Abstract
Background:
Surgical excision is considered one of the most effective treatments for secondary osteosarcoma (SO). It remains unclear whether the survival of patients with secondary osteosarcoma (SO) could be associated with their surgical willingness.
Materials and methods:
The statistics of the patients diagnosed with SO between 1975 and 2008 were gathered from the surveillance epidemiology and end results (SEER) database. The patients were divided into three subgroups according to their surgical compliance. The authors used the multivariable Logistic regression analysis and cox regression method to reveal the influence of surgical compliance on prognosis and the risk factors of surgical compliance. Additionally, the authors formulated a nomogram model to predict the overall survival (OS) of patients. The concordance index (C-index) was used to evaluate the accuracy and practicability of the above prediction model.
Results:
Sixty-three (9.2%) of the 688 patients with SO who were recommended for surgical treatment refused to undergo surgery. Lower surgical compliance can be ascribed to an earlier time of diagnosis and refusal of chemotherapy. The lower overall survival (OS) {[hazard ratio (HR)] 1.733, [CI] 1.205-2.494, P value [P]=0.003} of not surgical compliant patients was verified by the multivariate cox regression method, compared with surgical compliant patients. In addition, the discernibility of the nomogram model was proven to be relatively high (C-index=0.748), by which we can calibrate 3-year- and 5-year OS prediction plots to obtain good concordance to the actual situation.
Conclusions:
Surgical compliance was proved to be an independent prognostic factor in the survival of patients with SO.
Keywords: Nomogram, overall survival (OS), secondary osteosarcoma (SO), SEER, surgical compliance
Introduction
Highlights
The factors affecting the overall survival of patients with secondary osteosarcoma are still unknown.
Surgical compliance is associated with survival outcomes in patients with secondary osteosarcoma.
Patients who were diagnosed earlier or refused chemotherapy are more likely to refuse surgery.
For the first time, we have established a nomogram model that includes surgical compliance to effectively predict 3-year to 5-year survival.
Osteosarcoma is the most familiar malignant bone tumour. Therefore, the majority of the patients are suffering from bone pain and tumour, resulting in inconvenience in daily life1. Secondary osteosarcoma (SO) could occur after other primary malignancy or cancer treatment2, and accounts for ~10% of osteosarcoma patients3. However, the mortality of osteosarcoma has been dramatically decreased from 80% to less than 40% by surgical resection associated with preoperative and postoperative chemotherapies4. Patients with SO still may suffer from a much worse prognosis than primary osteosarcoma5,6, and thus SO has become a major concern. SO was formally included in the classification of soft tissue and osteosarcoma by WHO in 2013 and remains included in the recently published 2020 WHO Classification7.
The worst prognosis of SO mainly stems from its typical characteristics, which consist of local invasion, high-grade and distant metastasis, as well as not-in-time treatment and the intolerance of patients8. In addition, the prior malignancy history may make it more complicated to conform diagnosis and accurate fight against osteosarcoma cells9–12. To conclude, SO could pose a great challenge to long-term survival.
Currently, surgical resection is adopted into the standard treatment of SO. According to Zhan Wang’s study, surgery can be considered an independent factor after analysing 444 older patients with SO13. Another similar conclusion can be drawn by Yanqi et al. 14, they provided a thorough report on the epidemiology and tumour characteristics to verify cancer-specific survival (CSS) and overall survival (OS) improvement of surgical resection.
Clinically, due to the poor physical condition and the repeated treatment of the primary tumour, especially after previous radiotherapy, chemotherapy or surgical treatment, most patients are physically and psychologically unwilling to undergo surgery again to treat SO, worrying about serious consequences resulting from the surgical failure.
In the state of arts, surgery compliance is regarded as a key factor to the prognosis of various diseases15–17, and thus becomes the focus of social healthcare research18,19. It is non-negligible that SO rarely happens, according to the clinical statistics. To our knowledge, there is few research on the surgery compliance of SO. Only a few case studies have been done in previous literature9–11. Other studies with slightly larger samples focused on the overall assessment and did not specifically reveal determinants of surgical compliance13,14.
Nevertheless, the above achievements encourage us to reveal an intrinsic relationship between surgical compliance and tumour characteristics of SO, so as to further assess the factors associated with surgical compliance and predict prognosis. Moreover, we developed a detailed nomogram to assess the individual prognoses of OS for the patients with SO. The results of this study may help clinicians conduct more effective monitoring of patients with SO facing surgical decisions.
Materials and methods
Data sources and study population
The osteosarcoma cases in this work have been officially reported on the SEER database. We obtained the clinical data by using SEER*Stat application (version 8.4.0) between 1975 and 2018, which was submitted to SEER database on November 2021, and issued in April 2022. The informed consent can be waived thanks to the data-sharing policy of SEER database. In our study, all the specimens were derived from the patients whose biopsy or surgical results were histologically confirmed. We refer to “SO” as the osteogenic sarcoma that occurred after the primary malignancy. We differentiate patients with SO from other ones by allocating them with serial numbers greater than or equal to 2 (n=939). Meanwhile, the patients with level IV tumours in the original diagnosis, or the interval between primary and secondary malignancy diagnoses was less than 6 months, were excluded from our study. Besides, patients with missing or incomplete data on key characteristics or covariates were also filtered out. There are 4 patients with unknown survival time and 23 patients with uncertain surgical conditions, among which 3 of both cases are repeated. Finally, 915 patients with SO were worthy to collect the demographic and clinicopathological data covering the overall course.
Variable definition
The essential information of all follow-up patients was extracted from the database, consisting of the diagnosis time, age, sex, race, marital status, area, economic income, pathological grading, location, tumour metastasis, radiotherapy, chemotherapy, Primary malignancy site and surgery status as well. These 14 variables are confirmed as independent of each other and suitable for modelling analysis in previous works20,21. We classified ‘race’ of patients into ‘white’ and ‘non-white’, ‘marital status’ into ‘married’ and ‘non-married’ subgroups, ‘Rural_urban’ into ‘urban’ and ‘non-urban’, ‘location’ into ‘appendicular’, ‘axial’, and ‘other’, and ‘primary_malignancy_site’ into ‘bone and joint’, ‘soft tissue’, ‘internal organ’ and ‘others’. Radiotherapy or tumour resection were considered an ordinary treatment for primary tumours. The time duration of diagnoses of the selected patients was 43 years. We classify the patients into three subgroups, that is the surgical compliance group consisting of those patients recommended for surgery who show good compliance, the non-compliant group with patients not willing to undergo surgery, and the non-surgical group with patients were not recommended for surgery. All the deaths were restricted to the cases resulting from SO. The OS refers to the period from the diagnosed time to the death resulting from any causes or last follow-up.
