Abstract
Introduction:
The blood-brain barrier (BBB) is a critical neurovascular unit regulating substances' passage from the bloodstream to the brain. Its selective permeability poses significant challenges in drug delivery for neurological disorders. Conventional methods often fail due to the BBB's complex structure.
Aim:
The study aims to shed light on their pivotal role in revolutionizing neurotherapeutics and explores the transformative potential of BBB-on-a-Chip technologies in drug delivery research to comprehensively review BBB-on-a-chip technologies, focusing on their design, and substantiate advantages over traditional models.
Methods:
A detailed analysis of existing literature and experimental data pertaining to BBB-on-a-Chip technologies was conducted. Various models, their physiological relevance, and innovative design considerations were examined through databases like Scopus, EbscoHost, PubMed Central, and Medline. Case studies demonstrating enhanced drug transport through BBB-on-a-Chip models were also reviewed, highlighting their potential impact on neurological disorders.
Results:
BBB-on-a-Chip models offer a revolutionary approach, accurately replicating BBB properties. These microphysiological systems enable high-throughput screening, real-time monitoring of drug transport, and precise localization of drugs. Case studies demonstrate their efficacy in enhancing drug penetration, offering potential therapies for diseases like Parkinson's and Alzheimer's.
Conclusion:
BBB-on-a-Chip models represent a transformative milestone in drug delivery research. Their ability to replicate BBB complexities, offer real-time monitoring, and enhance drug transport holds immense promise for neurological disorders. Continuous research and development are imperative to unlock BBB-on-a-Chip models' full potential, ushering in a new era of targeted, efficient, and safer drug therapies for challenging neurological conditions.
Keywords: blood-brain barrier, chip, delivery, drug, neurotherapeutics, neuro-vasculature
Introduction
Highlights
The blood-brain barrier (BBB) is a critical neurovascular unit regulating substances’ passage from the bloodstream to the brain. Its selective permeability poses significant challenges in drug delivery for neurological disorders. Conventional methods often fail due to the BBB’s complex structure.
Microphysiological systems, employing microfluidic technology, intricately emulate the complex peripheral and central nervous systems, among other organs. These systems, encompassing organoids, three-dimensional (3D) printed tissues, and organ-on-a-chips, amalgamate in-vivo and in-vitro models, facilitating dynamic fluid flow within a 3D environment for precise tissue mimicry.
For the treatment of neurological illnesses, the use of BBB-on-a-Chip models in drug delivery experiments shows great promise. Researchers may be able to create treatments for diseases that were previously thought to be challenging to target, such as Parkinson’s disease, Alzheimer’s disease, and brain tumours, by enhancing medication transport over the BBB.
The central nervous system relies on the intricate biochemical processes involving macronutrients such as carbohydrates, fats, and proteins, as well as essential micronutrients including B vitamins and coenzymes for its sustenance and physiological stability. These processes are meticulously regulated by the blood-brain barrier, as illustrated in Figure 1 1. This intricate barrier serves as a gatekeeper, controlling the influx of various compounds from the bloodstream into the brain tissue, thus safeguarding the delicate neural environment2.
Figure 1.
History of blood-brain barrier.
The central nervous system is susceptible to pathological damage arising from infections, vascular diseases, immunological dysregulation, and degenerative processes, necessitating efficient therapeutic intervention3. Drug delivery, the method of administering medicinal compounds, is crucial for achieving therapeutic or preventive effects in humans. This process involves four fundamental stages: absorption, influenced by drug dosage and gut pH; distribution, affected by drug binding to plasma proteins, perfusion, diffusion; metabolism, regulated by enzyme systems; and excretion through organs such as the kidneys, lungs, saliva, and bile.
Drug delivery to the brain encounters a significant obstacle in the form of the blood-brain barrier (BBB). This barrier selectively permits entry only to lipophilic molecules with low molecular weight, rendering ~98% of pharmaceutical compounds unable to traverse it4,5. Enhancing this process holds paramount importance for several reasons. Physically, optimizing drug delivery enables smoother access to managing brain disorders by increasing drug distribution within the brain parenchyma. It allows for precise localization of drugs to specific sites of action, reducing systemic toxicity and enhancing treatment efficiency. Moreover, it mitigates the risk of clinical failure for potentially effective therapeutic agents. Psychologically, prolonged illness due to inefficient treatment correlates with an elevated risk of psychiatric disorders, hindering both the recovery process and overall quality of life. Neurodegenerative diseases, in particular, are linked to disruptions in emotions, cognition, and social behaviour6. Economically, inefficiencies in pharmacokinetic processes within brain tissues lead to protracted and financially burdensome treatment journeys. Therefore, improving drug delivery to the brain is inversely proportional to the duration and affordability of treatment.
This article aims to comprehensively review the existing literature pertaining to the development of a BBB on a chip for optimizing drug delivery to the brain.
