Abstract
Understanding cannabis-drug interactions is critical given regulatory changes that have increased access to and use of cannabis. Cannabidiol (CBD) and Δ-9-tetrahydrocannabinol (Δ9-THC), the most abundant phytocannabinoids, are in vitro reversible and time-dependent (CBD only) inhibitors of several cytochrome P450 (CYP) enzymes. Cannabis extracts were used to evaluate quantitatively potential pharmacokinetic cannabinoid-drug interactions in 18 healthy adults. Participant received, in a randomized cross-over manner (separated by ≥ 1 week), a brownie containing (i) no cannabis extract (ethanol/placebo), (ii) CBD-dominant cannabis extract (640 mg CBD + 20 mg Δ9-THC), or (iii) Δ9-THC-dominant cannabis extract (20 mg Δ9-THC and no CBD). After 30 minutes, participants consumed a cytochrome P450 (CYP) drug cocktail consisting of caffeine (CYP1A2), losartan (CYP2C9), omeprazole (CYP2C19), dextromethorphan (CYP2D6), and midazolam (CYP3A). Plasma and urine samples were collected (0–24 hours). The CBD + Δ9-THC brownie inhibited CYP2C19 > CYP2C9 > CYP3A > CYP1A2 (but not CYP2D6) activity, as evidenced by an increase in the geometric mean ratio of probe drug area under the plasma concentration-time curve (AUC) relative to placebo (AUCGMR) of omeprazole, losartan, midazolam, and caffeine by 207%, 77%, 56%, and 39%, respectively. In contrast, the Δ9-THC brownie did not inhibit any of the CYPs. The CBD + Δ9-THC brownie increased Δ9-THC AUCGMR by 161%, consistent with CBD inhibiting CYP2C9-mediated oral Δ9-THC clearance. Except for caffeine, these interactions were well-predicted by our physiologically-based pharmacokinetic model (within 26% of observed interactions). Results can be used to help guide dose adjustment of drugs co-consumed with cannabis products and the dose of CBD in cannabis products to reduce interaction risk with Δ9-THC.
The use of cannabis products for medicinal and non-medicinal purposes continues to increase worldwide. As of 2023, 37 US states and the District of Columbia have legalized the use of cannabis through regulated retail outlets, and many other countries have enacted similar changes that have either legalized or decriminalized cannabis use.1 The abundant phytocannabinoid cannabidiol (CBD) is approved as a pharmaceutical preparation in many countries for the treatment of certain types of epilepsy, with doses ranging from 2.5–25 mg/kg twice daily.2 CBD is also widely available in non-pharmaceutical formulations and is commonly used to self-treat a variety of health conditions, including anxiety, insomnia, and pain. Another abundant phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC), is approved in many countries for the treatment of anorexia related to advanced HIV/AIDS, cachexia, or adverse effects associated with chemotherapy, with recommended doses ranging from 2.5–1 5 mg for up to 4–6 doses daily.3 The Δ9-THC and the in vivo metabolite, 11-hydroxy-Δ9-THC (11-OH-Δ9-THC), produce notable pharmacodynamic (PD) effects, including a subjective “high” and impairment of cognitive functioning at higher doses, which are commonly associated with use of Δ9-THC-dominant cannabis products.4,5 In contrast, prior studies showed that CBD does not produce strong interoceptive acute drug effects or impair functioning and has very low abuse liability.6,7
The widespread use of CBD- and Δ9-THC-containing products carries risk for pharmacokinetic (PK) and PD interactions with pharmaceutical drugs, which could result in altered toxicity or efficacy. For example, case reports have documented potentially dangerous increases in the international normalized ratio (INR) in patients taking the cytochrome P450 (CYP) 2C9 substrate, warfarin, when CBD treatment or recreational use of cannabis was initiated.8–10 Thus, there is a compelling need to determine whether CBD and Δ9-THC precipitate PK drug interactions. In vitro studies with CBD and Δ9-THC conducted by our group11–14 and others15–19 predict a high likelihood for CBD to precipitate CYP-mediated13 and UDP-glucuronosyltransferase (UGT)-mediated12 drug interactions at the observed maximum unbound plasma concentration (Cmax,u) achieved after a single oral CBD dose (700 mg). CBD is a reversible inhibitor of CYP2B6, CYP2C8, CYP2C9, CYP2D6, UGT1A9, UGT2B4, and UGT2B712 and a time-dependent inhibitor of CYP1A2, CYP2C19, and CYP3A.13 In contrast, Δ9-THC at the observed Cmax,u after a single oral dose (20 mg) was predicted to precipitate modest interactions with drugs primarily metabolized by CYP2C9.13
The primary goal of the current work was to evaluate the aforementioned in vitro to in vivo predictions by conducting an in vivo study in humans to determine the magnitude of PK interactions between two different whole-plant cannabis extracts (one CBD-dominant and one Δ9-THC-dominant) and a validated oral CYP cocktail consisting of the probe drugs caffeine (CAF; CYP1A2), losartan (LOS; CYP2C9), omeprazole (OMP; CYP2C19), dextromethorphan (DXM; CYP2D6), and midazolam (MDZ; CYP3A).20 One secondary goal was to capitalize on the CBD-dominant extract to determine whether CBD can precipitate a PK/PD interaction with Δ9-THC. Another secondary goal was to determine whether our validated physiologically-based PK (PBPK) model for CBD21 could quantitatively predict the observed CBD-precipitated interactions with the CYP probe drugs. Results from the PK interaction study and PBPK model predictions are presented. Results from the PD changes for Δ9-THC, in the presence of CBD, are reported elsewhere.22
METHODS
Materials
See Supplementary Information for the materials used in this study.
In vivo pharmacokinetic interaction study
The study was conducted in cannabis-experienced healthy participants who signed the Johns Hopkins Medicine Institutional Review Board-approved informed consent document (see Zamarripa et al.22 for details of inclusion and exclusion criteria as well as the CONSORT checklist; Table S1). The study is registered with the ClinicalTrials.gov database (NCT04201197). Briefly, the participants were instructed to abstain from using cannabis products for 30 days prior to the first study arm and to abstain from any medication likely to confound interpretation of study results (e.g., interaction with CBD, Δ9-THC, or the CYP cocktail drugs) for at least 14 days prior to the study. Participants were also instructed to abstain from CAF or CAF-containing beverages 24 hours before the study sessions and to abstain from using cannabis/cannabinoid and tobacco products, grapefruit, and grapefruit juice, or other juices, or change any approved medication or dietary supplement during the study. All participants underwent a urine drug test, which included Δ9-THC, to ensure compliance, and female participants underwent a pregnancy test immediately prior to each test session; only those testing negative could participate in the study.
Cannabis brownies.
CBD-dominant (59% CBD; 2% Δ9-THC) and Δ9-THC-dominant (70% Δ9-THC; no CBD) whole plant cannabis extracts were obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program (see Zamarripa et al.22 for additional details). Unfortunately, a CBD-dominant extract devoid of Δ9-THC was not available. The cannabis extracts were subjected to decarboxylation to convert naturally occurring Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and cannabidiolic acid to Δ9-THC and CBD, respectively. The cannabis extracts were dissolved in ethanol (USP 190 proof; Spectrum Chemical, New Brunswick, NJ) for ease of dose measurement and cannabinoid content assessed. Based on in vitro to in vivo predictions, the composition of the extracts, and prior human research, target doses of 20 mg Δ9-THC and 640 mg CBD were selected for the present study. The ethanol solution was added to commercial brownie batter in individual baking trays to deliver precise dosing of 640 mg CBD + 20 mg Δ9-THC (CBD + Δ9-THC), 20 mg Δ9-THC and no CBD (Δ9-THC), or no cannabinoids (placebo). The ethanol was evaporated from the batter prior to baking for all dose conditions. The target dose of 20 mg Δ9-THC and/or 640 mg CBD was confirmed by analyzing sample brownies (ElSohly Laboratories, University, MS). The placebo brownies were prepared by adding an equal volume of ethanol, which was allowed to evaporate to ensure the same consistency of brownies among dose conditions.
Study design and procedures.
Based on a power analysis (see below), 18 healthy adult participants were administered a CBD + Δ9-THC brownie, a Δ9-THC brownie, and a placebo brownie in a double-blind randomized crossover manner; each of the 3 arms was separated by at least 1 week (Figure S1). Thirty minutes after brownie administration (to allow dissolution and absorption of the cannabinoids), each participant was administered an oral CYP probe drug (Inje) cocktail20 consisting of 100 mg CAF (CYP1A2), 25 mg LOS (CYP2C9), 20 mg OMP (CYP2C19), 30 mg DXM (CYP2D6), and 2 mg MDZ (CYP3A). One hour prior to consuming a brownie, each participant was provided a standard low-fat breakfast. Blood (10 mL via an intravenous catheter) was collected into 10-mL EDTA-containing vacutainer tubes prior to administering the placebo brownie (baseline) and at 0.25 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, and 24 hours after CYP cocktail administration. Pooled urine samples were collected 0–6 hours, 6–12 hours, and 12–24 hours post-cocktail administration. PD outcomes measuring cognitive and psychomotor performance and subjective drug effects were recorded at baseline and 0–24 hours post-cocktail administration as described previously.22 Blood samples were centrifuged for 10 minutes at 1200 g (4°C) to harvest plasma. Total urine volume from 0–6 hours, 6–12 hours, and 12–24 hours was recorded. Aliquots (~ 2 mL) of plasma and urine were added to two cryovials and stored at −80°C pending analysis for CYP probe drugs, cannabinoids, and relevant metabolites by ultra-high-performance liquid chromatography-tandem accurate mass spectrometry (UHPLC–MS/MS).
