Abstract
Background/Aim
Hair-follicle keratinocytes contain high levels of cysteine, which is derived from methionine, rapidly proliferate, and form the hair shaft. The high proliferation rate of hair-follicle keratinocytes resembles that of aggressive cancer cells. In the present study, we determined the effect of a methionine-deficient diet on hair loss (alopecia) in mice with or without homocysteine supplementation.
Materials and Methods
Mice were fed a normal rodent diet (2020X, ENVIGO) (Group 1); a methionine-choline-deficient diet (TD.90262, ENVIGO) (Group 2); a methionine-choline-deficient diet with a 10 mg/kg/day supply of homocysteine administered by intra-peritoneal (i.p.) injection for 2 weeks (Group 3). In Group 2, mice were fed a methionine-choline-deficient diet for an additional 2 weeks but with 10 mg/kg/day of i.p. l-homocysteine and the mice were observed for two additional weeks. Subsequently, the mice were fed a standard diet that included methionine. Hair loss was monitored by photography.
Results
After 14 days, hair loss was observed in Group 2 mice on a methionine-restricted diet but not in Group 3 mice on the methionine-restricted diet which received i.p. homocysteine. In Group 2, at 2 weeks after methionine restriction, hair loss was not rescued by homocysteine supplementation. However, after restoration of methionine in the diet, hair growth resumed. Thus, after 2 weeks of methionine restriction, only methionine restored hair loss, not homocysteine.
Conclusion
Hair maintenance requires methionine in the diet. Future experiments will determine the effects of methionine restriction on hair-follicle stem cells.
Keywords: Hair loss, alopecia, methionine, homocysteine, methionine restriction
In mammals, hair growth is cyclical with an anagen growth phase, catagen cessation of hair growth and a long telogen phase with loss of the hair shaft (1-4). Permanent hair loss or alopecia occurs during aging in males and females, but much more frequently in males (5,6). In alopecia the hair follicle miniaturizes and produces a very small vellus hair shaft (7). Severe hair loss also occurs due to cancer chemotherapy in both males and females.
Cancer cells of all types arrest or lose viability by methionine restriction due to methionine addiction for transmethylation reactions (8-13).
Hair-follicle-keratinocyte proliferation is among the most rapid in the mammalian body (14). Keratinocytes form the hair shaft (4,15,16). The loss of rapidly proliferating keratinocytes accounts for hair loss during cytotoxic chemotherapy, which targets rapidly-cycling cells. The present report describes the effect of methionine restriction on hair maintenance in young C57BL/6 mice.
Materials and Methods
Mice. C57BL/6 mice (AntiCancer Inc., San Diego, CA, USA) aged 4-6 weeks were used in the present study. The mice were housed in a controlled environment facility equipped with a High Efficiency Particulate Air (HEPA)-filtered rack, adhering to standard light/dark cycles of 12 h. The ethical committee of the AntiCancer Institutional Animal Care and Use Committee approved the use of animals, in accordance with the National Institutes of Health Guide. The studies were conducted using the Animal Research Reporting of In Vivo studies (ARRIVE) 2.0 standards.
Diets. During the experimental period, three groups of 5 mice each were subjected to different dietary conditions. Group 1 received a standard rodent diet (2020X, ENVIGO, Indianapolis, IN, USA); Group 2 was fed a methionine-choline-deficient diet (TD.90262, ENVIGO); and Group 3 was also fed a methionine-choline-deficient diet but received intra-peritoneal (i.p.) injection of l-homocysteine at a dose of 10 mg/kg/day. This dietary intervention was maintained for a duration of two weeks. In Group 2, mice continued a methionine-choline-deficient diet for another 2 weeks but with l-homocysteine administered i.p. at 10 mg/kg/day. The effects on the dorsal mouse hair were monitored for an additional two-week period. Subsequently, the mice were reintroduced to a standard diet that included methionine.
Blood collection. Mouse blood samples were collected through the tail vein between the hours of 9 and 10 AM on day 15.
Statistics. GraphPad Prism 9.4.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to conduct the statistical analyses. All data are presented as the mean and standard deviation. The level of significance was set at 0.05 or lower.
