Abstract
A widely held dogma in the preclinical addiction field is that females are more vulnerable than males to drug craving and relapse. Here, we first review clinical studies on sex differences in psychostimulant and opioid craving and relapse. Next, we review preclinical studies on sex differences in psychostimulant and opioid reinstatement of drug seeking after extinction of drug self-administration, and incubation of drug craving (time-dependent increase in drug seeking during abstinence). We also discuss ovarian hormones’ role in relapse and craving in humans and animal models and speculate on brain mechanisms underlying their role in cocaine craving and relapse in rodent models. Finally, we discuss imaging studies on brain responses to cocaine cues and stress in men and women.The results of the clinical studies reviewed do not appear to support the notion that women are more vulnerable to psychostimulant and opioid craving and relapse. However, this conclusion is tentative because most of the studies reviewed were correlational, not sufficiently powered, and not a priori designed to detect sex differences. Additionally, imaging studies suggest sex differences in brain responses to cocaine cues and stress. The results of the preclinical studies reviewed provide evidence for sex differences in stress-induced reinstatement and incubation of cocaine craving but not cue- or cocaine-induced reinstatement of cocaine seeking. These sex differences are modulated in part by ovarian hormones. In contrast, the available data do not support the notion of sex differences in craving and relapse/reinstatement for methamphetamine or opioids in rodent models.
Significance Statement
This systematic review summarizes clinical and preclinical studies on sex differences in psychostimulant and opioid craving and relapse. Results of the clinical studies reviewed do not appear to support the notion that women are more vulnerable to psychostimulant and opioid craving and relapse. Results of preclinical studies reviewed provide evidence for sex differences in reinstatement and incubation of cocaine seeking but not for reinstatement or incubation of methamphetamine or opioid seeking.
I. Introduction
Substance use disorders are characterized by high relapse rates during abstinence (Hunt et al., 1971; Sinha, 2011; SAMHSA, 2016; Marsh et al., 2018). Over the last decades, investigators examined sex differences in human drug use and relapse (Griffin et al., 1989). In the early 1990s, Kosten et al. (1993) reported that women have more severe cocaine use problems, more cocaine use days, and shorter abstinence periods. In contrast, women had better outcomes at 6-month follow-up (Kosten et al., 1993). In the early 2000s, Elman et al. (2002) reported that cue-induced cocaine craving is higher in women. In parallel, many preclinical studies since the 1990s reported that female rats are more sensitive than males to the reinforcing effects of cocaine, as assessed by drug self-administration and conditioned place preference models (Lynch et al., 2002; Roth and Carroll, 2004; Lynch, 2006; Becker and Hu, 2008; Quinones-Jenab and Jenab, 2012). For example, female rats acquire cocaine self-administration faster than male rats (Lynch and Carroll, 1999). Additionally, investigators reported sex differences in relapse to cocaine seeking, as assessed by extinction-reinstatement and incubation of drug craving models (Becker, 2016; Carroll and Lynch, 2016; Becker et al., 2017) (see below). These findings have led to the widely held dogma especially in the preclinical addiction field that across drug classes females are more vulnerable to initiation and escalation of drug use and to relapse to drug use during abstinence (Fattore et al., 2008; Becker, 2016; Carroll and Lynch, 2016; Becker and Chartoff, 2019).
The goal of our review is to critically examine evidence for sex differences in psychostimulant and opioid craving and relapse in humans and in reinstatement of drug seeking and incubation of drug craving in rat models. We refer readers to excellent comprehensive reviews of the preclinical literature on sex differences in initiation and escalation of drug self-administration and withdrawal (Lynch et al., 2002; Roth et al., 2004; Becker and Koob, 2016; Becker and Chartoff, 2019). Notably, our review does not cover sex differences in humans and animal models of legal drugs (nicotine and alcohol). We refer readers to excellent papers and reviews on this topic (Walitzer and Dearing, 2006; Rahman et al., 2016; Goenaga et al., 2020).
We first review clinical studies on sex differences in psychostimulant (cocaine, methamphetamine) and opioid (heroin) craving and relapse. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for the literature search strategy and searched Embase, PsycINFO, and MEDLINE databases. The details of the search strategy for MEDLINE are provided below, which was adapted for the other two databases using similar terms (see methods of the systematic review in the Supplemental Material for more details):
(“craving”[MeSH] OR “craving”[tiab] OR “recurrence”[MeSH] OR “relapse”[tiab] OR “abstinence”[tiab] OR “remission”[tiab]) AND (“analgesics opioid”[Pharmacological Action] OR “Central Nervous System Stimulants”[Pharmacological Action] OR “Cocaine”[MeSH] OR “Amphetamine-Related Disorders”[MeSH] OR “Opioid-Related Disorders”[MeSH] OR “Cocaine-Related Disorders”[MeSH] OR “opioid”[tiab] OR “opiate”[tiab] OR “opium”[tiab] OR “heroin”[tiab] OR “morphine”[tiab] OR “stimulants”[tiab] OR “amphetamine”[tiab] OR “dextroamphetamine”[tiab] OR “methamphetamine”[tiab] OR “Cocaine”[tiab] OR “caffeine”[tiab]) AND (“sex characteristics”[MeSH] OR “sex factors”[MeSH] OR “sex characteristics”[tiab] OR “sex differences”[tiab] OR “gender”[tiab] OR “gender differences”[tiab]). Filters: Humans
Next, we review preclinical studies on sex differences in psychostimulant and opioid (heroin, fentanyl, oxycodone) reinstatement of drug seeking and incubation of drug craving. We also discuss the role of ovarian hormones in cocaine craving and relapse/reinstatement in humans and animal models. As with the clinical search, our preclinical literature search followed PRISMA guidelines, and we searched Embase, PsycINFO, and MEDLINE datasets using keywords and controlled vocabulary terms relevant to our review. Below we provide the details of the research strategy for MEDLINE, which was adapted for the other two databases:
((craving[Title/Abstract] OR relapse[Title/Abstract] OR recurrence[Title/Abstract] OR abstinence[Title/Abstract] OR recovery[Title/Abstract] OR remission[Title/Abstract]) AND (“sex differences”[Title/Abstract] OR “sex difference”[Title/Abstract] OR “gender differences”[Title/Abstract] OR “gender difference”[Title/Abstract])) AND (opioid[Title/Abstract] OR methamphetamine[Title/Abstract] OR estrous cycle[Title/Abstract] OR estradiol[Title/Abstract] OR progesterone[Title/Abstract]). Filters: Other Animals
Next, we propose a mechanistic model of the role of ovarian hormones in mediating sex differences in cocaine relapse in rat models. Finally, we summarize results from several human imaging studies on the brain response to drug cues and stress in men and women. In Supplemental Tables S1–S5 and Figs. 1 and 2, we provide a summary of the studies reviewed. In Table 1, we provide a glossary of terms (italic font in the text), and in Table 2, we outline the methodological considerations in studying ovarian hormones in human and rodent models of drug craving and relapse. In the Supplemental Material, we describe the methodological details of the systematic review of both the clinical (Supplemental Fig. 1) and preclinical (Supplemental Fig. 2) literature [note: Our review does not include studies on sex differences in extinction responding 1 day after the last self-administration session because this procedure does not meet the operational definition of relapse (resumption of drug-seeking behavior after a period of abstinence)].
TABLE 1.
Glossary of terms
| Behavioral Term | Definition |
|---|---|
| Conditioned place preference | A Pavlovian conditioning procedure in which one distinct context is paired with noncontingent drug injections while another context is paired with vehicle injections. During subsequent drug-free tests, increased preference for the drug context serves as a measure of the drug’s reinforcing effects. |
| Craving | An affective state described as an urge for drug; it can be induced in human drug users by exposure to the self-administered drug, drug cues and contexts, or stress. |
| Cue-induced reinstatement | An experimental condition in which laboratory animals are first trained to self-administer a drug or non–drug reinforcer, and each reinforcer delivery is temporally paired with a discrete cue (e.g., tone or light). Operant responses are then extinguished in the absence of the reinforcer and the cue. During reinstatement testing, exposure to the cue, which is earned contingently during testing, reinstates responding. |
| Discrete cue | An experimental condition in which an environmental cue (e.g., light, tone) is contingently paired with the reinforcer delivery. |
| Discriminative cue | An experimental condition in which an environmental cue (e.g., light, tone) predicts the availability of a reinforcer. |
| Drug-induced reinstatement | Resumption of drug seeking after extinction following noncontingent priming injections of the self-administered drug or related drugs immediately prior to the test session. |
| Drug self-administration | An operant procedure in which laboratory animals lever press (or nose poke) for drug injections or oral drug delivery. Most (but not all) drugs self-administered by humans are self-administered by rodents and nonhuman primates. |
| Early abstinence | The beginning of abstinence phase when people with substance use disorders experience somatic withdrawal symptoms. In studies using rat models, this term refers to the first several days after cessation of drug self-administration. |
| Extinction | The decrease in the frequency or intensity of learned responses after the removal of the unconditioned stimulus (e.g., drug) that has reinforced the learning. In studies on incubation of drug craving and relapse after forced or voluntary abstinence, extinction responding (in the presence of the drug-paired contextual and discrete cues) is the operational measure of drug seeking. |
| Forced abstinence | Experimental conditions in which abstinence after drug self-administration is imposed by the experimenter. In animal models, forced abstinence can be achieved by (1) extinction training in the drug self-administration context or a nondrug context or (2) keeping the experimental animals in their home cage during the abstinence period. |
| Incubation of drug craving | A hypothetical psychologic process inferred from the findings of time-dependent increases in nonreinforced drug seeking after cessation of drug self-administration in rodents. |
| Intermittent-access drug self-administration | A drug self-administration procedure in which the drug is repeatedly available for short periods that are separated by long timeout periods (typically 12 cycles of 5-min drug access, 25-min timeout). Exposure to this procedure induces binge-like self-administration behavior and spiking brain drug levels. |
| Long-access drug self-administration | A drug self-administration procedure in which the drug is continuously available for extended daily sessions (6 h/day or more). This procedure results in escalation of drug intake and high and stable drug concentrations in the brain. |
| Reinstatement of drug seeking | Postextinction resumption of operant behavior that had previously been maintained by a drug. Reinstatement is induced by a drug priming injection, stressors, contexts previously paired with drug self-administration, or response-contingent presentation of drug-associated cues. |
| Relapse | Resumption of drug-taking behavior during self-imposed (voluntary) or forced abstinence in humans and laboratory animals. |
| Sex | Characterization of an individual as female or male from biologic and morphologic features. |
| Short-access drug self-administration | A drug self-administration procedure in which the drug is continuously available during short daily sessions (3 h/day or less). |
| Stress-induced reinstatement | Resumption of drug seeking after extinction following exposure to environmental or pharmacological stressors. |
| Voluntary abstinence | Experimental conditions in which the self-administered drug is available in the self-administration chamber but the laboratory animal either stops or significantly decreases drug self-administration behavior. In animal models, voluntary abstinence can be achieved by introducing 1) mild foot shock punishment after the drug-reinforced operant response, 2) an electric barrier that delivers mild shock near the drug-paired lever, 3) mutually exclusive alternative palatable food reward, and 4) mutually exclusive alternative social reward. |
TABLE 2.
Methodological considerations in studying the role of ovarian hormones in drug craving and relapse in humans and animal models
| Method Used | Limitations | References |
|---|---|---|
| Clinical studies | ||
| Identification of the phases based on self-report of menses onset combined with radioimmunoassay from estradiol and progesterone plasma concentrations | 1. Assumption of similar cycle length between women. 2. Does not take in consideration the variation of hormones concentration within each phase. 3. Cycle can be disrupted by drug intake. 4. Only one or two blood samples per participant, which is restricted to a specific time point and does not fully represent estradiol and progesterone concentrations across the full cycle. |
(Collins et al., 2007; Evans et al., 2002; Lukas et al., 1996; Sofuoglu et al., 1999) |
| Saliva sample of estradiol and progesterone | Only one or two blood samples per participant, which is restricted to a specific time point and does not fully represent estradiol and progesterone concentrations across the full cycle. | (Fox et al., 2008) |
| Preclinical studies | ||
| Vaginal swab | 1. Based on the proportion of leukocytes, cornified and nucleated cells in the vaginal sample, which can be subjective. 2. Some studies pool different phases together based on similar behavior responses, which excludes the role of hormones variations between the different phases. 3. Does not take in consideration the variation of hormone concentration within each phase. |
(Bechard et al., 2018; Cox et al., 2013; Feltenstein et al., 2009; Feltenstein and See, 2007; Fuchs et al., 2005; Kerstetter et al., 2008; Kippin et al., 2005; Nicolas et al., 2019) |
| Radioimmunoassay for plasma levels of estradiol and progesterone | Only one or two blood samples per animal, which is restricted to a specific time-point and does not fully represent estradiol and progesterone concentrations across the full cycle. | (Feltenstein et al., 2009; Feltenstein and See, 2007) |
Fig. 1.