Bioanalyses.
All CYP probes, their conjugated and unconjugated metabolites, CBD, and the conjugated metabolites of Δ9-THC were analyzed as follows: 50 μL of plasma or 40 μL of pooled urine were mixed with acetonitrile (150 μL) containing internal standards (tolbutamide (25 ng/mL), CBD-d3 (10 ng/mL), and 11-COOH Δ9-THC glucuronide-d3 (10 ng/ml)) to precipitate proteins. After centrifugation at 18,000 g for 10 minutes, the supernatant was analyzed by UHPLC–MS/MS (Supplementary Materials; Table S2). Δ9-THC, 11-OH Δ9-THC, and 11-COOH Δ9-THC plasma concentrations were quantified after liquid–liquid extraction to enhance sensitivity. Briefly, 1 mL of plasma was spiked with a mixture of internal standards (6 ng/mL of Δ9-THC-d3, 11-OH Δ9-THC-d3, and 11-COOH Δ9-THC-d3) and formic acid (final concentration 0.15%) in a glass tube. Five milliliters of hexane:ethylacetate (5:1) were added to the tube and covered with parafilm prior to vigorous shaking for 30 minutes. After centrifugation (200 g) at room temperature for 10 minutes, the organic layer was collected and added to a clean glass tube and evaporated to dryness at 40°C under nitrogen. Acetonitrile (100 L) was added to the tube, vortex-mixed at high speed for 30 seconds, and the resulting concentrate was transferred to a glass insert for UHPLC–MS/MS analysis (Supplementary Materials; Table S2). Further details about the bioanalyses methods are provided in the Supplementary Materials.
Pharmacokinetic analyses.
The area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) for each CYP probe and corresponding metabolites was estimated using Phoenix WinNonlin (version 8.1; Certara, Princeton, NJ). Due to their long half-lives, the true terminal phases for Δ9-THC and its metabolites could not be accurately estimated from the 24-hour collection period. Therefore, the AUC from time zero to the last measured concentration (AUC0-t ) was determined. CAF and paraxanthine (PXN) were detected in 12 participants at baseline. The concentrations of these two analytes in the subsequent plasma samples were corrected by subtracting the calculated decayed concentrations of the residual analyte using the terminal half-life (t1/2) observed in the respective individual. Two participants were deemed poor CYP2D6 metabolizers based on their phenotyping data; their DXM plasma AUC0-∞ and DXM (DOR)/DXM AUC ratio was 5,120% and 4,350% and 2% and 5%, respectively, of the mean values of the remaining subjects. Accordingly, they were excluded from further analyses. The t1/2 of the probe drugs, PXN, Δ9-THC, and Δ9-THC metabolites was estimated using at least the last 3 terminal data points. The formation clearance (CLf) of all probe drug metabolites or Δ9-THC metabolites was estimated as the ratio of the cumulative molar amount of the metabolite (including any sequential terminal glucuronide metabolites) excreted into urine from 0-t to parent plasma AUC0-t. This calculation assumes that over 24 hours, most (if not all) of the metabolite (including sequential terminal glucuronide metabolite) formed in the body is recovered in the urine (i.e., minimal non-renal excretion).
Power and statistical analyses.
Assuming 25% intra-individual variability in LOS AUC, a power analysis yielded a sample size of 18 based on 84% power, with a type I error of 0.05, to detect a 25% change in the geometric mean ratio (GMR) of LOS AUC (AUCGMR) in the presence vs. absence of CBD+Δ9-THC or Δ9-THC.22 PK end points were evaluated using a one-way analysis of variance with Dunnett’s multiple comparison test or a paired Student’s t-test (see footnotes to the tables for the statistical test used). If there was a P value < 0.05, the effect of treatment (CBD + Δ9-THC or Δ9-THC arm) on PK end points was considered significant vs. placebo or the Δ9-THC arm (for impact of CBD on the PK of Δ9-THC).
PBPK modeling and simulation.
We used our previously validated CBD PBPK model21 along with PBPK models for CAF, DXM, MDZ, and OMP available in the Simcyp library (Simcyp version 21) to predict the various CBD-drug interactions. Because the library did not contain a model for LOS, a previously reported model23 was used with minor modifications. Based on human liver microsomal studies24 and an in vitro intrinsic clearance of 18.1 μL/min/mg,23 the fraction metabolized (fm) for CYP2C9-mediated losartan carboxylic acid (LOS-COOH) formation was estimated to be 0.42. The fm for additional CYP2C9-mediated and CYP3A4-mediated pathways were estimated to be 0.38 and 0.20, respectively, based on the 80% contribution of CYP2C9 to LOS metabolism.24 As is customary, the models were considered validated if the predicted AUC and Cmax of the probe drug were within 0.5 to 2-fold of the observed mean values.
PBPK model simulations were conducted to predict the magnitude (AUCGMR) of CYP-mediated CBD-drug interactions after oral administration of a single dose of the CYP probe with a single oral dose of CBD (640 mg). Justification for excluding 20 mg Δ9-THC is provided in the discussion. Model predictive performance was evaluated by comparing the model-predicted and observed AUCGMR of CBD-drug interactions after a single oral dose of CBD (640 mg). The model was considered validated if the ratio of predicted to observed AUCGMR was within 0.5 to 2.0-fold. The effects of multiple dose administrations of oral CBD (640 mg twice daily for 7 days, i.e., to mimic CBD steady-state plasma concentration) on probe drug AUC and Cmax were next evaluated. The number of participants, their age, sex distribution, CBD dose, CYP probe dose, and prandial state in the simulations matched those in the clinical study.
RESULTS
Participants and safety
Eighteen healthy adults (11 men and 7 women) completed the study. Their age, weight, and body mass index (mean ± SD) were 30 ± 7 years, 78 ± 12 kg, and 25 ± 2 kg/m2, respectively. Twelve participants self-identified as White and non-Hispanic, three as Black and non-Hispanic, and three as Asian and non-Hispanic. At enrollment, subjects reported a median of 93 days since last cannabis use (range: 39–295 days). None of the participants reported taking medications known to alter the metabolism of the CYP probe drugs. The brownies and study drugs were well-tolerated; no participant experienced adverse effects other than those expected from Δ9-THC, and no serious adverse events occurred.
Effects of the CBD + Δ9-THC or Δ9-THC brownie on CYP probe drug PK
Unless indicated otherwise, the Δ9-THC brownie had no effects on the PKs of any of the probe drugs or their metabolites (Figures 1–5, Table S3). Compared with the placebo brownie, the CBD + Δ9-THC brownie significantly increased the AUCGMR and Cmax,GMR of LOS by 63–77% (Figure 1b,c, Table S3), decreased the LOS-COOH/LOS AUCGMR by 30% (Figure 1c), and had no effect on LOS t1/2. The CBD + Δ9-THC brownie increased OMP AUCGMR and Cmax,GMR by 207% and 81%, respectively (Figure 2b,c, Table S3), decreased the 5-hydroxy OMP (5-OH OMP)/OMP AUCGMR by 50% (Figure 2c, Table S3), and had no effect on OMP t1/2 (Table S3). The CBD + Δ9-THC brownie modestly increased MDZ AUCGMR and Cmax,GMR by 56% and 28%, respectively (Figure 3b,c, Table S3), and had no effect on MDZ t1/2 (Table S3). The CBD + Δ9-THC brownie increased the AUCGMR of 1′hydroxy MDZ (1′-OH MDZ)/MDZ by 72% and decreased 1′-OH MDZ CLf,GMR and MDZ 1′-O -glucuronide CLf,GMR by 53% and 74%, respectively (Figure 3c,d, Table S3). The CBD + Δ9-THC brownie modestly increased the AUCGMR and t1/2 of CAF by 32–39% and had no effect on Cmax,GMR (Figure 4b,c, Table S3) and PXN/CAF AUCGMR (Figure 4c). The CBD + Δ9-THC brownie had no effect on DXM AUCGMR, Cmax,GMR, and t1/2 (Figure 5b,c, Table S3). The CBD + Δ9-THC brownie had no effect on the AUCGMR of DOR/DXM and decreased the AUCGMR of DOR glucuronide/DOR and the DOR glucuronide CLf,GMR by 44% and 55%, respectively (Figure 5c,d). The Δ9-THC brownie decreased the DOR/DXM AUCGMR by 30% (Table S3). The dose-adjusted CBD (Figure S2) and Δ9-THC (Figure 6) plasma concentrations observed in this study were consistent with those published previously.25–29
Figure 1.
