Abstract
Background/Aim
To evaluate the long-term oncological outcomes in men with intermediate risk prostate cancer (PCa) enrolled in active surveillance (AS).
Patients and Methods
From April 2015 to December 2022, 30 men with Gleason score 3+4/ISUP Grade Group2 (GG2), greatest percentage of cancer (GPC) ≤50%, Gleason pattern 4 ≤10%, ≤3 positive biopsy cores were enrolled in AS. All patients underwent confirmatory transperineal saturation biopsy (SPBx: 20 cores) 12 months from diagnosis plus multiparametric magnetic resonance (mpMRI) evaluation. At the last follow-up, 68Ga prostate-specific membrane antigen (PSMA) positron-emission tomography (PET)/computed tomography (CT) was added: lesions with PIRADS score ≥3 and/or standardized uptake value (SUVmax) >5 were submitted to four targeted cores.
Results
Three out of 30 (10%) men with GG2 PCa were reclassified at confirmatory biopsy. At the last follow-up (median 5.2 years), only 2 of 27 (7.4%) men were reclassified and 23/30 (76.6%) continued AS.
Conclusion
Men with favorable GG2 PCa enrolled in AS have good long-term oncological results. The use of selective criteria (i.e., SPBx, mpMRI, PSMA PET/CT) reduces the risk of reclassification.
Keywords: Active surveillance, prostate cancer, intermediate risk Pca
Prostate cancer (PCa) is the most prevalent tumor among men and has a considerable impact on morbidity and mortality worldwide with more than 1.4 million new diagnoses in 2020 and 375,000 associated deaths worldwide (1). With an aging population, the number of patients with less aggressive and localized PCa has increased by the use of prostate-specific antigen (PSA) screening; although, the risk to detect not clinically significant PCa (Gleason score 6/ISUP Grade Group 1) is decreased following the introduction of multiparametric magnetic resonance (mpMRI) in clinical practice (2,3). As a result, many indolent PCas are diagnosed and conservatively treated using the Active Surveillance (AS) protocol because definitive treatments, such as radical prostatectomy (RP) and radiotherapy, can decrease one’s quality of life (QOL) in terms of urinary, sexual, and bowel functions without an increased survival rate. The management and monitoring of men enrolled in AS has improved by the use of mpMRI and transperineal prostate biopsy, and in clinical trials, by the genetic counselling and prostate-specific membrane antigen (PSMA) positron-emission tomography/computed tomography (PET/CT) evaluation (4,5).
Recently, men with intermediate risk PCa characterized by Gleason score 3+4/International Society of Urologic Pathology (ISUP) Grade Group (GG) 2 with Gleason pattern 4 ≤10%, a number of positive cores ≤3 with a greatest percentage of cancer (GPC) ≤50 have been considered suitable for AS with good long-term results in terms of overall survival and clinical progression (6,7).
We here report the long-term oncological outcomes of men with intermediate favorable PCa risk enrolled in the AS protocol.
Patients and Methods
From May 2013 to December 2022, 200 men aged between 52 and 73 (median age 63) with a very low risk PCa were enrolled in the AS protocol. After institutional review board and ethical committee approval were granted, informed consents were obtained from all participants included in the study. The presence of the following criteria defined eligibility: life expectancy greater than 10 years, clinical stage T1C, PSA below 10 ng/ml, PSA density (PSA-D) <0.20, ≤3 positive biopsy cores, Gleason score 6/ISUP GG1, and maximum core percentage of cancer (GPC) ≤50% (8). All the patients 12 months after the PCa diagnosis underwent mpMRI evaluation and confirmatory transperineal saturation prostate biopsy (SPBx: 20 cores): 45/200 (22.5%) men were upgraded, and 12/200 (6%) men autonomously decided to leave the AS protocol (9,10). At a median follow-up of 6.1 years (range=12-120 months) 139/200 (69.5%) very low risk patients continued the AS protocol.
