Abstract
Background/Aim
Warthin’s tumor, the second most frequent neoplasia of the parotid gland, is characterized by a proliferation of both epithelial and lymphoid components. In addition to epithelial and lymphoid cells, various other cell types are implicated to varying degrees in the immune response. Notably, mast cells have long been recognized as a consistent cell population within this tumor. Despite the historical acknowledgment of mast cell presence, their true distribution and significance within Warthin’s tumor remain unclear. In this study, we aimed to elucidate the distribution and significance of mast cells in Warthin’s tumor.
Materials and Methods
Histochemical and immunohistochemical methods were employed for the evaluation of mast cells within tumor specimens.
Results
Our study revealed a notable concentration of mast cells in the epithelial component of Warthin’s tumor. Microscopic examination showed predominant lymphoid and epithelial elements with occasional cystic formations. Immunohistochemical analysis identified mast cells in both components, emphasizing their role in the tumor microenvironment. Double immunostaining (mast cell tryptase and CD34) revealed no significant correlation between mast cells and blood vessels. Intraepithelial mast cells (IEMCs) had a significantly higher density in the epithelial component, suggesting a potential association with the tumor’s benign nature. The relationship between IEMCs and epithelial cells, especially in the presence of cystic structures, offers valuable insights into the unique features of Warthin’s tumor.
Conclusion
Our study contributes to the understanding of mast cells in Warthin’s tumor, highlighting a substantial concentration within the epithelial component. This knowledge may pave the way for further investigations into the roles of mast cells in the pathogenesis and treatment of Warthin’s tumor.
Keywords: Warthin tumor, mast cell, tryptase, immunohistochemistry
The structural and evolutionary complexity of head and neck tumors poses significant diagnostic and therapeutic challenges. The heterogeneity of these tumors, originating from various normal tissues in the head and neck region, is characterized by microscopic elements that are unique to this region and are not found in other organ systems. This distinctive pathology may be attributed to the distinct embryological origin of tissues in the head and neck area (1).
Salivary glands, particularly the parotid gland, are recognized as an important source of tumors with diverse histological characteristics. These tumors can present diverse structural features and may sometimes resemble malignant lesions, as is the case of pleomorphic adenoma. However, it is important to note that pleomorphic adenomas are benign tumors in most cases. Histogenetically, it is suggested that the majority of parotid tumors originate from the long-intercalated ducts within the gland that contain numerous progenitor cells and are susceptible to carcinogens (2).
Among salivary gland tumors, the Warthin tumor emerges as the second most frequent tumor in the parotid gland. Characterized by a distinctive tissue structure, Warthin’s tumor is generally benign but poses challenges in histopathological diagnosis. Despite being described over a century ago, certain aspects of this tumor’s characteristics remain unclassified, and their impact on tumor evolution is still unknown (3).
Warthin’s tumor, also known as cystadenoma lymphangio-matosum papilliferum, is a benign lesion primarily found in the parotid lobe, occasionally in the submandibular glands (4). It can be bilateral in 10% of cases, more common in males, and linked to smoking as a significant risk factor. Macroscopically, it exhibits a lobulated appearance, appearing fixed to surrounding tissues, resembling a malignant tumor. Microscopically, it features multicystic structures separated by connective bands (5).
Warthin’s tumor comprises two major components: lymphoid tissue and an epithelial component. The lymphoid tissue is often predominant, characterized by diffuse lymphoid infiltrates and germinal center lymphoid follicles. B lymphocytes are the primary cellular component, accompanied by variable numbers of protein-S100 positive cells, dendritic cells, mast cells, and T lymphocytes (6). The epithelial component, covering the tumor surface, consists of large oncocyte-like cells arranged in two rows with differing sizes and molecular profiles. This component expresses a range of molecular markers, both epithelial and non-epithelial, including unusual markers for this location, such as somatostatin. The diagnostic and evolutionary significance of these markers remains unknown (7).