History
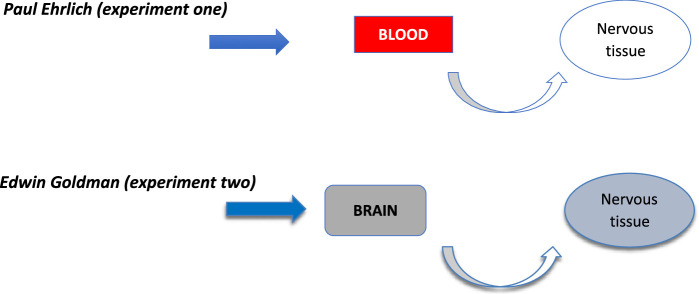
The origins of our understanding of the BBB can be traced back over a century ago. In a serendipitous turn of events, Nobel laureate Paul Ehrlich injected blue dye into mouse blood, leading to the revelation that the nervous tissue remained impermeable to the dye7. It was not until 50 years later that physician Edwin Goldman conducted a groundbreaking experiment, by injecting blue dye directly into the mouse brain, Goldman demonstrated that the nervous tissue indeed absorbed the dye, marking a pivotal moment in scientific inquiry7. This pivotal experiment laid the foundation for the concept of the blood-brain barrier, which is characterized as a selectively permeable barrier. It is composed of epithelial-like cells exhibiting high resistance tight junctions within the endothelium of capillaries.
The BBB
The BBB stands as the largest interface governing the exchange between the bloodstream and the brain, acting as a vital neurovascular unit which is composed of distinct elements8:
Endothelial cells
These cells form a monolayer of simple squamous epithelial cells lining the capillary walls. They are set apart from other vascular regions by the presence of tight junctions, luminal polarization, and a high abundance of mitochondria. Functionally, endothelial cells serve as a robust barrier, altering the physical properties of substances to control their passage.
Astrocytes
These star-shaped cells envelop the surface of cerebral capillaries. They function as potassium channels, contribute to the maintenance of water homoeostasis, and regulate ionic concentrations. Astrocytes play a pivotal role in the intricate workings of the blood-brain barrier.
Pericytes
Positioned between other cells, pericytes act as phagocytes, clearing foreign molecules and contributing significantly to maintaining the stability of the barriers. Their role is crucial in preserving the integrity of the BBB.
Basement membrane
This membrane serves as the structural foundation, connecting the cells of the barrier. It plays a vital role in regulating communication between the intracellular and extracellular environments, ensuring the coordinated functioning of the components.
Tight junctions
Representing the epitome of cellular barricades, tight junctions are apical cell-cell junctions characterized by their impermeability. These junctions consist of a group of proteins, including transmembrane proteins such as occludin and claudin-5, as well as various cytoplasmic proteins. Together, these components form a strong defense, allowing only select molecules to traverse the barrier, thus upholding the sanctity of the brain environment.
The BBB serves six critical functions: it maintains brain stability by safeguarding against pathogens and neurotoxins, ensures ionic homoeostasis and brain nourishment through specific ion channels and transporters, regulates neurotransmitters by guiding their influx, restricts plasma macromolecules from leaking into the brain—particularly controlling protein leakage to prevent neural apoptosis—and adjusts the permeability of cerebral capillaries. Additionally, it maintains brain volume by regulating the flow of water and salts9.The unique structure and functions of the blood-brain barrier pose three significant challenges for drug treatments targeting brain disorders10:
Structural challenge
The blood-brain barrier is characterized by a lipophilic plasma membrane allowing influx of only very small molecules and tight capillary junctions. These features, although obstacles, present opportunities for developing innovative drug delivery systems.
Chemical challenge
Multiple receptors, including transferrin and insulin receptors, are found on the surface of endothelial cells within the blood-brain barrier. Moreover, the barrier contains nuclear receptors expressing drug-metabolizing enzymes. Modulation of these receptors can enable drugs to traverse the barrier.
Transport-mediated challenge
The BBB features plasma membranes housing influx and efflux transporters that do not transport pharmaceuticals to the brain. Targeting these transporters offers a viable strategy to enhance drug delivery to the brain.
In response to these challenges, innovative methods and strategies have been devised for drug delivery to the brain. The current methodologies and their limitations are comprehensively documented and analyzed in Table 1 11.
Table 1.
Current methods and limitations in drug delivery
| Method | Type | Advantages | Challenges | Limitations |
|---|---|---|---|---|
| Nanoparticles | Noninvasive | Brain targeting using specific physiological status | Structural | Neurotoxicity and cytotoxicity |
| Bypass BBB | Noninvasive | Bypass the BBB through intranasal, interstitial and intrathecal | 3 CHALLENGES | Suitable for low dose only |
| Brain permeability enhancer | Noninvasive | Transiently open the BBB | Structural | Risk for human due to mismatch with rodents during clinical trials |
| Receptor mediated transcytosis, receptor antagonist and enzyme modulator | Noninvasive | High specificity, selectivity and affinity | Chemical | Toxicity |
| Carrier-mediated transport | Noninvasive | Ability to cross the BBB by intravenous injection | Transport mediated | Mainly for small molecules |
| Convection-enhanced delivery | Invasive | Reduces side effects to healthy brain tissue | 3 CHALLENGES | Leakage of drug leading to toxicity |
BBB, blood-brain barrier.
Introduction to microphysiological systems (Organ-on-a-chip)
Microphysiological systems, employing microfluidic technology, intricately emulate the complex peripheral and central nervous systems, among other organs. These systems, encompassing organoids, three-dimensional (3D) printed tissues, and organ-on-a-chips, amalgamate in-vivo and in-vitro models, facilitating dynamic fluid flow within a 3D environment for precise tissue mimicry12. Notably, they replicate patient-specific pathology, disease initiation, and progression timelines13. These systems boast 3D structures mirroring human organs, ensuring physiologically relevant cell-cell and cell-matrix interactions, and facilitating real-time monitoring of disease progression and organ responses to drugs14,15. Pharmaceutical companies leverage these advantages to enhance drug discovery outcomes, improving accuracy and reducing costs by evaluating drug toxicity, safety, and efficacy16. Furthermore, given the brain’s intricate nature, microphysiological systems offer invaluable insights into neuronal transport and regeneration. Their 3D environment makes them ideal for simulating the physical and physiological complexities of the blood-brain barrier17. Despite challenges in differentiation protocols, recent strides in deriving brain endothelium from human-induced pluripotent stem cells (iPSCs) expand the array of brain cell types available for clinically relevant models in central nervous system diseases18,19 (Table 2).
