Abstract
Background:
Acute alcohol withdrawal results in severe physiological disruption, including potentially lethal central nervous system (CNS) hyperexcitability. Although benzodiazepines successfully mitigate the symptoms of acute alcohol withdrawal, this treatment does not significantly reduce recidivism rates in post-dependent individuals. Instead, persistent affective disturbances that often emerge weeks to months after initial detoxification appear to play a significant role in relapse risk; however, it remains unclear whether genetic predispositions contribute to their emergence, severity, and/or duration. Interestingly, significant genotypic and phenotypic differences have been observed among distinct C57BL/6 (B6) substrains, and in particular, C57BL/6J (B6J) mice have been found to reliably exhibit higher voluntary ethanol intake and ethanol preference compared to several C57BL/6N (B6N) derived substrains. To date, however, B6 substrains have not been directly compared on measures of acute withdrawal severity or affective-behavioral disruption during extended abstinence.
Methods:
Male and female B6J and B6NJ mice were exposed to either a 7-day chronic-intermittent ethanol vapor (CIE) protocol or to ordinary room air in inhalation chambers. Subsequently, blood ethanol concentrations and handling-induced convulsions (HICs) were evaluated during acute withdrawal, and mice were then tested weekly for affective behavior on the sucrose preference test, light-dark box test, and forced swim test throughout 4 weeks of (forced) abstinence.
Results:
Despite documented differences in voluntary ethanol intake between these substrains, we found little evidence for substrain differences in either acute withdrawal or long-term abstinence between B6J and B6NJ mice.
Conclusions:
In B6J and B6NJ mice, both the acute and long-term sequelae of ethanol withdrawal are dependent on largely non-overlapping gene networks relative to those underlying voluntary ethanol drinking.
Keywords: CIE, affective behavior, alcohol abstinence, B6J, B6NJ
1. Introduction
Alcohol Use Disorder (AUD) is a complex, chronic-relapsing disorder characterized by clinically-defined symptoms including the presence of both metabolic and functional tolerance, uncontrollable compulsions to seek and consume alcohol, and the emergence of a negative affective state (e.g., irritability, anxiety, depression) that defines a withdrawal syndrome when consumption is terminated (American Psychological Association, 2013; Mason, 2017). While the acute physiological symptoms of alcohol withdrawal can be effectively managed with benzodiazepines in both rodents (Heilig, Egli, Crabbe, & Becker, 2010) and humans (Mayo-Smith, 1997), such treatments do not significantly reduce the likelihood of eventual relapse (Malcolm et al., 2002; Askgaard et al., 2016). Instead, longer-term affective-behavioral disruptions, which can arise weeks to months after initial detoxification and often persist across even prolonged periods of abstinence, are likely the key drivers behind relapse (Malcolm, 2003; Heilig et al., 2010).
There appears to be a substantial genetic contribution for development of AUD, as twin studies suggest that 50-60% of individual differences in risk are heritable (Enoch & Goldman, 2001). Accordingly, there have been many investigations of the underlying genetic bases for various AUD-related phenotypes in experimental animals, including both initial preference for ethanol in the two-bottle free-choice protocol and acute withdrawal severity. In mice, the most frequently studied index of acute withdrawal severity is the handling-induced convulsion (HIC) test, which serves as a behavioral index for central nervous system (CNS) hyperexcitability and effectively models specific aspects of the acute alcohol withdrawal syndrome often observed in alcohol-dependent individuals (Goldstein & Pal, 1971; Goldstein, 1972; Becker & Hale, 1993; Becker, Diaz-Granados, & Weathersby, 1997). Several studies have revealed a consistent negative genetic correlation between initial ethanol preference and withdrawal-induced HIC severity among inbred mouse strains, in that higher-preferring strains typically display relatively low sensitivity to acute ethanol withdrawal (Metten et al., 1998; Hitzemann et al., 2009). In contrast, however, selectively-bred Withdrawal Seizure-Prone (WSP) and Withdrawal Seizure-Resistant (WSR) mice, bred for high and low HIC scores during acute ethanol withdrawal, respectively (Crabbe, Kosobud, & Young, 1983; Crabbe, Kosobud, Young, Tam, & McSwigan, 1985), show little or no difference in two-bottle free-choice ethanol intake (Ford et al., 2011; Crabbe et al., 2013; Rosenwasser, Fixaris, Crabbe, Brooks, & Ascheid, 2013). Similarly, a recent study from our laboratory found little difference between WSP and WSR mice in anxiety- and/or depressive-like behavior during forced abstinence, providing evidence that acute and long-term consequences of ethanol withdrawal appear to be dependent on largely non-overlapping gene networks (Hartmann, Holbrook, Haney, Crabbe, & Rosenwasser, 2019). Nevertheless, there has been relatively little inquiry into the underlying genetic predispositions for the emergence and/or severity of affective-behavioral disruptions during extended periods of abstinence.
Since laboratory rodents rarely achieve appreciable blood ethanol concentrations (BECs) through voluntary consumption, various methods have been developed to experimentally induce ethanol dependence in rodents (Lovinger & Crabbe, 2005). In particular, inhalation of volatilized ethanol vapor exposure is capable of rapid and effective dependence induction, which is typically validated in mice through observation of heightened HIC scores during acute withdrawal (Goldstein & Pal, 1971; Goldstein, 1972; Becker & Hale, 1993; Becker et al., 1997). Studies of ethanol vapor inhalation have also provided useful insights into the complexities of behavioral disruptions during long-term (forced) ethanol abstinence, as increases in anxiety- and/or depressive-like behavior in rats (Valdez et al., 2002; Zhao, Weiss, & Zorrilla, 2007; Walker et al., 2010) and mice (Logan, McCulley, Seggio, & Rosenwasser, 2012; Sidhu, Kreifeldt, & Contet, 2018; Hartmann et al., 2019) have been observed weeks after termination of ethanol vapor exposure.
Interestingly, however, anxiety- and depressive-like behaviors in mice may follow distinct temporal trajectories during prolonged abstinence. Across diverse ethanol-administration protocols and behavioral test procedures, mice usually display significant anxiety-like behavior during the first 72 hours of abstinence, a phase referred to as “acute withdrawal” (Finn, Gallaher, & Crabbe, 2000; Kliethermes, Cronise, & Crabbe, 2004; Kash, Baucum, Conrad, Colbran, & Winder, 2009; Pleil et al., 2015; Perez & De Biasi, 2015; Rose et al., 2016; Gong et al., 2017); however, several negative findings have also been reported (Cox et al., 2013; Lee, Coehlo, McGregor, Waltermire, & Szumlinski, 2015; Daut et al., 2015; Metten et al., 2018). At four days to three weeks into abstinence, generally referred to as “early abstinence (Heilig et al., 2010), anxiety-like behavior becomes markedly less prominent in mice (Fukushiro et al., 2012; Pang, Renoir, Du, Lawrence, & Hannan, 2013; Lee et al., 2015; Holleran et al., 2016; Becker, Kieffer, & Le Merrer, 2017). Furthermore, and in contrast to studies in rats (Valdez et al., 2002; Zhao et al., 2007; Gillett, Harshberger, & Valdez, 2013), the presence of anxiety-like behavior does not appear to reemerge in mice during “protracted abstinence” (abstinence extending to greater than four weeks) (Lee, Coehlo, Solton, & Szumlinski, 2017; Becker et al., 2017).
