Supplemental Digital Content is Available in the Text.
Keywords: antiviral treatment, COVID-19, hospitalization, systematic literature review, real-world effectiveness
Abstract
Background:
Nirmatrelvir/ritonavir (NMV/r) is an oral antiviral drug used to treat mild-to-moderate coronavirus disease 2019 (COVID-19) in patients aged 12 years or older at high risk of progression to severe disease (eg, hospitalization and death). Despite being the preferred option for outpatient treatment in the majority of countries worldwide, NMV/r is currently underutilized in real-world clinical practice.
Areas of Uncertainty:
As numerous real-world studies have described patient outcomes following treatment with NMV/r, this systematic literature review provides a comprehensive summary of evidence on NMV/r effectiveness against hospitalization and mortality further organized by clinically meaningful categories, such as acute versus longer-term follow-up, age, underlying health conditions, and vaccination status, to help inform health care decision making.
Data Sources:
We searched Embase and PubMed (December 22, 2021–March 31, 2023) and congress abstracts (December 1, 2021–December 31, 2022) for reports describing NMV/r effectiveness.
Therapeutic Advances:
In total, 18 real-world studies met final selection criteria. The evidence showed that NMV/r significantly reduced postinfection risk of all-cause and COVID-19-related hospitalization and mortality in both acute (≤30 days) (21%–92%) and longer-term (>30 days) (1%–61%) follow-up. The reduction in postinfection risk was higher when treatment was received within 5 days of symptom onset. Real-world effectiveness of NMV/r treatment was observed regardless of age, underlying high-risk conditions, and vaccination status.
Conclusion:
The systematic literature review findings demonstrated the effectiveness of NMV/r against hospitalization and mortality during the Omicron period among individuals at high risk of progression to severe COVID-19 disease.
INTRODUCTION
Nirmatrelvir/ritonavir (NMV/r) is an oral antiviral medication used for the treatment of mild-to-moderate COVID-19 in individuals at high risk of progression to severe disease, including hospitalization and death. In the United States, NMV/r received Food and Drug Administration emergency use authorization on December 22, 2021, for the treatment of individuals aged 12 years or older1,2 and was shortly thereafter made available in Europe on January 28, 2022.3 Other authorizations and approvals followed across the globe. Globally, over 12.7 million treatment courses of NMV/r have been prescribed since January 2022.4 In many countries, NMV/r is recommended as the preferred outpatient COVID-19 treatment for individuals at high risk of progression to severe illness.5–7
The efficacy of NMV/r to prevent progression to COVID-19–related hospitalization or death was first demonstrated in the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial. This randomized, double-blind, placebo-controlled, multinational trial showed that NMV/r reduced the risk of COVID-19–related hospitalization or death from any cause by 86% when patients were treated within 5 days of symptom onset (0.9% vs. 6.5%, absolute risk in treated vs. placebo, respectively; P < 0.001).8 In contrast to EPIC-HR, which enrolled immunologically naive patients with COVID-19 (ie, unvaccinated and not previously infected with SARS-CoV-2), the Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR) trial included vaccinated patients.9 Although EPIC-SR enrollment was stopped early due to the low rate of hospitalization or death in the standard-risk population,9 the clinical trial found that NMV/r efficacy among vaccinated adults with ≥1 risk factor, while not statistically significant, was supportive of the efficacy data observed in the EPIC-HR study.8 Prespecified secondary end point results showed a nominally significant 62% decrease in COVID-19–related medical visits per day across all patients, relative to placebo in a subgroup analysis.9
However, questions still remain about the clinical relevance of these results for the treatment of contemporary patients in the real-world setting, given that the EPIC-HR trial was conducted in unvaccinated adults without a history of prior infection during a period when the Delta variant was the predominant one. This is in contrast to the current situation of higher levels of population immunity to SARS-CoV-2 (from vaccination, infection, or both) and a lower likelihood of severe illness stemming from infection with the Omicron variant and its sublineages.8
The current systematic literature review (SLR) was undertaken to understand the real-world effectiveness of NMV/r in reducing the acute and longer-term postinfection risk of hospitalization, mortality, or a composite of hospitalization or mortality among nonhospitalized patients with COVID-19 by age, underlying high-risk conditions (UHCs), and vaccination status.
METHODS
This SLR was conducted in accordance with Cochrane Handbook for Systematic Reviews of Interventions10 and Preferred Reporting Items for Systematic reviews and Meta-Analysis11 guidelines. The literature search was conducted in PubMed and Embase. Outcomes of interest were identified using prespecified search strategies in articles published between December 22, 2021, and March 31, 2023 (see Supplemental Digital Content 1, http://links.lww.com/AJT/A151) and supplemented by a review of gray literature from conference proceedings from December 1, 2021, to December 31, 2022 (see Supplemental Digital Content 2, http://links.lww.com/AJT/A152).
Two independent reviewers (D.G. and J.P.) evaluated titles and abstracts using the Population, Intervention, Comparison, Outcomes, and Study Design framework (see Supplemental Digital Content 3, http://links.lww.com/AJT/A153); a third adjudicator (G.M.) resolved any discrepancies. Studies available in English that included patients aged 12 years or older with COVID-19 treated with NMV/r as a single intervention (ie, not combined with other treatment options, such as other antivirals) and who were at high risk of progression to severe COVID-19 were eligible for inclusion in the SLR.
