Abstract
Background/Aim
The underlying processes of renal cell carcinoma (RCC), one of the deadliest malignancies of the urinary system, are still poorly understood. HECT domain E3 ubiquitin protein ligase 2 (HECTD2) is an E3 ubiquitin ligase implicated in the pulmonary inflammatory response. This study investigated the impact of HECTD2 on regulating inflammation in RCC cells and its potential mechanisms.
Materials and Methods
HECTD2 expression in RCC tissues was examined. Immunoprecipitation and western blot (WB) analysis confirmed that HECTD2 up-regulated euchromatic histone lysine methyltransferase 2 (EHMT2) protein degradation. ChIP experiments validated tumor necrosis factor α Inducing protein 1 (TNFAIP1) as a direct target of EHMT2. qRT-PCR determined HECTD2 and TNFAIP1 expression in RCC cells. Cell viability was assayed via CCK-8. ELISA was employed to measure the expression of IL-6, TNF-α, IL-8, and IL-1β. WB analysis was conducted to test p38/JNK pathway-related protein (p38, p-p38, JNK, and p-JNK) expression.
Results
HECTD2 and TNFAIP1 were significantly up-regulated in RCC patient tissues and cells. Subsequent investigations revealed that HECTD2 promoted an inflammatory response in RCC cells. Additionally, HECTD2 up-regulated TNFAIP1 expression, and high TNFAIP1 expression could reverse the repressive impact of low HECTD2 expression on the inflammatory response in RCC cells. Rescue experiments demonstrated that the addition of p38/JNK pathway inhibitors attenuated the impact of TNFAIP1 overexpression on the RCC inflammatory response.
Conclusion
Our findings establish a new mechanism by which HECTD2 exerts a pro-inflammatory role in RCC cells and present a prospective method for an anti-inflammatory intervention targeting the HECTD2/TNFAIP1 axis in malignancies.
Keywords: HECTD2, TNFAIP1, p38/JNK pathway, Renal cell carcinoma, Inflammatory response
One of the most prevalent malignancies of the genitourinary system, renal cell carcinoma (RCC), has a significant death rate (1). A prevalent subtype of RCC, clear cell RCC (ccRCC), accounts for around 75% of RCC cases (2,3). Due to high rates of RCC recurrence and metastasis, the prognosis for patients is still dismal despite advances in medical technology (3). Therefore, it is necessary to dissect molecular regulatory mechanisms underlying RCC development and identify new biomarkers for targeted therapy.
In recent years, the association between inflammation and cancer has become a focus of cancer research. Inflammation is a fundamental innate immune response of the body to disrupted tissue homeostasis (4). Inflammatory molecules have a crucial role in the development of several malignancies, including breast, lung, colon cancer and RCC, as shown by numerous studies (5-8). For instance, the inflammasome adaptor protein PYCARD is a protein involved in inflammation, pyroptosis, and apoptosis. The expression of PYCARD is associated with immune response and its overexpression is positively correlated with poor prognosis in ccRCC patients (9). This study aimed to further explore the mechanisms influencing inflammatory response in RCC cells and provide new directions for the treatment of RCC-associated inflammation.
The well-known BTB domain protein tumor necrosis factor α Inducing protein 1 (TNFAIP1) is essential for DNA synthesis, cell apoptosis, cell migration, and immune response (10,11). Recent studies have reported that TNFAIP1 down-regulation promotes malignant progression in cancers such as hepatocellular carcinoma and neuroblastoma (10,12). For instance, in lung cancer cells, down-regulation of Circ0001320 up-regulates TNFAIP1 and TPM1 by binding to miR-558, thus inhibiting tumor cell growth and invasion (13). Moreover, TNFAIP1 has been reported to regulate inflammatory responses. Zhao et al. (14) found that by stimulating NF-ĸB activity, TNFAIP1 functions as a crucial mediator of inflammation. Xu et al. (15) discovered that knockdown of lncRNA KCNQ1OT1 down-regulates TNFAIP1 by increasing the expression of miR-137, thereby inhibiting the inflammatory response and oxidative stress in ox-LDL-induced THP-1 macrophages. However, regarding the mechanism of the TNFAIP1-mediated inflammatory response in RCC cells, there is a dearth of information.
We examined HECT domain E3 ubiquitin protein ligase 2 (HECTD2) expression in RCC tissues through clinical analysis and revealed that HECTD2 was highly expressed in RCC tissues and associated with an inflammatory response. Previous studies have shown that HECTD2 can enhance the occurrence of pulmonary inflammation caused by Gram negative bacteria through ubiquitination of PIAS1 (16). Subsequently, we validated that low expression of HECTD2 inhibited the inflammatory response in RCC cells through cell-based experiments. Furthermore, functional analysis revealed that HECTD2 up-regulated euchromatic histone lysine methyltransferase 2 (EHMT2) protein degradation and identified TNFAIP1 as a direct target of EHMT2. Further investigation demonstrated that TNFAIP1 promoted the inflammatory response in RCC cells through the p38/JNK pathway. Thus, our findings suggest that HECTD2 modulates the inflammatory response in RCC cells by up-regulating TNFAIP1 expression and modulating the p38/JNK pathway. This study revealed a novel molecular mechanism associated with RCC progression and offers theoretical insights into RCC treatment.
Materials and Methods
Patients and samples. Between February 2021 and December 2022, a total of 10 pairs of fresh RCC tissues (Tumor) and their corresponding adjacent normal tissues (Normal) were collected from RCC patients who underwent nephrectomy at the department of urinary surgery of Deyang City People’s Hospital for qRT-PCR analysis. Immediately after surgical removal, all fresh tissue samples were quickly frozen in liquid nitrogen and kept at –80˚C until RNA extraction. Serum samples were collected from 10 RCC patients prior to surgery. Additionally, serum samples from 10 healthy individuals were used as controls for ELISA analysis. This study was authorized by the Ethics Committee of Deyang City People’s Hospital (2023-04-080-K01), was conducted following the Declaration of Helsinki, and obtained informed consent from all patients and volunteers.