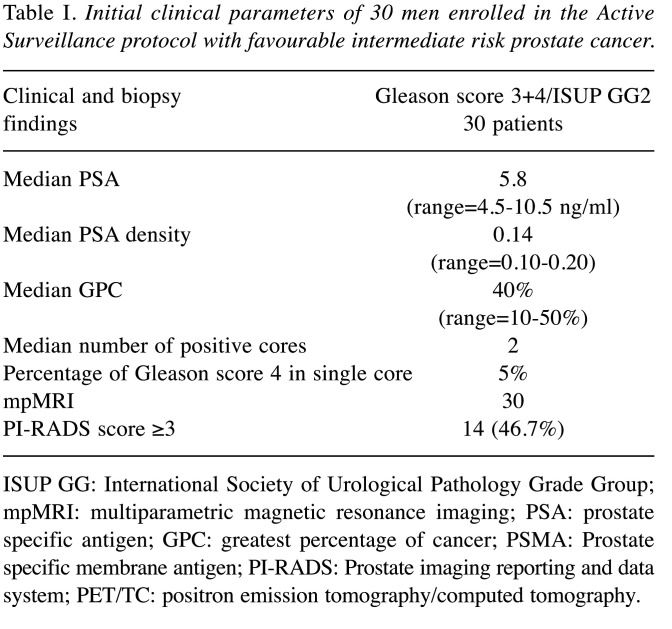
From April 2015 to December 2022, a subset of 30 men with intermediate risk PCa adequately informed, accepted to be enrolled in the AS protocol; the presence of the following biopsy histology parameters defined eligibility: Gleason score 3 + 4/ISUP GG2, GPC ≤50%, Gleason pattern 4 ≤10%, and ≤3 positive biopsy cores. During the follow-up (median 5.2 years; range=2-8 years), the 30 men with intermediate risk PCa (Table I) underwent the same scheduled protocol adding 68GaPSMA PET/CT to the mpMRI evaluation before last SPBx.
Table I. Initial clinical parameters of 30 men enrolled in the Active Surveillance protocol with favourable intermediate risk prostate cancer.
ISUP GG: International Society of Urological Pathology Grade Group; mpMRI: multiparametric magnetic resonance imaging; PSA: prostate specific antigen; GPC: greatest percentage of cancer; PSMA: Prostate specific membrane antigen; PI-RADS: Prostate imaging reporting and data system; PET/TC: positron emission tomography/computed tomography.
All mpMRI examinations were performed using a 1.5 and 3.0 Tesla scanner equipped with surface 16 channels phased-array coil placed around the pelvic area with the patient in the supine position; the mpMRI lesions characterized by Prostate Imaging Reporting and Data System (PI-RADS) version 2 (4) scores ≥3 were suspected to be cancer (11). PET/CT imaging was performed using a CT-integrated PET scanner (Biograph 6; Siemens, Knoxville, TN, USA); PSMA was prepared with a fully automated radiopharmaceutical synthesis device based on a modular concept (Eckert & Ziegler Eurotope, Berlin, Germany). Images were processed to obtain PET, CT, and PET-CT fusion sections in the axial, coronal, and sagittal planes with a thickness of approximately 0.5 ~ cm; the location of focal uptake on 68Ga-PSMA PET/TC, three-dimensional size, and standardized uptake value (SUVmax) values were reported on a per-lesion basis (12,13).
All the mpMRI (PI-RADS score ≥3) (10) and 68Ga-PET/TC index lesions (SUVmax ≥5) (4) underwent cognitive targeted cores (mpMRI-TPBx and PSMA-TPBx: four cores) combined with SPBx; the procedure was performed transperineally using a Hitachi 70 Arietta ecograph (Hitachi, Chiba, Japan) supplied by a bi-planar trans-rectal probe under sedation and antibiotic prophylaxis (14).
Results
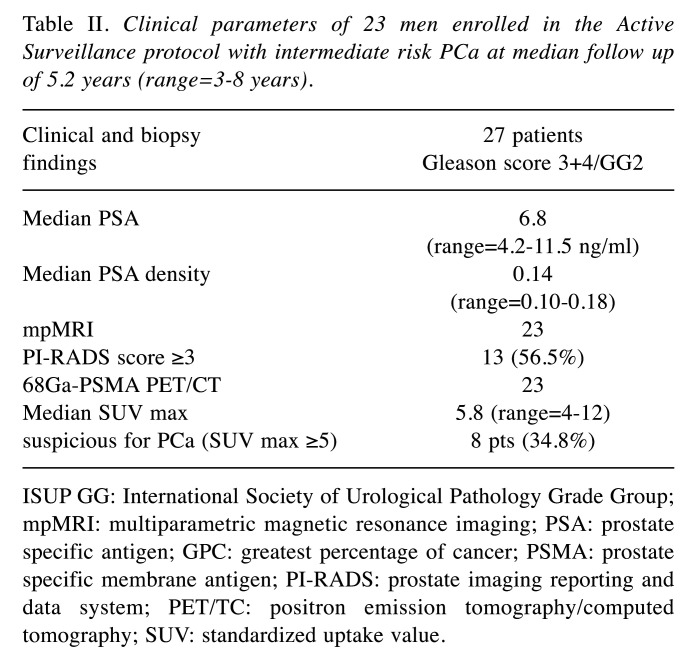
All the patients with a favorable GG2 PCa risk underwent 12 months after the diagnosis mpMRI evaluation plus confirmatory transperineal SPBx and 3/30 (10%) were reclassified: all the patients had a GG2 PCa characterized by a Gleason 4 pattern <10% with a GPC >50% in two cases (70 and 80%) and in 3/3 cases by a number of positive cores greater than 3 (4 cores in two cases and 5 cores in one case). During the follow-up (median 5.2 years) the men underwent scheduled transperineal SPBx combined with additional mpMRI/TRUS fusion biopsies (4 cores) of lesions with PI-RADS scores ≥3 and 2/27 (7.4%) of them were reclassified: 3 cores involved by Gleason score 4+3/ISUP GG3 with a percentage of cancer GPC of 50%; PSA density was 0.20, PIRADS score 4 and median SUVmax 8.2. The other 25/30 (83.3%) patients continued follow-up and clinical parameters are reported in Table II. Two men autonomously decided