Unlike with studies of anxiety-like behavior, inconsistent findings regarding depressive-like behavior during acute withdrawal have been reported, and results appear to be significantly dependent on the method of ethanol administration, and to some extent, the behavioral assay utilized. In mice, forced methods of ethanol administration (Karadayian, Busso, Feleder, & Cutrera, 2013; Arora & Vohora, 2016; Metten et al., 2018), as opposed to long-term voluntary consumption paradigms (Stevenson et al., 2009; Lee et al., 2015; Lee et al., 2016; Holleran et al., 2016; Lee et al., 2017), seem to more reliably produce depressive-like behavior during acute withdrawal. Nonetheless, there is abundant evidence for the presentation of depressive-like behavior in mice during early abstinence, across various methods of ethanol administration and behavioral assays (Stevenson et al., 2009; Pang et al., 2013; Holleran et al., 2016; Roni & Rahman, 2017; Kim et al., 2017; Gong et al., 2017). Currently, there is a relative dearth of studies analyzing mouse depressive-like behavior during protracted abstinence, and the few existing studies (Logan et al., 2012; Lee et al., 2017; Hartmann et al., 2019) have revealed mixed results.
Consistent with biomedical research in general, the majority of alcohol research in mice has utilized C57BL/6J (B6J) mice. B6J mice were selected for the original Mouse Genome Sequencing Consortium (Waterston et al., 2002; Church et al., 2009) and are favored among alcohol researchers due to their high levels of voluntary ethanol preference relative to other inbred strains (Belknap, Crabbe, & Young, 1993; Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008). Although B6J mice originated at The Jackson Laboratory (JAX), JAX sent a number of these mice to the National Institutes of Health (NIH) in 1951, and the NIH colony subsequently became recognized as C57BL/6N (B6N) mice, formally recognizing B6J and B6N as distinct substrains (Morse, 1978; Bryant, 2011). Further, over time, separate colonies of B6N have been established at several other breeding sites, including Charles River Laboratories in 1974 (C57BL/6NCrl; B6NCrl), Taconic Biosciences in 1991 (C57BL/6NTac; B6NTac), and JAX in 2005 (C57BL/6NJ; B6NJ). As a result of genetic drift (Kumar et al., 2013), significant genotypic and phenotypic differences have emerged among the various B6 substrains (Bryant et al., 2008; Mekada et al., 2009; Matsuo et al., 2010; Keane et al., 2011; Simon et al., 2013; Eisfeld, Gasper, Suresh, & Kawaoka, 2018; Åhlgren & Voikar, 2019). In particular, B6J mice have been found to display consistently higher voluntary ethanol intake and preference compared to several of the B6N derived substrains (Ramachandra, Phuc, Franco, & Gonzales, 2007; Mulligan et al., 2008; McCulley, Hartmann, Holbrook, Kumar, & Rosenwasser, 2016). On the other hand, with the exception of a single study examining potential differences in expression of the “alcohol deprivation effect” (ADE) (Khisti, Wolstenholme, Shelton, & Miles, 2006), B6J and B6N substrains have not been directly compared on other ethanol-related phenotypes. Such studies would be highly valuable and could help elucidate the complex phenotypic and genetic architecture linking multiple ethanol-related traits.
The present experiment was designed to compare the responses of B6J and B6NJ mice during distinct phases of ethanol withdrawal and abstinence. Male and female B6J and B6NJ mice were exposed to either to a 7-day chronic intermittent ethanol vapor (CIE) protocol or to ordinary room air in custom inhalation chambers. BECs and HIC scores were evaluated during acute withdrawal, and mice were then tested weekly on well-established assays of affective behavior (sucrose preference test, SPT; light-dark box test, LDT; forced swim test, FST) over the course of four post-treatment (post-Tx) weeks. Since previous research has shown that higher-preferring inbred strains typically display relatively low sensitivity to acute ethanol withdrawal (Metten et al., 1998; Hitzemann et al., 2009), we hypothesized that B6NJ mice would display higher HIC scores during acute withdrawal relative to B6J mice. While little is known concerning genetic (or physiological) linkages between acute withdrawal and long-term abstinence, we tentatively hypothesized that B6NJ mice would show increased affective disruption during abstinence relative to B6J.
2. Methods
2.1. Animals
Male and female C57BL/6J (B6J; M, n = 20; F, n = 20) and C57BL/6NJ (B6NJ; M, n = 18; F, n = 20) mice were shipped to the University of Maine from The Jackson Laboratory (Bar Harbor, ME). Mice arrived in the laboratory at approximately 6 weeks of age and were immediately group-housed, 5 per cage by substrain, sex, and assigned treatment (ethanol vapor vs. air-control; see below), in large mouse cages (32 x 20 x 14 cm). Animals were housed under a LD 12:12 lighting regimen (lights off at 1700) with food (Prolab RMH 3000; LabDiet, St. Louis, MO) and tap water available ad libitum throughout the experiment. All experimental procedures were approved by the University of Maine Institutional Animal Care and Use Committee (IACUC).
2.2. Procedures
Animals were group-housed in large mouse cages (32 x 20 x 14 cm) and kept within larger Plexiglas inhalation chambers (60 x 36 x 60 cm) constructed according to a design provided by Dr. Howard Becker (Medical University of South Carolina). During an initial 2-week acclimation period, the system fan circulated ordinary room air to all chambers, after which animals in the experimental group were exposed to a 7-day chronic-intermittent ethanol vapor exposure (CIE) protocol (see below), while air-exposed controls were handled identically but exposed only to air. Following CIE or air treatment, animals were single-housed in standard mouse cages (30 x 18 x 12 cm) and placed 3 per shelf within a light-shielded and sound-attenuating metal cabinet equipped with a standard 15W fluorescent bulb on each shelf. Immediately after termination of the final ethanol vapor exposure, blood ethanol concentrations (BECs) were measured in CIE-exposed animals, and five hours after termination of the final ethanol vapor exposure, all animals were administered the Handling-Induced Convulsions (HIC) Test (see below). Subsequently, animals underwent weekly behavioral testing (see below) during a 4-week period of (forced) abstinence, with all behavioral tests beginning at the onset of the dark phase. For each test week, the order of behavioral tests was as follows: (1) sucrose preference test (SPT), (2) light-dark box test (LDT), and (3) forced swim test (FST). 24 hours separated the SPT and LDT, 72 hours separated the LDT and FST, and 48 hours separated the final test of one week and the initial test of the following week. This order was intended to minimize the effects of repeated administration, with larger gaps between potentially more invasive behavioral tests. Experimental and control groups consisted of 9-10 animals per group for each sex/substrain; n for each group are available in Table 1.
Table 1.
