Abstract
The aim of this study was to evaluate the effect of five varieties on the quality of herbaceous peony tea by physicochemical analysis, sensory evaluation, antimicrobial capacity analysis and a combination of gas chromatography with quadruple time of flight mass spectrometry (GC-QTOF). Antibacterial and antioxidant analyses revealed that the ABTS free radical scavenging rate of HPT was high, ranging from 82.20% to 87.40% overall. ‘Madame Claude Tain’ had the strongest inhibitory ability against Staphylococcus aureus with an inhibitory effect of 12.65 mm. The sensory evaluation showed that ‘Angel cheeks’ had the highest overall sensory score. GC-QTOF combined with orthogonal projections to latent structures discriminant analysis showed that 22 volatile components were the key aroma components of herbaceous peony tea. Different varieties of herbaceous peony tea had a unique characteristic aroma. ‘Angel cheeks’ imparted lily-like and chestnut fragrances, which were attributed to linalool and 3,5-octadien-2-one. ‘Sea Shell’, ‘Mother's Choice’ and ‘Angel Cheek’ had a medicinal aroma, which may be due to the presence of o-cymene. Overall, ‘Angel cheeks’ was the most suitable for developing high-quality herbaceous peony tea in five varieties. This study provided a theoretical basis and technical guidance for the development of herbaceous peony.
The aim of this study was to evaluate the effect of five varieties on the quality of herbaceous peony tea.
1. Introduction
Herbaceous peony (Paeonia lactiflora pall) is renowned as one of the famous traditional flowers of China with excellent fragrance and color. Herbaceous peony can be planted on an area of 100 000 hectares.1–3 At present, the herbaceous peony industry is mainly ornamental and cut flowers, the single ornamental use is easy to waste. Making flower petals into scented tea is more conducive to storage and transportation, and adds value. With the rise of new style tea beverages, peony flower tea can be used as a high quality raw material to increase the characteristic flavor of tea beverages. In addition, the petals of herbaceous peony are rich in terpenoids, flavonoids and other nutrients, which have antibacterial and anticancer effects.4,5 It is a good way to extend the industrial chain and increase the income through the processing and utilization of flower petals. The technologies for peony tea mainly include direct hot air drying, blanching and scenting.3,4 Although the direct drying process is simple in operation, it greatly destroys the nutrients and aromatic substances of peony tea, and the quality is difficult to guarantee.6,7 Scented tea only uses tea leaves to absorb the fragrance of the petals, and then discard the petals, resulting in a serious waste of materials. Compared with traditional green tea and black tea, the tea directly dried after picking the petals has weak taste and weak commodity competitiveness. Therefore, the development of new herbaceous peony tea processing technology and new products is imminent.
More than 500 varieties of herbaceous peony have been cultivated around the world.2 Varieties affect functional composition and flavor of peony.8 Polyphenols are the most important components to affect the quality of tea. Tea polyphenols are oxidized to form polymerization products, such as theaflavin and theobromine, which are mainly responsible for the color and flavor of tea.9 Studies have shown that the decrease of polyphenols can cause the tea taste to become lighter and the flavor to decrease significantly. Flavonoids and their glycosides are the most abundant polyphenols.10 Flavonoids in tea are also important components that influence the flavor of tea, such as flavonols, can work with catechins and caffeine to affect the bitterness and astringency of tea.11 Total tea sugar is also important components that affect the flavor quality of tea. For some free monosaccharides, except to participating in taste formation, they can also promote the formation of nitrogen-containing heterocyclic aromatic substances through the Maillard reaction.12 However, the quality evaluation of herbaceous peony tea has not been reported.
Aroma is an important index to evaluate the quality of tea.13 The characteristic aroma substances of tea are mainly affected by variety, geography, processing technology and other factors.14 For example, Wuyi rock tea is known for its unique ‘rock charm and floral fragrance’, which is due to the suitable climate and typical tea tree varieties in the Wuyi Mountain region of Fujian, China.15 Advanced characteristic aroma can not only highlight the characteristics of tea, but also mark the grade of tea.14 Varietal factors significantly affected the aroma of peony. Researchers recently showed that the light color series had a stronger aroma than the dark color series, and the fragrance of ‘Yang Fei Chu Yu’ contained nerolidol, linalool and other components, while ‘Carina’ was odorless in peony.16 However, there are no relevant studies on the selection of the best varieties of herbaceous peony tea.
Therefore, different varieties of herbaceous peony petals were selected as raw materials to prepare herbaceous peony tea (HPT) according to the process of fixation, rolling and drying. The effects of different varieties on HPT were evaluated from the aspects of sensory evaluation, nutritional analysis and efficacy analysis. In addition, the aroma characteristics of HPT were analyzed by gas chromatography-quadruple time-of-flight mass spectrometry (GC-QTOF) combined with odor activity value (OAV). Finally, the most suitable processing varieties of HPT were selected. The purpose of this study is to provide theoretical basis and technical support for the comprehensive utilization of herbaceous peony and the sustainable development of tea industry.
2. Materials and methods
2.1. Chemicals
The primary chemicals used in this study included ethyl caprate (standard, Beijing Solarbio Science & Technology Co., Ltd) and dichloromethane (standard products, Merck, Darmstadt, Germany). Hexanal standard products was purchased from Shanghai yuanye Bio-Technology Co., Ltd. benzeneacetaldehyde, citronellol, nonanal, phenylethyl alcohol standard products were purchased from terpineol Tianjin Altascientific Technology Co., LTD. Potassium acetate, aluminum nitrate, and folin phenol of AR quality were purchased from Beijing Dingsheng Xingchuang Biotechnology Co., Ltd. (Beijing, China). Phenol, rutin, gallic acid and other conventional reagents were purchased from a local supplier.