to leave the AS protocol. At the last scheduled biopsy, mpMRI and 68PSMA PET/CT showed 14/23 (60.8%) and 8/23 (34.7%) lesions were suspicious for PCa and were submitted to targeted cores combined with SPBx. In detail, mpMRI PI-RADS score resulted in ≤2 vs. 3 vs. 4 in 10 (43.8%) vs. 8 (34.8%) vs. 5 (12.4%) men. The average intraprostatic SUVmax and tumor dimension were 4.6 g/ml (range=3.2-19.8 g/ml) and 7.0 mm (range=4-12 mm), respectively. Only 8/23 (34.8%) men had a SUVmax ≥5 (range=5.1-19.8). Moreover, 68Ga-PSMA PET/TC showed two suspicious lesions for metastases in correspondence with the iliac ala and spinal cord, which were not confirmed by MRI evaluation.
Table II. Clinical parameters of 23 men enrolled in the Active Surveillance protocol with intermediate risk PCa at median follow up of 5.2 years (range=3-8 years).
ISUP GG: International Society of Urological Pathology Grade Group; mpMRI: multiparametric magnetic resonance imaging; PSA: prostate specific antigen; GPC: greatest percentage of cancer; PSMA: prostate specific membrane antigen; PI-RADS: prostate imaging reporting and data system; PET/TC: positron emission tomography/computed tomography; SUV: standardized uptake value.
Discussion
The estimated treatment-free probability at 15 years from diagnosis of patients with GG1 PCa enrolled in the AS protocol is equal to 58% (15). At a median follow-up of 15 years, cancer-specific mortality of 1,610 patients with localized PCa (more than one third with intermediate or high-risk disease) was 2.7% irrespective of treatment. Moreover, 24.4% of men enrolled in the AS protocol were alive without any curative treatment (16). Gearman et al. (17) reported that 93.9% and 82.6% of 8,095 patients with GG1 or GG2 PCa who underwent RP showed an organ confined disease and the 10-year systemic progression-free survival rate equal was to 99% vs. 96.5%, respectively.
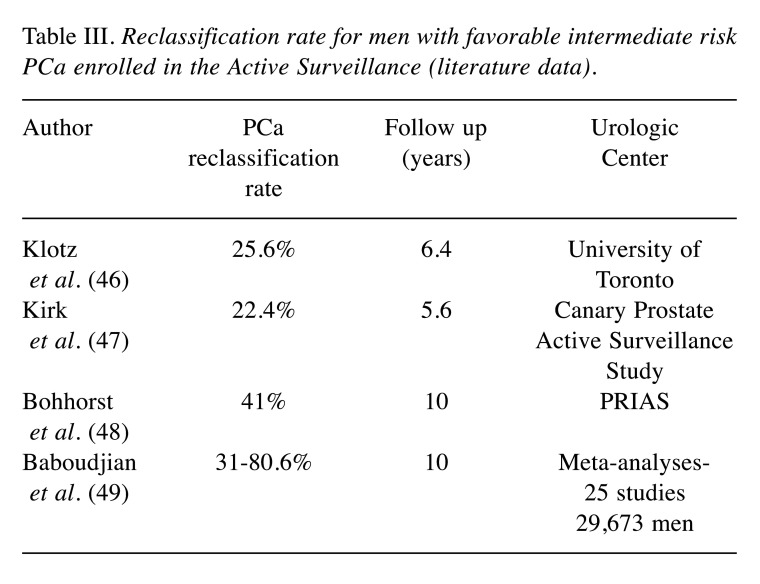
AS is endorsed by clinical guidelines as the preferred management strategy for low-risk prostate cancer; the American Urological Association (AUA) Quality (AQUA) Registry Rates report a sharply and consistently increased number of men enrolled in AS from 2014 (26.5%) to 2021 (59.6%) (18). Although favorable GG2 PCa are found to harbor adverse surgical pathology histology in about 25% of the cases (19), a low percentage of Gleason pattern 4 in biopsy is associated, in selected cases, with favorable prostatectomy histology and good oncological outcomes (Table III); men with GG2 cancer combined with Gleason pattern 4 <5% in a biopsy core had a definitive histology similar to patients with GG1 PCa (20). Conversely, the risk of upgrading at definitive histology in men with 1 or 2 cores with Gleason score 3+4 and PSA levels <20 ng/ml is higher in the case of a single core with Gleason pattern 4 >20% (21,22). Klotz et al. (23) reported that candidates with a better prognosis were men categorized as having intermediate-risk disease with a PSA level between 10 and 20 ng/ml, a GG2 disease with a small percentage of Gleason 4 pattern, a negative mpMRI or negative targeted biopsy. Musunuru et al. (24) reported that at a median follow-up of 6.7 years among the estimated 15-year metastasis-free survival of 213 patients with GG2 PCa was equal to 84%.