Mean (±SEM) body weight (BW) in male (M) and female (F) J and NJ mice immediately prior to and following CIE or air-control (CON) treatment and percent change in body weight during treatment. CIE, chronic-intermittent ethanol; J, C57BL/6J; NJ, C57BL/6NJ.
| B6 Substrain | Sex | Treatment | n | Pre-Tx BW (g) | Post-Tx BW (g) | % BW change |
|---|---|---|---|---|---|---|
| J | F | CON | 10 | 18.3 ± 0.45 | 19.1 ± 0.36 | +4.13 ± 0.89 |
| J | F | CIE | 10 | 19.1 ± 0.57 | 19.3 ± 0.55 | +1.02 ± 0.98 |
| J | M | CON | 10 | 24.2 ± 0.30 | 24.7 ± 0.35 | +2.15 ± 0.70 |
| J | M | CIE | 10 | 25.3 ± 0.25 | 25.3 ± 0.30 | +0.25 ± 0.78 |
| NJ | F | CON | 10 | 18.7 ± 0.32 | 19.8 ± 0.33 | +6.21 ± 1.60 |
| NJ | F | CIE | 10 | 18.6 ± 0.32 | 18.8 ± 0.34 | +1.22 ± 1.15 |
| NJ | M | CON | 9 | 23.6 ± 0.57 | 24.3 ± 0.52 | +2.87 ± 1.15 |
| NJ | M | CIE | 9 | 23.5 ± 0.49 | 23.5 ± 0.53 | −0.10 ± 0.89 |
2.2.1. Chronic-Intermittent Ethanol Protocol
A 7-day CIE protocol was employed in which ethanol vapor was delivered to the experimental chambers for 16 hours per day alternating with 8 hours of ordinary room air for 7 consecutive days, with each vapor exposure period beginning at dark onset. Air-exposed controls were handled identically but exposed only to air. Immediately prior to each vapor exposure period, CIE animals were administered a priming injection containing 1.6 g/kg ethanol and 68.1 mg/kg pyrazole HCl, an alcohol dehydrogenase inhibitor used to rapidly increase and BEC (Becker & Hale, 1993). Pyrazole was dissolved in 20% v/v ethanol solution and injected i.p. at a volume of 10 mL/kg. Air-exposed control animals were administered an identical dose of pyrazole in 0.9% saline solution, but without ethanol, at the same injection volume. At the conclusion of each exposure period, chamber doors for the ethanol vapor-exposed group were opened for 20 minutes to allow adequate vapor dissipation; chamber doors for the air-exposed control group remained closed to ensure no accidental exposure to ethanol vapor. All animals were weighed prior to and halfway through the 7-day CIE cycle to ensure appropriate injection volumes, and to monitor possible CIE-induced changes in body weight (see below). Ethanol was vaporized using a pressurized pump to filter air through a porous diffusing stone submerged in a 1.0-L bottle of 95% ethanol. A calibrated mixture of ethanol vapor and plain room air was continuously delivered during the daily 16-hour ethanol exposure period. Ordinary air was always provided at a flow rate between 10-12 L/min throughout the experiment in order to ensure adequate airflow to meet the animals’ respiratory requirements. Because prior studies had shown that metabolic tolerance to ethanol develops across multiple-day CIE protocols (Metten & Crabbe, 2005; Metten, Sorensen, Cameron, Yu, & Crabbe, 2010), we adopted methodologies from Metten et al. (2010) and Hartmann et al. (2019) and gradually increased the flow of ethanol vapor from 1.5 to 2.2 L/min across the 7 days of CIE in an effort to produce stable blood ethanol levels. Unlike Metten et al. (2010), however, we were only able to measure BECs at the end of the protocol, so we possess no objective measure of the success of this maneuver. Based on previous work (Metten & Crabbe, 2005; Metten et al., 2010), and considering the parameters employed here, we expected ethanol-exposed mice to reach pharmacologically relevant BECs of at least 130 mg/dL. Chamber ethanol concentrations were measured on a daily basis by extracting 5 mL air samples from the exposure chambers through a rubber stopper using a 60-mL syringe, mixing the sample with 55 mL of ambient air, and injecting the diluted sample into a breathalyzer (Lifeloc FC-10; Wheat Ridge, CO). Breathalyzer readings were then compared to a standardized calibration curve to determine chamber ethanol concentrations.
2.2.2. Measurement of Ethanol Concentrations in Tail Blood
BECs were measured in CIE-exposed animals immediately following the final treatment period. A small (approximately 20 μL) blood sample was collected from the tip of the tail of each mouse and centrifuged for 10 minutes at 1000 x g to separate plasma from whole blood. BECs were determined from 5 μL plasma samples using an AM-1 alcohol analyzer (Analox Instruments; Lunenburg, MA). Air-control animals also underwent the tail snip procedure to account for potential induced stress (though their blood samples were not analyzed).
2.2.3. Body Weights
Body weights (g) were obtained in CIE and air-exposed control animals at the beginning of the CIE protocol, on Day 4 of the CIE, and at the termination of the final CIE cycle. The effects of CIE on body weight were evaluated by computing percent body weight change from the beginning to the end of the CIE protocol.
2.2.4. Handling-Induced Convulsions Test
Animals were gently picked up by the tail, briefly held in place, and then rotated slowly along a 360° arc. Convulsive signs were rated by experimenters blind to treatment and group on a predefined scale (0-7) (Crabbe, Merrill, & Belknap, 1991; Metten & Crabbe, 2005), depending on the severity of the response along with the extent of the handling manipulation required to elicit the behavioral response. HIC scores typically correlate with other measures of ethanol withdrawal (Kosobud & Crabbe, 1986; Metten et al., 1998), and are known to be sensitive to both dose and duration of chronic ethanol exposure (Becker & Hale, 1993; Becker et al., 1997; Metten & Crabbe, 2005; Metten et al., 2010). All animals were administered the HIC test five hours following termination of the final ethanol vapor exposure since the highest HIC scores for post-dependent B6J mice typically occur at this time point (Metten & Crabbe, 2005).
2.2.5. Sucrose Preference Test
Animals were offered two-bottle, free-choice access to a 0.75% sucrose solution and plain water for 24 hours. This concentration of sucrose solution was employed in order to avoid any possibility of observing a “ceiling effect.” Based on previous observations in our laboratory, sucrose solutions as low as 1.0% have at times produced such an effect, whereas 0.75% has consistently produced 70-85% preference over plain water. Pre- and post-measurements of bottle weight (g) were used to obtain overall intake. Sucrose preference was determined by dividing the volume of sucrose solution consumed by total fluid intake. Decreases in sucrose preference are generally interpreted as “anhedonic behavior” (Katz, 1982), an inability to derive pleasure from normally pleasurable stimuli, which is one of the defining symptoms when diagnosing Major Depression Disorder (APA, 2013).
2.2.6. Light-Dark Box Test
Animals were placed in a two-compartment test chamber in which one compartment (27 x 17 x 27 cm) is kept darkened while the other (27 x 27 x 27 cm) is illuminated via an overhead lamp (550-650 lux). The compartments are separated by a divider with a small central opening (6 x 6 cm) through which the mouse can easily shuttle between the two compartments. Animals were initially placed in the dark compartment and were permitted to freely-move about the apparatus for 6 minutes. Behavior was video-recorded, and the following parameters were extracted: (1) percentage of time spent in the light compartment, (2) latency to first entry to the light compartment, and (3) the total number of transitions between compartments for the entire 6-minute period. Less time spent in, and longer latencies to initially enter, the light compartment are interpreted as anxiety-like behavior based in part on extensive pharmacological evidence that anxiolytic drugs increase the percentage of time an animal spends in the light compartment (Bourin & Hascoët, 2003). The number of transitions between compartments is used as an assay of general locomotor activity in the test environment and is typically reduced by anxiogenic treatments including ethanol withdrawal (Kliethermes, 2005).