Statistical analysis
We adapted Stata/MP 16.0 software and R software (version 4.1.2) to the data pre-processing and statistical analyses, respectively. The optimal age and economic income stratification boundaries were found by X-tile software (version 3.6.1). Univariate logistic regression was used for preliminary screening variables to analyze factors that influence surgical compliance. Only variables with P less than 0.05 were meaningful to Multivariate logistic regression analysis, which can identify independent risk factors for surgical non-compliance. Univariate cox was used for preliminary screening variables affecting survival outcome, and significant variables with P<0.2 would be included in multivariate cox regression analysis. The survival of patients with different surgical compliance was analyzed using the k-m curve. Then, the different survival curves were tested by log-rank method. The significant variables from multivariate analysis will be used in the nomogram formulation. We tested and verified the prognostic accuracy of the nomogram model through the consistency check. In addition to basic functions, the R software package used in this analysis also consisted of rio (for data import and export), survival (survival analysis), survminer (survival curve drawing), and rms (nomogram). The tests with P less than 0.05 would be considered as statistically significant, which were selected from all the two-tailed ones.
Results
Identification of cut-off values of age and economic income
Unreasonable cut-off points selection could make any analysis invalid. Therefore, we resort to the X-tile method as our cut-point optimization tool to determine the optimal stratifications for the patients with SO. From the numerical results shown in X-tile figure (Fig. 1A), the optimal age divisions of diagnosis should be younger than 38 years, 38–72 years, older than 72 years. Meanwhile, the optimal partitions of the economic income is identified by these optimal cut-points:less than 3323 $, 3323–3784 $, greater than 3784 $ (Fig. 1B).
Figure 1.
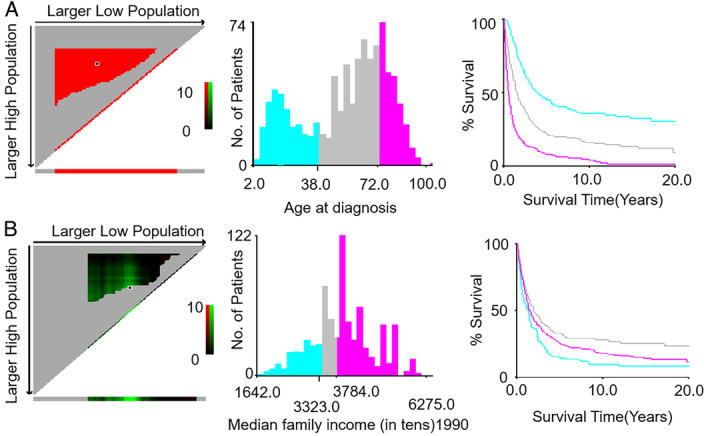
The X-tile analysis was used to identify the optimal cut-off values of age of diagnosis (A) and economic income (B).
Baseline characteristics of patients
The cohort study in this work consists of 915 SO patients, among which the numbers of the patients suggested for surgical excision and for non-surgical treatment are 688 and 227, respectively. It is worth mentioning that 63 out of 668 patients did not follow the surgical suggestion, accounting for 9.2%. Table 1 shows the relationship between patient baseline characteristics (including demographic and clinicopathologic characteristics) and surgical compliance.
Table 1.
Demographics characteristic for patients with secondary osteosarcomas (SO)
| [ALL], N (%) | Compliance, N (%) | Non-compliance, N (%) | Non-surgical, N (%) | ||
|---|---|---|---|---|---|
| N=915 | N=625 | N=63 | N=227 | p.overall | |
| Year_diagnosisa | <0.001 | ||||
| <1995 | 129 (14.10) | 91 (14.56) | 28 (44.44) | 10 (4.41) | |
| 1995–2005 | 256 (27.98) | 183 (29.28) | 19 (30.16) | 54 (23.79) | |
| >2005 | 530 (57.92) | 351 (56.16) | 16 (25.40) | 163 (71.81) | |
| Status | <0.001 | ||||
| Survival | 254 (27.76) | 215 (34.40) | 7 (11.11) | 32 (14.10) | |
| Death | 661 (72.24) | 410 (65.60) | 56 (88.89) | 195 (85.90) | |
| Time | 15.00 (5.00;35.00) | 20.00 (9.00;48.00) | 11.00 (2.00;22.50) | 6.00 (2.00;14.00) | <0.001 |
| Agea | <0.001 | ||||
| <38 | 243 (26.56) | 186 (29.76) | 19 (30.16) | 38 (16.74) | |
| 38–72 | 437 (47.76) | 315 (50.40) | 25 (39.68) | 97 (42.73) | |
| >72 | 235 (25.68) | 124 (19.84) | 19 (30.16) | 92 (40.53) | |
| Sex | 0.972 | ||||
| Female | 478 (52.24) | 327 (52.32) | 32 (50.79) | 119 (52.42) | |
| Male | 437 (47.76) | 298 (47.68) | 31 (49.21) | 108 (47.58) | |
| Race | 0.041 | ||||
| White | 716 (78.25) | 490 (78.40) | 57 (90.48) | 169 (74.45) | |
| non-White | 197 (21.53) | 134 (21.44) | 6 (9.52) | 57 (25.11) | |
| NA | 2 (0.22) | 1 (0.16) | 0 | 1 (0.44) | |
| Marital_status | 0.584 | ||||
| Married | 425 (46.45) | 284 (45.44) | 30 (47.62) | 111 (48.90) | |
| Non-married | 460 (50.27) | 322 (51.52) | 28 (44.44) | 110 (48.46) | |
| NA | 30 (3.28) | 19 (3.04) | 5 (7.94) | 6 (2.64) | |
| Rural_Urban | 0.005 | ||||
| Urban | 142 (15.52) | 96 (15.36) | 16 (25.40) | 30 (13.22) | |
| non-Urban | 614 (67.10) | 427 (68.32) | 43 (68.25) | 144 (63.44) | |
| NA | 159 (17.38) | 102 (16.32) | 4 (6.35) | 53 (23.35) | |
| Median_family_income | 0.001 | ||||
| <3323 | 164 (17.92) | 100 (16.00) | 17 (26.98) | 47 (20.70) | |
| 3323–3784 | 162 (17.70) | 120 (19.20) | 8 (12.70) | 34 (14.98) | |
| >3784 | 437 (47.76) | 308 (49.28) | 35 (55.56) | 94 (41.41) | |
| NA | 152 (16.61) | 97 (15.52) | 3 (4.76) | 52 (22.91) | |
| Grade | 0.008 | ||||
| I+II | 59 (6.45) | 49 (7.84) | 6 (9.52) | 4 (1.76) | |
| III+IV | 502 (54.86) | 378 (60.48) | 21 (33.33) | 103 (45.37) | |