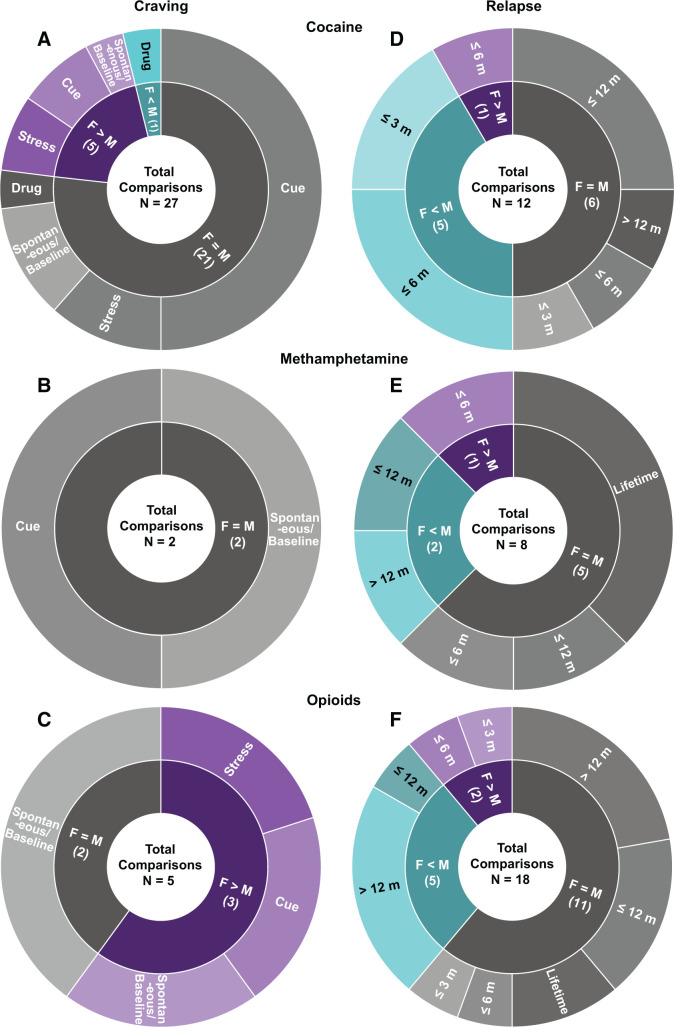
Summary of clinical studies on sex differences in psychostimulant and opioid craving and relapse. Both craving (left) and relapse (right) panels depict the proportion of observations and list the total number of comparisons of sex differences in which F > M, F = M, and F < M for cocaine (A and D), methamphetamine (B and E), and opioids (C and F). Conditions under which craving was measured, and the abstinence period when relapse was assessed is also displayed. F, females; M, males; m, months. Note: The number of comparisons does not equal the number of studies for a given category in Supplemental Table S1 because in some studies investigators assessed more than one dependent measure (e.g., both cue- and stress-induced craving). Spontaneous craving refers to baseline nonprovoked subjective craving.
II. Sex Differences in Drug Craving and Relapse: Clinical Studies
We review studies on sex differences in psychostimulant and opioid craving and relapse in human laboratory and treatment settings (Fig. 1; Supplemental Table S1). Drug craving is correlated, to some degree, with drug use (Bordnick and Schmitz, 1998; Da Silveira et al., 2006; Paliwal et al., 2008; Preston et al., 2009, 2018) and often predictive of future relapse (Bordnick and Schmitz, 1998; Sinha et al., 2006, 2011). In human laboratory studies, drug craving is measured by psychometric self-report assessments before and after exposure to the drug itself, drug-related cues, or stressors (Jaffe et al., 1989; Sinha et al., 2011). Cue-induced craving is achieved by exposing study participants to videos or pictures showing drug-associated cues (e.g., syringe), handling of drug paraphernalia, standardized scripts, or individualized guided imagery (Bedi et al., 2011). Stress-induced craving is achieved by exposing study participants to psychologic (e.g., personalized imagery, public speaking, mental arithmetic), physical (e.g., cold pressor), or pharmacological (e.g., α-2 adrenoreceptor antagonist yohimbine) stressors (Charney et al., 1983; Sinha et al., 1999, 2011; Stine et al., 2002).
In treatment settings, relapse is defined as resumption of regular drug use during outpatient treatment or after completion of inpatient or outpatient treatment. These studies rely on follow-up interviews at specific time points or ecological momentary assessment, real-time reporting of drug craving, and use in the natural environment (Shiffman et al., 1996; Epstein et al., 2009). We also review cross-sectional studies because they provide additional insight on sex differences in relapse vulnerability.
A. Psychostimulants
1. Craving
Several studies examined sex differences in cocaine craving (Fig. 1; Supplemental Table S1), and although some studies suggest greater craving in women, other studies do not.
2. Spontaneous Drug Craving
Cocaine (Fig. 1A): Elman et al. (2001) reported that when craving is assessed during early abstinence (12 hours) with a craving questionnaire of six items measuring aspects of craving, such as current intensity, projected intensity, resistance, and response to drug-associated cue, women reported higher spontaneous (nonprovoked) cocaine craving than men. However, other studies found no sex differences in cocaine craving 2 days into abstinence (Waldrop et al., 2010) or in nonabstaining users (Volkow et al., 2011) when craving was measured with the Craving/Distress/Mood scale or a brief version of the Cocaine Craving Questionnaire. Similarly, when craving is monitored at least a week into abstinence with a brief version of the Cocaine Craving Questionnaire or a 10-point visual analog scale, several studies reported no sex differences in spontaneous craving (Fox et al., 2006, 2013; Paliwal et al., 2008).
Methamphetamine (Fig. 1B): Galloway et al. (2010) reported no sex differences in spontaneous methamphetamine craving measured by a self-reported 0–100 scale (endpoint anchors of “no craving” and “most craving ever experienced”) during 4 months of abstinence.
3. Drug-Induced Craving
Cocaine (Fig. 1A): During early abstinence (<7 days), craving induced by cocaine injection was either similar in men and women when measured by multidimensional questionnaires (Elman et al., 2002) or higher in men when measured with 10-point visual analog scales (Evans et al., 1999).
4. Cue-Induced Craving
Cocaine (Fig. 1A): No sex differences in cue-induced cocaine craving were observed after 2 days of abstinence (Waldrop et al., 2010) or in nonabstaining users (Volkow et al., 2011). Similarly, Fox et al. (2006) and Potenza et al. (2012) reported no sex differences in cue-induced craving when craving was measured at least a week into abstinence using a 10-point visual analog scale. Additionally, using the same measurement tool, there were no sex differences in cocaine craving in individuals with comorbid substance use disorder undergoing long-term opioid agonist treatment (Avants et al., 1995; Kennedy et al., 2013). However, Fox et al. (2014) and Robbins et al. (1999) reported higher cue-induced cocaine craving measured with a 10-point visual analog scale in women after 14–21 days of inpatient treatment or long-term outpatient treatment, respectively.
Methamphetamine (Fig. 1B): Tolliver et al. (2010) reported no sex differences in cue-induced methamphetamine craving measured with a 10-point visual analog scale during early abstinence.
5. Stress-Induced Craving
Cocaine (Fig. 1A): Waldrop et al. (2010) reported stronger correlation between peak craving and peak response to a social stressor (Trier Social Stress Test) in women after 2 days of abstinence. Similarly, Moran-Santa Maria et al. (2014) reported that within 2–3 days of initiating abstinence, women report greater yohimbine-induced craving measured with a 10-point visual analog scale. In contrast, using the same craving scale method, Back et al. (2005) reported no sex differences in craving induced by psychological (Mental Arithmetic Task) or physical (Cold Pressor Task) stressors. Additionally, Brady et al. (2009) reported that after similar abstinence length, men and women show similar corticotropin-releasing factor (CRF)-induced craving. Finally, no sex differences in craving induced by script-guided stressful situations were observed in participants abstinent for at least 2 weeks using a 10-point visual analog scale (Li et al., 2005).
Together, these studies show that women are more sensitive to stress-induced craving only during early abstinence and that this effect is independent of the type of stressor (e.g., physical or psychosocial). However, our conclusion is tentative because of the few studies published, the divergent results, and the low statistical power to measure sex differences.
6. Relapse
Cocaine (Fig. 1D): Kosten et al. (1993) reported that upon entering treatment, women had higher relapse rates. However, they reported that there were no sex differences during the treatment study (desipramine or lithium carbonate), and at 6-month follow-up, relapse rates were lower in women (Kosten et al., 1993). In contrast, Kennedy et al. (2013) reported that in polydrug (cocaine + heroin) users, women undergoing opioid agonist therapy showed higher relapse rates over a 7-month period. However, during 3-month (Burch et al., 2015; Bashiri et al., 2017) and 6-month (Gallop et al., 2007) treatment follow-up, women showed lower relapse rates. Similarly, when relapse was assessed at treatment follow-up, women often exhibited better outcomes. Specifically, women had lower relapse rates at 6-month follow-up from both inpatient (Weiss et al., 1997) and outpatient (Kosten et al., 1993) treatment. In contrast, no sex differences in relapse rates were observed 9 months (Sterling et al., 2004) or 12 months (Negrete and Emil, 1992; McKay et al., 2003) after outpatient treatment. Additionally, during a 2-year period after a randomized clinical trial in polydrug (cocaine + opioid) users, men and women showed similar relapse rates (Schottenfeld et al., 1998).
Methamphetamine (Fig. 1E): Hillhouse et al. (2007) reported higher relapse rates in women during 4-month outpatient treatment but no sex differences in relapse at 6-month or 12-month post-treatment. In contrast, Lanyon et al. (2019) reported higher relapse rates in men at both 12-month and 5-year follow-ups. He et al. (2013) and Brecht et al. (2000, 2004) reported no sex differences in lifetime relapse rates among inpatients or after treatment completion.
B. Opioids
1. Craving
Back et al. (2011) reported greater spontaneous opioid craving in women, and Yu et al. (2007) reported higher cue-induced heroin craving in women during inpatient treatment (Fig. 1C). Additionally, Moran et al. (2018) reported that over 4 months of outpatient opioid maintenance therapy, women showed higher stress-induced opioid craving. However, Herbeck et al. (2016) reported no sex differences in opioid craving during 3 weeks of extended-release naltrexone treatment. Similarly, Kennedy et al. (2013) reported no sex differences in heroin craving during 6 months of outpatient opioid agonist treatment with methadone (100 mg/day).
2. Relapse
Maehira et al. (2013) reported higher relapse rates in women during the first 2 months of abstinence (Fig. 1F). Ignjatova and Raleva (2009) reported more heroin lapses in women during 6 months of opioid agonist treatment with methadone (dose not provided). In contrast, Kamal et al. (2007) and Kennedy et al. (2013) reported no sex differences in heroin relapse during the first 3 (methadone, average 74 mg/day) and 6 (methadone, 100 mg/day) months of opioid agonist treatment.
After more prolonged abstinence periods (>1 year), there is some evidence for lower relapse rates in women. Gordon et al. (2017) reported lower relapse rates for women at 1-year follow-up after buprenorphine treatment (average 8 mg/day). Zimmer-Höfler and Dobler-Mikola (1992) reported similar findings after 2 years of abstinence from different therapeutic programs (e.g., methadone maintenance therapy, therapeutic community program) and prisons. Additionally, during a 2-year follow-up period during opioid agonist treatment (buprenorphine, 4 mg/day) for polydrug (cocaine + opioids) use, women had more drug-free days (Schottenfeld et al., 1998). Within the same longitudinal study of individuals using heroin who were recruited from randomly selected treatment agencies delivering various treatments (e.g., methadone or buprenorphine maintenance treatment, drug-free residential rehabilitation, or detoxification), Darke et al. reported that women were less likely to relapse over 3-year (Darke et al., 2007) and 11-year (Darke et al., 2015) follow-up periods. These results contrast with previous studies reporting no sex differences in relapse at 7-year (Zimmer-Höfler and Dobler-Mikola, 1992) and 8-year (Brunswick and Messeri, 1986) follow-ups. Additionally, several studies reported no sex differences in relapse over 12-month follow-up during methadone treatment [average dose 130 mg/day in Adelson et al. (2018) and 40–70 mg/day in Levine et al. (2015) and Smyth et al. (2012)] and no sex differences in lifetime opioid relapse rates (Zhou et al., 2017; Moradinazar et al., 2020). Importantly, the different outcomes from these studies could be due to the divergent screening of the participants. Indeed, although some studies directly compared drug craving and relapse in men and women after the same opioid agonist treatment (e.g., methadone or buprenorphine maintenance therapy), others compared cohorts of participants with mixed therapy programs (e.g., opioid agonist therapy, detoxification, or drug-free residential rehabilitation), which may increase sample variability and decrease the power to detect sex differences.