Cell culture. All cell lines were accessed from ATCC (Manassas, Virginia, USA). The HK2 cells were cultured in K-SFM (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, MA, USA). The RCC cell lines ACHN, A498, and Caki-1 were in medium with 10% FBS, ACHN and A498 cells were in EMEM (Merck, NJ, USA), and Caki-1 cells were in McCoy’s 5A Medium (Thermo Fisher Scientific). To investigate the impact of the p38/JNK pathway, RCC cells were treated with 16 μM p38/JNK pathway inhibitor GS-444217 (MedchemExpress, Monmouth Junction, NJ, USA). All cells were cultured in a cell incubator at 37˚C with 5% CO2 (17,18).
Based on previous studies (19), an inflammatory cell model was induced using recombinant human TNF-α (PeproTech, Cranbury, NJ, USA) at the following concentrations: 0, 1, 10, and 20 ng/ml for a duration of 24 h.
Cell transfection. si-HECTD2, oe-TNFAIP1, and negative control were synthesized by Shanghai Genechem Co., Ltd. (Shanghai, PR China). Lipofectamine 3000 (Invitrogen) was utilized for cell transfection, and subsequent experiments were conducted 48 h post-transfection. Experiments were independently repeated three times.
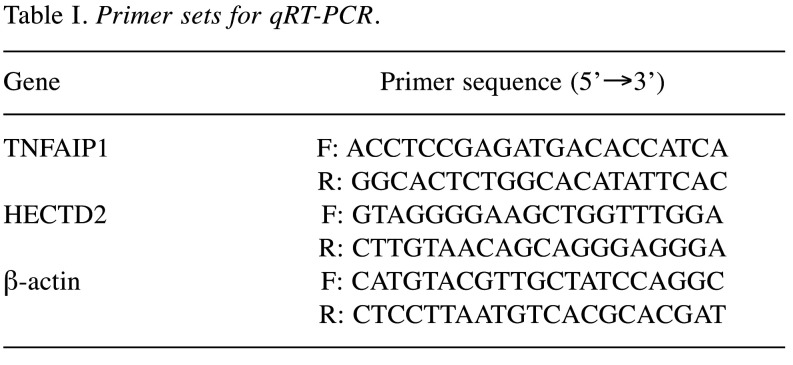
qRT-PCR. Total RNA extraction was done by using Trizol (Invitrogen). The PrimeScript™ RT reagent Kit (TaKaRa, Kyoto, Japan) was utilized for reverse transcription of 5 μg total RNA samples to synthesize cDNA. QuantiTect SYBR® Green RT-PCR Kit (Qiagen, Germantown, MD, USA) was employed for qRT-PCR on an ABI PRISM 7900 Sequence Detection System (Thermo Fisher Scientific). Relative expression of HECTD2 and TNFAIP1 were calculated by the 2-ΔΔCt method, with β-actin as internal control. Primer sequences are provided in Table I.
Table I. Primer sets for qRT-PCR.
Western blot (WB). Total protein isolation was done by using RIPA lysis buffer (Beyotime, Shanghai, PR China), and protein concentration was tested with a BCA protein concentration detection kit (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE gel and transferred onto PVDF membranes. After blocking with BSA blocking solution for 1 h at room temperature, membranes were incubated with the following antibodies at 4˚C overnight: anti-p-p38 [Phospho-p38 MAPK (Thr180/Tyr182) (D3F9) XP® Rabbit mAb #4511], anti-p38 [p38 MAPK (D13E1) XP® Rabbit mAb #8690], anti-p-JNK [Phospho-SAPK/JNK (Thr183/Tyr185) (81E11) Rabbit mAb #4668], anti-JNK [SAPK/JNK (E7R5D) Rabbit mAb #67096], anti-β-actin [β-Actin (13E5) Rabbit mAb #4970], all from Cell Signaling Technology, Danvers, MA, USA, as well as HECTD2 (ab173572) and EHMT2 (ab40542) from Abcam, Cambridge, UK. After incubation with goat anti-rabbit IgG-HRP (ab205718) (Abcam) secondary antibody at room temperature for 1 h, protein bands were visualized with the ECL detection kit (Pierce Biotechnology, Rockford, IL, USA) and imaged using a fluorescence and chemiluminescence imaging system (Clinx, Anhui, P.R. China).
CCK-8 assay. Cells were plated in a 96-well culture plate (2×103 cells/well). Following cell attachment at 0, 24, 48, 72, and 96 h, 10 μl of CCK-8 reagent was supplemented to each well, followed by 2 h of incubation at 37˚C. Absorbance was measured at 450 nm with a microplate reader (Bio-Rad, Hercules, CA, USA).
ELISA. The human TNF-α ELISA kit (ab181421), the IL-8 (ab214030), IL-1β (ab214025) and IL-6 (ab178013) ELISA kits were used to detect the inflammatory cytokines TNF-α, IL-1β, IL-8, and IL-6, respectively. All procedures were conducted according to the kits’ instructions (Abcam).
Immunoprecipitation. Cells were lysed using NP40 (Beyotime), and 1000 mg of protein was incubated with the specified antibody at 4˚C for 4 h. A/G agarose beads (Beyotime) were added to 50 μl of protein sample, and the mixture was incubated on a shaker at 4˚C for 2 h. The beads were collected and rinsed three times with lysis buffer. Protein immunoblotting analysis was performed using the specified antibodies.
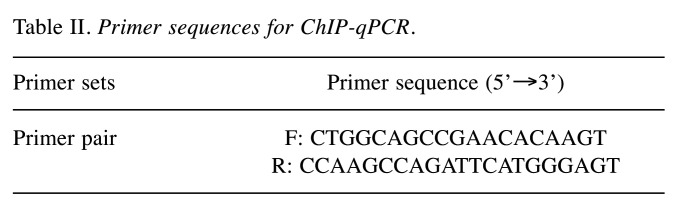
Chromatin immunoprecipitation (ChIP) assay. The ChIP assay was conducted with Magna ChIP A/G kit (Merck). RCC cells were transfected with si-NC and si-EHMT2 for 48 h, crosslinked with 1% formaldehyde (Merck) for 10 min at room temperature, and quenched with 1× glycine (Merck) for 5 min. Next, RCC cells were rinsed with cold 1× PBS containing 1× protease inhibitor cocktail II (MedchemExpress). Following nuclear extraction, the Bioruptor Pico sonicator was used for 15 cycles of 30 s ON and 30 s OFF to get chromatin fragments of 200-1000 bp. Chromatin was then immunoprecipitated overnight at 4˚C with 2 μg of H3K9me2 antibody (ab176882) (Abcam) or 2 μg of H3K9ac antibody (ab32129) (Abcam) along with 20 μl of Magna ChIP A/G magnetic beads. The complexes were incubated with ChIP elution buffer and RNase A mixture at 30˚C for 37 min and incubated with proteinase K at 62˚C for 2 h. Following DNA purification with spin columns, samples were measured by qRT-PCR. Primer sequences are provided in Table II.