| NA | 354 (38.69) | 198 (31.68) | 36 (57.14) | 120 (52.86) | |
| Location | <0.001 | ||||
| Appendicular | 278 (30.38) | 213 (34.08) | 23 (36.51) | 42 (18.50) | |
| Axial | 449 (49.07) | 272 (43.52) | 31 (49.21) | 146 (64.32) | |
| Others | 188 (20.55) | 140 (22.40) | 9 (14.29) | 39 (17.18) | |
| CS_mets | <0.001 | ||||
| No | 433 (47.32) | 336 (53.76) | 14 (22.22) | 83 (36.56) | |
| Yes | 112 (12.24) | 44 (7.04) | 3 (4.76) | 65 (28.63) | |
| NA | 370 (40.44) | 245 (39.20) | 46 (73.02) | 79 (34.80) | |
| Radiation for prior malignancies | 0.001 | ||||
| No | 749 (81.86) | 531 (84.96) | 49 (77.78) | 169 (74.45) | |
| Yes | 166 (18.14) | 94 (15.04) | 14 (22.22) | 58 (25.55) | |
| Chemotherapy for prior malignancies | <0.001 | ||||
| No | 412 (45.03) | 249 (39.84) | 37 (58.73) | 126 (55.51) | |
| Yes | 503 (54.97) | 376 (60.16) | 26 (41.27) | 101 (44.49) | |
| Primary_malignancy_site | <0.001 | ||||
| Bone and joint | 759 (82.95) | 492 (78.72) | 57 (90.48) | 210 (92.51) | |
| Soft tissue | 104 (11.37) | 89 (14.24) | 4 (6.35) | 11 (4.85) | |
| Internal organ | 21 (2.30) | 20 (3.20) | 0 | 1 (0.44) | |
| Others | 31 (3.39) | 24 (3.84) | 2 (3.17) | 5 (2.20) | |
| Surgical_for_primary_site | <0.001 | ||||
| No | 409 (44.70) | 120 (19.20) | 63 (100.00) | 226 (99.56) | |
| Yes | 506 (55.30) | 505 (80.80) | 0 | 1 (0.44) |
CS_mets, Metastasis; Grade I+II, well differentiated and moderately differentiated; Grade III+IV, poorly differentiated and undifferentiated; NA, unknown.
The cut-off values of age and economic income were determined by X-tile program.
AS can be seen from (Table 1), surgical compliance has improved over time. Before 1995, non-compliant patients accounted for about 44.44% of the overall number of patients. Fortunately, that ratio has decreased to 25.4% in the last 20 years. Table 1 also shows that the sex and marital status of the patients are irrelevant to their surgical compliance.
Multiple meaningful conclusions can be drawn from the patient baseline characteristics data. From a historical perspective, the patient behaved more compliant with the surgical treatment, which can be verified by the fact that the proportion of surgical compliance has increased from 14.56% before 1995 to 56.16% after 2005. In terms of patient age, the highest surgical compliance is contributed by patients from 38 to 72 years of age, and the lowest in patients older than 72 years.
We found that the patients who received radiation and chemotherapy treatment are poles apart in surgical compliance. Namely, the patients with radiation therapy had worse compliance than those without radiotherapy, while the patients with chemotherapy had better compliance that those without chemotherapy. Among the patients with different SEER tumour stages, the patients with non-metastatic SO could accept surgery more easily than metastasized ones (53.76% vs. 7.04%, P<0.001). Furthermore, the location of tumour also affected the surgical compliance and the surgical non-compliance of the patients with SO in axial parts (e.g. spine) than those with tumours in appendicular parts. Besides, the proportion of patients with axial tumour who are unable to have surgery is significantly higher than those with appendicular tumour. We also found that the patients from non-urban areas account for the highest proportion of surgical non-compliance, and among the patients who were unable to undergo surgery, they also accounted for the highest proportion. Considering the surgery cost is not affordable to all the patients, especially the group of Chinese families with a median family income of less than 3323 $. People with good financial condition are more inclined to comply with doctors’ surgical advice. Finally, it is important to mention that the pathological classification was not applicable to subsequent analysis in our study because most patients belonging to the mismatched group lack of effective information (57.14%).
Factors associated with poor surgical compliance
We resorted to the logistic regression to identify the factors significantly associated with surgical compliance (Fig. 2). Firstly, we took advantage of the univariate logistic regression method to reveal multiple relevant factors to surgical compliance (Fig. 2A), and then demine the dominant factors by the multivariate logistic regression method (Fig. 2B). By doing so, we found that there are three main factors could influence the compliance of the patients with SO in our study, that is year of diagnosis, race and chemotherapy. Patients who were diagnosed more recently, non-White and received chemotherapy were more willing to receive surgery. Namely, patients who were diagnosed in 1995–2005 [odds ratio (OR), 0.355; 95% CI, 0.184–0.673; P=0.0017], or after 2005 (OR, 0.156; 95% CI, 0.079–0.299; P<0.0001), non-White patients (OR, 0.377; 95% CI, 0.14–0.846; P=0.0303) and patients received chemotherapy (OR, 0.505; 95% CI, 0.29–0.867; P=0.014) behaved higher surgical compliance.
Figure 2.