C. Conclusions
The studies reviewed do not support the notion of sex differences in drug craving and relapse for either psychostimulants or opioids. However, these studies suggest areas for further examination, including potential sex differences in craving and relapse vulnerability during early abstinence wherein women may be more vulnerable.
III. Sex Differences in Drug Craving and Relapse: Preclinical Studies
Sex differences in drug relapse were examined using the reinstatement and incubation of drug-craving models (Fig. 2; Supplemental Table S2) (Venniro et al., 2016). In studies using the reinstatement model, investigators determined sex differences in resumption of drug seeking after extinction induced by exposure to the self-administered drug, discrete cues, contextual cues, and stress. For clarity, we refer to discrete cues as “cues” in the text below. In stress-induced reinstatement studies, the typical stressors investigators used in male rats were intermittent foot shock (Shaham and Stewart, 1995; Shaham et al., 2000; Mantsch et al., 2016) and yohimbine as a pharmacological stressor (Shepard et al., 2004; Mantsch et al., 2016). In opioid users, yohimbine has been shown to induce stress- and withdrawal-like symptoms and opioid craving (Stine et al., 2002). However, interpretation of results from studies using yohimbine in reference to stress-induced reinstatement has been challenged by Chen et al. (2015). They showed that at the dose range yohimbine is used in reinstatement studies, the drug’s effect on reinstatement is independent of the history of contingent self-administration and unrelated to the commonly assumed stress-like effects of yohimbine.
Fig. 2.
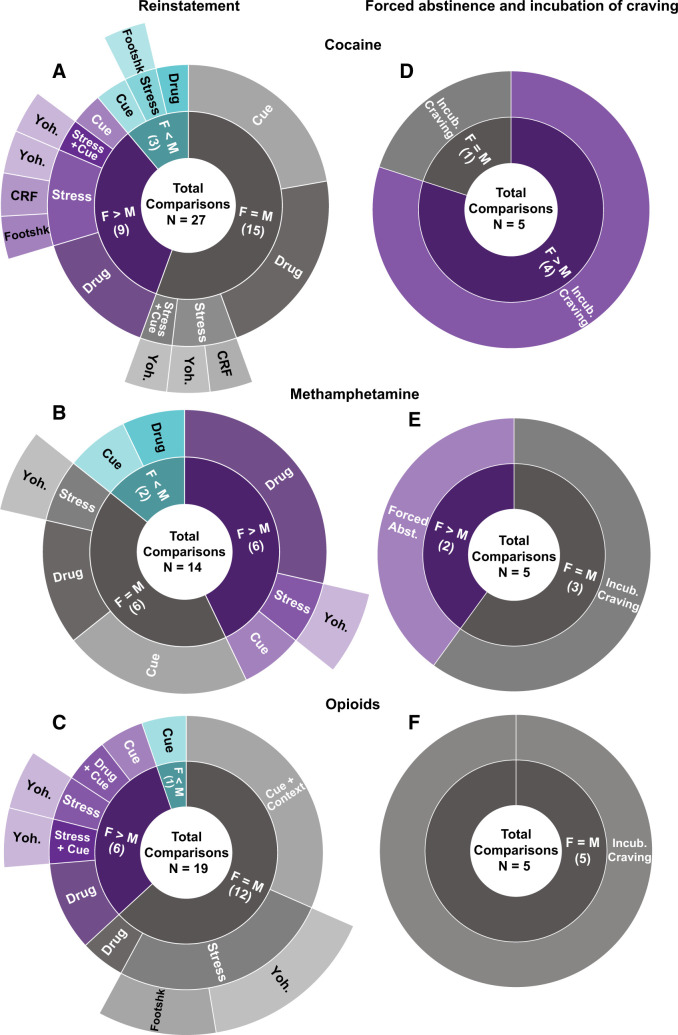
Summary of preclinical studies on sex differences in psychostimulant and opioid reinstatement and incubation of craving. Both reinstatement of drug-seeking behavior (left) and incubation of craving and drug seeking after forced abstinence (right) panels depict the proportion of observations and list the total number of comparisons of sex differences in which F > M, F = M, and F < M for cocaine (A and D), methamphetamine (B and E), and opioids (C and F). The conditions under which drug seeking was measured are also displayed. F, females; M, males; Yoh, yohimbine. Note: the number of comparisons does not equal the number of studies for a given category in Supplemental Table S2 because in some studies investigators assessed more than one dependent measure (e.g., both cue- and stress-induced reinstatement).
A. Psychostimulants
1. Drug-Induced Reinstatement
Cocaine (Fig. 2A): Lynch and Carroll (2000) reported that female rats showed higher drug-induced reinstatement (often termed drug priming-induced reinstatement) after short-access drug self-administration of cocaine at 1.0 and 3.2 mg/kg but not 0.32 mg/kg. This initial finding of sex differences in cocaine-induced reinstatement was confirmed in subsequent studies (Anker et al., 2009; Smith et al., 2012; Doncheck et al., 2020). In contrast, after continuous long-access drug self-administration of cocaine, Zlebnik et al. (2014) and Swalve et al. (2016) reported no sex differences in cocaine-induced reinstatement. Additionally, after intermittent-access drug self-administration of cocaine, Kawa and Robinson (2019) reported no sex differences in reinstatement induced by cocaine priming (<1.6 mg/kg, i.v.).
A tentative conclusion from these studies is that females are more sensitive to cocaine-induced reinstatement after short-access but not long-access cocaine self-administration, suggesting that cocaine history (e.g., quantity of drug intake) may be an important contributing factor to sex differences in cocaine-induced reinstatement.
Finally, Jordan and Andersen (2018) reported that after 30 days of short-access self-administration training with a high (0.75 mg/kg) but not low (0.25 mg/kg) cocaine unit dose (training started on P28) followed by 30 abstinence days, cocaine seeking was higher in males than in females after exposure to a noncontingent cocaine injection (10 mg/kg, i.p) immediately prior to a single relapse test session under extinction conditions. The relevance of these results to sex differences in cocaine-induced reinstatement after extinction is unknown because the rats did not undergo extinction training and the authors did not assess the effect of vehicle (saline) injections on drug seeking.
Methamphetamine (Fig. 2B): After both short- and long-access drug self-administration, methamphetamine (1 mg/kg)-induced reinstatement was higher in female rats (Holtz et al., 2012; Reichel et al., 2012; Cox et al., 2013; Cordie and McFadden, 2019). However, using the same dose, others reported either no sex differences in methamphetamine-induced reinstatement (Everett et al., 2020; Zlebnik et al., 2021) or higher reinstatement in males (Everett et al., 2021).
2. Cue-Induced Reinstatement
Cocaine (Fig. 2A): Zhou et al. (2014) reported higher cue-induced reinstatement in females after short-access cocaine self-administration. In contrast, under similar training conditions, several studies reported no sex differences in cue-induced reinstatement (Fuchs et al., 2005; Swalve et al., 2016; Bechard et al., 2018; Weber et al., 2018). Similarly, no sex differences in cue-induced reinstatement were observed after long-access (Zlebnik et al., 2014) or intermittent-access (Kawa and Robinson, 2019) cocaine self-administration.
Methamphetamine (Fig. 2B): Five studies examined sex differences in cue-induced reinstatement of methamphetamine seeking, and, as with cocaine, the results are inconclusive. After short-access self-administration, Cox et al. (2013) reported higher cue-induced reinstatement in female rats. In contrast, Bernheim et al. (2017) and Everett et al. (2020a) reported no sex differences. Additionally, Takashima et al., (2018) reported higher cue-induced reinstatement in male rats after long-access self-administration. Zlebnik et al. (2021) used a methamphetamine self-administration protocol of two 2-hour sessions/day and reported no sex differences in cue-induced reinstatement.
3. Stress-Induced Reinstatement
Cocaine (Fig. 2A): After short-access self-administration, Anker and Carroll (2010b) reported higher yohimbine-induced reinstatement, and Feltenstein et al. (2011) reported higher yohimbine- and cue-induced reinstatement in females. In contrast, Zlebnik et al. (2014) reported no sex differences in either yohimbine-induced or yohimbine- and cue-induced reinstatement. Doncheck et al. (2020) reported no sex differences in restraint stress-induced potentiation of drug-induced reinstatement. However, they reported that intermittent footshock potentiates drug-induced reinstatement in males but not females. In contrast, Connelly et al. (2020) reported higher foot shock–induced reinstatement in females.
In male rats, the effect of intermittent footshock stress on reinstatement of drug seeking is inhibited by extrahypothalamic CRF, and ventricular CRF injections mimic the effect of intermittent footshock on reinstatement (Shaham et al., 2000; Erb et al., 2001; Mantsch et al., 2016). Based on these results, Buffalari et al. (2012) examined sex differences in CRF-induced reinstatement of cocaine seeking after short-access self-administration. The main finding was that although ventricular injections of CRF reinstated cocaine seeking in both sexes, the response to CRF was more variable in females than in males.
Methamphetamine (Fig. 2B): Cox et al. (2013) reported higher yohimbine-induced reinstatement in females, whereas Everett et al. (2020a) reported no sex differences. The reasons for these different results are unclear but may be due to a long abstinence period (21 days) before the extinction phase in the Everett et al. (2020a) study. As discussed above, interpretation of data from yohimbine studies in reference to stress-induced reinstatement is problematic (Chen et al., 2015; Mantsch et al., 2016).
Together, these divergent results across different stressors do not indicate significant sex differences in stress-induced reinstatement of cocaine or methamphetamine seeking.
4. Incubation of Craving and Relapse after Abstinence
Cocaine (Fig. 2D): Kerstetter et al. (2008) reported longer-lasting and higher incubation of cocaine craving (up to 180 days of home-cage forced abstinence) in females after long-access drug self-administration. We extended these results and reported higher incubation of cocaine craving on abstinence day 29 in females after both long-access and intermittent-access cocaine self-administration (Nicolas et al., 2019) (Fig. 3). Most recently, Corbett et al. (2021) reported similar results (i.e., higher incubation in females) after both 15 and 48 abstinence days. Johnson et al. (2019) reported similar results with higher cocaine seeking in female rats tested at 1 or 30 abstinence days after short-access cocaine self-administration. However, these results should be interpreted with caution because in both sexes the incubation effect was variable and statistically nonsignificant, likely because of the use of the short-access procedure [incubation is less robust with this procedure (Lu et al., 2004)]. Additionally, Madangopal et al. (2019) reported that incubation of the response to cocaine discriminative cues (e.g., light, tone) that predict availability of the drug is more persistent in female rats and lasts for up to 200 abstinence days. However, these results should also be interpreted with caution because the study was not powered to detect sex differences.
Fig. 3.
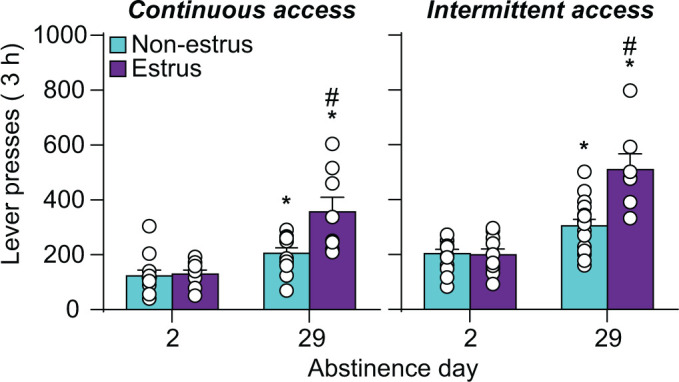
Effect of estrous cycle on incubation of craving after long-access and intermittent-access cocaine self-administration in female rats. Relapse (incubation) test. Mean ± S.E.M. number of active lever presses per session after continuous (nonestrus: n = 12 for day 2 and 29, estrus: n = 9/8 for day 2/29) and intermittent (nonestrus: n = 13/16 for day 2/29, estrus n = 10/7 for days 2/29) access drug self-administration. * Different from day 2 within each estrous phase, P < 0.05; # Different from nonestrus on day 29, P < 0.05. Adapted from Nicolas et al. (2019). Figure 3 was reproduced with permission from Elsevier.
Fig. 4.