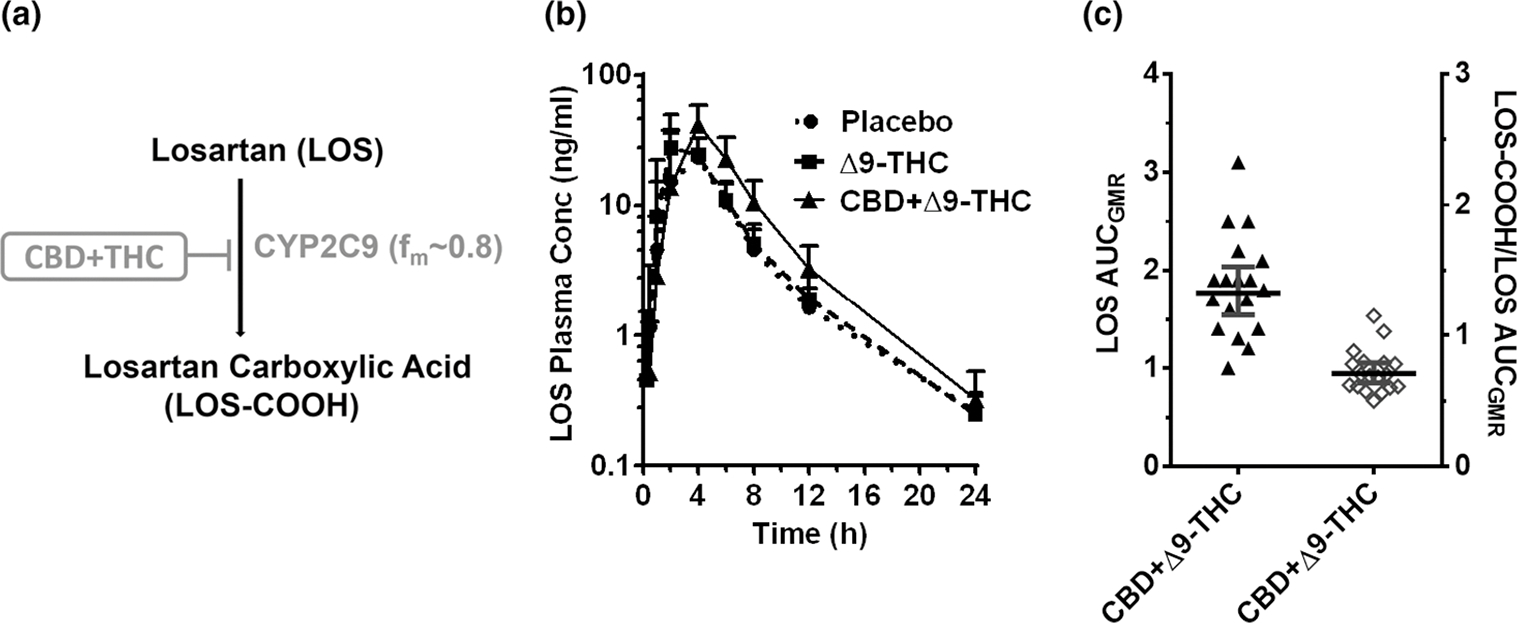
CYP2C9-mediated CBD-LOS interaction. (a) LOS biotransformation pathway inhibited by the CBD + Δ9-THC brownie. (b) LOS plasma PK profile after administration of a brownie containing Δ9-THC (20 mg; dashed line), CBD + Δ9-THC (640 mg + 20 mg; continuous line), or placebo (dotted line). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 18 participants). For depiction, nominal timepoints were used. (c) Effect of the CBD + Δ9-THC brownie on LOS PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent or metabolite/parent in the presence of the CBD + Δ9-THC brownie vs. the placebo brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis while the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CBD, cannabidiol; CI, confidence interval; fm, fraction metabolized by the indicated enzyme; LOS, losartan;
Figure 5.
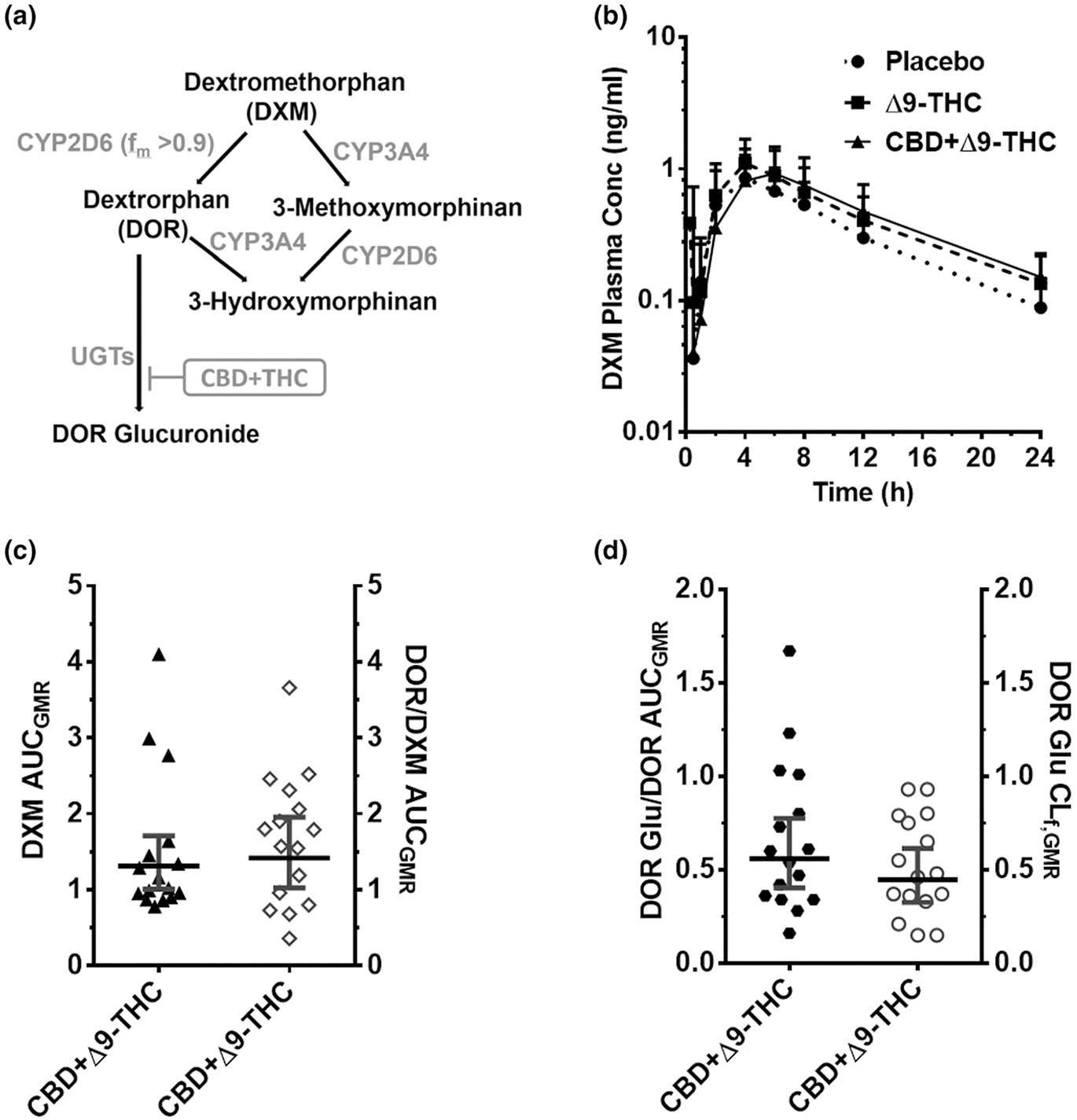
CYP2D6-mediated CBD-DXM interaction. (a) DXM biotransformation pathway. DXM is subsequently metabolized primarily to its glucuronide. (b) DXM plasma PK profile after administration of a brownie containing Δ9-THC (20 mg; dashed line), CBD + Δ9-THC (640 mg + 20 mg; continuous line), or placebo (dotted line). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 16 participants; two were poor metabolizers and therefore excluded). For depiction, nominal timepoints were used. (c, d) Effects of the CBD + Δ9-THC brownie on PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent, metabolite/parent, or metabolite CLf in the presence of the CBD + Δ9-THC brownie vs. the placebo brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis, whereas the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; CBD, cannabidiol; CI, confidence interval; CLf,GMR, formation clearance geometric mean ratio; DXM, dextromethorphan; fm, fraction metabolized by the indicated enzyme; PK, pharmacokinetic.
Figure 2.