Table III. Reclassification rate for men with favorable intermediate risk PCa enrolled in the Active Surveillance (literature data).
Clinical, genomic, and radiological biomarkers including the Decipher Genomic Classifier, circulating miRNAs and urinary biomarkers, have been reported to improve risk stratification and patient selection (25-29). Regarding the genetic markers, Carter et al. (30) reported that BRCA2 mutations in men enrolled in AS were associated with a higher risk of reclassification. Imaging has improved AS criteria especially in cases of intermediate risk PCa; although mpMRI is strongly recommended in AS, it is not yet practical to omit scheduled prostate biopsy and replace it entirely with mpMRI alone because the non-negligible percentage of false negative rate (31). However, mpMRI combined with other clinical parameters has reduced sampling errors and underdiagnosis during systematic biopsy (32). Huang et al. (33) showed that that lower apparent diffusion coefficient (ADC) values of the index lesion are significantly associated with an increased risk of reclassification in men on AS with GG1 PCa (34). Recently, PSMA PET/CT demonstrated good accuracy in the diagnosis of clinically significant PCa being not inferior to mpMRI evaluation (35-37); in fact, SUVmax value has been correlated with PCa aggressiveness (12). Roscigno et al. (38) reported that in the presence of negative mpMRI a PSA density higher than 0.20 allowed to diagnose 16% of GG2 PCa; moreover, Saout et al. (39) reported that negative mpMRI and PSA density ≤0.10, during follow-up had an excellent negative predictive value for treatment. Dai et al. (40) reported in multivariable analysis that age, number of positive cores and perineural invasion in biopsy histology were independent predictors of reclassification. Other studies focused on approaches of prostate biopsy procedure to reduce the risk of upgrading; Zattoni et al. (41) reported that transperineal targeted biopsy improves the concordance of biopsy and final histology; in addition, transperineal SPBx reduces the risk of upgrading during AS follow-up (9,42). Recently, the introduction in clinical practice of digital pathology has improved the accuracy of biopsy histology in selecting men candidates for AS allowing to better identify the percentage of GG2 PCa using the needle core (43).
Despite being a noninvasive treatment strategy, AS may subject some patients to active examinations, as it fails to consider the speed of individual disease progression; the use of risk-calculator that includes factors, such as age, PSA level, PSA kinetics, biopsy results, mpMRI findings, and genetic testing may influence the risk classification and selection for each man in AS (44-50).
In our series, only 5/30 (16.6%) men with favorable GG2 PCa were reclassified during the follow-up (median 5.2 years) and 2/30 (6.7%) decided to leave AS; our results demonstrated an accurate selection of the patients by performing transperineal SPBx, mpMRI and, recently, PSMA PET/CT that allowed to continue AS in 23/30 (76.6%) men.
Some considerations should be made regarding our results. First, the number of patients is low, and a greater number of patients should be prospectively evaluated. Second, the low reclassification rate at confirmatory biopsy of GG2 vs. GG1 PCa (16.6 vs. 22.5%) could be correlated with the accurate clinical inclusions criteria (i.e., PSA and PSAD values, SPBx) that allowed the diagnosis of small volume GG2 PCa. Finally, a long-term follow-up is needed to confirm oncological outcomes.
In conclusion, men with intermediate favorable GG2 PCa could be enrolled in the AS protocol with good oncological long-term results. The use or selective criteria (SPBx, mpMRI, PSAD, genetic counselling, digital pathology, PSMA PET/CT) could reduce the risk of reclassification during the follow-up.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization, P.P.; Methodology, P.P.; Software, P.L.; Validation, P.P., P.L.; Formal Analysis, P.P, P.L.; Investigation, P.P.; Resources, P.P.; Data Curation, P.P., P.L.; Writing – Original Draft Preparation, P.P., P.L.; Writing – Review & Editing, P.P., P.L.; Visualization, P.P, P.L., P.M., F.F.; Supervision, P.P.