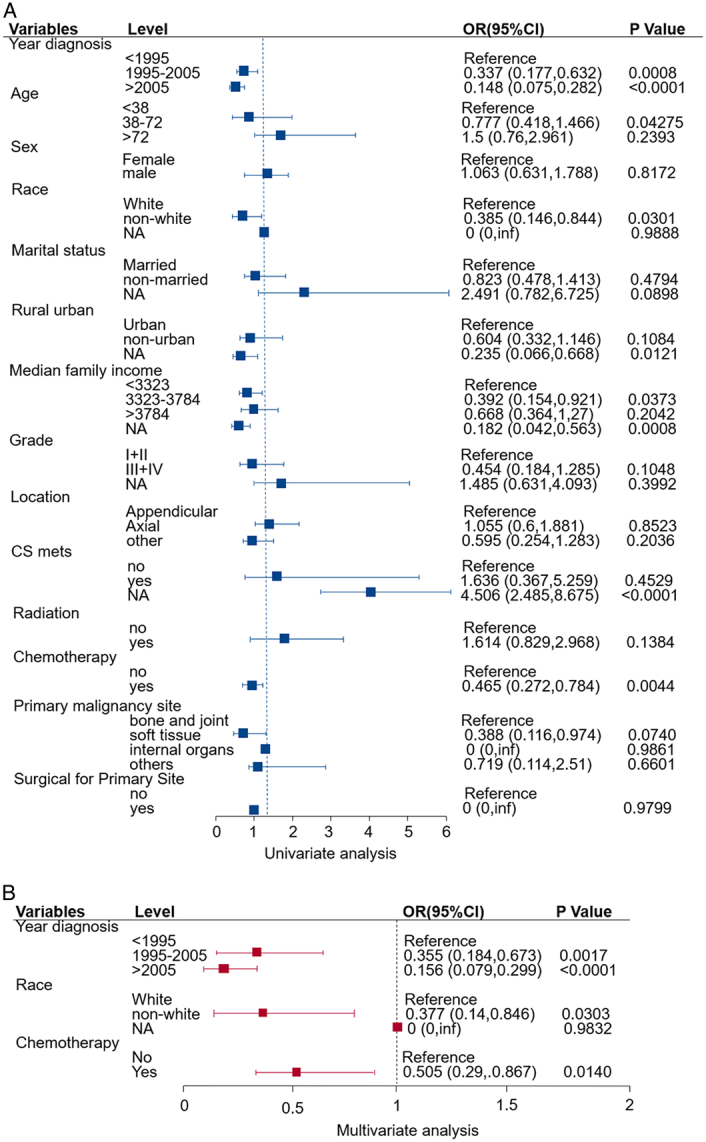
Forest plot of univariate (A) logistic analysis of surgical non-compliance adjusted by year of diagnosis, age, sex, race, marital status, area, economic income, grade, location, metastasis, radiation, chemotherapy, primary maligancy site and surgical. The variables with P<0.05 were included in multivariate (B) logistic analysis. The squares on the transverse lines represent the hazard ratio (HR), and the transverse lines represent 95% CI. The cut-off values of age and economic income were determined by X-tile program. OR, odds ratio.
Survival analysis
Comparison of the survival outcome among different groups
The stratification of patients according to their surgical conditions is shown in Fig. 3. Based on the comparison of the surgical compliance and the non-compliance groups, we can conclude that the better surgical compliance, the higher patients’ OS probability, that is the surgical non-compliance group has worse OS (P<0.0001). The same conclusion can also be drawn from the OS of the surgical compliance group compared with the non-surgical group (P<0.0001). The difference in survival curves between the surgical compliance and non-surgical groups is negligible.
Figure 3.
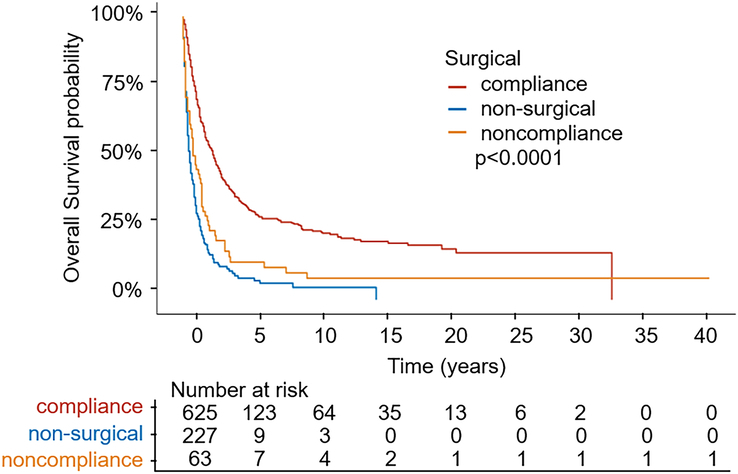
Kaplan–Meier estimates of the overall survival for the total cohort among three groups (surgical compliance group; surgical non-compliance group; non-surgical group).
Cox regression analysis of the prognostic factors
Cox regression can be utilized to conduct prognostic factors analysis for the OS (Table 2). Specifically, the univariate analysis indicated that surgical compliance, diagnosis time, age, marital status, economic income, grade, location, metastasis, radiotherapy, chemotherapy and surgery for the primary site were significant factors for OS. The patients diagnosed in 1995–2005 and after 2005 had a better OS [hazard ratio (HR)=0.748, 95% CI:0.597–0.937, P=0.0116; HR=0.746, 95% CI:0.603–0.923, P=0.0069] than those diagnosed before 1995.
Table 2.