2.2.7. Forced Swim Test
Animals were placed in a clean, glass cylinder (20 cm diameter, 30 cm tall) filled with tap water (maintained at a temperature of 23-25°C) to a depth of 15 cm. Animals were permitted to freely move about for 6 minutes, while dim red light (<5 lux) from an overhead lamp provided sufficient illumination for video recording. The percentage of time spent actively swimming (as opposed to passively floating) was determined via ANY-maze software (Stoelting Co., Wood Dale, IL). Since animals almost universally display little immobility during the initial 2 minutes upon being placed in water, it is convention to exclusively analyze the final 4 minutes of the total 6-minute period (Petit-Demouliere, Chenu, & Bourin, 2005; Can, Dao, Arad, Terrillion, Piantadosi, & Gould, 2012). After each session, animals were removed from the water, lightly dried via paper towel, and individually kept in a standard cage positioned under a separate red-light lamp (<5 lux) for 10 minutes (to ensure adequate drying) before returning to their respective home cage. Water in the cylinder was emptied and replaced with fresh water after each session. This test is primarily thought to reflect the balance between active and passive coping strategies in response to an acute stressor (Can et al., 2012), with swimming interpreted as active coping and floating interpreted as passive coping (i.e., depressive-like behavior). However, some posit that increased immobility (i.e. floating) can be interpreted as an adaptive response and should not necessarily be interpreted as “depressive-like behavior” since it misrepresents the utility and limitations of the assay (de Kloet & Molendijk, 2016; Commons, Cholanians, Babb, & Ehlinger, 2017). Nevertheless, there is an abundance of pharmacological evidence that antidepressant administration increases the percentage of time spent actively swimming (Porsolt, Bertin, & Jalfre, 1977; Porsolt, Bertin, & Jalfre, 1978; Porsolt, Bertin, Blavet, Deniel, & Jalfre, 1979; Petit-Demouliere et al., 2005).
2.2.8. Statistics
Initial body weight, percent body weight change, and HIC test data were analyzed using 3-factor (treatment, substrain, sex) analysis of variance (ANOVA), while BECs were analyzed in ethanol-exposed animals using 2-factor (substrain, sex) ANOVA. Behavioral data were initially analyzed using 4-factor (treatment, substrain, sex, post-Tx day) mixed-design ANOVA, followed where appropriate by separate 3- or 2-factor ANOVAs and subsequent Bonferroni-corrected post-hoc tests. Specifically, when significant 3-factor interactions involving sex were detected, the initial analysis was followed by either 1) separate 2-factor ANOVAs (treatment, substrain) in males and females for each individual post-Tx day, or 2) separate 3-factor ANOVAs (treatment, substrain, post-Tx day) for males and females. When significant main effects of post-Tx day were detected, Bonferroni-corrected t-tests were used to make all possible pairwise comparisons among test days. When significant interactions involving post-Tx day were seen, these were followed-up by conducting separate ANOVAs for each individual test day including any relevant factors (treatment, substrain, and/or sex). Full ANOVA results (F, df, p, partial η2) are provided for the initial 4-factor analysis, but only p-values are indicated for follow-up tests. Data are presented as means ± SEM and effects were considered to be statistically significant when p < .05. Data analysis was performed using SPSS 25.0 (IBM Inc., Armonk, NY) and figures were generated using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Body Weights, BECs, and HIC Test
Prior to treatment, males weighed significantly more than females (F1,70 = 339.84, p < .001, partial η2 = .829) and B6J mice weighed significantly more than B6NJ (F1,101 = 22.40, p = .046, partial η2 = .056), but there were no significant differences between assigned treatment groups (Table 1). Expressed as percent change from pre-Tx weight, ethanol-exposed mice gained significantly less weight than air-exposed controls (F1,70 = 18.88, p < .001, partial η2 = .212) and males gained significantly less weight than females (F1,70 = 6.16, p = .015, partial η2 = .081) over the course of treatment. All groups showed mean BECs well above the threshold for intoxication, and while there were no significant effects of sex or substrain, females showed somewhat higher BECs than males (Table 2). Lastly, ethanol-exposed mice exhibited significantly higher HIC scores than air-exposed controls (F1,70 = 14.40, p < .001, partial η2 = .171) five hours after termination of the final treatment period (Fig. 1), but there were no significant effects of substrain or sex.
Table 2.
Mean (±SEM) BECs for ethanol-exposed male and female J and NJ mice immediately following the 7-day CIE protocol. BEC, blood ethanol concentrations; other abbreviations as in Table 1.
| B6 Substrain | Sex | Treatment | n | BEC (mg/dL) |
|---|---|---|---|---|
| J | F | CIE | 10 | 155.7 ± 6.24 |
| J | M | CIE | 10 | 150.0 ± 8.74 |
| NJ | F | CIE | 10 | 155.7 ± 11.81 |
| NJ | M | CIE | 9 | 142.1 ± 8.67 |
Figure 1.
Mean (±SEM) handling-induced convulsions (HICs) scores six hours following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in C57BL/6J (J) and C57BL/6NJ (NJ) mice.
3.2. Sucrose Preference Test
ANOVA revealed significant main effects of treatment (F1,70 = 7.72, p = .007, partial η2 = .099) and post-Tx day (F3,210 = 4.52, p = .004, partial η2 = .061), as well as significant treatment x post-Tx day (F3,210 = 7.86, p < .001, partial η2 = .101) and substrain x treatment x sex (F1,70 = 4.41, p = .039, partial η2 = .059) interactions (Fig. 2). Ethanol-exposed mice exhibited significantly lower sucrose preference than air-exposed controls overall, and post-hoc analysis showed that this effect was significant on post-Tx days 8, 15, and 22 (ps < .05), but not post-Tx day 1. Pairwise comparisons among post-Tx days showed that sucrose preference was significantly lower on post-Tx day 15 than on post-Tx days 8 and 22 (ps < .05), but not post-Tx day 1. To explore the interaction involving sex and substrain, separate substrain x treatment ANOVAs were performed in males and females for each individual test day (Fig. 2). In females, a main effect of treatment was present on post-Tx days 1, 8, and 22 (ps < .05); however, ethanol-exposed mice displayed significantly higher sucrose preference on post-Tx day 1, but significantly lower sucrose preference on post-Tx days 8 and 22. In males, sucrose preference was significantly higher in B6J mice relative to B6NJ on post-Tx day 15 (p = .003) and significantly lower in ethanol-exposed mice relative to air-exposed controls on post-Tx day 22 (p = .018).
Figure 2.
Mean (±SEM) sucrose preference following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in all animals (top), female only (middle), and male only (bottom) C57BL/6J (J) and C57BL/6NJ (NJ) mice.
3.3. Light-Dark Box Test
Percentage of time in light:
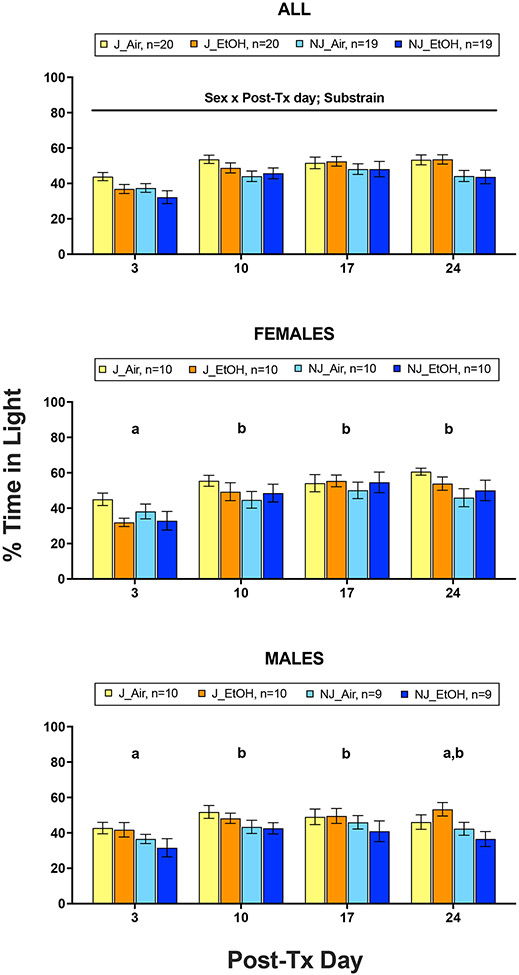
ANOVA revealed significant main effects of substrain (F1,70 = 8.97, p = .004, partial η2 = .114) and post-Tx day (F3,210 = 23.03, p < .001, partial η2 = .248), as well as a significant post-Tx day x sex interaction (F3,210 = 3.18, p = .025, partial η2 = .043), but no significant effects involving treatment (Fig. 3). B6J mice spent a significantly greater percentage of time in light than B6NJ. Females displayed a significantly lower percentage of time in light on post-Tx day 3 than all other test days (ps < .001), while males showed a significantly lower percentage of time in light on post-Tx day 3 than post-Tx test days 10 and 17 (ps < .05), but not post-Tx day 24 (Fig. 3).