Described nearly a century ago by pathologist Aldred Scott Warthin (8), publications on Warthin’s tumor are infrequent, with limited studies on tumor cellularity. Existing data primarily emphasize the dense lymphoid component and bilayered oncocytic epithelium with papillary and cystic architecture. The etiology remains unknown, with factors such as smoking, autoimmune diseases, Epstein Barr virus infection, ionizing radiation, and chronic infections implicated (9). Malignancy is exceedingly rare, occurring in only 0.3% of cases (10). Identifying additional benignity criteria is crucial. The molecular profile, including markers of striatal ducts in the parotid gland, justifies the tumor’s exclusive location (11). The overall appearance determined by the two components of the tumor is monomorphic, with a clear distinction between the two tissue components. The presence of other cell types is typically incidental except for mast cells, which we study in the present research. Numerous studies have demonstrated the presence of mast cells in Warthin tumor. It is appreciated by some authors that the presence of mast cells reflects the benign nature of the lesions. In almost all available publications, mast cells are presented as cells associated with the lymphoid component (12,13). This aspect is not surprising if we consider the known data on these cells. The mast cell is one of the cellular components of the connective tissue, but it is highlighted with particular staining methods. We assume that for this reason they have been studied little or not at all in Warthin’s tumor. Additionally, the functional significance of these cells in Warthin’s tumor remains unknown, despite the relatively well-characterized markers of mast cell granules. Mast cell accumulation has been reported in a wide variety of tumors, both benign and malignant. For decades, arguments have been presented both in favor of and against the idea that mast cells exert antitumor effects. Recently, the accumulation of mast cells has been correlated with microvascular density in malignant tumors, but this correlation is not confirmed in benign tumors, regardless of their nature (14). Not all mast cells in the human body contain all the substances identified in granules under normal and pathological conditions. The variations include the concentration of glycosaminoglycans, biogenic amines, and growth factors. Notably, the only enzyme without major changes appears to be mast cell tryptase (15), which we used as the primary method of identification through immuno-histochemistry. In the present study, we report for the first time a significant accumulation of intraepithelial mast cells (IEMCs), which exhibit intense tryptase positivity but are deficient of passenger granular mediators.
Materials and Methods
Primary processing. We retrospectively investigated 11 cases with Warthin tumor; the specimens were obtained from the archive of the Histopathology Laboratory of the Arad County Hospital. All surgically excised formations were located in the parotid lobe or in its immediate vicinity. Nine of the 11 cases studied were male, aged between 44 and 65 years. Fragments intended for histopathological study were fixed in buffered formalin and embedded in paraffin, according to the usual histological technique. The entire inclusion procedure was performed with the Thermo Fisher Scientific work system (Citadel 2000 Tissue Processor; Thermo Fisher Scientific, Waltham, MA, USA). Initial sections from each case were stained with Mayer’s variant hematoxylin-eosin for histopathological diagnosis and microscopic structural details.
Histochemical methods. We applied the most sensitive histochemical methods to identify mast cells, targeting some characteristic substances in their cytoplasmic granules. Thus, we performed the staining with toluidine blue at pH2.2, which can highlight metachromatically or orthochromatically the glycosaminoglycans in the mast cell granules. In addition, we applied alcian blue staining at pH2.5, which can stain mast cells deep blue, especially in the presence of sulphated glycosaminoglycans, such as heparin, chondroitin sulphate, and dermatan sulphate. Sections stained by these two methods were previously dehydrated in increasing concentrations of ethyl alcohol, cleared in toluene, and mounted with Canada balsam.
Immunohistochemical methods. Serial sections from each case were processed according to the immunohistochemical standard procedure. The following primary antibodies were used: CD34 clone QBend10 (Leica Biosystems, Deer Park, IL USA), mast cell tryptase clone AA1 (Dako, Santa Clara, CA, USA), mast cell chymase clone CC1 (Thermo Fisher Scientific, Waltham, MA, USA), CD117 clone ACK2 (Dako). Antigen unmasking was performed with Bond Epitope Retrieval Solution 2 (Leica Biosystems) for 20 min followed by endogenous peroxidase blocking for 5 min, incubation with primary antibody for 20 min, and visualization with Bond Polymer Refine Detection System (Thermo Fisher Scientific) for 15 min. The main chromogen was diaminobenzidine, which stains the final reaction product in brown. In the double immunostaining process, the procedure was reiterated following treatment with the primary antibodies. To highlight the second final reaction product in red, the Bond Polymer Refine Red Detection System from Leica Biosystems was employed. The entire immunohistochemical procedure was performed automatically with the Bond Max Autostainer (Leica Biosystems).
