Abstract
Application of 1-aminoocyclopropane-1-carboxylic acid, an ethylene precursor, decreased nodulation of Macroptilium atropurpureum by Bradyrhizobium elkanii. B. elkanii produces rhizobitoxine, an ethylene synthesis inhibitor. Elimination of rhizobitoxine production in B. elkanii increased ethylene evolution and decreased nodulation and competitiveness on M. atropurpureum. These results suggest that rhizobitoxine enhances nodulation and competitiveness of B. elkanii on M. atropurpureum.
The symbiotic interactions between a legume and (brady)rhizobia result in a unique, nitrogen-fixing plant organ, the nodule. Recent studies have shown that the phytohormone ethylene inhibits nodule formation in some legumes (8, 9, 16, 24, 25). Application of 1-aminoocyclopropane-1-carboxylic acid (ACC), a precursor of ethylene, inhibits nodulation in Medicago truncatula (24).
Rhizobitoxine [2-amino-4-(2-amino-3-hydropropoxy)-trans-but-3-enoic acid] is an ethylene synthesis inhibitor that is produced by the legume symbiont Bradyrhizobium elkanii (15, 17–19, 22, 39). It is thought that production of this compound enhances nodulation of the host legume because of its inhibitory effect on ethylene synthesis. However, some reports have shown that there is not a significant difference in nodule number between plants inoculated with B. elkanii USDA61 and plants inoculated with rhizobitoxine-deficient mutants during nodulation of Glycine max, Glycine soja, Vigna unguiculata, and Macroptilium atropurpureum (26, 39). Recently, Duodu et al. observed a significant difference in nodule number between plants inoculated with isogenic variants of USDA61 during nodulation of Vigna radiata (7). Although these findings do not seem to be consistent with the hypothesis that rhizobitoxine has a positive effect on nodulation, the inconsistency can be explained by differences in the ethylene sensitivity of nodulation among leguminous species; nodulation of G. max is generally not sensitive to ethylene (10, 31, 38), while nodulation of V. radiata is sensitive (7). The inconsistency could also result from differences in the abilities of the strains used in the experiments to produce rhizobitoxine; strain USDA61 is a weak producer of rhizobitoxine (39).
In addition to G. max, the leguminous plant M. atropurpureum is a nodulating host for B. elkanii and Bradyrhizobium japonicum (12, 15). Although the effect of ethylene on nodulation has been studied in many leguminous host plants so far, the effect of ethylene in M. atropurpureum is not known. B. elkanii was found to be more competitive than B. japonicum for nodulation of M. atropurpureum in a multistrain environment when a field soil was inoculated with a mixture of several strains isolated from the field soil (21). In general, B. elkanii accumulates rhizobitoxine in cultures and in nodules, while B. japonicum does not (5, 15, 18, 19). These results led us to investigate the role of rhizobitoxine production on the nodulation and competitiveness of B. elkanii on M. atropurpureum by using a B. elkanii strain that produces high levels of rhizobitoxine, B. elkanii USDA94.
Siratro (M. atropurpureum Urb. cv. Siratro) seeds were obtained from Yukijirushi Shubyo Co. (Hokkaido, Japan). The seeds were surface sterilized with 70% ethanol for 5 min and then with 3% hydrogen peroxide for 1 min; they were washed 10 times at 1-min intervals with sterile distilled water after each treatment. The surface-sterilized seeds were sown in sterile plastic growth pouches that were watered with a nitrogen-free plant nutrient solution (1) and incubated at 25°C for 2 days in the dark. Two days after sowing, the germinated young seedlings in the pouches were transferred to a chamber and grown by using the following cycle: 14 h of light at 28°C and 10 h of darkness at 23°C.
