Abstract
Introduction and importance:
Cytarabine, a pyrimidine analogue, is commonly used to treat multiple haematological conditions, such as acute leukaemias and lymphomas. One of the rare and less reported complications of cytarabine is peripheral neuropathy, in which peripheral nerves are damaged, often causing weakness, numbness, and pain, usually in the hands and feet.
Case presentation:
The authors report the case of a 17-year-old male who developed a gradual onset of weakness and sensory loss in all four limbs during treatment with a conventional dose of cytarabine for acute myeloid leukaemia. Cytarabine was discontinued after the development of symptoms, and his motor and sensory functions gradually improved over the course of 3 months.
Clinical discussion:
Alongside some well-known side effects of cytarabine, including bone marrow suppression, cerebellar syndrome, and cardiotoxicity, peripheral neuropathy is one of the uncommon side effects of cytarabine. Diagnosis includes identifying and grading the severity of chemotherapy-induced peripheral neuropathy (CIPN) through clinical assessment and nerve conduction studies. Management includes withdrawing the chemotherapeutic agent and supportive treatment with drugs such as duloxetine. Recent studies also favour the use of acupuncture and sensorimotor-based exercise intervention for the management of CIPN.
Methods:
This case report has been prepared in line with the SCARE 2023 criteria.
Conclusion:
Although rare, even a conventional dosage of cytarabine can cause peripheral neuropathy, and routine neuromuscular examinations can help in the early diagnosis and intervention to limit further progression and reverse the course of the disease.
Keywords: acute myeloid leukaemia, cytarabine, peripheral neuropathy
Introduction
Highlights
Peripheral neuropathy is an uncommon complication of cytarabine.
This is the first reported case of cytarabine-induced peripheral neuropathy from Nepal.
Early diagnosis through patient education, thorough neurological examination, and nerve conduction studies are essential to prevent significant morbidity.
Cytarabine, an anti-cancer drug, is used to treat a variety of haematological malignancies, including acute leukaemia and lymphoma. It is also known as cytosine arabinoside (ara-C) and is a widely used pyrimidine analogue that inhibits DNA polymerase α, which is incorporated into the DNA and terminates DNA chain elongation. Alongside some well-known side effects of cytarabine including bone marrow suppression, cerebellar syndrome, and cardiotoxicity, peripheral neuropathy is one of the uncommon side effects of the conventional dose of cytarabine. Peripheral neuropathy can occur especially infrequently with high doses of cytarabine and when it is used in combination with daunorubicin and asparaginase1. We report a case of cytarabine-induced peripheral neuropathy affecting all four limbs in a 17-year-old male patient diagnosed with Acute Myeloid Leukemia in whom only cytarabine was given without the aforementioned agents in its conventional dose. Peripheral neuropathy in our patient underwent complete recovery following discontinuation of the drug. This case report has been prepared in line with the SCARE 2023 criteria.2
Presentation of case
A 17-year-old male presented to a tertiary care hospital complaining of persistent high-grade fever for 5 days with rigors and night sweats. In the last seven months, the patient had four similar instances when he had persistent fever lasting for 5–6 days associated with night sweats. Upon examination, the patient was found to have multiple petechial rashes and ecchymosis over his upper limbs and back. After a thorough examination and a series of investigations, the patient was diagnosed with acute myeloid leukaemia high risk (FLT3 mutation positive; NPM mutation-negative), with abnormal karyotypes 48, XXY, +4, and +5(8).