Table 2.
A comparative analysis of physiological and anatomical design consideration, cells and biomaterial, and their usage in the nervous system
| System | Physiological and anatomical design consideration | Cells and biomaterial | Usage | Refs |
|---|---|---|---|---|
| Microvasculature | Endothelium lining BBB using fluidic shear stress Blood vessels using fluid shear stress |
Cells: HUVECs, hCMEC/D3 Fabric: Microfluidic Cells: HUVECs/mouse fibroblasts/mouse smooth muscle cells Fabric: microextrusion bioprinting, Microfluidic |
Drug testing Drug testing System development |
20,21 |
| Brain | Neural tissue Blood-brain barrier |
Cells: 1-rat embryonic neurons and astrocytes,mouse neural stem cells, mouse primary cortical neurons 2-hESCs, hPSCs Fabric: 1-microextrusion bioprinting 2- microwell array Cells: human limbal cells Fabric: human amniotic membrane Cells: endothelial/ astrocytic cell lines; hBMVEC/pericytes/astrocytes Fabric: Microfluidics |
System development and drug testing Drug testing |
22,23
24 |
| Eye | Corneal epithelium Corneal stroma using cell alignment Corneal endothelial layer using mechanical properties full-thickness cornea |
Cells: human limbal cells Fabric: human amniotic membrane Cells: human and rabbit corneal fibroblasts, hCSSCs/hCFs Human keratocytes Fabrics: silk microgrooves; microextrusion bioprinting Cells: primary human corneal endothelial cells, human, sheep or bovine corneal endothelial cell lines Fabric: hydrogel substrates rabbit corneal epithelial cells/keratocytes/endothelial cells hESC-derived limbal epithelial cell-like cells/corneal endothelial cell-like cells Fabric: 1- fibrin-agarose hydrogels 2- decellularized porcine cornea |
Epithelium transplantation System development System development Cornea transplantation System development Cornea transplantation |
25
26,27 28,29 30,31 |
| Tumour | Vascular interface using fluidic shear stress Stromal interaction using fluidic shear stress Glioblastoma using oxygen gradient |
Cells: HDMEC/ breast cancer cells HUVECs, lung fibroblasts, monocytes, melanoma cancer cells Bone marrow stromal cells/ liver tumour cells Patient-derived glioblastoma and vascular endothelial cells Fabric: microfluidics, microextrusion bioprinting |
Drug testing and system development | 32–35 |
BBB, blood-brain barrier; Refs, references.
Design and construction of BBB-on-a-chip
The organ-on-a-chip technique integrates in-vivo and in-vitro models, enhancing physiological microenvironments, imaging systems, and real-time sensor outputs36. To replicate the human BBB, models are exposed to physiological fluid flow, establishing realistic dimensions. Various BBB models include static, Dynamic in-vitro model (DIV), and BBB-on-a-chip models. BBB-on-a-chip models, which employ microfluidic technology, have excelled, considering blood flow effects and enabling specific molecule transport screening37. These models reconstruct tight junctions in monoculture and co-culture settings, incorporating endothelial cells with astrocytes and pericytes in 2D and 3D microenvironments24,38.
Microvasculature endothelial cells from animals or humans are subjected to physiological shear stress. BBB models on chips also allow the study of complex mechanisms for leucocyte recruitment39. Additionally, the glymphatic pathway, clearing brain solutes potentially linked to neurodegenerative diseases, can be intensively studied using microfluidic devices, applying physiologically relevant blood pressure, intracranial pressure, and flows40,41. Transepithelial electrical resistance (TEER) serves as a quick, noninvasive measure of brain tightness, comparable between devices. Recording electrical and biochemical signals of sensory neurons presents challenges, addressed using multiple microelectrodes and compatible microimaging systems on PDMS chips. Microfluidic combined microelectrodes facilitate subcellular structure visualization and neuronal electrical activity measurement on BBB chips42 (Table 3).
Table 3.