2.2. HPT samples
Five varieties of herbaceous peony petals from ‘Sea Shell’, ‘Angel Cheeks’, ‘YangFeiChuYu’, ‘Mothers Choice’ and ‘Madame Claude Tain’ were picked from 7:00 am to 9:00 am on June 4, 2021 in Yanqing, Beijing, China (4027′21.92′′N, 11554′19.40′′E). Three baskets of flowers in full bloom were randomly picked for each variety, forty flowers per basket, flowers between 11 and 13 cm in diameter. Freshly picked petals were temporarily kept in 2–4 °C cold storage. The petals were processed on the following day. The fresh petals were put into a wok and heated at 80 °C for 10 min. The petals were cooled for 2 min and then rolled into a cord with a rolling machine. The thin funicular petals were dried in a hot air-drying oven at 60 °C until the petals had reached a constant weight. Finally, different varieties of HPT, including SS (raw material ‘Sea Shell’), AC (raw material ‘Angel Cheeks’), YF (raw material ‘YangFeiChuYu’), MC (raw material ‘Mothers Choice’) and MT (raw material ‘Madame Claude Tain’) were obtained.
2.3. Physicochemical analysis
The turbidity of tea infusion was measured using a portable turbidity meter (WZB-175, Rex Electric Chemical). The color of tea samples was measured by utilizing a Digieye digital imaging system (Verivide, Leicester, UK). The color of tea samples was expressed according to the L*, a*, b* color system.17 The total sugar content (TSC), total polyphenol content (TPC), and total flavonoid content (TFC) were determined as previously described.4 The phenol–sulfuric acid method was selected to evaluate the TSC. Glucose was used as a standard. The TPC was determined by the Folin–Ciocalteu method and expressed as gallic acid equivalent (mg GAE/g DW). The TFC was evaluated based on the chromogenic reaction of aluminum salt, and the data were expressed as the rutin equivalent (mg RE/g DW).
2.4. Sensory analysis
Eight trained panelists (four males and four females) evaluated the HPT using the traditional sensory evaluation of flower tea (GB/T 23776-2018). All panelists have more than 1 year experience in tea sensory analysis. Accurately weigh 3 g tea sample, add boiling water at 1 : 50 tea-water ratio. After brewing for 5 minutes, 100 mL of tea infusion was immediately taken and transferred to a standard white ceramic cup for sensory evaluation, each tea infusion was subjected to a sensory test and repeated three times. The panelists scored five factors, including shape, liquor color, aroma, taste, and leaf bottom. Each sensory attribute was scored on a 100-point scale.
2.5. Analysis of antioxidant activities
The antioxidant activities of HPT were analyzed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) free radical scavenging tests.19 The DPPH and ABTS radical scavenging activity (%) was calculated using the following equation:Scavenging activity = [1 − (A − Ai)/A0] × 100%where A is the absorbance of the sample; Ai is the absorbance of control group, and A0 is the absorbance of blank group.
2.6. Antimicrobial activity assay
A 20% solution of tea infusion (v/v) was heated in a 70 °C water bath for 5 min and then centrifuged at 12 000 rpm for 5 min. The supernatant was removed for additional study. The strains used were Staphylococcus aureus, methicillin-resistant S. aureus, and Escherichia coli. They were obtained from the China General Microbiological Culture Collection Center (CGMCC).
The antimicrobial activity of HPT made from different varieties was assayed using the agar well diffusion method. Briefly, the assay plates consisted of two layers. The bottom layer contained 10 mL of 2% sterile agar. After agar curing, a sterile Oxford cup were lightly placed on top. A volume of 10 mL LB medium was mixed with approximately 105 CFU mL−1 of Staphylococcus aureus, methicillin-resistant S. aureus, and Escherichia coli and poured on the upper layer. After solidification, the oxford cups were removed, and 50 μL of a 20% sample of tea (v/v) was added to each well. The control was sterile water. The samples were incubated at 37 °C for 24 h, and the bacteriostatic zone was determined to determine the bacteriostatic activity.18,19
2.7. GC-QTOF analysis
The GC-QTOF analytical method was used as described by Li et al.4,20 A total of 0.20 g of tea powder was placed in a 20 mL vial, and 600 μL of 0.9% sodium chloride and 1 mL of boiling water were added. Finally, 5 μL of a 0.02 mg mL−1 standard of ethyl caprate was added while waiting for the GC-QTOF determination.
The volatile compounds of HPT were analyzed on a 7890B-7200 model GC-MS system (Agilent Technologies, Santa Clara, CA, USA) with an HP-5MS UI column (30 m × 0.25 mm × 0.25 μm). The arrow of HS – SPME was retained at 250 °C for 30 min, incubated at 60 °C for 10 min, extracted for 40 min at 60 °C, and desorbed in the GC injection port at 250 °C for 5 min. In addition, the injection temperature of GC-QTOF was 250 °C.21 The initial oven temperature was 60 °C, increased to 120 °C at a rate of 10 °C min−1 and increased to 200 °C at 2 °C min−1. It was finally heated to the 230 °C at 20 °C min−1 and held for another 3 min. Helium was used as the carrier gas, and the shunt ratio of injection was a 30 : 1 split mode. The mass spectrometer was operated in the electron ionization mode at 70 eV, and the temperatures for the ion source and transfer-line were 230 °C and 250 °C, respectively. The mass spectra were obtained in the full scan mode (30–600 amu). The confirmation of identification was completed by comparing the linear retention indices (RI) with a standard solution of n-alkanes (C7–C40). Quantitation analysis of volatile compounds was performed by the internal standard method, using the ratio of GC peak area to internal standard peak area.22 The analysis was carried out in triplicate.
2.8. OAV calculation
OAVs were used to assess the contributions of volatile compounds to the aroma of tea samples. OAVs were obtained by dividing the calculated concentration of volatile compounds by their odor threshold in water.
Numerically, OAV was equal to the ratio of the compound concentration (Ci) to the odor threshold (OT) in water, and compounds with an OAV > 1 are generally considered to substantially contribute to the aroma characteristics.23
The OAV calculation equation was listed as follows: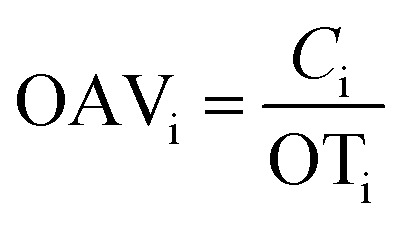
Note: Ci (μg L−1) was the relative content of volatile compounds; OTi (μg L−1) was the aroma threshold in water for volatile compounds.