References
- 1.Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, Morgan TM, Carlsson SV. 2022 Update on Prostate Cancer epidemiology and risk factors—a systematic review. Eur Urol. 2023;84(2):191–206. doi: 10.1016/j.eururo.2023.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harder FN, Heming CAM, Haider MA. mpMRI Interpretation in Active Surveillance for Prostate Cancer—An overview of the PRECISE score. Abdom Radiol (NY) 2023;48(7):2449–2455. doi: 10.1007/s00261-023-03912-2. [DOI] [PubMed] [Google Scholar]
- 3.Timilshina N, Alibhai SMH, Tomlinson G, Sander B, Cheung DC, Finelli A. Long-term outcomes following active surveillance of low-grade prostate cancer: a population-based study using a landmark approach. J Urol. 2023;209(3):540–548. doi: 10.1097/JU.0000000000003097. [DOI] [PubMed] [Google Scholar]
- 4.Pepe P, Pepe L, Tamburo M, Marletta G, Pennisi M, Fraggetta F. 68Ga-PSMA PET/CT evaluation in men enrolled in prostate cancer active surveillance. Arch Ital Urol Androl. 2023;95(2):11322. doi: 10.4081/aiua.2023.11322. [DOI] [PubMed] [Google Scholar]
- 5.Pepe P, Roscigno M, Pepe L, Panella P, Tamburo M, Marletta G, Savoca F, Candiano G, Cosentino S, Ippolito M, Tsirgiotis A, Pennisi M. Could 68Ga-PSMA PET/CT evaluation reduce the number of scheduled prostate biopsies in men enrolled in active surveillance protocols. J Clin Med. 2022;11(12):3473. doi: 10.3390/jcm11123473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell JR, Siddiqui MM. Active surveillance in favorable intermediate risk prostate cancer: outstanding questions and controversies. Curr Opin Oncol. 2022;34(3):219–227. doi: 10.1097/CCO.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 7.Preisser F, Cooperberg MR, Crook J, Feng F, Graefen M, Karakiewicz PI, Klotz L, Montironi R, Nguyen PL, D’Amico AV. Intermediate-risk prostate cancer: stratification and management. Eur Urol Oncol. 2020;3:270–280. doi: 10.1016/j.euo.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Pepe P, Cimino S, Garufi A, Priolo G, Russo GI, Giardina R, Reale G, Pennisi M, Morgia G. Confirmatory biopsy of men under active surveillance: extended versus saturation versus multiparametric magnetic resonance imaging/transrectal ultrasound fusion prostate biopsy. Scand J Urol. 2017;51(4):260–263. doi: 10.1080/21681805.2017.1313310. [DOI] [PubMed] [Google Scholar]
- 9.Pepe P, Pepe L, Pennisi M, Fraggetta F. Which prostate biopsy in men enrolled in active surveillance? Experience in 110 men submitted to scheduled three-years transperineal saturation biopsy combined with fusion targeted cores. Clin Genitourin Cancer. 2021;19(4):305–308. doi: 10.1016/j.clgc.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Pepe P, Pepe L, Pennisi M, Fraggetta F. Confirmatory transperineal saturation prostate biopsy combined with mpMRI decrease the reclassification rate in men enrolled in Active Surveillance: Our experience in 100 men submitted to eight-years scheduled biopsy. Arch Ital Urol Androl. 2022;94(3):270–273. doi: 10.4081/aiua.2022.3.270. [DOI] [PubMed] [Google Scholar]
- 11.Pepe P, Garufi A, Priolo G, Pennisi M. Can MRI/TRUS fusion targeted biopsy replace saturation prostate biopsy in the re-evaluation of men in active surveillance. World J Urol. 2016;34(9):1249–1253. doi: 10.1007/s00345-015-1749-3. [DOI] [PubMed] [Google Scholar]
- 12.Pepe P, Pepe L, Tamburo M, Marletta G, Savoca F, Pennisi M, Fraggetta F. (68)Ga-PSMA PET/CT and prostate cancer diagnosis: which SUVmax value. In Vivo. 2023;37(3):1318–1322. doi: 10.21873/invivo.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepe P, Garufi A, Priolo GD, Galia A, Fraggetta F, Pennisi M. Is it time to perform only magnetic resonance imaging targeted cores? Our experience with 1,032 men who underwent prostate biopsy. J Urol. 2018;200(4):774–778. doi: 10.1016/j.juro.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Pepe P, Pennisi M. Morbidity following transperineal prostate biopsy: Our experience in 8.500 men. Arch Ital Urol Androl. 2022;94(2):155–159. doi: 10.4081/aiua.2022.2.155. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson S, Benfante N, Alvim R, Sjoberg DD, Vickers A, Reuter VE, Fine SW, Vargas HA, Wiseman M, Mamoor M, Ehdaie B, Laudone V, Scardino P, Eastham J, Touijer K. Long-term outcomes of active surveillance for prostate cancer: The Memorial Sloan Kettering Cancer Center experience. J Urol. 2020;203(6):1122–1127. doi: 10.1097/JU.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, Martin RM, Young GJ, Walsh EI, Bryant RJ, Bollina P, Doble A, Doherty A, Gillatt D, Gnanapragasam V, Hughes O, Kockelbergh R, Kynaston H, Paul A, Paez E, Powell P, Rosario DJ, Rowe E, Mason M, Catto JWF, Peters TJ, Oxley J, Williams NJ, Staffurth J, Neal DE, ProtecT Study Group Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547–1558. doi: 10.1056/NEJMoa2214122. [DOI] [PubMed] [Google Scholar]
- 17.Gearman DJ, Morlacco A, Cheville JC, Rangel LJ, Karnes RJ. Comparison of pathological and oncologic outcomes of favorable risk Gleason score 3 + 4 and low risk Gleason score 6 prostate cancer: considerations for active surveillance. J Urol. 2018;199(5):1188–1195. doi: 10.1016/j.juro.2017.11.116. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Meeks W, Fang R, Gaylis FD, Catalona WJ, Makarov DV. Time trends and variation in the use of active surveillance for management of low-risk prostate cancer in the US. JAMA Netw Open. 2023;6(3):e231439. doi: 10.1001/jamanetworkopen.2023.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel HD, Tosoian JJ, Carter HB, Epstein JI. Adverse pathologic findings for men electing immediate radical prostatectomy: defining a favorable intermediate-risk group. JAMA Oncol. 2018;4(1):89–92. doi: 10.1001/jamaoncol.2017.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CC, Kong MX, Zhou M, Rosenkrantz AB, Taneja SS, Melamed J, Deng FM. Gleason score 3+4=7 prostate cancer with minimal quantity of Gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am J Surg Pathol. 2014;38(8):1096–1101. doi: 10.1097/PAS.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 21.Cole AI, Morgan TM, Spratt DE, Palapattu GS, He C, Tomlins SA, Weizer AZ, Feng FY, Wu A. Siddiqui J: Prognostic value of percent Gleason Grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J Urol. 2016;196:405–411. doi: 10.1016/j.juro.2016.01.120. [DOI] [PubMed] [Google Scholar]
- 22.Patel HD, Tosoian JJ, Carter HB, Epstein JI. Adverse pathologic findings for men electing immediate radical prostatectomy: Defining a favorable intermediate-risk group. JAMA Oncol. 2018;4(1):89–92. doi: 10.1001/jamaoncol.2017.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klotz L. Active surveillance in intermediate-risk prostate cancer. BJU Int. 2020;125(3):346–354. doi: 10.1111/bju.14935. [DOI] [PubMed] [Google Scholar]
- 24.Musunuru HB, Yamamoto T, Klotz L, Ghanem G, Mamedov A, Sethukavalan P, Jethava V, Jain S, Zhang L, Vesprini D, Loblaw A. Active surveillance for intermediate risk prostate cancer: survival outcomes in the sunnybrook experience. J Urol. 2016;196(6):1651–1658. doi: 10.1016/j.juro.2016.06.102. [DOI] [PubMed] [Google Scholar]
- 25.Press BH, Jones T, Olawoyin O, Lokeshwar SD, Rahman SN, Khajir G, Lin DW, Cooperberg MR, Loeb S, Darst BF, Zheng Y, Chen RC, Witte JS, Seibert TM, Catalona WJ, Leapman MS, Sprenkle PC. Association between a 22-feature genomic classifier and biopsy Gleason upgrade during active surveillance for prostate cancer. Eur Urol Open Sci. 2022;37:113–119. doi: 10.1016/j.euros.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandellini P, Ciniselli CM, Rancati T, Marenghi C, Doldi V, El Bezawy R, Lecchi M, Claps M, Catanzaro M, Avuzzi A. Prediction of grade reclassification of prostate cancer patients on active surveillance through the combination of a three-miRNA signature and selected clinical variables. Cancers (Basel) 2021;13:2433. doi: 10.3390/cancers13102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonergan PE, Washington SL 3rd, Cowan JE, Zhao S, Nguyen HG, Shinohara K, Cooperberg MR, Carroll PR. Risk factors for biopsy reclassification over time in men on active surveillance for early stage prostate cancer. J Urol. 2020;204(6):1216–1221. doi: 10.1097/JU.