The bacterial strains and plasmids used in this study are listed in Table 1. Bradyrhizobium strains were maintained in HM medium (4) containing 0.1% arabinose and 0.025% yeast extract or in Tris-YMRT (20). Escherichia coli strains were maintained in Luria-Bertani medium (29). Before inoculation, Bradyrhizobium strains were cultured in HM medium containing 0.1% arabinose and 0.025% yeast extract at 30°C for 6 days. The cells were collected by centrifugation and washed twice with sterile water, and the concentration was adjusted to 107 cells · ml−1 by direct counting with a Thoma hemocytometer. One milliliter of the bacterial suspension was inoculated onto 2-day-old seedlings in sterile growth pouches.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Bradyrhizobium elkanii | ||
| USDA94 | Wild type, RT+ | Keyserb |
| RTS2 | USDA94 RT− mutant, rtxA::KIXX, Kmr | This study |
| MA941 | USDA94 labelled with gusA by mTn5SSgusA20, Spr | This study |
| Bradyrhizobium japonicum | ||
| USDA110 | Wild type, RT− | Keyserb |
| MA106 | USDA110 labelled with gusA by mTn5SSgusA20, Spr | This study |
| Escherichia coli | ||
| JM109 | Cloning strain | Toyobo |
| S17-1 λ-pir | Strain used for conjugation and gene disruption | 24 |
| Plasmids | ||
| pCR2.1 | Cloning vector, Apr Kmr | Invitrogen |
| pUC18 | Cloning vector, Apr | Toyobo |
| pUC4-KIXX | Plasmid carrying 1.6-kb aph cassette, Apr Kmr | Pharmacia |
| pSUP202 | Plasmid used for gene disruption, Apr Cmr Tcr | 20 |
| pmTn5SSgusA20 | Plasmid used for transposon insertion, Apr Spr | 24 |
| pCR7-2950.8 | pCR2.1 carrying a 3.0-kb PCR fragment, Apr Kmr | This study |
| pUC7-2950.1 | pUC18 carrying a 3.0-kb EcoRI fragment from pUC7-2950.8, Apr | This study |
| pUC7-2950::KIXX.15 | pUC7-2950.1 carrying a 1.6-kb XhoI fragment from pUC4-KIXX, Apr Kmr | This study |
| pSUPrtx::KIXX.7 | pSUP202 carrying a 4.6-kb EcoRI fragment from pUC7-2950::KIXX.15, Apr Cmr Kmr | This study |
| pαHD7 | pCNTR carrying a 1.2-kb ISB12 (RSα) fragment from B. japonicum HRS isolate NK5 | 4 |
RT, rhizobitoxine production; Kmr, kanamycin resistance; Spr, spectinomycin resistance; Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance; aph, aminoglycoside-3′-O-phosphotransferase gene.
Keyser, H. H. Keyser, U. S. Department of Agriculture, Beltsville, Md.
To see if production of ethylene in M. atropurpureum inhibits nodule formation by B. elkanii, siratro seedlings were inoculated with USDA94 in the presence and in the absence of ACC (Sigma-Aldrich Japan, Tokyo, Japan). It is thought that applying ACC to plants increases the ethylene level because this compound is a precursor of ethylene. ACC Powder was diluted in nitrogen-free plant nutrient solutions to final concentrations of 1 and 10 μM. The nutrient solutions containing ACC were added to plant roots just after inoculation and every day during plant growth. Siratro seedlings that received a nitrogen-free nutrient solution that did not contain ACC were used as controls. For a time course study of nodulation, nodules were counted by counting the visible nodules with diameters greater than 0.2 mm and large globular nodules with diameters greater than 1 mm. Student's t test was used to assess the statistical significance of differences in nodule number at a confidence level of 0.05.
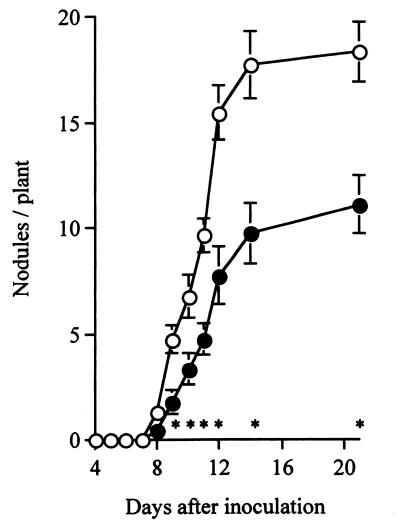
Ethylene synthesis was measured by the method of Suganuma et al. (34). After the nutrient solution was removed with paper towels, plant roots were incubated in a 5-ml glass container at 30°C for 2 h (three to five plants per container). The ethylene concentration in the container was measured by using a model GC-7A gas chromatograph (Shimadzu, Tokyo, Japan) equipped with a flame ionization detector and a Porapak Q column (2.2 mm by 2 m; Waters Associates Inc.). We calculated the rate of ethylene synthesis by using the concentrations obtained. As determined in the absence of ACC, the rate of ethylene synthesis in siratro roots 3 days after inoculation with USDA94 (0.79 pmol of ethylene · plant−1 · h−1) was less than the rate of ethylene synthesis in uninoculated control roots (5.46 pmol of ethylene · plant−1 · h−1). These results suggest that inoculation with B. elkanii suppressed ethylene synthesis in the M. atropurpureum roots, and they are consistent with previous findings that rhizobitoxine application inhibits ethylene synthesis and the enzymatic activity of ACC synthase in the ethylene synthesis pathway in other plants (23, 40). When 1 μM ACC was applied to USDA94-inoculated siratro roots just after inoculation and during plant growth, the ethylene synthesis rate increased from 0.79 pmol of ethylene · plant−1 · h−1 (no ACC) to 3.29 (1 μM ACC) or 3.75 (10 μM ACC) pmol of ethylene · plant−1 · h−1 within 3 days after inoculation. These results indicate that ACC treatment increased ethylene synthesis in M. atropurpureum roots inoculated with rhizobitoxine-producing B. elkanii. Using 1 μM ACC, we assessed the effect of ethylene on nodulation of M. atropurpureum inoculated with B. elkanii. The number of nodules formed in the presence of ACC 8 days after inoculation and later were significantly less than the numbers of nodules formed in the absence of ACC (Fig. 1). This finding suggests that in M. atropurpureum ethylene-induced inhibition of nodulation is similar to inhibition of nodulation in Pisum sativum (8, 16), Trifolium repens (8), Medicago sativa (25), Vicia sativa (9), M. truncatula (24), and V. radiata (7).