Univariate and multivariate analysis of overall survival (OS) rates
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Level | HR (95% CI) | P | HR (95% CI) | P | |
| Surgery_compliance | |||||
| Compliance | Reference | Reference | |||
| Non-compliance | 1.982 (1.497, 2.624) | <0.0001 | 1.733 (1.205, 2.494) | 0.003 | |
| Non-surgical | 2.943 (2.469, 3.507) | <0.0001 | 1.975 (1.385, 2.817) | 0.0002 | |
| Year_diagnosisa | |||||
| <1995 | Reference | Reference | |||
| 1995–2005 | 0.748 (0.597, 0.937) | 0.0116 | 0.791 (0.564, 1.11) | 0.1753 | |
| >2005 | 0.746 (0.603, 0.923) | 0.0069 | 0.747 (0.485, 1.149) | 0.184 | |
| Agea | |||||
| <38 | Reference | Reference | |||
| 38–72 | 1.991 (1.625, 2.44) | <0.0001 | 2.323 (1.834, 2.943) | <0.0001 | |
| >72 | 4.291 (3.445, 5.345) | <0.0001 | 4.221 (3.236, 5.506) | <0.0001 | |
| Sex | |||||
| Female | Reference | ||||
| Male | 0.991 (0.851, 1.155) | 0.9097 | |||
| Race | |||||
| White | Reference | ||||
| Non-White | 1.04 (0.864, 1.251) | 0.6822 | |||
| NA | 0 (0, Inf) | 0.9863 | |||
| Marital_status | |||||
| Married | Reference | Reference | |||
| Non-married | 0.762 (0.652, 0.89) | 0.0006 | 1.199 (1.004, 1.433) | 0.0453 | |
| NA | 0.699 (0.44, 1.111) | 0.1297 | 0.792 (0.488, 1.288) | 0.3478 | |
| Rural_Urban | |||||
| Urban | Reference | Reference | |||
| Non-Urban | 0.953 (0.775, 1.173) | 0.6495 | 1.187 (0.923, 1.527) | 0.1819 | |
| NA | 0.814 (0.6, 1.105) | 0.1873 | 1.064 (0.442, 2.562) | 0.8903 | |
| Median_family_income | |||||
| <3323 | Reference | Reference | |||
| 3323–3784 | 0.614 (0.479, 0.786) | 0.0001 | 0.608 (0.462, 0.799) | 0.0004 | |
| >3784 | 0.763 (0.627, 0.928) | 0.0069 | 0.706 (0.556, 0.896) | 0.0042 | |
| NA | 0.65 (0.48, 0.879) | 0.0051 | 0.697 (0.276, 1.758) | 0.4439 | |
| Grade | |||||
| I+II | Reference | Reference | |||
| III+IV | 2.01 (1.392, 2.902) | 0.0002 | 2.143 (1.472, 3.121) | 0.0001 | |
| NA | 2.402 (1.653, 3.49) | <0.0001 | 2.041 (1.386, 3.007) | 0.0003 | |
| Location | |||||
| Appendicular | Reference | Reference | |||
| Axial | 1.516 (1.267, 1.813) | <0.0001 | 1.348 (1.111, 1.636) | 0.0025 | |
| Others | 1.316 (1.056, 1.641) | 0.0146 | 0.946 (0.743, 1.204) | 0.6514 | |
| CS_mets | |||||
| No | Reference | Reference | |||
| Yes | 3.318 (2.616, 4.207) | <0.0001 | 2.456 (1.901, 3.173) | <0.0001 | |
| NA | 1.555 (1.313, 1.842) | <0.0001 | 1.171 (0.884, 1.552) | 0.2719 | |
| Radiation for prior malignancies | |||||
| No | Reference | Reference | |||
| Yes | 1.406 (1.161, 1.703) | 0.0005 | 0.915 (0.748, 1.119) | 0.386 | |
| Chemotherapy for prior malignancies | |||||
| No | Reference | Reference | |||
| Yes | 0.549 (0.471, 0.64) | <0.0001 | 0.754 (0.632, 0.899) | 0.0017 | |
| Primary_malignancy_site | |||||
| Bone and joint | Reference | ||||
| Soft tissue | 0.881 (0.686, 1.131) | 0.3209 | |||
| Internal organ | 0.847 (0.515, 1.393) | 0.5128 | |||
| Others | 0.812 (0.53, 1.244) | 0.3397 | |||
| Surgical_for_primary_site | |||||
| No | Reference | Reference | |||
| Yes | 0.448 (0.384, 0.523) | <0.0001 | 0.887 (0.629, 1.25) | 0.4926 | |
After univariate analysis, we selected variables with P<0.2 for further multivariate analysis.
CS_mets, Metastasis; Grade I+II, well differentiated and moderately differentiated; Grade III+IV, poorly differentiated and undifferentiated; HR, hazard ratio; NA, unknown.
The cut-off values of age and economic income were determined by X-tile program.
Elder patients (aged from 38 to 72, or higher than year-old, compared with less than 38) seemed more probably have a worse OS (HR=1.991, 95% CI:1.625–2.44, P<0.0001; HR=4.291, 95% CI:3.445, 5.345, P<0.0001). Patients with non-married status could have a good prognosis (HR=0.762, 95% CI:0.652, 0.89, P=0.0006). With regards to OS, race, sex and area appear to have low association with survival (Table 2).
We found that the economic status of patients is inversely proportional to the prognosis (>3784 vs.<3323, HR=0.763, 95% CI: 0.627–0.928, P=0.0069). It can be seen from Table 2, that the higher the pathological grade is, the worse prognosis could be (P<0.05).
Patients with axial tumours have a higher survival risk than those with appendicular tumours in OS (HR=1.516, 95% CI: 1.267–1.813, P<0.0001). Moreover, the same conclusion can also be made for metastasis cases, a distant tumour, compared with no metastasis one, had a worse effect on OS (HR=3.318, 95% CI: 2.616–4.207, P<0.0001).
Through the univariate analysis, we found that patients with surgical non-compliance had a worse OS than those who complied with surgical recommendations (HR=1.982, 95% CI: 1.497–2.624, P<0.0001). Poor compliance means a higher risk. Previous radiation therapy is a prognostic risk factor (HR=1.406, 95% CI: 1.161–1.703, P=0.0005). Moreover, the patients received the chemotherapy could more likely obtain better OS (HR=0.549, 95% CI: 0.471–0.64, P<0.0001). In addition, the prognosis of patients who did not undergo surgery were significantly worse than those who received primary surgery (HR=0.448, 95% CI: 0.384–0.523, P<0.0001).
We are committed to determining which factors would influence the OS of patients with SO. According to univariate analysis results, variables with P value less than 0.2 were selected for multivariate analysis combined with clinical treatment methods. With the aid of the multivariate analysis model, we can determine whether surgical compliance was an independent prognostic factor for survival. Patients with poor surgical compliance were at increased risk. In addition, through the multivariate analysis, we found that the older patient, the axially located tissue, or the advanced tumour would increase the risk. Finally, the economical income and chemotherapy were protective factors. In comparison, the time of diagnosis, areas, radiotherapy and primary surgery were not statistically significant.
Construction and verification of the Nomogram
We proposed a predicting nomogram for OS of patients with SO, whose parameters include compliance, age, marriage, year of diagnosis, region, income, grade, tumour location, metastasis, radiotherapy chemotherapy, and primary surgery. The nomogram indicated that among all the factors that influence prognosis, the tumour location (Skeletal or extraosseous tumours) and age play the largest role, followed by tumour grade and the diagnosis time. According to the contribution of each parameter to prognosis in the multivariate regression analysis model, each parameter can be scored quantitatively (Table 2). Given all the parameter scores of any patient, we can add them up to obtain the total score, which was then placed on the total point line. According to the mapping relationship between the total point line and the 3-year and 5-year OS probability, we can obtain the predicted survival probability for the patients (Fig. 4A).
Figure 4.
