Abstract
Background:
Impaired kidney function is frequently observed in patients following cardiopulmonary bypass (CPB). Our group has previously linked blood transfusion to acute declines in S-nitroso haemoglobin (SNO-Hb; the main regulator of tissue oxygen delivery), reductions in intraoperative renal blood flow, and postoperative kidney dysfunction. While not all CPB patients receive blood, kidney injury is still common. We hypothesized that the CPB procedure itself may negatively impact SNO-Hb levels leading to renal dysfunction.
Materials and methods:
After obtaining written informed consent, blood samples were procured immediately before and after CPB, and on postoperative day (POD) 1. SNO-Hb levels, renal function (estimated glomerular filtration rate; eGFR), and plasma erythropoietin (EPO) concentrations were quantified. Additional outcome data were extracted from the patients’ medical records.
Results:
Twenty-seven patients were enroled, three withdrew consent, and one was excluded after developing bacteremia. SNO-Hb levels declined after surgery and were directly correlated with declines in eGFR (R=0.48). Conversely, plasma EPO concentrations were elevated and inversely correlated with SNO-Hb (R=−0.53) and eGFR (R=−0.55). Finally, ICU stay negatively correlated with SNO-Hb concentration (R=−0.32).
Conclusion:
SNO-Hb levels are reduced following CPB in the absence of allogenic blood transfusion and are predictive of decreased renal function and prolonged ICU stay. Thus, therapies directed at maintaining or increasing SNO-Hb levels may improve outcomes in adult patients undergoing cardiac surgery.
Keywords: Cardiopulmonary bypass, kidney injury, nitric oxide, S-nitroso, S-nitroso haemoglobin
Introduction
Highlights
S-nitroso haemoglobin (SNO-Hb) within red blood cells is a major regulator of micro-vascular oxygen deliver. We found that SNO-Hb decreased during adult bypass and that this decline was directly linked to declines in kidney function and increases in length of hospital stay. Thus SNO-Hb can serve as novel biomarker of early postoperative dysfunction and could also be a drugable target to improve surgical outcomes.
Each year in the United States, hundreds of thousands of patients undergo cardiopulmonary bypass (CPB)1,2; coronary artery bypass grafting (CABG) alone accounts for ~400 000 of these procedures3. Developed in the 1960s, use of CPB is now viewed as routine by the lay public. And yet, despite significant advances in surgical techniques, extra-corporeal circulation technology and related perioperative care algorithms, going “on pump” is not a benign event. Instead, CPB has the potential to induce a myriad of adverse postoperative sequelae, independent of the cardiac condition that prompted the surgical intervention4–6.
One of the most common detrimental consequences of CPB is acute kidney injury (AKI), which may be severe enough to require renal replacement therapy5,7. The reported rates of postoperative kidney injury vary widely, mainly as a result of changing diagnostic criteria8. Yet even at the low end (estimated at 5–10% of all patients undergoing CPB), kidney injury remains a major contributor to poor postoperative outcomes including significant increases in 30-day mortality9. Equally important is the growing recognition that an episode of AKI may confer long-term risk. A large meta-analysis focused on prolonged survival after discharge determined that in-hospital AKI significantly increased mortality rates from 4.3 to 8.9 per 100 person-years (relative risk 2.59; 95% CI between 1.97 and 3.42)7. Similarly, it was determined that 15% of patients who experienced an in-hospital episode of AKI progressed to Stage 3 chronic kidney disease (CKD) within 2.5 years; the incidence rate was only 3% in a matched patient group without AKI10. A particularly notable finding from this latter study was that the AKI patients were identified at the time of discharge as “completely recovered”, defined as having a serum creatinine less than 1.1 times their baseline (admission) value.
AKI frequently occurs following CPB because the procedure and surgical manipulations can reduce or interrupt kidney blood flow11,12. Systemic regulators of oxygen delivery can be interrupted by renal hypo-perfusion that decreases local production of endogenous vasodilators while stimulating the build-up of vasoconstrictive inflammatory agents. Consequently, the period of oxygen deficiency in the renal microvasculature may be prolonged, with blood flow (and potential delivery of protective agents) being impaired even after restoration of large vessel flow13–15. Despite the primacy of AKI to negatively impact coronary patient outcomes, there are no accepted therapies to prevent injury or rapidly restore renal function, a consequence of the poor understanding of the events that initiate and/or propagate kidney damage.