FIG. 1.
Effect of ACC on nodulation of M. atropurpureum cv. Siratro inoculated with B. elkanii USDA94. Each point represents the mean number of nodules per plant. The bars indicate standard errors. Nine plants were treated and assessed for each data point. Symbols: ○, no ACC; ●, 1 μM ACC. Asterisks indicate significant differences in mean nodule number between treatments at a confidence level of 0.05.
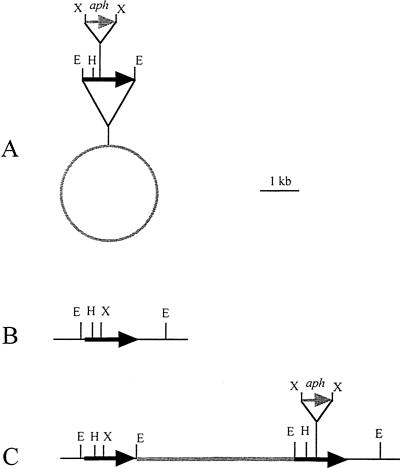
B. elkanii USDA61 is a weak producer of rhizobitoxine (39), so a strain that produces high levels of rhizobitoxine, B. elkanii USDA94, was used to construct a rhizobitoxine-deficient mutant. The rtxA gene reportedly is responsible for rhizobitoxine production by B. elkanii USDA61 (27). This gene encodes two regions similar to a rat serine:pyruvate aminotransferase and a yeast O-acetylhomoserine sulfhydrolase (27, 28). The rhizobitoxine-deficient mutant was constructed by homologous DNA recombination by using the homologous rtxA gene DNA from USDA94 and a kanamycin resistance gene. To obtain a DNA fragment homologous to the rtxA gene, we designed two primers (5′-TAG AAT TCT CCA ACG AGT GAC AGT ATG CGA-3′ and 5′-CTA ACT GAA CAG CCT CAT AAC G-3′) and used them for PCR amplification of total DNA of B. elkanii USDA94 with the following temperature program: 94°C for 2 min, followed by 40 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 3 min. The PCR products were cloned into pCR2.1 (Invitrogen, San Diego, Calif.). The DNA sequences of the clones were determined by using a model 373A DNA sequencer (Perkin-Elmer Japan, Chiba, Japan). One of the clones obtained contained a 2,948-bp insert whose sequence was 99.6% identical to the DNA sequence of the rtxA gene of USDA61. This plasmid was designated pCR7-2950.8. Plasmid pCR7-2950.8 was digested with EcoRI, and a 2.9-kb EcoRI fragment was cloned into pUC18. The plasmid obtained was designated pUC7-2950.1. Plasmid pUC7-2950.1 was digested with XhoI, and a 1.6-kb fragment containing the aminoglycoside 3′-phosphotransferase (aph) gene from pUC4-KIXX (Amersham-Pharmacia Biotech, Uppsala, Sweden) was inserted. The resulting plasmid was designated pUC7-2950::KIXX.15. Plasmid pUC7-2950::KIXX.15 was digested with EcoRI. A 4.6-kb EcoRI fragment from pUC7-2950::KIXX.15 was ligated into pSUP202 (33). The resulting plasmid was designated pSUPrtx::KIXX.7 (Fig. 2A). The restriction map of the region around rtxA in USDA94 is shown in Fig. 2B. These cloning experiments were performed by using E. coli JM109.
FIG. 2.
Construction of rhizobitoxine production-deficient mutant RTS2. RTS2 was derived from B. elkanii USDA94 by homologous recombination with a 2.9-kb PCR product (Table 1). (A) Plasmid pSUPrtx::KIXX.7. This plasmid contains DNA homologous to rtxA from USDA61 (length, 2.9 kb) and a 1.6-kb kanamycin resistance cassette (aph). The vector region of pSUP202 is 7.8 kb long. (B) Restriction map of the region around rtxA in the USDA94 genome. (C) Restriction map of the region around rtxA in the RTS2 genome. Black arrows, rtxA; gray arrow, aph; gray line, pSUP202; E, EcoRI site; H, HindIII site; X, XhoI site.
