Abstract
Background/Aim
Hidradenitis suppurativa (HS) is linked to immune dysregulation and systemic inflammation. While previous studies indicate a higher prevalence of ocular manifestations in HS, the specific risk of keratopathy and keratitis remains unclear. The primary aim of this study was to assess the risk of keratitis and keratopathy in individuals with HS.
Patients and Methods
In this retrospective cohort study conducted with data from the TriNetX database, 53,716 patients with HS were matched to an equivalent number of non-HS controls using propensity score matching. The study covered the period from January 1st, 2005, to December 31st, 2017. Hazard ratios and their respective 95% confidence intervals (CIs), were computed to evaluate the occurrences of keratitis and keratopathy over a 5-year duration in patients with HS, compared to non-HS controls.
Results
HS was associated with a 1.52 times higher risk of keratitis over a 5-year period (95%CI=1.24-1.86) and a 1.47 times higher risk of keratopathy (95%CI=1.18-1.84). These risks remained consistent in sensitivity analyses. The elevated risk of keratitis was observed across both sexes. However, the risk of keratopathy was significantly higher in women with HS (HR=1.61, 95%CI=1.24-2.10) and individuals aged 18-64 years (HR=1.32, 95%CI=1.04-1.68).
Conclusion
HS was linked to an elevated risk of both keratitis and keratopathy over a 5-year period. Ophthalmologic manifestations are recommended to be considered in HS standard care.
Keywords: Hidradenitis suppurativa, keratitis, keratopathy, cohort, epidemiology, electronic medical records
Hidradenitis suppurativa (HS) is a persistent debilitating inflammatory cutaneous condition marked by painful inflamed nodules, abscesses, and pus- discharging tunnels known as sinus tracts and fistulas on intertriginous skin like the axillae, inguinal, and gluteal areas (1,2). Follicular hyperkeratosis leading to rupture of follicles and inflammation of apocrine glands is believed to contribute to the pathogenesis of HS, resulting in this follicular occlusive disease (1). Pain and discharge from lesions significantly reduce quality of life in patients with HS (1,3). Smoking and obesity have been importantly identified as risk factors associated with HS (1,4).
Immune system dysregulation in HS produces elevated levels of pro-inflammatory cytokines like anti-tumor necrosis factor (TNF)-alpha, Interleukin (IL)-1β, and IL-17 (5-11). These pro-inflammatory factors can trigger systemic inflammation and comorbid conditions including inflammatory bowel disease (IBD), axial spondyloarthropathy (SpA) and metabolic disorders (4,12,13). Ophthalmic manifestations in patients with HS have been reported in some cohort studies and case reports. Two previous cohort studies revealed the coexistence in HS and inflammatory eye diseases (14,15), and anterior uveitis was the most common ocular manifestation among patients with HS, followed by episcleritis, optic neuritis, and keratitis (15). In 1967, the HS-corneal disease association was first proposed in a case report, when 4 cases of interstitial keratitis were observed in 62 patients with HS (16). Bilateral sides of interstitial keratitis were later documented in two case reports of patients with HS, responding well to immunotherapy like adalimumab (3,17). Apart from non-ulcerating inflammation of the cornea, peripheral ulcerative keratitis may also specifically be linked to HS according to prior two case reports of male patients with HS (18,19). Although Rosalynn et al. (20) found a significantly increased rate of keratitis in the HS group but not of interstitial keratitis, there is a need to elucidate clearer the relationship because the relationship to interstitial keratitis was not significant and confounding factors were not fully addressed.
Furthermore, a recent systematic review and meta-analysis indicated that psoriasis and HS share a common inflammatory pathway and show co-occurrence in (21). Given the increased keratopathy incidence in patients with psoriasis, observed by Lee et al. (22), we assume that patients with HS may also have heightened keratopathy risk. Current HS screening guidelines do not include assessment of ocular comorbidities, showing limited evidence of ophthalmic comorbidities in HS (23). Further research is warranted to elucidate the relationship between HS and keratopathy risk and determine if ophthalmologic screening should be incorporated into standard HS care. In this study, we aimed to assess whether the presence of HS elevates the risk of keratopathy or keratitis. We performed a longitudinal cohort study using the real-world data from TriNetX and conducted multivariate model to analyze the potential related comorbidities.
Patients and Methods
This is a retrospective cohort study. We utilized data from the TriNetX research network, a global federated health network that aggregates electronic health records from over 100 collaborative healthcare organizations (HCOs) worldwide. TriNetX is a widely utilized platform in epidemiological and clinical dermatology research (24,25). The records within the TriNetX database undergo a comprehensive de-identification process, negating the requirement for informed consent in studies utilizing this database. For this particular study, we focused on the “US collaborative network”, comprising 60 HCOs in the United States with a repository of over 80 million patient records for our analysis. This study followed the STROBE guideline and was approved by the Institutional Review Board of Tungs’ Taichung MetroHarbor Hospital (IRB TTMHH No.:112208N).