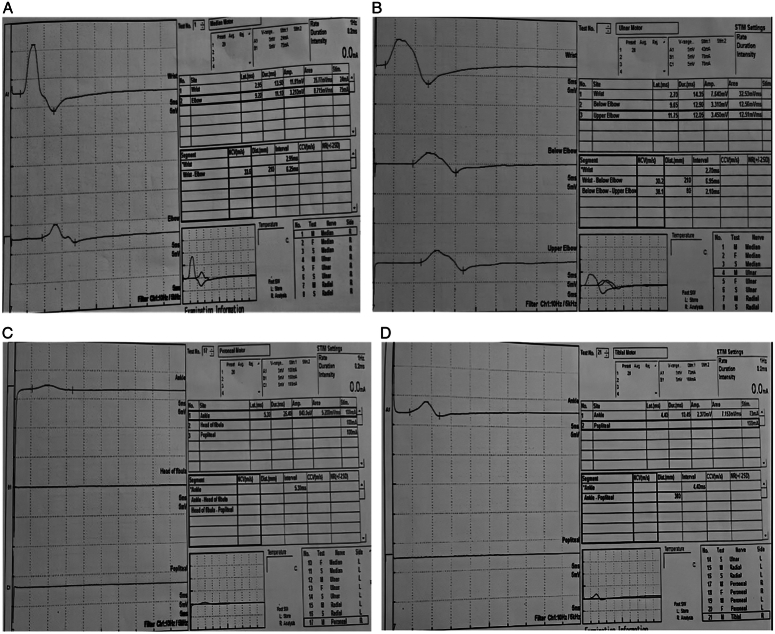
The patient was treated with 7+3 induction chemotherapy (cytarabine 200 mg/m2 for 7 days, along with daunorubicin 60 mg/m2 for 3 days). Due to a poor response to initial induction therapy (on day 28, bone marrow aspiration—47% blast), the patient received re-induction with the FLAG protocol (fludarabine 220 mg total and cytarabine 1500 mg/m2—10g total). In addition to the FLAG protocol, the patient received Venetoclax 100 mg daily for 14 days. He developed bilateral lower limb numbness on the 11th day of the FLAG regimen chemotherapy. Over the next few days, the patient developed a burning sensation in the lower limb, bilateral (right>left) foot drop, and bilateral sensory neuropathy of the lower limb over the L5 and S1 dermatomes. He also showed diminished hand grip strength and decreased sensation along the ulnar and medial nerve distributions on both sides. Peripheral neuropathy was grade 3 according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) (version 5.0). MRI of the brain and spinal cord screening were performed to rule out any other possible cause of the weakness, which showed normal results. A cerebrospinal fluid (CSF) study was performed, which was clear for active CNS disease and any possible infection. The results of the nerve conduction study were consistent with those of the motor and sensory neuropathies (Fig. 1).
Figure 1.
Nerve conduction study shows conduction slowing, latency prolongation and reduction of amplitude consistent with neuropathy in (A) median nerve, (B) ulnar nerve, (C) peroneal nerve, (D) tibial nerve.
Chemotherapy was discontinued after a diagnosis of neuropathy was made. Physiotherapy and acupuncture were performed to help the patient deal with the painful neuropathy. Supportive medications, such as duloxetine and pregabalin, were added to the patient’s therapy. His motor and sensory functions gradually improved and completely resolved after 3 months.
Discussion
To the best of our knowledge, this is the first reported case of cytarabine-induced peripheral neuropathy in Nepal, which is a rare complication of cytarabine when used alone. While other chemotherapy agents such as paclitaxel, cisplatin, oxaliplatin, thalidomide, bortezomib, and vincristine are commonly associated with polyneuropathy, only a few cases of peripheral neuropathy for cytarabine have been reported3–5. Cytarabine neurotoxicity has no known mechanism, although axonal degeneration and scattered demyelination could be involved, as is supported by a single histologically reported case following cytarabine treatment6.
Diagnosis includes identifying and grading the severity of chemotherapy-induced peripheral neuropathy (CIPN). Methods for identifying and grading the severity of CIPN can generally be separated into tools that utilize patient-reported outcomes, composite scoring systems with a functional assessment component, and quality-of-life tools7. The National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE), a clinician-led patient-reported tool, is the one that is most frequently used, although others are also used, including the Eastern Cooperative Oncology Group (ECOG) criteria and the WHO neurotoxicity scale. The latest version of the NCI-CTCAE (version 5.0) grades both motor and sensory neuropathy according to asymptomatic (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4) neurotoxicity, and death (grade 5)8. The reference standard for the diagnosis of big fibre involvement in CIPN, the nerve conduction study (NCS), provides an objective measure of large fibre function9. Peripheral nerve demyelination shows conduction slowing and latency prolongation, while axonal loss is characterized by a reduction in amplitude in NCS.
Currently, there is no approved preventive therapy for CIPN, and physicians should instead consider whether it is prudent to delay, reduce, substitute, or discontinue chemotherapy in patients with intolerable neuropathy and/or functional impairment10. According to the American Society of Clinical Oncology (ASCO) guidelines, duloxetine is the only agent with sufficient evidence to justify its use in patients with CIPN who have established severe symptoms10.