Based on a robust combination of data from pre-clinical interventional experiments16–19 and observational clinical studies20, we postulate that renal injury results (at least in part) from CPB-mediated disruptions in S-nitrosothiol (SNO) homoeostasis. Protein S-nitrosylation is a major mechanism through which the cellular influences of nitric oxide (NO) are exerted21,22. Haemoglobin (Hb) is the prototypical S-nitrosylated protein23,24, with SNO-Hb deploying vasodilatory NO bioactivity as red blood cells (RBCs) transit the circulatory system. Low oxygen tension in the periphery promotes the graded-release of SNO-based vasodilatory activity from the beta-Cys 93 thiol within RBCs—oxygen delivery is thus linked to local metabolic demand25. Blood flow, inflammation, and cell signalling are all impacted by surgical interventions26–30. This inter-connection suggests CPB may induce a combination of systemic and focal (e.g. kidney) impairments in S-nitrosylation. As a first proof of concept test of this hypothesis, we monitored SNO-Hb levels in a cohort of adult heart surgery patients to determine if changes in NO/SNO bioactivity could be linked to subsequent kidney dysfunction and outcome. Reporting of the results of this case series follows the SCARE31 and PROCESS Guidelines32.
Patients and methods
Population
This single-site investigation was a prospective observational case series conducted at University Hospitals-Cleveland Medical Center, a tertiary care and research institution. The protocol was approved by the hospital’s institutional review board and the trial was registered with a public database in accordance with the Declaration of Helsinki. Inclusion criteria were patients over the age of 18 undergoing CPB. Patients were excluded if it was a repeat CPB procedure, they had any blood-borne infection, pre-existing kidney disease, they received an intraoperative or postoperative allogenic RBC transfusion, or if they were enroled in an interventional study. Written informed consent to participate was obtained from each patient prior to surgery. Subjects consented to offline analysis of blood samples and review of their medical records along with the plan to publish aggregate de-identified results.
Surgery
A standard perioperative routine was followed: premedication with iv midazolam; surgical anaesthesia with inhaled isoflurane and iv fentanyl; and iv rocuronium for neuromuscular relaxation. Autologous RBCs and crystalloid pump prime were employed to maintain haematocrit at greater than 18%. Vasodilators, vasoconstrictors, and inotropes were employed as clinically indicated. The CPB system consisted of a Terumo oxygenator (Terumo Medical), and Sorin S5 roller pump (Sorin Medical). Blood cardioplegia using the Quest MPS system (Quest Medical) was utilized on all cases. CPB used non-pulsatile perfusion at 2.4 l/min/m2. Nasopharyngeal temperature was maintained at 32°C during CPB; rewarming to 36°C occurred at bypass conclusion.
Arterial blood samples were obtained from an indwelling catheter prior to going on CPB (T1), immediately after discontinuation of the CPB circuit (T2), and on postoperative day one (POD 1; T3)—arterial access typically ended on POD 1 or 2 with catheter removal. Samples were transported on ice to the research laboratory for quantification of SNO-Hb levels. Postoperative patient management was directed by the ICU staff who were unaware of the study goals. RBC SNO-Hb levels were quantified offline; the resultant values were not used to direct anyone’s clinical care. Additional blood chemistry values were extracted from the subjects’ medical records.
Blood samples analysis
RBC SNO-Hb concentrations were determined using a validated photolysis/chemiluminescence method33. Hb was isolated from the RBCs and then stored at −80°C for batch analysis. Measurements of plasma erythropoietin (EPO) were performed using an ELISA kit according to the manufacturer’s protocol. Estimated glomerular filtration rate (eGFR) was calculated for each individual, based on levels of serum creatinine, serum albumin, blood urea nitrogen (BUN), sex, and demographics, using the following equation:34
Statistics
Analyses were conducted using R-statistical analysis software, Version 4.1.2. Standard parametric methods were used to assess for change in specific parameters over time. After confirming the assumption of normality and sphericity, paired t-tests were used for comparing before/after differences while repeated-measures analysis of variance was employed for the longitudinal data comparisons. Linear relationships were assessed for using Pearson correlation coefficients. In all cases, P values less than 0.05 were considered statistically significant.
Results
Twenty-seven patients provided pre-operative written informed consent to be enroled in the study; their demographic details are presented in Table 1. Three patients withdrew their consent after enroling while one patient developed bacteremia and was therefore withdrawn. The final cohort had an average baseline eGFR of 76.7 ± 24.3 ml/min and an average total Hb level of 11.8 ± 1.5 g/dl. General surgical data for the 23 subjects are presented in Table 2. Most procedures were valve repairs yet across the group, surgical parameters were relatively consistent. Patients were well-oxygenated but experienced a decline in arterial blood oxygen content resulting from a 2 g/dl decline in Hb levels at the end of surgery; ICU time and hospital stay were comparable with historical norms. Perioperative clinical chemistry data for kidney function and NO components are presented in Table 3. Creatinine, albumin, and BUN fluctuated with only the latter parameter significantly increasing during the study period. The rise in EPO level by POD 1 was indicative of a period of decreased kidney oxygenation. Note that the inclusion of all three markers in the calculation of GFR provided a clearer picture of postoperative kidney function (Fig. 2) than creatinine alone. SNO-Hb levels declined by the end of surgery and then further declined on POD 1, which accounted for the matching decline in totalNO-Hb as the amount of FeNO-Hb stayed constant.