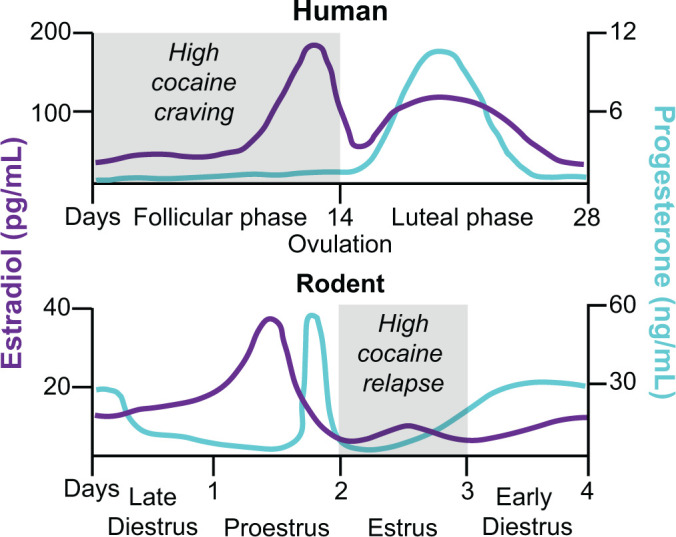
Schematic comparison of the menstrual cycle in humans and the estrous cycle in rodents and fluctuation of estradiol and progesterone levels across the cycle phases. In humans, the menstrual cycle is divided into the follicular and luteal phases separated by ovulation (Sherman and Korenman, 1975; Anker and Carroll, 2010a). The cycle begins with menses, and the onset of the follicular phase is characterized by high levels of estradiol, with a peak during the preovulation period before decreasing to a moderate level during the luteal phase. Conversely, progesterone is at its lowest level during the follicular phase and starts increasing during the preovulation period to peak at the midluteal phase. In female rodents, the estrous cycle is divided into late diestrus, proestrus, estrus, and early diestrus phases, with ovulation occurring between proestrus and estrus (Cora et al., 2015; Krentzel and Meitzen, 2018). Estradiol peaks twice at the middle of the early diestrus and proestrus phases and drops by ovulation. Progesterone peaks at the end of the early diestrus and proestrus phases, and low stable levels remain for the rest of the cycle. In many studies, investigators pool together early and late diestrus phases to a single phase called diestrus because of similar hormonal and cytologic characteristics. Additionally, when no behavioral differences are observed during proestrus and diestrus, investigators combine these phases and call the combined phase nonestrus. There are major differences in the reproductive cycle of women and female rodents: the duration of the cycle, the cycle pattern of estradiol and progesterone, and the amplitude of hormone level variations. However, when the hormonal ratio over the phases is compared, some analogies are conventionally made: the follicular phase is comparable to the estrus phase (progesterone < estradiol) and the luteal phase to the nonestrus phase (progesterone > estradiol) (Anker and Carroll, 2010a; Krentzel and Meitzen, 2018).
In contrast, Venniro et al. (2021) reported that after either long-access (12 hour/day) or intermittent long-access (12 hour/day, 5 minutes ON, 25 minutes OFF ×24), there were no sex differences in incubation of cocaine craving after home-cage forced abstinence or after voluntary abstinence induced by providing rats with a mutually exclusive choice between cocaine or rewarding social interaction. The different results of Venniro et al. (2021) versus the studies of Kerstetter et al. (2008), Nicolas et al. (2019), and Corbett et al. (2021) may be because of two reasons. The first is that Venniro et al. (2021) trained their rats for 12 hours/day, whereas in the other studies, the session duration was 6 hours/day. The second is that the male and female rats in Venniro et al. (2021) study were first trained to lever press for social interaction for 6 days, whereas the rats in the other studies were not.
Methamphetamine (Fig. 2D): Venniro et al. (2017) (long-access self-administration) and Everett et al. (2020a) (short- and long-access self-administration) reported no sex differences in incubation of methamphetamine craving after either forced abstinence in home cage or voluntary abstinence in the drug self-administration chambers, the latter being achieved by providing rats with mutually exclusive choices between the self-administered drug and an alternative nondrug reinforcer (e.g., palatable food or social interaction) (Caprioli et al., 2015; Caprioli et al., 2017; Venniro et al., 2018; Venniro and Shaham, 2020; Fredriksson et al., 2021). Similarly, Daiwile et al. (2019) reported no sex differences in incubation of methamphetamine craving after forced abstinence after a self-administration protocol of two 3-hour sessions/day. In contrast, Ruda-Kucerova et al. (2015, 2017) reported that in a single relapse test after 15 days of forced abstinence from short-access methamphetamine self-administration, drug seeking was higher in female rats. In the context of sex differences in incubation of drug craving, these results are difficult to interpret because the authors did not establish that incubation had occurred under their experimental conditions.
B. Opioids
1. Drug-Induced Reinstatement (Fig. 2C)
In an early study, Klein et al. (1997) reported that reacquisition of oral fentanyl self-administration after extinction is higher in female rats. However, these results are difficult to interpret without ruling out potential confounds related to the oral route of drug administration (i.e., sex differences in pharmacokinetics and taste sensitivity). In a recent study, Malone et al. (2021) tested both sexes for fentanyl-induced reinstatement after short- (1 hour) or long- (6 hours) access fentanyl self-administration training. They reported higher fentanyl-induced reinstatement in females after short- but not long-access fentanyl self-administration. Similarly, Smethells et al. (2020) reported higher heroin-induced reinstatement and heroin- and cue-induced reinstatement after short-access self-administration.
2. Cue- and Context-Induced Reinstatement (Fig. 2C)
Cue-induced reinstatement: Vazquez et al. (2019) reported higher extinction responding and cue-induced reinstatement of heroin seeking in females after short-access drug self-administration in food-restricted rats. In contrast, Phillips et al. (2020) reported no sex differences in cue-induced reinstatement of oxycodone self-administration after 3 hours oral oxycodone self-administration in mice. Additionally, Smethells et al. (2020) reported no differences in cue-induced reinstatement after short-access heroin self-administration. Bakhti-Suroosh et al. (2021) tested both sexes for cue-induced reinstatement after 6 hours of extinction in a single daily session performed 14 days after intermittent-access fentanyl self-administration (24 hours/day) under two training conditions: fixed ratio 1 reinforcement schedule with or without 1-second timeout. There were no sex differences in either extinction responding or cue-induced reinstatement in the no-timeout training condition. In contrast, sex differences (higher responding in females) emerged in the 1-second timeout training condition for extinction responding but not cue-induced reinstatement. The reasons for the sex-specific effect of the timeout manipulation on extinction responding are unknown. Most recently, Malone et al. (2021) tested both sexes for cue-induced reinstatement after short- (1 hour) or long- (6 hours) access fentanyl self-administration training. They reported lower cue-induced reinstatement in females after long- but not short-access self-administration.
Context-induced reinstatement: Bossert et al. (2020, 2021) reported no sex differences in context-induced reinstatement of oxycodone or heroin seeking after long-access (6 hours/day) drug self-administration training.
Together, there is limited evidence for sex differences in cue- or context-induced reinstatement of opioid seeking. Unexpectedly, under certain experimental conditions, female rats can be either more sensitive [intermittent access (24 hour/day) plus 1-second timeout] or less sensitive [long-access (6 hours/day) drug self-administration] to cue-induced reinstatement of fentanyl seeking.
3. Stress-Induced Reinstatement (Fig. 2C)
Smethells et al. (2020) reported no sex differences in extinction responding but higher yohimbine-induced reinstatement in females; they also reported higher yohimbine- and cue-induced reinstatement in females after short-access heroin self-administration. In contrast, Fulenwider et al. (2020) reported no sex differences in footshock–induced reinstatement of oxycodone seeking after short-access oral oxycodone self-administration training. Malone et al. (2021) tested male and female rats for yohimbine-induced reinstatement after short- (1 hour) or long- (6 hours) access fentanyl self-administration training. There were no sex differences in either extinction responding or yohimbine-induced reinstatement; however, independent of the access condition, yohimbine’s effect on reinstatement was weak and statistically significant only after injections of the low (1 mg/kg) but not higher (2 mg/kg) dose. In the context of sex differences in stress-induced reinstatement, these data are difficult to interpret because of the weak effect of yohimbine on reinstatement in both sexes. In a follow-up study, the same group studied the effect of systemic injections of the glucocorticoid receptor antagonist PT150 on stress-induced reinstatement of fentanyl seeking (Hammerslag et al., 2021). They reported that PT150 decreased intermittent footshock– but not yohimbine-induced reinstatement in male but not female rats. However, these results should also be interpreted with caution because they are 1) based on post hoc analyses in the absence of a significant interaction between the footshock stress condition and PT150 dose, and 2) the weak effect of yohimbine on reinstatement in both sexes and the weak effect of footshock on reinstatement in females. Finally, the data on the inhibitory effect of glucocorticoid antagonism on stress-induced reinstatement of opioid seeking is unexpected based on previous endocrinological and pharmacological studies showing that the effect of intermittent footshock on reinstatement of drug seeking is independent of activation of the hypothalamic-pituitary-adrenal axis and glucocorticoid systems (Shaham et al., 2000; Erb et al., 2001; Mantsch et al., 2016).
Together, the limited number of studies reviewed above do not support the notion of increased vulnerability to stress-induced reinstatement of opioid seeking in females.
4. Incubation of Craving and Relapse during Abstinence (Fig. 2F)
Unlike the mixed evidence for reinstatement of opioid seeking, there is no evidence for sex differences in incubation of opioid craving after either forced or voluntary abstinence achieved via either providing the rats with alternative non–drug reinforcer in a choice procedure or by introducing an electric barrier of increasing shock intensity near the drug-paired lever (Cooper et al., 2007).
Using a long-access self-administration protocol, Venniro et al. (2017) reported no sex differences in either incubation of heroin seeking after forced abstinence or reversal of incubation of heroin craving after food choice–induced abstinence. Venniro et al. (2019) replicated the findings for forced abstinence and also reported no sex differences in the inhibition of incubation of heroin seeking after social choice–induced abstinence. Reiner et al. (2020) reported no sex differences in relapse to fentanyl seeking after 2 weeks of food choice–induced abstinence. Fredriksson et al. (2020) reported no sex differences in incubation of oxycodone seeking after forced abstinence or potentiation of incubation of oxycodone seeking after electric barrier–induced abstinence. Finally, Bossert et al. (2021) reported no sex differences in incubation of heroin seeking after home-cage forced abstinence. In this study, the authors performed the incubation-related tests after 1 and 8 abstinence days (within-subjects assessment) in a nondrug context because the same rats were also tested for context-induced reinstatement after extinction of the operant response in the nondrug context.
C. Conclusions
The studies reviewed demonstrate sex differences for stress-induced reinstatement of cocaine seeking and incubation of cocaine craving after forced abstinence. In contrast, there is mixed evidence for sex differences in cue- or drug-induced reinstatement of cocaine seeking. In the case of cocaine-induced reinstatement, it appears that females are more vulnerable after short-access but not long-access cocaine self-administration. The literature on sex differences in cocaine pharmacokinetics is mixed, but sex differences in cocaine-induced reinstatement could be at least in part due to sex differences in first-pass cocaine metabolism after intraperitoneal noncontingent cocaine injections (Bowman et al., 1999; Festa et al., 2004). Similarly, the evidence supporting sex differences in reinstatement of methamphetamine and opioid seeking across different reinstating stimuli is mixed at best. Additionally, there is consistent evidence for lack of sex differences in incubation of methamphetamine and opioid craving after forced or voluntary abstinence.
However, these conclusions should be confirmed in future studies considering the relatively small number of sex differences studies, particularly for stress-induced reinstatement, wherein investigators primarily used yohimbine as the stress manipulation. In this regard, the subjects in the first intermittent footshock stress–induced reinstatement study were mostly female rats (Shaham and Stewart, 1995), but with few exceptions (Buffalari et al., 2012; Sedki et al., 2015; Connelly et al., 2020; Doncheck et al., 2020), only males were used in subsequent studies on reinstatement induced by intermittent footshock or other stressors like restraint, food restriction, or forced swim (Mantsch et al., 2016; Reiner et al., 2019). In addition, the studies on sex differences in opioid relapse and craving did not assess opioid dependence by parametric measures of spontaneous or naloxone-precipitated withdrawal symptoms. Consequently, we used the duration of opioid self-administration training as a proxy for nondependent (short-access, 1- to 3-hour daily session) and dependent (long-access, 6 hours or longer daily session) conditions. In this regard, session duration has been shown to be a critical factor in determining the magnitude of relapse to opioid seeking (Ahmed et al., 2000; Reiner et al., 2019).
Finally, studies on reinstatement of food seeking also provide little evidence for sex differences. No sex differences were observed for sucrose-, yohimbine-, or cue-induced reinstatement (Cox et al., 2013; Bernheim et al., 2017; Hernandez et al., 2020) after palatable food self-administration. However, Cox et al. (2013) reported higher cue-induced reinstatement of sucrose seeking in female rats.
IV. Role of Menstrual and Estrous Cycle
Several studies examined the role of ovarian hormones and menstrual/estrous cycle (Figs. 3 and 4) in drug (primarily cocaine) craving and relapse in humans and rat models (Supplemental Tables S3–S4). The methodological aspects and limitations of menstrual and estrous cycle tracking are summarized in Table 2.