Different types of BBB-on-chip models
| Types of BBB models | Materials and components | Cell types | Culture techniques | Application and limitation | Refs |
|---|---|---|---|---|---|
| Vertical 2D culture | PDMs | HBMEs, pericytes, astrocytes, hiNPCs | PC | Modelling of the BBB function and drug testing ( toxicity) and permeability of CNS | 43 |
| Parallel 3D chambers | PDMS | RBE4 cells and astrocytes | Pores generated by lithography between two chambers | Intergration of human BBB microfluidic model into a high-throughput plate-based format for drug screening purposes | 44 |
| Static 2D models (in vitro ) | PLGA nanofiber mesh Collagen gel covered with monolayer of brain microvascular endothelium |
HIPSC-EC and astrocytes Brain endothelial cells Pericytes and MSCs IPSC-BMECs, astrocytes, pericytes and neurons |
Polymer trans well membrane model Membrane free hydrogel BBB model |
Human BBB physiology and pathology with higher TEER value and good barrier functions Nanoparticule transcytosis quantification and transendothelial PEG-P (CL-g-TMC)polumersomes delivery. BBB phenotypes with TJ and permeability and up-regulating pericytes mark. |
45
46 47 48 |
| 3D biomimetic multichannels culture | NA | BEnd.3, U87 gliobalstoma cells | PC | Formation of a 1:1 scale biomimetic BBB model with satisfied TEER and capability for drug screening. | 49 |
| Parallel 3D multichannels culture | PDMS | hUVEC, rat astrocytes in gel, rat neurons in gel | NA | New platform for the development of a more sophisticated and complex 3D in-vitro neurovascular model and has good observation of neurons. | 50 |
| 2D vertical tandem multi chamber | PDMS | HBMECs, astrocytes, pericytes | PC | Mimicking the effects of intravascular administration of the psychoactive drug methamphetamine and determines metabolic coupling between the BBB and neurons. | 51 |
| DIV-model | Transmural microholes | Astrocytes and hECs PBMECs |
3D vasculogenic model QV-600 chamber multi chamber perfusion system |
Studies BBB Enhance and maintain TEER for longer Used to investigate penetration of anti-epileptic drugs. |
52
53 54 |
| Microfluidic 3D model | Collagen I gel | ECs, astrocytes and pericytes | 3D vasculogenic hydrogel model | Simple, new, cost effective in-vitro model for targeting neuroinflammatory conditions | 55 |
2D, two dimensional; 3D, three dimensional; BBB, blood-brain barrier; BMEC, brain microvascular endothelial cell; CNS, central nervous system; EC, endothelial cell; h, human; hiPSC, human-induced pluripotent stem cell; iNPCs, induced neuron progenitor cells; m, mouse; NA, not applicable; NSC, neuron stem cell; PC, polycarbonate; PDMS, polydimethylsiloxane; PET, polyethylene terephthalate; r, rat; TEER, transepithelial electrical resistance; UVEC, umbilical vein endothelial cords.
Mimicking BBB properties recapitulating BBB-specific features
Mimicking the intricate qualities of the BBB involves replicating its unique characteristics, such as tight junctions between endothelial cells acting as a physical barrier, specialized transporters controlling molecule transit, enzymatic metabolism of certain chemicals, and complex cellular signalling processes regulating permeability56,57. Researchers utilize cutting-edge models like BBB-on-a-Chip systems and co-culture approaches to replicate these BBB features, enabling the creation of specialized treatments for neurological illnesses and enhancing understanding of drug interactions with the BBB.
Testing and validation of BBB-on-a-chip models
The development of BBB-on-a-Chip models offers a potential strategy for imitating BBB features37,58. These microfluidic systems incorporate cells, tissues, and biomimetic components, mimicking the fundamental properties of the BBB59. Essential steps in evaluating these models involve rigorous testing and validation. Researchers assess the permeability of the Brain-on-a-Chip to various compounds, including medicines and nanoparticles, to gauge its resemblance to the in-vivo BBB60. Furthermore, they evaluate the model’s receptivity to diverse stimuli and its ability to reproduce unique traits specific to particular diseases. This evaluation process is crucial for researching neurological disorders and conducting medication screening61,62.
Advantages of BBB-on-a-chip models over traditional in-vitro and in-vivo models
Compared to traditional in-vitro and in-vivo models, BBB-on-a-Chip models offer numerous advantages63. They enhance drug behaviour prediction at the barrier, increase physiological relevance by simulating dynamic cell interactions within the BBB, and mitigate ethical concerns associated with animal testing. Additionally, these models are more economical and resource-efficient, addressing ethical issues and significantly reducing research timeframes64. In conclusion, BBB-on-a-Chip models present a promising method for simulating BBB characteristics. They offer substantial precision, effectiveness, and ethical considerations in neuroscientific and pharmaceutical research, ultimately advancing our comprehension of the blood-brain barrier and neurological diseases37,65 (Table 4).
Table 4.
Comparison between traditional in-vivo models, traditional in-vitro models, and BBB-on-a-chip models in different aspects
| Aspect/areas | Traditional in-vitro models | Traditional in-vivo models | BBB-on-a-chip models |
|---|---|---|---|
| Physiological relevance | Limited capacity to accurately imitate BBB physiology. | Although in-vivo models frequently mimic the BBB, they might not accurately reflect human biology. | High quality replication of BBB features, such as tight junctions, transporters, and cellular connections. |
| High throughput | Slower and less suited to high-throughput screening. | Expensive and time-consuming, particularly for animal investigations. | Allow for the testing of numerous drug candidates in parallel, speeding up the drug discovery process. |
| Real-time monitoring | Most conventional in-vitro models have a limited ability for real-time monitoring. | In-vivo real-time data collecting is feasible but could be intrusive and complicated. | Real-time evaluation of drug transport and BBB responses with the provision of dynamic data. |
| Disease modelling | Accuracy in reproducing illness conditions is limited. | Diseases can be replicated in in-vivo models, although they may be difficult to modify or control. | Can be used to examine drug distribution in the setting of neurological illnesses and add disease-specific components. |
| Ethical considerations | Involves using animals, which raises ethical issues and regulatory difficulties. | Involves animal testing, which might lead to logistical and ethical issues. | Reducing the necessity for animal testing will help to address ethical issues. |
| Cost-effectiveness | The price may vary, but it might be less than for in-vivo experiments. | Frequently more resource-intensive, including upkeep and care for animals. | Cost-effective because fewer resources are needed. |
BBB, blood-brain barrier.