Full-text articles were examined by 2 reviewers using the predefined inclusion and exclusion criteria. Final studies for data extraction were performed by a single reviewer (D.G.), while a second reviewer (J.P.) checked each data point. In cases where a study was available both as a preprint and as a published peer-reviewed publication within the date range of the literature search, data from the peer-reviewed publication were reported in this SLR. Study variables captured included study design and population characteristics, including acute (≤30 days) versus longer-term (>30 days) postinfection follow-up period, COVID-19 vaccination status, circulating/dominant variant and sublineages, history of prior infection, time from symptom onset to treatment, and variables associated with hospitalization, mortality, and composite end points.12 Study outcomes of hospitalization, mortality, and a composite of hospitalization or mortality were stratified by acute and longer-term follow-ups. The results were further stratified by age, UHCs, and vaccination status, when available.
Studies reporting adjusted estimates of treatment effectiveness with corresponding 95% confidence intervals (CIs) were selected for inclusion. Adjusted hazard ratios (aHRs) and odds ratios (aORs) were converted to effectiveness (ie, relative reduction) by subtracting the aHR or aOR from 1 and multiplying by 100. No statistical analysis was conducted across the studies. Bias assessment was performed by 2 independent reviewers (L.C. and C.R.) using the Risk of Bias in Non-randomized Studies of Interventions tool (see Supplemental Digital Content 4, http://links.lww.com/AJT/A154).13
RESULTS
Study characteristics
Of 1880 records returned in the initial search (PubMed, n = 296; Embase, n = 78; and gray literature, n = 1506), 18 studies reported on NMV/r effectiveness as a single intervention for hospitalization or mortality and were included in this SLR (Figure 1, see Supplemental Digital Content 5, http://links.lww.com/AJT/A155).14–31 Of these, 15 studies evaluated effectiveness outcomes during acute follow-up ≤30 days after a positive COVID-19 test, diagnosis, or treatment initiation14,15,19–31 and 3 evaluated effectiveness outcomes during longer-term follow-up (>30 days) following diagnosis or treatment initiation.16–18 Two studies that assessed outcomes 35 days after COVID-19 diagnosis were included as studies with acute outcomes.21,28
FIGURE 1.
SLR Preferred Reporting Items for Systematic reviews and Meta-Analysis diagram. Eighteen studies reported on NMV/r effectiveness as a single intervention for hospitalization or mortality and were included in this SLR. *Refer to supplemental material 5 for a list of reference citations for the 66 publications.
The 18 studies included a total of 343,197 patients receiving NMV/r treatment.14–31 All studies reported data for the outcome of hospitalization; 16 (88.9%) of these studies provided data on the composite of hospitalization or mortality.15–26,28–31 Most of these studies were conducted in North America (n = 9),14,17,18,20,24–26,30,31 followed by Europe (n = 3),23,26,27 Asia Pacific (n = 2),15,19 and Middle East/Africa (n = 2)21,28; 2 studies had a global reach.16,22
All of the studies were retrospective in nature.14–31 Only 1 study was a single-arm study.23 The main comparator in most studies was no NMV/r (n = 15),14–22,24–26,28,30,31 followed by molnupiravir (n = 5),15,18,27–29 sotrovimab (n = 2),27,29 remdesivir (n = 1),27 and others (n = 1).30 Among the 18 studies, 7 reported administering NMV/r treatment ≤5 days after the onset of symptoms19,20,22,26–28,31; however, only one of these studies, by Lewnard et al, described the exact timing of NMV/r treatment from symptom onset.27 Most (n = 17) studies captured vaccination status,14,15,17–31 but only 11 reported the actual number of vaccine doses received by patients.17,18,20,23,24,26–31 The predominant COVID-19 variant discussed in 12 studies was Omicron15,19–21,23,24,26–31; 6 studies included details about the sublineages of the virus, namely BA.1/BA.2 and BA.4/BA.5 (see Supplemental Digital Content 6, http://links.lww.com/AJT/A156).19,23,24,27,29,31
Among the 18 studies, 8 (44.4%) reported outcomes by age.14,20,21,23,24,26,28,30 In addition, 61.1% of the studies included data on race/ethnicity related to NMV/r treatment.14,16–18,20,22,24,25,29–31 The majority of these patients were White (68.6%), with Black/African American and Hispanic ethnicities making up 12.6% and 11.4%, respectively.
Acute follow-up
Hospitalization
In 6 studies of all-cause hospitalization, NMV/r effectiveness compared with no NMV/r ranged from 21% to 89% (Figure 2).14,15,22–25 In 3 studies of COVID-19-related hospitalization, effectiveness of NMV/r versus no NMV/r ranged from 24% to 60% (Figure 3).18,20,24
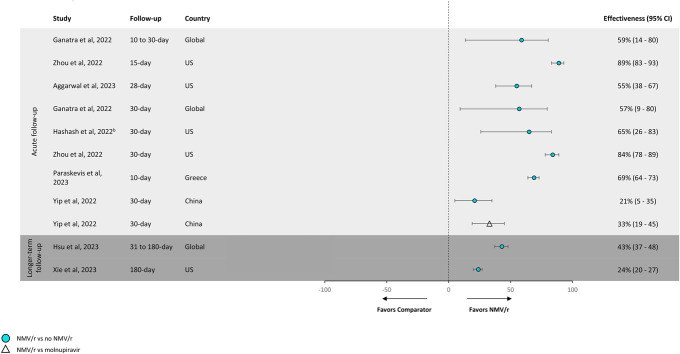
FIGURE 2.