Patients diagnosed with HS and having a visit record between January 1st, 2005, and December 31st 2017, were identified as the HS cohort. Due to the prospective updating of the TriNetX database, all individuals in this study were subjected to a follow-up period exceeding five years. All individuals who deceased before the index date or had a prior history of neoplasms, keratitis, or keratopathy were removed from the current design. Non-HS controls were selected from individuals with a visit record for a general examination and no previous HS diagnosis. The patient selection process is reported in Figure 1. Propensity score matching for critical confounders was implemented in the analytic models. In the main analysis, 53,716 patients with HS and 53,716 non-HS controls were enrolled. We assessed the risk of keratitis and keratopathy over different follow-up periods. Sensitivity models incorporated various wash-out periods and matching algorithms to mitigate potential bias and validate the results. Stratification analyses were conducted to examine the observed association in different age and sex subgroups.
Figure 1. Participant selection flowchart.
The analytical system in TriNetX research network was utilized to perform all analyses in this study. Propensity score matching was implemented in each analysis, encompassing stratification and sensitivity analyses. Hazard ratios (HR) for outcomes were calculated, and simultaneous computation of 95% confidence intervals (95%CI) was performed to denote the significance of the results. Standardized differences (SD) were employed to illustrate differences in baseline characteristics between groups. An SD value exceeding 0.1 indicated a significant distinction between the groups under comparison.
Results
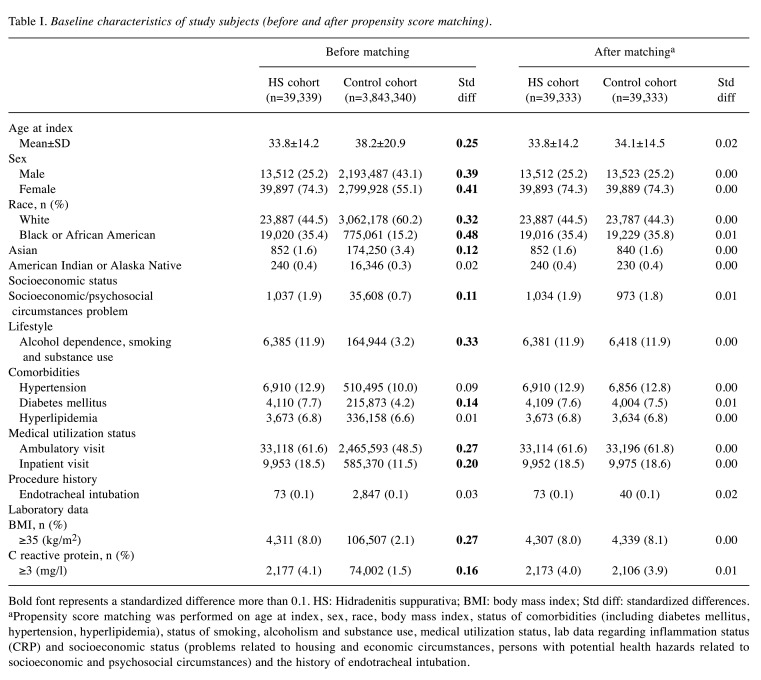
After propensity score matching, the HS cohort (n=53,676) and control cohort (n=53,676) were balanced on baseline demographics, comorbidities, healthcare utilization, and other measured characteristics as shown in Table I. Detailed codes for these covariates were reported in Table II. The average age of individuals in the HS cohort was 33.8 years old and 74% of them were female.
Table I. Baseline characteristics of study subjects (before and after propensity score matching).
Bold font represents a standardized difference more than 0.1. HS: Hidradenitis suppurativa; BMI: body mass index; Std diff: standardized differences. aPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
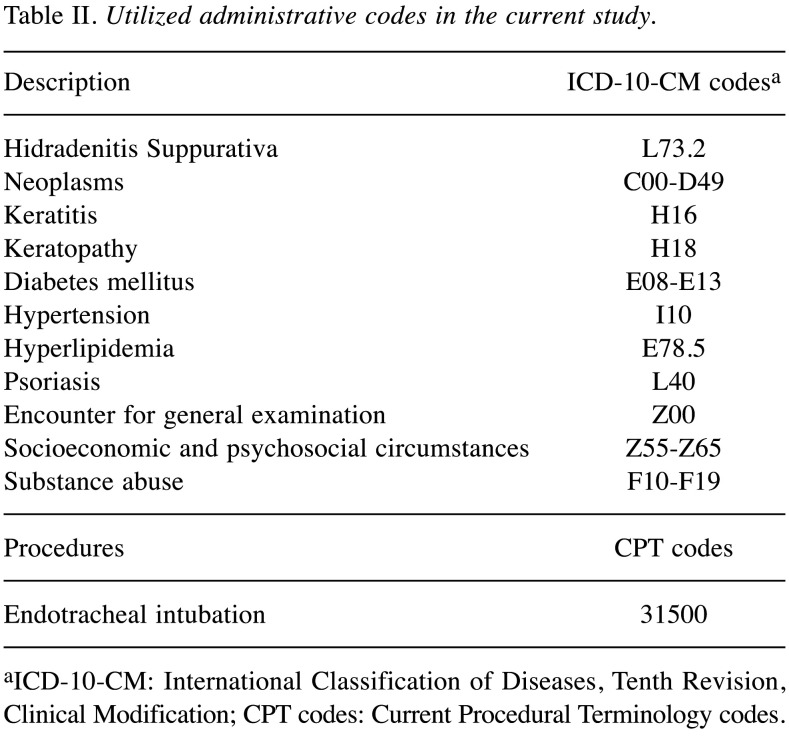
Table II. Utilized administrative codes in the current study.
aICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; CPT codes: Current Procedural Terminology codes.
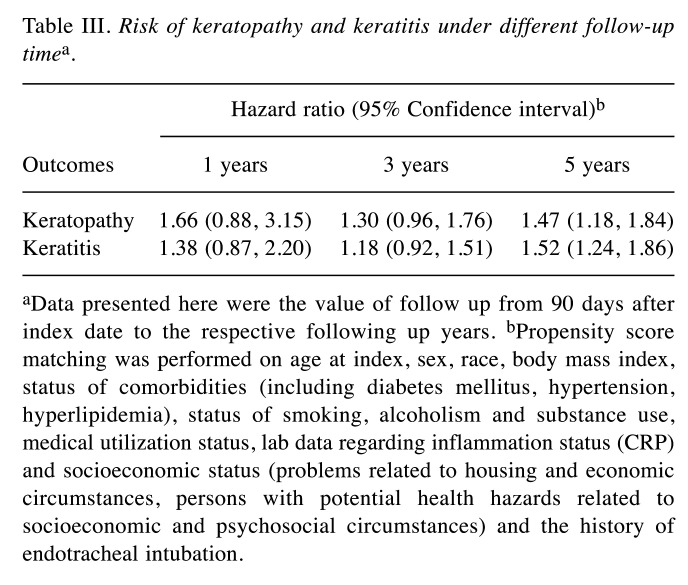
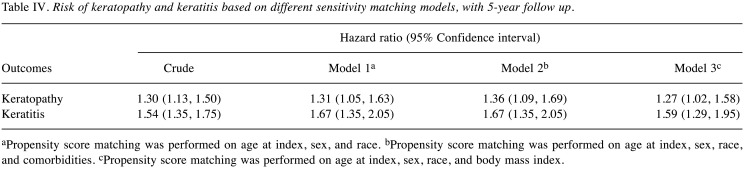
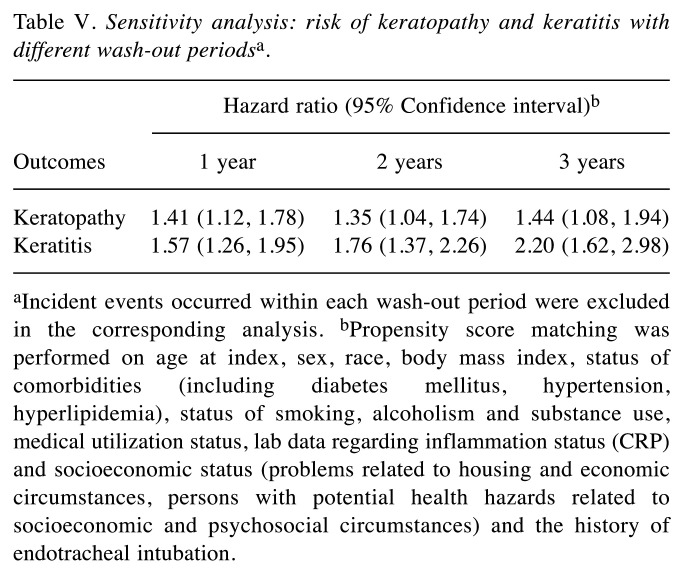
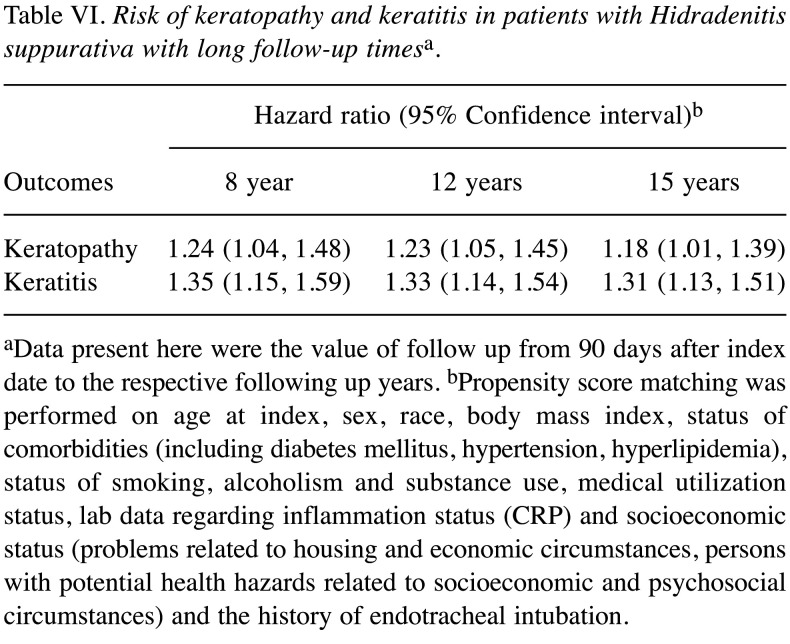
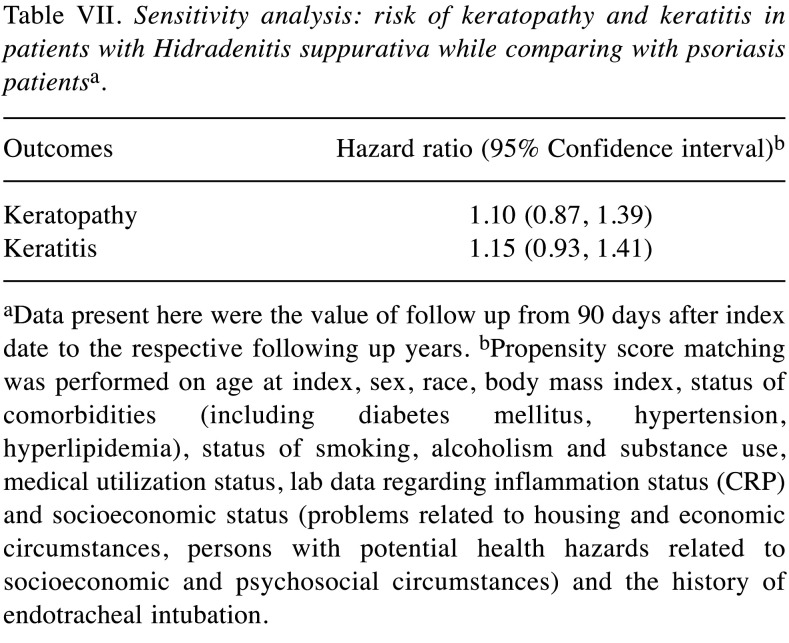
Patients with HS presented an elevated risk of developing the two ocular conditions over time relative to matched controls without HS. Specifically, HS was linked to a 47% and 52% higher 5-year risk of keratopathy and keratitis, respectively (Table III). The observed association remained in sensitivity models (Table IV and Table V). In the crude model, risk of keratopathy was 1.30 (95%CI=1.13-1.50) and risk of keratitis was 1.54 (95%CI=1.35-1.75). Moreover, a similar trend was also observed in longer follow-up time (Table VI). Comparing with people with psoriasis, the significance of 5-year risk of keratitis and keratopathy in patients with HS has not been observed (Table VII).
Table III. Risk of keratopathy and keratitis under different follow-up timea.
aData presented here were the value of follow up from 90 days after index date to the respective following up years. bPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
Table IV. Risk of keratopathy and keratitis based on different sensitivity matching models, with 5-year follow up.
aPropensity score matching was performed on age at index, sex, and race. bPropensity score matching was performed on age at index, sex, race, and comorbidities. cPropensity score matching was performed on age at index, sex, race, and body mass index.
Table V. Sensitivity analysis: risk of keratopathy and keratitis with different wash-out periodsa.
aIncident events occurred within each wash-out period were excluded in the corresponding analysis. bPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
Table VI. Risk of keratopathy and keratitis in patients with Hidradenitis suppurativa with long follow-up timesa.
aData present here were the value of follow up from 90 days after index date to the respective following up years. bPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
Table VII. Sensitivity analysis: risk of keratopathy and keratitis in patients with Hidradenitis suppurativa while comparing with psoriasis patientsa.