Figure 3.
Mean (±SEM) percentage of time spent in light following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in all animals (top), female only (middle), and male only (bottom) C57BL/6J (J) and C57BL/6NJ (NJ) mice. Post-Tx days with no shared letters are significantly different (p < .05) from one another.
Latency to enter light:
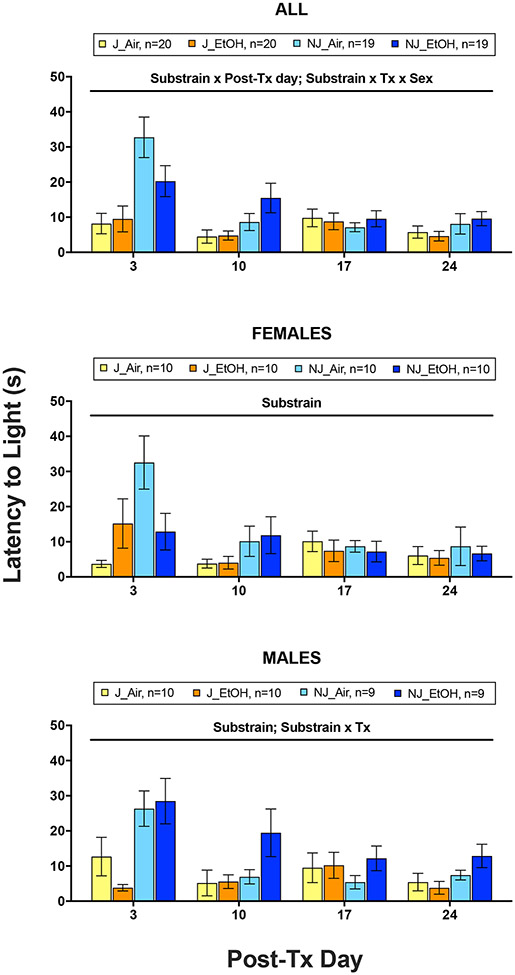
ANOVA revealed significant main effects of substrain (F1,70 = 19.08, p < .001, partial η2 = .214) and post-Tx day (F3,210 = 11.23, p < .001, partial η2 = .138), as well as significant substrain x post-Tx day (F3,210 = 7.32, p < .001, partial η2 = .095) and substrain x treatment x sex (F1,70 = 7.44, p = .008, partial η2 = .096) interactions (Fig. 4). B6NJ mice exhibited significantly longer latencies to enter the light than B6J mice overall, and post-hoc tests showed that this effect was significant on post-Tx days 3 and 10 (ps < .05). Pairwise comparisons among post-Tx days showed that latency to enter light was significantly longer on post-Tx day 3 than all other test days. To investigate the interaction involving sex, separate substrain x treatment ANOVAs were performed in males and females (Fig. 4). While a significant main effect of substrain was present in both sexes (females: p = .024; males: p < .001), a significant substrain x treatment interaction was observed only in males (p = .027). Both male and female B6NJ mice exhibited significantly longer latencies than B6J mice, but, in B6NJ mice, only ethanol-exposed males showed longer latencies relative to air-exposed controls.
Figure 4.
Mean (±SEM) latency of first transition to light following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in all animals (top), female only (middle), and male only (bottom) C57BL/6J (J) and C57BL/6NJ (NJ) mice.
Total transitions:
ANOVA revealed significant main effects of substrain (F1,70 = 106.99, p < .001, partial η2 = .605), sex (F1,70 = 11.76, p = .001, partial η2 = .144), and post-Tx day (F3,210 = 4.57, p = .004, partial η2 = .061), but no treatment effects (Fig. 5). B6J mice and females displayed significantly greater total transitions than B6NJ mice and males, respectively. Pairwise tests among post-Tx days showed that total transitions were significantly lower on post-Tx day 3 than post-Tx day 10, but no other significant differences were present.
Figure 5.
Mean (±SEM) total transitions following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in C57BL/6J (J) and C57BL/6NJ (NJ) mice.
3.4. Forced Swim Test
ANOVA revealed significant main effects of substrain (F1,70 = 8.20, p = .006, partial η2 = .105), treatment (F1,70 = 4.79, p = .032, partial η2 = .064), and post-Tx day (F3,210 = 70.58, p < .001, partial η2 = .502), as well as a significant substrain x treatment x post-Tx day interaction (F3,210 = 3.50, p = .016, partial η2 = .026), but no significant effects involving sex (Fig. 6). Overall, B6J and ethanol-exposed mice displayed significantly higher immobility than B6NJ and air-exposed controls, respectively, while immobility was significantly lower on post-Tx day 6 than all other test days (ps < .001). To examine the interaction involving post-Tx day, separate substrain x treatment ANOVAs were performed for individual test days. Significant main effects of substrain were present on post-Tx days 13 (p = .003) and 27 (p = .015), significant main effects of treatment were seen on post-Tx days 13 (p = .018) and 20 (p = .039), and a significant substrain x treatment interaction was noted on post-Tx day 20 (p = .047). B6J mice displayed significantly higher immobility than B6NJ mice on post-Tx days 13 and 27 (ps < .05). Ethanol-exposed mice displayed significantly higher immobility than air-exposed controls on post-Tx day 13 (p < .05); however, on post-Tx day 20, this effect was only present in B6NJ mice.
Figure 6.
Mean (±SEM) time spent immobile (s) following 7-day chronic-intermittent ethanol (EtOH) and control (Air) treatments in C57BL/6J (J) and C57BL/6NJ (NJ) mice. Asterisk symbol indicates p < .05 for treatment pairwise comparisons.
4. Discussion
As expected, ethanol-exposed mice reached pharmacologically relevant BECs (approximately 150 mg/dL), similar to those seen in previous studies of ethanol vapor exposure (Becker et al. 1997; Metten & Crabbe, 2005). Further, ethanol-exposed mice displayed significantly higher HIC scores than air-exposed controls, thus confirming that the CIE protocol employed here effectively induced sufficient tolerance to produce CNS hyperexcitability upon withdrawal. While several studies have utilized longer ethanol vapor exposure paradigms (Becker et al., 1997; Logan et al., 2012, McCool & Chappell, 2015), even a 3-day CIE treatment is sufficient to elevate HIC scores (Becker & Hale, 1993). Importantly, however, no substrain differences in either BECs or HIC scores were observed, suggesting that B6J and B6NJ mice show similar physiological adaptation to CIE treatment. Nevertheless, it remains possible that substrain differences in HIC scores could emerge with longer CIE exposures and/or at higher BECs.