Plasmid pSUPrtx::KIXX.7 was used to transform E. coli S17-1 λ-pir (37) for conjugative transfer to Bradyrhizobium cells. The transformant was grown at 37°C overnight in Luria-Bertani medium containing 50 mg of kanamycin per liter, 50 mg of ampicillin per liter, and 20 mg of tetracycline per liter. B. elkanii USDA94 was grown in HM medium at 30°C for 6 days. Cells of the E. coli transformant and B. elkanii USDA94 were collected, washed with sterile 0.8% NaCl, and resuspended in sterile 0.8% NaCl containing 0.01% Tween 20. The suspension was applied to a sterile filter and incubated at 30°C overnight. The cells on the filter were suspended in sterile water, spread onto HM medium containing 150 mg of kanamycin per liter and 50 mg of polymyxin B per liter, and incubated at 30°C. Kanamycin- and polymyxin B-resistant colonies were selected and maintained in HM medium containing 150 mg of kanamycin per liter and were used for Southern analysis. The probes used in the Southern analysis were the 3.0-kb EcoRI rtxA homologue fragment from pCR7-2950.8, the EcoRI-digested pSUP202 plasmid vector (length, 7.8 kb), the 1.6-kb XhoI kanamycin-resistant aph gene of pUC4KIXX, and the 1.2-kb BamHI fragment of pαHD7 containing Bradyrhizobium insertion element RSα (11, 14) for DNA fingerprinting. One of the appropriate strains, a kanamycin-resistant mutant that was designated RTS2 and was obtained from USDA94, was used in this study. Rhizobitoxine concentrations in cultures were determined as described by Yasuta et al. (40).
Southern analyses of RTS2 performed with the pSUP202 plasmid vector, the aph gene fragment of pUC4KIXX, and RSα probes revealed that the mutant produced unique signals characteristic of both the plasmid vector and the aph gene and produced the same signal pattern as the parent strain when the RSα probe was used (data not shown). The mutant contained a DNA insertion with a kanamycin resistance cassette downstream of the rtxA gene in the genomic DNA (Fig. 2C). The DNA insertion might have occurred through a single crossover recombination event involving the USDA94 genome and the introduced plasmid pSUPrtx::KIXX.7 in the downstream region of rtxA. The rhizobitoxine concentrations in the mutant RTS2 and wild-type strain USDA94 cultures were compared. The limit of the detection was 0.01 μM rhizobitoxine. No rhizobitoxine was detected in the mutant RTS2 culture (concentration, <0.01 μM), whereas the rhizobitoxine concentration in the wild-type strain USDA94 culture was 17.5 μM. Inoculation with mutant RTS2 did not induce foliar chlorosis in G. max cv. Lee (data not shown), a finding similar to a previous finding for a rhizobitoxine mutant of B. elkanii USDA61 (26). Because the single-crossover mutant RTS2 did not produce rhizobitoxine, at least one additional gene (downstream of rtxA and in the same operon) may be required for rhizobitoxine production.
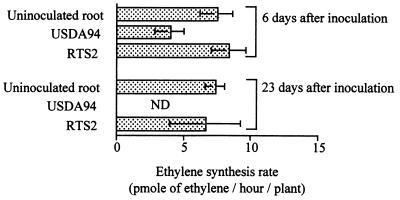
Using isogenic variants of rhizobitoxine-deficient mutant RTS2 and wild-type strain USDA94, we assessed the effect of rhizobitoxine production by B. elkanii on ethylene synthesis in M. atropurpureum. Ethylene synthesis in plant roots was measured as described above by examining plants on days 6 and 23 after inoculation. On days 6 and 23 after inoculation in the absence of ACC, the rates of ethylene synthesis in siratro roots inoculated with USDA94 were less than the rates of ethylene synthesis in uninoculated control roots (Fig. 3). When siratro was inoculated with RTS2, the ethylene synthesis rate was greater than the rate of synthesis in roots inoculated with USDA94 and equivalent to the rate of synthesis in uninoculated roots. The lack of rhizobitoxine production by B. elkanii USDA94 resulted in a loss of ethylene synthesis suppression in siratro, indicating that rhizobitoxine production by B. elkanii suppressed ethylene synthesis in M. atropurpureum. When we examined siratro roots inoculated with USDA94 in the absence of ACC, the ethylene synthesis rate was 3.24 ± 0.68 pmol/h/plant on day 6 after inoculation, while on day 23 after inoculation ethylene synthesis was not detectable, suggesting that more rhizobitoxine accumulated over time in the presence of B. elkanii. Rhizobitoxine is produced in nodules and is transported to the roots and shoots (17, 39). Because the number of nodules on day 23 after inoculation with USDA94 was about 13 times more than the number of nodules on day 6 after inoculation (data not shown), the increase in nodule number should have resulted in a higher concentration of rhizobitoxine in plants inoculated with B. elkanii.
FIG. 3.
Effects of B. elkanii strains on ethylene synthesis in M. atropurpureum roots. Plants were inoculated with B. elkanii USDA94 and rhizobitoxine production-deficient mutant RTS2. For each treatment, the mean ethylene evolution rate was determined 6 days after inoculation (15 plants) and 23 days after inoculation (9 plants). The bars indicate standard errors. ND, not detected.