Table 1.
Demographic data
| Parameter | Value |
|---|---|
| Population | |
| Age (years) | 71±10 |
| Sex (M/F) | 19/8 |
| Ethnicity | |
| White | 23 |
| African American | 2 |
| Undeclared | 2 |
| BMI (kg/m2) | 31±6 |
| eGFR (arbitrary units) | 76.7±24.3 |
| Hb (g/dl) | 11.9±1.5 |
| HbA1c (%) | 5.7±0.5 |
eGFR, estimated glomerular filtration rate; F, female; Hb, haemoglobin; M, male.
Table 2.
General surgical data
| Parameter | Value | |
|---|---|---|
| Procedure | ||
| AVR | 8 | |
| MVR | 7 | |
| TVR | 2 | |
| CABG | 4 | |
| ASDC | 1 | |
| AAA | 1 | |
| Pump time (min) | 144±60 | |
| Clamp time (min) | 123±67 | |
| Surgery time (min) | 333±97 | |
| Estimated blood loss (ml) | 643±327 | |
| Autologous transfusion (ml) | 572±345 | |
| Oxygenation | Pre | Post |
| Arterial saturation (SaO2; %) | 99.4±2.3 | 98.3±5.1 |
| Arterial oxygen content (CaO2; ml/dl) | 16.0±2.1 | 12.9±2.4a |
| Total haemoglobin (g/dl) | 11.9±1.5 | 9.7±1.7a |
| ICU stay (h) | 71±61 | |
| Hospital stay (days) | 11±6 | |
Significantly different from the Pre value, P<0.05.
AAA, abdominal aortic aneurysm; ASDC, atrial septal defect closure; AVR, atrial valve replacement; CABG, coronary artery bypass grafting; MVR, mitral valve replacement; TVR, tricuspid valve replacement.
Table 3.
Perioperative clinical chemistries
| Parameter | Start | End | POD 1 | POD 2 | POD 3 |
|---|---|---|---|---|---|
| Creatinine (mg/dl) | 0.99±0.29 | 1.04±0.32 | 1.01±0.27 | 0.98±0.33 | 0.90±0.31 |
| Albumin (g/dl) | 3.3±0.5 | 3.7±1.0 | 3.3±0.3 | 3.1±0.4 | 3.4±1.4 |
| BUN (mg/dl) | 18±6 | 18±5 | 20±6 | 24±9 | 25±9 |
| EPO (mU/ml) | 17.8±15.0 | 21.5±21.0 | 47.3±28.7a | — | — |
| NO components (X per Hb × 103) | |||||
| SNO-Hb | 5.9±2.1 | 4.8±1.7 | 4.1±1.2a | — | — |
| FeNO-Hb | 2.5±1.0 | 2.3±1.3 | 2.2±1.0a | — | — |
| totalNO-Hb | 8.5±2.6 | 7.1±2.4 | 6.3±1.8a | — | — |
Significantly different from the Start value, P<0.05.
BUN, blood urea nitrogen; EPO, erythropoietin; FeNO-Hb, iron nitrosyl haemoglobin; POD, postoperative day; SNO-Hb, S-nitroso haemoglobin; totalNO-Hb, total nitric oxide bound to haemoglobin.
Figure 2.