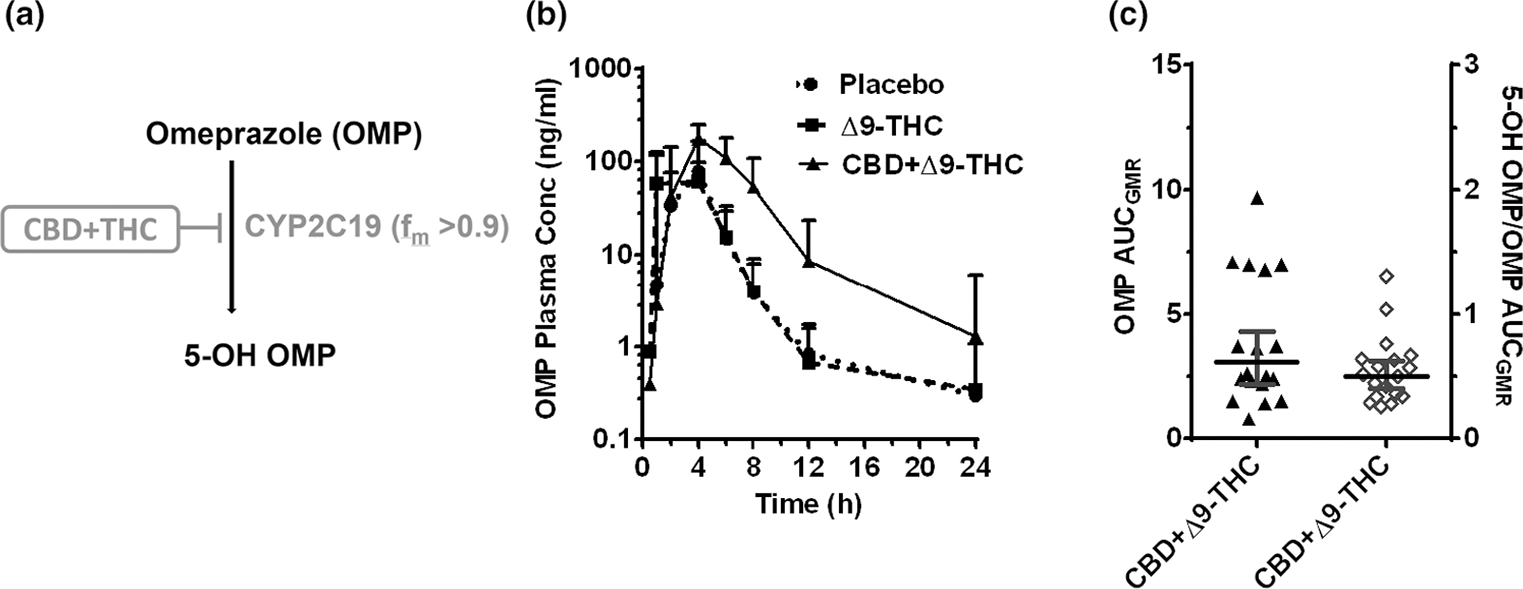
CYP2C19-mediated CBD-OMP interaction. (a) OMP biotransformation pathway inhibited by the CBD + Δ9-THC brownie. (b) OMP plasma PK profile after oral administration of a brownie containing Δ9-THC (20 mg; dashed line), CBD + Δ9-THC (640 mg + 20 mg; continuous line), or placebo (dotted line). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 18 participants). For depiction, nominal timepoints were used. (c) Effect of the CBD + Δ9-THC brownie on OMP PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent or metabolite/parent in the presence of the CBD + Δ9-THC brownie vs. the placebo brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis, whereas the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CBD, cannabidiol; CI, confidence interval; fm, fraction metabolized by the indicated enzyme; OMP, omeprazole; PK, pharmacokinetic.
Figure 3.
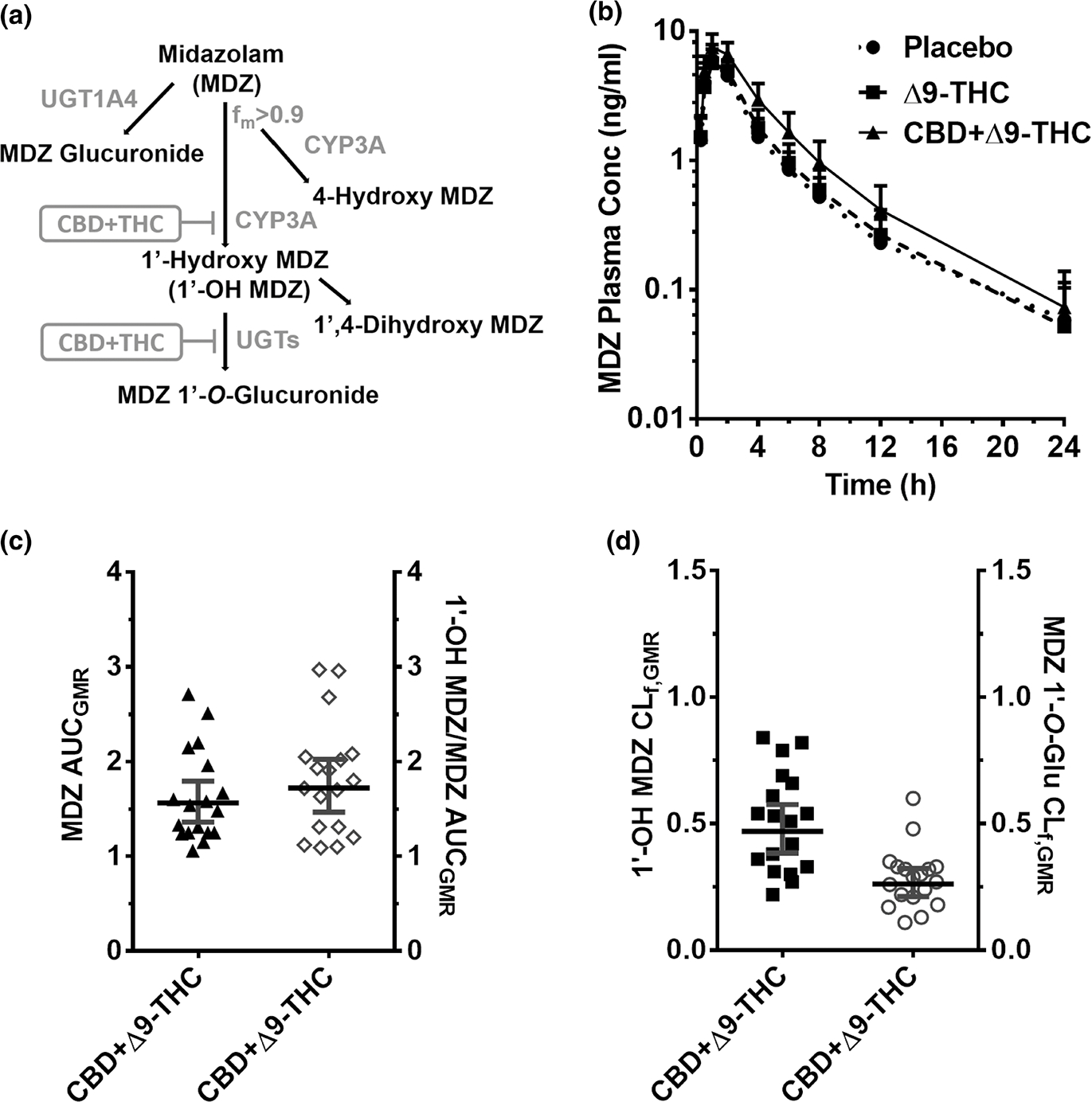
CYP3A-mediated CBD-drug interactions. (a) MDZ biotransformation pathway inhibited by the CBD + Δ9-THC brownie. 1′-OH MDZ is subsequently metabolized primarily to its glucuronide. (b) MDZ plasma PK profile after oral administration of a brownie containing Δ9-THC (20 mg; dashed line), CBD + Δ9-THC (640 mg + 20 mg; continuous line), or placebo (dotted line). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 18 participants). For depiction, nominal time points were used. (c, d) Effect of the CBD + Δ9-THC brownie on MDZ and MDZ metabolite PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent, metabolite/parent, or metabolite CLf in the presence of the CBD + Δ9-THC brownie vs. the placebo brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis, whereas the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CBD, cannabidiol; CI, confidence interval; CLf, formation clearance; fm, fraction metabolized by the indicated enzyme; MDZ, midazolam; PK, pharmacokinetic.
Figure 4.