Overall, this experiment detected significant assay-dependent effects of CIE treatment, substrain, sex, and post-Tx day, but revealed few substrain differences in the expression of abstinence-induced affective disturbances (i.e., substrain x CIE treatment interactions). Therefore, we found little evidence for differences between B6J and B6NJ mice in the affective consequences of long-term forced abstinence. Indeed, the one significant substrain x treatment interaction found in this study was embedded in a 3-way interaction involving sex. Thus, in the LDT, ethanol-exposed B6NJ mice exhibited significantly longer latencies than ethanol-exposed B6J mice, but only in males. Although this is evidence of a substrain difference in abstinence-induced anxiety-like behavior (at least in males), such an interaction was seen only for this single dependent variable of the LDT. Similarly, in our measures of depressive-like behavior, effects of substrain occurred in complex interactions with CIE treatment (FST), post-Tx day (SPT, FST), and sex (SPT). In the SPT, substrain differences (B6J > B6NJ) were seen only in males and were significant only on post-Tx day 15, but the effects of CIE treatment were driven mostly by females. In contrast, the FST data revealed a similar initial emergence of depressive-like behavior (during early abstinence) in ethanol-exposed mice of both substrains, but a suggestive substrain difference in the persistence of depressive-like behavior over time. Ethanol-exposed mice displayed significantly higher immobility than air-exposed controls on post-Tx day 13, but on post-Tx day 20 this effect was only present in B6NJ mice, suggesting that CIE treatment may induce somewhat longer lasting behavioral depression in B6NJ mice than B6J mice. Nevertheless, since this was observed only in the FST, and not the SPT, we are unable to provide definitive conclusions regarding possible substrain differences in abstinence-induced depressive-like behavior. Future work is needed in order to confirm whether this subtle difference in behavioral depression susceptibility is indeed assay specific.
Interestingly, despite significantly elevated BECs, effects of CIE treatment were seen more reliably on assays for depressive-like (SPT, FST), than anxiety-like (LDT), behavior. These findings support two emerging generalizations within the literature: 1) induction of physical dependence, specifically through high dose forced ethanol administration paradigms, does not necessarily result in global affective disruption, and 2) mice, unlike rats, display more consistent manifestation of depressive- versus anxiety-like behaviors during ethanol withdrawal (Holleran & Winder, 2017). While the literature is complex, the emergence of affective disturbances in post-dependent mice has been shown to follow distinct temporal trajectories which depend on several factors, including the specific ethanol-administration protocols and behavioral assays utilized (Kliethermes, 2005; Heilig et al., 2010). Whereas significant effects of post-Tx day were seen throughout all measures of each behavioral assay, in the absence of post-Tx day x CIE treatment interactions, effects of post-Tx day are unlikely to be directly related to ethanol exposure. Therefore, we focus our discussion on significant post-Tx day x treatment interactions.
In the SPT, ethanol-exposed mice showed significantly greater sucrose preference on post-Tx day 1 (albeit only in females), but exhibited lower sucrose preference (i.e., suggestive of anhedonia) on post-Tx days 8 and 22. This reversal in the effects of CIE between post-Tx days 1 and 8 illustrates the complex temporal dynamics of affective processing in post-dependent mice (Heilig et al., 2010; Holleran & Winder, 2017). Similarly, in the FST, ethanol-exposed mice exhibited greater depressive-like behavior (i.e., greater time spent immobile) than air-exposed controls overall, and specifically on post-Tx days 13 and 20. Together, the SPT and FST data suggest that CIE vapor exposure induced a delayed and sustained display of behavioral depression, which is consistent with previous work employing either long-term free-choice drinking or maintenance on an ethanol liquid diet to induce tolerance (Stevenson et al., 2009; Pang et al., 2013; Holleran et al., 2016; Roni & Rahman, 2017; Kim et al., 2017; Gong et al., 2017). Thus, our data provide novel evidence for the ability of CIE vapor exposure to induce delayed emergence and sustained expression of depressive-like behavior during long-term forced abstinence in mice.
In contrast to the assays for depressive-like behavior, however, CIE treatment evoked only minor effects of anxiety-like behavior in the LDT. Of the three dependent variables measured in the LDT, only latency to the first transition to light revealed any effect of CIE treatment. Prior work suggests that anxiety-like behaviors are most often observed during acute withdrawal (Finn et al., 2000; Kliethermes et al., 2004; Kash et al., 2009; Pleil et al., 2015; Perez & De Biasi, 2015; Rose et al., 2016; Gong et al., 2017), but wane during early abstinence and are generally absent during protracted abstinence (Fukushiro et al., 2012; Pang et al., 2013; Lee et al., 2015; Holleran et al., 2016; Lee et al., 2017; Becker et al., 2017). Assays for anxiety-like behavior, such as the LDT, are usually based on the animal’s innate tendency to balance exposure to light (“threatening”) and dark (“safe”) environments, and are widely considered to provide a valid model for risk-assessment behavior (Hascoët, Bourin, & Nic Dhonnchadha, 2001). However, it has been suggested that “non-anxious” behaviors, such as general locomotion, may confound the assessment of anxiety-like behavior in these types of assays. Moreover, some evidence suggests that general locomotion and anxiety-related behaviors are inherently negatively correlated, and thus likely impossible to dissociate (Kliethermes, 2005; Milner & Crabbe, 2008). Therefore, in line with previous research, we found highly correlated effects on percentage of time spent in light and total transitions, a marker of general locomotion (Kliethermes, 2005), while neither variable was affected by CIE exposure.
As aforementioned, we found little evidence that B6J and B6NJ mice differed in their responses to ethanol withdrawal and/or abstinence; however, these two substrains appeared to exhibit differences in anxiety-like behavior independent of ethanol exposure. For example, B6J mice exhibited a significantly higher percentage of time in light and a greater number of transitions than B6NJ mice. These data suggest B6NJ mice may be genetically predisposed to greater baseline levels of anxiety-like behavior than B6J mice, a finding consistent with previous work (Åhlgren & Voikar, 2019).
Several factors that could potentially limit the generalizability of these findings should also be considered. For example, a repeated-measures design was employed in which individual animals were tested for affective behavior at multiple time points, though the different assays were administered in a fixed sequence. While this design allows for the possibility of sequence and/or practice effects (Cryan & Holmes, 2005; Paylor, Spencer, Yuva-Paylor, & Pieke-Dahl, 2006), it also conserves animal resources and typically increases statistical power. Further, previous studies in mice suggest that practice effects for the light-dark box test are essentially absent when administered at weekly intervals (Bouwknecht & Paylor, 2002; Bouwknecht, van der Gugten, Groenink, Olivier, & Paylor, 2004), and that inter-test sequence effects are minimal when separated by intervals as short as one day (Paylor et al., 2006). As in our previous study using a similar testing schedule (Hartmann et al., 2019), there were no obvious trends toward increasing or decreasing levels of anxiety- or depressive-like behavior in the present results, with the possible exception of the SPT. Nonetheless, we acknowledge that different results may have been obtained if independent groups for each post-Tx time point were instead utilized.