Table II. Primer sequences for ChIP-qPCR.
Data analysis. All experiments were performed at least 3 times. Data were presented as mean±standard deviation (SD) and analyzed on GraphPad Prism 8.0 (GraphPad, La Jolla, CA, USA) software. Student’s t-test, log-rank test, and analysis of variance (ANOVA) were used. p<0.05 was considered statistically significant.
Results
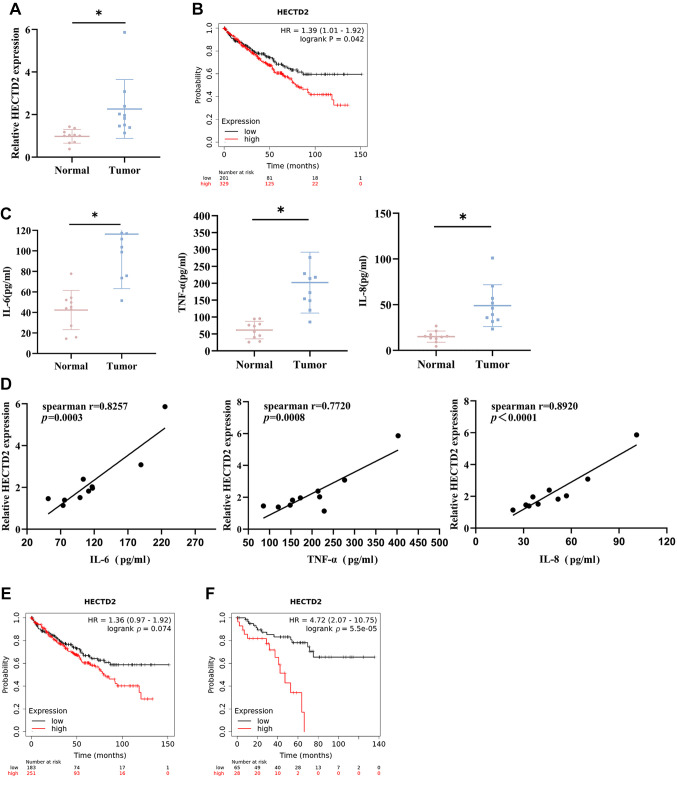
High HECTD2 expression in RCC tissues is positively correlated with inflammatory markers. To investigate whether HECTD2 contributes to RCC progression, we recruited 10 RCC patients who underwent surgical resection and obtained tumor tissues and adjacent non-cancerous tissues. HECTD2 levels in these tissue samples were detected using qRT-PCR. HECTD2 expression was significantly higher in RCC tumor tissues compared to adjacent non-cancerous tissues (Figure 1A). ccRCC is the most common subtype of RCC (1). We obtained pan-cancer data using the Kaplan Meier Plotter web tool and found that high expression of HECTD2 was associated with shorter survival time in patients with ccRCC (Figure 1B). Furthermore, expression of inflammatory markers (IL-6, TNF-α, IL-8) in the blood of 10 RCC patients and healthy volunteers were assayed using ELISA. Their expression was higher in the blood of RCC patients than in healthy volunteers (Figure 1C). Subsequently, correlation between HECTD2 expression and IL-6, TNF-α, and IL-8 expression was performed. The data showed a positive correlation between the expression of HECTD2 and inflammatory markers (Figure 1D).
Figure 1. HECTD2 is highly expressed in renal cell carcinoma (RCC) tissues and is positively correlated with the levels of inflammatory response markers. A: Expression of HECTD2 in RCC tissues. B: Kaplan-Meier Plotter survival analysis of patients with clear cell renal cell carcinoma (ccRCC) in high and low expression HECTD2 groups. C: Expression of inflammatory response markers (IL-6, TNF-α, IL-8) in patient blood samples. D: Correlation analysis between HECTD2 expression and IL-6, TNF-α and IL-8 expression. E: Kaplan Meier Plotter survival analysis of ccRCC patients with macrophage enriched in high and low expression HECTD2 groups. F: Kaplan Meier Plotter survival analysis of ccRCC patients with reduced macrophages in high and low expression HECTD2 groups. *p<0.05 using t-test and log-rank test.
The tumor microenvironment is composed of various types of immune cells, among which tumor-associated macrophages are an important type of immune cells. Tumor-associated macrophages have two phenotypes, namely the M1-type (highly lethal macrophages) and the M2-type (immuno-suppressive macrophages) (20). Similarly, we used Kaplan Meier Plotter to investigate the survival rate of ccRCC patients with macrophage enrichment and found no statistically significant difference in survival rate (p=0.074) between patients with high and low expression of HECTD2 (Figure 1E). In patients with reduced macrophages, there was a significant difference in the survival rate between patients with high and low expression of HECTD2 (p=5.5×10–5) (Figure 1F). We speculated whether there was a difference in the survival rate caused by the high and low expression of HECTD2 due to different numbers of macrophages. The above research results indicated that HECTD2 was highly expressed in RCC tissue, and its high expression was associated with low survival rates in ccRCC patients. HECTD2 was positively correlated with the content of inflammatory markers. There was a significant difference in survival rates between ccRCC patients with high and low expression of HECTD2, which was related to the number of macrophages.