Drug delivery optimization
How BBB-on-a-chip enhances drug delivery studies
Drug delivery studies have been completely transformed by BBB-on-a-Chip models, especially in the context of neurological illnesses. For enhancing drug delivery, these microfluidic devices, which mimic the BBB, provide numerous benefits: (Table 5).
Table 5.
Advantages/usages of microfluidic devices (BBB-on-a-chip models) in different areas
| Areas | Advantages/usage |
|---|---|
| Physiological relevance | Tight connections between endothelial cells and the existence of transporters are two distinguishing characteristics of the BBB that BBB-on-a-Chip models accurately mimic62,66. Because of this physiological relevance, scientists/researchers may examine how medications interact with the barrier in a controlled setting, giving them information about whether they can pass the BBB67. |
| High throughput | Potential drug candidates can be screened in a high-throughput manner using these models. Multiple substances can be tested at once by researchers hastening the drug development process68. |
| Real-time monitoring | Drug transport and cellular responses can be observed in real-time using BBB-on-a-Chip technologies, hence, this dynamic examination offers insightful information on medication permeability and potential BBB side effect69–71. |
| Disease modelling | These models allow for the incorporation of disease-specific components, enabling researchers to examine drug delivery in the setting of neurological diseases like Alzheimer’s or glioblastoma. This aids in identifying prospective treatments and evaluating their effectiveness71–73. |
BBB, blood-brain barrier.
Case studies demonstrating improved drug transport
The increased drug transport abilities of BBB-on-a-Chip models are highlighted in several case studies. For instance, researchers have tested potential treatments for brain tumours using these systems and have seen better drug penetration through the BBB, resulting in more successful therapies69,74. Additionally, BBB-on-a-Chip models have contributed in the discovery of compounds that can improve medicine delivery to the brain, potentially reducing the progression of neurodegenerative illness75,76.
Potential impact on neurological disorders
For the treatment of neurological illnesses, the use of BBB-on-a-Chip models in drug delivery experiments shows great promise. Researchers may be able to create treatments for diseases that were previously thought to be challenging to target, such Parkinson’s disease, Alzheimer’s disease, and brain tumours, by enhancing medication transport over the BBB77,78. The use of medications that are specifically targeted to afflicted brain regions while sparing healthy tissue may result in more effective therapy with fewer adverse effects.
In the context of neurological illnesses, BBB-on-a-Chip models have opened up new opportunities for drug delivery optimization. They may speed up the development of novel therapies by simulating BBB features and enabling high-throughput screening, thereby enhancing the prognosis for patients with difficult neurological disorders.
Addressing challenges and future directions
Despite significant progress, challenges persist in the utilization of BBB-on-a-chip, hindering its widespread adoption. Accurately replicating the intricate BBB within microfluidic chips remains a challenge. Commonly used Polydimethylsiloxane (PDMS) cannot combine with hydrophobic compounds, impacting drug concentration and efficacy. Addressing this, thermoplastic-based chip fabrication emerges as an optimal solution due to superior compatibility with biomimetic materials, enhanced stability, light permeability, and electrical conductivity78. Economical concerns also loom large. Scaling up production and ensuring cost-effectiveness for widespread use in pharmaceutical research and clinical applications demand attention. While PDMS is prevalent in academic research, its unsuitability for large-scale commercial production necessitates exploration of alternatives like thermoplastics, despite their manipulation challenges65.
Preserving BBB-on-a-chip stability and consistency is paramount. Replicating mechanical forces influencing BBB functions, such as pulsatile blood flow, cues from neighbouring tissues, and shear stress, remains complex yet vital for accurate drug delivery optimization. Human-induced pluripotent stem cells (iPSCs) offer a promising solution. They generate Brain Microvascular Endothelial Cell (BMEC)-like monolayers expressing tight junctions and BBB-related markers. Further differentiation protocol modifications could yield transcriptomically closer iBMECs, enhancing BBB modelling. iPSC-based BBB-on-a-chip exhibits physiologically significant Transepithelial/Transendothelial Electrical Resistance (TEER) values, crucial for maintaining BBB function under elevated shear stress79.
Emerging technologies, such as advanced nanoparticle formulations and biomimetic nanoparticles, are revolutionizing drug transport across the BBB80,81. Noninvasive modulation through focused ultrasound techniques82 and cutting-edge imaging methods for real-time monitoring83 and assessing novel delivery methods84 further enhance BBB-on-a-chip capabilities. Interdisciplinary collaborations among pharmacology, biology, engineering, and materials science experts are pivotal in designing and optimizing these microfluidic systems85. BBB-on-a-chip holds tremendous potential to enhance drug delivery to the brain, addressing various neurological conditions and central nervous system diseases86.
Limitations
The study encountered several noteworthy limitations. The authors faced logistical challenges arising from disparate geographical locations, diverse time zones, varying educational backgrounds and degrees, as well as distinct work responsibilities. These inherent differences impeded the scheduling and participation in discussion meetings, thereby affecting the efficiency of follow-up and revision processes. Additionally, the insufficient number of articles pertinent to the research topic posed a challenge, as it fell short of the targeted coverage.
The study content faced limitations aligned with the three specific objectives of this review paper. Firstly, in addressing the BBB and the associated drug delivery process, challenges were encountered in the creation of a visually impactful illustration elucidating the intricate structure of the BBB. Moreover, the extensive array of methods for drug delivery to the brain led to a somewhat congested presentation of data. Secondly, while exploring organ-on-chip technologies, limitations arose from the difficulty in providing a concise explanation of the prior applications of this technology due to the scarcity of comprehensive research papers on the topic. Thirdly, in outlining the drug delivery optimization process through the BBB on a chip, limitations emerged during the validation of its significance through case studies, with only four studies identified, resulting in a less robust justification for the intervention. Additionally, the broad scope of discussion regarding the validation of BBB-on-a-chip models presented challenges.