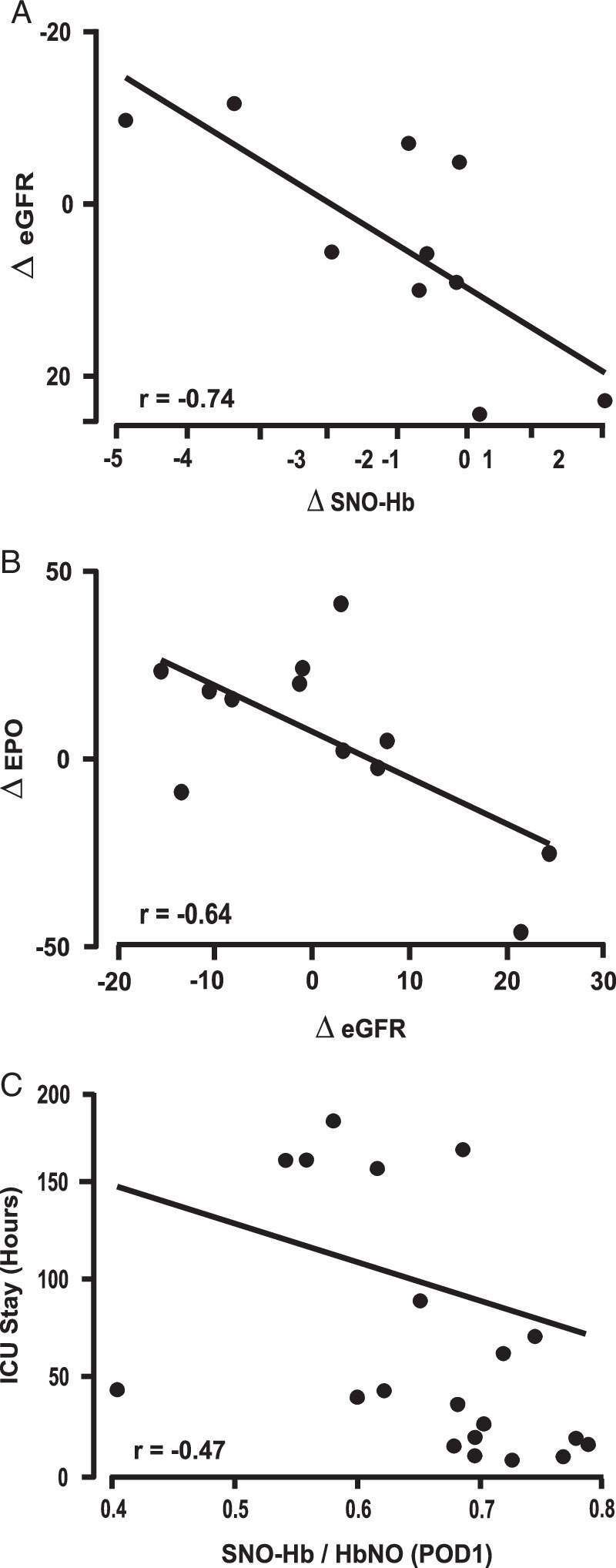
Relationship between clinical blood markers and outcome. (A) Change in estimated glomerular filtration rate (eGFR) from pre-cardiopulmonary bypass (CPB) (T1) to postoperative day 1 (POD 1) was plotted versus change in S-nitroso haemoglobin (SNO-Hb) during CPB (from T1 to T2). Decline in eGFR after CPB correlated with decline in SNO-Hb during CPB (r=0.74, P=0.01). (B) Change in serum erythropoietin (EPO) from pre-CPB to POD 1 (T3-T1) was plotted versus change in eGFR from pre-CPB (T1) to POD 1 (T3). The increase in EPO correlated with the decline in eGFR after CPB (r=−0.65, P=0.02). (C) Length of ICU stay after CPB was plotted versus SNO-Hb level at POD 1 (T3). As an overall measure of outcome, declines in SNO-Hb at T3 (POD 1) were directly correlated with increased ICU stay.
We next tested for correlations between SNO status, kidney function, and outcome (i.e. ICU stay). Based on Cohen35, in our linear regression model with n=23 and to see effect size of 0.5 at a significance level of 0.05, we have achieved 89% power. Figure 1A graphically-depicts the reduction in NO bioactivity. As noted, this reduction was almost entirely accounted for by the loss of SNO-Hb (P=0.00087) as haem nitrosyl Hb (FeNO-Hb) levels remained constant (P=0.36). Figure 1B graphically-depicts the increase in EPO (P=0.0024). Strengthening the link between SNO-Hb and kidney oxygenation, there was a direct inverse correlation between change in EPO levels and SNO-Hb (Fig. 1C, P=0.03), that is lower SNO-Hb was associated with higher EPO concentration.
Figure 1.