aData present here were the value of follow up from 90 days after index date to the respective following up years. bPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
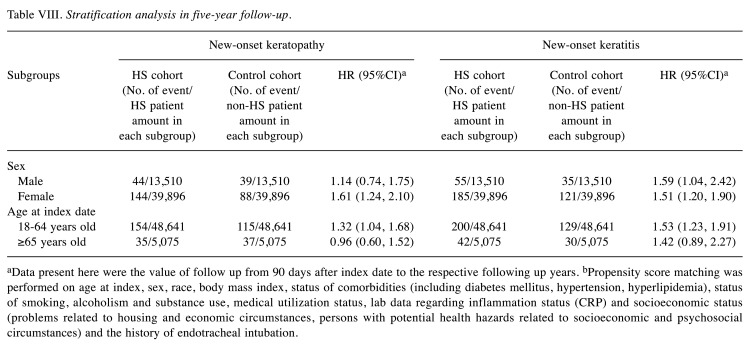
Further stratified analyses indicated the heightened keratitis risk among those with HS persisted across sexes. In the male subgroup, the risk of keratitis was 1.59 (95%CI=1.04-2.42) in patients with HS, whereas in the female subgroup, the risk of keratitis was 1.51 (95%CI=1.20-1.90). As for the risk of keratopathy, female patients with HS presented significantly higher risk than non-HS female individuals (HR=1.61; 95%CI=1.24-2.10). Male patients with HS did not present significant keratopathy risk compared with male non-HS controls. Patients with HS aged 18-64 years had significantly greater risk for developing keratitis and keratopathy, with the hazard ratio of 1.53, (95%CI=1.23-1.91) and 1.32 (95%CI=1.04-1.68), respectively (Table VIII).
Table VIII. Stratification analysis in five-year follow-up.
aData present here were the value of follow up from 90 days after index date to the respective following up years. bPropensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and socioeconomic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances) and the history of endotracheal intubation.
Discussion
We report an elevated risk for developing keratitis and keratopathy in patients with HS during a 5-year follow-up. The association remained in sensitivity models. Risk of keratitis was observed in all age and sex subgroups, except from the population older than 65 years. Moreover, the risk of keratopathy was especially observed in the female HS population, with the risk of greater than 1.6-fold compared with non-HS female controls (HR=1.61; 95%CI=1.24-2.10).
Previous studies have reported different inflammatory eye diseases (IED) in the context of HS, among which uveitis was the most common manifestation of IED (14,15). Keratitis only accounted for 23.5% (15) and 5% (14) of cases in these reports. Different forms of IED were also documented by case reports of patients with HS, such as peripheral ulcerative keratitis (18,19), interstitial keratitis (3,16,17), keratoconjunctivitis sicca (26), and phlyctenular keratoconjunctivitis (27). However, there is lack of statistical-methodological studies specifically examining the risk of corneal diseases in HS. In a recent case-control study in the United States, Conic et al. reported a 1.54-fold odds ratio having keratitis in HS patients (95%CI=1.43-1.66). However, in this study, risk of interstitial keratitis was not significant in HS patients (20). However, their study only adjusted sex and race in the multivariate logistic regression. In our current report, a longer disease follow-up period and wider range of matching covariates were applied and an elevated likelihood for in developing keratitis and keratopathy among patients with HS was observed.
According to our study, we suggest that HS could be a potential risk factor for keratitis and keratopathy in patients without prior major corneal disease after multivariate adjustment for several risk factors. Various systemic diseases are reported to be associated with HS (4,12,13), and some of them also cooccur with corneal diseases. Lee et al. previously revealed that psoriasis, a chronic inflammatory systemic disease, is associated with the development of keratopathy (22). Ophthalmic manifestations of IBD also include keratopathy, which serves as an important clinical clue before IBD diagnosis (28). In our study, we adjusted for C-reactive protein (CRP) level, the most sensitive indicator of inflammation, to deal with the variance of patients’ inflammation status (29). In view of the low specificity of CRP to different inflammation diseases (30), potential confounding bias may still exist in the study. Moreover, CRP not only reflected inflammation status but also infection status (31). We included all types of keratitis regardless of their etiology as long as they were recorded by the ICD code. We speculate the possibility of increased risk of not only immune-mediated keratitis but also infectious keratitis in HS. Chronic old ruptured lesions on patients with HS are prone to be infected due to lack of natural skin barrier (32). A theory also suggested that bacterial infection can induce HS by activating inflammatory response (5). Whether infectious or non-infectious keratitis contribute to the relation with HS needs to be examined by further stratification analysis.
Among all the different types of keratitis, peripheral ulcerative keratitis (PUK) and interstitial keratitis were those most documented to be associated with HS. Cornea is a five-layered structure consisting of a bulk of collagen fibers and extracellular matrix (ECM) in the stromal layer (33). The levels of corneal ECM is known to be regulated by matrix metalloproteinases (MMPs) and tissue inhibitor of MMP (TIMP) (34). The pathogenesis of PUK remains unclear, but both humoral and cellular immune mechanisms were believed to be involved (35). When the immune responses are activated, the plasma cells will release antibodies, activate complement pathways and result in the accumulation of inflammatory cells. Proteases are then released by the cells and impair the regulation of ECM production, leading to the lysis of corneal stroma (34). The peripheral cornea also has a good vascular and lymphatic supply compared to the central cornea, which results in bringing more inflammatory cells and cytokines to the cornea (34,36). According to recent reports, patients with HS have a relative over-activating immune regulatory pathway with elevation of TNF-α, IL-1β, IL-17, and other inflammatory cytokines (1,37). Increased expression of MMPs induced by TNF-α, IL-1β, and IL-17 was also observed in skin lesions in HS patients and could be associated with tissue injuries (1,26). Therefore, the dysregulated MMPs and the increased levels of circulating cytokines may give rise to a higher prevalence of keratitis in the HS group of our study.