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 28.Jairath NK, Dal Pra A, Vince R Jr, Dess RT, Jackson WC, Tosoian JJ, McBride SM, Zhao SG, Berlin A, Mahal BA, Kishan AU, Den RB, Freedland SJ, Salami SS, Kaffenberger SD, Pollack A, Tran P, Mehra R, Morgan TM, Weiner AB, Mohamad O, Carroll PR, Cooperberg MR, Karnes RJ, Nguyen PL, Michalski JM, Tward JD, Feng FY, Schaeffer EM, Spratt DE. A systematic review of the evidence for the decipher genomic classifier in prostate cancer. Eur Urol. 2021;79(3):374–383. doi: 10.1016/j.eururo.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb LF, Zheng Y, Faino AV, Bianchi-Frias D, Cooperberg MR, Brown MD, Brooks JD, Dash A, Fabrizio MD, Gleave ME, Liss M, Morgan TM, Thompson IM, Wagner AA, Carroll PR, Nelson PS, Lin DW. Performance of PCA3 and TMPRSS2:ERG urinary biomarkers in prediction of biopsy outcome in the Canary Prostate Active Surveillance Study (PASS) Prostate Cancer Prostatic Dis. 2019;22(3):438–445. doi: 10.1038/s41391-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter HB, Helfand B, Mamawala M, Wu Y, Landis P, Yu H, Wiley K, Na R, Shi Z, Petkewicz J, Shah S, Fantus RJ, Novakovic K, Brendler CB, Zheng SL, Isaacs WB, Xu J. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol. 2019;75(5):743–749. doi: 10.1016/j.eururo.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesnut GT, Vertosick EA, Benfante N, Sjoberg DD, Fainberg J, Lee T, Eastham J, Laudone V, Scardino P, Touijer K, Vickers A, Ehdaie B. Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for men with prostate cancer on active surveillance. Eur Urol. 2020;77(4):501–507. doi: 10.1016/j.eururo.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saout K, Zambon A, Nguyen TA, Lucas C, Payrard-Starck C, Segalen T, Tissot V, Doucet L, Marolleau J, Deruelle C. Impact of multiparametric MRI and PSA density on the initial indication or the maintaining in active surveillance during follow-up in low-risk prostate cancer. Clin Genitourin Cancer. 2022;20(3):e244–e252. doi: 10.1016/j.clgc.2022.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Huang MM, Macura KJ, Landis P, Epstein JI, Gawande R, Carter HB, Mamawala M. Evaluation of apparent diffusion coefficient as a predictor of grade reclassification in men on active surveillance for prostate cancer. Urology. 2020;138:84–90. doi: 10.1016/j.urology.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Pepe P, D’Urso D, Garufi A, Priolo G, Pennisi M, Russo G, Sabini MG, Valastro LM, Galia A, Fraggetta F. Multiparametric MRI apparent diffusion coefficient (ADC) accuracy in diagnosing clinically significant prostate cancer. In Vivo. 2017;31(3):415–418. doi: 10.21873/invivo.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow KM, So WZ, Lee HJ, Lee A, Yap DWT, Takwoingi Y, Tay KJ, Tuan J, Thang SP, Lam W, Yuen J, Lawrentschuk N, Hofman MS, Murphy DG, Chen K. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: a systematic review and meta-analysis. Eur Urol. 2023;84(1):36–48. doi: 10.1016/j.eururo.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Heetman JG, Lavalaye J, Polm PD, Soeterik TFW, Wever L, Paulino Pereira LJ, van der Hoeven EJRJ, van Melick HHE, van den Bergh RCN. Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography in active surveillance for prostate cancer trial (PASPoRT) Eur Urol Oncol. 2023 doi: 10.1016/j.euo.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Pepe P, Pepe L, Tamburo M, Marletta G, Pennisi M, Fraggetta F. Targeted prostate biopsy: 68Ga-PSMA PET/CT vs. mpMRI in the diagnosis of prostate cancer. Arch Ital Urol Androl. 2022;94(3):274–277. doi: 10.4081/aiua.2022.3.274. [DOI] [PubMed] [Google Scholar]