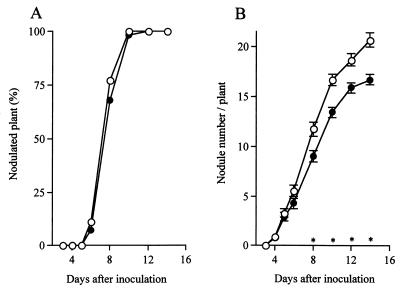
The effect of rhizobitoxine-deficient strain RTS2 on nodulation of siratro was also examined. When we compared inoculation with mutant RTS2 and inoculation with wild-type strain USDA94, we found no difference in the percentages of nodulated plants that had the first visible nodules (Fig. 4A). The same trend was observed for the percentages of nodulated plants having the first large nodules (data not shown). These results suggest that a lack of rhizobitoxine production in B. elkanii does not result in a delay in nodulation of M. atropurpureum, which is consistent with the findings of Ruan and Peters (26), who used B. elkanii USDA61 and isogenic rhizobitoxine mutants. A lack of rhizobitoxine production did not affect emergence of the first nodules, but the numbers of nodules were significantly different over time after inoculation when the isogenic variants of USDA94 were used (Fig. 4B). When the numbers of nodules were compared, the numbers of visible nodules on siratro roots after inoculation with the different isogenic variants were not different before day 6 after inoculation. From 8 days after inoculation, however, the numbers of visible nodules on siratro roots inoculated with RTS2 were less than the numbers of visible nodules on roots USDA94. Fewer nodules after inoculation with RTS2 were also observed when the numbers of large nodules were compared (data not shown). These results suggest that rhizobitoxine production by B. elkanii enhances nodulation of M. atropurpureum. A similar effect of rhizobitoxine production was described by Duodu et al. (7), who examined ethylene-sensitive nodulation of V. radiata. The reason(s) for the delayed effect of rhizobitoxine production on the number of siratro nodules is not clear. One possible explanation is that a higher concentration of rhizobitoxine in plants is necessary before there is a visible effect on nodulation. This seems logical because there was no difference in the time of appearance of the first nodules when inoculation with the rhizobitoxine mutant and inoculation with the wild type were compared (Fig. 4A). The conclusion that rhizobitoxine production by B. elkanii enhances nodulation of M. atropurpureum seems to contradict the data obtained with the isogenic variants of USDA61 (26). The latter data might have resulted from differences in the ability to produce rhizobitoxine; Xiong and Fuhrmann (39) reported that USDA94 produced more rhizobitoxine than USDA61 produced in plants.
FIG. 4.
Nodulation of M. atropurpureum cv. Siratro inoculated with B. elkanii USDA94 and rhizobitoxine production-deficient mutant RTS2. Each point represents a mean based on six independent experiments. (A) Time course for percentages of nodulated plants. (B) Time course for number of nodules per plant. Symbols: ○, plants inoculated with B. elkanii USDA94 (73 plants); ●, plants inoculated with rhizobitoxine production-deficient mutant RTS2 (72 plants). The bars indicate standard errors. The asterisks indicate significant differences in mean numbers of nodules between the treatments at a confidence level of 0.05.
To investigate competitiveness for nodulation in the wild-type and rhizobitoxine-deficient strains, mTn5SSgusA20 (37) was used to label B. elkanii and B. japonicum strains. Recently, gusA-marked transposons, including mTn5SSgusA20, have been developed (37), and using these transposons has some advantages over other techniques (2, 3, 6, 13, 30, 36). The gusA marking system results in marked (Brady)rhizobium cells with competitive ability indistinguishable from the competitive ability of the parent cells; this makes screening for the competitive ability of strains of interest simple and rapid (32). Bradyrhizobium strains marked with gusA by using mTn5SSgusA20 were constructed as described by Yuhashi et al. (41). In this study, 3 weeks after coinoculation nodules whose diameters were greater than 1 mm were harvested and used in a GUS assay performed as described by Yuhashi et al. (41). Each of the harvested nodules was cut in half with a razor blade. The hemispheric nodule segments were immersed in a GUS assay solution (50 mg of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid [X-Gluc] per liter, 2 g of sodium dodecyl sulfate per liter, 20% methanol, 20 mM sodium phosphate buffer [pH 7.0]), subjected to a vacuum for 15 min, and incubated at 30°C overnight. Samples were fixed with 0.5% glutaraldehyde–0.2 M sodium cacodylate (pH 7.2) for 1 h, washed twice with distilled water, and observed with a stereoscopic microscope. When uniform GUS activity was observed in the infected region of a nodule, the nodule was considered occupied only by a gusA-marked strain. When there was no GUS activity in the infected region of a nodule, we assumed that the nodule was formed only by the unmarked strain. When GUS activity was observed as a spattered pattern in the infected region of a nodule, the nodule was considered cooccupied by both strains. In the case of cooccupation, each strain was scored as if it occupied one-half of a nodule in order to calculate nodule occupancy values. The chi-square test was used to assess the statistical significance of differences in the numbers of occupied nodules at a confidence level of 0.05.