A. Clinical Studies
Sofuoglu et al. (1999) reported lower cocaine desire (craving) during the luteal than the follicular phase. Evans et al. (2002) reported lower cocaine-induced subjective positive drug effects and craving during the luteal than the follicular phase. These results suggest a protective effect of progesterone (high during luteal phase) on cocaine-induced cocaine craving. In agreement with this idea, several studies reported that women with high endogenous progesterone levels (comparable to luteal phase) are less sensitive to stress- and cue-induced cocaine craving than women with low progesterone levels (Sinha et al., 2007; Moran-Santa Maria et al., 2018; Sherman et al., 2020). Additionally, several studies reported that exogenous progesterone reduces positive subjective cocaine effects and craving in both women and men (Sofuoglu et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006; Fox et al., 2013; Milivojevic et al., 2016), confirming progesterone’s protective effects.
In contrast, in occasional intranasal cocaine users, Lukas et al. (1996) reported no effect of the menstrual cycle on cocaine-induced subjective positive drug effects. The results of this negative study agree with those from other studies in cocaine-dependent women (Collins et al., 2007; Potenza et al., 2012). Additionally, Fox et al. (2008) reported no variations in craving over the menstrual cycle during the first month of abstinence from smoked cocaine, but the ratio of estradiol/progesterone was stable across the cycle, which could explain these negative results.
However, both the negative and positive results reviewed above should be interpreted with caution because of relatively small sample sizes and other factors that may play a role in the behavioral outcomes under investigation (e.g., severity of cocaine use disorder, route of administration).
B. Preclinical Studies
The results of studies on the role of estrous cycle and ovarian hormones in reinstatement and incubation of craving are summarized in Supplemental Table S4.
Cocaine: Cocaine-induced reinstatement is higher during estrus than diestrus or proestrus (Kippin et al., 2005 Feltenstein and See, 2007; Kerstetter et al., 2008; Feltenstein et al., 2009). The expression of incubation of cocaine craving is higher during estrus than nonestrus (Fig. 3) (Kerstetter et al., 2008; Nicolas et al., 2019; Corbett et al., 2021). In contrast, evidence for the role of estrous cycle in cue- and stress-induced reinstatement is mixed. Fuchs et al. (2005) reported lower cue-induced reinstatement of cocaine seeking in females in estrus than nonestrus. Feltenstein et al. (2011) reported that yohimbine- and cue-induced reinstatement is lower in estrus and diestrus than proestrus. In contrast, they reported that the estrus phase has no effect on yohimbine-induced reinstatement of cocaine seeking. Peterson et al. (2014) and Bechard et al. (2018) also reported similar cue-induced reinstatement of cocaine seeking during different phases of the estrous cycle.
Suppression of ovarian hormones by ovariectomy decreases cocaine-induced reinstatement, whereas chronic estradiol treatment in ovariectomized rats restores this reinstatement to levels similar to those of sham rats (Larson et al., 2005; Anker et al., 2007). Additionally, acute proestrus-level estradiol in ovariectomized rats potentiates cocaine-induced reinstatement (Doncheck et al., 2018). In contrast, exogenous progesterone treatment in free-cycling rats decreases cocaine-induced reinstatement (Anker et al., 2007; Feltenstein et al., 2009). Anker et al. (2007) also reported that exogenous progesterone treatment in ovariectomized rats inhibits the facilitating effect of estradiol on cocaine-induced reinstatement. Together, these results suggest a role of ovarian hormones in cocaine relapse with estradiol increasing relapse vulnerability and progesterone having an opposite effect.
Methamphetamine, heroin, and fentanyl: The role of ovarian hormones in relapse/reinstatement to methamphetamine and opioid seeking is largely unknown. Cox et al. (2013) reported that the estrous cycle has no effect on methamphetamine-induced reinstatement. Vazquez et al. (2019) reported that estradiol or progesterone treatment has no effect on cue-induced reinstatement of heroin seeking. Sedki et al. (2015) reported that food restriction for 2 weeks (a chronic stressor) increases relapse to heroin seeking after home-cage forced abstinence in female rats. This effect is not decreased by ovariectomy, and unexpectedly, in ovariectomized rats, estradiol replacement but not progesterone injections decrease the potentiation effect of chronic food restriction on relapse. These results highlight the necessity to better characterize the potential role of the estrous cycle in heroin relapse.
Bakhti-Suroosh et al. (2021) reported higher extinction responding and cue-induced reinstatement of drug seeking after 14 days of forced abstinence from intermittent-access fentanyl self-administration during estrus versus nonestrus. In contrast, Malone et al. (2021) reported that the estrous cycle has no effect on cue-, yohimbine-, and fentanyl-induced reinstatement after short- or long-access fentanyl self-administration training. These different results may be due to the use of the intermittent access self-administration procedure for 23 hours/day and imposing 14 days abstinence before the relapse/reinstatement tests in the study of Bakhti-Suroosh et al. (2021) versus the use of continuous long-access self-administration procedures for 6 hours/day without an abstinence period in the study conducted by Malone et al. (2021).
C. Conclusions
The clinical and preclinical studies reviewed above suggest that under certain conditions, cocaine craving and relapse are dependent on the menstrual/estrous cycle, with higher vulnerability during the follicular/estrus phase. The hypothesis that emerges from the studies reviewed is that progesterone decreases cocaine craving and relapse vulnerability, whereas estradiol has opposite effects. Specifically, the menstrual/estrous cycle influences incubation of cocaine craving and cocaine-induced reinstatement. However, the review of the literature does not provide clear evidence for sex differences in the magnitude of cocaine-induced reinstatement, indicating that the influence of hormonal cycle on relapse-related behaviors in females does not necessarily lead to higher relapse vulnerability in females compared with males.
Many of the studies reviewed were designed to specifically assess the influence of the menstrual/estrous cycle on cocaine relapse and craving with statistical power to detect the effect of hormonal fluctuations and manipulation on relapse-related behaviors if such effect exists. However, some limitations of the methods to identify menstrual and estrous cycles phases need to be taken into consideration in the interpretation of the results. We describe these limitations in Table 2.
Finally, the results of Sedki et al. (2015) described above on the “protective” effect of estradiol suggest that this hypothesis may not generalize to opioid drugs [but see Bakhti-Suroosh et al. (2021) for results congruent with the notion of opposing roles of estradiol and progesterone in drug relapse].
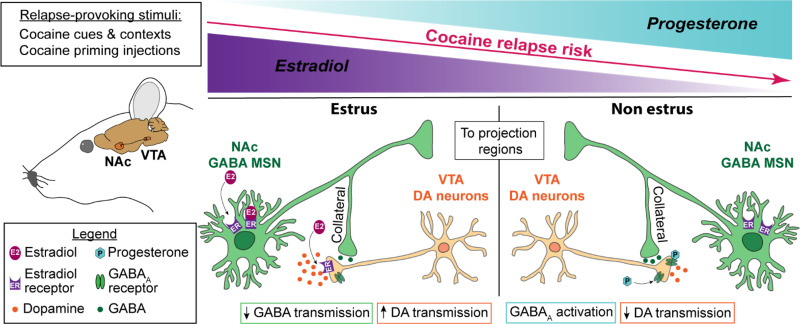
V. Brain Mechanisms
The brain mechanisms involved in the putative opposite effects of estradiol and progesterone on cocaine craving and relapse are unknown. We speculate that the mesolimbic dopamine system, with its projections from ventral tegmental area (VTA) to nucleus accumbens (NAc), is critically involved. This system plays important roles in both reinstatement of cocaine seeking after extinction (Shalev et al., 2002; Kalivas and McFarland, 2003; Schmidt et al., 2005) and incubation of cocaine craving (Wolf, 2016; Dong et al., 2017). Many studies reported that amphetamine-induced striatal and accumbal dopamine release is increased during estrus in free-cycling females (Becker and Ramirez, 1981; Becker and Cha, 1989; Castner et al., 1993) and by estradiol treatment in ovariectomized rats (Becker, 1990; Thompson and Moss, 1994; Becker and Rudick, 1999; Cummings et al., 2014; Song et al., 2019). In contrast, progesterone has an opposite effect by inhibiting striatal dopamine release in estradiol-primed ovariectomized females (Dluzen and Ramirez, 1984; Becker and Rudick, 1999). Calipari et al. (2017) also reported that dopamine activity in the VTA-to-NAc projection is higher during estrus than nonestrus.
We propose that in females estradiol and progesterone exert opposite effects on dopamine neurotransmission in the VTA-to-NAc projection, leading to increased cocaine craving and relapse by estradiol and decreased cocaine craving and relapse by progesterone (Fig. 5). Other neurobiological mechanisms of reinstatement and incubation of cocaine craving, including mesocorticolimbic glutamate transmission (Cornish and Kalivas, 2000; McFarland and Kalivas, 2001; Kalivas et al., 2009; Wolf, 2016; Dong et al., 2017), may also contribute to the sex differences described above. As discussed elsewhere, there are sex differences in brain glutamate systems and drug-induced neuroadaptations in glutamate transmission that may play a role in this regard (Giacometti and Barker, 2020). To date, no evidence has been provided on the role of estradiol and progesterone in relapse-related behavior in males.
Fig. 5.
A proposed brain mechanism model of the role of estradiol and progesterone in cocaine relapse in rodent models. There is evidence that estradiol potentiates striatal and NAc dopamine release by modulating GABAergic neurotransmission of medium spiny neurons (MSNs) through collaterals synapsing on dopamine neurons (Krentzel and Meitzen, 2018; Yoest et al., 2018). This effect is likely mediated by membrane estrogen receptors (ERs) (membrane-associated ERα and membrane-associated ERβ) expressed in MSNs (Almey et al., 2012). ERα and ERβ antagonists prevent estradiol enhancement of amphetamine-induced dopamine release (Xiao et al., 2003), and overexpression of ERα in the striatum increases the effect of estradiol on K+ induced GABA release (Schultz et al., 2009). Additionally, ERα and ERβ are expressed on VTA dopamine neurons terminals in NAc (Yoest et al., 2018), which play a critical role in reinstatement of cocaine seeking (Shaham et al., 2003; Schmidt et al., 2005). Together, these results suggest a role of ERα and ERβ in estradiol regulation of dopamine neurotransmission and, by implication, in reinstatement of cocaine seeking. Additionally, increased dopamine release by estradiol would be at least in part due to its action on dopamine receptors: striatal dopamine receptor 2 (Drd2) affinity decreases during estrus (Di Paolo et al., 1988), and estradiol injections in ovariectomized rats decrease Drd2 binding in striatum (Bazzett and Becker, 1994). In contrast to estradiol, progesterone decreases striatal dopamine release, as shown in estradiol-primed ovariectomized females treated with progesterone (Dluzen and Ramirez, 1984; Becker and Rudick, 1999). Progesterone and its metabolites are positive allosteric modulators of GABAA receptors (Schumacher et al., 1989; Schumacher and McEwen, 1989; Lambert et al., 1995). Drugs that promote GABAA function (e.g., imidazenil, diazepam) decrease cocaine-induced increases in dopamine release in NAc shell (Giorgetti et al., 1998). Consequently, progesterone could protect against estradiol-induced increases in cocaine seeking by inhibiting NAc dopamine release via increased GABAA receptor transmission. Together, we propose that during the estrus/follicular phase, cocaine- or cocaine cue-induced NAc dopamine release is increased by estradiol through its action on ER in GABAergic medium spiny striatal neurons and dopamine neurons terminals, leading to disinhibition of dopamine neurons and directly enhanced VTA dopamine cell firing via decreased Drd2 signaling, resulting in increased cocaine reinstatement/relapse. In contrast, during nonestrus/luteal phase, high progesterone levels may inhibit dopamine release induced by drug or drug cues through its action on GABAA receptors expressed in VTA dopamine terminals (Brodnik et al., 2019; Lopes et al., 2019), resulting in decreased cocaine relapse. DA, dopamine.
A. Human Imaging Studies on Sex Differences in Response to Cocaine Cues and Stress
During the last several decades, numerous studies have used positron emission tomography (PET) (Volkow et al., 1991) and functional magnetic resonance imaging (fMRI) (London et al., 1999) to identify brain regions activated (or inhibited) during craving induced by exposure to drug injections (Stein et al., 1998), drug cues (Grant et al., 1996), and stress (Sinha et al., 2005). However, with rare exceptions, these studies were either performed only in male participants or included both sexes but were not designed (lacked power) to assess sex differences. Indeed, in our systematic literature search, we only identified six studies that statistically evaluated sex differences in brain activity during cue- or stress-induced cocaine craving (Supplemental Table S5). We describe these studies below. In the Supplemental Material we also describe results from several studies wherein investigators separately analyzed imaging data within each sex. The data from these studies are inconclusive and difficult to interpret without knowing whether the interactions between sex and cue or stress conditions are significant (see Nieuwenhuis et al., 2011).