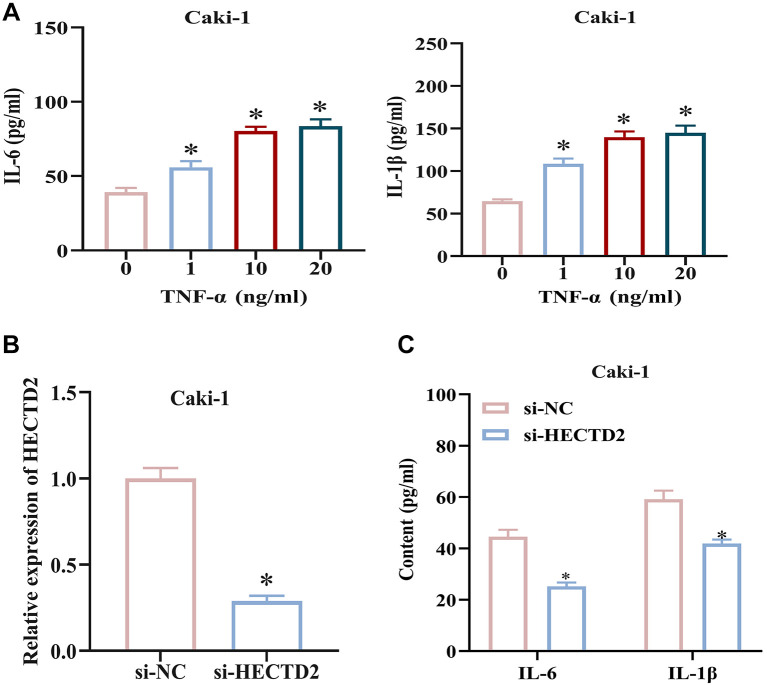
Knockdown of HECTD2 suppresses the inflammatory response in RCC cells. TNF-α can induce an inflammatory response in tumors. In this study, cells were treated with TNF-α at 0, 1, 10, and 20 ng/ml to induce an inflammatory response, and expression of inflammatory factors (IL-6, IL-1β) were assessed using ELISA. The results revealed that IL-6 and IL-1β levels in the culture medium increased with increasing concentrations of TNF-α (Figure 2A). Subsequently, we generated Caki-1 cell lines with HECTD2 knockdown and measured the transfection efficiency. HECTD2 expression was reduced in Caki-1 cells with HECTD2 knockdown compared to control group (Figure 2B). Furthermore, cells transfected with si-HECTD2 were treated with the optimal concentration (10 ng/ml) of TNF-α; IL-6 and IL-1β expression was then tested using ELISA. The results demonstrated a significant reduction in IL-6 and IL-1β expression in RCC cells after knockdown of HECTD2 compared to control cells (Figure 2C). These findings demonstrate that knockdown of HECTD2 can suppress the inflammatory response in RCC cells.
Figure 2. Knockdown of HECTD2 suppresses the inflammatory response in renal cell carcinoma (RCC) cells. A: Induction of inflammatory response in RCC cells by treatment with 0, 1, 10 and 20 ng/ml of TNF-α, and detection of IL-6 and IL-1β expression. B: Construction of si-HECTD2 RCC cell line and measurement of transfection efficiency. C: Construction of si-HECTD2 RCC cell line and detection of IL-6 and IL-1β expression. *p<0.05 using ANOVA (A) and t-test (B-C).
Up-regulation of TNFAIP1 expression by HECTD2. Previous studies have found that in colorectal cancer, propionate up-regulates HECTD2 to down-regulate EHMT2; more specifically, HECTD2 promotes the proteasomal degradation of EHMT2, reducing EHMT2 expression. The down-regulation of EHMT2 promotes TNFAIP1 expression, i.e., EHMT2 up-regulates TNFAIP1 expression through modulation of H3K9 acetylation and dimethylation), thereby inhibiting the growth of colorectal cancer cells (21). This indicates that the expression of HECTD2 could affect TNFAIP1 expression by influencing EHMT2-mediated regulation of TNFAIP1 expression.
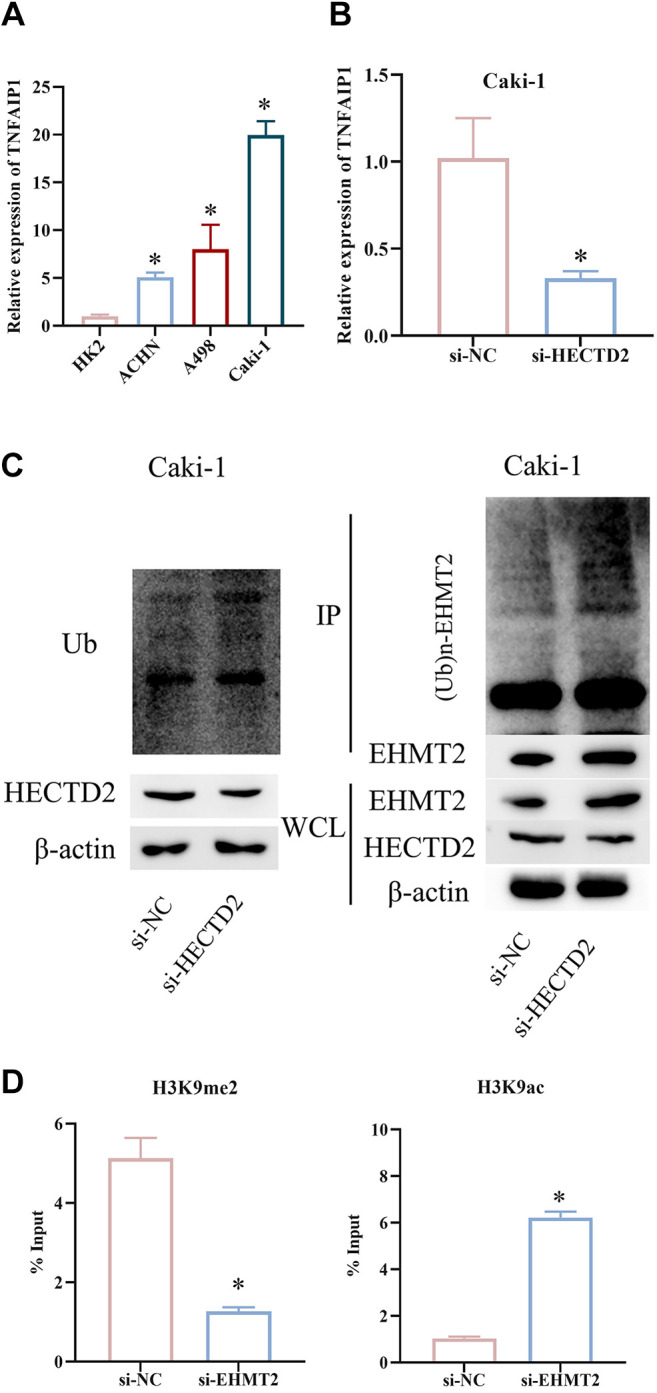
Therefore, we examined TNFAIP1 expression in HK2, ACHN, A498, and Caki-1 cells using qRT-PCR. TNFAIP1 was up-regulated in RCC cells (Figure 3A). Next, we generated Caki-1 cells with knockdown of HECTD2 expression. qRT-PCR of TNFAIP1 expression showed that TNFAIP1 expression was lower in cells with HECTD2 knockdown compared to control cells (Figure 3B). This suggested that expression of TNFAIP1 was also influenced by HECTD2 in RCC. To investigate