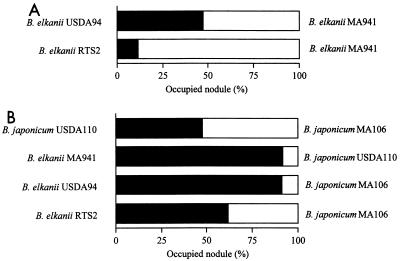
The gusA-marked strains B. elkanii MA941 and B. japonicum MA106 were obtained. When MA941 was coinoculated with parent strain USDA94 onto siratro (cell ratio, 1:1), the nodule occupancy value for the marked strain was almost the same as the value for the parent (47.4% USDA94 and 52.6% MA941) (Fig. 5A). Similar results were obtained when B. japonicum MA106 and USDA110 were coinoculated (47.5% USDA110 and 52.5% MA106) (Fig. 5B). These findings indicate that the competitive ability of the gusA-marked strains was indistinguishable from that of the parent strain.
FIG. 5.
Effect of rhizobitoxine production-deficient phenotype on competitiveness of B. elkanii USDA94 during nodulation of M. atropurpureum cv. Siratro. A chi-square test was used for statistical analyses at a confidence level of 0.05. (A) Competition between B. elkanii USDA94 and RTS2. B. elkanii MA941 is a gusA-marked USDA94 variant. RTS2 is a rhizobitoxine production-deficient mutant of USDA94. In the first experiment 228 nodules from 18 plants that were coinoculated with USDA94 and MA941 were used (47.4% USDA94 and 52.6% MA941). The data were consistent with the hypothesis that the occupied nodule ratio was 1:1. In the second experiment 159 nodules from nine plants that were coinoculated with RTS2 and MA941 were used (11.6% RTS2 and 88.4% MA941). The data were not consistent with the hypothesis that the occupied nodule ratio was 1:1. Statistical analysis showed that the difference in nodule occupancy values between USDA94 and RTS2 is significant. (B) Competition between B. elkanii USDA94 and B. japonicum USDA110. B. japonicum MA106 is a gusA-marked USDA110 variant. In the first experiment 141 nodules from 17 plants that were coinoculated with USDA110 and MA106 were used (47.5% USDA110 and 52.5% MA106). The data were consistent with the hypothesis that the occupied nodule ratio was 1:1. In the second experiment 141 from nine plants that were coinoculated with MA941 and USDA110 were used (91.5% MA941 and 8.5% USDA110). In the third experiment 193 nodules from 17 plants that were coinoculated with USDA94 and MA106 were used (91.2% USDA94 and 8.8% MA106). In the fourth experiment 177 nodules from 18 plants that were coinoculated with RTS2 and MA106 were used (61.9% RTS2 and 38.1% MA106). The MA941-USDA110 values and the USDA94-MA106 values are not significantly different. All other pairs of values are significantly different.
When rhizobitoxine production-deficient mutant RTS2 was coinoculated with MA941 (a gusA-marked USDA94 derivative) onto siratro roots, the nodule occupancy values for RTS2 and MA941 were 11.6 and 88.4%, respectively (Fig. 5A). Compared to the results obtained after coinoculation of USDA94 and MA941, the loss of rhizobitoxine production by USDA94 resulted in lower nodulation competitiveness in siratro roots. This suggests that rhizobitoxine production enhances the competitiveness of B. elkanii during nodulation of M. atropurpureum. As summarized by Triplett and Sadowsky (35), previous research has indicated that several phenotypes of (Brady) rhizobium strains play significant roles in nodulation competitiveness; these phenotypes include antibiosis, cell surface characteristics, motility, speed of nodulation, and symbiotic effectiveness. Among the phenotypes involved in nodulation competitiveness, rhizobitoxine production is unique because it suppresses ethylene synthesis in inoculated plants.
When B. elkanii MA941 and B. japonicum USDA110 were coinoculated onto siratro roots, the nodule occupancy values were 91.5 and 8.5%, respectively (Fig. 5B). Similar results were obtained when B. elkanii USDA94 and B. japonicum MA106 were coinoculated (91.2% USDA94 and 8.8% MA106) (Fig. 5B). In our preliminary studies, similar results were also obtained when gusA-marked strains of B. elkanii USDA76 and USDA31 were used in coinoculation experiments involving USDA110 (data not shown). These results suggest that B. elkanii exhibits greater competitiveness than B. japonicum in M. atropurpureum roots. This is consistent with the observation of Minamisawa et al. that the nodule occupancy value for B. elkanii was greater than the nodule occupancy value for B. japonicum when siratro was inoculated with a field soil and a mixture of several strains isolated from the field soil (21).
When we examined competitive nodulation by using the rhizobitoxine production-deficient mutant B. elkanii RTS2 and B. japonicum MA106 (a gusA-marked USDA110 derivative), the nodule occupancy values were 61.9% RTS2 and 38.1% MA106 (Fig. 5B). Compared with the results obtained in experiments performed with the parent strains (91.5% MA941 and 8.5% USDA110; 91.2% USDA94 and 8.8% MA106) the competitiveness of RTS2 with USDA110 was less than the competitiveness of wild-type strain USDA94. These results suggest that a lack of rhizobitoxine production affects the competitiveness of B. elkanii and B. japonicum during M. atropurpureum nodulation. During M. atropurpureum nodulation, rhizobitoxine production by B. elkanii is one of the factors that contribute to high occupancy values for the species that are in competition with Bradyrhizobium field strains.