Stratification analysis results of this study indicated a higher risk of developing keratopathy in female patients with HS (HR=1.61; 95%CI=1.24-2.10) and higher keratitis risk in both male (HR=1.59; 95%CI=1.04-2.42) and female patients with HS (HR=1.51; 95%CI=1.20-1.90). Similarly, Lee et al. reported a higher coexistence of HS and IED in women (15). Female sex is also an important risk factor for the development of dry eye disease (DED), a corneal disease presented with loss of homeostasis in tear film (42). DED is known to affect the corneal sensitivity, which can destruct the ocular surface and increase the risk of keratopathy (22,43,44). Up-regulation of MMPs and proinflammatory genes were found in immortalized cells under the exposure of estrogen, and such hormone-related situation could potentially explain the sex differences in patients with DED (44). It is worth noting that HS, PUK, and DED are all related to dysregulation of MMPs induced by inflammatory cytokines.
In previous reports, it was stated that age >60 years old could be a risk factor for keratopathy in people with psoriasis (22). The cell senescence in ageing can lead to increased epithelial permeability, reduced fibroblasts in the stroma, and reduction of cell density in corneal endothelium (45). The aforementioned mechanism represents poor wound healing of cornea under infection or inflammation in elders. Nevertheless, in the current study, patients with HS aged more than 65 years did not present significant association with keratitis and keratopathy compared with non-HS controls, whereas only younger patients with HS presented the observed association. Likewise, in previous case reports documenting keratitis as complications of HS, the reported patients were also mainly young adults, with their ages ranging between 18 to 33 years old (3,17-19). In spite of common pathogenesis between psoriasis and HS, there is need for more large-population studies to determine the differences in age distribution of corneal diseases with HS.
The primary strength of this research lies in its extensive sample size and the utilization of a robust dataset, enabling the identification of significant risk differences between the HS group and the non-HS group. Nevertheless, there are several limitations to our study. First, the definition of exposure, outcomes, and covariates relied on ICD-10 codes within the electronic medical record database, potentially missing patients with keratitis or keratopathy not captured in a hospital-based dataset due to detection bias. Second, considering that patients with HS might have heightened healthcare accessibility, ophthalmologic detections could be more frequently identified, introducing potential medical surveillance bias. Despite setting medical utilization status as a covariate for propensity score matching, medical surveillance bias may still impact outcome assessments. Third, the majority of individuals included in the study are either White or Black/African American. Despite multiple matchings and adjustments, generalizing our findings across diverse racial populations is limited, given clinical variations. Fourth, misclassification bias and residual confounders may persist, necessitating cautious interpretation of the results. Finally, our observational study design does not allow for definitive causation explanation regarding the relationship between HS and keratitis or keratopathy.
Conclusion
Our study reports a real-world association between HS and the occurrence of keratitis and keratopathy. The observed association may offer valuable insights for clinicians when addressing ocular comorbidities in patients with HS.
Funding
This work was supported by Tungs’ Taichung MetroHarbor Hospital (TTMHH-R1130084).
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
All the Authors were involved in drafting or revising the article and approved the submitted version. Study conception and design: Gau SY, Liu PY, Chen SN, Chiu TM, Tsai RY, Chang HC, Li CP. Data acquisition: Chang HC and Gau SY. Data analysis and demonstration: Chang HC, Chen SN, Chiu TM, Gau SY. Original draft preparation: Gau SY, Liu PY, Chen SN, Chiu TM, Tsai RY, Chang HC, Li CP.