whether HECTD2 regulates TNFAIP1 expression through EHMT2 in RCC, we performed WB and immunoprecipitation experiments for validation. Firstly, we examined influence of HECTD2 on total protein ubiquitination in Caki-1 cells. Knockdown of HECTD2 did not change overall level of protein ubiquitination. However, immunoprecipitation experiments revealed a significant decrease in EHMT2 ubiquitination after HECTD2 knockdown (Figure 3C). To unveil direct target genes of EHMT2, we did additional ChIP assays following si-EHMT2 treatment. We designed ChIP primers targeting the TNFAIP1 promoter region and conducted ChIP assays using antibodies against H3K9me2 and H3K9ac. The data showed a significant decrease in H3K9 dimethylation and an increase in H3K9 acetylation at the TNFAIP1 gene promoter region after EHMT2 knockdown compared to si-NC group (Figure 3D). These findings indicate that HECTD2 up-regulates TNFAIP1 expression.
Figure 3. HECTD2 up-regulates TNFAIP1 expression. A: Expression of TNFAIP1 in HK2 and RCC cell lines ACHN, A498, and Caki-1. B: Expression of TNFAIP1 in Caki-1 cells. C: Interaction between HECTD2 and EHMT2. D: Detection of the direct target gene TNFAIP1 of EHMT2. *p<0.05 using ANOVA (A) and t-test (B, D).
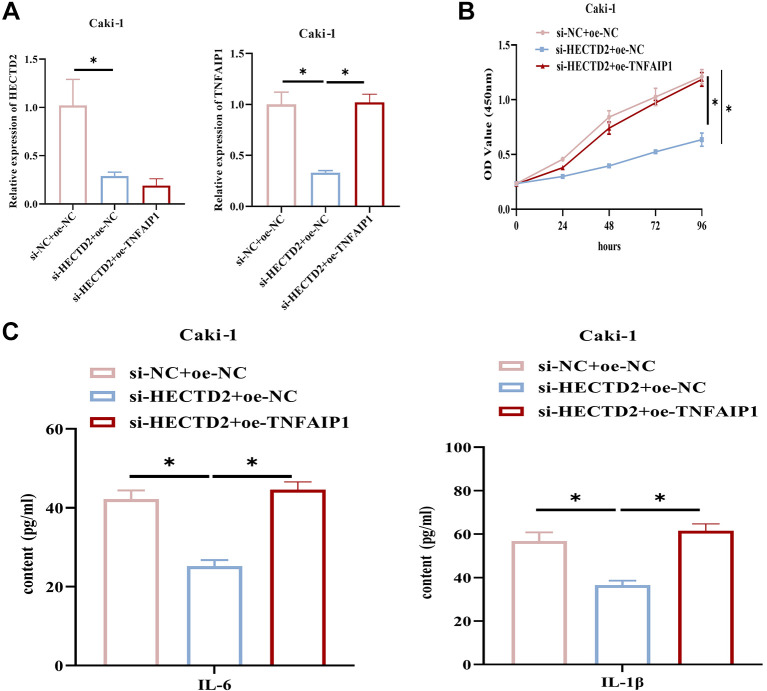
HECTD2 promotes a RCC cell inflammatory response through up-regulation of TNFAIP1 expression. To clarify the role of HECTD2 in RCC cell progression through up-regulation of TNFAIP1, we established the following groups based on Caki-1 cells: si-NC+oe-NC, si-HECTD2+oe-NC, si-HECTD2+oe-TNFAIP1. qRT-PCR results showed that knockdown of HECTD2 significantly down-regulated HECTD2 and TNFAIP1 in Caki-1 cells, while up-regulation of TNFAIP1 restored TNFAIP1 expression to the control group level (Figure 4A). CCK-8 unraveled that silencing of HECTD2 significantly repressed the viability of Caki-1 cells, while overexpressing TNFAIP1 reversed the impact of low HECTD2 expression on RCC cell viability (Figure 4B). Cells were treated with 10 ng/ml TNF-α, and expression of IL-6 and IL-1β was measured by ELISA. Knockdown of HECTD2 in Caki-1 cells led to a significant reduction in IL-6 and IL-1β expression, while up-regulation of TNFAIP1 reversed the repressive impact of low HECTD2 expression on the expression of inflammation-related factors (Figure 4C). These findings indicate that HECTD2 promotes RCC cell inflammation through up-regulation of TNFAIP1 expression.
Figure 4. HECTD2 promotes the inflammatory response in renal cell carcinoma (RCC) cells by up-regulating TNFAIP1 expression. A: Expression of HECTD2 and TNFAIP1. B: Measurement of RCC cell viability. C: Detection of IL-6 and IL-1β expression. *p<0.05 using ANOVA (A-C).