In the studies described below, cue-induced craving was provoked by either presentation of videos or pictures showing cocaine-associated cues or by standardized or personalized scripts (Kilts et al., 2004; Volkow et al., 2011; Zhang et al., 2020). Stress-induced craving was induced by standardized or personalized scripts (Sinha et al., 1999; Li et al., 2005). Brain activity changes induced by cocaine or stress cues were compared with those induced by neutral cues. Participants in these studies were either abstinent and underwent inpatient or outpatient treatment (Kilts et al., 2004; Li et al., 2005; Zhang et al., 2020) or were non–treatment-seeking active cocaine users (Volkow et al., 2011).
Kilts et al. (2004) used PET with [15O]H2O to measure regional cerebral blood flow (an index of brain activity) after cue exposure during early abstinence (days 1–14) in eight women. They compared the women to five men from their previous study (Kilts et al., 2001) and three new men. There were no sex differences in cue-induced cocaine craving. Exposure to cocaine cues induced stronger activation (increased glucose utilization) in right amygdala, left insula, right postcentral gyrus, and left caudate nucleus in men. In contrast, women showed increased activation of right precentral gyrus, middle frontal gyrus, and posterior cingulate gyrus. These results should be interpreted with caution because of the low number of participants and the use of male data from a previous study.
Volkow et al. (2011) used PET with 2-deoxy-2[18F]fluoro-d-glucose to measure brain glucose metabolism in response to cocaine cues in 10 women and 16 men. There were no sex differences in cue-induced cocaine craving. In contrast, sex differences were detected in cue-induced whole-brain metabolism (glucose utilization) with significantly decreased activity in women and modest increased activity in men. Additionally, analyses of specific brain areas showed decreased glucose utilization in frontal, cingulate, and parietal cortices, thalamus, and midbrain in women. In contrast, men showed increased glucose utilization in right inferior frontal gyrus. Direct comparisons of men and women showed sex by cue (cocaine cue, neutral cue) interaction due to greater decrease in glucose utilization among women in frontal (broca areas 8, 9, 10), anterior cingulate, posterior cingulate, inferior parietal, and dorsomedial thalamus.
The reasons for the different pattern of results in the two aforementioned studies, cue-induced brain activation in women in Kilts et al. (2004) versus cue-induced inhibition in Volkow et al. (2011), are unknown. One potential reason is that participants in the first study (Kilts et al., 2004) were treatment seekers tested during abstinence, whereas those in the second study (Volkow et al., 2011) were non–treatment-seeking tested during active cocaine use.
Kober et al. (2016) investigated fMRI-based changes in brain activity in response to videos depicting cocaine use, gambling, or sad scenarios in participants with cocaine dependence or pathologic gambling. There were no sex differences in cocaine craving or urges between men (n = 18) and women (n = 12) with cocaine-dependence. However, men showed greater dorsomedial prefrontal cortex and superior frontal gyrus activation in response to the cocaine videos.
Joseph et al. (2019) used fMRI to investigate the effects of oxytocin on cocaine craving and cocaine cue-induced activity in right amygdala and dorsomedial prefrontal cortex (PFC) among cocaine-dependent participants with (24 men, 16 women) or without (19 men, 8 women) childhood trauma history. Independent of the trauma condition, there were no sex differences in cue-induced cocaine craving, and in both sexes, oxytocin had no effect on this measure. Independent of the trauma condition, there were no sex differences in the placebo groups for cue-induced activation of dorsomedial PFC, a finding different from that of Kober et al. (2016), and oxytocin decreased this activation in all groups in a sex-independent manner. A different pattern of results was observed for cue-induced activation of right amygdala, wherein sex differences (activation in men but not women) were observed in the trauma but not nontrauma participants. Additionally, in the trauma groups, oxytocin increased cue-induced right amygdala activity in women but decreased this activity in men. In contrast, oxytocin had no effect on cue-induced right amygdala activity in men and women without trauma. The small sample size of women with prior trauma limits the interpretation of the sex-specific effects of oxytocin on amygdala activity in this study.
Zhang et al. (2020) used fMRI to investigate periaqueductal gray (PAG) activity and connectivity between PAG and ventromedial prefrontal cortex (vmPFC) after craving induced by cue exposure during early abstinence (7–10 days) in 10 women and 42 men. The PAG is known for its role in pain, avoidance, and defensive behaviors (Basbaum and Fields, 1984; Graeff, 1994). There were no sex differences in cue-induced cocaine craving, cue-induced PAG activity, or cue-induced increased PAG-vmPFC connectivity. However, PAG-vmPFC connectivity strength was positively correlated with cue-induced craving in men but negatively correlated in women. These results suggest sex differences in the role of PAG-vmPFC connectivity in cue-induced cocaine craving. However, these results should be interpreted with caution because of the low number of women participants and other significant sex differences in the sample (age and depression score).
Li et al. (2005) investigated brain activation using fMRI during stress imagery in 10 women and 17 males who were abstinent for 2–3 weeks. There were no sex differences in stress-induced cocaine craving. Additionally, there were no sex differences in the observed negative correlations between stress-induced craving and activation of the anterior and posterior cingulate. However, sex differences (higher activation in women) were observed for stress-induced activation of left frontolimbic areas, including anterior cingulate, insula, dorsolateral and medial PFC, inferior frontal cortices, and posterior cingulate cortex.
B. Conclusions
The results of the studies reviewed demonstrate strong sex differences in the effect of cue- and stress-induced cocaine craving manipulations on brain activity, with both quantitative (different degrees of activation) and, unexpectedly, qualitative (opposite effects) differences (Fig. 6). These differential brain responses occurred despite the consistent lack of sex differences in cue- or stress-induced subjective craving. Thus, a tentative conclusion from these studies is that to the degree that correlational results from imaging studies reflect causes rather than consequences of drug craving, the brain circuits controlling cocaine craving in men and women are likely different. The studies reviewed also suggest that in both sexes the brain mechanisms of cue-induced versus stress-induced cocaine craving are largely dissociable. This conclusion is consistent with results from preclinical studies on differences in brain circuits of cue- versus stress-induced reinstatement of drug seeking (Shalev et al., 2002; Bossert et al., 2013; Reiner et al., 2019). Another observation from the studies reviewed is that cue-induced brain activation versus inhibition is dependent on the substance use disorder phase (active use vs. abstinence) (Kilts et al., 2004; Volkow et al., 2011).
Fig. 6.
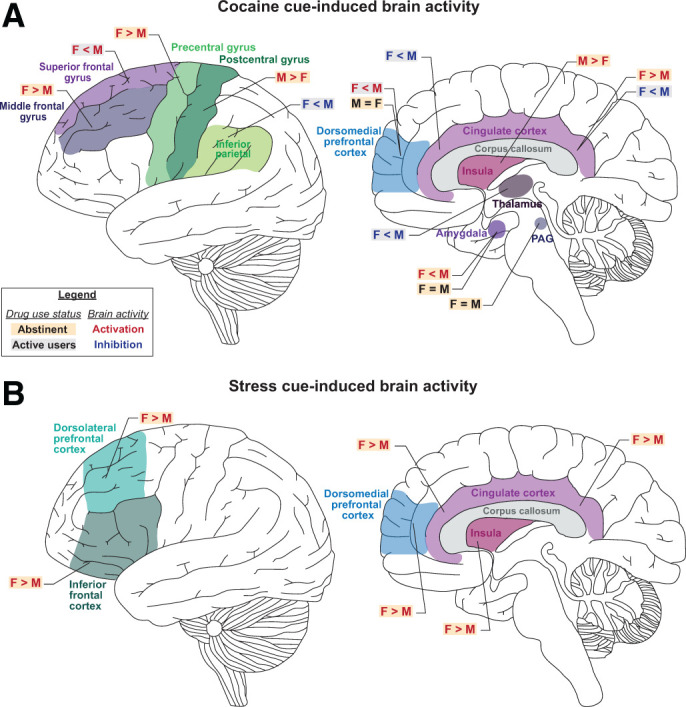
Sex differences in cue- and stress-induced brain activation in humans who use cocaine. Schematic illustration of sex differences in (A) cocaine cue-induced and (B) stress-induced brain activation (assessed by PET or fMRI). F, female; M, male.
A limitation of the studies reviewed, particularly in reference to an important clinical outcome—relapse after prolonged abstinence—is that they were performed during early abstinence or ongoing cocaine use. Thus, the pattern of brain activation in both sexes in response to drug cues and stress during protracted abstinence is unknown. Finally, for the fMRI studies, a common problem in the studies reviewed was small sample sizes that are well below the recommended number of human participants required for reproducible task-based fMRI studies (Turner et al., 2018). Thus, future research should replicate these studies with larger sample sizes.
Finally, the implications of the sex differences in brain activity during cue- and stress-induced cocaine craving to the development of new medications are unknown, specifically in regard to the general conclusion of lack of sex differences in response to opioid agonist therapy. However, these sex differences in brain activity during cue- and stress-induced cocaine craving could be potentially used as brain markers to predict relapse in men and women.
VI. Conclusion and Clinical Implications
A prevailing notion in the preclinical addiction field is that females are more vulnerable than males to drug self-administration, withdrawal, and relapse across drug classes (Fattore et al., 2008; Becker, 2016; Carroll and Lynch, 2016). Here, we critically reviewed this notion with respect to psychostimulant and opioid craving and relapse in humans and rodent models. Unexpectedly, as our own research was guided by this notion (Zlebnik et al., 2014, 2021; Nicolas et al., 2019), our review does not support ubiquitous female susceptibility to craving and relapse.
Our main conclusion of the clinical literature is that the published studies do not support the idea that women are more vulnerable to psychostimulant and opioid craving and relapse. However, this conclusion is tentative because many of the studies reviewed were either correlational or not sufficiently powered to detect sex differences.
Our main conclusion of the preclinical literature is that there are sex differences in stress-induced reinstatement of cocaine seeking and incubation of cocaine craving, which in the latter case are modulated in part by ovarian hormones. In contrast, there is minimal evidence for sex differences for either cue- or cocaine-induced reinstatement of cocaine seeking. Additionally, the studies with methamphetamine and heroin (and other opioid drugs) do not support the notion of sex differences in reinstatement of drug seeking or incubation of craving for these drugs. However, for reinstatement of methamphetamine and heroin seeking, our conclusion is tentative because only a few studies were published, and the results are mixed.
The reasons for the drug-specific evidence of sex differences in rodent models are unknown. Potential reasons could be the distinct neurobiological mechanisms of relapse between opioids versus psychostimulants and cocaine versus methamphetamine (Badiani et al., 2011; Bossert et al., 2013) and the differential interaction of these drugs with ovarian hormones or organizational sex effects.
Another main conclusion is that fluctuations in ovarian hormones appear to play a role in cocaine craving in humans as well as cocaine-induced reinstatement and incubation of craving in rat models. The hypothesis that emerges from these studies is that progesterone decreases drug craving and relapse, whereas estradiol has an opposite effect (see Fig. 5 for a proposed brain mechanism for these effects).
An issue to consider from a translational perspective is the apparent lack of concordance between preclinical and clinical studies with cocaine, with some evidence supporting sex differences in rat models but not in humans. This discrepancy is not surprising because of the complex social, legal, cultural, and language-related factors that contribute to substance use disorders in humans that cannot be modeled in rodents (Heilig et al., 2016; de Wit et al., 2018; Venniro et al., 2020). However, a close inspection of the human results suggests some similarities, opportunities for future research, and potential treatment implications. Specifically, in some studies women report greater cocaine craving than men during early abstinence (Elman et al., 2001; Waldrop et al., 2010; Moran-Santa Maria et al., 2014). Additionally, several laboratory studies report decreased cocaine craving during the luteal versus follicular phase (Sofuoglu et al., 1999; Evans et al., 2002). Thus, abstinence attempts during the luteal phase may be more successful than those during the follicular phase. Indeed, similar approaches have led to favorable cessation outcomes in tobacco smokers (Allen et al., 2008, 2009a,b). Additionally, treatment with exogenous progesterone reduced cocaine craving (Sofuoglu et al., 2002, 2004; Evans and Foltin, 2006; Fox et al., 2013; Milivojevic et al., 2016). Together, these results suggest that in both humans and rodent models, the menstrual/estrous cycle contributes to cocaine seeking and highlights progesterone as a potential adjunct pharmacotherapy to reduce cocaine relapse.
The influence of gonadal hormones on drug craving is an example of how different mechanisms may differentially promote susceptibility to relapse in males and females. Additionally, as discussed above, despite the apparent lack of sex differences in self-reported craving and rates of relapse in humans, neuroimaging studies identified sex differences in regional brain activation associated with cue- and stress-induced cocaine craving (Fig. 6) (Kilts et al., 2004; Li et al., 2005; Volkow et al., 2011; Potenza et al., 2012; Zhang et al., 2020). These results suggest sex differences in brain mechanisms of cocaine (and potentially other drugs) craving and possibly relapse. This conclusion is supported by preclinical studies suggesting sex-specific mechanisms of cocaine-seeking behaviors (Calipari et al., 2017; Doncheck et al., 2020).