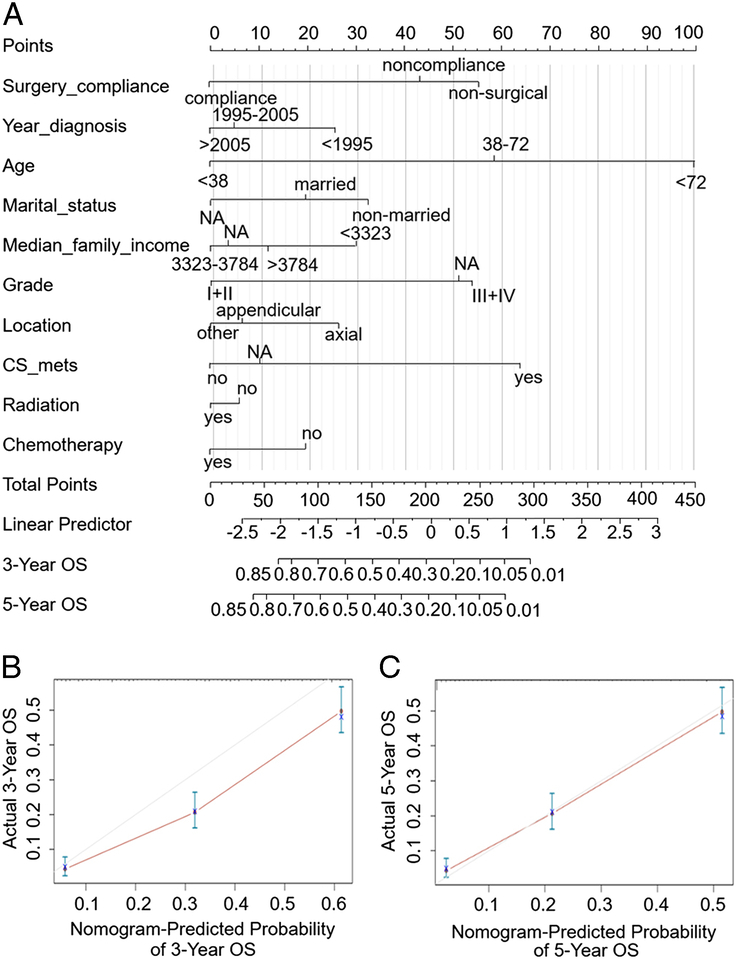
Nomogram predicting 3-year and 5-year overall survival (OS) of patients with secondary osteosarcomas. The nomogram summed the points identified on the scale for each variable. The total points projected on the button scale indicate the likelihood of 3-year and 5-year OS (A). The calibration curves for predicting patient survival at 3-year (B) and 5-year (C). OS overall survival.
The C-index of our prediction nomogram was bootstrapping validated as 0.748 (95% CI: 0.729–0.766), which was greater than 0.7 and indicated good discriminant performance (Fig. 4B, C). It can be seen from the calibration chart that the OS probability of the prediction and the observation groups were consistent, which verified the good predictability of our prediction nomogram. In most ranges, the decision curve is significantly higher than the ALL line and the None line, which verifies the high decision value of the model (Figure S1A, B, Supplemental Digital Content 1, http://links.lww.com/MS9/A397).
Survival trends stratified by the time of diagnosis, age and chemotherapy
In order to investigate the survival trend further, we stratify the patients according to the diagnosis time, age and chemotherapy (Figure S2, Supplemental Digital Content 1, http://links.lww.com/MS9/A397). Before 1995, the difference of the OS probability between the surgical non-compliance and the non-surgical groups is almost negligible (Figure S2A, B, Supplemental Digital Content 1, http://links.lww.com/MS9/A397). After 2005, in terms of survival probability, the surgical compliance group was better than the other two groups (Figure S2C, Supplemental Digital Content 1, http://links.lww.com/MS9/A397). The OS probability curves of the latter two groups have the same trend.
Regardless of how old of the patients (Figure S2D, E, F, Supplemental Digital Content 1, http://links.lww.com/MS9/A397) or whether they were treated with chemotherapy (Figure S2G, H, Supplemental Digital Content 1, http://links.lww.com/MS9/A397), the surgical compliance group was better than the other two groups, and the non-surgical group was the worst from the perspective of survival prognosis.
Discussion
In recent decades, SO has become a major concern, the 5-year OS probability is only 14.6%22. Surgery is an important treatment for osteosarcoma. Patients can only be cured with complete surgical removal13,23. Even with SO, surgery also can significantly prolong the survival of patients24,25. However, due to the particularity of SO, which usually occurs after the initial malignancy with a long incubation period, many patients refuse surgery due to poor physical condition, the long and complex process of multidrug chemotherapy, the side effects of previous surgical treatment, and a lack of proper understanding of the disease and its treatment. We believe that a patient’s surgical compliance plays a crucial role in the long-term and complex treatment process, and it is necessary to study the surgical compliance of SO. This is the first study on the survival of SO and the impact of surgical compliance on this particular patient cohort. Our research has confirmed that surgical compliance is an independent prognostic factor for SO.
We found that nearly 10% of patients with SO did not receive surgery, which is similar to the previous results3. Surgical compliance is defined as the situation where a doctor recommends surgery for a patient’s condition, and the patient either chooses to refuse or accept it16. It is not clear whether patients in the non-compliant group were also treated with other adjuvant therapies. However, it is clear that they should follow the National Comprehensive Cancer Network Guidelines (NCCN) guidelines to undergo surgery.
As expected, the prognosis of the surgical compliance group was significantly better than that of the non-compliant group. The non-surgical group had the worst prognosis because patients in the non-surgical group had advanced tumours, severe complications or contraindications. Our study demonstrated that surgical compliance was an independent factor in improving OS. Next, studies are needed to identify relevant demographic and clinicopathological factors.
Our logistic regression model found that the diagnosis time indeed affected surgical compliance. During the past two decades, patient compliance has significantly improved from that in the 1970s to 1990s, which is similar to other cancers. Liu et al. 16 analyzed the surgical compliance of patients with gastric cancer and showed that patients’ surgical compliance gradually improved over time. The same results were seen in lung cancer17.
We speculate that the advances in social care, insurance and education have made a difference. Modern patients are significantly more aware of their disease than those in the past. The advances of modern medicine (such as clinical, genetic and functional research levels) also help improve patients’ compliance with surgery26. For instance, Mary F. Wedekind suggests that modulating the immune response may benefit osteosarcoma, thereby improving existing therapies for osteosarcoma27. Elizabeth Thoenen demonstrates that modern techniques including whole genome sequencing have recently revealed undetected changes in TP53 in osteosarcoma28.
