Abstract
By far the largest contribution to ion detectability in liquid chromatography-driven mass spectrometry-based proteomics is the efficient generation of peptide molecular ions by the electrospray source. To maximize the transfer of peptides from the liquid to gaseous phase and allow molecular ions to enter the mass spectrometer at microspray flow rates, an efficient electrospray process is required. Here we describe the superior performance of newly design vacuum insulated probe heated electrospray ionization (VIP-HESI) source coupled to a Bruker timsTOF PRO mass spectrometer operated in microspray mode. VIP-HESI significantly improves chromatography signals in comparison to electrospray ionization (ESI) and nanospray ionization using the captivespray (CS) source and provides increased protein detection with higher quantitative precision, enhancing reproducibility of sample injection amounts. Protein quantitation of human K562 lymphoblast samples displayed excellent chromatographic retention time reproducibility (<10% coefficient of variation (CV)) with no signal degradation over extended periods of time, and a mouse plasma proteome analysis identified 12% more plasma protein groups allowing large-scale analysis to proceed with confidence (1,267 proteins at 0.4% CV). We show that the Slice-PASEF VIP-HESI mode is sensitive in identifying low amounts of peptide without losing quantitative precision. We demonstrate that VIP-HESI coupled with microflow rate chromatography achieves a higher depth of coverage and run-to-run reproducibility for a broad range of proteomic applications. Data and spectral libraries are available via ProteomeXchange (PXD040497).
Keywords: vacuum insulated probe heated electrospray ionization (VIP-HESI) source, captivespray (CS), electrospray ionization (ESI), microflow liquid chromatography (μLC), data-independent acquisition-parallel accumulation and serial fragmentation (dia-PASEF), slice-parallel accumulation and serial fragmentation (Slice-PASEF)
Graphical Abstract
INTRODUCTION
Large scale proteomic analysis requires consistent interrogation of assembled cohort samples in a highly reproducible and high-throughput fashion to obtain quantitative differences with strong statistical significance of perturbed networks of proteins. Nanoflow liquid chromatography-tandem mass spectrometry (nLC-MS/MS), a mainstay of proteomics analysis, provides high-sensitivity with minimal sample usage, but its quantitative proteomic application is restricted by poor robustness over extended periods of time, leading to low sample throughput and additional batch effect considerations.1 As next-generation mass spectrometry (MS) instruments become increasingly faster and more sensitive, microflow liquid chromatography (μLC) workflows circumvent such issues by attaining stable electrospray ionization and higher signal stability, resulting in improved data reproducibility coupled with the ease of use as compared to low-flow nanospray ionization.2–8 Consequently, μLC-tandem mass spectrometry (μLC-MS/MS) has gained an advantage over nLC-MS/MS in large-scale proteome research experiments where sample amounts are not limited and have been shown to achieve significant proteome coverage.7 Peptide measurements by μLC-MS/MS are further improved with the advancements in the data-independent acquisition (DIA) methods that can achieve high protein depth and coverage, with excellent reproducibility compatible with μLC-MS/MS.9
Different high-flow rate chromatography methods have been used with a variety of mass spectrometer instruments. For instance, we and several others have applied capillary flow rates (1–10 μL/min) coupled with TripleTOF mass spectrometers (SCIEX), accelerating data measurements for increased sample throughput.3,10–15 Lately, several studies have shown the application of μLC1,16 and capillary flow9 chromatography workflows in robustly quantifying thousands of proteomes using Thermo-Fisher Orbitrap mass spectrometers. More recently, the Bruker timsTOF MS system with added ion mobility, has gained popularity due to its higher duty cycle in parallel accumulation and serial fragmentation (PASEF) mode that provides an additional dimension of peptide ion separation, resulting in a reduction in the sample complexity due to fewer cofragmentation events, significantly improving the proteome coverage.17 Furthermore, Bruker has recently introduced a new ion source, known as vacuum insulated probe heated electrospray ionization (VIP-HESI), for operation on the timsTOF is capable of handling analytical flow rates and is primarily used for small molecule studies such as lipidomics18,19 and quantitative analysis of environmental samples.20 Additionally, Lindemann et al. compared the performance of VIP-HESI source with the standard electrospray ionization (ESI) source in quadrupole time-of-flight (QTOF) MS using mycotoxins samples and found that the former source exhibited increased sensitivity, which utilized additional heated drying gases to enhance desolvation.20 However, there has been no thorough assessment of the comparative efficiency of these ion sources in proteomics to date. Szyrwiel et al. benchmarked the Slice-PASEF method using analytical flow (500 μL/min) coupled with a VIP-HESI source and timsTOF MS;21 however, to achieve the balance between performance and robustness, μLC-MS/MS could also be suitable for proteomics applications.7 Here we evaluate the performance of microflow chromatography for quantitative proteome analysis in Slice-PASEF and dia-PASEF modes using a sensitive timsTOF ion mobility MS. For most proteomics applications, the Bruker timsTOF MS is coupled with nanoflow rate chromatography with a captivespray (CS) ion source. To achieve more robust operation and ease of use, we investigated the performance of three ionization sources from Bruker including the new VIP-HESI ion source as well as the standard ESI operated in the μLC-MS/MS mode and compared it with a CS ion source using nanoflow rate workflows.
In our comparative analysis, we standardized the VIP-HESI source with a microflow chromatography workflow with two different analytical column dimensions (i.e., 0.5 mm and 1.0 mm internal diameter (ID)) operating at 20 μL/min and 40 μL/min flow rates, to investigate the efficiency of the proteomic analysis of synthetic peptide mixtures, HeLa and K562 cell line tryptic digests, and tryptic digests of undepleted mouse plasma using dia-PASEF. The acquired data illustrates higher sensitivity and higher reproducibility in peptide ion MS and MS/MS collection using VIP-HESI than compared to standard ESI and CS applications. These results suggest that the VIP-HESI source with microflow chromatography using DIA methods in a timsTOF MS has the potential of processing thousands of samples in an automated unattended operation with superior higher performance characteristics.
EXPERIMENTAL PROCEDURES
Preparation of a Synthetic Peptide Mixture
An isotopically light version of the SCIEX PepCalMix solution containing 20 non-naturally occurring synthetic peptides that cover a wide mass and retention time range (SynPeptide, Shanghai, China) was used. A stock solution of 1 pmol/μL was prepared in 5% v/v acetic acid in 2% v/v acetonitrile (ACN)-containing water, and aliquots of 10 μL each were stored at −80 °C until further use. For ESI and CS-MS measurements, 1 μL of the PepCalMix aliquot was diluted in 39 μL of the same solvent to achieve the final peptide concentration: 25 fmol/μL. For ESI and VIP-HESI MS linearity measurements, 10 μL of the PepCalMix aliquot was diluted in 790 μL in the same solvent to achieve a final concentration of 12.5 fmol/μL. To obtain the sample amount of 12.5 fmol on column, we injected 1 μL of sample. The volume of sample injected was linearly doubled for each sample amount of 25 fmol, 50 fmol, 100 fmol, 200 fmol, 400 fmol, and 800 fmol.
MOUSE TISSUE AND PLASMA SAMPLE PREPARATION
Mouse Husbandry and Interventions
All the mouse samples were prepared at the University of Michigan in a specific pathogen-free colony kindly supplied by Dr. R. Miller. Genetic background and husbandry conditions for the Snell dwarf and GHRKO mice were described previously.22 Mice used for rapamycin (encapsulated, used at 14 ppm), canagliflozin (180 ppm), 17α-estradiol (17aE2) (14.4 ppm), acarbose (1000 ppm), and calorie-restricted samples were of the UM-HET3 stock, produced as the offspring of CByB6F1/J mothers and C3D2F1/J fathers, as described previously.23 The base diet was Purina 5LG6. To monitor specific-pathogen status, sentinel mice were exposed to spent bedding for 2 weeks prior to testing, and all tests were negative for the entire aging colony during the experimental period. The protocols were reviewed and approved by the University of Michigan’s Institutional Animal Care and Use Committee. The metadata of the mouse liver, kidney, gastrocnemius muscle tissues, and plasma samples are provided in Supplementary Table 1.
Sample Harvesting
Mice were humanely euthanized at 12 months of age. Euthanasia occurred between 8 am and 11 am (lights cycled on at 6 am and off at 6 pm) by rapid asphyxiation in an enclosed container containing CO2 gas. Mice were unconscious within 5 s and stopped breathing at about 10 s. The interval between removal from the home cage to death was less than 1 min. Mice were removed as soon as breathing ceased, and blood was harvested by closed-chest cardiac puncture.
Sample Preparation
Tissue sections from kidney, liver, and gastrocnemius muscle were processed for DIA LC-MS/MS analysis as follows. Frozen tissue was lysed in a 50 mM ammonium bicarbonate buffer with 5% sodium dodecyl sulfate (SDS) and homogenized on a Precellys Evolution (Bertin Instruments, France) for 9 rounds of 20 s at 8500 rpm with 30 s of rest in between. Protein concentration of the lysate was determined using the bicinchoninic acid (BCA) protein assay (Pierce, Cat# 23227). Lysate containing 200 μg of protein was aliquoted out and denatured for 2 min at 90 °C. After denaturation, samples were reduced with 5 mM TCEP ((tris(2-carboxyethyl) phosphine) Sigma Cat# 4706) for 30 min at 60 °C and then alkylated with 10 mM α-iodoacetamide (Millipore Cat# 407710) for 30 min at room temperature in the dark. Samples were acidified with phosphoric acid to a final concentration of 1.2% and S-Trap buffer (1 00 m M a m monium bicarbonate buffer +90% methanol) was added at 1:7 ratio prior to loading on S-Trap wells (96-well plate format, ProtiFi, USA) according to the manufacturer’s instructions. Using a positive pressure manifold (Resolvex M10, Tecan, USA), we washed twice with the S-Trap buffer before overnight digestion at 37 °C with Trypsin (Promega Cat# V511X) in digestion buffer (50 mM ammonium carbonate) at a 1:50 ratio. Digested peptides were eluted as per the manufacturer’s instructions (S-Trap 96-well plate protocol version 1.4). Importantly for normalization, all plates contained an even mix of samples from all tissues, arranged systematically to avoid bias across either rows or columns. In nearly all cases all three tissue samples from the same donor were loaded onto the same plate.