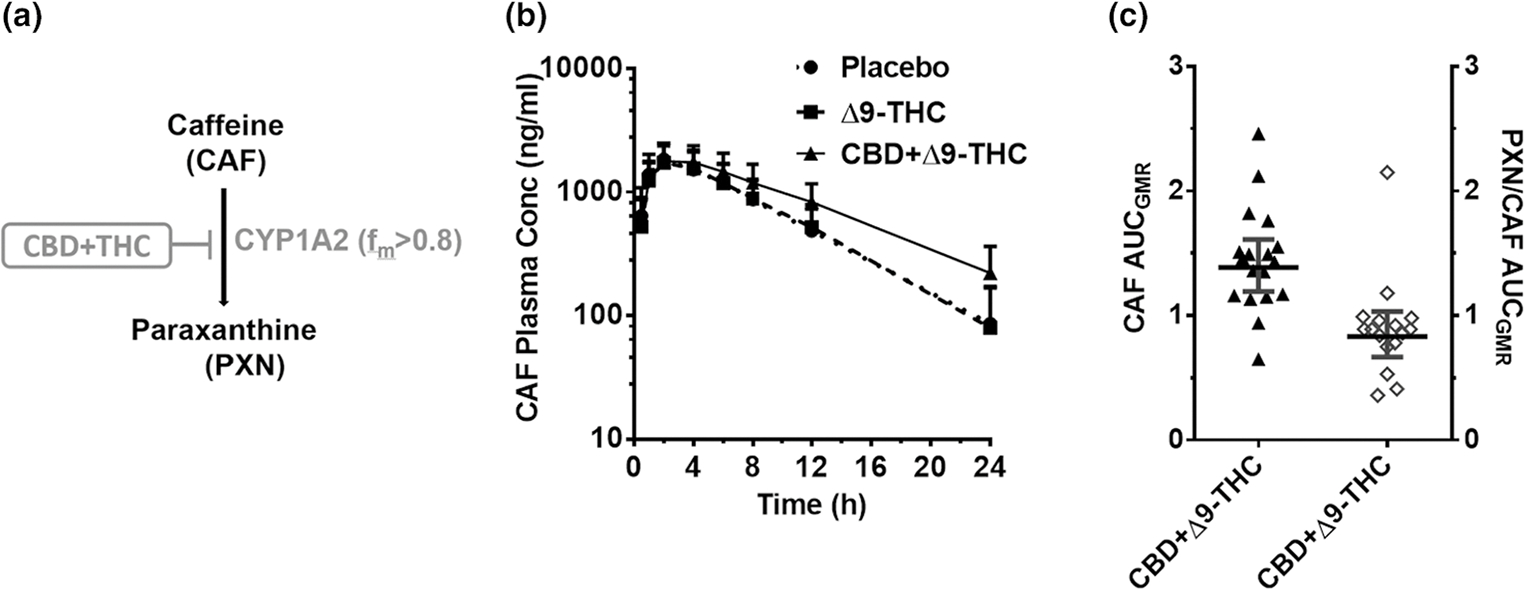
CYP1A2-mediated CBD-CAF interaction. (a) CAF biotransformation pathway inhibited by the CBD + Δ9-THC brownie. (b) CAF plasma PK profile after administration of a brownie containing Δ9-THC (20 mg; dashed line), CBD + Δ9-THC (640 mg + 20 mg; continuous line), or placebo (dotted line). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 18 participants). For depiction, nominal timepoints were used. (c) Effect of the CBD + Δ9-THC brownie on PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent or metabolite/parent in the presence of CBD + Δ9-THC brownie vs. the placebo brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis while the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CBD, cannabidiol; CAF, caffeine; CI, confidence interval; fm, fraction metabolized by the indicated enzyme; PK, pharmacokinetic.
Figure 6.
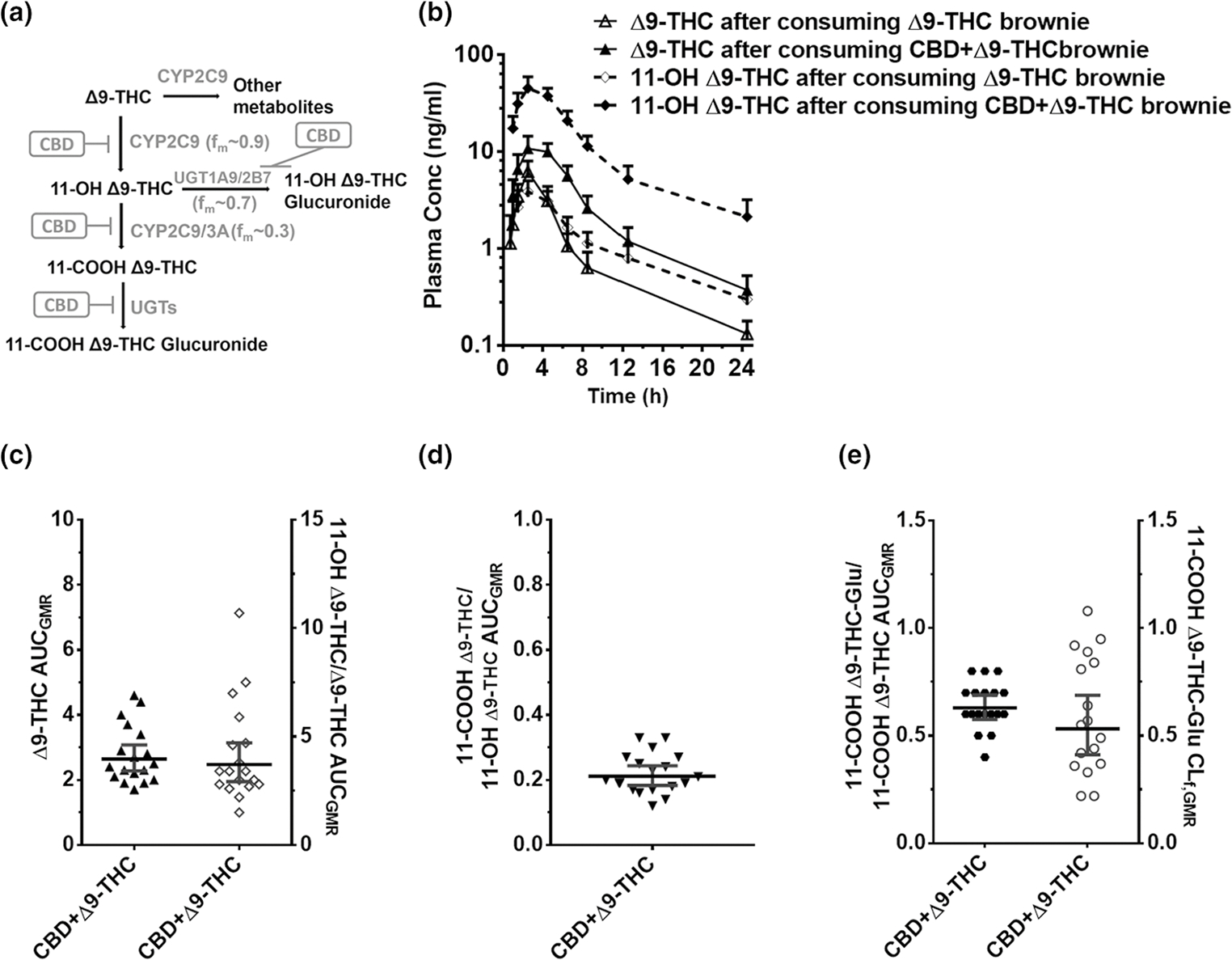
CBD-Δ9-THC PK interactions. (a) Δ9-THC biotransformation pathways inhibited by CBD. (b) Δ9-THC (continuous line) or 11-OH Δ9-THC (dashed line), plasma PK profile after administration of a brownie containing Δ9-THC (20 mg) or CBD + Δ9-THC (640 mg + 20 mg). Symbols and error bars denote geometric means and 90% CIs (shown in only one direction for visual purposes), respectively (n = 18 participants). For depiction, nominal timepoints were used. (c, d, e) Effect of the CBD + Δ9-THC brownie on Δ9-THC and Δ9-THC metabolite PK end points. Horizontal bars and vertical bars denote geometric mean ratios and 90% CIs, respectively, of the parent, metabolite/parent, or metabolite CLf in the presence of the CBD + Δ9-THC brownie vs. the Δ9-THC brownie. Symbols denote values for each participant, filled symbols refer to the left Y-axis, whereas the open symbols refer to the right Y-axis. Δ9-THC, Δ9-tetrahydrocannabinol; AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CBD, cannabidiol; CI, confidence interval; CLf,GMR, formation clearance geometric mean ratio; fm, fraction metabolized by the indicated enzyme; PK, pharmacokinetic.
Effects of the CBD + Δ9-THC brownie on Δ9-THC pharmacokinetics
Compared with the Δ9-THC brownie, the CBD + Δ9-THC brownie significantly increased Δ9-THC AUCGMR and Cmax,GMR by 161% and 92%, respectively (Figure 6b,c, Table S4). The CBD + Δ9-THC brownie increased the AUCGMR of 11-OH-Δ9-THC/Δ9-THC by 313% and decreased the 11-COOH-Δ9-THC/11-OH-Δ9-THC by 79% (Figure 6c,d, Table S4). The CBD + Δ9-THC brownie significantly decreased the 11-COOH-Δ9-THC glucuronide/11-COOH Δ9-THC AUCGMR and 11-COOH Δ9-THC glucuronide CLf,GMR by ~ 40–50% (Figure 6e, Table S4).
PBPK modeling and simulation.