Microscopic evaluation and data analysis. All sections were scanned with Desk Panoramic Scanner (3D Histech, Budapest, Hungary) and stored at the Histology Department Digital Slides Library Case Center. Section evaluation, imaging, and processing were performed using Pannoramic Viewer pathology software (3D Histech). Mast cell density was calculated according to the modified Weidner method, choosing three fields with maximum mast cell density, the arithmetic mean was the final result for each case. The modification from the original method, employed for counting blood microvessels, involves assessing mast cell density at x400 magnification. Mast cell density was calculated separately for intraepithelial and lymphoid tissue.
Data analysis. Statistical analyses were performed using Statistical Package for Social Sciences version 22.0 (SPSS Inc., Champaign, IL, USA). The results were statistically analyzed using Student’s test and a p-value of <0.05 was considered as significant.
Results
Microscopic examination of hematoxylin-eosin-stained sections revealed the existence of the two types of tissues that predominantly make up Warthin’s tumor. The lymphoid component was predominant in only 4 of the 11 cases (36.36%) and consisted of diffuse high-density lymphoid tissue and occasional lymphoid follicles with a germinal center. The lymphoid component was arranged in the spaces between the epithelial cords that were often deformed, depending mostly on the size of the germinal center.
In 8 of the 11 cases, we observed cystic formations with variable shape and size, containing an unstructured acidophilic material, located in the immediate vicinity of the epithelial component. The intracystic material did not include cellular detritus or exfoliated individual cells.
The epithelial component consisted of cells arranged in two rows. All epithelial cells had centrally arranged, small nuclei with no atypical features. The cytoplasm was intensely acidophilic, with a homogeneous appearance. The cystic aspect was also suggested by the reduced accumulations of unstructured material observed in different stages of evolution, particularly within the epithelial component. In both the epithelial and lymphoid components, we did not observe cells with distinctive morphologies in the sections stained with hematoxylin-eosin. When the epithelial component predominated, the lymphoid component was characterized by diffuse lymphoid infiltrates, and lymphoid follicles were absent. In toluidine blue staining, few mast cells were identified, most of them orthochromatic and located especially in the lymphoid tissue. IEMCs stained with toluidine blue were identified in 3 cases, and were orthochromatic. Metachromatic granulations with reduced density were observed only occasionally in the anticipated area where IEMCs are located. Applying alcian blue staining, we observed IEMCs in only two cases, in the form of rare, isolated, intensely blue-stained cells.
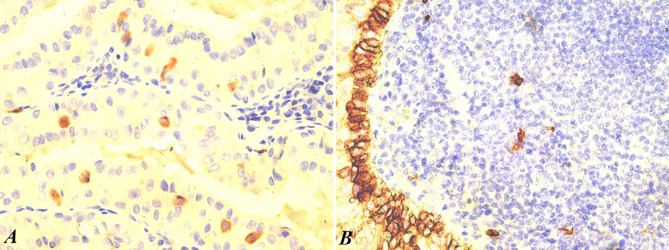
Immunohistochemistry for mast cell chymase identified mast cells in all cases, but in low numbers, and most of them were located in diffuse lymphoid tissue, but not in lymphoid follicles. Immunoreaction for CD117 was intensely positive in isolated cells with morphology suggestive of mast cells. Furthermore, it was intense and continuous in the deep cell layer of the epithelial component. Mast cells were positive for CD117 in all cases studied, but their number was lower than that obtained with mast cell tryptase (Figure 1A).
Figure 1. Intraepithelial mast cells (brown) identified with CD117 staining (A, ×400). Deep epithelial cell layer (brown) intensely positive for CD117 (B, ×200).
The basal cell layer of the epithelial component of the Warthin’s tumor was intensely and homogeneously positive for CD117, an aspect that signals the potential progenitor role of these cells (Figure 1B). In the adjacent lymphoid tissue, isolated mast cells were present in small numbers, positioned in a manner that respected the germinal center.