Results
Effect of two-week dietary methionine restriction on hair loss in C57BL/6 mice. Mice on the methionine-containing diet in Group 1 had normal hair (Figure 1A). After 14 days on methionine restriction, hair loss was observed in Group 2 mice (Figure 1B). Hair loss was avoided by supplying homocysteine i.p. to mice on the methionine-restricted diet in Group 3 (Figure 1C). In Group 2, after 4 weeks of continued methionine restriction, hair loss increased (Figure 2).
Figure 1. Mouse photos in each group after 2 weeks of treatments. A) Group 1; normal diet. B) Group 2; methionine-deficient diet for 2 weeks. C) Group 3; methionine-deficient diet with i.p. homocysteine supply for 2 weeks.
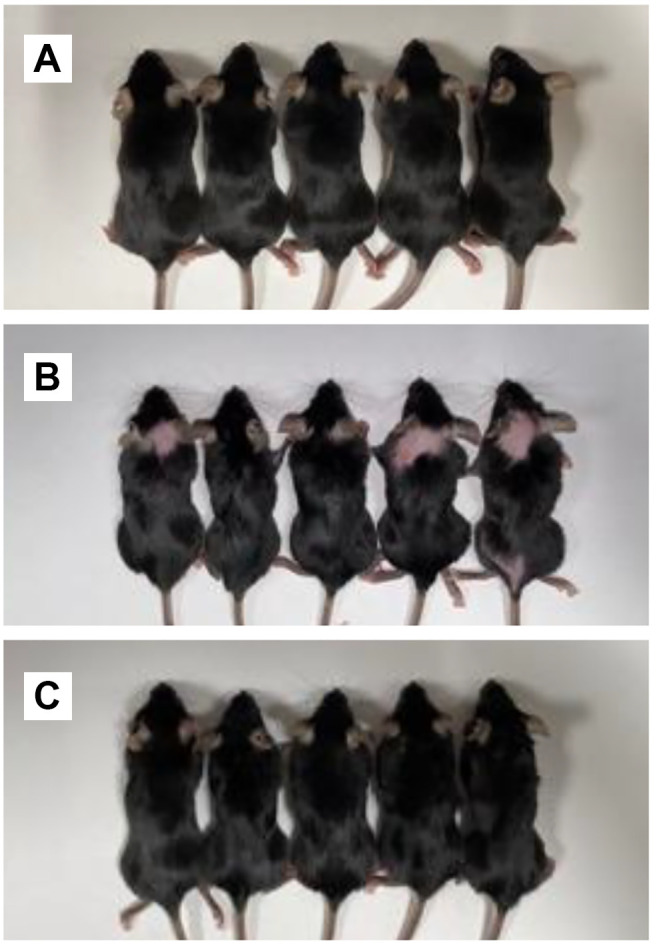
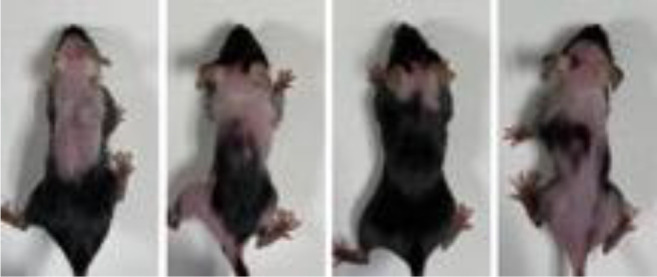
Figure 2. Mouse photo of Group 2 after 4 weeks of methionine restriction.
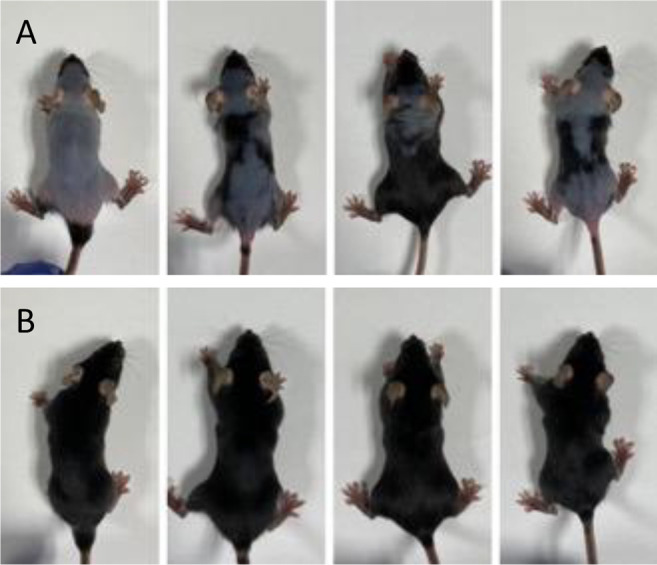
Rescue of hair loss after methionine restriction. After two weeks of methionine restriction, hair growth in C57BL/6 mice could not be rescued by homocysteine (Figure 3). After two weeks of methionine restriction a methionine-containing diet rescued hair growth (Figure 4).