Historically, consistent negative correlations have been seen between ethanol preference drinking and HIC withdrawal severity among inbred strains (Metten et al., 1998; Metten et al., 2014). At the extremes of this spectrum, B6J mice exhibit high ethanol preference drinking and low HIC severity, whereas DBA/2J (D2J) mice show low ethanol preference drinking and high HIC severity (Metten et al., 1998; Metten & Crabbe, 2005; Metten et al., 2010). These variables have also been postulated to be predictive of the manifestation of anxiety- and/or depressive-like behavior during forced ethanol abstinence. For instance, 72 hours following termination of a 2-cycle CIE vapor exposure, D2J mice showed greater anxiety-like behavior on the LDT than B6J, compared to their respective air-exposed controls (McCool & Chappell, 2015). Similarly, C3H mice, who display intermediate levels of ethanol preference drinking and HIC withdrawal severity demonstrated more dramatic and sustained hypolocomotion following a 3-cycle CIE exposure than did B6J mice (Logan et al., 2012). Intriguingly, our data demonstrate that similar associations do not appear to manifest between B6J and B6NJ substrains, a finding consistent with previous work from our laboratory involving selectively bred WSP and WSR lines (Hartmann et al., 2019). Despite prior data consistently demonstrating significant differences in ethanol preference among B6 substrains (Blum, Briggs, DeLallo, Elston, & Ochoa, 1982; Ramachandra et al., 2007; Mulligan et al., 2008), B6J and B6NJ mice do not differ in HIC severity, nor, as we have now shown, in abstinence-induced anxiety- or depressive-like behavior.
Perhaps the principal finding of this study, however, is that despite significant effects of both substrain and CIE treatment in various behavioral assays, there was little overall evidence for substrain x CIE treatment interactions. As discussed, such findings in the SPT and FST were embedded within complex interactions among other factors, and a clear substrain x CIE treatment interaction was seen only for one of three relevant dependent variables in the LDT, and only in males. Together, these observations suggest that despite substrain differences in voluntary ethanol intake, no mutations significantly influential for abstinence-induced affective disruption have arisen in either B6J or B6N mice since initial colony separation in 1951.
Acknowledgments:
This research was supported by departmental and institutional sources; no funding was received from public, commercial, or not-for-profit agencies.
Footnotes
Conflicts of interest: none.
5. References
- American Psychiatric Association, DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc. [Google Scholar]
- Åhlgren J, & Voikar V (2019). Experiments done in Black-6 mice: what does it mean. Lab Anim (NY), 48(6), 171–180. [DOI] [PubMed] [Google Scholar]
- Arora S, & Vohora D (2016). Comparative Evaluation of Partial α2 -Adrenoceptor Agonist and Pure α2 -Adrenoceptor Antagonist on the Behavioural Symptoms of Withdrawal after Chronic Alcohol Administration in Mice. Basic Clin Pharmacol Toxicol, 119(2), 202–209. [DOI] [PubMed] [Google Scholar]
- Askgaard G, Hallas J, Fink-Jensen A, Molander AC, Madsen KG, & Pottegård A (2016). Phenobarbital compared to benzodiazepines in alcohol withdrawal treatment: A register-based cohort study of subsequent benzodiazepine use, alcohol recidivism and mortality. Drug Alcohol Depend, 161, 258–264. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, & Weathersby RT (1997). Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol, 14(4), 319–326. [DOI] [PubMed] [Google Scholar]
- Becker HC, & Hale RL (1993). Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res, 17(1), 94–98. [DOI] [PubMed] [Google Scholar]
- Becker JAJ, Kieffer BL, & Le Merrer J (2017). Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addict Biol, 22(5), 1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, & Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl), 112(4), 503–510. [DOI] [PubMed] [Google Scholar]
- Blum K, Briggs AH, DeLallo L, Elston SF, & Ochoa R (1982). Whole brain methionine-enkephalin of ethanol-avoiding and ethanol-preferring c57BL mice. Experientia, 38(12), 1469–1470. [DOI] [PubMed] [Google Scholar]
- Bourin M, & Hascoët M (2003). The mouse light/dark box test. Eur J Pharmacol, 463(1-3), 55–65. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, & Paylor R (2002). Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res, 136(2), 489–501. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der Gugten J, Groenink L, Olivier B, & Paylor RE (2004). Effects of repeated testing in two inbred strains on flesinoxan dose-response curves in three mouse models for anxiety. Eur J Pharmacol, 494(1), 35–44. [DOI] [PubMed] [Google Scholar]
- Bryant CD (2011). The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann N Y Acad Sci, 1245, 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, & McRoberts JA (2008). Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. Journal of Neurogenetics, 22(4), 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, & Gould TD (2012). The mouse forced swim test. J Vis Exp, 59, e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DM, Goodstadt L, Hillier LW, Zody MC, Goldstein S, She X, Bult CJ, Agarwala R, Cherry JL, DiCuccio M, Hlavina W, Kapustin Y, Meric P, Maglott D, Birtle Z, Marques AC, Graves T, Zhou S, Teague B, Potamousis K, Churas C, Place M, Herschleb J, Runnheim R, Forrest D, Amos-Landgraf J, Schwartz DC, Cheng Z, Lindblad-Toh K, Eichler EE, Ponting CP, & Mouse Genome Sequencing Consortium (2009). Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS biology, 7(5), e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, & Ehlinger DG (2017). The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci, 8(5), 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, & Thiele TE (2013). Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcoholism, clinical and experimental research, 37(10), 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, & Young ER (1983). Genetic selection for ethanol withdrawal severity: differences in replicate mouse lines. Life Sci, 33(10), 955–962. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, & McSwigan JD (1985). Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet, 15(6), 521–536. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, & Belknap JK (1991). Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res, 550(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Huang LC, Cameron AJ, Schlumbohm JP, Barkley-Levenson AM, & Metten P (2013). Ethanol drinking in withdrawal seizure-prone and -resistant selected mouse lines. Alcohol, 47(5), 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, & Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov, 4(9), 775–790. [DOI] [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, Camp M, & Holmes A (2015). Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addiction biology, 20(2), 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, & Molendijk ML (2016). Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast, 2016, 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld AJ, Gasper DJ, Suresh M, & Kawaoka Y (2018). C57BL/6J and C57BL/6NJ Mice Are Differentially Susceptible to Inflammation-Associated Disease Caused by Influenza A Virus. Front Microbiol, 9, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, & Goldman D (2001). The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep, 3(2), 144–151. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, & Crabbe JC (2000). Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther, 292(1), 394–405. [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Anacker AM, Crabbe JC, Mark GP, & Finn DA (2011). The influence of selection for ethanol withdrawal severity on traits associated with ethanol self-administration and reinforcement. Alcohol Clin Exp Res, 35(2), 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushiro DF, Saito LP, Mári-Kawamoto E, Aramini TC, Costa JM, Josino FS, Uehara RA, & Frussa-Filho R (2012). Withdrawal from repeated treatment with ethanol induces a protracted decrease in novelty-seeking behavior and enhancement of environmental habituation in mice. Pharmacology, biochemistry, and behavior, 101(1), 132–137. [DOI] [PubMed] [Google Scholar]
- Gillett K, Harshberger E, & Valdez GR (2013). Protracted withdrawal from ethanol and enhanced responsiveness stress: regulation via the dynorphin/kappa opioid receptor system. Alcohol, 47(5), 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB (1972). Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther, 180(2), 203–215. [PubMed] [Google Scholar]
- Goldstein DB, & Pal N (1971). Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science, 172(3980), 288–290. [DOI] [PubMed] [Google Scholar]
- Gong MF, Wen RT, Xu Y, Pan JC, Fei N, Zhou YM, Xu JP, Liang JH, & Zhang HT (2017). Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology, 234(20), 3143–3151. [DOI] [PubMed] [Google Scholar]
- Hartmann MC, Holbrook SE, Haney MM, Crabbe JC, & Rosenwasser AM (2019). Affective Behavior in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant Mice during Long-Term Alcohol Abstinence. Alcohol Clin Exp Res, 43(7), 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoët M, Bourin M, & Nic Dhonnchadha BA (2001). The mouse light-dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry, 25(1), 141–166. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked. Addict Biol, 15(2), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R, Edmunds S, Wu W, Malmanger B, Walter N, Belknap J, Darakjian P, & McWeeney S (2009). Detection of reciprocal quantitative trait loci for acute ethanol withdrawal and ethanol consumption in heterogeneous stock mice. Psychopharmacology, 203(4), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LE, Patel S, & Winder DG (2016). Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology, 41(8), 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, & Winder DG (2017). Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav, 16(1), 8–14. [DOI] [PubMed] [Google Scholar]
