al-Amshany Z.M. Nanocomposites containing polyvinyl alcohol and reinforced carbon-based nanofiller: a super effective biologically active material. Nanobiomedicine. 2018;5 doi: 10.1177/1849543518794818. [DOI] [PMC free article] [PubMed] [Google Scholar]