In leguminous plants in which ethylene-induced inhibition of nodulation occurs, rhizobitoxine production is an effective strategy for enhancing competitive nodulation. However, the mechanism that results in a higher occupancy value for a rhizobitoxine producer is still unclear. In our preliminary analysis, the rate of ethylene evolution in siratro roots that were coinoculated with B. japonicum USDA110 and B. elkanii USDA94ΔNOD (42) lacking nodD1D2KABC genes was significantly lower than the rate of ethylene evolution in siratro roots inoculated with USDA110 alone but similar to the rate of ethylene evolution in siratro roots inoculated with USDA94 (3 and 6 days after inoculation) (data not shown). Lower rates of ethylene synthesis could be expected in whole roots in a multistrain environment containing rhizobitoxine-producing rhizobia and non-rhizobitoxine-producing rhizobia. One possible explanation for a higher occupancy value for a rhizobitoxine producer in a multistrain environment is the local effect of the rhizobitoxine produced at infection sites of the producer.
Acknowledgments
This work was supported in part by grants to K.M. from the Ministry of Education, Science, and Culture of Japan (grant 11556012) and the Joint Research Program of the Institute of Genetic Ecology, Tohoku University (grant 981002). K.Y. acknowledges a research fellowship from the Japan Society for the Promotion of Science for Young Scientists.
We thank W. Barraquio (University of Philippines, Quezon City, Philippines) for helpful comments on the manuscript.
REFERENCES
- 1.Akao S, Kouchi H. Light microscopic observation of root hair curling of soybean induced by Rhizobium infection. Jpn J Soil Sci Plant Nutr. 1989;60:53–55. . (In Japanese.) [Google Scholar]
- 2.Berger J A, May S N, Berger L R, Bohlool B B. Colorimetric enzyme-linked immunosorbent assay for the identification of strains of Rhizobium in culture and in the nodules of lentils. Appl Environ Microbiol. 1979;37:642–646. doi: 10.1128/aem.37.3.642-646.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushby H V A. Quantitative estimation of rhizobia in non-sterile soil using antibiotics and fungicides. Soil Biol Biochem. 1981;13:237–239. [Google Scholar]
- 4.Cole M A, Elkan G H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973;4:248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine T E, Kuykendall L D, O'Neill J J. DNA homology group and the identity of bradyrhizobial strains producing rhizobitoxine-induced foliar chlorosis on soybean. Crop Sci. 1988;28:938–941. [Google Scholar]
- 6.Dudman W F. Antigenic analysis of Rhizobium japonicum by immunodiffusion. Appl Microbiol. 1971;21:973–985. doi: 10.1128/am.21.6.973-985.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duodu S, Bhuvaneswari T V, Stokkermans T J, Peters N K. A positive role for rhizobitoxine in Rhizobium-legume symbiosis. Mol Plant-Microbe Interact. 1999;12:1082–1089. [Google Scholar]
- 8.Goodlass G, Smith K A. Effects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.) Plant Soil. 1979;51:387–395. [Google Scholar]
- 9.Heidstra R, Yang W C, Yalcin Y, Peck S, Emons A M, van Kammen A, Bisseling T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development. 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- 10.Hunter W J. Ethylene production by root nodules and effect of ethylene on nodulation in Glycine max. Appl Environ Microbiol. 1993;59:1947–1950. doi: 10.1128/aem.59.6.1947-1950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isawa T, Sameshima R, Mitsui H, Minamisawa K. IS1631 occurrence in Bradyrhizobium japonicum highly reiterated sequence-possessing strains with high copy numbers of repeated sequences RSα and RSβ. Appl Environ Microbiol. 1999;65:3493–3501. doi: 10.1128/aem.65.8.3493-3501.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan C D. Genus II. Bradyrhizobium Jordan 1982, 137VP. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams and Wilkins; 1984. pp. 242–244. [Google Scholar]
- 13.Jorsey D P, Beynon J L, Johnston A W, Beringer J E. Strain identification of Rhizobium using intrinsic antibiotic resistance. J Appl Bacteriol. 1979;46:343–350. [Google Scholar]
- 14.Kaluza K, Hahn M, Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985;162:535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuykendall L D, Saxena B, Devine T E, Udell S E. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol. 1992;38:501–505. [Google Scholar]
- 16.Lee K H, LaRue T A. Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 1992;100:1759–1763. doi: 10.1104/pp.100.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamisawa K, Kume N. Determination of rhizobitoxine and dihydrorhizobitoxine in soybean plants by amino acid analyzer. Soil Sci Plant Nutr. 1987;33:645–649. [Google Scholar]