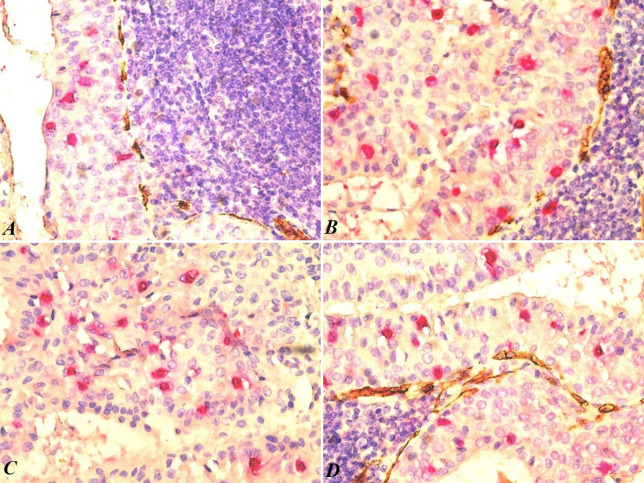
We applied double immunostaining with mast cell tryptase and CD34 to evaluate a possible relationship with the blood vessels located at the border between the two tissue components. Mast cells are known to be involved in various physiological and pathological processes, including angiogenesis – the formation of new blood vessels. Therefore, it was possible to numerically evaluate mast cells and blood vessels in the same microscopic fields. In the lymphoid component, mast cells were absent from the lymphoid follicles, particularly in the germinal centers, and were identified by the red-stained signal in the diffuse lymphoid tissue adjacent to the epithelial component. In two of the cases, we did not find mast cells in the lymphoid component with any of the applied staining methods. In the interfollicular diffuse lymphoid tissue, we observed a few mast cells in the immediate vicinity of capillary blood vessels. Our analysis revealed no statistically significant correlation between the number of blood vessels and the number of mast cells associated with the lymphoid and epithelial components of the tumor.
Through double immunostaining with mast cell tryptase and CD34, we identified mast cells in all 11 cases of Warthin’s Tumor included in the present study. However, their localization varied across different anatomical areas of the tumor. The number of IEMCs was significantly higher in the epithelial component than in the lymphoid component. In the epithelial component, the average density of mast cells was 20.54/×400 microscopic field (with values between 12 and 26). In the lymphoid component, the average mast cell density was 2.63/×400 microscopic field (with values between 0 and 4). The statistical analysis showed a strong correlation between the location of IEMCs and the benign character of the tumor (p<0.0001).
In the epithelial component, mast cells were distributed along the entire height of the epithelium, sometimes extending close to the free surface (Figure 2A and B). Notably, in two cases, we observed free mast cells on the surface of the epithelium within the lumen of small cystic structures, a distinctive feature of Warthin’s tumors. In all cases, we observed an intimate relationship between the IEMCs and the epithelial cells in the two layers of the tumor (Figure 2C and D). Specifically, the two layers refer to the epithelial layer and the adjacent lymphoid tissue layer. In areas where mast cells were relatively numerous in the lymphoid tissue, they were rarer in the epithelial component.
Figure 2. Intraepithelial mast cells (red) and small blood vessels (brown) at the epithelium-lymphoid tissue interface. Note the absence of tryptasepositive cells in the lymphoid tissue and the immature nature of the small vessels at the interface (A). High density of intraepithelial mast cells and rare mast cells in the lymphoid component (B). The distribution of mast cells throughout the height of the epithelium, including at the level of the free pseudocystic surface (C). Small cysts with epithelium containing mast cells in the superficial epithelial layer (D). CD34/mast cell tryptase double immunostaining, magnification ×400.
Discussion
Mast cells are classically described as connective tissue cells, although they are formed in the hematogenous bone marrow from a common CD34-positive precursor (16). The presence of IEMCs is unusual for several reasons. First, they do not establish tight junctions with the surrounding epithelial cells, suggesting a distinct mechanism for their migration across the basement membrane. Second, the precise mechanism by which they traverse the basement membrane remains unclear. Finally, the significance of their presence within the epithelium is currently unknown (17). Literature on this topic is limited, with a search of electronic databases using the term "intraepithelial mast cell" revealing only 10 articles published over the years that explicitly mention these terms in the title (18,19). In this context, the topic we have chosen for the present study seems justified, but from the point of view of practical importance, we cannot make specific statements at the present time.
Intriguingly, the significance of IEMCs has been largely overlooked, leading to their characterization as incidental findings in various pathological lesions across different organs (18,20-22). Moreover, existing data are inconsistent and often lack reference to normal tissues overall, particularly those in the oral cavity. Across the identified studies on this topic, mast cell density has commonly been limited to 1-2 cells per microscopic field, with a predominant presence in inflammatory lesions. IEMCs have been reported in a wide variety of lesions, including squamous cell carcinoma, gastric microcarcinoidosis (23), lichen planus gingiva (24), damaged intestinal epithelium due to infection with parasites of the Strongyloides family (25), and interstitial cystitis, where the presence of IEMCs is a microscopic diagnostic criterion (26).