Two of the three trials in a systematic review of acupuncture for the treatment of CIPN revealed that it was helpful in improving self-reported CIPN measures11–13. An analysis of 19 randomized controlled trials involving 1174 patients revealed that acupuncture considerably reduced pain and, surprisingly, increased nerve conduction velocity14. According to a recent systematic review and meta-analysis, sensorimotor-based exercise intervention reduced the loss of postural stability caused by CIPN, and exercise interventions significantly improved CIPN symptoms15.
Conclusion
Although it rarely occurs, peripheral neuropathy caused by cytarabine can seriously hinder a patient’s recovery and increase morbidity in a patient population that is already at risk owing to their pre-existing conditions. Therefore, the incidence and/or severity of cytarabine-induced peripheral neuropathy can be reduced through patient education regarding potential symptoms, integration of detailed neuromuscular examinations into patient care, and early intervention following the initial emergence of signs and symptoms. Patient education should be given regarding early reporting of neurological symptoms, and special attention should be given to the findings of routine neuromuscular examinations to aid in the early diagnosis of neurotoxicity. Upon suspicion of neurotoxicity, appropriate medical intervention should be promptly started with physiotherapy to ensure early rehabilitation.
Ethical approval
Not required.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
None.
Author contribution
K.K., S.A. and A.B. were involved in the study concept, data collection, and writing of the manuscript. S.S., V.B. and J.B. were involved in the treatment and reviewing of the manuscript. All the authors were involved in the final review of the manuscript.
Conflicts of interest disclosure
None to declare.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Dr Kshitiz Karki.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 12 March 2024
Contributor Information
Kshitiz Karki, Email: dr.kshitizkarki@gmail.com.
Sugat Adhikari, Email: sugat.adhikari@gmail.com.
Suraj Shrestha, Email: multisurazz@gmail.com.
Jenish Bhandari, Email: drjenishbhandari17@gmail.com.
Bidisha Baral, Email: bidishabaral9@gmail.com.
Aastha Baral, Email: baralaastha@gmail.com.
References
- 1.Powell BL, Capizzi RL, Lyerly ES, et al. Peripheral neuropathy after high-dose cytosine arabinoside, daunorubicin, and asparaginase consolidation for acute nonlymphocytic leukemia. J Clin Oncol 1986;4:95–97. [DOI] [PubMed] [Google Scholar]
- 2.Sohrabi C, Mathew G, Maria N, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl 2023;109:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nevill TJ, Benstead TJ, McCormick CW, et al. Horner’s syndrome and demyelinating peripheral neuropathy caused by high-dose cytosine arabinoside. Am J Hematol 1989;32:314–315. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Asai O, Dobashi N, et al. Peripheral neuropathy caused by high-dose cytosine arabinoside treatment in a patient with acute myeloid leukemia. J Infect Chemother 2006;12:148–151. [DOI] [PubMed] [Google Scholar]
- 5.Burgess J, Ferdousi M, Gosal D, et al. Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol Ther 2021;9:385–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgeat A, De Muralt B, Stalder M. Peripheral neuropathy associated with high-dose Ara-C therapy. Cancer 1986;58:852–854. [DOI] [PubMed] [Google Scholar]
- 7.Park SB, Alberti P, Kolb NA, et al. Overview and critical revision of clinical assessment tools in chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst 2019;24(Suppl 2):S13–S25. [DOI] [PubMed] [Google Scholar]
- 8.Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 2010;46:479–494. [DOI] [PubMed] [Google Scholar]
- 9.Kandula T, Farrar MA, Kiernan MC, et al. Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer. Clin Neurophysiol 2017;128:1166–1175. [DOI] [PubMed] [Google Scholar]
- 10.Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO Guideline Update. J Clin Oncol 2020;38:3325–3348. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Moody J, Marx BL, et al. Treatment of chemotherapy-induced peripheral neuropathy in integrative oncology: a survey of acupuncture and oriental medicine practitioners. J Altern Complement Med 2017;23:964–970. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Giustini D, Seely D. A systematic review of acupuncture for chemotherapy-induced peripheral neuropathy. Curr Oncol 2019;26:e147–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Wang L, Shi H, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer 2017;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Wang Y, Zhang J, et al. Efficacy and safety of acupuncture against chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2020;2020:8875433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin WL, Wang RH, Chou FH, et al. The effects of exercise on chemotherapy-induced peripheral neuropathy symptoms in cancer patients: a systematic review and meta-analysis. Support Care Cancer 2021;29:5303–5311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.