References
- 1.Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa. J Am Acad Dermatol. 2020;82(5):1045–1058. doi: 10.1016/j.jaad.2019.08.090. [DOI] [PubMed] [Google Scholar]
- 2.Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 3.Alzaga Fernandez AG, Demirci H, Darnley-Fisch DA, Steen DW. Interstitial keratitis secondary to severe hidradenitis suppurativa: a case report and literature review. Cornea. 2010;29(10):1189–1191. doi: 10.1097/ICO.0b013e3181d4fd5c. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TV, Damiani G, Orenstein LAV, Hamzavi I, Jemec GB. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35(1):50–61. doi: 10.1111/jdv.16677. [DOI] [PubMed] [Google Scholar]
- 5.van der Zee HH, van der Woude CJ, Florencia EF, Prens EP. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Results of a pilot study. Br J Dermatol. 2010;162(1):195–197. doi: 10.1111/j.1365-2133.2009.09430.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012;21(10):735–739. doi: 10.1111/j.1600-0625.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- 7.Roussomoustakaki M, Dimoulios P, Chatzicostas C, Kritikos HD, Romanos J, Panayiotides JG, Kouroumalis EA. Hidradenitis suppurativa associated with Crohn’s disease and spondylo-arthropathy: response to anti-TNF therapy. J Gastroenterol. 2003;38(10):1000–1014. doi: 10.1007/s00535-003-1185-9. [DOI] [PubMed] [Google Scholar]
- 8.Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur J Pharmacol. 2011;672(1-3):1–8. doi: 10.1016/j.ejphar.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-Bourboulis EJ, Nagy I, Bechara FG, Sartorius K, Lapins J, Krahl D, Altmeyer P, Revuz J, Zouboulis CC. What causes hidradenitis suppurativa. Exp Dermatol. 2008;17(5):455–456. doi: 10.1111/j.1600-0625.2008.00712_1.x. discussion 457-472. [DOI] [PubMed] [Google Scholar]
- 10.Kelly G, Hughes R, McGarry T, van den Born M, Adamzik K, Fitzgerald R, Lawlor C, Tobin AM, Sweeney CM, Kirby B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173(6):1431–1439. doi: 10.1111/bjd.14075. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis EJ, Antonopoulou A, Petropoulou C, Mouktaroudi M, Spyridaki E, Baziaka F, Pelekanou A, Giamarellou H, Stavrianeas NG. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br J Dermatol. 2007;156(1):51–56. doi: 10.1111/j.1365-2133.2006.07556.x. [DOI] [PubMed] [Google Scholar]
- 12.Pescitelli L, Ricceri F, Prignano F. Hidradenitis suppurativa and associated diseases. G Ital Dermatol Venereol. 2018;153(3 Suppl 2):8–17. doi: 10.23736/s0392-0488.17.05772-8. [DOI] [PubMed] [Google Scholar]
- 13.Garcovich S, Genovese G, Moltrasio C, Malvaso D, Marzano AV. PASH, PAPASH, PsAPASH, and PASS: The autoinflammatory syndromes of hidradenitis suppurativa. Clin Dermatol. 2021;39(2):240–247. doi: 10.1016/j.clindermatol.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Saygın D, Syed AU, Lowder CY, Srivastava S, Maya JJ, Hajj-Ali RA. Characteristics of inflammatory eye disease associated with hidradenitis suppurativa. Eur J Rheumatol. 2018;5(3):165–168. doi: 10.5152/eurjrheum.2018.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DJ, Desai S, Laurent E, Kopplin LJ. Characterization and management of inflammatory eye disease in patients with hidradenitis suppurativa. Ocul Immunol Inflamm. 2021;29(7-8):1318–1323. doi: 10.1080/09273948.2020.1739718. [DOI] [PubMed] [Google Scholar]
- 16.Bergeron JR, Stone OJ. Interstitial keratitis associated with hidradenitis suppurativa. Arch Dermatol. 1967;95(5):473–475. [PubMed] [Google Scholar]
- 17.Quigley C, Butler T, Murphy C, Power W. Perforation in interstitial keratitis associated with hidradenitis suppurativa: medical and surgical management. BMJ Case Rep. 2023;16(1):e251928. doi: 10.1136/bcr-2022-251928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abid N, Opran A, Rosner F. Hidradenitis suppurativa complicated by erosive arthropathy and ulcerative keratitis. J Clin Rheumatol. 1999;5(1):29–31. doi: 10.1097/00124743-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Dallalzadeh LO, Ang MJ, Beazer AP, Spencer DB, Afshari NA. Peripheral ulcerative keratitis secondary to severe hidradenitis suppurativa. Am J Ophthalmol Case Rep. 2022;25:101403. doi: 10.1016/j.ajoc.2022.101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conic RRZ, Fabbrocini G, Marasca C, Bragazzi NL, Watad A, Adawi M, Damiani G. Burden of ocular comorbidities in patients with hidradenitis suppurativa. JAMA Dermatol. 2021;157(2):226–227. doi: 10.1001/jamadermatol.2020.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gau SY, Preclaro IAC, Wei JC, Lee CY, Kuan YH, Hsiao YP, Juang SE, Ma KS. Risk of psoriasis in people with hidradenitis suppurativa: A systematic review and meta-analysis. Front Immunol. 