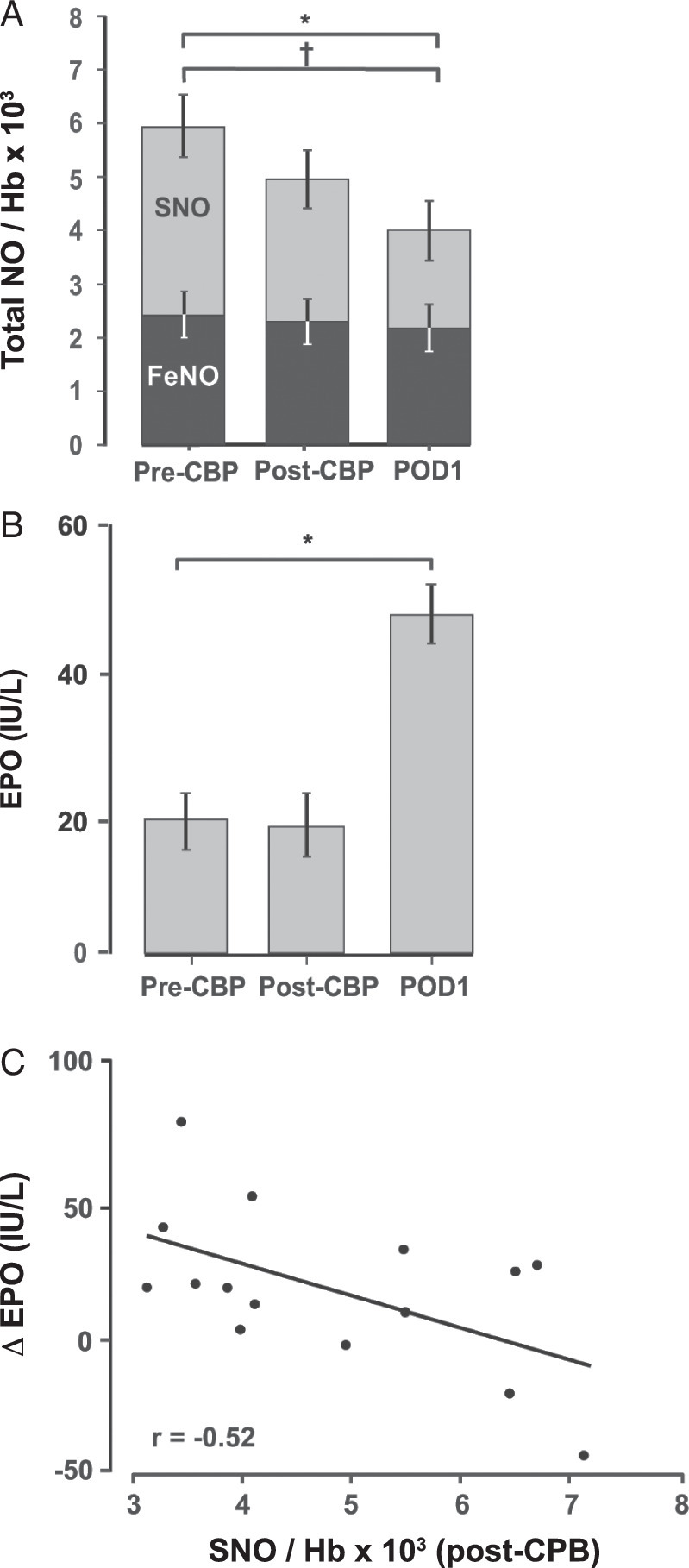
Change in clinical markers after cardiopulmonary bypass (CPB). (A) Amounts of FeNO, S-nitroso haemoglobin (SNO-Hb) and total nitric oxide (NO) (expressed per Hb × 103) in pre-CPB (T1), immediate post-CPB (T2) and postoperative day 1 (POD 1, T3) blood samples. SNO-Hb and total NO declined from pre-CPB (T1) to post-CPB (T2) and POD 1 (T3), while FeNO levels remained constant. (B) Erythropoietin (EPO) concentrations in pre-CPB, immediate post-CPB and POD 1 blood samples. EPO increased after CPB, from T1/T2 to T3. (C) Scatter plot depicting the inverse correlation between SNO-Hb level post-CPB and change in EPO (∆ EPO) from completion of CPB to POD 1 (T3-T2). The level of SNO inversely correlated with the EPO concentration (r=−0.53, P=0.037).
The functional consequences of changes in SNO-Hb and EPO were reflected in worsened kidney function and increased hospitalization time. Both the magnitude of the decline in SNO-Hb and the increase in EPO correlated with declines in eGFR (Fig. 2A and Fig. 2B, respectively). Finally, as an overall measure of outcome, declines in SNO-Hb were directly correlated with increased ICU length of stay (Fig. 2C, P=0.03).
Discussion
The results from this observational study link CPB-mediated disruptions in SNO homoeostasis to impaired kidney function and worse outcomes (defined as increased ICU stay) in adult cardiac surgical patients. These findings add to a growing appreciation that, in general, depletion of NO bioactivity can reduce tissue oxygenation and induce organ dysfunction and, specifically, that kidneys exhibit an enhanced sensitivity to conditions that reduce circulating RBC SNO-Hb levels12,20.
The unique anatomical make-up and functional activity of the kidneys confers an enhanced susceptibility to oxygen deprivation14. The kidneys receive ~20–25% of cardiac output (even though they constitute only 2% of body mass), which, along with the small difference between the arterial and venous oxygen content, would suggest significant reserve capacity. Instead, the presence of preglomerular countercurrent shunts (key for sodium resorption) entails that, even under normal conditions, the kidney functions in a hypoxic environment; the pO2 in the cortex is between 40 and 45 mmHg, and it gets progressively lower in the internal regions of the medulla and papilla. As a result, there is little oxygen reserve, so even small reductions in blood flow, and thus oxygen delivery, can have outsized effects on renal cellular activity compared to other organs36,37.
Various nonspecific efforts directed towards limiting the intraoperative initiating factors of AKI (viz. renal hypo-perfusion) have been implemented; these include modifying surgical techniques to reduce kidney ischaemia time and limiting volume depletion. However, fluid management recommendations present the dichotomy of avoiding both anaemia and blood transfusion. In addition, compliance with such efforts can be thwarted by a patient’s pre-existing condition(s) and/or intraoperative changes in status (e.g. Haemorrhage, difficult dissection, etc.)38–40.