- 38.Roscigno M, Stabile A, Lughezzani G, Pepe P, Galosi AB, Naselli A, Naspro R, Nicolai M, La Croce G, Aljoulani M, Perugini G, Guazzoni G, Montorsi F, Balzarini L, Sironi S, Da Pozzo LF. The use of multiparametric magnetic resonance imaging for follow-up of patients included in active surveillance protocol. Can PSA density discriminate patients at different risk of reclassification? Can PSA density discriminate patients at different risk of reclassification. Clin Genitourin Cancer. 2020;18(6):e698–e704. doi: 10.1016/j.clgc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Saout K, Zambon A, Nguyen TA, Lucas C, Payrard-Starck C, Segalen T, Tissot V, Doucet L, Marolleau J, Deruelle C, Joulin V, Fourcade A, Fournier G, Valeri A. Impact of multiparametric MRI and PSA density on the initial indication or the maintaining in active surveillance during follow-up in low-risk prostate cancer. Clin Genitourin Cancer. 2022;20(3):e244–e252. doi: 10.1016/j.clgc.2022.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Dai C, Ganesan V, Nyame YA, Almassi N, Greene DJ, Hettel D, Magi-Galluzzi C, Gong M, Jones JS, Stephenson AJ, Berglund RK, Klein EA. Older age at diagnosis and initial disease volume predict grade reclassification risk on confirmatory biopsy in patients considered for active surveillance. Urology. 2019;130:106–112. doi: 10.1016/j.urology.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 41.Zattoni F, Marra G, Martini A, Kasivisvanathan V, Grummet J, Harkin T, Ploussard G, Olivier J, Chiu PK, Valerio M, Marquis A, Gontero P, Guo H, Zhuang J, Frydenberg M, Moon D, Morlacco A, Kretschmer A, Barletta F, Heidegger I, Tilki D, van den Bergh R, Dal Moro F, Briganti A, Montorsi F, Novara G, Gandaglia G, EAU-YAU Prostate Cancer Working Party Is there an impact of transperineal versus transrectal magnetic resonance imaging-targeted biopsy on the risk of upgrading in final pathology in prostate cancer patients undergoing radical prostatectomy? A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group multi-institutional study. Eur Urol Focus. 2023;9:621–628. doi: 10.1016/j.euf.2023.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Pepe P, Garufi A, Priolo GD, Pennisi M. Multiparametric MRI/TRUS fusion prostate biopsy: advantages of a transperineal approach. Anticancer Res. 2017;37:3291–3294. doi: 10.21873/anticanres.11695. [DOI] [PubMed] [Google Scholar]
- 43.Salvi M, Caputo A, Balmativola D, Scotto M, Pennisi O, Michielli N, Mogetta A, Molinari F, Fraggetta F. Impact of stain normalization on pathologist assessment of prostate cancer: a comparative study. Cancers (Basel) 2023;15(5):1503. doi: 10.3390/cancers15051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vos II, Luiting HB, Roobol MJ. Active surveillance for prostate cancer: past, current, and future trends. J Pers Med. 2023;13(4):629. doi: 10.3390/jpm13040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seibert TM, Garraway IP, Plym A, Mahal BA, Giri V, Jacobs MF, Cheng HH, Loeb S, Helfand BT, Eeles RA, Morgan TM. Genetic risk prediction for prostate cancer: Implications for early detection and prevention. Eur Urol. 2023;83:241–248. doi: 10.1016/j.eururo.2022.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Salemi M, Pettinato A, Fraggetta F, Calogero AE, Pennisi M, Pepe L, Pepe P. Expression of miR-132 and miR-212 in prostate cancer and metastatic lymph node: Case report and revision of the literature. Arch Ital Urol Androl. 2020;92(3) doi: 10.4081/aiua.2020.3.209. [DOI] [PubMed] [Google Scholar]
- 47.Kirk PS, Zhu K, Zheng Y, Newcomb LF, Schenk JM, Brooks JD, Carroll PR, Dash A, Ellis WJ, Filson CP, Gleave ME, Liss M, Martin F, McKenney JK, Morgan TM, Nelson PS, Thompson IM, Wagner AA, Lin DW, Gore JL. Treatment in the absence of disease reclassification among men on active surveillance for prostate cancer. Cancer. 2022;128(2):269–274. doi: 10.1002/cncr.33911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, Roobol MJ, PRIAS study group A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70(6):954–960. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Baboudjian M, Breda A, Rajwa P, Gallioli A, Gondran-Tellier B, Sanguedolce F, Verri P, Diana P, Territo A, Bastide C, Spratt DE, Loeb S, Tosoian JJ, Leapman MS, Palou J, Ploussard G. Active surveillance for intermediate-risk prostate cancer: a systematic review, meta-analysis, and metaregression. Eur Urol Oncol. 2022;5(6):617–627. doi: 10.1016/j.euo.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Mazzucchelli R, Nesseris I, Cheng L, Lopez-Beltran A, Montironi R, Scarpelli M. Active surveillance for low-risk prostate cancer. Anticancer Res. 2010;30(9):3683–3692. [PubMed] [Google Scholar]