Effectivenessa against all-cause hospitalization. NMV/r effectiveness against all-cause hospitalization compared with no NMV/r ranged from 21% to 89%. aTreatment effectiveness percentages were calculated by subtracting hazard ratios or odds ratios from 1 and multiplying by 100. bThis study was limited to patients with inflammatory bowel disease. Note: The definition of “no NMV/r” varied across studies. The following definitions were used for “no NMV/r”: Yip et al,15 Hashash et al,25 and Zhou et al14 used “not prescribed NMV/r or molnupiravir”; Ganatra et al22 used “did not receive ritonavir, nirmatrelvir, tixagevimab-cilgavimab, bebtelovimab, bamlanivimab, molnupiravir, or convalescent plasma transfusion”; Xie et al17 used “did not receive other outpatient COVID-19 antiviral or antibody treatments within 30 days of the index date”; and Hsu et al,16 Paraskevis et al,23 and Aggarwal et al24 stated “not receiving NMV/r.”
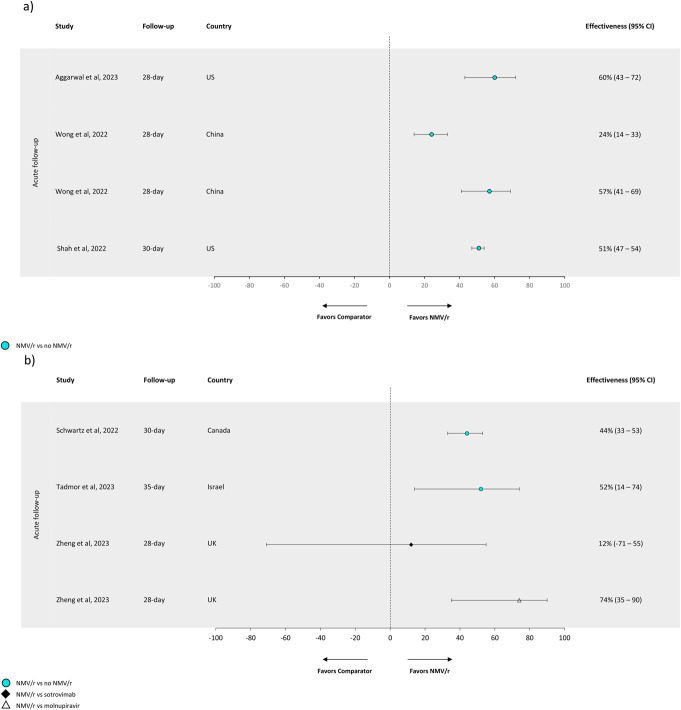
FIGURE 3.
Effectivenessa against COVID-19-related outcomes. Effectiveness of NMV/r against COVID-19-related hospitalization versus no NMV/r ranged from 24% to 60%. (A) Effectiveness against COVID-19-related hospitalization. (B) Effectiveness against COVID-19-related hospitalization or mortality. (A) Treatment effectiveness percentages were calculated by subtracting hazard ratios or odds ratios from 1 and multiplying by 100. Note: The definition of “no NMV/r” varied across studies. The following definitions were used for “no NMV/r”: Tadmor et al28 and Wong et al19 used “did not receive NMV/r or molnupiravir”; and Shah et al,20 Schwartz et al,26 and Aggarwal et al24 stated “not receiving NMV/r.” There are 2 estimates for Wong et al19 because the authors reported 2 different measures of association: aHR (0.76; 0.67, 0.86) and aOR (0.43; 0.31, 0.59).