Figure 3. Mouse photo of Group 2, on a methionine-restricted diet two weeks after supplying homocysteine, which began two weeks after the start of methionine restriction.

Figure 4. Photos of group 2 mice after re-introducing a normal diet containing methionine. A) Normal methionine-containing diet introduced after 1 week of methionine restriction. B) Normal methionine-containing diet introduced after 2 weeks of methionine restriction.
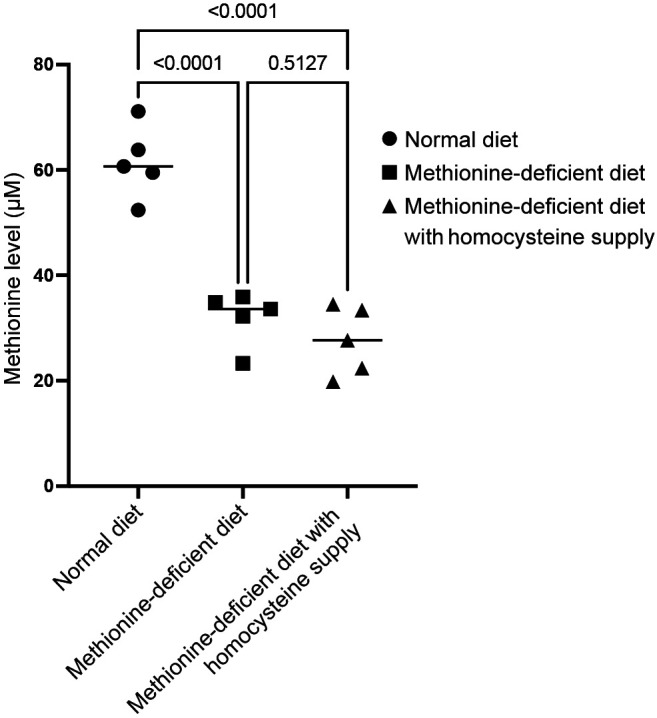
Blood methionine after methionine restriction. Two weeks after methionine restriction, blood methionine decreased 48.0%, and decreased 55.2% during which homocysteine replaced methionine (Figure 5).
Figure 5. Blood methionine level of each group on day 15.
Discussion
The present report demonstrates that hair maintenance in C57BL/6 mice is methionine dependent, similar to cancer cells. Homocysteine administered i.p., in place of methionine in the diet, rescued hair growth in the mice during methionine restriction for 2 weeks. After 2 weeks of methionine restriction hair growth was rescued only by methionine and not by i.p. injection of homocysteine, which could prevent hair loss during the first 2 weeks of methionine restriction. Homocysteine did not raise the blood level of methionine and therefore could possibly directly rescue hair growth during the first 2 weeks of methionine restriction, but not after longer periods of methionine restriction. It appears that after two weeks of methionine restriction an irreversible step occurs after which homocysteine cannot rescue hair growth. The mechanism of homocysteine rescue of hair growth during early stages of methionine restriction needs to be investigated. Homocysteine is derived from methionine and is converted to cysteine (17) and the keratinocytes of the hair shaft require a high level of cysteine.
Future experiments will investigate if hair maintenance in the mouse requires high levels of methionine in hair-follicle keratinocyte stem cells (4) or nestin expressing hair-follicle associated pluripotent (HAP) stem cells (15,16). The effect of methionine restriction will also be investigated on the different follicle cycle stages, anagen, catagen, and telogen (2-4). Future experiments will also determine if a high-methionine diet can reverse age-related alopecia.