We recommend several directions for further exploration of this topic. These include a heightened focus on experimental research and case studies, a dedicated investigation into the efficacy of BBB-on-a-chip in neurodegenerative diseases, and an enhancement of the scope of discussion through the incorporation of illustrative elements and straightforward justifications.
Conclusion
The advent of BBB-on-a-Chip models marks a pivotal breakthrough in drug delivery research. These microphysiological systems faithfully replicate the intricacies of the BBB, providing a controlled setting to study drug interactions and assess medication permeability in neurological disorders. Unlike traditional in-vitro and in-vivo models, BBB-on-a-Chip models confer notable advantages. They facilitate high-throughput screening, swiftly evaluating multiple drug candidates, and offer real-time monitoring of drug transport and cellular responses, yielding invaluable insights into medication delivery. This innovative approach not only deepens our understanding of neurological disorders but also heralds targeted, more efficient, and safer drug therapies for challenging conditions like Parkinson’s disease, Alzheimer’s disease, and brain tumours.
Rigorous testing and validation are imperative to ensure the accuracy and reliability of BBB-on-a-Chip models. Researchers meticulously evaluate these models’ permeability to various compounds, replicating real-world conditions. Through meticulous testing, scientists can assess the models’ responsiveness to stimuli and disease-specific traits, augmenting their utility in neurological research and drug screening. While BBB-on-a-Chip models exhibit astounding potential, persistent challenges need addressing. Accurately replicating the BBB’s complexity within microfluidic chips, ensuring stability and consistency, and tackling economic concerns are pivotal tasks. Additionally, emerging technologies like advanced nanoparticles, focused ultrasound techniques, and cutting-edge imaging methods present new avenues for exploration. Interdisciplinary collaborations among experts in diverse fields are imperative for optimizing these microfluidic systems, paving the way for groundbreaking advances in brain drug delivery.
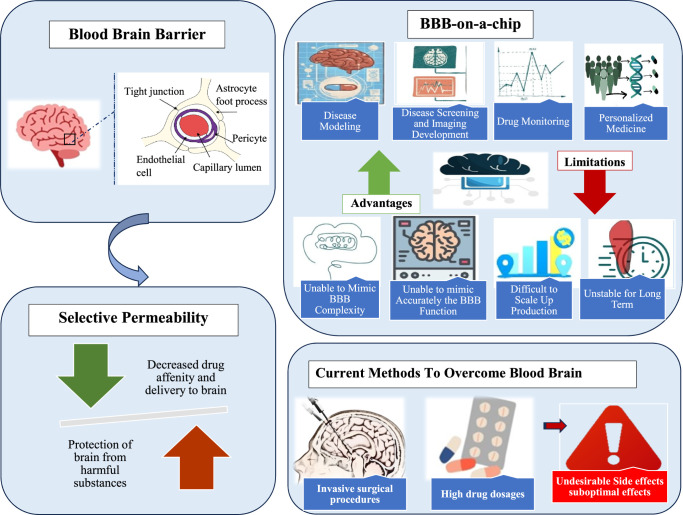
As these promising advancements unfold, a compelling need for ongoing research and development becomes apparent. Collaborative efforts across scientific disciplines are vital to refining BBB-on-a-Chip models, overcoming challenges related to replicating BBB complexity, and ensuring long-term stability. This call for continuous research underscores the urgency in unlocking the full potential of BBB-on-a-Chip models, seamlessly integrating them into mainstream drug delivery strategies, and significantly improving patient outcomes in the realm of neurological diseases (Fig. 2).
Figure 2.
Summary of the various key points discussed in the manuscript.
Ethics approval
Not applicable.
Consent
Informed consent was not required for this article.
Source of funding
We have not received any financial support for this manuscript.
Author contribution
All authors have approved the final manuscript for submission. B.K.: supervising the draft, reviewing and editing, project administration. O.U.: conceptualization, writing-review and designing, project administration. S.S.: writing the first draft and revising. N.R.: writing the first draft and revising. V.F.: writing the first draft and revising. Y.H.: writing the first draft and revising. N.M.: writing the first draft and revising. K.Y.: writing the first draft and revising.
Conflicts of interest disclosure
The author declared no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Abubakar Nazir.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
The authors appreciate the Journal Editors’s valuable feedback.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Published online 4 March 2024
Contributor Information
Burhan Kantawala, Email: burhanuddin72@hotmail.com.
Sanobar Shariff, Email: sanobarshariff@gmail.com.
Nagham Ramadan, Email: naghamramadan2001@gmail.com.
Violette Fawaz, Email: violette.fawaz@hotmail.com.
Youmna Hassan, Email: youmna.h00@gmail.com.
Nadine Mugisha, Email: munadine@gmail.com.
Konstantin Yenkoyan, Email: enkoyan@yahoo.com.
Abubakar Nazir, Email: ABU07909@GMAIL.COM.
Olivier Uwishema, Email: uwolivier1@ktu.edu.tr.