Effectiveness by age and UHCs
All-cause hospitalizations among patients aged ≥65 years treated with NMV/r versus no NMV/r were lower at 10–30 days (aHR range: 0.11–0.17; aOR range: 0.31–0.37) following COVID-19 diagnosis.14,23,24 In patients aged <65 years, all-cause hospitalizations were lower for NMV/r versus no NMV/r at 15–30 days (aHR range: 0.16–0.19 and aOR: 0.53; 0.34–0.80).14,24
For COVID-19-related hospitalizations, Shah et al found that NMV/r versus no NMV/r was effective against 30-day COVID-19-related hospitalization following diagnosis across age-groups (18–49 years: aHR = 0.59, 95% CI, 0.48–0.71; 50–64 years: 0.40, 0.34–0.48; ≥65 years: 0.53, 0.48–0.58).20 Arbel et al reported significantly lower 35-day COVID-19-related hospitalization for patients aged ≥65 years treated with NMV/r versus no NMV/r (aHR: 0.27; 0.15–0.49), but not in patients aged 40–64 years (aHR: 0.74; 0.35–1.58).21
No studies reported on all-cause hospitalization of patients with UHCs; however, COVID-19-related hospitalization at 30 days was significantly lower in patients with UHCs who received NMV/r versus no NMV/r. Shah et al reported a significant reduction in 30-day hospitalization in patients aged ≥18 years treated with NMV/r versus no NMV/r with 1 UHC or ≥2 UHCs (aHR: 0.57; 0.45–0.71 and 0.47; 0.44–0.51, respectively). This reduction in hospitalization among NMV/r-treated patients with ≥2 UHCs was seen across all age-groups, including 18–49 (aHR: 0.54; 0.43–0.67), 50–64 (aHR: 0.40; 0.34–0.48), and ≥65 years (aHR: 0.51; 0.47–0.56).20
Effectiveness by vaccination status
Patients treated with NMV/r versus no NMV/r had lower all-cause and COVID-19-related hospitalization regardless of vaccination status. Three studies (Zhou et al, Ganatra et al, and Aggarwal et al) reported significantly lower 30-day all-cause hospitalization among patients treated with NMV/r versus no NMV/r across vaccination groups, including unvaccinated (aOR: 0.46; 0.27–0.77), ≥1 vaccine dose (aOR: 0.43; 0.20–0.91 and aHR: 0.18; 0.12–0.28), 1–2 vaccine doses (aOR: 0.40; 0.20–0.79), and ≥3 vaccine doses (aOR: 0.47; 0.29–0.74).14,22,24 In addition, Zhou et al14 reported significantly lower all-cause hospitalization among vaccinated (≥1 dose) patients who were treated with NMV/r versus no NMV/r at 15 days (aHR: 0.11; 0.06–0.20).
For COVID-19-related hospitalizations, Shah et al reported a lower 30-day risk in patients treated with NMV/r versus no NMV/r across all vaccination groups, including unvaccinated (aHR: 0.50; 0.43–0.59), 2 vaccine doses (aHR: 0.50; 0.42–0.58), and ≥3 vaccine doses (aHR: 0.50; 0.45–0.55).20
Mortality
Two studies by Wong et al and Aggarwal et al reported NMV/r versus no NMV/r effectiveness of 66% and 85% against all-cause mortality (Figure 4),19,24 at 28 days (aHR: 0.34; 0.22–0.52; aOR: 0.15; 0.03–0.50) following confirmed COVID infection.19,24 The 30-day COVID-19-related mortality for patients following treatment with NMV/r versus no NMV/r was significantly lower (aOR: 0.49; 0.40–0.60).26
FIGURE 4.
Effectivenessa against all-cause mortality. During acute follow-up, the effectiveness of NMV/r against all-cause mortality versus no NMV/r ranged from 66% to 85%. aTreatment effectiveness percentages were calculated by subtracting hazard ratios or odds ratios from 1 and multiplying by 100. Note: The definition of “no NMV/r” varied across studies. The following definitions were used for “no NMV/r”: Xie et al17 used “did not receive other outpatient COVID-19 antiviral or antibody treatments within 30 days of the index date”; Bajema et al18 and Wong et al19 used “did not receive NMV/r or molnupiravir”; and Aggarwal et al24 and Hsu et al16 stated “not receiving NMV/r.” There are 2 estimates for Wong et al19 because the authors reported 2 different measures of association: aOR (0.23; 0.11, 0.49) and aHR (0.34; 0.22, 0.52).
Effectiveness by age and UHCs
Adjusted estimates of NMV/r effectiveness stratified by age, UHCs, or vaccination status were available for COVID-19-related mortality but not for all-cause mortality.
Arbel et al showed a significant reduction in 35-day COVID-19-related mortality risk among older (≥65 years) patients treated with NMV/r versus no NMV/r (aHR: 0.21; 0.05–0.82); however, the reduction was not significant among those aged 40–64 years (aHR: 1.32; 0.16–10.75), potentially due to low event rates.21 Schwartz et al26 reported significantly lower 30-day COVID-19-related mortality in patients aged ≥70 and <70 years with NMV/r versus no NMV/r (aOR: 0.48; 0.39–0.59 and 0.13; 0.03–0.57, respectively). Furthermore, Schwartz et al26 observed a significant reduction in 30-day COVID-19-related mortality in patients treated with NMV/r versus no NMV/r with <3 and ≥3 UHCs, including chronic heart disease and advanced liver disease (aOR: 0.50; 0.39–0.64 and 0.48; 0.34–0.67, respectively).
Effectiveness by vaccination status
Schwartz et al26 reported significantly reduced 30-day COVID-19-related mortality following treatment dispense date in patients treated with NMV/r versus no NMV/r across all vaccination groups, including unvaccinated (aOR: 0.34; 0.16–0.74), 1–2 vaccine doses (aOR: 0.23; 0.11–0.51), and ≥3 vaccine doses (aOR: 0.54; 0.43–0.67).