- 18.Minamisawa K. Comparison of extracellular polysaccharide composition, rhizobitoxine production, and hydrogenase phenotype among various strains of Bradyrhizobium japonicum. Plant Cell Physiol. 1989;30:877–884. [Google Scholar]
- 19.Minamisawa K. Division of rhizobitoxine-producing and hydrogen-uptake positive strains of Bradyrhizobium japonicum by nifDKE sequence divergence. Plant Cell Physiol. 1990;31:81–89. [Google Scholar]
- 20.Minamisawa K, Fukai K. Production of indole-3-acetic acid by Bradyrhizobium japonicum: a correlation with genotype grouping and rhizobitoxine production. Plant Cell Physiol. 1991;32:1–9. [Google Scholar]
- 21.Minamisawa K, Onodera S, Tanimura Y, Kobayashi N, Yuhashi K, Kubota M. Preferential nodulation of Glycine max, Glycine soja and Macroptilium atropurpureum by two Bradyrhizobium species, japonicum and elkanii. FEMS Microbiol Ecol. 1997;24:49–56. [Google Scholar]
- 22.Owens L D, Wright D A. Rhizobial-induced chlorosis in soybeans: isolation, production in nodules, and varietal specificity of the toxin. Plant Physiol. 1964;40:927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens L D, Lieberman M, Kunishi A. Inhibition of ethylene production by rhizobitoxine. Plant Physiol. 1971;48:1–4. doi: 10.1104/pp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penmetsa R V, Cook D R. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 25.Peters N K, Crist-Estes D K. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1989;91:690–693. doi: 10.1104/pp.91.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan X, Peters N K. Isolation and characterization of rhizobitoxine mutants of Bradyrhizobium japonicum. J Bacteriol. 1992;174:3467–3473. doi: 10.1128/jb.174.11.3467-3473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan X, Zhang C, Peters N K. Bradyrhizobium japonicum rhizobitoxine genes and putative enzyme functions: expression requires a translational frameshift. Proc Natl Acad Sci USA. 1993;90:2641–2645. doi: 10.1073/pnas.90.7.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan X, Peters N K. Author's correction. Isolation and characterization of rhizobitoxine mutants of Bradyrhizobium japonicum. Proc Natl Acad Sci USA. 1993;90:12054–12055. [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Schmidt E L, Bakole R O, Bohlool B B. Fluorescent antibody approach to the study of rhizobia in soil. J Bacteriol. 1968;95:1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt J S, Harper J E, Hoffman T K, Bent A F. Regulation of soybean nodulation independent of ethylene signaling. Plant Physiol. 1999;119:951–959. doi: 10.1104/pp.119.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sessitsch A, Jjemba P K, Hardarson G, Akkermans A D L, Wilson K J. Measurement of the competitiveness index of Rhizobium tropici strain CIAT899 derivatives marked with the gusA gene. Soil Biol Biochem. 1997;29:1099–1110. [Google Scholar]
- 33.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 34.Suganuma N, Yamauchi H, Yamamoto K. Enhanced production of ethylene by soybean roots after inoculation with Bradyrhizobium japonicum. Plant Sci. 1995;111:163–168. [Google Scholar]
- 35.Triplett E W, Sadowsky M J. Genetics of competition for nodulation of legumes. Annu Rev Microbiol. 1992;42:399–428. doi: 10.1146/annurev.mi.46.100192.002151. [DOI] [PubMed] [Google Scholar]
- 36.Turco R F, Moorman T B, Bezdicek D F. Effectiveness and competitiveness of spontaneous antibiotic resistance marked strains of Rhizobium leguminosarum and Rhizobium japonicum. Soil Biol Biochem. 1986;18:259–262. [Google Scholar]
- 37.Wilson K J, Sessitsch A, Corbo J C, Giller K E, Akkermans A D L, Jefferson R A. β-Glucronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology. 1995;141:1691–1705. doi: 10.1099/13500872-141-7-1691. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z-P, Staehelin C, Wiemken A, Bolle T. Ethylene responsiveness of soybean cultivars characterized by leaf senescence, chitinase induction and nodulation. J Plant Physiol. 1996;149:690–694. [Google Scholar]
- 39.Xiong K, Fuhrmann J J. Soybean response to nodulation by wild-type and an isogenic Bradyrhizobium elkanii mutant lacking rhizobitoxine production. Crop Sci. 1996;36:1267–1271. [Google Scholar]
- 40.Yasuta T, Satoh S, Minamisawa K. New assay for rhizobitoxine based on inhibition of 1-aminocyclopropane-1-carboxylate synthase. Appl Environ Microbiol. 1999;65:849–852. doi: 10.1128/aem.65.2.849-852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuhashi K, Minamisawa K, Minakawa Y, Tobias D J, Kubota M, Akao S. Nodulation and competitiveness of gusA-marked Bradyrhizobium japonicum A1017 in soybean. Soil Sci Plant Nutr. 1997;43:473–478. [Google Scholar]
- 42.Yuhashi K, Akao S, Fukuhara H, Tateno E, Chun J-Y, Stacey G, Hara H, Kubota M, Asami T, Minamisawa K. Bradyrhizobium elkanii induces outer cortical root swelling in soybean. Plant Cell Physiol. 1995;36:1571–1577. [Google Scholar]