Many studies have shown that age is a negative factor for surgical compliance due to significant differences in surgical options among patients of different ages15. Our study also showed that surgical compliance was lowest when patients were older than 72 years. Many studies have shown that SO often occurs in the elderly and has a worse prognosis than primary osteosarcoma3. The 5-year survival probability of elderly patients is low, and the prognosis is worse than that of younger patients29.
However, our study did not prove that age was a factor in surgical compliance. Inadequate risk analysis of surgical compliance in patients with osteosarcoma might lead to poor prognosis, especially in older patients. Aggressive tumours and poor response to treatment may result in such poor prognosis13. Older people should be treated more positively. More specific cancer management strategies for older patients have multiple benefits, including helping clinicians build confidence and improving patient compliance with surgery.
Studies have shown that economic income is a factor affecting patient compliance19. Coats et al. 30 found that financial accessibility and acceptance were inversely correlated with treatment abandonment, i.e., the higher income means better compliance, which is consistent with our results.
By comparing the surgical compliance of high and low-income groups, we found that high-income group had significantly higher surgical compliance. However, our study did not find that living area and economic income were significantly related to surgical compliance, which was inconsistent with our expectations.
This result may be due to the small size of SO samples and, thus, the lack of information on relevant variables. As refers to economic income, Medical expenditure may lead to delays in treatment for low-income people who are unable or unwilling to seek treatment a timely manner.
In our study, tumour characteristics were considered as irrelated with patients’ surgical compliance from logistic regression analysis, while cox analysis indicated advanced, axial, metastasizing SOs were more prone to poor survival. Duchman.31 reported that tumour grade is a prognostic factor for osteosarcoma. Zheng et al. 32 determined that tumour grade and histology are independent prognostic indicators for patients with osteosarcoma. Ferrari et al. 22 reported that the 5-year OS of patients with osteosarcoma over the age of 40 and those with synchronous metastasis was 22%. Iwata S. found that 25% of patients with distant metastases, when diagnosed, showed poor surgery due to a lack of confidence33. Such a situation suggests that early detection and treatment are beneficial for survival. In our study, we also found an interesting phenomenon, namely, patients with Axial SO are more common and accounted for a high proportion of non-surgical cases, which indicated the Axial tumour is a risk factor for prognosis and deteriorate the survival of patients.
In addition, previous treatments, including surgery, radiotherapy and chemotherapy, also influence surgical compliance. We found that prior chemotherapy was a highly relevant factor for surgical compliance and an independent prognostic factor. No association was found in compliance or prognosis with radiotherapy. Patients with SO who had not previously received chemotherapy were less likely to undergo surgery due to their probable lack of knowledge of the treatment of SO.
From the perspective of comprehensive treatment, SO is less sensitive to conventional chemotherapy than primary osteosarcoma34, and surgery becomes a radical treatment. In addition, some researchers found that biophysical therapy, immunotherapy and Regorafenib may be adjunctive therapies affecting patients’ surgical survival10. Therefore, we believe that assisted surgical treatment can also improve patients’ understanding of the treatment of SO, thus improving patients’ surgical compliance, whose effectiveness clearly needs more investigation.
In cox multivariate analysis, gender and primary site were not found to be significant, which is different from previous reports. However, race was not significant in the prognosis of SO, but was a highly correlated factor for surgical compliance. Previous studies have found that there are differences in cancer treatment choices among different races, but the specific differences are inconsistent among different studies35, which may be a result of the different selected research objects and research groups. In addition, due to the lack of available information on histological subtypes, they were not included in the analysis, as other small sample studies were16,35.
Previous research has documented the impact of nomograms on predicting survival rates in SO. Yanqi et al. 14 developed a validated prognostic nomogram with a C-index of 0.826 to forecast the prognosis of SO. However, no evaluation of factors related to treatment choices was conducted. Presently, there is a dearth of prognostic nomograms that consider surgical compliance for SO. Consequently, we have amalgamated more familiar clinical variables to construct a nomogram tailored to surgical non-compliant patients with SO. Our study is pioneering in revealing that surgical compliance is a pivotal prognostic determinant for SO. Insufficient surgical compliance can ultimately contribute to unfavourable outcomes. Our proposed risk assessment model offers an opportunity for enhanced clinical decision-making and patient counselling, particularly for individuals who are not actively involved in treatment selection. Subsequently, we can assist patients in developing a proper understanding of the disease and the necessity of treatment through nutritional support, psychological intervention, follow-up examinations, complication management, and effective communication. This will help patients make informed decisions and choose the most suitable treatment plan.
There are several limitations in this study. First, although we attempted to control the heterogeneous features of characteristics by using multivariate analysis, there may be other unknown confounding factors that cannot be taken into account. Secondly, we can’t gather information such as family background, education level, insurance and so on, which may also influence surgical compliance. Third, due to the particularity of SO, the long-term and complex treatment process of multidrug chemotherapy may affect surgical compliance. In addition, the nomogram of the prognosis of SO requires external validation, and we believe that more clinical studies have to be conducted so as to obtain more impressive results.
Conclusions
In conclusion, our study confirmed that surgical compliance is an independent prognostic factor affecting the survival of SO. Lower surgical compliance was strongly correlated with early diagnosis and rejection of chemotherapy. We constructed a nomogram that provides an individual prediction of survival for patients. This can help clinicians quickly respond and identify patients with poor prognosis so as to reverse the prognosis and formulate effective prevention and treatment measures for this invasive disease.
Ethics statements
Not applicable.
Consent
Not applicable.
Source of funding
This work was supported by the National Natural Science Foundation of China [82072725 and 81872042 to X.C., 81702442 to Z.L., 81972332 to Y.C., 82002591 to G.F.], the Key Program of Jiangsu Provincial Health Commission [ZD2021039 to X.C.], the Natural Science Foundation of Jiangsu province [BK20170623 to Z.L., BK20200273 to G.F.], the China Postdoctoral Science Foundation [2020M670090ZX to Z.L., 2021MD703958 to G.F.], the Postdoctoral Science Found of Jiangsu province [2018K090B to Z.L.].
Author contribution
X.C. and Z.L. designed the study. J.W., G.F. and Z.Z. wrote the manuscript. L.D., Y.C., H.L., D.X., Z.D., J.Z. and L.J. helped with the final revision of the review. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors have no relevant financial or non-financial interests to disclose.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Jing Wang.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Patient consent for publication
Not applicable.