Composite of hospitalization or mortality
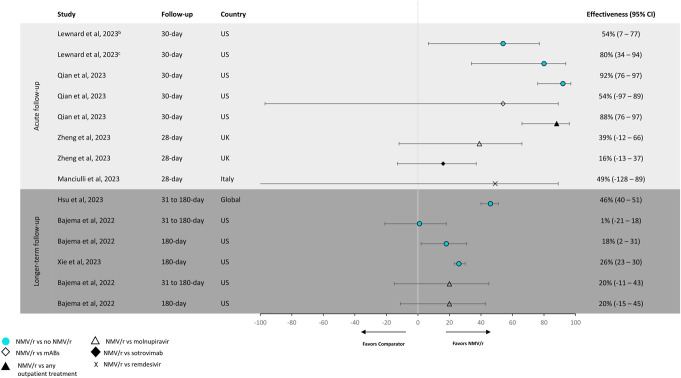
Four studies evaluated NMV/r effectiveness against all-cause hospitalization or mortality (Figure 5).27,29,31 In 2 of these studies that compared NMV/r with no NMV/r, NMV/r showed 54%–92% effectiveness in reducing the risk of the composite end point of hospitalization or mortality: this included 1 study (Qian et al) with a population of patients with systemic autoimmune rheumatic disease exhibiting the highest effectiveness and the other study (Lewnard et al) with a population of patients aged 12 years or older with effectiveness as high as 80%.30,31 In the 3 studies that assessed the effectiveness of NVM/r versus comparators (vs. monoclonal antibodies, molnupiravir, sotrovimab, and remdesivir), the effectiveness ranged from 16% to 88%.27,29,30
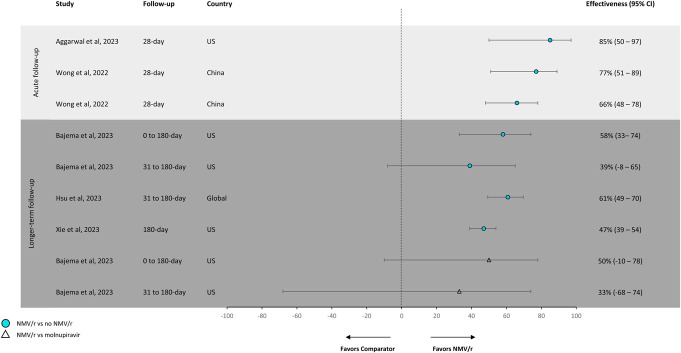
FIGURE 5.
Effectivenessa against all-cause hospitalization or mortality. NMV/r showed 54%–92% effectiveness in reducing the risk of the composite end point of hospitalization or mortality compared with no NMV/r during acute follow-up. aTreatment effectiveness percentages were calculated by subtracting hazard ratios or odds ratios from 1 and multiplying by 100. Lewnard et al (2023)31 was the only study to have effectiveness percentages already calculated. bPopulation includes all patients, regardless of treatment timing relative to symptom onset. cPopulation includes patients who received NMV/r treatment within 5 days of symptom onset. Note: The definition of “no NMV/r” varied across studies. The following definitions were used for “no NMV/r”: Qian et al30 used “no NMV/r, molnupiravir, monoclonal antibodies, remdesivir, or combination”; Xie et al17 used “did not receive other outpatient COVID-19 antiviral or antibody treatments within 30 days of the index date”; Bajema et al18 used “did not receive NMV/r or molnupiravir”; and Lewnard et al31 and Hsu et al16 stated “not receiving NMV/r.”
The effectiveness of NMV/r versus no NMV/r for the composite end point of COVID-19-related hospitalization or mortality was 44% and 52% in 2 studies (Figure 3).26,28 One other study assessed NMV/r versus molnupiravir or sotrovimab and reported an NMV/r effectiveness of 12% and 74%, respectively.29
Effectiveness by age and UHCs
Compared with no NMV/r treatment, NMV/r was associated with lower odds at 30 days across age-groups, including 18–64 years (aOR: 0.07; 0.02–0.31) and ≥65 years (aOR: 0.11; 0.02–0.54).30 NMV/r was associated with significantly lower composite 30-day all-cause hospitalization or mortality compared with no NMV/r (aOR: 0.08; 0.03–0.24) following a positive COVID-19 test among adults aged ≥18 years with a systemic rheumatic disease.30
Regarding the COVID-19-related composite end point of hospitalization or mortality, NMV/r was associated with significantly lower odds at 30 days following treatment dispense date compared with no NMV/r across age-groups, including patients aged <70 and ≥70 years (aOR: 0.34; 0.15–0.79 and 0.55; 0.45–0.66, respectively).26
For UHCs, Tadmor et al28 reported significantly lower composite 35-day COVID-19-related hospitalization or COVID-19-related mortality following a positive COVID-19 test among patients with chronic lymphocytic leukemia treated with NMV/r versus no NMV/r (aOR: 0.48; 0.13–0.63).