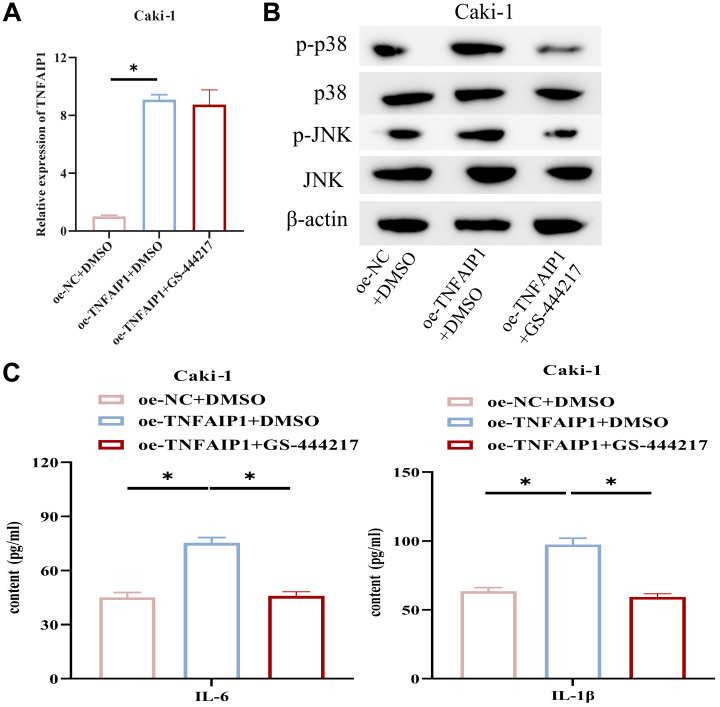
TNFAIP1 promotes RCC cell inflammatory response through the p38/JNK pathway. TNFAIP1 plays a key role in the p38/JNK pathway (22). Hence, we speculated that TNFAIP1 may foster RCC cell inflammation via the p38/JNK pathway. By constructing cell lines treated with oe-TNFAIP1 and GS-444217 (a p38/JNK pathway inhibitor), we performed qRT-PCR to measure TNFAIP1 expression. Overexpressing TNFAIP1 increased TNFAIP1 expression in Caki-1 cells (Figure 5A). WB analysis of p38, JNK, and their phosphorylation levels, which were related to the p38/JNK signaling pathway, revealed that p-p38 and p-JNK expression were significantly elevated in Caki-1 cells with up-regulated TNFAIP1, while the addition of GS-444217 restored p-p38 and p-JNK expression to control group levels (Figure 5B). ELISA results illustrated that up-regulation of TNFAIP1 significantly promoted IL-6 and IL-1β expression, while the addition of GS-444217 attenuated this effect (Figure 5C). These findings suggest that TNFAIP1 promotes RCC cell inflammation through the p38/JNK pathway.
Figure 5. TNFAIP1 promotes the inflammatory response in renal cell carcinoma (RCC) cells through the p38/JNK pathway. A: Expression of TNFAIP1. B: Expression of P38 and JNK proteins, as well as their phosphorylation levels, related to the P38/JNK signaling pathway. C: Detection of IL-6 and IL-1β expression. *p<0.05 using ANOVA (A, C).
Discussion
RCC is a frequently diagnosed malignancy, and its high incidence is negatively correlated with survival in patients (23). With the development of medical research, more diagnostic and treatment methods for RCC have been explored. Long-term prognosis and treatment results are still lacking despite substantial advancements in the detection and treatment of human malignancies. Therefore, the study of the mechanisms underlying RCC remains a significant research task for medical researchers today.
E3 ubiquitin ligase (HECTD) members homologous to E6APC terminus (HECT) are involved in tumor progression through ubiquitin modification (24). HECTD1 down-regulation elevates the migration and EMT of cervical cancer cells by stabilizing SNAIL through ubiquitination-mediated regulation (25). HECTD2, an E3 ubiquitin ligase, promotes melanoma proliferation and immune evasion (26). Kobelyatskaya et al. (27) predicted through bioinformatics methods that HECTD2 can serve as a potential therapeutic target in the main molecular subtype TMPRSS2-ERG of prostate cancer. According to previous research results, HECTD2 has been shown to be involved in the occurrence and development of cancer. In colorectal cancer cells, HECTD2 overexpression induces LPCAT1 ubiquitination and degradation, inhibiting tumor cell proliferation (28). In RCC cells, by weakening miR-320a, HIF-1α increases the expression of HECTD2, boosting tumor cell proliferation, migration, invasion, and EMT while decreasing apoptosis and hastening the malignant development of cancer cells (29). We saw up-regulated HECTD2 in RCC through clinical analysis, which is consistent with previous research. Furthermore, cellular experiments demonstrated that up-regulation of HECTD2 promoted the inflammatory response of RCC cells. These findings confirmed the pro-inflammatory and oncogenic properties of HECTD2 in RCC progression.
Additionally, WB and immunoprecipitation confirmed that up-regulation of HECTD2 promotes proteasomal degradation of the EHMT2 protein, and ChIP experiments validated TNFAIP1 as a direct target of EHMT2. This suggests that HECTD2 can regulate the expression of TNFAIP1 by influencing EHMT2, which is similar to the findings reported by Ryu et al. (21). TNFAIP1 is a protein induced early in inflammation by TNF-α (30). Research has demonstrated that TNFAIP1 plays a pivotal role in the regulation of numerous physiological and pathological processes, including tumor development and the migration of cancer cells. The CRL3BTBD9 E3 ubiquitin ligase complex causes TNFAIP1 degradation to prevent cancer cell migration when TNFAIP1 is overexpressed in human lung carcinoma (12). Xu et al. (31) found that down-regulation of miRNA-15a in human osteosarcoma inhibits cancer cell malignant behaviors by targeting TNFAIP1. We found high expression of TNFAIP1 in RCC cells. To investigate whether TNFAIP1 is critical in inflammatory response of RCC cells promoted by HECTD2, we further up-regulated TNFAIP1 based on knocking down HECTD2 in RCC. Up-regulation of TNFAIP1 significantly reversed the repressive impact of low HECTD2 expression on the inflammatory response of RCC cells. These data reveal that HECTD2/TNFAIP1 may serve as therapeutic targets for RCC.