IEMCs in allergic conditions have been reported by several authors, and some proposed a mechanism of action (27). Considering the major involvement of mast cells in allergic diseases, several groups have observed the presence of IEMCs in such cases. In tumors, they may contribute to tumor growth, angiogenesis, and immune suppression. Based on the data published so far, it was concluded that IEMCs appear only in pathological conditions (28).
Mast cells are immune cells that play a crucial role in various physiological and pathological processes, including inflammation, immunity, and tissue homeostasis. The migration of mast cells from the connective tissue into the epithelial compartment is a critical component of the mucosal response to various stimuli, including allergens, pathogens, and tissue damage. This migration process is regulated by a complex interplay of chemokines, adhesion molecules, and proteolytic enzymes. Enerback et al. (1986) showed that IEMCs derive from connective tissue and not from basophilic granulocytes. In patients with "hay fever", IEMCs were observed only on biopsies taken during the season of allergen circulation. Some of the migrated mast cells had typical connective mast cell ultrastructure, including lamellar and spiral granulations, and lipid inclusions. The ultrastructural heterogeneity of IEMCs may be the consequence of the epithelial microenvironment, but there is no definite evidence for this (29).
Mast cell mediators are well known for their major implications in the pathogenesis of asthma. In this context, the accumulation of mast cells with and without morphological changes in the tissues of the respiratory system in these patients is predictable (30). There are still controversies regarding the absolute number of mast cells in patients with asthma, which most likely derive from technical deficiencies, but also from the impossibility of identifying completely degranulated mast cells during attacks using usual methods (31). Gibson et al. (1993) developed a technique for evaluating IEMCs on bronchial washing preparations. The results were not compared with the histological ones, because for ethical reasons mucosal biopsy is not performed in these patients. The number of mast cells was significantly higher in patients with asthma, and most were identified in patients with allergic asthma. The presence of mast cells in the bronchial washing is synonymous for these authors with the presence of IEMCs. The connective tissue origin of mast cells in the lamina propria is demonstrated by mast cell tryptase positive reactions and toluidine blue metachromasia (32).
Mast cells have been shown to be associated with Warthin’s tumor largely following cytological examination of May Grunwald Giemsa-stained aspirates. With this method the mast cells are metachromatic. It is easy to understand that the cytological observations cannot specify the tissue localization of mast cells, but only signal the increased incidence of these cells and their association with the benign nature of the proliferation (33,34). Despite these limitations, the presence of mast cells in Warthin’s tumor has been associated with several clinicopathological features: increased mast cell density was correlated with favorable prognosis, mast cell degranulation was associated with lymphoid follicle formation, and mast cells were associated with the benign nature of Warthin’s tumor (35).
In recent decades, heightened interest in mast cell biology has stemmed from the identification of substances exerting significant impacts on cells and tissues, both in normal physiological conditions and various pathologies. Notably, growth factors like vascular endothelial growth factor have been recognized in this context. Over the years, mast cell identification methods have evolved, with mast cell tryptase emerging as the most sensitive and specific marker to date, surpassing thiazide dyes proposed by Ehrlich. Surprisingly, most intraepithelial mast cells in the current series were negative for histochemical reactions with toluidine blue and alcian blue. This outcome is likely attributed to the loss of sulphated glycosaminoglycans during the intraepithelial migration process. Our results regarding the specificity and sensitivity of the identification methods signal several important points. Mast cell chymase is a highly specific marker, but it identifies only a subset of mast cells. Furthermore, CD117 (c-kit) is a sensitive but less specific marker that is expressed in various cell types, including the basal ones in Warthin’s tumor. The mechanism by which mast cells migrate from the lymphoid tissue to the epithelial component remains unclear, particularly in cases that present a high density of these cells. The presence of mast cells in the epithelial component can be observed on aspirate smears and is a useful indicator for the benign nature of the proliferation (36-40).
Warthin’s tumor has a favorable prognosis in most cases, and recurrences have only rarely been reported (41). The differential diagnosis includes only a few lesions, such as lymphoepithelial cyst, sebaceous lymphadenomas, or salivary gland cystadenoma. Malignant transformation can lead to the evolution of the lymphoid component into malignant lymphoma (42); the epithelial component can give rise to adenocarcinoma, mucoepidermoid adenocarcinoma (43,44), squamous cell carcinoma, oncocytic carcinoma, and surprisingly, Merkel cell carcinoma.