Effectiveness by vaccination status
Compared with no NMV/r treatment, a significantly lower composite end point of hospitalization or mortality within 30 days of a positive test for COVID-19 was seen among patients treated with NMV/r across various vaccinated groups, including vaccinated with 2 mRNA vaccine doses or 1 adenovirus dose (aOR: 0.03; 0.01–0.81) and boosted (>2 mRNA vaccine doses or >1 adenovirus dose; aOR: 0.09; 0.03–0.32) populations.30
In Lewnard et al, NMV/r versus no NMV/r had an overall estimated effectiveness of 53.6% (95% CI, 6.6–77.0) in preventing all-cause hospital admission or mortality within 30 days of a positive test for COVID-19 in a highly vaccinated patient population, which increased to 79.6% (95% CI, 33.9–93.8) when NMV/r was dispensed within 5 days of symptom onset.31 Effectiveness increased further to 89.6% (95% CI, 50.2–97.8) in analyses restricted to patients who were dispensed treatment on the same day as their positive test. Similar results were observed in a subgroup analysis of vaccinated patients, where an effectiveness of 83.1% (95% CI, 30.4–95.9) and 92.2% (95% CI, 52.0–98.7) was observed when NMV/r was dispensed within 5 days of symptom onset, and 55.3% (95% CI, 6.6–78.7) and 66.5% (95% CI, 24.0–85.3) when given at any time in individuals who received ≥2 or ≥3 doses of COVID-19 vaccine, respectively.31
Schwartz et al26 observed a significantly lower composite end point of hospitalization or mortality at 30 days among patients treated with NMV/r versus no NMV/r across vaccinated groups, including unvaccinated (aOR: 0.44; 0.23–0.84), 1–2 vaccine doses (aOR: 0.25; 0.12–0.50), and ≥3 vaccine doses (aOR: 0.62; 0.51–0.75).
Longer-term follow-up
Three studies evaluated outcomes of longer-term NMV/r treatment.16–18 No studies were identified that assessed longer-term all-cause or COVID-19-related outcomes by age, UHCs, or vaccination status.
Hospitalization
Two studies that evaluated all-cause hospitalization at 31–180 days following diagnosis in patients treated with NMV/r versus no NMV/r reported an effectiveness of 24% and 43%, respectively (Figure 2).16,17 No studies of NMV/r versus other treatments for COVID-19-related hospitalization were identified.
Mortality
Three studies reported aHR/aOR for NMV/r versus no NMV/r for all-cause mortality up to 180 days following diagnosis or NMV/r treatment with effectiveness ranging from 39% to 61% (Figure 4).16–18 Bajema et al18 reported similar results for NMV/r versus molnupiravir with an effectiveness of 33% at 0–180 days and 50% at 31–180 days.
Composite of hospitalization or mortality
Three studies of the NMV/r effectiveness against all-cause hospitalization or mortality within 180 days following diagnosis or NMV/r treatment are shown in Figure 5.16–18 All 3 compared NMV/r with no NMV/r and found a range of effectiveness of 1%–46%16–18; 1 study assessed the effectiveness of NVM/r versus molnupiravir within 180 days with an effectiveness of 20%.18
Therapeutic Advances
This SLR identified a total of 18 articles that assessed the real-world effectiveness of NMV/r in the prevention of acute and longer-term severe outcomes of COVID-19 from December 22, 2021, through March 31, 2023. These studies spanned Delta and Omicron variant predominance and consistently showed that NMV/r was associated with significantly lower hospitalization compared with no NMV/r treatment,14–17,19,20,22–25 regardless of age, UHCs, and vaccination history.14,20–24 In addition, the evidence indicates that NMV/r is associated with a significantly lower likelihood of a composite of all-cause or COVID-19-related hospitalization or mortality compared with no NMV/r treatment, across multiple UHCs and vaccination statuses.26,30
Three studies evaluated the real-world effectiveness of NMV/r in the 30–180 days posttreatment initiation or diagnosis,16–18 a follow-up period that has not been studied in a randomized clinical trial setting. NMV/r effectiveness outcomes beyond 30 days consistently demonstrated directionally positive results in reducing the risk of hospitalization, mortality, and the composite of hospitalization or mortality. COVID-19 postacute outcomes have been observed to peak in the first 3 months following acute infection32–35; therefore, events captured after this point may be less likely related to COVID-19 or treatment.
Real-world data have been an essential source of information for understanding the clinical relevance and generalizability of NMV/r efficacy demonstrated in the pivotal EPIC-HR trial. The findings from this SLR support the effectiveness of NMV/r across geography, SARS-CoV-2 variants, age, presence of UHCs, and vaccination status.8 We found that only 1 study, conducted by Lewnard et al, took into consideration the timing of symptom onset relative to treatment initiation, in alignment with the EPIC-HR trial and current treatment guidance.31 In this study, a substantial increase in effectiveness against all-cause hospitalization or mortality was observed among those where NVM/r was administered within 5 days of symptom onset (79.7%), compared with those where NVM/r was administered at any time (53.6%), and further increased (89.6%) in those whose treatment was dispensed on the day of their test, highlighting the importance of timely treatment.31 In retrospective studies using real-world data, accurate symptom onset data are hard to come by and positive SARS-CoV-2 tests or COVID-19 diagnosis dates are often used as a proxy for symptom onset, which, in most cases, occur later than the actual symptom onset. Thus, studies without information on symptoms and onset timing may be more likely to include patients with a longer interval between symptom onset and treatment initiation than those included in EPIC-HR and in the study of Lewnard et al.
Despite being the preferred option for outpatient treatment in the majority of countries, NMV/r is currently underutilized in real-world clinical practice.36–38 The reasons for this have not been established, but may be linked to unawareness of the treatment or uncertainty around SARS-CoV-2 rebound after NVM/r treatment, which has been reported widely in the media. Two recent studies—a literature review of randomized trial and observational studies conducted by the Centers for Disease Control and Prevention39 and an analysis of viral RNA shedding data from EPIC-HR and EPIC-SR conducted by the U.S. Food and Drug Administration40—reported similar virologic rebound rates between NVM/r treated and untreated patients.39,40 Moreover, virologic rebound after NVM/r treatment was not associated with COVID-19–related hospitalization or death.40 These findings continue to support the safety and efficacy of NMV/r in eligible patients and underscores national guidelines advising that rebound should not deter providers from prescribing this highly effective antiviral treatment when indicated to prevent severe COVID-19.