In addition, this study also disclosed that up-regulated TNFAIP1 promotes p-p38 and p-JNK expression in RCC cells, and the addition of P38/JNK pathway inhibitors can reverse this phenomenon. It is worth noting that the JNK/P38 signaling pathway is an important component of the MAPK family, and evidence suggests that activation of p38 and JNK signaling pathways promotes inflammation and cell death (17). For example, hypaphorin inactivates p38/JNK signaling by increasing DUSP1, thereby preventing lipopolysaccharide-induced acute lung injury and pro-inflammatory responses (32). In this study, we found, through rescue experiments, that up-regulation of TNFAIP1 could promote the inflammatory response of RCC cells, while the addition of P38/JNK pathway inhibitors weakened the effect of TNFAIP1 on the inflammatory response of RCC cells. These findings gave important information on the mechanisms of HECTD2/TNFAIP1.
In summary, this study primarily investigated the influence of HECTD2 on the inflammatory response in RCC cell lines and explored its potential mechanisms. HECTD2 was highly expressed in RCC and promoted activation of p38/JNK signaling pathway by regulating TNFAIP1 expression, thus increasing the inflammatory response of RCC cells. However, there are still some limitations in this study. For example, the functional validation of HECTD2 in animal models has not been performed.
Conclusion
In summary, this study mainly explores and verifies that HECTD2, which is highly expressed in RCC cells, can target the up-regulation of TNFAIP1 expression, mediate p38/JNK signaling, and enhance the inflammatory response of RCC cells. Interestingly, there has been no literature reporting that the HECTD2/TNFAIP1 regulatory axis can affect the inflammatory response of RCC cells. In the future, HECTD2/TNFAIP1 could have important clinical implications for RCC diagnosis and therapy. In addition, targeted regulation of the p38/JNK signaling pathway related to inflammatory response can serve as a breakthrough point for studying the mechanism of inflammatory response in RCC cells.
Conflicts of Interest
All Authors declare that they have no potential conflicts of interest.
Authors’ Contributions
All Authors contributed to data analysis, drafting, and revising the article, gave their final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgements
The study was supported by Hospital incubation project of Deyang People’s Hospital (No. FHS202301).
References
- 1.Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17(4):245–261. doi: 10.1038/s41581-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song E, Song W, Ren M, Xing L, Ni W, Li Y, Gong M, Zhao M, Ma X, Zhang X, An R. Identification of potential crucial genes associated with carcinogenesis of clear cell renal cell carcinoma. J Cell Biochem. 2018;119(7):5163–5174. doi: 10.1002/jcb.26543. [DOI] [PubMed] [Google Scholar]
- 3.Wolf MM, Kimryn Rathmell W, Beckermann KE. Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene. 2020;39(17):3413–3426. doi: 10.1038/s41388-020-1234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374(6571):1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S, Charan M, Shukla RK, Agarwal P, Misri S, Verma AK, Ahirwar DK, Siddiqui J, Kaul K, Sahu N, Vyas K, Garg AA, Khan A, Miles WO, Song JW, Bhutani N, Ganju RK. cPLA2 blockade attenuates S100A7-mediated breast tumorigenicity by inhibiting the immunosuppressive tumor microenvironment. J Exp Clin Cancer Res. 2022;41(1):54. doi: 10.1186/s13046-021-02221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anitha R, Subashini R, Kannayiram G, Gayathri D. Chronic inflammatory-modulating potential of Cassia auriculata with proinflammatory cytokine IL-1beta and its anticancer effect on lung cancer cell line. Anticancer Agents Med Chem. 2021;21(3):343–354. doi: 10.2174/1871520620666200811111114. [DOI] [PubMed] [Google Scholar]
- 7.Seetha A, Devaraj H, Sudhandiran G. Indomethacin and juglone inhibit inflammatory molecules to induce apoptosis in colon cancer cells. J Biochem Mol Toxicol. 2020;34(2):e22433. doi: 10.1002/jbt.22433. [DOI] [PubMed] [Google Scholar]
- 8.Kruk L, Mamtimin M, Braun A, Anders HJ, Andrassy J, Gudermann T, Mammadova-Bach E. Inflammatory networks in renal cell carcinoma. Cancers (Basel) 2023;15(8):2212. doi: 10.3390/cancers15082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su JQ, Tian X, Xu WH, Anwaier A, Ye SQ, Zhu SX, Wang Y, Gu J, Shi GH, Qu YY, Zhang HL, Ye DW. The inflammasomes adaptor protein PYCARD is a potential pyroptosis biomarker related to immune response and prognosis in clear cell renal cell carcinoma. Cancers (Basel) 2022;14(20):4992. doi: 10.3390/cancers14204992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Huang S, Qiu F, Ding X, Sun Y, Wei C, Hu X, Wei K, Long S, Xie L, Xun Y, Chen W, Zhang Z, Liu N, Xiang S. Tumor necrosis factor α-induced protein 1 as a novel tumor suppressor through selective downregulation of CSNK2B blocks nuclear factor-ĸB activation in hepatocellular carcinoma. EBioMedicine. 2020;51:102603. doi: 10.1016/j.ebiom.2019.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang X, Tangkham T, Aljahdali B, Lee S, Su M, Dibart S. The role of TNF-α induced protein 1 in the activation of pro-apoptotic proteins. Hum Cell. 2021;34(4):1123–1129. doi: 10.1007/s13577-021-00529-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Tang Y, Xue Z, Jin X, Ma G, Zhao P, Chu X. SUVmax ratio on PET/CT may differentiate between lung metastases and synchronous multiple primary lung cancer. Acad Radiol. 2020;27(5):618–623. doi: 10.1016/j.acra.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Mao Y, He JX, Zhu M, Dong YQ, He JX. Circ0001320 inhibits lung cancer cell growth and invasion by regulating TNFAIP1 and TPM1 expression through sponging miR-558. Hum Cell. 2021;34(2):468–477. doi: 10.1007/s13577-020-00453-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Li S, Xia N, Shi Y, Zhao CM. Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model. J Cell Physiol. 2018;233(5):4307–4316. doi: 10.1002/jcp.26254. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Chen L, Wang RJ, Meng J. LncRNA KCNQ1OT1 knockdown inhibits ox-LDL-induced inflammatory response and oxidative stress in THP-1 macrophages through the miR-137/TNFAIP1 axis. Cytokine. 2022;155:155912. doi: 10.1016/j.cyto.2022.155912. [DOI] [PubMed] [Google Scholar]