A significant number of pharmacologic agents have been utilized in attempts to prevent or ameliorate AKI. These include dopaminergic agonists, antioxidants and free radical scavengers, atrial natriuretic peptide, anti-inflammatories, diuretics, volume expanders, statins, NO donors, etc38,41,42. Specific drugs in each category (alone or as combination therapy) have demonstrated benefit in pre-clinical models of AKI, but this success has not translated to clinical practice for a variety of reasons:
Nonspecific vasoactive agents produce dose-limiting hypo- or hypertension;
Previous lack of consensus on defining AKI with respect to both appearance and resolution;
Therapy initiation curtailed due to patient status (e.g. volume expansion/fluid loading in the setting of cardiovascular or pulmonary disease); and/or
Failure to improve outcome in clinical trials (e.g. diuretics)38,41.
The mechanism(s) that precipitated the decline in RBC SNO-Hb we observed here remain to be determined. As noted previously, in other settings, surgical manipulation has been shown to reduce SNO-Hb. There is also the inflammatory response and haemolysis that occurs as the RBCs circulate through the bypass circuit. Finally, it is important to recognize the current CPB protocol involves non-pulsatile flow43, which is a very artificial setting compared to the innate movement of blood through the vasculature. As a result, an interval without pressure cycling (and no variable sheer stress) could impact the generation of NO/SNO from endothelial nitric oxide synthase. Whatever the cause(s), our findings suggest a new contributor to AKI; aberrant S-nitrosylation.
This discovery provides for a novel putative therapeutic target—restoration of RBC NO/SNO bioactivity. Using swine, we previously determined that surgical manipulations can deplete circulating SNO-Hb levels, leading to reductions in kidney blood flow and eGFR, and increases in enzymatic markers of tissue injury, all of which were resolved by administration of an S-nitrosylating agent17. We have observed similar benefits following intraoperative transfusion with SNO-Hb loaded RBCs19. We have also determined that administration of a SNO-donor can improve tissue oxygenation in humans44 and we are initiating a clinical trial to determine if the same agent (ethyl nitrite) can improve the efficacy of blood transfusion (NCT03999229). These previous translational and clinical findings, along with the current clinical results, strongly suggest that increasing the intraoperative circulating pool of NO/SNO bioactivity could be both prophylactic and therapeutic against AKI.
This study supports the current body of evidence that CPB can induce postoperative AKI and worsen patient outcomes, and adds to it by identifying a potential mediator of injury (reductions in SNO-Hb). We recognize that the correlative analysis of the prospectively collected data coupled with the small sample size are weaknesses of this study—additional studies in bypass patients with more significant pathologies as well contributions from other organ dysfunction on SNO-Hb and outcomes are certainly warranted. However, the matching findings regarding AKI from other adult bypass studies lessen this concern12,45,46. There was an insufficient number of patients to sub-divide the cohort by surgical intervention. Nonetheless, the surgical data (Table 2) indicates this was a relatively homogenous group. Moreover, as noted, identifying SNO-Hb as a determinant of renal function and, ultimately, patient outcome, is consistent with a growing body of literature connecting deficits in RBC SNO-Hb to pathologies of kidney oxygenation.
In summary, we have linked CPB-mediated dysregulated SNO homoeostasis to kidney dysfunction and worse patient outcomes. SNO-Hb levels are inversely correlated with kidney function, in terms of eGFR, EPO, and ICU stay, suggesting its utility as a target for therapeutic intervention and as a prognostic biomarker. While we focused here on CPB, we believe that reductions in RBC SNO-Hb may be the major contributor to the incidence and severity of AKI in a variety of medical settings (e.g. sepsis, blood transfusion, laparoscopic surgery, etc). As such, therapeutics that can prevent or correct reductions in the ability of RBCs to deliver oxygen would have widespread clinical applications—and are currently undergoing human testing44—applications that could improve patient outcomes and reduce medical care costs by reducing hospital length of stay and ameliorating progression to chronic kidney disease.
Ethical approval
Circulating S-Nitrosothiols in Human Health and Disease Study was approved by the University Hospitals-Cleveland Medical Center Institutional Review Board. Protocol Number 06-10-04.
Consent
Written informed consent was obtained from each subject prior to surgery. Subjects consented to offline analysis of blood samples and review of their medical records along with the plan to publish aggregate de-identified results. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
This work was supported in part by grants from the National Institutes of Health (HL075443 and HL126900), the AHA-Allen Brain Health Initiative (19PABHI34580006), the Eppley Foundation (New York, NY), Enduring Hearts (Marietta, GA), and Additional Ventures (Palo Alto, CA), as well as by contract W81XWH-16-1-0520 from the US Army Medical Research and Materiel Command.