Several limitations of this SLR should be noted: first, several studies leveraged the same datasets. For example, 3 datasets were used across multiple studies—the US Department of Veterans Affairs COVID-19 Shared Data Resource,17,18 the TriNetX Analytics Network,16,22,25 and the Hospital Authority of Hong Kong.15,16,19 While these studies had differences in study design, including recruitment methods, inclusion/exclusion criteria, and length of follow-up, the study cohorts may not be mutually exclusive. Therefore, effectiveness estimates could have been influenced by patients represented in >1 study. Second, heterogeneity in cohort selection criteria of included studies (eg, UHCs, reporting of vaccination status, and treatment timing in relation to symptom onset), measurement of study end points, and differences in follow-up periods (eg, 3 studies assessed outcomes up to 180 days)16–18 precluded the conduct of a meta-analysis. Furthermore, most studies included in this analysis were evaluated as having moderate bias due to the observational nature of the studies and inherent limitations stemming from unmeasured confounders and analyses of heterogeneous real-world patient cohorts.
There have been 6 meta-analyses recently published with data generated during the Omicron period; findings of these studies are in line with previously published data.41–46 Li et al summarized 7 studies of vaccinated, high-risk patients and showed that NMV/r treatment reduced the incidence of all-cause hospitalization or mortality within 30 days for vaccinated patients (risk ratio: 0.53; 0.40–0.70).41 This benefit was higher in patients aged >65 years when compared with those aged 50–65 years. Another study by Tian et al42 evaluated 12 studies comparing NMV/r treatment with other antivirals and reported a reduction in longer-term mortality and disease progression. The meta-analysis by Souza et al43 summarized 14 studies of vaccinated and unvaccinated high-risk patients and found a reduction in the risk of hospitalization, mortality, or a composite of hospitalization and/or mortality with NMV/r versus standard treatment without antivirals of 53% (OR: 0.47; 0.36–0.60), 59% (OR: 0.41; 0.35–0.52), or 56% (OR: 0.44; 0.31–0.64), respectively. Subgroups <60 years and >60 years showed similar results. This SLR was intended to encompass a broader assessment of NMV/r effectiveness further organized by clinically meaningful categories, such as acute versus longer-term follow-up, age, UHCs, and vaccination status, thereby serving as the most comprehensive SLR of NMV/r effectiveness to date to the best of our knowledge. The scope of this SLR did not include nonsevere outcomes of COVID-19, such as health care resource utilization, emergency department visits, and long COVID.
CONCLUSION
This SLR summarized the evidence on NMV/r effectiveness against severe outcomes of COVID-19 reported between December 22, 2021, and March 31, 2023. The evidence showed that NMV/r effectively reduced hospitalization and mortality across geography, SARS-CoV-2 variants, age groups, UHCs, and COVID-19 vaccination status. Effectiveness was higher when NMV/r was received within 5 days of symptom onset in alignment with current treatment guidance and increased further when treatment was given on the same day as testing, emphasizing the importance of timely treatment for the prevention of severe illness.
ACKNOWLEDGMENTS
The authors thank Louise Crathorne, MSc, and Claudia Roeder, PhD, of AESARA who performed the bias assessment.
Footnotes
Funding for this study was provided by Pfizer, Inc.
A. S. Cha-Silva, T. Bergroth, R. Alexander-Parrish, J. Yang, F. Draica, J. M. McLaughlin, and J. L. Nguyen are employees and stockholders of Pfizer Inc. J. Patel, D. A. Garner, G. Meier and R. H. Stanford are paid consultants and M. B. Gavaghan is an independent contractor working on behalf of Pfizer Inc.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.americantherapeutics.com).
REFERENCES
- 1.Pfizer Press Release. Pfizer received U.S. FDA emergency use authorization for novel COVID-19 oral antiviral treatment. New York, NY: Pfizer Inc.; 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-receives-us-fda-emergency-use-authorization-novel. Accessed April 1, 2024. [Google Scholar]
- 2. PAXLOVID™. Highlights of Prescribing Information. New York, NY: Pfizer Inc.; 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf. Accessed July 12, 2023. [Google Scholar]
- 3.Paxlovid. European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid#authorisation-details-section. Accessed October 10, 2023.