Conclusion
The study of Warthin tumor-associated mast cells provides compelling evidence for the substantial presence of tryptase-positive intraepithelial mast cells. The density of intraepithelial mast cells is 10-12 times higher than that of the lymphoid tissue. The remarkable density of IEMCs, significantly exceeding that of the lymphoid tissue, highlights their potential significance in the tumor microenvironment. The observation of defective IEMCs with depleted glycosaminoglycans underscores the limitations of conventional histochemical methods for their identification. These findings contribute to a growing body of evidence implicating mast cells in tumor biology. Further research is warranted to elucidate the precise mechanisms by which mast cells contribute to Warthin’s tumor development and progression.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization, R.C.; methodology, R.C.; software, C.S.D.; validation, R.A.C. and N.P.G.; formal analysis, R.A.C. and N.P.G.; investigation, R.C., R.A.C. and N.P.G.; data curation, E.M.V. and I.O.; writing – original draft preparation, R.C.; writing – review and editing, C.S.D.; visualization, C.S.D.; supervision, R.A.P.; project administration, M.R. All Authors have read and agreed to the published version of the manuscript.
Acknowledgements
The Authors are grateful to all colleagues who contributed with cases and critically revised the manuscript. Many thanks to Ciprian Oniga for his excellent processing of specimens.
Funding
This research received no external funding.
References
- 1.Canning M, Guo G, Yu M, Myint C, Groves MW, Byrd JK, Cui Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front Cell Dev Biol. 2019;7:52. doi: 10.3389/fcell.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer J, Hariharan A, Cao UMN, Mai CTT, Wang A, Khayambashi P, Nguyen BH, Safi L, Tran SD. An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches. Cancers (Basel) 2021;13(15):3910. doi: 10.3390/cancers13153910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzenko YV, Romanuk AM, Dyachenko OO, Hudymenko O. Pathogenesis of Warthin’s tumors. Interv Med Appl Sci. 2016;8(2):41–48. doi: 10.1556/1646.8.2016.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldblum JR, Lamps LW, Myers J. Elsevier. 2017. Head and Neck Pathology. In: Rosai and Ackerman’s surgical pathology, 11th Edition. , -884, 2017. pp. pp. 883–884. [Google Scholar]
- 5.Orabona GDA, Abbate V, Piombino P, Romano A, Schonauer F, Iaconetta G, Salzano G, Farina F, Califano L. Warthin’s tumour: Aetiopathogenesis dilemma, ten years of our experience. J Craniomaxillofac Surg. 2015;43(4):427–431. doi: 10.1016/j.jcms.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Nagao T, Sato E, Inoue R, Oshiro H, H Takahashi R, Nagai T, Yoshida M, Suzuki F, Obikane H, Yamashina M, Matsubayashi J. Immunohistochemical analysis of salivary gland tumors: application for surgical pathology practice. Acta Histochem Cytochem. 2012;45(5):269–282. doi: 10.1267/ahc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheuk W, Chan JKC. Advances in salivary gland pathology. Histopathology. 2007;51(1):1–20. doi: 10.1111/j.1365-2559.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 8.Warthin AS. Papillary cystadenoma lymphomatosum. A rare teratoid of the parotid region. J Cancer Res. 1929;13:116–125. doi: 10.1158/jcr.1929.116. [DOI] [Google Scholar]
- 9.Gallo O, Bocciolini C. Warthin’s tumour associated with autoimmune diseases and tobacco use. Acta Otolaryngol. 1997;117(4):623–627. doi: 10.3109/00016489709113449. [DOI] [PubMed] [Google Scholar]