2022;13:1033844. doi: 10.3389/fimmu.2022.1033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CY, Chen HC, Lin HW, Huang JY, Lin TL, Yang CH, Yeh CB, Lin HY, Yang SF. Increased risk of keratopathy after psoriasis: A nationwide population-based study. PLoS One. 2018;13(7):e0201285. doi: 10.1371/journal.pone.0201285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, Alikhan A, Daveluy SD, Delorme I, Goldfarb N, Gulliver W, Hamzavi I, Jaleel T, Kimball AB, Kirby JS, Kirchhof MG, Lester J, Lev-Tov H, Lowes MA, Micheletti R, Orenstein LA, Piguet V, Sayed C, Tan J, Naik HB. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86(5):1092–1101. doi: 10.1016/j.jaad.2021.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HC, Lin CY, Guo YC, Lu HY, Lee CY, Wu MC, Gau SY. Association between hidradenitis suppurativa and atopic diseases: a multi-center, propensity-score-matched cohort study. Int J Med Sci. 2024;21(2):299–305. doi: 10.7150/ijms.90086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang HC, Wu CL, Chiu TM, Liao WC, Gau SY. Risk of osteoarthritis in patients with hidradenitis suppurativa: a global federated health network analysis. Front Immunol. 2023;14:1285560. doi: 10.3389/fimmu.2023.1285560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schargus M, Langhorst CA, Joachim S, Frings A, Krause K, Reifenberger J, Geerling G, Frings VG. Hidradenitis suppurativa is associated with symptoms of keratoconjunctivitis sicca. Curr Eye Res. 2021;46(1):23–30. doi: 10.1080/02713683.2020.1775259. [DOI] [PubMed] [Google Scholar]
- 27.Gargallo-Benedicto A, Clemente-Tomás R, Pastor-Espuig M, Ballano-Ruiz A, Garzarán-Teijeiro A, Alias-Alegre E, Navarro-Casado MN. Bilateral phlyctenular keratoconjunctivitis in the context of hidradenitis suppurativa: a case report and literature review. Ocul Immunol Inflamm. 2022;30(4):992–994. doi: 10.1080/09273948.2020.1833223. [DOI] [PubMed] [Google Scholar]
- 28.Andriano TM, Benesh G, Babbush KM, Hosgood HD, Lin J, Cohen SR. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61(10):1270–1275. doi: 10.1111/ijd.16244. [DOI] [PubMed] [Google Scholar]
- 29.Vadakayil AR, Dandekeri S, Kambil SM, Ali NM. Role of C-reactive protein as a marker of disease severity and cardiovascular risk in patients with psoriasis. Indian Dermatol Online J. 2015;6(5):322–325. doi: 10.4103/2229-5178.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso D, Padoan A, D’Incà R, Arrigoni G, Scapellato ML, Contran N, Franchin C, Lorenzon G, Mescoli C, Moz S, Bozzato D, Rugge M, Plebani M. Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis. Clin Chem Lab Med. 2020;58(6):968–979. doi: 10.1515/cclm-2019-1125. [DOI] [PubMed] [Google Scholar]
- 31.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapins J, Jarstrand C, Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140(1):90–95. doi: 10.1046/j.1365-2133.1999.02613.x. [DOI] [PubMed] [Google Scholar]
- 33.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37(3):588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Gupta Y, Kishore A, Kumari P, Balakrishnan N, Lomi N, Gupta N, Vanathi M, Tandon R. Peripheral ulcerative keratitis. Surv Ophthalmol. 2021;66(6):977–998. doi: 10.1016/j.survophthal.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology. Cornea. 2000;19(5):625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmology. 1988;95(4):463–472. doi: 10.1016/s0161-6420(88)33164-7. [DOI] [PubMed] [Google Scholar]
- 37.Lowe MM, Naik HB, Clancy S, Pauli M, Smith KM, Bi Y, Dunstan R, Gudjonsson JE, Paul M, Harris H, Kim E, Shin US, Ahn R, Liao W, Hansen SL, Rosenblum MD. Immunopathogenesis of hidradenitis suppurativa and response to anti-TNF-α therapy. JCI Insight. 2020;5(19):e139932. doi: 10.1172/jci.insight.139932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canoui-Poitrine F, Le Thuaut A, Revuz JE, Viallette C, Gabison G, Poli F, Pouget F, Wolkenstein P, Bastuji-Garin S. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133(6):1506–1511. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- 39.Sen HN, Davis J, Ucar D, Fox A, Chan CC, Goldstein DA. Gender disparities in ocular inflammatory disorders. Curr Eye Res. 2015;40(2):146–161. doi: 10.3109/02713683.2014.932388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa and associated factors: Still unsolved problems. J Am Acad Dermatol. 2009;61(2):362–365. doi: 10.1016/j.jaad.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 41.Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77(1):118–122. doi: 10.1016/j.jaad.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. doi: 10.3238/arztebl.2015.0071. quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valim V, Trevisani VF, de Sousa JM, Vilela VS, Belfort R Jr. Current approach to dry eye disease. Clin Rev Allergy Immunol. 2015;49(3):288–297. doi: 10.1007/s12016-014-8438-7. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, Hampel U, McDermott AM, Schaumberg DA, Srinivasan S, Versura P, Willcox MD. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15(3):284–333. doi: 10.1016/j.jtos.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Faragher RG, Mulholland B, Tuft SJ, Sandeman S, Khaw PT. Aging and the cornea. Br J Ophthalmol. 1997;81(10):814–817. doi: 10.1136/bjo.81.10.814. [DOI] [PMC free article] [PubMed] [Google Scholar]