2.9. Statistical analysis
All the determinations were performed in triplicate, and the data were analyzed as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used for the significance tests determined by SPSS 23 (IBM, Inc., Armonk, NY, USA). Multivariate analysis techniques were performed using SIMCA 14.1, including a principal component analysis (PCA) and a orthogonal partial least-squares discriminant analysis (OPLS-DA). The R Programming Language was selected to process the GC-QTOF data using a multivariate statistical hierarchical cluster analysis (HCA).
3. Results and discussion
3.1. Physicochemical analysis
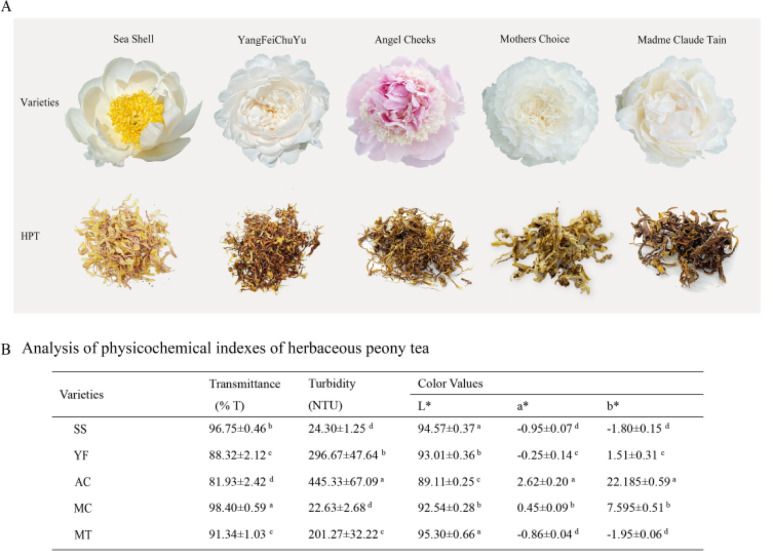
The physicochemical quality was the typical factor used to evaluate the appearance of tea with different varieties. Five varieties of HPT, including SS, MC, YF, AC and MT, were selected to evaluate the physicochemical effects on HPT (Fig. 1A).
Fig. 1. Appearance (A) and physicochemical parameters (B) of HPT from different varieties. Different letters indicate significant differences at the 0.05 level.
As shown in Fig. 1, the different varieties significantly affected the turbidity and color of tea infusion. Varietal factors have a substantial influence on the color of tea. SS, YF, MC and MT had white series of petals. Herbaceous peony petals were light yellow or brown after unified processing into HPT. The AC originated from pink petals, which were also brown. This could be because the contents of anthocyanins, flavonols, and other substances of the different varieties varied (pink series > white series). The L* values of the five tea infusions ranged from 89.11% to 95.30%, the a* values and b* value of AC was significantly higher than those of the others, and the color tended to be redder and yellower (Fig. 1B). This could also be related to the plant compounds described earlier.
The degree of turbidity was a concern in tea. Varietal factors significantly affected the turbidity of HPT. The turbidity of HPT with different varieties ranged from 22.63 NTU (MC) to 445.33 NTU (AC). AC has the highest turbidity, which could be caused by the higher levels of components in AC, such as flavonoids. In addition, the petals of ‘Angel Cheeks’ were thinner, and the rolling process was easily broken, which increased the turbidity. The light transmittance of all the tea infusions was higher than 80%. This shown that the overall appearance quality of HPT was good.
Overall, variety had a substantial impact on the physiochemistry of HPT, particularly the turbidity and the color, which indicated that the selection of variety was very important for the quality of HPT.
3.2. Nutritional analysis
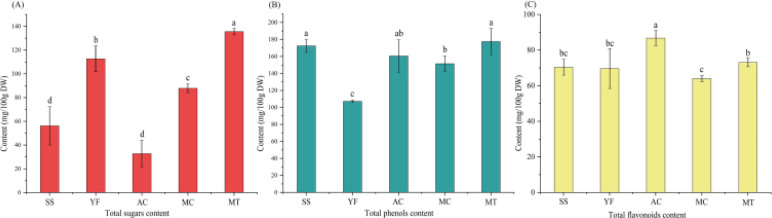
Total sugars, total phenols and total flavonoids were the three important active components in tea infusion, which were important indicators used to evaluate the grade of tea.24 Total sugars not only affected the taste qualities of the tea, such as freshness, bitterness and astringency, but also has antibacterial and antioxidant effects.25–27 As shown in Fig. 2A, different varieties of HPT had significant effects on the content of total sugars. Remarkbly, MT had the highest total sugar content of 135.65 mg/100 g DW, which was nearly four times that of AC (32.78 mg/100 g DW). There were significant differences between SS, YF, and MC, which ranged from 56.21 mg/100 g DW to 112.7 mg/100 g DW.
Fig. 2. Total sugars content (A), total phenols content (B) and total flavonoids content (C) analyses of different varieties of HPT.
Tea phenols were bioactive compounds with various functions, such as antioxidant and antibacterial effects.24,28 Additionally, tea polyphenols also determine the aroma and taste of tea, which affects its quality.4 As shown in Fig. 2B, except for YF, the total phenol content of the other four varieties had no significant difference and ranged from 151.49 mg/100 g DW to 177.56 mg/100 g DW. These results indicated that the different varieties of herbaceous peony had little effect on the total phenol content of HPT. The lowest total phenol content of YF was 107.19 mg/100 g DW. This could be owing to the low content of phenols in the flower petals.