- 16.Coon TA, McKelvey AC, Lear T, Rajbhandari S, Dunn SR, Connelly W, Zhao JY, Han S, Liu Y, Weathington NM, McVerry BJ, Zhang Y, Chen BB. The proinflammatory role of HECTD2 in innate immunity and experimental lung injury. Sci Transl Med. 2015;7(295):295ra109. doi: 10.1126/scitranslmed.aab3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amos LA, Ma FY, Tesch GH, Liles JT, Breckenridge DG, Nikolic-Paterson DJ, Han Y. ASK1 inhibitor treatment suppresses p38/JNK signalling with reduced kidney inflammation and fibrosis in rat crescentic glomerulonephritis. J Cell Mol Med. 2018;22(9):4522–4533. doi: 10.1111/jcmm.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Cui S, Li SY, Cui LY, Nan QY, Lin XJ, Xuan MY, Jin J, Piao SG, Jiang YJ, Zheng HL, Jin JZ, Chung BH, Yang CW, Cui JH, Li C. The angiotensin receptor neprilysin inhibitor LCZ696 attenuates renal fibrosis via ASK1/JNK/p38 MAPK-mediated apoptosis in unilateral ureteral obstruction. PLoS One. 2023;18(6):e0286903. doi: 10.1371/journal.pone.0286903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Chatterjee I, Gujral T, Alakkam A, Coffing H, Anbazhagan AN, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Activation of nuclear factor-ĸB by tumor necrosis factor in intestinal epithelial cells and mouse intestinal epithelia reduces expression of the chloride transporter SLC26A3. Gastroenterology. 2017;153(5):1338–1350.e3. doi: 10.1053/j.gastro.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen QY, Gao B, Tong D, Huang C. Crosstalk between extracellular vesicles and tumor-associated macrophage in the tumor microenvironment. Cancer Lett. 2023;552:215979. doi: 10.1016/j.canlet.2022.215979. [DOI] [PubMed] [Google Scholar]
- 21.Ryu TY, Kim K, Han TS, Lee MO, Lee J, Choi J, Jung KB, Jeong EJ, An DM, Jung CR, Lim JH, Jung J, Park K, Lee MS, Kim MY, Oh SJ, Hur K, Hamamoto R, Park DS, Kim DS, Son MY, Cho HS. Human gut-microbiome-derived propionate coordinates proteasomal degradation via HECTD2 upregulation to target EHMT2 in colorectal cancer. ISME J. 2022;16(5):1205–1221. doi: 10.1038/s41396-021-01119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Zhang W, Wang S, Cai L, Jiang Y, Pan Y, Liang Y, Xian J, Jia L, Li L, Zhao H, Zhang Y. Cullin3-TNFAIP1 E3 ligase controls inflammatory response in hepatocellular carcinoma cells via ubiquitination of RhoB. Front Cell Dev Biol. 2021;9:617134. doi: 10.3389/fcell.2021.617134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YY, Hu HH, Wang YN, Liu JR, Liu HJ, Liu JL, Zhao YY. Metabolomics in renal cell carcinoma: From biomarker identification to pathomechanism insights. Arch Biochem Biophys. 2020;695:108623. doi: 10.1016/j.abb.2020.108623. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Li F, Cheng Z, Kong Y, Chen C. The role of E3 ubiquitin ligase HECTD3 in cancer and beyond. Cell Mol Life Sci. 2020;77(8):1483–1495. doi: 10.1007/s00018-019-03339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, De Geyter C, Jia Z, Peng Y, Zhang H. HECTD1 regulates the expression of SNAIL: Implications for epithelial mesenchymal transition. Int J Oncol. 2020;56(5):1186–1198. doi: 10.3892/ijo.2020.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottina E, Panova V, Doglio L, Kazachenka A, Cornish G, Kirkpatrick J, Attig J, Young GR, Litchfield K, Lesluyes T, Van Loo P, Swanton C, MacRae J, Tüting T, Kassiotis G. E3 ubiquitin ligase HECTD2 mediates melanoma progression and immune evasion. Oncogene. 2021;40(37):5567–5578. doi: 10.1038/s41388-021-01885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobelyatskaya AA, Pudova EA, Katunina IV, Snezhkina AV, Fedorova MS, Pavlov VS, Kotelnikova AO, Nyushko KM, Alekseev BY, Krasnov GS, Kudryavtseva AV. Transcriptome profiling of prostate cancer, considering risk groups and the TMPRSS2-ERG molecular subtype. Int J Mol Sci. 2023;24(11):9282. doi: 10.3390/ijms24119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Li DH, Xu Z. [HECTD2 represses cell proliferation in colorectal cancer through driving ubiquitination and degradation of LPCAT1] Mol Biol (Mosk) 2022;56(4):574–584. doi: 10.31857/s0026898422040073. [DOI] [PubMed] [Google Scholar]
- 29.Lv D, Shen T, Yao J, Yang Q, Xiang Y, Ma Z. HIF-1α induces HECTD2 up-regulation and aggravates the malignant progression of renal cell cancer via repressing miR-320a. Front Cell Dev Biol. 2021;9:775642. doi: 10.3389/fcell.2021.775642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, Yuan Y. Tumor necrosis factor alpha-induced proteins in malignant tumors: progress and prospects. Onco Targets Ther. 2020;13:3303–3318. doi: 10.2147/OTT.S241344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Zhang J, Yan L, Dong JM, Guo Q. Mirna-15a inhibits proliferation, migration and invasion by targeting tnfaip1 in human osteosarcoma cells. Int J Clin Exp Pathol. 2015;8(6):6442–6449. [PMC free article] [PubMed] [Google Scholar]
- 32.Ding YH, Miao RX, Zhang Q. Hypaphorine exerts anti-inflammatory effects in sepsis induced acute lung injury via modulating DUSP1/p38/JNK pathway. Kaohsiung J Med Sci. 2021;37(10):883–893. doi: 10.1002/kjm2.12418. [DOI] [PubMed] [Google Scholar]