The ratio of the PBPK model-predicted to observed (P/O) AUCGMR for the CYP2C19-, CYP2C9-, CYP3A-, and CYP2D6-mediated interactions after a single oral dose of CBD (640 mg) was within the range for validation (0.5–2.0; Figure 7a, Table S4). The model slightly overpredicted the CYP1A2-mediated CBD-CAF interaction (P/O AUCGMR 2.2; Figure 7a, Table S4). Following chronic oral CBD administration, the AUCGMR for the CYP2C19-, CYP3A-, and CYP1A2-mediated interactions were predicted to increase (vs. single dose) further by 250%, 290%, and 80%, respectively (Figure 7b). In contrast, chronic CBD administration was predicted not to further increase the magnitude of the CYP2C9- and CYP2D6-mediated interactions (Figure 7b). Because consumers often take CBD at lower doses, the model predicted CBD at 20 mg (twice daily for 7 days) to interact (AUCGMR > 1.25) with drugs metabolized by CYP2C19, CYP3A, and CYP1A2 (Figure 7b). The lowest oral CBD dose predicted to be devoid of interactions with the CYP probe drugs (AUCGMR < 1.25) was 5 mg twice daily.
Figure 7.
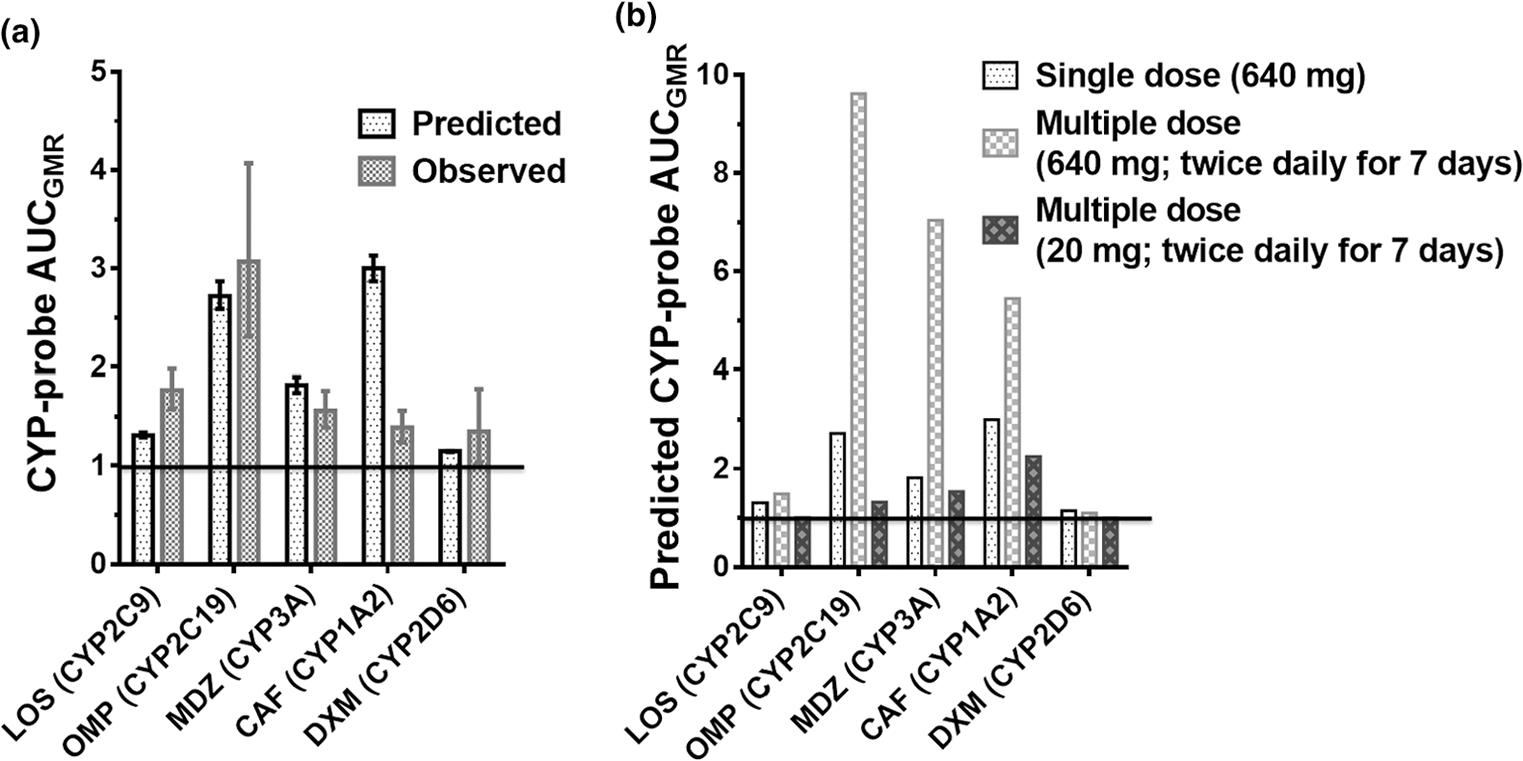
Physiologically-based pharmacokinetic model-predicted and observed magnitude (AUCGMR) of CYP-mediated CBD-drug interactions after oral administration of single dose of the CYP probe with (a) a single oral dose of CBD (640 mg) or (b) on day 7 (steady-state) following multiple oral doses of CBD (640 mg or 20 mg) twice daily. Error bars denote 90% confidence intervals. AUCGMR, area under the plasma concentration-time curve geometric mean ratio; CAF, caffeine; CBD, cannabidiol; DXM, dextromethorphan; GMR, geometric mean ratio; LOS, losartan; MDZ, midazolam; OMP, omeprazole.
DISCUSSION
Prior to initiating the clinical study, both the Δ9-THC-dominant and CBD-dominant extracts were tested for CYP inhibition (including time-dependent inhibition (TDI)) using human liver microsomes. The extracts showed a similar extent of reversible and/or TDI of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A activity at the equivalent molar concentration of pure CBD or Δ9-THC, suggesting that other phytoconstituents in these extracts do not contribute to CYP inhibition (data not shown). Accordingly, all the interactions observed in the current study were interpreted to be precipitated by either CBD + Δ9-THC or Δ9-THC alone.
Of the two brownies containing a cannabis extract, the CBD + Δ9-THC brownie precipitated interactions with all the CYP probes, except DXM (CYP2D6). The order of magnitude of the interaction was CYP2C19 > CYP2C9 > CYP3A > CYP1A 2, with the AUCGMR ranging from ~ 3.1 to ~ 1.4 (Table S3). The Δ9-THC brownie did not interact with any of the CYP probes. All these interactions resulted in modest to no change in t½, suggesting that the interactions reflected mostly inhibition by CBD of first-pass metabolism of the probe drugs when the intestinal and hepatic inlet concentrations of CBD and the probe drugs are expected to be high.13 The lack of interactions in the presence of the Δ9-THC brownie suggested that interactions precipitated by the CBD + Δ9-THC brownie were due to CBD alone. The Δ9-THC PBPK model predictions (discussed below) further support this conclusion.
Despite significant inhibition of MDZ oral clearance, the AUCGMR of the primary metabolite, 1′-OH MDZ, or that of 1′-OH MDZ/MDZ, did not decrease as expected (Figure 3, Table S3). Instead, the AUCGMR of 1′-OH MDZ/MDZ increased and CLf of MDZ 1′-O-glucuronide decreased (Table S3), indicating CBD inhibited the sequential UGT-mediated (UGT2B4 and/or UGT2B7)30,31 metabolism of 1′-OH MDZ. Similarly, CBD inhibited the metabolism of DOR to its glucuronide, mediated by UGT2B4, 2B7, 2B15, and/or 2B1732 and possibly to 3-hydroxymorphinan (CYP3A), resulting in an increased AUCGMR of DOR/DEX and reduced DOR-glucuronide CLf (Figure 5, Table S3). The in vitro inhibition of recombinant UGTs (UGTA1, 1A3, 1A4, 1A6, 1A9, 2B4, 2B7, 2B10, 2B15, and 2B17) by CBD-dominant extract (data not shown) supports these results. Moreover, these results are consistent with CBD being a potent in vitro inhibitor of UGT2B4, UGT2B7, and UGT2B15 (half-maximal inhibitory concentration (IC50,u) < 0.6 μM).12
Except for the interaction with MDZ, literature reports support our conclusions. Case studies have reported an increase in INR when oral CBD (titrated to 40 mg/kg/day) or cannabis was co-consumed with warfarin, suggesting inhibition of CYP2C9-mediated metabolism of warfarin.8–10 In addition, oral CBD (750 mg twice daily for 14 days) inhibited the CYP2C19-mediated metabolism of N-desmethylclobazam, resulting in a 240% increase in plasma N-desmethylclobazam AUC.33 Chronic CBD administration (750 mg twice daily for 16 days) also inhibited CYP1A2-mediated CAF metabolism, as evidenced by a near doubling of CAF plasma AUC and t½.34 In contrast to our results, chronic CBD administration (750 mg twice daily for 15 days) had no effect on MDZ plasma AUC, possibly due to simultaneous CYP3A induction. However, consistent with our data, a 100% increase in 1′-OH MDZ plasma AUC was observed.35 There are no literature reports of UGT-based in vivo drug interactions with CBD; the data presented here are the first reports of such potential interactions.