Flavonoids primarily determine the taste and color of tea and were thought to be the primary factors that were responsible for the positive health effects of drinking tea.29,30 The content of flavonoids was influenced by the tea variety,31 and the total flavonoid content of HPT with different varieties was shown in Fig. 2C. Among them, AC had the highest content of total flavonoids, which was 86.77 mg/100 g DW. This may be due to the pink series of AC petals, which have higher anthocyanin and flavonol content than white series petals.32 In addition, the AC petals were thinner, and the rolling process was easier to break cells, making flavonoids more dissolved into the tea. The total flavonoid contents of SS (70.38 mg/100 g DW), YF (69.65 mg/100 g DW) and MT (73.07 mg/100 g DW) were not significantly different at P < 0.05. MT had the lowest total flavonoid content of 64.02 mg/100 g DW. It was apparent that the tea made from herbaceous peony petals with the same color has little effect on the total flavonoid content.
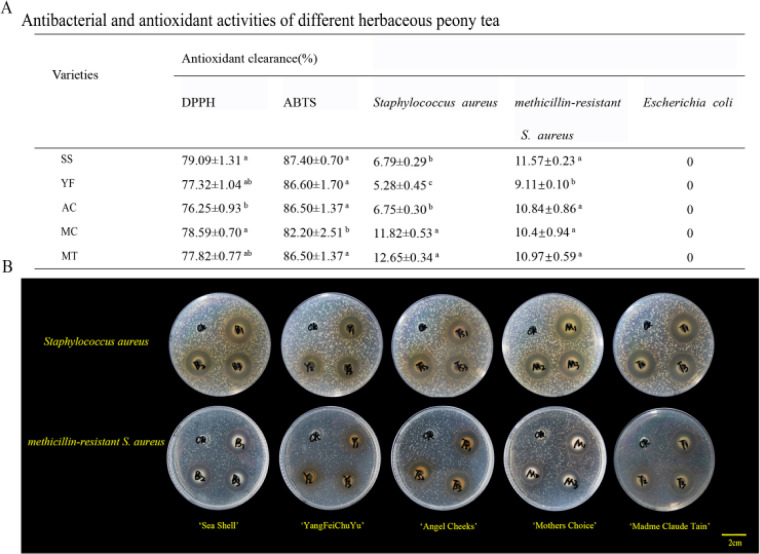
3.3. Antioxidant assays
DPPH and ABTS scavenging free radicals have been widely used to determine the antioxidant capacity of various samples.33,34 The type of variety significantly affected the scavenging ability of HPT on DPPH as shown in Fig. 3B. The total antioxidant capacity of DPPH in the HPT was in the range of 76.24–79.09%, which was weaker than those of oolong tea and Japanese green tea. This could be owing to the lower content of tea polyphenols in the petals than in leaves. The ABTS scavenging rate was 82.20–87.40%. The MC variety had the lowest rate of ABTS scavenging (82.20%) and was not suitable for the development of HPT.
Fig. 3. Antioxidant ability (A) and antibacterial ability (B) of different varieties of HPT.
3.4. Antibacterial assays
The antibacterial activities of HPT on Staphylococcus aureus, methicillin-resistant S. aureus, and Escherichia coli as shown in Fig. 3. The inhibitory effect of SS against methicillin-resistant Staphylococcus aureus was 11.57 mm, which was not significantly different from MT (10.97 mm). Remarkably, MT showed the strongest inhibitory effect on Staphylococcus aureus (12.65 mm), which was two times higher than the SS (6.79 mm). However, the HPT had no effect on Escherichia coli. In addition, different varieties of HPT also differed significantly in their ability to inhibit the same type of bacteria. Compared to other varieties of HPT, YF had the lowest degree of antibacterial activity against Staphylococcus aureus with a zone of inhibition of 5.28 mm. This could be because YF has the lowest amount of TPC, the primary antibacterial compounds, which led to the weakest antibacterial ability.
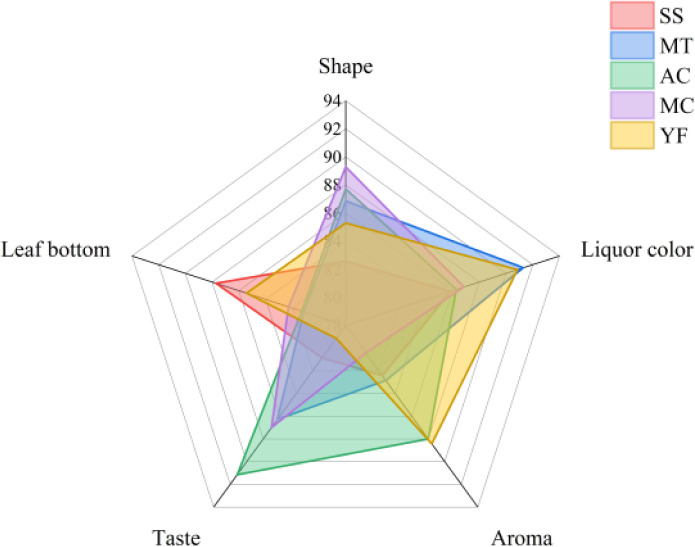
3.5. Sensory evaluation
Variety was considered to be the primary factor that affects the taste, aroma, and color of tea compared with the season, ecological environment and processing technology.35 A sensory evaluation of HPT was conducted as shown in Fig. 4, each of the five different varieties of HPT had its own sensory characteristics. Aroma attribute (25%) was the most important variable in the sensory score.4 AC had a higher aroma score (88.0) and taste score (91.14). This may be due to the thinner petals of AC, and the enzymes in the petals were easily deactivated during fixation, thus inhibiting the enzymatic oxidation reactions. This was more conduced to the formation of aroma and other qualities. MT had the lowest taste score (79.14) and was not suitable as a raw material for HPT. YF was excellent in color (86.85) and taste (89.28). However, it performed significantly poorly in aroma (82.85). Although YF has been reported to contain up to 92.67% of aroma components in full bloom, it lost more aroma greater after it was processed into herbaceous peony tea. In general, AC was a suitable raw material for HPT.