Author contribution
This was a multi-departmental effort that required coordination, input, and hands-on involvement from several individuals. With respect to specific contributions: J.D.R. conceived the study; J.D.R. designed the research studies in consultation with M.C., J.K., and J.S.S.; A.M. led the subject sampling and monitoring with assistance from R.N., E.P.C., L.Z., N.R.P., M.C., and J.K.; A.M., R.N., E.P.C., L.Z., N.R.P., and A.H. conducted the blood sample analyses; A.M., R.N., E.P.C., L.Z., R.B., N.R.P., and A.H. collated and analyzed the data; A.M., J.K., and J.D.R. wrote the manuscript with critical insights provided by R.B., R.T.P., and J.S.S.; A.H. contributed new reagents/analytical tools; and all authors provided editorial input to the final submission.
Conflicts of interest disclosure
J.S.S. and J.D.R. hold patents related to renitrosylation, some of which may be licensed for commercial development. In addition, J.S.S. has an equity interest in SNO Bio, a company developing nitrosylation-related therapeutics, and is a consultant to NNOXX, a company developing devices to measure SNO-Hb. Their institutions are aware of these potential conflicts and appropriate management plans are in place. None of the other authors have relevant conflicts to disclose.
Research registration unique identifying number (UIN)
Research Registry researchregistry9291 Circulating S-Nitrosothiols in Human Health and Disease https://www.researchregistry.com/browse-theregistry#home/registrationdetails/64b833ef8d1cd900276c9f7d/.
Guarantor
James Reynolds.
Data access statement
Research data supporting this publication are available from the corresponding author.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Presentation
Moyal et al. Potential relationship between nitric oxide bioactivity and kidney function following cardiopulmonary bypass. AHA BCVS 2018 Conference, Grand Hyatt, San Antonio, Tx.
Acknowledgements
The authors thank the perioperative clinical staff for assisting with obtaining blood samples as well as the subjects who volunteered to participate in this study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 March 2024
Contributor Information
Andrew Moyal, Email: ajm218@case.edu.
Ryan Nazemian, Email: mxn288@case.edu.
Edwin Pacheco Colon, Email: exp199@case.edu.
Lin Zhu, Email: lin.zhu3@unsw.edu.au.
Ruth Benzar, Email: rbenzar@gmail.com.
Nicole R. Palmer, Email: nrp63@case.edu.
Martha Craycroft, Email: Martha.Craycroft@uhhospitals.org.
Alfred Hausladen, Email: alfred.hausladen@case.edu.
Richard T. Premont, Email: rtp27@case.edu.
Jonathan S. Stamler, Email: jss156@case.edu.
John Klick, Email: johnklick@mac.com.
James D. Reynolds, Email: jxr343@case.edu.
References
- 1.Alkhouli M, Alqahtani F, Kalra A, et al. Trends in Characteristics and Outcomes of Hospital Inpatients Undergoing Coronary Revascularization in the United States, 2003-2016. JAMA Network Open 2020;3:e1921326, e1921326. [DOI] [PubMed] [Google Scholar]
- 2.Melly L, Torregrossa G, Lee T, et al. Fifty years of coronary artery bypass grafting. J Thorac Dis 2018;10:1960–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachar BJ, Manna B. Coronary Artery Bypass Graft. StatPearls; 2023. [PubMed] [Google Scholar]
- 4.Murphy GJ, Angelini GD. Side effects of cardiopulmonary bypass: what is the reality? J Card Surg 2004;19:481–488. [DOI] [PubMed] [Google Scholar]
- 5.Milne B, Gilbey T, De Somer F, et al. Adverse renal effects associated with cardiopulmonary bypass. Perfusion 2024;39:452–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessel EA, II. What’s new in cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2019;33:2296–2326. [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Liu B, Liang Z, et al. Acute kidney injury following cardiopulmonary bypass: a challenging picture. Oxidat Med Cell Longev 2021;2021:8873581. doi: 10.1155/2021/8873581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuura R, Iwagami M, Moriya H, et al. The clinical course of acute kidney disease after cardiac surgery: a retrospective observational study. Sci Rep 2020;10:6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu RK, Hsu CY. The role of acute kidney injury in chronic kidney disease. Semin Nephrol 2016;36:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Q, Colgan SP, Shelley CS. Hypoxia: the force that drives chronic kidney disease. Clin Med Res 2016;14:15–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Liu J, Li L, et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: a retrospective cohort study. J Cardiothorac Surg 2019;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day JR, Taylor KM. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005;3:129–140. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, Ricksten SE, Bragadottir G, et al. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin Exp Pharmacol Physiol 2013;40:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater JM, Orszulak TA, Cook DJ. Distribution and hierarchy of regional blood flow during hypothermic cardiopulmonary bypass. Ann Thorac Surg 2001;72:542–547. [DOI] [PubMed] [Google Scholar]