Mouse undepleted plasma from 284 mice was processed for DIA LC-MS/MS analysis as follows. Frozen plasma aliquots were thawed at 4 °C for 3 h followed by a hard spin to pellet insoluble particles (5 min, 10000g, 4 °C). Protein concentration was determined using the BCA assay (Pierce, Cat# 23227) as described above. Samples were normalized by aliquoting 400 μg in phosphate-buffered saline (PBS) into a final volume of 37.5 μ L. Denaturation was performed by adding a 12.5 μL lysis buffer (200 mM triethylammonium bicarbonate (TEAB), 20% SDS) to a final concentration of 5% SDS and heating for 5 min at 90 °C with 800 rpm shaking. All mixing and shaking steps were performed in a ThermoMixer C orbital thermal controlled shaker (Eppendorf) with the Smartblock for deep-well plates. After denaturation, samples were reduced with 5 mM TCEP (Sigma Cat# 4706) for 15 min at 55 °C and then alkylated with 10 mM iodoacetamide (Millipore-Sigma Cat# 407710) for 10 min in the dark at room temperature, both with 800 rpm shaking. Samples were acidified with neat orthophosphoric acid to a final concentration of 2.7% (v/v). The S-trap buffer (100 mM TEAB pH 8.5/90% methanol) was added at a 1:7 ratio prior to loading on the S-trap 96 well-plate format (ProtiFi, USA) by 1 min spin at 4000g. Once loaded, samples were washed six times with 400 μL of S-trap buffer and spun at 4000g for 5 min to ensure complete dryness.
Tryptic digestion was performed at 37 °C with 125 μL of trypsin (Promega Cat# V511X) in a digestion buffer (100 mM TEAB pH 8.5) at a 1:25 ratio. After the first hour of incubation at 37 °C, an additional 75 μL of digestion buffer was added to prevent the drying out of the samples. After overnight incubation at 37 °C, the digested peptides were eluted with 80 μL of digestion buffer and centrifugation at 4000g for 1 min, and then with 80 μL of 50% ACN in a digestion buffer and centrifugation at 4000g for 1 min. The final eluate was ~250 μL. Quantification of the digested peptides was performed by the fluorescamine fluorescent peptide as say24 (Pierce, Cat# 23290) before drying down to completion.
HeLa Cell Culture and Protein Digestion
HeLa S3 immortalized human epithelial cells (ATCC Cat# CCL-2.2) cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) were grown at 37 °C in 5% CO2 to confluency. Cells were rinsed with cold PBS three times, frozen on ethanol/dry ice in pellets, and stored at −80 °C. Cells were lysed in a urea buffer (8 M urea, 0.1 M Tris pH 8.2) by three rounds of flash-freezing in ethanol/dry ice followed by thawing/vortexing. The lysate was sonicated on ice for 8 rounds of the 30 s in a cup-horn sonicator (full power) to shear any DNA, then centrifuged for 15 min at 21,000g. Protein quantity was determined by a BCA assay. The lysate was reduced with 5 mM TCEP for 30 min at 37 °C in a benchtop incubator at 850 rpm, alkylated with 15 mM iodoacetamide for 30 min at 20 °C in the dark at 850 rpm, and then quenched with 5 mM TCEP for 30 min at 20 °C at 850 rpm. Protein was precipitated in 80% ethanol, and automated protein-aggregation capture (PAC) was performed on a BioSprint-96 (Qiagen) according to the following method: plate 1 - magnetic head comb, plate 2–1.6 μg/μL carboxylate beads (1:1 mixture of hydrophilic and hydrophobic beads [mass/mass], GE Healthcare Cat#’s 24152105050250 and 65152105050250) in 500 μL H2O per well, plate 3–500 μL 80% ethanol per well, plate 4–0.4 μg/μL lysate in 500 μL 80% ethanol per well, plate 5 to 7–500 μL 80% ethanol per well, plate 8–300 μL 50 mM ammonium bicarbonate per well. The program was set to collect the beads from plate 2, wash for 5 min at medium speed in plate 3, bind protein for 15 min at medium speed in plate 4, wash for 5 min per plate at medium speed for plates 5 through 7, and mix beads for 10 min at medium speed in plate 8. The beads were then left in plate 8, transferred to 1.5 mL microcentrifuge tubes, and trypsin (Promega) at a ratio of 1:100 (w/w) trypsin: protein lysate was added to the tubes. Digestion was carried out for 18 h at 37 °C on a tube rotator. Digestion was quenched with the addition of neat formic acid to 1%, beads were resolved on a magnet, and the supernatant was dried in a SpeedVac (Thermo-Fisher). Samples were resuspended in 0.1% formic acid/H2O (v/v) for liquid chromatography–mass spectrometry.
K562 Cell Culture and Protein Digestion
K562 cells (ATCC CCL-243, human bone marrow myeloid leukemia lymphoblast cell line) were cultured at 37 °C with 5% CO2 in Eagle’s minimum essential medium (EMEM, ATCC 30–2003) supplemented with 10% FBS and were grown to 70% confluence. Cells were lysed in urea buffer (8 M urea, 0.1% RapiGest, and 100 mM ammonium bicarbonate pH 8). Protein quantity was determined by a BCA assay. Proteins were reduced with 5 mM TCEP for 30 min at 37 °C in a benchtop incubator at 850 rpm, alkylated with 10 mM iodoacetamide for 30 min at room temperature in the dark, and digested using trypsin (1:50 w/w), and samples desalted with tC18 SepPak cartridges (Waters).
High-pH Fractionation
To maximize the proteome coverage from HeLa digest, peptides were fractionated using a reversed-phase Acquity CSH C18 1.7 μm 1 × 150 mm column (Waters, Milford, MA) on a UltiMate 3000 high-pressure liquid chromatography (HPLC) system (Dionex, Sunnyvale, CA) operating at 30 μL/min and SpotOn (Bruker). 5 mM ammonium bicarbonate as buffer A and 100% ACN as buffer B were used. Peptides were separated by a linear gradient from 5% B to 35% B in 55 min, followed by a linear increase to 70% B in 8 min. Twenty-four in-solution fractions were collected in a concatenated manner,25 acidified to pH < 2 with trifluoroacetic acid, and vacuum-dried prior to DDA (data dependent acquisition) LC-MS/MS.
Liquid Chromatography–Mass Spectrometry
All peptide samples were spiked in with iRT standard peptides (Biognosys AG, Schlieren, Switzerland) and applied to MS analysis using EasyLC nano HPLC (Thermo-Fisher Scientific), Vanquish Neo HPLC system (Thermo-Fisher Scientific), Evosep One system HPLC (EvoSep), and nano Elute HPLC (Bruker) configured in either nanoflow or microflow modes were coupled to a timsTOF PRO mass spectrometer (Bruker). Both modes were operated with 99.9% water, 0.1% formic acid/Milli-Q water (v/v, buffer A), and 99.9% ACN, 0.1% formic acid (v/v, buffer B). Four different HPLC systems, four different samples, three different ion sources, nine different analytical columns, and eight different gradient lengths were used in this study (Supplementary Table 2).
For CS measurements of PepCalMix and mouse plasma, the nanoflow mode was selected, and peptides were trapped on a 0.5 cm × 0.3 mm trap cartridge Chrom XP C18, 3 μm (Thermo-Fisher Scientific) at 10 μL/min and separated on a C18 UHP 15 cm × 0.15 mm × 1.5 μm column (Bruker/PepSep) at 1 μL/min using a 45 min stepwise gradient from 3% to 25% B in 37 min, 25% to 35% B in 8 min, 35% to 80% B in 1 min and isocratic flow at 80% B for 2 min followed by fast washing and equilibration for three column volumes. For generating a comprehensive HeLa sample library, peptides were separated on various columns including a C18, 8 cm × 0.15 mm × 1.5 μm column (Bruker/PepSep), various columns including a C18, 15 cm × 0.15 mm × 1.5 μm column (Bruker/PepSep), various columns including a C18, 15 cm × 0.15 mm × 1.9 μm column (Bruker/PepSep), various columns including a C18, 25 cm × 0.2 mm × 1.5 μm column (Bruker/PepSep), various columns including a C18, 25 cm × 0.15 mm × 1.9 μm column (Bruker/PepSep), a various columns including C18, 25 cm × 0.075 mm × 1.6 μm column (IonOpticks, Australia), and a C18, 40 cm × 0.075 mm × 1.9 μm column (Bruker/PepSep) using premade 200SPD, 100SPD, 60SPD, 30SPD, and 15SPD gradient methods using Evosep One and 90-minute linear gradient from 3% to 35% B in 90 min, 35% to 95% B in 20 min and isocratic flow at 95% B for 20 min followed by equilibration of the column for starting conditions for 9 min. The CS ion source was equipped with a 20 μm emitter (Bruker), and the parameters were as follows: 1700 V capillary voltage, 3.0 L/min dry gas, and temperature set to 180 °C.
For ESI and VIP-HESI measurements, the workflow was changed to microflow in the Vanquish Neo, peptides were trapped on a 50 × 1 mm ID trap cartridge Chrom XP C18, 3 μm (Thermo-Fisher Scientific) at 50 μL/min and separated on either a C18, 15 cm × 1 mm × 1.7 μm Kinetix column (Phenomenex, USA) at 40 μL/min using the same 45 min stepwise gradient as described above or a C18 20 cm × 0.5 mm × 1.9 μm column (Dr. Maisch GmbH) at 20 μL/min using a linear 21 min gradient from 3 to 35% B in 21 min, 35 to 80% B in 1 min and isocratic flow at 80% B for 2 min. The standard ESI source was equipped with a 100 μm electrode probe (Bruker), and the parameters were as follows: 4500 V capillary voltage, 10.0 L/min dry gas, and temperature 200 °C. The VIP-HESI source was equipped with a 50 μm electrode probe (Bruker), and the parameters were as follows: 4000 V capillary voltage, 3.0 L/min dry gas, and temperature 200 °C, probe gas flow 3.0 L/min and temperature 100 °C. The VIP-HESI source parameter settings were optimized for spray stability over extended periods of time using the background signal with 0.5 mm, 1.0 mm ID analytical columns and 20 μL/min, 40 μL/min flow rates, respectively.