Because Δ9-THC is metabolized in the liver primarily by CYP2C9 (fm ~ 0.9), Δ9-THC could possibly serve as a CYP2C9 probe. Unlike LOS, Δ9-THC likely can report first-pass interactions with respect to hepatic and intestinal CYP2C9 inhibition due to its high hepatic extraction ratio (> 0.9)36 and CYP2C9-mediated metabolism by human intestinal microsomes.37 Consistent with this hypothesis and the higher fm,CYP2C9 compared with LOS (~ 0.8),24 the Δ9-THC AUCGMR in the presence of CBD was higher than that of LOS (2.6 vs. 1.8; Figure 6 vs. Figure 1, Tables S3, S4). Like the CBD-LOS interaction, CBD had modest to no effect on the observed Δ9-THC t1/2, suggesting that the CBD-Δ9-THC interaction also occurred primarily during first pass. Moreover, the percent increase in Δ9-THC AUC by CBD correlated well with the percent decrease in LOS-COOH/LOS AUC (r = 0.93; data not shown), supporting the premise that Δ9-THC could serve as a CYP2C9 probe. In contrast to our observations at the higher CBD dose (640 mg), but consistent with our PBPK modeling results (discussed below), a lower oral dose of CBD (5.4 mg) had no effect on Δ9-THC PK.38 In addition, as expected, because the intravenous clearance of Δ9-THC is hepatic blood-flow limited,36 oral CBD (1,500 mg) had no effect on intravenous Δ9-THC PK.39
Contrary to expectation, inhibition of 11-OH Δ9-THC formation by CBD did not result in a reduction in AUCGMR of 11-OH Δ9-THC/Δ9-THC (Table S4). Instead, the 11-OH Δ9-THC/Δ9-THC AUCGMR increased and the 11-COOH Δ9-THC/11-OH Δ9-THC AUCGMR decreased, indicating that CBD inhibited metabolism of 11-OH Δ9-THC to 11-COOH Δ9-THC. The reduction in CLf of 11-COOH Δ9-THC supports this observation (Table S4). Consistent with the increase in AUCGMR of Δ9-THC and 11-OH Δ9-THC by CBD, the combined PD effects of these phytocannabinoids also increased.22
Our CBD PBPK model21 successfully predicted the magnitude of the interaction, or lack thereof, among CBD and OMP, LOS, MDZ, and DXM (Figure 7, Table S5). The predicted interactions among CBD and OMP, MDZ, or DXM also met the more stringent bioequivalence criterion. In contrast, the model overpredicted the CBD-CAF interaction assuming TDI of CYP1A2 (P/O AUCGMR = 2.2). The reason for this overprediction is not known but could be due to overprediction of the in vitro CBD-CYP1A2 interaction based on inhibition of phenacetin O-deethylation in human liver microsomes. As expected, all of these CYP-mediated interactions were overpredicted by the mechanistic static model, especially those involving TDI.11,13 This and related models predict worse-case scenarios as they do not take into consideration time-dependent changes in inhibitor concentrations in vivo.40
Because CBD is usually taken chronically and is a time-dependent inhibitor of CYP2C19, CYP3A, and CYP1A2,11 CBD-probe drug interactions after multiple CBD dosing were predicted using our CBD PBPK model. The interaction between CBD and OMP (CYP2C19), MDZ (CYP3A), and CAF (CYP1A2) vs. single dose CBD increased by 250%, 290%, and 80%, respectively (Figure 7). In contrast, because CBD does not accumulate significantly upon chronic administration,21,41 the magnitude of a reversible interaction with the CYP2C9 and CYP2D6 probes did not increase. The PBPK model further predicted that the CBD dose would have to be as low as 5 mg twice daily to avoid any interaction with the CYP probes.
To confirm that the 161% increase in Δ9-THC plasma AUC in the presence of the CBD + Δ9-THC brownie did not contribute to the observed CBD + Δ9-THC brownie-CYP interactions, a preliminary oral Δ9-THC PBPK model was developed based on our previous intravenous/inhalation Δ9-THC model.36 Using our in vitro inhibition data13 and this model, we confirmed that increasing the oral Δ9-THC dose by 200–300% (> 161% increase in Δ9-THC AUCGMR observed in the current study) did not predict any interactions with the CYP probes (data not shown). Moreover, the potential and magnitude of inhaled Δ9-THC to produce drug interactions can be predicted using our previously developed inhalation Δ9-THC PBPK model.36
Although the study used rigorous methods, there are limitations and areas of future research that warrant mention. First, the CYP probe drug-CBD interactions were evaluated after a single dose of CBD + Δ9-THC; interactions related to chronic CBD administration were predicted using PBPK models. A CBD multiple-dose study is needed to confirm these predictions because CBD may induce CYPs, particularly CYP3A.35 Second, although CBD-mediated inhibition of UGTs was observed, our data cannot deduce the magnitude of inhibition of each UGT isoform because multiple UGTs are involved in the metabolism of the probe drug metabolites. A standalone in vivo study using UGT probes is warranted to corroborate these findings. Third, other enzymes that were inhibited by CBD in vitro (e.g., CYP2A6 and CYP2B6),12,13,42 and transporters involved in drug disposition need to be evaluated with respect to CBD or Δ9-THC interactions in vivo. Fourth, the cannabis extracts used for the current study were administered 30 minutes prior to the probe drug cocktail. A replicated study in which the cocktail is administered simultaneously with the extracts is needed to assess the magnitude of the interactions change relative to administration time. Fifth, our study was based on NIDA-supplied plant extracts, and we demonstrated, in vitro, that other phytoconstituents do not contribute to the interaction produced by CBD or Δ9-THC. Because most (if not all) cannabis produced widely available over the counter are also plant-based, we speculate that our findings apply to these products as well. However, it is possible that these products may contain phytoconstituents that differ from our extracts. Therefore, we cannot rule out the possibility that these different phytoconstituents (if any) may contribute to drug interactions. Finally, the magnitudes of interactions would be higher or lower depending on the contribution of a given metabolic pathway (fm). For example, fm for the CYP2C9-mediated metabolism of Δ9-THC is greater than that for LOS (0.9 vs. 0.8), suggesting that Δ9-THC would be more sensitive than LOS to an interaction with CBD.
In summary, a single dose of a CBD-dominant cannabis extract Δ9-THC (640 mg CBD + 20 mg Δ9-THC in a brownie) significantly inhibited the activity of all CYPs tested except CYP2D6, whereas a single dose of a Δ9-THC-dominant cannabis extract containing the same Δ9-THC dose (20 mg Δ9-THC in a brownie) had no effect. As to whether the magnitude of CYP inhibition by CBD will necessitate a change in the dose of a drug co-administered with CBD will need to be evaluated on a case-by-case basis based on the following factors: (i) the therapeutic window of the victim drug. For example, a twofold increase in the plasma AUC of the victim drug caused by CBD will likely warrant a change in its dose only if it is a narrow therapeutic window drug; (ii) the fm of the victim drug via the affected CYP. The greater the fm via a CYP inhibited by CBD, the greater will be the increase in the plasma AUC of the victim drug resulting in higher likelihood of a clinically significant interaction; and (iii) the dose of CBD consumed and whether it is a single dose or multiple doses. All these factors can be modeled via our PBPK model. Indeed, except for CYP1A2, our oral CBD PBPK model successfully predicted the magnitude of CBD-CYP probe drug interactions. CBD increased the AUC0-t of Δ9-THC and the active metabolite 11-OH Δ9-THC, resulting in enhanced pharmacological effects of Δ9-THC.22 After completing our Δ9-THC PBPK model (in progress), the CBD and Δ9-THC models can be applied to predict CBD-Δ9-THC interactions, as well as CYP- or UGT-mediated CBD and/or Δ9-THC interactions for different combinations of Δ9-THC and CBD present in cannabis products. These in vivo and PBPK modeling results can help guide dose adjustment (or alternative therapy) of pharmaceutical drugs co-consumed with cannabis products and the content of CBD in cannabis products to avoid or minimize the potential for CBD-Δ9-THC interactions.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Based on in vitro studies with human liver microsomes, oral cannabidiol (CBD) and Δ-9-tetrahydrocannabinol (Δ9-THC) were both predicted to inhibit CYP1A2, CYP2C9, and CYP3A, in vivo in a time-dependent or reversible manner. CBD was also predicted to inhibit CYP2C19 and CYP2D6.
WHAT QUESTION DID THIS STUDY ADDRESS?
Do brownies containing a CBD-dominant (640mg CBD+20mg Δ9-THC) or Δ9-THC dominant (20mg Δ9-THC, no CBD) cannabis plant extract precipitate the above-predicted CYP-mediated drug interactions in healthy adult participants?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The CBD+ Δ9-THC brownie inhibited the activity of all CYPs tested except CYP2D6 (CYP2C19 > 2C9 > 3A> 1A2), whereas the Δ9-THC brownie did not inhibit any of the CYPs. The CBD+ Δ9-THC brownie also inhibited CYP2C9-mediated oral Δ9-THC clearance. Except for CYP1A2, an oral CBD physiologically-based pharmacokinetic model quantitatively predicted the observed CYP-mediated interactions.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These results can guide dose adjustment (or alternative therapy) of pharmaceutical drugs co-consumed with cannabis products, as well as the CBD content in cannabis products to prevent or minimize CBD-Δ9-THC interaction risk.