- 10.Therkildsen MH, Christensen N, Andersen LJ, Larsen S, Katholm M. Malignant Warthin’s tumour: a case study. Histopathology. 1992;21(2):167–171. doi: 10.1111/j.1365-2559.1992.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Raghu AR, Rehani S, Bishen KA, Sagari S. Warthin’s tumour: a case report and review on pathogenesis and its histological subtypes. J Clin Diagn Res. 2014;8(9):ZD37–ZD40. doi: 10.7860/JCDR/2014/8503.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theoharides TC, Conti P. Mast cells: the JEKYLL and HYDE of tumor growth. Trends Immunol. 2004;25(5):235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Nechushtan H. The complexity of the complicity of mast cells in cancer. Int J Biochem Cell Biol. 2010;42(5):551–554. doi: 10.1016/j.biocel.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Segura-Villalobos D, Ramírez-Moreno IG, Martínez-Aguilar M, Ibarra-Sánchez A, Muñoz-Bello JO, Anaya-Rubio I, Padilla A, Macías-Silva M, Lizano M, González-Espinosa C. Mast Cell-Tumor Interactions: Molecular mechanisms of recruitment, intratumoral communication and potential therapeutic targets for tumor growth. Cells. 2022;11(3):349. doi: 10.3390/cells11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rönnberg E, Melo FR, Pejler G. Mast cell proteoglycans. J Histochem Cytochem. 2012;60(12):950–962. doi: 10.1369/0022155412458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115(6):1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson CK, Mori M, Bjermer L, Löfdahl CG, Erjefält JS. Novel site-specific mast cell subpopulations in the human lung. Thorax. 2009;64(4):297–305. doi: 10.1136/thx.2008.101683. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125(5):1046–1053.e8. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb D, Lumsden A. Intra-epithelial mast cells in human airway epithelium: evidence for smoking-induced changes in their frequency. Thorax. 1982;37(5):334–342. doi: 10.1136/thx.37.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Y, Altemeier WA, Vandree J, Piliponsky AM, Johnson B, Appel CL, Frevert CW, Hyde DM, Ziegler SF, Smith DE, Henderson WR Jr, Gelb MH, Hallstrand TS. Increased density of intraepithelial mast cells in patients with exercise-induced bronchoconstriction regulated through epithelially derived thymic stromal lymphopoietin and IL-33. J Allergy Clin Immunol. 2014;133(5):1448–1455. doi: 10.1016/j.jaci.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder DJ, Mak N, Hurlbut DJ, Justinich CJ. Atopic and non-atopic eosinophilic oesophagitis are distinguished by immunoglobulin E-bearing intraepithelial mast cells. Histopathology. 2012;61(5):810–822. doi: 10.1111/j.1365-2559.2012.4303.x. [DOI] [PubMed] [Google Scholar]
- 22.Knöbber D, Agha-Mir-Salim P, Merker HJ, Jahnke V. Intraepithelial mast cells in the vocal cords and nasal mucosa. Laryngorhinootologie. 1993;72(12):590–594. doi: 10.1055/s-2007-997960. [DOI] [PubMed] [Google Scholar]
- 23.Auböck L. Intraepithelial mast cells in the human gastric mucosa in a case of microcarcinoidosis. Acta Morphol Acad Sci Hung. 1980;28(1-2):59–69. [PubMed] [Google Scholar]
- 24.Gelbmann CM, Schteingart CD, Thompson SM, Hofmann AF, Barrett KE. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J Clin Invest. 1995;95(6):2831–2839. doi: 10.1172/JCI117988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert-Bayo M, Paracuellos I, González-Castro AM, Rodríguez-Urrutia A, Rodríguez-Lagunas MJ, Alonso-Cotoner C, Santos J, Vicario M. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells. 2019;8(2):135. doi: 10.3390/cells8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen S, Thompson SA, Hald T, Barnard RJ, Gilpin CJ, Dixon JS, Gosling JA. Mast cells in interstitial cystitis. Br J Urol. 1982;54(3):283–286. doi: 10.1111/j.1464-410x.1982.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW, Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest. 2012;122(2):748–758. doi: 10.1172/JCI58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll T, Silva M, Sarker RSJ, Tschurtschenthaler M, Schnalzger T, Mogler C, Denk D, Schölch S, Schraml BU, Ruland J, Rad R, Saur D, Weichert W, Jesinghaus M, Matiasek K, Steiger K. Comparative study of the role of interepithelial mucosal mast cells in the context of intestinal adenoma-carcinoma progression. Cancers (Basel) 2022;14(9):2248. doi: 10.3390/cancers14092248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enerbäck L, Pipkorn U, Olofsson A. Intraepithelial migration of mucosal mast cells in hay fever: ultrastructural observations. Int Arch Allergy Appl Immunol. 1986;81(4):289–297. doi: 10.1159/000234152. [DOI] [PubMed] [Google Scholar]