PepCalMix, mouse liver, kidney, gastrocnemius muscle tissues, and plasma sample injections were acquired using Bruker timsTOF preformed dia-PASEF mode schema covering the m/z range of 400–1200 and 1/K0 range 0.6 to 1.42 in 32 × 25 Da windows with a mass overlap of 1 Da, resulting in a total cycle time of 1.8 s. A total of seven different sample amounts of PepCalMix peptides (12.5 fmol, 25 fmol, 50 fmol, 100 fmol, 200 fmol, 400 fmol, and 800 fmol), and six different injection amounts (0.4 μg, 2 μg, 4 μg, 10 μg, 20 μg, and 40 μg) of mouse plasma sample and 200 ng each of liver, kidney, gastrocnemius muscle tissues samples were measured. For the Slice-PASEF experiment, three different amounts of HeLa injections viz. 10 ng, 100 ng, and 1000 ng were measured. The 1Frame (1F) Slice-PASEF method was downloaded from the recently published study21 that covers the 400–1000 m/z and the 1/K0 range 0.75–1.2. The premade py8 dia-PASEF method (that was compared with the 1F Slice-PASEF method) was modified to set the precursor mass range to 400–1000 m/z and 1/K0 0.65–1.37, with 24 × 25 Da windows with ramp and accumulation time 72 ms and total cycle time estimated to 0.7 s. For both methods, the high sensitivity mode was enabled in the tims control acquisition software of the mass spectrometer.
For HeLa library generation, 24 fractions were measured using timsTOF MS (Bruker) set to dda-PASEF acquisition scan mode covering 100–1700 m/z with 10 PASEF ramps. The tims settings were 100 ms ramp and accumulation time (100% duty cycle), resulting in 1.1 s of total cycle time. Active exclusion was enabled with a 0.4 min release. The default collision energy with a base of 0.6 1/K0 [V s/cm2] is set at 20 eV and 1.6 1/K0 [V s/cm2] at 59 eV was used. Isolation widths were set at 2 m/z at <700 m/z and 3 m/z at >800 m/z. To achieve more comprehensive coverage, HeLa digest peptides were also acquired using dia-PASEF preformed py3, py5, and py8 (Bruker) schema based on the length of gradient used in the Evosep One HPLC.
Spectral Assay Library Generation
For detailed quantitative proteomic analysis in individual experiments, we generated two comprehensive hybrid sample-specific spectral libraries for both HeLa and mouse plasma samples using DDA and DIA runs in Spectronaut26 16.0.220606.53000 (Biognosys) respectively. The mouse spectral library was built using search archives (.psar) files from a published study27 and in-house sample-specific DIA acquisition files of mouse liver, kidney, gastrocnemius muscle tissues, and plasma samples. The HeLa library was generated using 24 fractionated DDA files from the HeLa sample and sample-specific DIA HeLa sample files as detailed in Supplementary Table 2. For respective libraries, related acquisition files were searched against the Mouse UniProt, one protein sequence per gene, FASTA (downloaded on March 07, 2021, containing 21,991 entries) and Human UniProt FASTA (downloaded on October 21, 2022, containing 20,375 entries). For each library iRT peptides (Biognosys) were appended and carbamidomethyl was used as fixed modification (C); acetyl (protein N-term) and oxidation (M) as variable modification; and enzyme digestion trypsin/P with up to two missed cleavages were allowed. Mass tolerances were automatically determined by Spectronaut, and for the rest of the processing, default settings were used. Identification search results were filtered at a 1% false discovery rate on the PSM, peptide, and protein levels. Sample-specific PepCalMix library was generated against the PepCalMix peptide FASTA sequence using the Spectronaut 17.0.221202.55965 (Biognosys) with the same settings described above.
Spectral Assay Library Quality Assessment Using DIALib-QC
Both comprehensive spectral assay libraries were assessed for their quality using DIALib-QC (v1.2).28 DIALib-QC evaluates 57 parameters of compliance and provides a detailed report of the library’s complexity, characteristics, modifications, completeness, and correctness. The assessment reports are provided in Supplementary Table 3. In the DIALib-QC assessment report, there were no problem assays found for both libraries, and were used as it is for DIA-MS analysis.
Data Analysis
Two different DIA software tools were used in this study, Spectronaut (Biognosys, Switzerland) to process PepCalMix data and mouse plasma samples, and DIA-NN29 to perform the targeted data extraction of Slice-PASEF HeLa data files.
Spectronaut
Quantification and DIA processing of PepCalMix and control UMHET mouse plasma samples were performed using Spectronaut DIA software (version 16.0.220606.53000 (Biognosys, Switzerland). The mouse assay library was used directly as generated and described above. For the nonlinear iRT calibration strategy, a dynamic window was used for both mass tolerance (MS1 and MS2), and to set up the extracted ion chromatogram (XIC) retention time (RT) window. Preprocessing of MS1 and MS2 calibration strategies was enabled. Decoy assays were dynamically generated using the scrambled decoy method with a set size of 0.1 as a fraction of the input library size. The identification was performed using the kernel density estimator with precursor and protein identification results filtered with a q-value of <0.01. For quantification, MS2 ion peak areas of quantified peptides were averaged to estimate the protein peak areas. Additional parameter settings were used as the default.
For PepCalMix sample data processing, the PepCalMix library was directly used and the identification was performed using a normal distribution density estimator with precursor and protein identification results filtered with a q-value of <0.01. For quantification, the Top N ranking order setting was disabled to include all 20 peptides to estimate PepCalMix protein quantity. In the linearity experiment, the analysis for all seven different sample amounts was conducted together for each source. Similarly, for the impact of flow rates analysis, all sample amounts with replicates were analyzed together. For the direct comparison of PepCalMix peptide abundances between different ion sources, joint processing of the respective measurements was performed.
Large-scale 284 plasma sample data analysis was performed using Spectronaut DIA software (version 17.0.221202.55965 (Biognosys, Switzerland) using both mouse library (as described above) and directDIA (library-free mode) to increase the proteome coverage and reduce the sparsity in the combined data matrix. For directDIA workflow, the database and parameter settings were kept the same as described above. Default settings were used without global normalization enabled. Trypsin specificity was set to two missed cleavages and a false discovery rate of 1% on both peptide and protein were used. Data filtering was set to q-value. For processing K562 samples, directDIA (library-free mode) was implemented within Spectronaut as described above.
DIA-NN
DIA-NN 1.8.2 beta 11 version21 was used for the targeted extraction of three replicates of each injection amount for both Slice-PASEF and dia-PASEF measurements. The comprehensive HeLa assay spectral library was provided to DIA-NN, and library precursors were annotated with the human FASTA database. The mass accuracies were set to 15 ppm (both MS1 and MS2), and the scan window was fixed to 5. The protein inference was disabled and spectral library protein grouping information was used. For Slice-PASEF analysis, --tims -scan was provided to DIA-NN in the additional options section. The measurements corresponding to each combination of the acquisition method and the injection amount were analyzed separately, and the output.tsv file was filtered at a 1% global protein q-value.
RESULTS AND DISCUSSION
Assessment of VIP-HESI Performance and Comparison with ESI and CS Ion Sources Coupled to timsTOF MS Using PepCalMix Peptides
The Bruker timsTOF MS offers two primary ion source configurations for proteomics applications, namely CS and ESI with nanoflow and microflow rates, respectively. Recently, a new ion source called VIP-HESI has been added to this lineup for proteomics to achieve greater robustness and stability in the ionization process as has been shown for metabolomics and pharmaceutical analysis, environmental analysis, food testing, and forensic drug investigations. The VIP-HESI source utilizes a vacuum-insulated heated probe and separate gases for nebulizing and desolvating the liquid sample using an ESI emitter. The nebulizer gas flows around the electrospray capillary at high velocity to assist in nebulization, while the heated probe gas (which can reach up to 500 °C) is used for effective desolvation, particularly at higher flow rates (Figure 1a). VIP-HESI source setup involves a ceramic heater with thermal profiling, which delivers the highest amount of heat near the probe tip and ensures that the fluid path remains cool before nebulization, thus protecting thermally fragile compounds. This results in an insulated flow path for the LC eluent, protecting it from temperature fluctuations that may occur during the LC gradient. The schematic representation of the difference between a passive drain port and the active exhaust technology used in the VIP-HESI source is shown in Figure 1a. A passive drain port can result in contamination and recirculation, while the active exhaust technology directs the flow to prevent liquid condensation in the source chamber and reduce nebulized gas recirculation which prevents the resampling of residual ions into the mass spectrometer, resulting in a cleaner system and improved source robustness.
Figure 1.