Fig. 4. Spider plot for the sensory profiles of different varieties of HPT.
3.6. Volatile component analysis
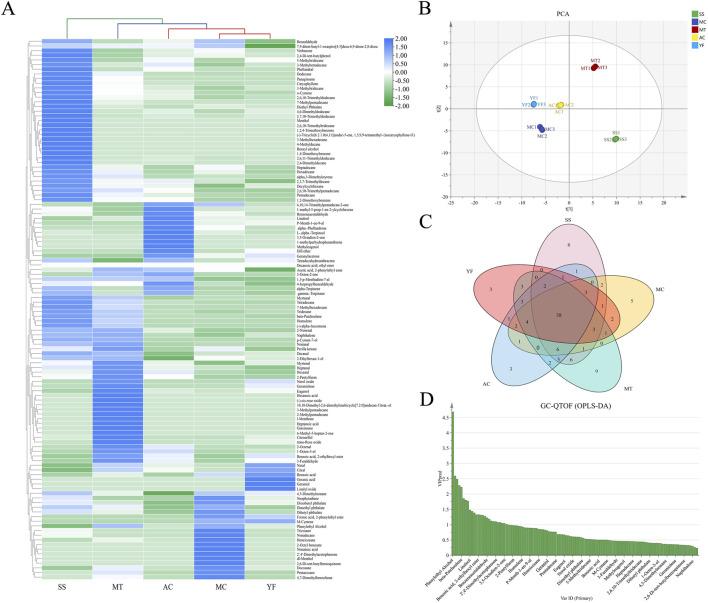
Aroma components were essential factors to determine the quality of tea20 and the change of aroma was very significant in the fixation process. The aroma of five varieties that were processed into HPT was investigated using the GC-QTOF with the NIST database (2017 Edition). Check the CAS number of volatile compounds on the NCBI website for verification and complete the volatile component calibration of HPT. After comparing data, 115 volatile components were obtained, including 10 alcohols, 12 aldehydes, 11 acids, 17 terpenoids, 42 hydrocarbons, 5 ketones, 6 heterocyclics and 12 other compounds (Table S2†).
To better understand the effects in the volatile components with different varieties of HPT, a complexheatmap analysis and PCA analysis were performed to provide statistical analyses, and the results were shown in Fig. 5. The results of PCA analysis clearly significantly differentiated between the different varieties (Fig. 5B). The complexheatmap analysis also grouped the five samples into three clusters. Remarkably, SS was significantly distinced, and its aroma was the most exceptional (Fig. 5A). SS and MT each comprised a class, while the third one included AC, MC and YF.
Fig. 5. Determination of the volatile components obtained from different varieties of herbaceous tea using GC-QTOF. (A) Complexheatmap analysis; (B) principal component analysis; (C) key compound VIP analysis of HPT samples with different varieties; (D) Venn analysis. GC-QTOF, quadruple time of flight mass spectrometry; HPT, herbaceous peony tea; VIP, variable importance projection.
Based on these results, OPLS – DA models were established to investigate the key differential compounds of 115 volatile components with HPT as shown in Fig. S1A.† It was found that different HPT cluster separation was obvious, indicating that there were significant differences in aroma between different varieties of HPT. After 200 tests of the model, it was found that the blue Q2 value on the left was lower than the origin, indicated that the model data was reliable (Fig. S1†). When the variable importance factor (VIP) value is greater than 1.0, the corresponding variable is defined as the key variable of the discriminant model (Yang et al., 2022). A total of 22 key volatile compounds were identified in HPT (Fig. 5C and Table 1). The mass-charge ratio diagram analysis of hexanal, benzeneacetaldehyde, citronellol, nonanal, phenylethyl alcohol and terpineol was shown in the Fig. S2.† These 22 key components were essential for the aroma quality of HPT. No aroma threshold was found for eight components, including 4,7-dimethylbenzofuran, benzoic acid, and 2-ethylhexyl ester, which were not discussed in this experiment (Table 1).
Odor quality, odor threshold, VIP and correlation coefficient with aroma of different HPT varietiesb,c.
| No. | Name | VIP | Aroma | OTa | OAV | Odor quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | MC | MT | AC | YF | ||||||
| 1 | l-α-Terpineol | 1.33 | 0.55 | 330.00 | 4.71 | 5.57 | 3.25 | 23.21 | 2.97 | Floral, sweet |
| 2 | Nonanal | 1.07 | 0.85 | 1.10 | 9211.00 | 4169.87 | 10 523.86 | 3701.72 | 4025.15 | Floral, lemon-like, fatty |
| 5 | Benzeneacetaldehyde | 1.23 | 0.78 | 4.00 | 1060.50 | 1079.79 | 545.22 | 2236.46 | 757.09 | Rose-like, cherry-like |
| 3 | Hexanal | 1.32 | 0.68 | 4.50 | 790.77 | 369.34 | 2132.10 | 1168.67 | 162.80 | Grassy, fatty |
| 4 | Caryophyllene | 2.49 | 0.51 | 64.00 | 1022.85 | 41.40 | 298.19 | 98.71 | 63.21 | Woody, acridity |
| 6 | Phenylethyl alcohol | 4.69 | 0.78 | 390.00 | 67.06 | 663.45 | 597.87 | 262.62 | 304.51 | Fruity, hyacinth-like, woody |
| 7 | Citronellol | 1.05 | 0.52 | 40.00 | 28.72 | 9.62 | 185.21 | 12.11 | 34.60 | Rose-like |
| 8 | o-Cymene | 1.81 | 0.50 | 11.40 | 2856.32 | 0.00 | 855.86 | 572.61 | 0.00 | Grassy, medicinal |
| 9 | 3,5-Octadien-2-one | 1.05 | 0.29 | 115.39 | 0.00 | 0.00 | 0.00 | 35.26 | 0.00 | Fruity, fatty, and chestnut |
| 10 | Linalool | 1.48 | 0.54 | 0.22 | 0.00 | 13 053.43 | 10 028.98 | 48 628.89 | 11 974.95 | Lily-like, sweet, woody |
| 11 | (−)-cis-Rose oxide | 1.39 | 0.35 | 0.50 | 0.00 | 0.00 | 23 399.03 | 0.00 | 0.00 | Rose-like, fruity, grassy |
| 12 | Nonanoic acid | 2.28 | 0.33 | 3000.00 | 0.00 | 13.33 | 0.00 | 0.00 | 0.00 | Musty, pungent |
| 13 | Geranic acid | 1.28 | 0.35 | 40.00 | 0.00 | 0.00 | 0.00 | 0.00 | 140.82 | Grassy |
| 14 | Phellandral | 1.02 | 0.42 | 3.00 | 3231.27 | 0.00 | 0.00 | 923.53 | 0.00 | Grassy, fatty |
| 15 | 4,7-Dimethylbenzofuran | 2.60 | 0.54 | — | — | — | — | — | — | Unknown |
| 16 | Beta-patchoulene | 2.23 | 0.55 | — | — | — | — | — | — | Unknown |