- 16.Shimazutsu K, Uemura K, Auten KM, et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci 2009;2:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yurcisin BM, Davison TE, Bibbs SM, et al. Repletion of S-nitrosohemoglobin improves organ function and physiological status in swine after brain death. Ann Surg 2013;257:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Qureshi A, Awad M, et al. A novel method to improve perfusion of ex vivo pumped human kidneys. Ann Surg 2021;274:e610–e615. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds JD, Bennett KM, Cina AJ, et al. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci 2013;110:11529–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matto F, Kouretas PC, Smith R, et al. S-nitrosohemoglobin levels and patient outcome after transfusion during pediatric bypass surgery. Clin Transl Sci 2018;11:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldar SM, Stamler JS. S-nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest 2013;123:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess DT, Matsumoto A, Kim SO, et al. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 2005;6:150–166. [DOI] [PubMed] [Google Scholar]
- 23.Doctor A, Stamler JS. Nitric oxide transport in blood: a third gas in the respiratory cycle. Comprehens Physiol 2011;1:541–568. [DOI] [PubMed] [Google Scholar]
- 24.Premont RT, Reynolds JD, Zhang R, et al. Role of nitric oxide carried by hemoglobin in cardiovascular physiology: developments on a three-gas respiratory cycle. Circ Res 2020;126:129–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 2005;67:99–145. [DOI] [PubMed] [Google Scholar]
- 26.Duebener LF, Sakamoto T, Hatsuoka S, et al. Effects of hematocrit on cerebral microcirculation and tissue oxygenation during deep hypothermic bypass. Circulation 2001;104(12 suppl 1):I260–I264. [DOI] [PubMed] [Google Scholar]
- 27.Kawahito K, Mohara J, Misawa Y, et al. Platelet damage caused by the centrifugal pump: in vitro evaluation by measuring the release of alpha-granule packing proteins. Artif Organs 1997;21:1105–1109. [DOI] [PubMed] [Google Scholar]
- 28.Murashima M, Nishimoto M, Kokubu M, et al. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci Rep 2019;9:20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro-Pérez J, Rodrigo R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep 2021;26:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senoner T, Schindler S, Stättner S, et al. Associations of oxidative stress and postoperative outcome in liver surgery with an outlook to future potential therapeutic options. Oxidative Med Cell Longev 2019;2019:3950818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agha RA, Franchi T, Sohrabi C, et al. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg 2020;84:226–230. [DOI] [PubMed] [Google Scholar]
- 32.Agha RA, Sohrabi C, Mathew G, et al. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) Guidelines. Int J Surg 2020;84:231–235. [DOI] [PubMed] [Google Scholar]
- 33.Hausladen A, Rafikov R, Angelo M, et al. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci U S A 2007;104:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed. L. Erlbaum Associates; 1988:567. [Google Scholar]
- 36.Epstein FH, Brezis M, Silva P, et al. Physiological and clinical implications of medullary hypoxia. Artif Organs 1987;11:463–467. [DOI] [PubMed] [Google Scholar]
- 37.Patinha D, Pijacka W, Paton JFR, et al. Cooperative oxygen sensing by the kidney and carotid body in blood pressure control. Front Physiol 2017;8:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Busse LW. Novel therapies for acute kidney injury. Kidney Int Rep 2017;2:785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman JM, Roth JV, Bjoraker DG. Maximum blood savings by acute normovolemic hemodilution. Anesth Analg 1995;80:108–113. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Spina S, Zadek F, et al. Effect of nitric oxide on postoperative acute kidney injury in patients who underwent cardiopulmonary bypass: a systematic review and meta-analysis with trial sequential analysis. Ann Intensive Care 2019;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 2016;15:568–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei C, Berra L, Rezoagli E, et al. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med 2018;198:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunagawa G, Koprivanac M, Karimov JH, et al. Is a pulse absolutely necessary during cardiopulmonary bypass? Expert Rev Med Devices 2017;14:27–35. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds JD, Jenkins T, Matto F, et al. Pharmacologic targeting of red blood cells to improve tissue oxygenation. Clin Pharmacol Ther 2018;104:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do-Nguyen CC, Sturmer DL, Yang G, et al. Oxygen delivery thresholds during cardiopulmonary bypass and risk for acute kidney injury. Ann Thorac Surg 2023;116:607–613. [DOI] [PubMed] [Google Scholar]
- 46.Krawczeski CD. Cardiopulmonary bypass and AKI: AKI Is bad, so let’s get beyond the diagnosis. Review. Front Pediatr 2019;7:492. [DOI] [PMC free article] [PubMed] [Google Scholar]