References
- 1.Muth A-K, Park SQ. The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr 2021;40:3999–4010. [DOI] [PubMed] [Google Scholar]
- 2.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview. Neurobiol Dis 2004;16:1–13. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari G, Tiwari R, Bannerjee S, et al. Drug delivery systems: an updated review. Int J Pharm Investig 2012;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam Y, Leach AG, Smith J, et al. Physiological and pathological factors affecting drug delivery to the brain by nanoparticles. Adv Sci (Weinh) 2021;8. Accessed October 18, 2023. https://pubmed.ncbi.nlm.nih.gov/34105297/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khawli LA, Prabhu S. Drug delivery across the blood–brain barrier. Mol Pharm 2013;10:1471–1472. [DOI] [PubMed] [Google Scholar]
- 6.Levenson RW, Sturm VE, Haase CM. Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu Rev Clin Psychol 2014;10:581–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davson H. History of the blood-brain barrier concept. In:. Implications of the Blood-Brain Barrier and Its Manipulation. Springer US; 1989:27–52. [Google Scholar]
- 8.Alahmari A. Blood-brain barrier overview: Structural and functional correlation. Neural Plast 2021;2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab 2012;32:1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achar A, Myers R, Ghosh C. Drug delivery challenges in brain disorders across the blood–brain barrier: novel methods and future considerations for improved therapy. Biomedicines 2021;9:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong X. Current strategies for brain drug delivery. Theranostics 2018;8:1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan DTT, Bender RHF, Andrejecsk JW, et al. Blood–brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood–central nervous system interface. Exp Biol Med (Maywood) 2017;242:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahadian S, Civitarese R, Bannerman D, et al. Organ‐on‐A‐chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater 2018;7. Accessed October 20, 2023. https://pubmed.ncbi.nlm.nih.gov/29034591/ [DOI] [PubMed] [Google Scholar]
- 14.Caplin JD, Granados NG, James MR, et al. Microfluidic organ‐on‐a‐chip technology for advancement of drug development and toxicology. Adv Healthc Mater 2015;4:1426–1450. [DOI] [PubMed] [Google Scholar]
- 15.Wagner I, Materne E-M, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013;13:3538. [DOI] [PubMed] [Google Scholar]
- 16.Wikswo JP. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med (Maywood) 2014;239:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cucullo L, Hossain M, Puvenna V, et al. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci 2011;12. Accessed October 20, 2023. https://pubmed.ncbi.nlm.nih.gov/21569296/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellin M, Marchetto MC, Gage FH, et al. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol 2012;13:713–726. [DOI] [PubMed] [Google Scholar]
- 19.Pamies D, Hartung T, Hogberg HT. Biological and medical applications of a brain-on-a-chip. Exp Biol Med (Maywood) 2014;239:1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeon JH, Na D, Choi K, et al. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices 2012;14:1141–1148. [DOI] [PubMed] [Google Scholar]
- 21.Shao X, Gao D, Chen Y, et al. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal Chim Acta 2016;934:186–193. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y-B, Polio S, Lee W, et al. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 2010;223:645–652. [DOI] [PubMed] [Google Scholar]
- 23.Lozano R, Stevens L, Thompson BC, et al. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015;67:264–273. [DOI] [PubMed] [Google Scholar]
- 24.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012;12:1784. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Chen J, Funderburgh JL, et al. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Molecular vision, 9:635. [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Rnjak-Kovacina J, Du Y, et al. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 2014;35:3744–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence BD, Marchant JK, Pindrus MA, et al. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009;30:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palchesko RN, Funderburgh JL, Feinberg AW. Engineered basement membranes for regenerating the corneal endothelium. Adv Healthc Mater 2016;5:2942–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palchesko RN, Lathrop KL, Funderburgh JL, et al. In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci Rep 2015;5:7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Du L, Sun P, et al. Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials 2017;124:180–194. [DOI] [PubMed] [Google Scholar]
- 31.Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat Methods 2013;10:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boussommier-Calleja A, Atiyas Y, Haase K, et al. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 2019;198:180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon NV, Chuah YJ, Cao B, et al. A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 2014;8:064118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi H-G, Jeong YH, Kim Y, et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng 2019;3:509–519. [DOI] [PubMed] [Google Scholar]
- 35.van der Helm MW, van der Meer AD, Eijkel JCT, et al. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016;4:e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadry H, Noorani B, Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herland A, van der Meer AD, FitzGerald EA, et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS One 2016;11:e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedergaard M. Garbage truck of the brain. Science 2013;340:1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 2012;4:159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke 2013;44(6_suppl_1):S93–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moutaux E, Charlot B, Genoux A, et al. An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 2018;18:3425–3435. [DOI] [PubMed] [Google Scholar]
- 42.Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015;9:054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deosarkar SP, Prabhakarpandian B, Wang B, et al. A novel dynamic neonatal blood-brain barrier on a chip. PLoS One 2015;10:e0142725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi D, Wu S, Lin H, et al. Establishment of a human iPSC- and nanofiber-based microphysiological blood–brain barrier system. ACS Appl Mater Interfaces 2018;10:21825–21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jong E, Williams DS, Abdelmohsen LKEA, et al. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J Control Release 2018;289:14–22. [DOI] [PubMed] [Google Scholar]