| 17 | 1-Methyl-5-prop-1-en-2-ylcyclohexene | 1.44 | 0.56 | — | — | — | — | — | — | Unknown |
| 18 | Benzoic acid, 2-ethylhexyl ester | 1.32 | 0.62 | — | — | — | — | — | — | Unknown |
| 19 | 2-Octyl benzoate | 1.19 | 0.33 | — | — | — | — | — | — | Unknown |
| 20 | 6,10,14-Trimethylpentadecan-2-one | 1.12 | 0.87 | — | — | — | — | — | — | Unknown |
| 21 | 2′,4′-Dimethylacetophenone | 1.08 | 0.33 | — | — | — | — | — | — | Unknown |
| 22 | (−)-Tricyclo[6.2.1.0(4,11)]undec-5-ene, 1,5,9,9-tetramethyl-(isocaryophyllene-I1) | 1.79 | 0.34 | — | — | — | — | — | — | Unknown |
OT, odor thresholds in water. All the odor thresholds and odor quality were obtained from: ‘Odour & Flavour Detection thresholds in water (in parts per billion, l μg L−1)’ (https://leffingwell.com/chirality/acyclics.htm); refs. 40–48.
— No threshold for the compound in water was found.
HPT, herbaceous peony tea; OAV, odor activity value; VIP, variable importance projection.
As shown in Fig. 5D and Table S2,† the specific components of five varieties with HPT were obtained by a Venn analysis. The specific component of MC was nonanoic acid (VIP = 2.28) with a stale and spicy aroma, which was not suitable for the development of HPT. (−)-cis-Rose oxide (VIP = 1.39) provides MT with a rose and unique cherry-like aroma, which contributes to the formation of a premium floral tea with flavor. 3,5-Octadien-2-one (VIP = 1.05) primarily endows AC with a fruity and fatty aroma. Interestingly, AC has a distinctive mushroom aroma. The unique component of YF was geranic acid (VIP = 1.28) with a grass flavor, but the aroma was not characteristic in HPT. Clearly, both MT and AC provide a characteristic aroma for high quality HPT.
3.7. The OAV and aroma quality of single odorant affect the aroma profile of HPT
The overall contribution of HPT to flavor depends not only on the concentration of key compounds but also on the OAV.23 The OAV and aroma characteristics significantly affect the aroma type of tea.36 As shown in Table 1, the key volatile compounds have similar types of aromas, and there were significant differences in their aroma among different varieties of HPT. By combining the odor quality, these 22 key components were divided into six aroma types, including floral, fruity, sweet, grass, woody, and fatty.
Notably, all five HPT have floral, sweet, fruity, grass, and woody flavors, which were primarily endowed by l-α-terpineol, phenylethyl alcohol, citronellol, benzeneacetaldehyde, nonanal, hexanal and caryophyllene. It was worth noting that phenylethyl alcohol, citronellol, benzeneacetaldehyde, nonanal and hexanal were significantly positively correlated with aroma scores. Among them, the aroma of phenylethyl alcohol and citronellol could result from herbaceous peony. They were an important representative of the fruity and rose scent cluster of herbaceous peony, and their contents in the petals was as high as 42.69 μg L−1 and 68.32 μg L−1.16 In particular, phenylethyl alcohol had a significant positive correlation with the aroma score of HPT (correlation coefficient 0.78). Phenylethyl alcohol and citronellol endow all the HPT with characteristic fruity, rose-like, and hyacinth-like aromas.37 It can be found that floral fragrance was the most dominant aroma type of HPT.
There were significant differences in the unique scents of the different varieties once the common scents were excluded. Interestingly, o-cymene, which had a medicinal aroma, was found in SS, MT, and AC. Herbaceous peony was a traditional Chinese medicine, and it was possible that this compound endows the petaled tea with medicinal fragrance. This was the first time that a characteristic aroma was identified that distinguishes HPT from other green teas. Remarkably, compared with other varieties in HPT, AC was unique in containing the aromatic compound 3,5-octadien-2-one (OAV = 4676.55 in AC), an exceptional chestnut aroma. 3,5-Octadien-2-one was the key aromatic substance that is only possessed by certain green tea varieties.38 This indicated that AC was the most characteristic variety in HPT. In addition, MC, MT, AC, and YF all contain linalool that has woody, sweet, and lily-like scents in contrast to SS. Linalool was not only a representative of lily scent in herbaceous peony but also considered to be the primary aromatic substance of high-quality green tea, such as Japanese green tea.39
Therefore, these results indicated that AC had the most characteristic aroma among the five varieties of HPT that was conducive to the formation of high-quality tea flavor. Additionally, although 2,6,11-trimethyldodecane, 2,7,10-trimethyldodecane, 2,3,7-trimethyldecane and 2,4-dimethyldecane were abundant in HPT, they do not contribute to the aroma.