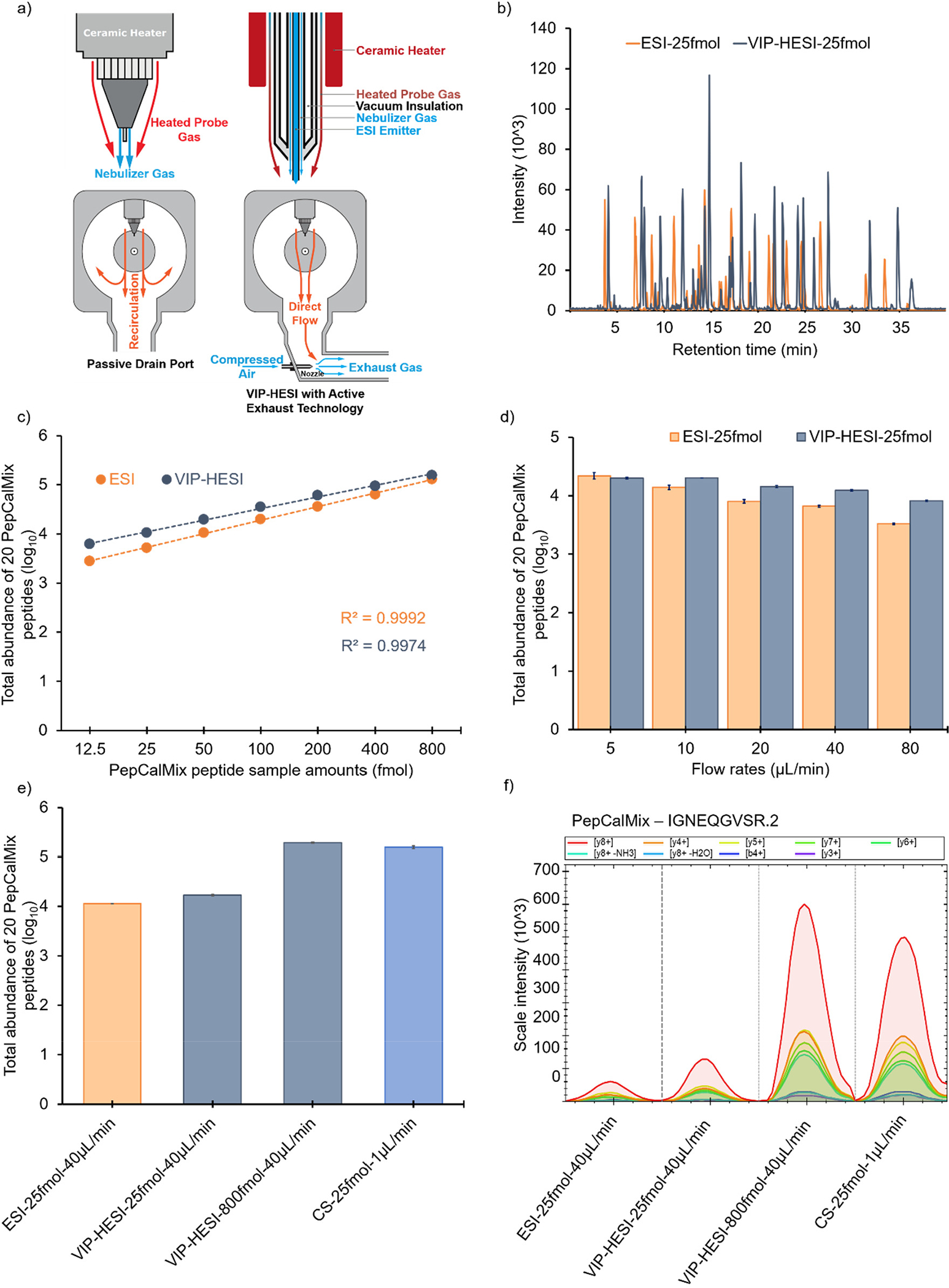
Comparative performance of VIP-HESI with ESI and CS sources setups using PepCalMix measurements. a) Schematic diagram of VIP-HESI ion source demonstrating its functionality with active exhaust technology. The red, orange and blue colored arrows indicate the direction of gas flow. b) Overlay of base peak chromatograms (BPC) of 25 fmol amount of PepCalMix peptide using VIP-HESI and ESI ion sources. c) Line graph of PepCalMix peptides to assess the linearity measured by dia-PASEF mode in timsTOF mass spectrometer using VIP-HESI and ESI ion sources. d) Effect of flow rates on the signal response of PepCalMix peptides obtained using ESI and VIP-HESI sources. The error bars indicate the variability within three replicates represented as the standard error of the mean at the log10 scale. These are calculated as the ratio of the standard deviation of the peptide intensities observed with each source setup replicate to the square root of the sample size (n = 3). e) Bar plots of total quantities of all 20 PepCalMix peptides observed with ESI, CS ion sources at 25 fmol injection amount at 40 μL/min and 1 μL/min respectively, and with VIP-HESI source at 25 and 800 fmol injection amounts at 40 μL/min. f) Extracted Ion Chromatogram (XIC) plots of a single precursor IGNEQGVSR.2 representing each source setup, colored by MS2 fragment intensity observed per measurement, as per the injection amount and flow rates.
Motivated by VIP-HESI source innovative technology, we evaluated its performance using μLC-MS/MS with a 1 mm ID column operating at a flow rate of 40 μL/min, coupled to a timsTOF mass spectrometer. We conducted an initial comparison between base peak chromatograms (BPCs) acquired with VIP-HESI and standard ESI ion sources using 25 fmol of a pool of 20 synthetic PepCalMix peptides and found that the VIP-HESI source provides higher sensitivity (Figure 1b). Further, the linear relationship between the signal response of the mass spectrometer to the sample amount was studied for both sources. We collected seven different sample amounts of PepCalMix peptides to cover a wide range from 12.5 fmol to 800 fmol (see Experimental Procedures). For both sources, an excellent linear gain in the total abundance of 20 PepCalMix peptides was observed as we increased the concentration with the goodness of fit R-Squared (R2) greater than 0.99 (Figure 1c and Supplementary Table 4a). These responses of the two sources show an excellent fit to linearity even at the individual peptide level across all sample amounts (Supplementary Figure 1 and Supplementary Table 4b). Source comparison shows the VIP-HESI exhibits higher sensitivity than the ESI source, as evident by the total abundance of PepCalMix peptides (Figure 1c). In addition, to assess the sensitivity of specific peptides from the VIP-HESI design over that of the ESI, we compared the fold change in peak areas of 20 individual peptides at each injection amount. We observed an improvement for 19 peptides (with an exception of VFTPLEVDVAK.3 peptide), with a range of 1-fold to 65-fold using the VIP-HESI source (Supplementary Table 4c). The most significant improvements were observed at lower sample amount measurements (12.5 fmol and 25 fmol), indicating that the VIP-HESI is more sensitive at these ranges. However, the sensitivity gains observed at the highest amounts (400 fmol and 800 fmol) were less noticeable, suggesting that the loss of sensitivity is being compensated by the higher injection amounts (Supplementary Table 4c).
The VIP-HESI ion source is designed to achieve maximum robustness across a wide range of flow rates. Next, we evaluated the impact of flow rates on the signal response obtained using ESI and VIP-HESI sources. To demonstrate this, we compared the performance of the two sources using 25 fmol on column of PepCalMix peptides at five different flow rates (5, 10, 20, 40, 80 μL/min). As expected, our results revealed that the signal response, as measured by the total abundance of all 20 PepCalMix peptides, increased with lower flow rates for both sources. For instance, reducing the flow rate from 80 μL/min to 5 μL/min led to 6.6 and 2.4-fold increase in signal response with ESI and VIP-HESI sources, respectively. The effect of flow rates is more pronounced with ESI than VIP-HESI due to the effective desolvation of the electrospray in the latter source, resulting in increased ion yield even at higher flow rates, such as 40 and 80 μL/min, as shown in Figure 1d and Supplementary Table 4d.
Furthermore, to evaluate the performance gains of the VIP-HESI source over the ESI and CS ion sources, we conducted a comparison of PepCalMix peptides at different dilution levels. We compared the total abundance of 20 peptides obtained with the ESI to VIP-HESI sources using 25 fmol injection amounts at 40 μL/min flow rates. The VIP-HESI yielded 48% more total abundance than the ESI source as indicated by higher peak areas (Figure 1e,f and Supplementary Table 4e). Subsequently, we acquired a 25 fmol amount of PepCalMix peptides using a CS source and compared it with the highest sample amount (800 fmol) measured using a VIP-HESI source to compensate for the loss of signal due to sample dilution at higher flow rates. The VIP-HESI provided 23% more total abundances than its CS counterpart, signifying better performance with the VIP-HESI source (Figure 1e and Supplementary Table 4e). Despite using an effective amount of only 0.8× (32-fold concentration at 40 μL/min flow rate), VIP-HESI exhibited greater sensitivity than the CS source. The results of different injected amounts of PepCalMix peptides using different ion sources on the signal intensity of extracted MS2 chromatograms is exemplified with precursor IGNEQGVSR.2 (Figure 1f). These observations show that enhanced sensitivity is achieved with the VIP-HESI source as the result of its probe gas-assisted thermal desolvation which improves the spray performance under microflow rate conditions. This experimental setup is extremely beneficial for biological applications where the protein sample amounts are in abundance.
Comparative Performance of the VIP-HESI and CS Ion Sources Using Undepleted Mouse Plasma Sample by dia-PASEF Analysis
To demonstrate the comparative performance of both electrospray ion source units on more challenging proteomics samples where the total sample amount was not limited, we measured two biological replicates (with a single technical injection each) for a control UMHET mouse plasma sample with six different injection amounts and two different flow rates (see Experimental Procedures). Here, we studied the effects of a sample amount of 0.4 μg at 1 μL/min using a 15 cm × 0.15 mm × 1.5 μm column coupled with CS and compared with 2 μg, 4 μg, 10 μg, 20 μg, and 40 μg sample amounts at 40 μL/min using a 15 cm × 1 mm × 1.7 μm Kinetix column (Phenomenex) coupled with the VIP-HESI source. For a fair comparison between the two electrospray types, the same length of the column was used, packed with similar C18 particle sizes, and the flow rates were optimized based on the internal diameter of these columns.
dia-PASEF analysis of replicates using Spectronaut resulted in the identification of 5,795–8,610 precursors and 493–802 unique proteins at a <1% protein FDR (Figure 2a,b and Supplementary Table 5). The VIP-HESI source performance results in an increase in precursors and protein identifications with more sample amounts being injected, although the gain was less prominent beyond the 20 μg injection amount, indicating a signal saturation was attained. Interestingly, injecting 50 times (20 μg) more sample amounts (with 40 times more sample dilution) using VIP-HESI, outperforms CS 0.4 μg measurement by identifying 3% more precursors and 12% more proteins (Figure 2a,b and Supplementary Table 5). Next, we evaluated the robustness and reproducibility of quantitative measurements by comparing the precursor abundances of two biological replicate injections from these sources as estimated by Spectronaut. As expected for high flow rate chromatography with the VIP-HESI source and a 20 μg measurement, a total of 7,074 precursors were quantified by both biological replicates with a high positive correlation (R2 = 0.94). With CS 0.4 μg replicate measurements, 6,387 precursors were quantified with a positive correlation (R2 = 0.89), indicating more variation was observed with nanoflow rates, compared to VIP-HESI-microflow runs (Figure 2c,d). Subsequently, we estimated the number of data points measured across the elution profiles and base peak widths for precursors identified in both VIP-HESI 20 μg and CS 0.4 μg measurements that used 1 mm and 0.15 mm ID columns, respectively. Similar median base peak widths of 0.16 and 0.14 min were observed with VIP-HESI 20 μg and CS 0.4 μg measurements, respectively. For both electrospray units, a median value of 5 data points per elution peak was obtained that provides an optimal quantification of an identified precursor using Spectronaut analysis (Figure 2e,f). Similar results were obtained by injecting one hundred times (40 μg) more sample amount for analysis coupled with VIP-HESI (Figure 2a,b and Supplementary Figure 2). These results demonstrate that the VIP-HESI source, combined with a 1 mm microbore column using μLC-MS/MS, can be applied to complex tryptic digests that are not sample-limited, such as blood plasma, to achieve higher sensitivity and higher quantification precision of precursors and proteins.
Figure 2.