- 4.Data on File. New York, NY: Pfizer Inc.; 2023. [Google Scholar]
- 5.Clinical management of adults summary NIH. Accessed July 12, 2023. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/clinical-management-of-adults-summary/
- 6.Final Draft Guidance: Nirmatrelvir Plus Ritonavir for Treating COVID-19 (Partial Review of TA878). London, England: NICE; 2023. Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta11324. Accessed October 4, 2023. [Google Scholar]
- 7.Bhimraj A Morgan RL Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2022;ciac724. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond J Leister-Tebbe H Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. New Engl J Med. 2022;386:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S. FDA. New York, NY: Pfizer Inc.; 2022. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting. Accessed October 19, 2023. [Google Scholar]
- 10.Higgins J Thomas J Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. London, England: Cochrane; 2023. Available at: https://training.cochrane.org/handbook/current. Accessed July 12, 2023. [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Atlanta, GA: Centers for Disease Control and Prevention; 2023. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed November 1, 2023. [PubMed] [Google Scholar]
- 13.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X Kelly SP Liang C, et al. Real-world effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID-19 at high risk for severe disease in the United States: a nationwide population-based cohort study. Clin Infect Dis. 2022;77:805–815. [Google Scholar]
- 15.Yip TC Lui GC Lai MS, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19). Clin Infect Dis. 2023;76:e26–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu WH Tsai YW Wu JY, et al. Post-acute hospitalization and mortality of nirmatrelvir plus ritonavir for COVID-19 survivors. J Infect. 2023;86:e107–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 2023;183:554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajema KL Berry K Streja E, et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med. 2022;176:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah MM Joyce B Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbel R Wolff Sagy Y Hoshen M, et al. Nirmatrelvir use and severe covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganatra S Dani SS Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019. Clin Infect Dis. 2023;76:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paraskevis D Gkova M Mellou K, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 community, highly vaccinated patients with high risk for severe disease: evidence that both antivirals reduce the risk for disease progression and death. medRxiv. 2023;02.09.23285737. doi: 10.1101/2023.02.09.23285737. [DOI] [Google Scholar]
- 24.Aggarwal NR Molina KC Beaty LE, et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis. 2023;23:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashash JG Desai A Kochhar GS, et al. Efficacy of paxlovid and lagevrio for COVID-19 infection in patients with inflammatory bowel disease: a propensity-matched study. Clin Gastroenterol Hepatol. 2023;21:841–843 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz KL Wang J Tadrous M, et al. Population-based evaluation of the effectiveness of nirmatrelvir–ritonavir for reducing hospital admissions and mortality from COVID-19. Can Med Assoc J. 2023;195:E220–E226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manciulli T, Spinicci M, Rossetti B, et al. Safety and efficacy of outpatient treatments for COVID-19: real-life data from a regionwide cohort of high-risk patients in Tuscany, Italy (the FEDERATE cohort). Viruses. 2023;15:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadmor T, Alapi H, Rokach L. Effectiveness of nirmatrelvir plus ritonavir treatment for patients with chronic lymphocytic leukemia during the Omicron surge. Blood. 2023;141:2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng B, Tazare J, Nab L, et al. Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform. medRxiv. 2023;2023:01.20.23284849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian G Wang X Patel NJ, et al. Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: a retrospective cohort study. Lancet Rheumatol. 2023;5:e139–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewnard JA McLaughlin JM Malden D, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023;23:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taquet M Dercon Q Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizrahi B, Sudry T, Flaks-Manov N, et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380:e072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiemken TL McGrath LJ Andersen KM, et al. Coronavirus disease 2019 severity and risk of subsequent cardiovascular events. Clin Infect Dis. 2023;76:e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin R. Paxlovid is effective but underused-here's what the latest research says about rebound and more. Jama. 2024;331:548. [DOI] [PubMed] [Google Scholar]
- 37.Kozlov M. COVID drug Paxlovid was hailed as a game-changer. What happened? Nature. 2023;613:224–225. [DOI] [PubMed] [Google Scholar]
- 38.Scott A Puzniak LA Murphy MV, et al. 522. Real-world, retrospective descriptive study of COVID-19 outpatient therapeutic uptake in high-risk patients subsequently hospitalized for COVID-19 during the Omicron period in the US. Open Forum Infect Dis. 2023;10:ofad500.591. [Google Scholar]
- 39.Smith DJ, Lambrou A, Patel P. SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals. MMWR Morb Mortal Wkly Rep. 2023;72:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington PR Cong J Troy SB, et al. Evaluation of SARS-CoV-2 RNA rebound after nirmatrelvir/ritonavir treatment in randomized, double-blind, placebo-controlled trials—United States and international sites, 2021–2022. MMWR Morb Mortal Wkly Rep. 2023;72:1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Xiang H, He B, et al. Nirmatrelvir plus ritonavir remains effective in vaccinated patients at risk of progression with COVID-19: a systematic review and meta-analysis. J Glob Health. 2023;13:06032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian F, Chen Z, Feng Q. Nirmatrelvir-ritonavir compared with other antiviral drugs for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95:e28732. [DOI] [PubMed] [Google Scholar]
- 43.Souza KM, Carrasco G, Rojas-Cortés R, et al. Effectiveness of nirmatrelvir-ritonavir for the treatment of patients with mild to moderate COVID-19 and at high risk of hospitalization: systematic review and meta-analyses of observational studies. PLoS One. 2023;18:e0284006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheema HA Jafar U Sohail A, et al. Nirmatrelvir-ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95:e28471. [DOI] [PubMed] [Google Scholar]
- 45.Amani B, Amani B. Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis. J Med Virol. 2023;95:e28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu CT Yin JY Chen XH, et al. Appraisal of evidence reliability and applicability of Paxlovid as treatment for SARS-COV-2 infection: a systematic review. Rev Med Virol. 2023;33:e2476. [DOI] [PubMed] [Google Scholar]