Conflicts of Interest
The Authors declare no competing interests regarding this work.
Authors’ Contributions
YK, NV, and KK performed experiments. YK, and RMH wrote the article. TT provided critical suggestions.
Acknowledgements
This article is dedicated to the memory of A. R. Moossa, MD, Sun Lee, MD, Professor Li Jiaxi, Masaki Kitajima, MD, Shigeo Yagi, Ph.D., Jack Geller, MD, Joseph R Bertino, MD, and J.A.R. Mead, Ph.D. The Robert M. Hoffman Foundation for Cancer Research provided funds for this study.
References
- 1.Davis BK. Phases of the hair-growth cycle. Nature. 1962;194(4829):694–694. doi: 10.1038/194694a0. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci U.S.A. 1993;90(15):7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-C. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes T, Girman CJ, Savin RC, Kaufman KD, Guo S, Lilly FR, Siervogel RM, Chumlea WC. Prevalence of male pattern hair loss in 18-49 year old men. Dermatol Surg. 1998;24(12):1330–1332. doi: 10.1111/j.1524-4725.1998.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 6.Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10(3):184–189. doi: 10.1111/j.1087-0024.2005.10102.x. [DOI] [PubMed] [Google Scholar]
- 7.Whiting DA. Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J Am Acad Dermatol. 2001;45(3):S81–S86. doi: 10.1067/mjd.2001.117428. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto J, Han Q, Inubushi S, Sugisawa N, Hamada K, Nishino H, Miyake K, Kumamoto T, Matsuyama R, Bouvet M, Endo I, Hoffman RM. Histone methylation status of H3K4me3 and H3K9me3 under methionine restriction is unstable in methionine-addicted cancer cells, but stable in normal cells. Biochem Biophys Res Commun. 2020;533(4):1034–1038. doi: 10.1016/j.bbrc.2020.09.108. [DOI] [PubMed] [Google Scholar]
- 9.Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro. 1984;20(8):663–670. doi: 10.1007/BF02619617. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Yip LY, Lee JHJ, Wu Z, Chew HY, Chong PKW, Teo CC, Ang HY, Peh KLE, Yuan J, Ma S, Choo LSK, Basri N, Jiang X, Yu Q, Hillmer AM, Lim WT, Lim TKH, Takano A, Tan EH, Tan DSW, Ho YS, Lim B, Tam WL. Methionine is a metabolic dependency of tumor-initiating cells. Nat Med. 2019;25(5):825–837. doi: 10.1038/s41591-019-0423-5. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto J, Inubushi S, Han Q, Tashiro Y, Sugisawa N, Hamada K, Aoki Y, Miyake K, Matsuyama R, Bouvet M, Clarke SG, Endo I, Hoffman RM. Linkage of methionine addiction, histone lysine hypermethylation, and malignancy. iScience. 2022;25(4):104162. doi: 10.1016/j.isci.2022.104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto J, Aoki Y, Inubushi S, Han Q, Hamada K, Tashiro Y, Miyake K, Matsuyama R, Bouvet M, Clarke SG, Endo I, Hoffman RM. Extent and instability of trimethylation of histone H3 lysine increases with degree of malignancy and methionine addiction. Cancer Genomics Proteomics. 2022;19(1):12–18. doi: 10.21873/cgp.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki Y, Han Q, Tome Y, Yamamoto J, Kubota Y, Masaki N, Obara K, Hamada K, Wang JD, Inubushi S, Bouvet M, Clarke SG, Nishida K, Hoffman RM. Reversion of methionine addiction of osteosarcoma cells to methionine independence results in loss of malignancy, modulation of the epithelial-mesenchymal phenotype and alteration of histone-H3 lysine-methylation. Front Oncol. 2022;12:1009548. doi: 10.3389/fonc.2022.1009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104(2):233–245. doi: 10.1016/S0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U.S.A. 2003;100(17):9958–9961. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005;102(15):5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehman T, Shabbir MA, Inam-Ur-Raheem M, Manzoor MF, Ahmad N, Liu ZW, Ahmad MH, Siddeeg A, Abid M, Aadil RM. Cysteine and homocysteine as biomarker of various diseases. Food Sci Nutr. 2020;8(9):4696–4707. doi: 10.1002/fsn3.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]