ACKNOWLEDGMENTS
The authors thank the staff at the Johns Hopkins Behavioral Pharmacology Research Unit for study support, the NIDA Drug Supply Program for providing cannabis extracts for this research, and ElSohly Laboratories for analyzing the finished drug products, and Ms. Deena Hadi at Washington State University for her help with administrative aspects of the study.
FUNDING
This work was supported by the National Institutes of Health National Center for Complementary and Integrative Health (grant U54 AT008909; M.F.P.) and in part by the National Institute on Drug Abuse (grant P01 DA032507; J.D.U. and grant T32DA07209; C.A.Z.).
Footnotes
CONFLICT OF INTEREST
T.S. has served as a consultant to Canopy Health Innovations. R.V. has received compensation as a consultant or advisory board member from Canopy Health Innovations, Mira1a Therapeutics Inc., MyMD Pharmaceuticals, Syqe Medical Ltd., Jazz Pharmaceuticals, WebMD, and Radicle Science Inc. E.M.W. has contract funding from Mira1a Therapeutics Inc., and MyMD Pharmaceuticals, and support as a co-I on contract with Canopy Health Innovations. M.P. is a member of the Scientific Advisory Board of Simcyp, Certara. All other authors declared no competing interests for this work.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References
- 1.Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. <https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf> (2020). Accessed 04 December 2022.
- 2.Epidiolex® Package Insert. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf> (2018). Accessed December 04, 2022.
- 3.Marinol® Package Insert. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf> (2017). Accessed December 04, 2022.
- 4.Mechoulam R & Gaoni Y The absolute configuration of δ1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 8, 1109–1111 (1967). [DOI] [PubMed] [Google Scholar]
- 5.Lemberger L, Martz R, Rodda B, Forney R & Rowe H Comparative pharmacology of Delta9-tetrahydrocannabinol and its metabolite, 11-OH-Delta9-tetrahydrocannabinol. J. Clin. Invest. 52, 2411–2417 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spindle TR et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 211, 107937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoedel KA et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-b lind, controlled trial. Epilepsy Behav. 88, 162–171 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Grayson L, Vines B, Nichol K, Szaflarski JP & UAB CBD Program An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav. Case Rep. 9, 10–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortopassi J Warfarin dose adjustment required after cannabidiol initiation and titration. Am. J. Health Syst. Pharm. 77, 1846–1 851 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Damkier P, Lassen D, Christensen MMH, Madsen KG, Hellfritzsch M & Pottegård A Interaction between warfarin and cannabis. Basic Clin. Pharmacol. Toxicol. 124, 28–31 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Bansal S, Maharao N, Paine MF & Unadkat JD Predicting the potential for cannabinoids to precipitate pharmacokinetic drug interactions via reversible inhibition or inactivation of major cytochromes P450. Drug Metab. Dispos. 48, 1008–1017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal S, Paine M & Unadkat J Can cannabinoids precipitate UGT-mediated drug interactions? FASEB J. 35, 1 (2021). [Google Scholar]
- 13.Bansal S, Paine MF & Unadkat JD Comprehensive predictions of cytochrome P450 (P450)-mediated In vivo cannabinoid-drug interactions based on reversible and time-dependent P450 inhibition in human liver microsomes. Drug Metab. Dispos. 50, 351–360 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox EJ et al. A marijuana-drug interaction primer: precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 201, 25–38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaori S, Kushihara M, Yamamoto I & Watanabe K Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem. Pharmacol. 79, 1691–1698 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Yamaori S, Okamoto Y, Yamamoto I & Watanabe K Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab. Dispos. 39, 2049–2056 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Arnold WR, Weigle AT & Das A Cross-talk of cannabinoid and endocannabinoid metabolism is mediated via human cardiac CYP2J2. J. Inorg. Biochem. 184, 88–99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasrin S, Watson CJW, Perez-Paramo YX & Lazarus P Cannabinoid metabolites as inhibitors of major hepatic CYP450 enzymes, with implications for cannabis-drug interactions. Drug Metab. Dispos. 49, 1070–1080 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasrin S, Watson CJW, Bardhi K, Fort G, Chen G & Lazarus P Inhibition of UDP-glucuronosyltransferase enzymes by major cannabinoids and their metabolites. Drug Metab. Dispos. 49, 1081–1089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu JY et al. Development of the ‘Inje cocktail’ for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin. Pharmacol. Ther. 82, 531–540 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Bansal S, Ladumor MK, Paine MF & Unadkat JD A physiologically-based pharmacokinetic model for cannabidiol in healthy adults, hepatically-impaired adults, and children. Drug Metab. Dispos. 51, 743–752 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamarripa CA et al. Assessment of orally administered Δ9-tetrahydrocannabinol when Coadministered with cannabidiol on Δ9-tetrahydrocannabinol pharmacokinetics and pharmacodynamics in healthy adults: a randomized clinical trial. JAMA Netw. Open 6, e2254752 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen HQ, Lin J, Kimoto E, Callegari E, Tse S & Obach RS Prediction of losartan-active carboxylic acid metabolite exposure following losartan administration using static and physiologically based pharmacokinetic models. J. Pharm. Sci. 106, 2758–2770 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Sica DA, Gehr TWB & Ghosh S Clinical pharmacokinetics of losartan. Clin. Pharmacokinet. 44, 797–814 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lunn S et al. Human pharmacokinetic parameters of orally administered Δ9-tetrahydrocannabinol capsules are altered by fed versus fasted conditions and sex differences. Cannabis Cannabinoid Res. 4, 255–264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A & Agurell S Do plasma concentrations of Δ9-tetrahydrocannabinol reflect the degree of intoxication? J. Clin. Pharmacol. 21, 171S–177S (1981). [DOI] [PubMed] [Google Scholar]
- 27.Nadulski T et al. Simultaneous and sensitive analysis of THC, 11-OH-THC, THC-C OOH, CBD, and CBN by GC-MS in plasma after Oral application of small doses of THC and cannabis extract. J. Anal. Toxicol. 29, 782–789 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Taylor L, Crockett J, Tayo B & Morrison G A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J. Clin. Pharmacol. 59, 1110–1119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayo B, Taylor L, Sahebkar F & Morrison G A phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin. Pharmacokinet. 59, 747–755 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B, Bush D, Doss GA, Vincent S, Franklin RB & Xu S Characterization of 1′-hydroxymidazolam glucuronidation in human liver microsomes. Drug Metab. Dispos. 36, 331–338 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Seo K-A, Bae SK, Choi YK, Choi CS, Liu KH & Shin JG Metabolism of 1′- and 4-hydroxymidazolam by glucuronide conjugation is largely mediated by UDP-glucuronosyltransferases 1A4, 2B4, and 2B7. Drug Metab. Dispos. 38, 2007–2013 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Lutz JD & Isoherranen N Prediction of relative in vivo metabolite exposure from in vitro data using two model drugs: dextromethorphan and omeprazole. Drug Metab. Dispos. 40, 159–1 68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison G, Crockett J, Blakey G & Sommerville K A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, Stiripentol, or valproate and cannabidiol in healthy subjects. Clin. Pharmacol. Drug Dev. 8, 1009–1031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thai C, Tayo B & Critchley D A phase 1 open-l abel, fixed-sequence pharmacokinetic drug interaction trial to investigate the effect of cannabidiol on the CYP1A2 probe caffeine in healthy subjects. Clin. Pharmacol. Drug Dev. 10, 1279–1289 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epidiolex® New Drug Application 210365Orig1s000-Clinical Pharmacology and Biopharmaceutics Review (s). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000ClinPharmR.pdf> (2018). Accessed December 04, 2022.
- 36.Patilea-Vrana GI & Unadkat JD Development and verification of a linked Δ 9-THC/11-OH-THC physiologically based pharmacokinetic model in healthy, nonpregnant population and extrapolation to pregnant women. Drug Metab. Dispos. 49, 509–520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar AR, Patilea-V rana GI, Anoshchenko O & Unadkat JD Characterizing and quantifying extrahepatic metabolism of (−)-Δ9-tetrahydrocannabinol (THC) and its psychoactive metabolite, (±)-11-hydroxy-Δ9-THC (11-OH-THC). Drug Metab. Dispos. 50, 734–7 40 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadulski T et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther. Drug Monit. 27, 799–810 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Hunt CA, Jones RT, Herning RI & Bachman J Evidence that cannabidiol does not significantly alter the pharmacokinetics of tetrahydrocannabinol in man. J. Pharmacokinet. Biopharm. 9, 245–260 (1981). [DOI] [PubMed] [Google Scholar]
- 40.Filppula AM, Parvizi R, Mateus A, Baranczewski P & Artursson P Improved predictions of time-d ependent drug-drug interactions by determination of cytosolic drug concentrations. Sci. Rep. 9, 5850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devinsky O et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90, e1204–e1211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasrin S, Coates S, Bardhi K, Watson C, Muscat JE & Lazarus P Inhibition of nicotine metabolism by cannabidiol (CBD) and 7-Hydroxycannabidiol (7-OH-CBD). Chem. Res. Toxicol. 36, 177–187 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