- 30.Méndez-Enríquez E, Hallgren J. Mast cells and their progenitors in allergic asthma. Front Immunol. 2019;10:821. doi: 10.3389/fimmu.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banafea GH, Bakhashab S, Alshaibi HF, Natesan Pushparaj P, Rasool M. The role of human mast cells in allergy and asthma. Bioengineered. 2022;13(3):7049–7064. doi: 10.1080/21655979.2022.2044278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson PG, Allen CJ, Yang JP, Wong BJO, Dolovich J, Denburg J, Hargreave FE. Intraepithelial mast cells in allergic and nonallergic asthma: Assessment using bronchial brushings. Am Rev Respir Dis. 1993;148(1):80–86. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 33.Santucci M, Gallo O, Calzolari A, Bondi R. Detection of Epstein-Barr viral genome in tumor cells of Warthin’s tumor of parotid gland. Am J Clin Pathol. 1993;100(6):662–665. doi: 10.1093/ajcp/100.6.662. [DOI] [PubMed] [Google Scholar]
- 34.Aguirre JM, Echebarría MA, Martínez-Conde R, Rodriguez C, Burgos JJ, Rivera JM. Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(1):60–63. doi: 10.1016/s1079-2104(98)90399-7. [DOI] [PubMed] [Google Scholar]
- 35.Jaafari-Ashkavandi Z, Ashraf MJ. Increased mast cell counts in benign and malignant salivary gland tumors. J Dent Res Dent Clin Dent Prospects. 2014;8(1):15–20. doi: 10.5681/joddd.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Köybaşioğlu FF, Önal B, Han Ü, Adabağ A, Şahpaz A. Cytomorphological findings in diagnosis of Warthin tumor. Turk J Med Sci. 2020;50(1):148–154. doi: 10.3906/sag-1901-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi TK, Ueda M, Nishino T, Kushima R, Nakajima S, Kaneko C. Association of mast cells with Warthin’s tumor in fine needle aspirates of the salivary gland. Acta Cytol. 1999;43(6):1052–1058. doi: 10.1159/000331353. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto H, Caselitz J, Seifert G. Cystadenolymphoma: an immunohistochemical study with special reference to Ig E and mastcells. Pathol Res Pract. 1985;180(4):364–368. doi: 10.1016/S0344-0338(85)80107-2. [DOI] [PubMed] [Google Scholar]
- 39.Caselitz J, Salfelder A, Seifert G. Mast cells in cystadenolymphomas. Klin Wochenschr. 1984;62(6):284–286. doi: 10.1007/BF01721890. [DOI] [PubMed] [Google Scholar]
- 40.Bottles K, Löwhagen T, Miller TR. Mast cells in the aspiration cytology differential diagnosis of adenolymphoma. Acta Cytol. 1985;29(4):513–515. [PubMed] [Google Scholar]
- 41.Quer M, Hernandez-Prera JC, Silver CE, Casasayas M, Simo R, Vander Poorten V, Guntinas-Lichius O, Bradley PJ, Tong-Ng W, Rodrigo JP, Mäkitie AA, Rinaldo A, Kowalski LP, Sanabria A, de Bree R, Takes RP, López F, Olsen KD, Shaha AR, Ferlito A. Current trends and controversies in the management of warthin tumor of the parotid gland. Diagnostics (Basel) 2021;11(8):1467. doi: 10.3390/diagnostics11081467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi Y, Ma Y, Yao Z, Qin Y, Zhao M. Warthin tumor complicated with T-lymphoblastic lymphoma: a case report. Hua Xi Kou Qiang Yi Xue Za Zhi. 2022;40(6):727–730. doi: 10.7518/hxkq.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishibashi K, Ito Y, Masaki A, Fujii K, Beppu S, Sakakibara T, Takino H, Takase H, Ijichi K, Shimozato K, Inagaki H. Warthin-like mucoepidermoid carcinoma. Am J Surg Pathol. 2015;39(11):1479–1487. doi: 10.1097/PAS.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 44.Park S, Lee YC, Lim SJ, Kim C. Malignant transformation of Warthin tumor in the cervical lymph node. Clin Nucl Med. 2023;48(4):342–344. doi: 10.1097/RLU.0000000000004571. [DOI] [PubMed] [Google Scholar]