- 46.Tian X, Brookes O, Battaglia G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci Rep 2017;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stebbins MJ, Gastfriend BD, Canfield SG, et al. Human pluripotent stem cell–derived brain pericyte–like cells induce blood-brain barrier properties. Sci Adv 2019;5:eaau7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho C-F, Wolfe JM, Fadzen CM, et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun 2017;8:15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adriani G, Ma D, Pavesi A, et al. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip 2017;17:448–459. [DOI] [PubMed] [Google Scholar]
- 50.Maoz BM, Herland A, FitzGerald EA, et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 2018;36:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Li Z, Yu Y, et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci Rep 2016;6:36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Partyka PP, Godsey GA, Galie JR, et al. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials 2017;115:30–39. [DOI] [PubMed] [Google Scholar]
- 53.Cucullo L, Hossain M, Rapp E, et al. Development of a humanized in vitro blood–brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia 2007;48:505–516. [DOI] [PubMed] [Google Scholar]
- 54.Santa-Maria AR, Walter FR, Figueiredo R, et al. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J Cereb Blood Flow Metab 2021;41:2201–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eilenberger C, Rothbauer M, Selinger F, et al. A microfluidic multisize spheroid array for multiparametric screening of anticancer drugs and blood–brain barrier transport properties. Adv Sci (Weinh) 2021;8:e2004856.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Liu C, Muok L, et al. Dynamic 3D on-chip BBB model design, development, and applications in neurological diseases. Cells 2021;10:3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noorani B, Cucullo L, Ahn Y, et al. Advanced microfluidic vascularized tissues as platform for the study of human diseases and drug development. Curr Neuropharmacol 2023;21:599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang L, Li S, Zheng J, et al. Recent progress in microfluidic models of the blood-brain barrier. Micromachines (Basel) 2019;10:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi J-H, Santhosh M, Choi J-W. In vitro blood–brain barrier-integrated neurological disorder models using a microfluidic device. Micromachines (Basel) 2019;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee CS, Leong KW. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr Opin Biotechnol 2020;66:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q, Liu J, Wang X, et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online 2020;19. 10.1186/s12938-020-0752-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Łach A, Wnuk A, Wójtowicz AK. Experimental models to study the functions of the blood–brain barrier. Bioengineering (Basel) 2023;10:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Y, Fan K, Lin J, et al. Advances in BBB on chip and application for studying reversible opening of blood–brain barrier by sonoporation. Micromachines (Basel) 2022;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wevers NR, Kasi DG, Gray T, et al. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jagtiani E, Yeolekar M, Naik S, et al. In vitro blood brain barrier models: an overview. J Control Release 2022;343:13–30. [DOI] [PubMed] [Google Scholar]
- 67.Miccoli B, Braeken D, Li Y-CE. Brain-on-a-chip devices for drug screening and disease modeling applications. Current Pharmaceutical Design 2022;24:5419–5436. [DOI] [PubMed] [Google Scholar]
- 68.Sethi B, Kumar V, Mahato K, et al. Recent advances in drug delivery and targeting to the brain. J Control Release 2022;350:668–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong X, Shah B. Current status of in vitro models of the blood-brain barrier. Curr Drug Deliv 2022;19:1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma A, Fernandes DC, Reis RL, et al. Cutting-edge advances in modeling the blood–brain barrier and tools for its reversible permeabilization for enhanced drug delivery into the brain. Cell Biosci 2023;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sunildutt N, Parihar P, Chethikkattuveli Salih AR, et al. Revolutionizing drug development: harnessing the potential of organ-on-chip technology for disease modeling and drug discovery. Front Pharmacol 2023;14:1139229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holloway PM, Willaime-Morawek S, Siow R, et al. Advances in microfluidic in vitro systems for neurological disease modeling. J Neurosci Res 2021;99:1276–1307. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-Zepeda D, Taghi M, Scherrmann J-M, et al. ABC transporters at the blood–brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2019;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knox EG, Aburto MR, Clarke G, et al. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry 2022;27:2659–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alajangi HK, Kaur M, Sharma A, et al. Blood–brain barrier: emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol Brain 2022;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W, Mehta A, Tong Z, et al. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv Sci (Weinh) 2021;8:2003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zidarič T, Gradišnik L, Velnar T. Astrocytes and human artificial blood-brain barrier models. Bosn J Basic Med Sci 2022;22:651–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Hou Y, Ai X, et al. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed Pharmacother 2020;132:110822. [DOI] [PubMed] [Google Scholar]
- 79.Vatine GD, Barrile R, Workman MJ, et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019;24:995–1005.e6. [DOI] [PubMed] [Google Scholar]
- 80.Li Q-Y, Lee J-H, Kim H-W, et al. Research models of the nanoparticle-mediated drug delivery across the blood–brain barrier. Tissue Eng Regen Med 2021;18:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sushnitha M, Evangelopoulos M, Tasciotti E, et al. Cell membrane-based biomimetic nanoparticles and the immune system: Immunomodulatory interactions to therapeutic applications. Front Bioeng Biotechnol 2020;8:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin J, Kong C, Cho JS, et al. Focused ultrasound–mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus 2018;44:E15. [DOI] [PubMed] [Google Scholar]
- 83.Li Z, Zhao Y, Lv X, et al. Integrated brain on a chip and automated organ‐on‐chips systems. Interdisciplinary Med 2023;1. 10.1002/inmd.20220002 [DOI] [Google Scholar]
- 84.Noorani B, Bhalerao A, Raut S, et al. A quasi-physiological microfluidic blood-brain barrier model for brain permeability studies. Pharmaceutics 2021;13:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma C, Peng Y, Li H, et al. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol Sci 2021;42:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reshma S, Megha KB, Amir S, et al. Blood brain barrier-on-a-chip to model neurological diseases. J Drug Deliv Sci Technol 2023;80:104174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.