Variation in the underlying neurobiology of craving and relapse between men and women may result in differential treatment effects. For example, women experienced a greater reduction in stress- and cue-mediated cocaine craving than men after administration of the α2-adrenergic agonist guanfacine (Fox et al., 2014). Sex differences in “treatment” effects were also demonstrated for drug-seeking behavior in rodent models (Anker et al., 2009; Anker and Carroll, 2010b; Holtz et al., 2012; Zhou et al., 2012; Swalve et al., 2016; Witt and Reissner, 2020). For example, in a recent study Bossert et al. (2020) reported that female rats are less sensitive than males to the effect of the μ opioid receptor partial agonist TRV130 on context-induced reinstatement and reacquisition of oxycodone self-administration.
Finally, our review focused on degree and prevalence of self-reported craving and rates of relapse and found no clear evidence for sex differences. However, the etiology of these phenomena may differ between men and women (Back et al., 2011), and future studies should examine the complex factors that may differentially promote craving and relapse in both sexes.
Abbreviations
- CRF
corticotropin-releasing factor
- Drd2
dopamine receptor 2
- fMRI
functional magnetic resonance imaging
- NAc
nucleus accumbens
- PAG
periaqueductal gray
- PET
positron emission tomography
- PFC
prefrontal cortex
- vmPFC
ventromedial prefrontal cortex
- VTA
ventral tegmental area.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Nicolas, Zlebnik, Farokhnia, Leggio, Ikemoto, Shaham.
Footnotes
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the text of the paper. The write-up of the review was supported by funds to National Institutes of Health National Institute on Drug Abuse (NIDA) Intramural Research Program (to Y.S., L.L., M.F., and S.I.), National Institute on Alcohol Abuse and Alcoholism (NIAAA) Division of Intramural Clinical and Biological Research (to L.L., M.F.), NIDA [Grant K99 DA047419] (to N.E.Z.), and Fondation pour la recherche médicale fellowship [Grant ARF201909009147] (to C.N.).
The authors declare that they do not have any competing interests or conflicts of interest (financial or otherwise) related to the material presented in this manuscript. An earlier version of this paper appears in MedRxiv: https://www.medrxiv.org/content/10.1101/2021.03.30.21254644v1.
C.N. and N.E.Z. contributed equally to this work.
 This article has supplemental material available at pharmrev.aspetjournals.org.
This article has supplemental material available at pharmrev.aspetjournals.org.
References
- Adelson M, Linzy S, Peles E (2018) Characteristics and outcome of male and female methadone maintenance patients: MMT in Tel Aviv and Las Vegas. Subst Use Misuse 53:230–238. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421. [DOI] [PubMed] [Google Scholar]
- Allen AM, Allen SS, Widenmier J, Al’absi M (2009a) Patterns of cortisol and craving by menstrual phase in women attempting to quit smoking. Addict Behav 34:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Lunos S, Hatsukami DK (2009b) Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav 34:928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D (2008) Menstrual phase effects on smoking relapse. Addiction 103:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG (2012) Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153:5373–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2010a) The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev 35:315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2010b) Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend 107:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME (2009) Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 203:63–72. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME (2007) Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol 15:472–480. [DOI] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL (1995) Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav 20:215–224. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H (2005) Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 180:169–176. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W (2011) Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011) Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti-Suroosh A, Towers EB, Lynch WJ (2021) A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology (Berl) 238:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338. [DOI] [PubMed] [Google Scholar]
- Bashiri M, Mancino MJ, Stanick VA, Thostenson J, Kosten TR, Oliveto AH (2017) Moderators of response to sertraline versus placebo among recently abstinent, cocaine dependent patients: a retrospective analysis of two clinical trials. Am J Addict 26:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB (1994) Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res 637:163–172. [DOI] [PubMed] [Google Scholar]
- Bechard AR, Hamor PU, Schwendt M, Knackstedt LA (2018) The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology (Berl) 235:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (1990) Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118:169–171. [DOI] [PubMed] [Google Scholar]
- Becker JB (2016) Sex differences in addiction. Dialogues Clin Neurosci 18:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Cha JH (1989) Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res 35:117–125. [DOI] [PubMed] [Google Scholar]
- Becker JB, Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD (1981) Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res 204:361–372. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN (1999) Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64:53–57. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H (2011) Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A, Leong KC, Berini C, Reichel CM (2017) Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacol Biochem Behav 161:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordnick PS, Schmitz JM (1998) Cocaine craving: an evaluation across treatment phases. J Subst Abuse 10:9–17. [DOI] [PubMed] [Google Scholar]
- Bossert JMKiyatkin EAKorah HHoots JKAfzal APerekopskiy DThomas SFredriksson IBlough BENegus SS, et al. (2020) In a rat model of opioid maintenance, the G-protein-biased MOR agonist TRV130 decreases relapse to oxycodone seeking and taking, and prevents oxycodone-induced brain hypoxia. Biol Psychiatry 88:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229:453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Townsend EA, Altidor L, Fredriksson I, Shekara A, Husbands S, Sulima A, Rice KC, Banks ML, Shaham Y (2021) Sex differences in the effect of chronic delivery of the buprenorphine analog BU08028 on heroin relapse and choice in a rat model of opioid maintenance. Br J Pharmacol accepted pending revisions. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM (1999) Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther 290:1316–1323. [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ (2009) Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry 66:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD (2004) Methamphetamine use behaviors and gender differences. Addict Behav 29:89–106. [DOI] [PubMed] [Google Scholar]
- Brecht ML, von Mayrhauser C, Anglin MD (2000) Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs 32:211–220. [DOI] [PubMed] [Google Scholar]
- Brodnik ZD, Batra A, Oleson EB, España RA (2019) Local GABAA receptor-mediated suppression of dopamine release within the nucleus accumbens. ACS Chem Neurosci 10:1978–1985. [DOI] [PubMed] [Google Scholar]
- Brunswick AF, Messeri PA (1986) Pathways to heroin abstinence: a longitudinal study of urban black youth. Adv Alcohol Subst Abuse 5:111–135. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch AE, Rash CJ, Petry NM (2015) Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 23:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ESJuarez BMorel CWalker DMCahill MERibeiro ERoman-Ortiz CRamakrishnan CDeisseroth KHan MH, et al. (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli DVenniro MZeric TLi XAdhikary SMadangopal RMarchant NJLucantonio FSchoenbaum GBossert JM, et al. (2015) Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry 78:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y (2017) Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci 37:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ (2016) How to study sex differences in addiction using animal models. Addict Biol 21:1007–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB (1993) Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE Jr (1983) Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci 33:19–29. [DOI] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ (2015) Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol 20:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M (2007) Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav 86:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly KL, Wolsh CC, Barr JL, Bauder M, Hausch F, Unterwald EM (2020) Sex differences in the effect of the FKBP5 inhibitor SAFit2 on anxiety and stress-induced reinstatement following cocaine self-administration. Neurobiol Stress 13:100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A (2007) A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology (Berl) 194:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, Travlos G (2015) Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 43:776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett CM, Dunn E, Loweth JA (2021) Effects of sex and estrous cycle on the time-course of incubation of cue-induced craving following extended-access cocaine aelf-administration. eNeuro 8:ENEURO.0054-21.2021 10.1523/ENEURO.0054-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordie R, McFadden LM (2019) Optogenetic inhibition of the medial prefrontal cortex reduces methamphetamine-primed reinstatement in male and female rats. Behav Pharmacol 30:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB (2014) Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend 135:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silveira DX, Doering-Silveira E, Niel M, Jorge MR (2006) Predicting craving among cocaine users. Addict Behav 31:2292–2297. [DOI] [PubMed] [Google Scholar]
- Daiwile AP, Jayanthi S, Ladenheim B, McCoy MT, Brannock C, Schroeder J, Cadet JL (2019) Sex differences in escalated methamphetamine self-administration and altered gene expression associated with incubation of methamphetamine seeking. Int J Neuropsychopharmacol 22:710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Marel C, Slade T, Ross J, Mills KL, Teesson M (2015) Patterns and correlates of sustained heroin abstinence: findings from the 11-year follow-up of the Australian Treatment Outcome Study. J Stud Alcohol Drugs 76:909–915. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Mills KL, Williamson A, Havard A, Teesson M (2007) Patterns of sustained heroin abstinence amongst long-term, dependent heroin users: 36 months findings from the Australian Treatment Outcome Study (ATOS). Addict Behav 32:1897–1906. [DOI] [PubMed] [Google Scholar]
- de Wit H, Epstein DH, Preston KL (2018) Does human language limit translatability of clinical and preclinical addiction research? Neuropsychopharmacology 43:1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T, Falardeau P, Morissette M (1988) Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci 43:665–672. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD (1984) Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology 39:149–155. [DOI] [PubMed] [Google Scholar]
- Doncheck EMLiddiard GTKonrath CDLiu XYu LUrbanik LAHerbst MRDeBaker MCRaddatz NVan Newenhizen EC, et al. (2020) Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology 45:1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck EM, Urbanik LA, DeBaker MC, Barron LM, Liddiard GT, Tuscher JJ, Frick KM, Hillard CJ, Mantsch JR (2018) 17β-Estradiol potentiates the reinstatement of cocaine seeking in female rats: role of the prelimbic prefrontal cortex and cannabinoid type-1 receptors. Neuropsychopharmacology 43:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Taylor JR, Wolf ME, Shaham Y (2017) Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci 37:10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR (2001) Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse 27:193–202. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR, Chabris CF, Breiter HC (2002) Cocaine-primed craving and its relationship to depressive symptomatology in individuals with cocaine dependence. J Psychopharmacol 16:163–167. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL (2009) Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J (2001) Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress 4:289–303. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW (2006) Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31:659–674. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW (1999) Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology 21:445–454. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159:397–406. [DOI] [PubMed] [Google Scholar]
- Everett NA, Baracz SJ, Cornish JL (2020) The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology 45:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett NA, Turner AJ, Costa PA, Baracz SJ, Cornish JL (2021) The vagus nerve mediates the suppressing effects of peripherally administered oxytocin on methamphetamine self-administration and seeking in rats. Neuropsychopharmacology 46:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W (2008) Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond) 4:51–65. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE (2009) Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology 34:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE (2011) Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 216:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2007) Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46:672–687. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R (2006) Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 185:348–357. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Paliwal P, Morgan PT, Sinha R (2008) Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology (Berl) 195:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R (2014) Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 39:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R (2013) The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology 38:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson I, Applebey SV, Minier-Toribio A, Shekara A, Bossert JM, Shaham Y (2020) Effect of the dopamine stabilizer (-)-OSU6162 on potentiated incubation of opioid craving after electric barrier-induced voluntary abstinence. Neuropsychopharmacology 45:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson I, Venniro M, Reiner DJ, Chow JJ, Bossert JM, Shaham Y (2021) Animal models of drug relapse and craving after voluntary abstinence: a review. Pharmacol Rev 73:1050–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 179:662–672. [DOI] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Hafeez H, Price ME, Baruffaldi F, Pravetoni M, Cheng K, Rice KC, Manvich DF, Schank JR (2020) Sex differences in oral oxycodone self-administration and stress-primed reinstatement in rats. Addict Biol 25:e12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop RJ, Crits-Christoph P, Ten Have TR, Barber JP, Frank A, Griffin ML, Thase ME (2007) Differential transitions between cocaine use and abstinence for men and women. J Consult Clin Psychol 75:95–103. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE; Methamphetamine Treatment Project Corporate Authors (2010) An examination of drug craving over time in abstinent methamphetamine users. Am J Addict 19:510–514. [DOI] [PubMed] [Google Scholar]
- Giacometti LL, Barker JM (2020) Sex differences in the glutamate system: Implications for addiction. Neurosci Biobehav Rev 113:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Javaid JI, Davis JM, Costa E, Guidotti A, Appel SB, Brodie MS (1998) Imidazenil, a positive allosteric GABAA receptor modulator, inhibits the effects of cocaine on locomotor activity and extracellular dopamine in the nucleus accumbens shell without tolerance liability. J Pharmacol Exp Ther 287:58–66. [PubMed] [Google Scholar]
- Goenaga JPowell GLLeyrer-Jackson JMPiña JPhan SPrakapenka AVKoebele SVNamba MDMcClure EABimonte-Nelson HA, et al. (2020) N-acetylcysteine yields sex-specific efficacy for cue-induced reinstatement of nicotine seeking. Addict Biol 25:e12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE, Fitzgerald TT, Vocci FJ (2017) A randomized clinical trial of buprenorphine for prisoners: Findings at 12-months post-release. Drug Alcohol Depend 172:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG (1994) Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res 27:811–829. [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126. [DOI] [PubMed] [Google Scholar]
- Hammerslag LR, Denehy ED, Carper B, Nolen TL, Prendergast MA, Bardo MT (2021) Effects of the glucocorticoid receptor antagonist PT150 on stress-induced fentanyl seeking in male and female rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JXie YTao JSu HWu WZou SZhang JZhang JZhang HYang X, et al. (2013) Gender differences in socio-demographic and clinical characteristics of methamphetamine inpatients in a Chinese population. Drug Alcohol Depend 130:94–100. [DOI] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, Shaham Y (2016) Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci 17:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Jeter KE, Cousins SJ, Abdelmaksoud R, Crèvecoeur-MacPhail D (2016) Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. J Addict Dis 35:305–314. [DOI] [PubMed] [Google Scholar]