- Karadayian AG, Busso MJ, Feleder C, & Cutrera RA (2013). Alterations in affective behavior during the time course of alcohol hangover. Behav Brain Res, 253, 128–138. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, Conrad KL, Colbran RJ, & Winder DG (2009). Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology, 34(11), 2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ (1982). Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav, 16(6), 965–968. [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellåker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assunção JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, & Adams DJ (2011). Mouse genomic variation and its effect on phenotypes and gene regulation. Nature, 477(7364), 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, & Miles MF (2006). Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol, 40(2), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Park SD, Lee RM, Lee BH, Choi SH, Hwang SH, Rhim H, Kim HC, & Nah SY (2017). Gintonin attenuates depressive-like behaviors associated with alcohol withdrawal in mice. Journal of affective disorders, 215, 23–29. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL (2005). Anxiety-like behaviors following chronic ethanol exposure. Neuroscience & Biobehavioral Reviews, 28(8), 837–850. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, & Crabbe JC (2004). Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res, 28(7), 1012–1019. [DOI] [PubMed] [Google Scholar]
- Kosobud A, & Crabbe JC (1986). Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther, 238(1), 170–177. [PubMed] [Google Scholar]
- Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, Vitaterna MH, de Villena FP, Churchill G, Bonci A, & Takahashi JS (2013). C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science (New York, N.Y.), 342(6165), 1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, & Szumlinski KK (2015). Binge alcohol drinking elicits persistent negative affect in mice. Behavioural Brain Research, 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo MA, Solton NR, & Szumlinski KK (2017). Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice. Front Psychol, 8, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, & Szumlinski KK (2016). Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front Cell Neurosci, 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, McCulley WD, Seggio JA, & Rosenwasser AM (2012). Effects of withdrawal from chronic intermittent ethanol vapor on the level and circadian periodicity of running-wheel activity in C57BL/6J and C3H/HeJ mice. Alcohol Clin Exp Res, 36(3), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, & Crabbe JC (2005). Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci, 8(11), 1471–1480. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, & Ballenger JC (2002). The effects of carbamazepine and lorazepam on single versus multiple previous alcohol withdrawals in an outpatient randomized trial. J Gen Intern Med, 17(5), 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm RJ (2003). GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry, 64 Suppl 3, 36–40. [PubMed] [Google Scholar]
- Mason BJ (2017). Emerging pharmacotherapies for alcohol use disorder. Neuropharmacology, 122, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, & Miyakawa T (2010). Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Smith MF (1997). Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA, 278(2), 144–151. [DOI] [PubMed] [Google Scholar]
- McCool BA, & Chappell AM (2015). Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol, 49(2), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley III WD, Hartmann MC, Holbrook SE, Kumar V, Rosenwasser AM (2016, May). Substrain differences in ethanol preference and running wheel activity in C57BL/6J and C57BL/6N mice. Abstract presented at the 18th annual Genes, Brain and Behavior meeting, Bar Harbor, ME. [Google Scholar]
- Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, & Yoshiki A (2009). Genetic differences among C57BL/6 substrains. Experimental animals, 58(2), 141–149. [DOI] [PubMed] [Google Scholar]
- Metten P, & Crabbe JC (2005). Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci, 119(4), 911–925. [DOI] [PubMed] [Google Scholar]
- Metten P, Iancu OD, Spence SE, Walter NA, Oberbeck D, Harrington CA, Colville A, McWeeney S, Phillips TJ, Buck KJ, Crabbe JC, Belknap JK, & Hitzemann RJ (2014). Dual-trait selection for ethanol consumption and withdrawal: genetic and transcriptional network effects. Alcoholism, clinical and experimental research, 38(12), 2915–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, & Belknap JK (1998). High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mammalian genome, 9(12), 983–990. [DOI] [PubMed] [Google Scholar]
- Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, & Crabbe JC (2018). An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice. Alcohol, 68, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, & Crabbe JC (2010). Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol Clin Exp Res, 34(9), 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner LC, & Crabbe JC (2008). Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav, 7(4), 496–505. [DOI] [PubMed] [Google Scholar]
- Morse HC (1978). Origins of Inbred Mice, Morse HC, eds. (Academic Press, NY: ). [Google Scholar]
- Mulligan MK, Ponomarev I, Boehm SL 2nd, Owen JA, Levin PS, Berman AE, Blednov YA, Crabbe JC, Williams RW, Miles MF, & Bergeson SE (2008). Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes, brain, and behavior, 7(6), 677–689. [DOI] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, & Hannan AJ (2013). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci, 37(11), 1803–1810. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, & Pieke-Dahl S (2006). The use of behavioral test batteries, II: effect of test interval. Physiol Behav, 87(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Perez EE, & De Biasi M (2015). Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol, 49(3), 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, & Bourin M (2005). Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl), 177(3), 245–255. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, & Kash TL (2015). Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology, 99, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, & Jalfre M (1979). Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol, 57(2-3), 201–210. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, & Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther, 229(2), 327–336. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, & Jalfre M (1978). “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol, 51(3), 291–294. [DOI] [PubMed] [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, & Gonzales RA (2007). Ethanol Preference Is Inversely Correlated With Ethanol-Induced Dopamine Release in 2 Substrains of C57BL/6 Mice. Alcoholism: Clinical and Experimental Research, 31(10), 1669–1676. [DOI] [PubMed] [Google Scholar]
- Roni MA, & Rahman S (2017). Lobeline attenuates ethanol abstinence-induced depression-like behavior in mice. Alcohol, 61, 63–70. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, & Jones SR (2016). Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. The International Journal of Neuropsychopharmacology, 19(5), pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, & Ascheid S (2013). Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol, 18(3), 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M, & Contet C (2018). Affective Disturbances During Withdrawal from Chronic Intermittent Ethanol Inhalation in C57BL/6J and DBA/2J Male Mice. Alcoholism: Clinical and Experimental Research, 42(7), 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H, Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates H, Gerdin AK, Golini E, Hancock JM, Hans W, Hölter SM, Hough T, Jurdic P, Keane TM, Morgan H, Müller W, Neff F, Nicholson G, Pasche B, Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C, Southwell A, Tocchini-Valentini GP, Vancollie VE, Westerberg H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, de Angelis MH, Herault Y, & Brown SD (2013). A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome biology, 14(7), R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, & Hodge CW (2009). Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology, 34(5), 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, & Koob GF (2002). Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism, clinical and experimental research, 26(10), 1494–1501. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathé AA, & Ehlers CL (2010). Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol, 44(6), 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigó R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, & Lander ES (2002). Initial sequencing and comparative analysis of the mouse genome. Nature, 420(6915), 520–562. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, & Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol, 42(3), 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, & Zorrilla EP (2007). Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res, 31(9), 1505–1515. [DOI] [PubMed] [Google Scholar]