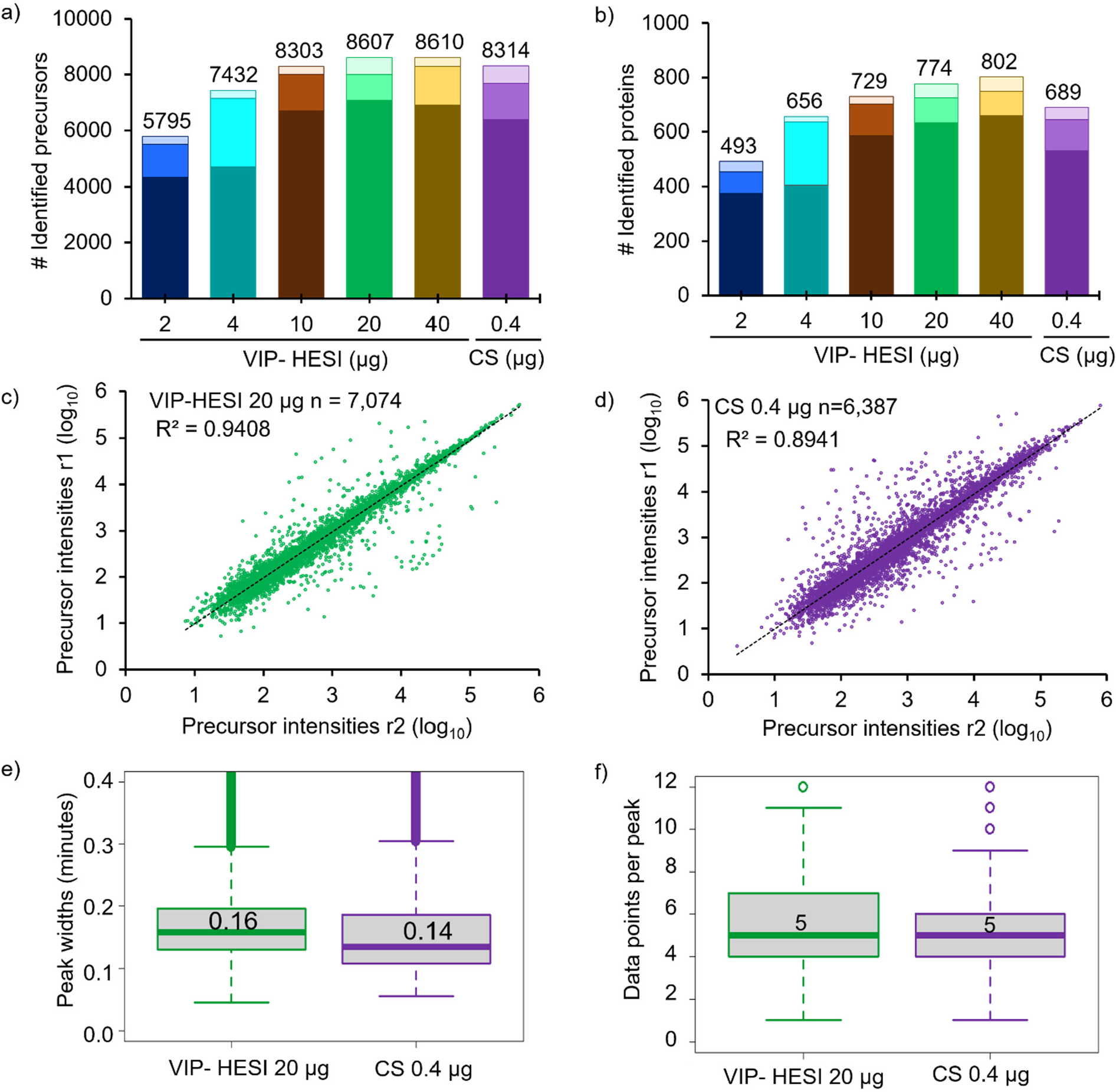
Comparative performance assessment of VIP-HESI and CS ion source setups using different injection amounts of undepleted mouse plasma samples. a) Number of precursors and b) protein groups identified with different injection amounts of mouse plasma samples analyzed in duplicate with a 45 min 40 μL/min microflow gradient. The darker color bar represents common identification between the replicates whereas the two lighter color bars at the top represents exclusive precursors and proteins identified in each biological replicate at the different sample amounts. Pearson correlation of precursor intensity values obtained from 7,074 and 6,387 precursors that were quantified in both replicates (r1 and r2) using c) VIP-HESI and d) CS source, respectively. e) Distribution of the base peak widths in minutes of precursors identified for VIP-HESI 20 μg and CS 0.4 μg measurements estimated by Spectronaut software. A median value of 0.16 (9.6 s) and 0.14 (8.4 s) peak widths were observed for VIP-HESI 20 μg and CS 0.4 μg injections, respectively. The first and third quartiles are marked by a box with a whisker marking a minimum/maximum value ranging to 0.6 interquartile, and the median is depicted as a solid line. f) Distribution of data points per elution peak for the VIP-HESI 20 μg and CS 0.4 μg measurements estimated by Spectronaut. The first and third quartiles are marked by a box with a whisker marking a minimum/maximum value ranging to 3 interquartile, and the median is depicted as a solid line.
Robust and Quantitative Analysis of Hundreds of Plasma Proteomes by VIP-HESI Source Coupled with Microflow Liquid Chromatography
Plasma proteomics is challenging due to the high dynamic range of protein concentrations that could lead to the identification of mostly high abundant proteins and poor detection for low abundant proteins using mass spectrometry resulting in less proteome coverage of these samples.30,31 To demonstrate the reproducible high-throughput application of the VIP-HESI source, we analyzed 284 undepleted mouse plasma samples. Two consecutive blanks after each sample were injected for column washing purposes, and one quality control (QC) sample, K562 digested peptides, was acquired after every thirty-two plasma samples to assess the technical variation in instrument performance. In total there were 891 consecutive injections over the course of ~20 days in this experimental setup. Once established that the 20 μg of mouse plasma sample is the optimal amount for this setup from our earlier experiments (Figure 2), we injected this amount for each sample using a 45 min (3–35% ACN) HPLC gradient.
Large-scale dia-PASEF analysis with Spectronaut software resulted in the identification of a total of 17,628 precursors and 1,267 mouse plasma protein groups at a <1% global protein FDR (Supplementary Table 6). Overall, we observed similar identification of precursors (8,627–11,885) and proteins (788–1,067) across all the samples, signifying the stable performance of the microflow LC system (Figure 3a). The retention times (RTs) of the spiked-in iRT peptides in 284 samples showed very high chromatographic reproducibility with a median coefficient of variation (CV) of 0.4% (Figure 3b,c). In addition, we checked the stability of the instrument setup by estimating the variation in the nine K562 QC samples. With 2 μg of K562 sample injection using a 21 min gradient (3–35% ACN) identified an average of 4541 ± 5 protein groups based on 50,657 ± 437 precursors, signifying robust qualitative performance observed across QC samples (Supplementary Figure 3 and Supplementary Table 7). Subsequently, we evaluated the quantitative precision between these QC technical replicates by estimating the CV for the obtained protein quantities. The median CVs of the proteins quantified in nine technical replicates were below 10%, indicating an excellent reproducibility in quantitation across the whole experiment (891 injections) (Supplementary Figure 3 and Supplementary Table 7). Further, we explored the variation of raw protein abundances across 284 mouse plasma samples and demonstrated that the distribution of protein quantities was similar across all samples even before normalization (Figure 3d and Supplementary Table 6). The medians were well-aligned across all the samples, indicating most proteins contributed equally to the observed total quantity in any sample, signifying consistency in the data acquisition with good ionization spray stability. As expected, protein quantities showed a large dynamic range across 5 orders of magnitude (Figure 3d). These results confirm the robust performance of VIP-HESI source in conjunction with microflow chromatography for high-throughput applications that demands consistent results over extended periods of time.
Figure 3.
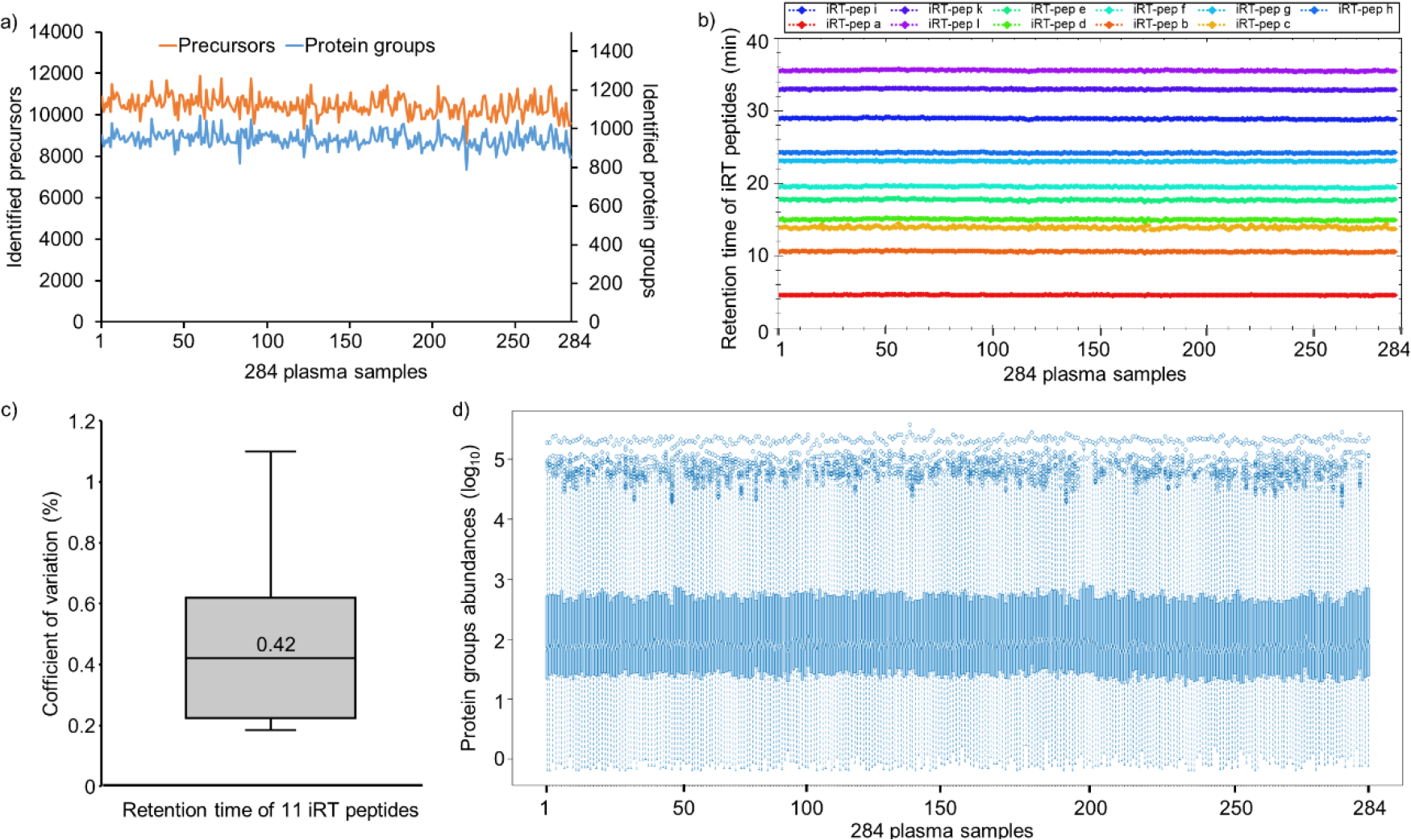
Large-scale quantitative analysis of undepleted mouse plasma samples using VIP-HESI coupled with microflow LC-MS/MS system. a) Number of precursors (orange) and protein groups (blue) quantified with 284 mouse plasma samples analyzed in a 45 min 40 μL/min microflow gradient on Vanquish Neo HPLC coupled to Bruker timsTOF Pro. b) A retention time QC plot for 11 iRT peptides spiked in each sample. c) Distribution of CV in retention time of iRT peptides across 284 samples, and the median is depicted as a solid line. d) Distribution plot of raw (un-normalized) protein quantities estimated in dia-PASEF analysis for 284 undepleted mouse plasma samples. Log10 protein quantities are plotted on the y-axis, sample number on the x-axis. Overall, protein quantities were similar across all samples with good alignment of sample medians and distributions over the first and third quartiles.