Supplementary Material
Footnotes
J.W., G.F. and Z.Z.: These authors wish it to be known that, in their opinion, the first three authors should be regarded as joint First Authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 15 March 2024
Contributor Information
Jing Wang, Email: laabc2021@163.com.
Gongbo Fu, Email: mhksfgb@126.com.
Zhongxiu Zhu, Email: 634278577@qq.com.
Lan Ding, Email: 523441365@qq.com.
Yitian Chen, Email: yitianchen@126.com.
Huiyu Li, Email: 271254258@qq.com.
Dan Xiang, Email: 460782849@qq.com.
Zhe Dai, Email: daizhe1991319@163.com.
Jialong Zhu, Email: Zhu_jialong@163.com.
Linlin Ji, Email: jll199801@163.com.
Zengjie Lei, Email: leizengjie@163.com.
Xiaoyuan Chu, Email: chuxiaoyuan000@163.com.
References
- 1.De Miguel GC, Abrantes AM, Laranjo M, et al. A new therapeutic proposal for inoperable osteosarcoma: photodynamic therapy. Photodiagnosis Photodyn Ther 2018;21:79–85. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The Etiology of Osteosarcoma In: Jaffe N, Bruland OS, Bielack S, eds. Pediatric and Adolescent Osteosarcoma. Springer US; 2010:15–32. [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casali PG, Bielack S, Abecassis N, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv79–iv95. [DOI] [PubMed] [Google Scholar]
- 5.Dray MS, Miller MV. Paget’s osteosarcoma and post-radiation osteosarcoma: secondary osteosarcoma at Middlemore Hospital, New Zealand. Pathology 2008;40:604–610. [DOI] [PubMed] [Google Scholar]
- 6.Hamre MR, Severson RK, Chuba P, et al. Osteosarcoma as a second malignant neoplasm. Radiother Oncol 2002;65:153–157. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv Anat Pathol 2021;28:119–138. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S. Second malignant neoplasms following radiotherapy. Int J Environ Res Public Health 2012;9:4744–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuermann A, Phelan R, Browning M. Development of secondary osteosarcoma after TBI and allogeneic bone marrow transplant: a case series of 3 patients. J Pediatr Hematol Oncol 2020;42:e100–e103. [DOI] [PubMed] [Google Scholar]
- 10.Pierobon M, Mercolini F, Affinita MC, et al. Secondary osteosarcoma after bone marrow transplant: an aggressive disease. J Adolesc Young Adult Oncol 2020;9:672–675. [DOI] [PubMed] [Google Scholar]
- 11.Kebudi R, Ozger H, Kizilocak H, et al. Osteosarcoma after hematopoietic stem cell transplantation in children and adolescents: case report and review of the literature. Pediatr Blood Cancer 2016;63:1664–1666. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima H, Ogose A, Hotta T, et al. Secondary osteosarcoma arising from osteochondroma following autologous stem cell transplantation with total-body irradiation for neuroblastoma: a case report. Oncol Lett 2015;10:1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Wu B, Zhou Y, et al. Predictors of the survival of primary and secondary older osteosarcoma patients. J Cancer 2019;10:4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Liu H, Wang S, et al. A nomogram for predicting cancer-specific survival in patients with osteosarcoma as secondary malignancy. Sci Rep 2020;10:12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faisham WI, Mat Saad AZ, et al. Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia Pac J Clin Oncol. 2017;13:e104–e110. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Xu M, Gao T, et al. Surgical Compliance and Outcomes in Gastric Cancer: a population-based cohort study. J Cancer 2019;10:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Mao W, Wang Y, et al. Surgical compliance and survival outcomes for patients with stage T1-2 non-small-cell lung cancer. Cancer Manag Res 2020;12:3597–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz-Largacha JA, Steiling KA, Kathuria H, et al. Initial surgical experience following implementation of lung cancer screening at an urban safety net hospital. J Thorac Cardiovasc Surg 2018;155:2674–2681. [DOI] [PubMed] [Google Scholar]
- 19.Burgoon ML, Miller PA, Hoover-Hankerson B, et al. Challenges to understanding and compliance among surgical patients in low-income urban teaching hospitals. Am Surg 2021;87:818–824. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Ji L, Wang X, et al. Nomogram predicts risk and prognostic factors for bone metastasis of pancreatic cancer: a population-based analysis. Front Endocrinol 2022;12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Hu J, Yang J, et al. Selection of optimal candidates forcytoreductive nephrectomy in patients with metastatic clear cell renal cell carcinoma: a predictive model based on SEER database. Front Oncol 2022;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari S, Bielack SS, Smeland S, et al. EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma. Tumori 2018;104:30–36. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018;18:39–50. [DOI] [PubMed] [Google Scholar]
- 24.Briccoli A, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol 2010;19:193–199. [DOI] [PubMed] [Google Scholar]
- 25.Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019;109:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anwar MA, El-Baba C, Elnaggar MH, et al. Novel therapeutic strategies for spinal osteosarcomas. Semin Cancer Biol 2020;64:83–92. [DOI] [PubMed] [Google Scholar]
- 27.Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer 2018;65:e27227. [DOI] [PubMed] [Google Scholar]
- 28.Thoenen E, Curl A, Iwakuma T. TP53 in bone and soft tissue sarcomas. Pharmacol Ther 2019;202:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am 2016;47:283–292. [DOI] [PubMed] [Google Scholar]
- 30.Coats V, Maltais F, Simard S, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J 2013;20:e10–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol 2015;39:593–599. [DOI] [PubMed] [Google Scholar]
- 32.Zheng W, Huang Y, Chen H, et al. Nomogram application to predict overall and cancer-specific survival in osteosarcoma. Cancer Manag Res 2018;10:5439–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwata S, Ishii T, Kawai A, et al. Prognostic factors in elderly osteosarcoma patients: a multi-institutional retrospective study of 86 cases. Ann Surg Oncol 2014;21:263–268. [DOI] [PubMed] [Google Scholar]
- 34.Giannini L, Incandela F, Fiore M, et al. Radiation-Induced sarcoma of the head and neck: a review of the literature. Front Oncol 2018;8:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu N, Molena D, Stem M, et al. Underutilization of treatment for regional gastric cancer among the elderly in the USA. J Gastrointest Surg 2018;22:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article.