- Hernandez JS, Binette AN, Rahman T, Tarantino JD, Moorman DE (2020) Chemogenetic inactivation of orbitofrontal cortex decreases cue-induced reinstatement of ethanol and sucrose seeking in male and female Wistar rats. Alcohol Clin Exp Res 44:1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA; Methamphetamine Treatment Project Corporate Authors (2007) Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction 102 (Suppl 1):84–95. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME (2012) Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend 120:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG (1971) Relapse rates in addiction programs. J Clin Psychol 27:455–456. [DOI] [PubMed] [Google Scholar]
- Ignjatova L, Raleva M (2009) Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy 110:285–289. [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989) Cocaine-induced cocaine craving. Psychopharmacology (Berl) 97:59–64. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, Kutlu MG, Calipari ES (2019) Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology 44:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Andersen SL (2018) Working memory and salivary brain-derived neurotrophic factor as developmental predictors of cocaine seeking in male and female rats. Addict Biol 23:868–879. [DOI] [PubMed] [Google Scholar]
- Joseph JE, McRae-Clark A, Sherman BJ, Baker NL, Moran-Santa Maria M, Brady KT (2019) Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H (2009) Glutamate transmission in addiction. Neuropharmacology 56 (Suppl 1):169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56. [DOI] [PubMed] [Google Scholar]
- Kamal F, Flavin S, Campbell F, Behan C, Fagan J, Smyth R (2007) Factors affecting the outcome of methadone maintenance treatment in opiate dependence. Ir Med J 100:393–397. [PubMed] [Google Scholar]
- Kawa AB, Robinson TE (2019) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology (Berl) 236:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL (2013) Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend 132:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008) Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 198:63–75. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP (2004) The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry 161:233–241. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252. [DOI] [PubMed] [Google Scholar]
- Klein LC, Popke EJ, Grunberg NE (1997) Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Exp Clin Psychopharmacol 5:99–106. [DOI] [PubMed] [Google Scholar]
- Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN (2016) Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology 41:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10:63–66. [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Meitzen J (2018) Biological sex, estradiol and striatal medium spiny neuron physiology: a mini-review. Front Cell Neurosci 12:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA (1995) Neurosteroids and GABAA receptor function. Trends Pharmacol Sci 16:295–303. [DOI] [PubMed] [Google Scholar]
- Lanyon C, Nambiar D, Higgs P, Dietze P, Quinn B (2019) Five-year changes in methamphetamine use, dependence, and remission in a community-recruited cohort. J Addict Med 13:159–165. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME (2005) Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav 82:98–108. [DOI] [PubMed] [Google Scholar]
- Levine AR, Lundahl LH, Ledgerwood DM, Lisieski M, Rhodes GL, Greenwald MK (2015) Gender-specific predictors of retention and opioid abstinence during methadone maintenance treatment. J Subst Abuse Treat 54:37–43. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R (2005) Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 57:487–494. [DOI] [PubMed] [Google Scholar]
- London ED, Bonson KR, Ernst M, Grant S (1999) Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol 13:227–242. [DOI] [PubMed] [Google Scholar]
- Lopes EF, Roberts BM, Siddorn RE, Clements MA, Cragg SJ (2019) Inhibition of nigrostriatal dopamine release by striatal GABAA and GABAB receptors. J Neurosci 39:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 (Suppl 1):214–226. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH (1996) Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 125:346–354. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 148:196–200. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164:121–137. [DOI] [PubMed] [Google Scholar]
- Madangopal R, Tunstall BJ, Komer LE, Weber SJ, Hoots JK, Lennon VA, Bossert JM, Epstein DH, Shaham Y, Hope BT (2019) Discriminative stimuli are sufficient for incubation of cocaine craving. eLife 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehira Y, Chowdhury EI, Reza M, Drahozal R, Gayen TK, Masud I, Afrin S, Takamura N, Azim T (2013) Factors associated with relapse into drug use among male and female attendees of a three-month drug detoxification-rehabilitation programme in Dhaka, Bangladesh: a prospective cohort study. Harm Reduct J 10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SG, Keller PS, Hammerslag LR, Bardo MT (2021) Escalation and reinstatement of fentanyl self-administration in male and female rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41:335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JC, Park K, Lin YA, Bersamira C (2018) Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007-2014. J Subst Abuse Treat 87:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Lynch KG, Pettinati HM, Shepard DS (2003) An examination of potential sex and race effects in a study of continuing care for alcohol- and cocaine-dependent patients. Alcohol Clin Exp Res 27:1321–1323. [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R (2016) Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 65:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradinazar M, Farnia V, Alikhani M, Asadi A, Marzbani B, Najafi F (2020) The effects of anxiety on relapse of patients with opioid use disorders under methadone maintenance treatment: control of the confounding variables. J Subst Use 25:34–39. [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT (2014) Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 231:4157–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Sherman BJ, Brady KT, Baker NL, Hyer JM, Ferland C, McRae-Clark AL (2018) Impact of endogenous progesterone on reactivity to yohimbine and cocaine cues in cocaine-dependent women. Pharmacol Biochem Behav 165:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Kowalczyk WJ, Phillips KA, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, Preston KL (2018) Sex differences in daily life stress and craving in opioid-dependent patients. Am J Drug Alcohol Abuse 44:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete JC, Emil S (1992) Cue-evoked arousal in cocaine users: a study of variance and predictive value. Drug Alcohol Depend 30:187–192. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, McCarthy MM, Shaham Y, Ikemoto S (2019) Incubation of cocaine craving after intermittent access cocaine self-administration: sex differences and estrous cycle. Biol Psychiatry 85:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ (2011) Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14:1105–1107. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R (2008) Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend 93:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ (2014) Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 231:2661–2670. [DOI] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, Fegan KN, Root DH (2020) Oral prescription opioid-seeking behavior in male and female mice. Addict Biol 25:e12828. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH (2018) Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology 43:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH (2009) Cocaine craving and use during daily life. Psychopharmacology (Berl) 207:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S (2012) Influence of sex differences and gonadal hormones on cocaine addiction. ILAR J 53:14–22. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Edward KL, Montgomery L, McEvedy S, Wilson A, Worrall-Carter L (2016) Is there any gender difference for smoking persistence or relapse following diagnosis or hospitalization for coronary heart disease? Evidence from a systematic review and meta-analysis. Nicotine Tob Res 18:1399–1407. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 223:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y (2019) Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology 44:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Lofaro OM, Applebey SV, Korah H, Venniro M, Cifani C, Bossert JM, Shaham Y (2020) Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J Neurosci 40:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53:223–230. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28:533–546. [DOI] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A (2015) Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front Psychiatry 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Babinska Z, Amchova P, Stark T, Drago F, Sulcova A, Micale V (2017) Reactivity to addictive drugs in the methylazoxymethanol (MAM) model of schizophrenia in male and female rats. World J Biol Psychiatry 18:129–142. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2016) 2015 National Survey on Drug Use and Health: Detailed Tables, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC (2005) Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol 526:65–76. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Kosten TR (1998) Prognostic factors in buprenorphine- versus methadone-maintained patients. J Nerv Ment Dis 186:35–43. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB (2009) Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29:1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, McEwen BS (1989) Regulation of high-affinity GABAa receptors in specific brain regions by ovarian hormones. Neuroendocrinology 50:315–320. [DOI] [PubMed] [Google Scholar]
- Schumacher M, McEwen BS (1989) Steroid and barbiturate modulation of the GABAa receptor. Possible mechanisms. Mol Neurobiol 3:275–304. [DOI] [PubMed] [Google Scholar]
- Sedki F, Gardner Gregory J, Luminare A, D’Cunha TM, Shalev U (2015) Food restriction-induced augmentation of heroin seeking in female rats: manipulations of ovarian hormones. Psychopharmacology (Berl) 232:3773–3782. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J (1995) Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 119:334–341. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089. [DOI] [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, Brady KT, Joseph JE, Nunn LM, McRae-Clark A (2020) The effect of oxytocin, gender, and ovarian hormones on stress reactivity in individuals with cocaine use disorder. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG (1975) Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD (1996) Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol 15:455–461. [DOI] [PubMed] [Google Scholar]
- Sinha R (2011) New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 13:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S (1999) Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 142:343–351. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR (2007) Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 190:569–574. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE (2005) Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 183:171–180. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M (2011) Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 218:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Greer A, Dougen B, Carroll ME (2020) Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology (Berl) 237:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC (2012) Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend 121:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, Fagan J, Kernan K (2012) Outcome of heroin-dependent adolescents presenting for opiate substitution treatment. J Subst Abuse Treat 42:35–44. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK (2002) Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 72:431–435. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7:274–283. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR (2004) Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav 78:699–705. [DOI] [PubMed] [Google Scholar]
- Song Z, Yang H, Peckham EM, Becker JB (2019) Estradiol-induced potentiation of dopamine release in dorsal striatum following amphetamine administration requires estradiol receptors and mGlu5. eNeuro 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS (1998) Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155:1009–1015. [DOI] [PubMed] [Google Scholar]
- Sterling RC, Dean J, Weinstein SP, Murphy J, Gottheil E (2004) Gender differences in cue exposure reactivity and 9-month outcome. J Subst Abuse Treat 27:39–44. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH (2002) Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry 51:642–651. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Zlebnik NE, Carroll ME (2016) Sex differences in reinstatement of cocaine-seeking with combination treatments of progesterone and atomoxetine. Pharmacol Biochem Behav 145:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Tseng J, Fannon MJ, Purohit DC, Quach LW, Terranova MJ, Kharidia KM, Oliver RJ, Mandyam CD (2018) Sex differences in context-driven reinstatement of methamphetamine seeking is associated with distinct neuroadaptations in the dentate gyrus. Brain Sci 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TL, Moss RL (1994) Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem 62:1750–1756. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, Baker NL, Brady KT (2010) Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse 36:106–113. [DOI] [PubMed] [Google Scholar]
- Turner BO, Paul EJ, Miller MB, Barbey AK (2018) Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol 1:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez M, Frazier JH, Reichel CM, Peters J (2019) Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learn Mem 27:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y (2020) Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21:625–643. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224:25–52. [DOI] [PubMed] [Google Scholar]
- Venniro M, Panlilio LV, Epstein DH, Shaham Y (2021) The protective effect of operant social reward on cocaine self-administration, choice, and relapse is dependent on delay and effort for the social reward. Neuropsychopharmacology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, Shaham Y (2019) Operant social reward decreases incubation of heroin craving in male and female rats. Biol Psychiatry 86:848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Shaham Y (2020) An operant social self-administration and choice model in rats. Nat Protoc 15:1542–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D (2017) Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 42:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A (1991) Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148:621–626. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C (2011) Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One 6:e16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, Spratt EG, Kreek MJ, Brady KT (2010) Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology 35:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Dearing RL (2006) Gender differences in alcohol and substance use relapse. Clin Psychol Rev 26:128–148. [DOI] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM (2018) Regionally specific effects of oxytocin on reinstatement of cocaine seeking in male and female rats. Int J Neuropsychopharmacol 21:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C (1997) Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 44:35–40. [DOI] [PubMed] [Google Scholar]
- Witt EA, Reissner KJ (2020) The effects of nicotinamide on reinstatement to cocaine seeking in male and female Sprague Dawley rats. Psychopharmacology (Berl) 237:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB (2003) The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology 77:239–245. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB (2018) Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav 104:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L (2007) Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav 86:485–492. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Wang W, Dhingra I, Le TM, Li CR (2020) Cue-elicited functional connectivity of the periaqueductal gray and tonic cocaine craving. Drug Alcohol Depend 216:108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE (2012) Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther 340:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE (2014) Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct 219:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yi Z, Yang X, Wang Z, Lyu X, Li J (2017) Gender differences and correlated factors of heroin use among heroin users. Subst Use Misuse 52:25–32. [DOI] [PubMed] [Google Scholar]
- Zimmer-Höfler D, Dobler-Mikola A (1992) Swiss heroin-addicted females. Career and social adjustment. J Subst Abuse Treat 9:159–170. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Holtz NA, Lepak VC, Saykao AT, Zhang Y, Carroll ME (2021) Age-specific treatment effects of orexin/hypocretin-receptor antagonism on methamphetamine-seeking behavior. Drug Alcohol Depend 224:108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME (2014) Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology (Berl) 231:3787–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]