Performance of VIP-HESI Microflow Setup in a Slice-PASEF Mode for Analysis of Low Sample Amounts
Lastly, we assessed the performance of the microflow coupled with a VIP-HESI source with the recently introduced Slice-PASEF, a continuous fragmentation mode of all precursor slices using trapped ion mobility,21 and compared it to a standard dia-PASEF mode. Specifically, we evaluated its performance on the dilution series of a tryptic digest standard produced from the HeLa cell line, acquired in triplicates. We separated HeLa sample amounts of 10 ng, 100 ng, and 1000 ng at 20 μL/min flow rate with a 20 cm × 0.5 mm ID, ReproSil Pur 120 C18-AQ, dP 1.9 μm (Dr. Maisch, GmbH) column setup and operated with a 21 min chromatographic gradient (3–35% ACN). For contrast, we used the 1F Slice-PASEF scheme and compared it to a preformed dia-PASEF scheme featuring 25 Da isolation windows covering the 400–1000 m/z range (see Experimental Procedures).
Slice-PASEF and dia-PASEF analysis with DIA-NN with different HeLa sample amounts resulted in the identification of 6,592–49,130 precursors at a <1% precursor FDR and 1,769–6,343 protein groups at a <1% protein FDR for the range of 10–1000 ng sample amounts. The Slice-PASEF with VIP-HESI microflow shows a significant increase in the number of precursors and protein groups, especially with low sample amount measurements (Figure 4a,b, and Supplementary Table 8). For 10 ng sample amounts, Slice-PASEF yielded 63% more precursors and 38% more protein groups than dia-PASEF. This is the result of a high MS/MS duty cycle in the 1F Slice-PASEF acquisition method,21 enhancing the sensitivity of the measurement. In contrast, with the higher injection amounts of 1000 ng, more ions are trapped and released by the TIMS device, and the dia-PASEF method with a lower duty cycle gains more identifications than its counterpart as it optimally balances between the time spent on accumulating the spectra and selecting new spectra (Figure 4a,b and Supplementary Table 8).
Figure 4.
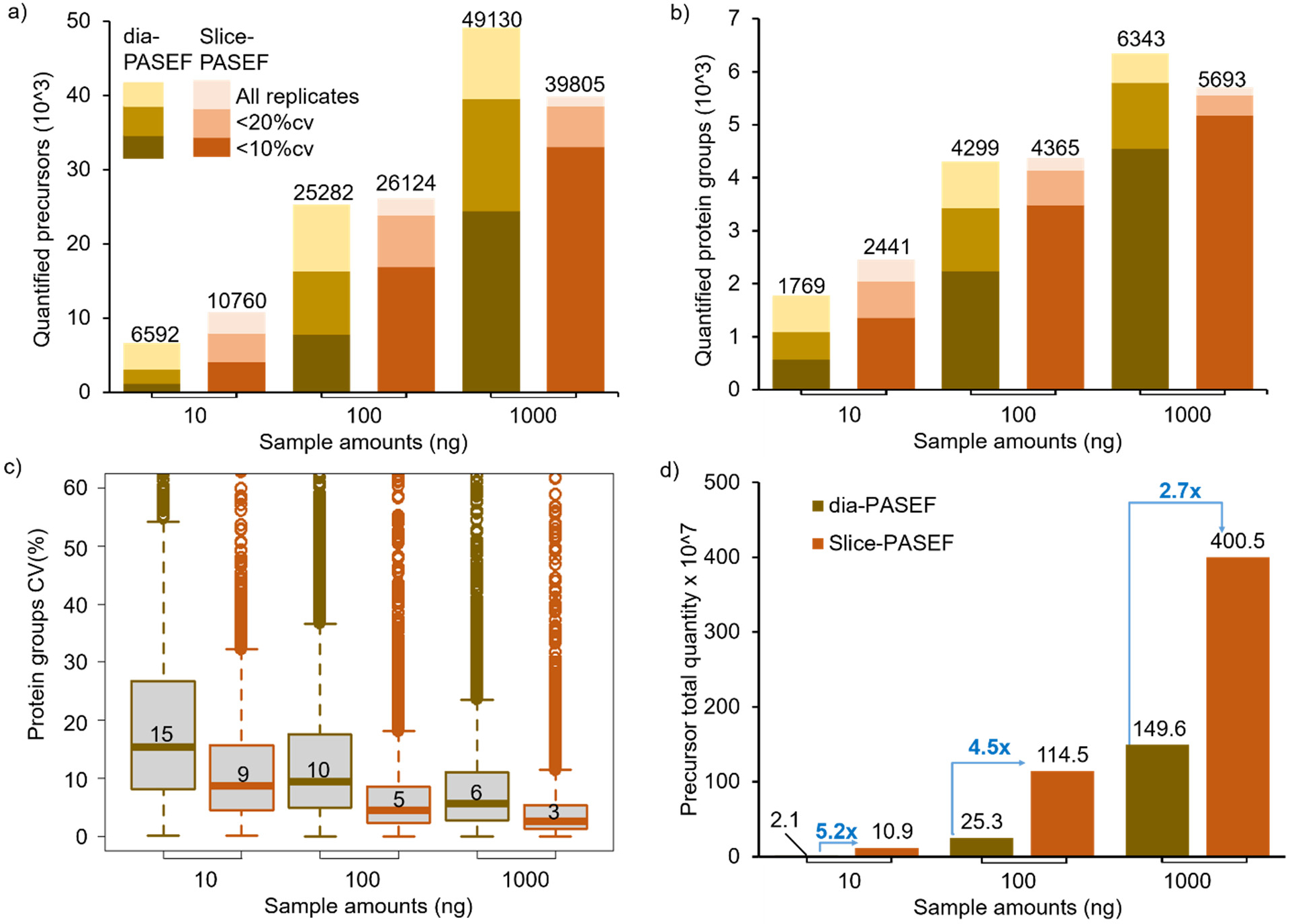
Performance of VIP-HESI-microflow setup with low sample amounts measured using dia-PASEF and Slice-PASEF acquisition methods. a) The number of precursors and b) protein groups quantified with different injection amounts of a HeLa tryptic digest analyzed in triplicate with a 21 min 20 μL/min microflow gradient on Vanquish Neo HPLC coupled to a Bruker timsTOF Pro. The precursors and protein groups that were observed in all three replicates of each measurement were represented as all replicates, and quantification precisions expressed as CV are illustrated at 10% and 20% levels. The Slice-PASEF method outperformed the dia-PASEF method in a 10 ng sample amount by quantifying more precursors and proteins at both the 10% and 20% CV levels. c) Distribution of the CVs of protein groups identified in all three replicates of different measurements at 1% protein FDR estimated by DIA-NN. The first and third quartiles are marked by a box with a whisker marking a minimum/maximum value ranging to 18 interquartile and the median is depicted as a solid line. d) The total quantity of all precursors identified in each measurement estimated by DIA-NN. The signal boost in the total quantity of all precursors between the same injection amounts is marked by the blue-colored arrows. The maximum boost is observed in the lowest sample amount of 10 ng measurements using the Slice-PASEF method.
Further, at all injection amount measurements, the 1F Slice-PASEF method demonstrated more precise quantitation. This effect is more profound at low sample amount measurements of 10 ng where the Slice-PASEF method precisely quantified 2.4 times more protein groups with a CV < 10% and 1.8 times more protein groups with a CV < 20% than dia-PASEF acquisition (Figure 4b). Additionally, we evaluated the quantitative precision among different sample amount measurements using Slice-PASEF and dia-PASEF acquisition methods. The median CVs of the proteins quantified in three technical replicates of Slice-PASEF measurements were 8.7%, 4.6%, and 2.7% for 10 ng, 100 ng, and 1000 ng, respectively. The dia-PASEF quantitative variation among the replicates was higher as median CVs were 15.3%, 9.7%, and 5.7% for 10 ng, 100 ng, and 1000 ng, respectively (Figure 4c). On comparing the total precursor quantity estimated by DIA-NN for both acquisition methods, Slice-PASEF provided a significant signal boost from a 5.2-fold increase for 10 ng to a 2.7-fold increase for 1000 ng amounts than its counterpart (Figure 4d). These results indicate that an optimized VIP-HESI source in conjunction with microflow chromatography is capable of measuring low sample amounts with superior quantitation using the Slice-PASEF mode in timsTOF MS.
CONCLUSION
Here, we demonstrate the performance boost of the VIP-HESI source coupled with microflow chromatography flow rates and compared it to ESI and CS ion source units using a Bruker timsTOF MS. The optimized VIP-HESI configuration for high-sensitivity proteomics, in particular, demonstrated a higher number of identifications, precise quantitation, and improved signal stability during the acquisition of a large plasma data set over extended periods of time. With the enhanced sensitivity, high ion-spray stability, and robust quantitative performance characteristics, VIP-HESI source is an efficient alternative to the nanoflow liquid chromatography approach for a wide range of proteomic applications.
Supplementary Material
Supplementary Figure 1. Bar graph of peak areas of individual 20 PepCalMix peptides to assess linearity measured at different sample amounts. a) ESI source, b) VIP-HESI source. In the ESI source measurements, AVYFYAPQIPLYANK.2 peptide ion was not detected in 12.5 fmol, 25 fmol, and 50 fmol samples, and TVESLFPEEAETPGSAVR.2 peptide ion was not detected in the 12.5 fmol sample. However, using the VIP-HESI source, all 20 PepCalMix peptides were detected for all sample amounts.