In general, the aroma of AC was the most characteristic among the five varieties. AC contains high-quality aromatic substances, including phenylethyl alcohol, citronellol, o-cymene and 3,5-octene-2-one, which provide AC with its characteristic fruity, lily-like, rose-like, medicinal, and chestnut scents. Compared with the other varieties, AC was more conducive to the formation of flavor in HPT and has more potential for commercial development.
4. Conclusions
The aim of this study was to analyze the physicochemical properties, sensory evaluation, antioxidant capacity and aroma quality of different varieties of herbaceous peony tea. The results showed that the transmittance of herbaceous peony tea with different varieties were high, with the overall above 80%. Meanwhile, the overall ABTS radical scavenging rate was 82.20–87.40%. The sensory evaluation showed that the highest overall sensory score for ‘Angel Cheeks’, with taste score of 91.14 and an aroma score of 88.0. This result suggests that ‘Angel Cheeks’ contributes to the formation of high-quality HPTs. Remarkably, 22 volatile components were the key aroma components of HPT. ‘Angel cheeks’ exhibited the best performance in aroma quality, which can be attributed to o-cymene, 3,5-octadien-2-one, and linalool, which provided the exceptional aromas of medicinal, chestnut and lily-like fragrance, respectively. However, the unknown threshold of composition limits the evaluation of aroma. Therefore, the characteristic aroma of HPT can be analyzed in more detail using molecular senses. Overall, ‘Angel cheeks’ was suitable for the processing and production of high-level HPT owing to its excellent aroma and efficacy qualities. This study promotes the green all-round development of herbaceous peony tea.
Author contributions
Xiaoxiao Wang: data curation, writing – original draft. Kairong Sun: data curation, software. Xueping Liao: methodology. Yanli Zhang: investigation. Yuqian Ban: investigation. Xiuxin Zhang: supervision, funding acquisition. Zihan Song: conceptualization, funding acquisition, writing – reviewing and editing.
Conflicts of interest
The authors declare no competing financial interest.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32102043); China Agriculture Research System (CARS-21); Central Public-interest Scientific Institution Basal Research Fund (No. Y2022XK30); Local Finance of Chengdu Agricultural Science & Technology Center (NASC2020KR03) and National Key R&D Program of China (2021YFE0110700). We thank the public laboratory of the Biotechnology Research Institute, Chinese Academy of Agricultural Sciences for use of the GC and TOF MS instrument and providing technical assistance.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08144c
Notes and references
- Li S. S. Chen L. G. Xu Y. J. Wang L. J. Wang L. S. Sci. Hortic. 2012;142:158–165. [Google Scholar]
- Feng L. Li Y. Sheng L. Li T. Zhao D. Tao J. J. Essent. Oil Bear. Plants. 2016;19:167–175. [Google Scholar]
- Xu H. Wu M. Zhang X. Wang B. Wang S. Zheng Z. Li D. Wang F. Ind. Crop Prod. 2022;188:115663. [Google Scholar]
- Wang X. Xiong H. Wang S. Zhang Y. Song Z. Zhang X. Ind. Crop Prod. 2023;193:116159. [Google Scholar]
- Wang S. L. Xue J. Q. Zhang S. F. Zheng S. N. Xue Y. Q. Xu D. H. Zhang X. X. Plant Physiol. Biochem. 2020;155:1–12. doi: 10.1016/j.plaphy.2020.06.029. [DOI] [PubMed] [Google Scholar]
- Le X. Hu S. Zheng J.-L. Cui E.-L. Zhu Y.-H. Zhu M. Ind. Crop Prod. 2022:114469. [Google Scholar]
- Yuan J. Hao L. J. Wu G. Wang S. Duan J. A. Xie G. Y. Qin M. J. J. Funct. Foods. 2015;19:786–795. [Google Scholar]
- Lv M. Yang Y. Choisy P. Xu T. Pays K. Zhang L. Zhu J. Wang Q. Li S. Wang L. Ind. Crop Prod. 2023;200:116707. [Google Scholar]
- Kumar P. V. S. Basheer S. Ravi R. Thakur M. S. J. Food Sci. Technol. 2011;48:440–446. doi: 10.1007/s13197-010-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Gao C. Zhao J. Zhang J. Zheng Z. Huang Y. Sun W. Food Chem. 2024:439. doi: 10.1016/j.foodchem.2023.138133. [DOI] [PubMed] [Google Scholar]
- Ye J. H. Ye Y. Yin J. F. Jin J. Liang Y. R. Liu R. Y. Tang P. Xu Y. Q. Trends Food Sci. Technol. 2022;123:130–143. [Google Scholar]
- Wen M. C. Cui Y. Q. Dong C. X. Zhang L. Food Res. Int. 2021;148:110588. doi: 10.1016/j.foodres.2021.110588. [DOI] [PubMed] [Google Scholar]
- Zeng L. T. Watanabe N. Yang Z. Y. Crit. Rev. Food Sci. 2019;59:2321–2334. doi: 10.1080/10408398.2018.1506907. [DOI] [PubMed] [Google Scholar]
- Zhai X. T. Zhang L. Granvogl M. Ho C. T. Wan X. C. Compr. Rev. Food Sci. Food Saf. 2022;21:3867–3909. doi: 10.1111/1541-4337.12999. [DOI] [PubMed] [Google Scholar]
- Xiao K. J. Mater. Cult. 2016;22:3–18. [Google Scholar]