Supplementary Figure 2. Performance assessment of VIP-HESI and CS ion sources using mouse plasma samples. a) Pearson correlation of precursor intensity values obtained from 6,984 precursors that were quantified in both replicates using VIP-HESI 0.4 μg measurements of mouse plasma samples. b) Distribution of the base peak widths of precursors identified for VIP-HESI 40 μg and CS 0.4 μg measurements estimated by Spectronaut. A median value of 0.16 (9.6s) and 0.14 (8.4s) peak widths were observed for VIP-HESI 40 μg and CS 0.4 μg injections, respectively. c) Distribution of data points per elution peak for the VIP-HESI 40 μg and CS 0.4 μg measurements estimated by Spectronaut.
Supplementary Figure 3. Estimation of MS instrument variation across the 891 VIP-HESI injections using K562 QC samples. The average a) number of precursors and b) protein groups identified with nine technical replicates. c) Distribution of CVs of proteins identified in all nine replicates at 1% protein FDR estimated by Spectronaut. The median CV of 10% correlates well with the signal stability achieved using the VIP-HESI source setup. The first and third quartile are marked by a box with a whisker marking a minimum/maximum value ranging to 10 interquartile and the median is depicted as a solid line.
Supplementary Table 1. Metadata of the mouse liver, kidney, gastrocnemius muscle tissues, and plasma samples.
Supplementary Table 2. Acquisition and LC separation details used in this study.
Supplementary Table 3. DIALib-QC report of spectral assay libraries “HeLa_timsTOF_DDA_DIA_Library” and “Mouse_tissue_plasma_DDA_DIA_Library”.
Supplementary Table 4. Performance of ESI and VIP-HESI sources with different sample amounts of PepCalMix peptides. a) Assessing linearity using total abundance of 20 PepCalMix peptides detected in three replicates at seven different sample amounts using ESI and VIP-HESI sources. b) Linearity assessment of ESI and VIP-HESI source using individual PepCalMix peptides across eight injected sample amounts. c) Sensitivity gains in abundance of 20 PepCalMix peptides using VIP-HESI compared to the ESI source at different sample amounts. Fold change was estimated by taking the ratio of average abundance for each PepCalMix peptide at seven sample amounts. The gradient of colors in the visualization denotes the degree of change in fold increase to decrease. Dark green denotes higher gains, light blue indicates lower gains, and dark blue indicates no observed gains with VIP-HESI. NA represents missing abundance values in ESI source. d) Impact of different flow rates on the PepCalMix peptides abundances using ESI and VIP-HESI sources. e) Comparative performance of VIP-HESI with standard ESI and CS ion sources using 20 synthetic PepCalMix peptides.
Supplementary Table 5. Comparative performance of the VIP-HESI and CS ion sources using mouse plasma sample by dia-PASEF analysis. a) VIP-HESI Mouse plasma measurements. b) CS Mouse plasma measurements.
Supplementary Table 6. Quantitative analysis of hundreds of plasma proteomes by VIP-HESI source coupled with microflow liquid chromatography. a) Mouse Plasma Precursor matrix. b) Mouse Plasma Protein matrix. c) Mouse Plasma RT-Chart Data.
Supplementary Table 7. Assessment of MS instrument variation across the 891 VIP-HESI injections using K562 QC samples analyzed by Spectronaut. a) Protein groups and b) Precursor sample matrices.
Supplementary Table 8. Comparative performance of VIP-HESI microflow setup using Slice-PASEF and dia-PASEF modes. a) dia-PASEF-10 ng, b) dia-PASEF-100 ng, c) dia-PASEF-1000 ng, d) Slice-PASEF-10 ng, e) Slice-PASEF-100 ng, f) Slice-PASEF-1000 ng. (PDF)
ACKNOWLEDGMENTS
This work was funded in part by the National Institutes of Health grants from the National Heart, Lung, and Blood Institute R01HL133135, the Office of the Director S10OD026936, the National Institute on Aging U19AG023122, and the National Science Foundation award 1920268. We thank Dr. Gary Kruppa (Bruker) and the technical resource team at Bruker for access to the VIP-HESI source used in this work. We thank Dr. Stephanie Kaspar-Schoenefeld (Bruker) for collecting MS data of fractionated HeLa lysate tryptic digest for spectral library generation and Dr. Eike Mucha (Bruker) for providing the schematic figure of VIP-HESI ion source presented in this study. Figure colors in this paper are optimized for color-impaired sight.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jproteome.3c00305
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.3c00305.
Contributor Information
Mukul K. Midha, Institute for Systems Biology, Seattle, Washington 98109, United States
Charu Kapil, Institute for Systems Biology, Seattle, Washington 98109, United States.
Michal Maes, Institute for Systems Biology, Seattle, Washington 98109, United States.
David H. Baxter, Institute for Systems Biology, Seattle, Washington 98109, United States
Seamus R. Morrone, Institute for Systems Biology, Seattle, Washington 98109, United States
Timothy J. Prokop, Institute for Systems Biology, Seattle, Washington 98109, United States
Robert L. Moritz, Institute for Systems Biology, Seattle, Washington 98109, United States
Data Availability Statement
All mass spectrometry dia-PASEF raw folders corresponding to PepCalMix, K562, and HeLa experiments (.d), dda-PASEF raw folders of HeLa fractionated samples (.d), FASTA database used to generate the assay libraries (.fasta), spectral assay libraries (.txt), their DIALib-QC reports (.tsv), and output information from Spectronaut and DIA-NN as supplementary tables (.xls) have been PRIDE deposited with the ProteomeXchange Consortium via the PRIDE partner32,33 repository with the data set identifier PXD040497 (http://www.ebi.ac.uk/pride).
REFERENCES
- (1).Bian Y; Zheng R; Bayer FP; Wong C; Chang YC; Meng C; Zolg DP; Reinecke M; Zecha J; Wiechmann S; et al. Robust, Reproducible and Quantitative Analysis of Thousands of Proteomes by Micro-Flow LC–MS/MS. Nat. Commun. 2020, 11 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Vowinckel J; Zelezniak A; Bruderer R; Mülleder M; Reiter L; Ralser M Cost-Effective Generation of Precise Label-Free Quantitative Proteomes in High-Throughput by MicroLC and Data-Independent Acquisition. Sci. Rep. 2018, 8 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sun R; Hunter C; Chen C; Ge W; Morrice N; Liang S; Zhu T; Yuan C; Ruan G; Zhang Q; et al. Accelerated Protein Biomarker Discovery from FFPE Tissue Samples Using Single-Shot, Short Gradient Microflow SWATH MS. J. Proteome Res. 2020, 19 (7), 2732–2741. [DOI] [PubMed] [Google Scholar]
- (4).Kuster B; Bian Y; Bayer FP; Chang YC; Meng C; Hoefer S; Deng N; Zheng R; Boychenko O Robust Microflow LC-MS/MS for Proteome Analysis: 38 000 Runs and Counting. Anal. Chem. 2021, 93 (8), 3686–3690. [DOI] [PubMed] [Google Scholar]
- (5).Gao H; Liu Y; Demichev V; Tate S; Chen C; Zhu J; Lu C; Ralser M; Guo T; Zhu Y Optimization of Microflow LC Coupled with Scanning SWATH and Its Application in Hepatocellular Carcinoma Tissues. J. Proteome Res. 2022, 21 (7), 1686–1693. [DOI] [PubMed] [Google Scholar]
- (6).Sui X; Wu Q; Cui X; Wang X; Zhang L; Deng N; Bian Y; Xu R; Tian R Robust Capillary- and Micro-Flow Liquid Chromatography-Tandem Mass Spectrometry Methods for High-Throughput Proteome Profiling. J. Proteome Res. 2022, 21 (10), 2472–2480. [DOI] [PubMed] [Google Scholar]
- (7).Bian Y; Gao C; Kuster B On the Potential of Micro-Flow LC-MS/MS in Proteomics. Expert Rev. Proteomics 2022, 19 (3), 153–164. [DOI] [PubMed] [Google Scholar]
- (8).Bian Y; The M; Giansanti P; Mergner J; Zheng R; Wilhelm M; Boychenko A; Kuster B Identification of 7 000–9 000 Proteins from Cell Lines and Tissues by Single-Shot Microflow LC-MS/MS. Anal. Chem. 2021, 93 (25), 8687–8692. [DOI] [PubMed] [Google Scholar]
- (9).Bruderer R; Muntel J; Müller S; Bernhardt OM; Gandhi T; Cominetti O; Macron C; Carayol J; Rinner O; Astrup A; et al. Analysis of 1508 Plasma Samples by Capillary-Flow Data-Independent Acquisition Profiles Proteomics of Weight Loss and Maintenance. Mol. Cell. Proteomics 2019, 18 (6), 1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Midha MK; Kusebauch U; Shteynberg D; Kapil C; Bader SL; Reddy PJ; Campbell DS; Baliga NS; Moritz RL A Comprehensive Spectral Assay Library to Quantify the Escherichia Coli Proteome by DIA/SWATH-MS. Sci. Data 2020, 7 (1), 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ludwig C; Gillet L; Rosenberger G; Amon S; Collins BC; Aebersold R Data-independent Acquisition-based SWATH-MS for Quantitative Proteomics: A Tutorial. Mol. Syst. Biol. 2018, 14 (8), e8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Poulos RC; Hains PG; Shah R; Lucas N; Xavier D; Manda SS; Anees A; Koh JMS; Mahboob S; Wittman M; et al. Strategies to Enable Large-Scale Proteomics for Reproducible Research. Nat. Commun. 2020, 11 (1), 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mueller LN; Rinner O; Schmidt A; Letarte S; Bodenmiller B; Brusniak M-Y; Vitek O; Aebersold R; Müller M SuperHirn - a Novel Tool for High Resolution LC-MS-Based Peptide/Protein Profiling. Proteomics 2007, 7 (19), 3470–3480. [DOI] [PubMed] [Google Scholar]
- (14).Zhang Y; Cai X; Ge W; Wang D; Zhu G; Qian L; Xiang N; Yue L; Liang S; Zhang F; et al. Potential Use of Serum Proteomics for Monitoring COVID-19 Progression to Complement RT-PCR Detection. J. Proteome Res. 2022, 21 (1), 90–100. [DOI] [PubMed] [Google Scholar]
- (15).Gao H; Zhang F; Liang S; Zhang Q; Lyu M; Qian L; Liu W; Ge W; Chen C; Yi X; et al. Accelerated Lysis and Proteolytic Digestion of Biopsy-Level Fresh-Frozen and FFPE Tissue Samples Using Pressure Cycling Technology. J. Proteome Res. 2020, 19 (7), 2732–2741. [DOI] [PubMed] [Google Scholar]