- Song C. Wang Q. Teixeira da Silva J. A. Yu X. J. Am. Soc. Hortic. 2018;143:248–258. [Google Scholar]
- Xu M. Wang J. Zhu L. Y. Food Chem. 2019;289:482–489. doi: 10.1016/j.foodchem.2019.03.080. [DOI] [PubMed] [Google Scholar]
- Li J. Huang S. Y. Deng Q. Y. Li G. L. Su G. C. Liu J. W. Wang H. M. D. Food Chem. Toxicol. 2020;136:111050. doi: 10.1016/j.fct.2019.111050. [DOI] [PubMed] [Google Scholar]
- Huang T. H. Chen C. L. Hung C. J. Kao C. T. J. Dent Sci. 2012;7:336–341. [Google Scholar]
- Li Y. C. He C. Yu X. L. Zhou J. T. Ntezimana B. Yu Z. Chen Y. Q. Ni D. J. LWT Food Sci. Technol. 2022;154:112597. [Google Scholar]
- Li Y. Ran W. He C. Zhou J. Chen Y. Yu Z. Ni D. Food Chem.: X. 2022;14:100289. doi: 10.1016/j.fochx.2022.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z. Jingwen L. Fankun Z. J. Food Sci. 2021;86:3467–3479. [Google Scholar]
- Wang M. Q. Ma W. J. Shi J. Zhu Y. Lin Z. Lv H. P. Food Res. Int. 2020;130:108908. doi: 10.1016/j.foodres.2019.108908. [DOI] [PubMed] [Google Scholar]
- Wen M. Han Z. Cui Y. Ho C.-T. Wan X. Zhang L. Food Chem. 2022;368:130803. doi: 10.1016/j.foodchem.2021.130803. [DOI] [PubMed] [Google Scholar]
- Troszynska A. Narolewska O. Robredo S. Estrella I. Hernandez T. Lamparski G. Amarowicz R. Food Qual. Prefer. 2010;21:463–469. [Google Scholar]
- Wang H. Shen S. Wang J. Jiang Y. Li J. Yang Y. Hua J. Yuan H. LWT Food Sci. Technol. 2022;155:112939. [Google Scholar]
- Lee Y. E. Yoo S. H. Chung J. O. Park M. Y. Hong Y. D. Park S. H. Park T. S. Shim S. M. J. Sci. Food Agric. 2020;100:3979–3986. doi: 10.1002/jsfa.10442. [DOI] [PubMed] [Google Scholar]
- Feng Z. Li Y. Li M. Wang Y. Zhang L. Wan X. Yang X. Food Chem. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- Liu F. Wang Y. Corke H. Zhu H. LWT Food Sci. Technol. 2022;170:114073. [Google Scholar]
- Zhu H. K. Liu F. Ye Y. Chen L. Li J. Y. Gu A. H. Zhang J. Q. Dong C. W. J. Food Eng. 2019;263:165–172. [Google Scholar]
- Chen D. Sun Z. Gao J. J. Peng J. K. Wang Z. Zhao Y. N. Lin Z. Dai W. D. Food Chem. 2022:377. doi: 10.1016/j.foodchem.2021.131976. [DOI] [PubMed] [Google Scholar]
- Wang S. L. Xue J. Q. Zhang S. F. Zheng S. N. Xue Y. Q. Xu D. H. Zhang X. X. Plant Physiol. Biochem. 2020;155:1–12. doi: 10.1016/j.plaphy.2020.06.029. [DOI] [PubMed] [Google Scholar]
- Sridhar K. Charles A. L. Food Chem. 2019;275:41–49. doi: 10.1016/j.foodchem.2018.09.040. [DOI] [PubMed] [Google Scholar]
- von Staszewski M. Pilosof A. M. R. Jagus R. J. Food Chem. 2011;125:186–192. [Google Scholar]
- Li Y. Ran W. He C. Zhou J. Chen Y. Yu Z. Ni D. Food Chem. X. 2022;14:100289. doi: 10.1016/j.fochx.2022.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Cao X. Yuan Z. Guo G. Food Chem. 2021;352:129359. doi: 10.1016/j.foodchem.2021.129359. [DOI] [PubMed] [Google Scholar]
- Sun J. Y. Li Q. Y. Luo S. Q. Zhang J. L. Huang M. Q. Chen F. Zheng F. P. Sun X. T. Li H. H. RSC Adv. 2018;8:23757–23767. doi: 10.1039/c8ra02727g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Lv H.-P. Shao C.-Y. Kang S. Zhang Y. Guo L. Dai W.-D. Tan J.-F. Peng Q.-H. Lin Z. Food Res. Int. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Wang J. Li M. Wang H. Huang W. Li F. Wang L. Ho C. T. Zhang Y. Zhang L. Zhai X. Wan X. J. Agric. Food Chem. 2022;70:10571–10583. doi: 10.1021/acs.jafc.2c02249. [DOI] [PubMed] [Google Scholar]
- Guo X. Ho C. T. Schwab W. Wan X. Food Chem. 2021;363:130328. doi: 10.1016/j.foodchem.2021.130328. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Lv H. P. Shao C. Y. Kang S. Zhang Y. Guo L. Dai W. D. Tan J. F. Peng Q. H. Lin Z. Food Res. Int. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Luo J. Zeng F. J. Food Sci. 2021;86:3467–3479. doi: 10.1111/1750-3841.15790. [DOI] [PubMed] [Google Scholar]
- Sanchez-Palomo E. Trujillo M. Garcia Ruiz A. Gonzalez Vinas M. A. Food Res. Int. 2017;100:201–208. doi: 10.1016/j.foodres.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Pan X. Wu J. Zhang W. Liu J. Yang X. Liao X. Hu X. Lao F. Food Chem. 2021;338:128117. doi: 10.1016/j.foodchem.2020.128117. [DOI] [PubMed] [Google Scholar]
- Dalpathadu K. A. P. Rajapakse H. U. K. D. Z. Nissanka S. P. Jayasinghe C. V. L. Arab. J. Chem. 2022;15:104147. [Google Scholar]
- Joshi R. Gulati A. Food Chem. 2015;167:290–298. doi: 10.1016/j.foodchem.2014.06.112. [DOI] [PubMed] [Google Scholar]
- Noguerol-Pato R. Gonzalez-Barreiro C. Cancho-Grande B. Martinez M. C. Santiago J. L. Simal-Gandara J. Food Chem. 2012;135:2771–2782. doi: 10.1016/j.foodchem.2012.06.104. [DOI] [PubMed] [Google Scholar]
- Liu Y.-X. Li W.-D. Wang Y. Zhong K. Zhao L. Gao H.-Y. Chin. J. Anal. Chem. 2021;49:e21104–e21111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.