- (16).He Y; Rashan EH; Linke V; Shishkova E; Hebert AS; Jochem A; Westphall MS; Pagliarini DJ; Overmyer KA; Coon JJ Multi-Omic Single-Shot Technology for Integrated Proteome and Lipidome Analysis. Anal. Chem. 2021, 93 (9), 4217–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Meier F; Brunner A; Koch S; Koch H; Lubeck M; Krause M; Goedecke N; Decker J; Kosinski T; Park MA; et al. Online Parallel Accumulation-Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteomics 2018, 17 (12), 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Alsuraih M; Laviolette B; Lin G; Kovi R; Daurio N; Ahn Y; Jiang Z; Ortiz R; Li S; Cheng Y; et al. Targeting ABCB4 Using MRNA-LNP for the Treatment of Rare Liver Diseases. bioRxiv 2023, No. 2023.04.11.535868. [Google Scholar]
- (19).Luethi D; Maier J; Rudin D; Szöllősi D; Angenoorth TJF; Stankovic S; Schittmayer M; Burger I; Yang JW; Jaentsch K; et al. Phosphatidylinositol 4,5-Bisphosphate (PIP2) Facilitates Norepinephrine Transporter Dimerization and Modulates Substrate Efflux. Commun. Biol. 2022, 5 (1), 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lindemann V; Schmidt J; Cramer B; Humpf HU Detection of Mycotoxins in Highly Matrix-Loaded House-Dust Samples by QTOF-HRMS, IM-QTOF-HRMS, and TQMS: Advantages and Disadvantages. Anal. Chem. 2022, 94 (10), 4209–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Szyrwiel L; Sinn L; Ralser M; Demichev V Slice-PASEF: Fragmenting All Ions for Maximum Sensitivity in Proteomics. bioRxiv 2022, No. 2022.10.31.514544. [Google Scholar]
- (22).Dominick G; Berryman DE; List EO; Kopchick JJ; Li X; Miller RA; Garcia GG Regulation of MTOR Activity in Snell Dwarf and GH Receptor Gene-Disrupted Mice. Endocrinology 2015, 156 (2), 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Miller RA; Harrison DE; Astle CM; Fernandez E; Flurkey K; Han M; Javors MA; Li X; Nadon NL; Nelson JF; et al. Rapamycin-Mediated Lifespan Increase in Mice Is Dose and Sex Dependent and Metabolically Distinct from Dietary Restriction. Aging Cell 2014, 13 (3), 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Udenfriend S; Stein S; Böhlen P; Dairman W; Leimgruber W; Weigele M Fluorescamine: A Reagent for Assay of Amino Acids, Peptides, Proteins, and Primary Amines in the Picomole Range. Science 1972, 178 (4063), 871–872. [DOI] [PubMed] [Google Scholar]
- (25).Kelstrup CD; Bekker-Jensen DB; Arrey TN; Hogrebe A; Harder A; Olsen JV Performance Evaluation of the Q Exactive HF-X for Shotgun Proteomics. J. Proteome Res. 2018, 17 (1), 727–738. [DOI] [PubMed] [Google Scholar]
- (26).Bruderer R; Bernhardt OM; Gandhi T; Miladinović SM; Cheng LY; Messner S; Ehrenberger T; Zanotelli V; Butscheid Y; Escher C; et al. Extending the Limits of Quantitative Proteome Profiling with Data-Independent Acquisition and Application to Acetaminophen-Treated Three-Dimensional Liver Microtissues. Mol. Cell. Proteomics 2015, 14 (5), 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Krasny L; Bland P; Burns J; Lima NC; Harrison PT; Pacini L; Elms ML; Ning J; Martinez VG; Yu Y; et al. A Mouse SWATH-Mass Spectrometry Reference Spectral Library Enables Deconvolution of Species-Specific Proteomic Alterations in Human Tumour Xenografts. Dis. Model. Mech. 2020, 13 (7), No. dmm044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Midha MK; Campbell DS; Kapil C; Kusebauch U; Hoopmann MR; Bader SL; Moritz RL DIALib-QC an Assessment Tool for Spectral Libraries in Data-Independent Acquisition Proteomics. Nat. Commun. 2020, 11 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Demichev V; Messner CB; Vernardis SI; Lilley KS; Ralser M DIA-NN: Neural Networks and Interference Correction Enable Deep Proteome Coverage in High Throughput. Nat. Methods 2020, 17 (1), 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Geyer PE; Wewer Albrechtsen NJ; Tyanova S; Grassl N; Iepsen EW; Lundgren J; Madsbad S; Holst JJ; Torekov SS; Mann M Proteomics Reveals the Effects of Sustained Weight Loss on the Human Plasma Proteome. Mol. Syst. Biol. 2016, 12 (12), 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kaur G; Poljak A; Ali SA; Zhong L; Raftery MJ; Sachdev P Extending the Depth of Human Plasma Proteome Coverage Using Simple Fractionation Techniques. J. Proteome Res. 2021, 20 (2), 1261–1279. [DOI] [PubMed] [Google Scholar]
- (32).Perez-riverol Y; Bai J; Bandla C; Garc D; Hewapathirana S; Kamatchinathan S; Kundu DJ; Prakash A; Frericks-zipper A; Eisenacher M; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50 (D1), D543–D552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Vizcaíno JA; Côté R; Reisinger F; Foster JM; Mueller M; Rameseder J; Hermjakob H; Martens L A Guide to the Proteomics Identifications Database Proteomics Data Repository. Proteomics 2009, 9 (18), 4276–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Bar graph of peak areas of individual 20 PepCalMix peptides to assess linearity measured at different sample amounts. a) ESI source, b) VIP-HESI source. In the ESI source measurements, AVYFYAPQIPLYANK.2 peptide ion was not detected in 12.5 fmol, 25 fmol, and 50 fmol samples, and TVESLFPEEAETPGSAVR.2 peptide ion was not detected in the 12.5 fmol sample. However, using the VIP-HESI source, all 20 PepCalMix peptides were detected for all sample amounts.
Supplementary Figure 2. Performance assessment of VIP-HESI and CS ion sources using mouse plasma samples. a) Pearson correlation of precursor intensity values obtained from 6,984 precursors that were quantified in both replicates using VIP-HESI 0.4 μg measurements of mouse plasma samples. b) Distribution of the base peak widths of precursors identified for VIP-HESI 40 μg and CS 0.4 μg measurements estimated by Spectronaut. A median value of 0.16 (9.6s) and 0.14 (8.4s) peak widths were observed for VIP-HESI 40 μg and CS 0.4 μg injections, respectively. c) Distribution of data points per elution peak for the VIP-HESI 40 μg and CS 0.4 μg measurements estimated by Spectronaut.
Supplementary Figure 3. Estimation of MS instrument variation across the 891 VIP-HESI injections using K562 QC samples. The average a) number of precursors and b) protein groups identified with nine technical replicates. c) Distribution of CVs of proteins identified in all nine replicates at 1% protein FDR estimated by Spectronaut. The median CV of 10% correlates well with the signal stability achieved using the VIP-HESI source setup. The first and third quartile are marked by a box with a whisker marking a minimum/maximum value ranging to 10 interquartile and the median is depicted as a solid line.
Supplementary Table 1. Metadata of the mouse liver, kidney, gastrocnemius muscle tissues, and plasma samples.
Supplementary Table 2. Acquisition and LC separation details used in this study.
Supplementary Table 3. DIALib-QC report of spectral assay libraries “HeLa_timsTOF_DDA_DIA_Library” and “Mouse_tissue_plasma_DDA_DIA_Library”.
Supplementary Table 4. Performance of ESI and VIP-HESI sources with different sample amounts of PepCalMix peptides. a) Assessing linearity using total abundance of 20 PepCalMix peptides detected in three replicates at seven different sample amounts using ESI and VIP-HESI sources. b) Linearity assessment of ESI and VIP-HESI source using individual PepCalMix peptides across eight injected sample amounts. c) Sensitivity gains in abundance of 20 PepCalMix peptides using VIP-HESI compared to the ESI source at different sample amounts. Fold change was estimated by taking the ratio of average abundance for each PepCalMix peptide at seven sample amounts. The gradient of colors in the visualization denotes the degree of change in fold increase to decrease. Dark green denotes higher gains, light blue indicates lower gains, and dark blue indicates no observed gains with VIP-HESI. NA represents missing abundance values in ESI source. d) Impact of different flow rates on the PepCalMix peptides abundances using ESI and VIP-HESI sources. e) Comparative performance of VIP-HESI with standard ESI and CS ion sources using 20 synthetic PepCalMix peptides.
Supplementary Table 5. Comparative performance of the VIP-HESI and CS ion sources using mouse plasma sample by dia-PASEF analysis. a) VIP-HESI Mouse plasma measurements. b) CS Mouse plasma measurements.
Supplementary Table 6. Quantitative analysis of hundreds of plasma proteomes by VIP-HESI source coupled with microflow liquid chromatography. a) Mouse Plasma Precursor matrix. b) Mouse Plasma Protein matrix. c) Mouse Plasma RT-Chart Data.
Supplementary Table 7. Assessment of MS instrument variation across the 891 VIP-HESI injections using K562 QC samples analyzed by Spectronaut. a) Protein groups and b) Precursor sample matrices.
Supplementary Table 8. Comparative performance of VIP-HESI microflow setup using Slice-PASEF and dia-PASEF modes. a) dia-PASEF-10 ng, b) dia-PASEF-100 ng, c) dia-PASEF-1000 ng, d) Slice-PASEF-10 ng, e) Slice-PASEF-100 ng, f) Slice-PASEF-1000 ng. (PDF)
Data Availability Statement
All mass spectrometry dia-PASEF raw folders corresponding to PepCalMix, K562, and HeLa experiments (.d), dda-PASEF raw folders of HeLa fractionated samples (.d), FASTA database used to generate the assay libraries (.fasta), spectral assay libraries (.txt), their DIALib-QC reports (.tsv), and output information from Spectronaut and DIA-NN as supplementary tables (.xls) have been PRIDE deposited with the ProteomeXchange Consortium via the PRIDE partner32,33 repository with the data set identifier PXD040497 (http://www.ebi.ac.uk/pride).


