Abstract
The rapid growth in the global energy demand for space cooling requires the development of more efficient environmental chillers for which adsorption-based cooling systems can be utilized. Here, in this contribution, we explore sorbents for chiller use via a pore-engineering concept to construct analogs of the 1-dimensional pore metal−organic framework MOF-74 by using elongated organic linkers and stereochemistry control. The prepared pore-engineered MOFs show remarkable equilibrium adsorption of the selected fluorocarbon refrigerant that is translated to a modeled adsorption-based refrigeration cycle. To probe molecular level interactions at the origin of these unique adsorption properties for this series of Ni-MOFs, we combined in situ synchrotron X-ray powder diffraction, neutron powder diffraction, X-ray absorption spectroscopy, calorimetry, Fourier transform infrared techniques, and molecular simulations. Our results reveal the coordination of fluorine (of CH2F in R134a) to the nickel(II) open metal centers at low pressures for each Ni-MOF analog and provide insight into the pore filling mechanism for the full range of the adsorption isotherms. The newly designed Ni-TPM demonstrates exceptional R134a adsorption uptake compared to its parent microporous Ni-MOF-74 due to larger engineered pore size/volume. The application of this adsorption performance toward established chiller conditions yields a working capacity increase for Ni-TPM of about 400% from that of Ni-MOF-74, which combined with kinetics directly correlates to both a higher coefficient of performance and a higher average cooling capacity generated in a modeled chiller.
INTRODUCTION
The International Energy Agency (IEA) estimated that c.a. 20% of the total electricity used in buildings around the world today is consumed by climate control units. This value is expected to triple by 2050 due to the rapid growth of global energy demand for space cooling.1 Such significant energy consumption is mostly industrially supplied via electricity. Adsorption cooling, a thermally driven alternative to the traditional compression-based refrigeration cycle, has the potential for significantly reducing this electricity demand.2 Sorption-based cooling relies on the cyclic transfer of refrigerant gas from a high energy state to a low energy state by adsorption and desorption to provide the pseudocompression effect that a traditional mechanical compressor would, where the driving force for energy transfer relies on heat rather than electricity.3,4 However, this process has currently shown limited applicability given its lower potential cooling performance due to a lack of highly effective sorbent/refrigerant pairs. For example, existing thermally driven sorption chillers typically use halogen salts (LiBr) or porous materials like silica gels, activated carbons, and zeolites as the sorbents.5,6 These sorbents suffer from either risk of toxicity (LiBr), lack of tunability and functionalization, or poor sorption capacity. The advancement and optimization of these pairs can lead to the enhancement of heat and mass recovery thereby improving the overall performance of such systems.
The performance of a sorption chiller is largely influenced by the refrigerant adsorption capacity of the porous material which directly translates into a more compact and efficient cooling system. Compared to traditional adsorbents, metal−organic frameworks (MOFs) typically possess much higher porosity and tunability which provide great benefits for refrigerant-based adsorption.7–10 Recent achievements have started to highlight the unique adsorption behavior and enhanced loading capacities of MOFs for potential use in adsorption chiller systems.11–21 Strategies to enhance the gas adsorption capacity in MOFs include (i) introduction of unsaturated metal sites,22–24 (ii) postmodification of the inorganic node or organic linker with appropriate functional groups,25,26 or (iii) increase of the MOF porosity via pore-engineering.27,28 This pore-engineering concept garnered recent attention in the MOF community by pioneering contributions from the Yaghi group,8,29 the Long group,30–32 and others.33,34 These works have shown the possibility of controlling and tuning the expansion of pore apertures from micropore sizes to mesopores. More recently, Zheng et al. showed that Ni-MOF-74 could also be engineered in a similar manner to provide larger pore analogs with enhanced adsorption uptakes for target molecules including water and fluorocarbon.35
To this end, we further extend this pore expansion methodology to design analogs of Ni-MOF-74, not only by using dihydroxyterephthalic acid ligands with 1 to 2 and 3 phenylene rings but also by adjusting the relative locations of the hydroxyl- and carboxylate moieties within these ligand analogs. In fact, this has led us to design a novel framework containing 4,4“-dihydroxy-2′,5′-dimethyl-[1,1’:4′,1”-terphenyl]-3,3′’-dicarboxylate-“meta” in combination with a nickel node cluster to form an enlarged pore analog of MOF-74 (tri-phenyl-“meta”, Ni-TPM). Accordingly, using these approaches, three more Ni-MOF-74 analogues were developed for comparison including Ni-BPP (3,3′-dioxido-4,4′-biphenyldicarboxylate, or simply bi-phenyl-“para”), Ni-BPM (biphenyl-“meta”), and Ni-TPP (tri-phenyl-“para”). Herein, we explore how these pore-engineered MOFs interact with a model fluorocarbon refrigerant, R134a (HFC-134a or 1,1,1,2-tetrafluoroethane) on a molecular level providing insights that are important for the design and applicability of such materials in industrial adsorption chiller systems.
Among appealing refrigerants that have already shown applicability with traditional mechanical compression-based chillers, hydrofluoroalkanes possess favorable properties including chemical stability and associated thermodynamic parameters such as boiling point, saturation pressures, enthalpy of evaporation, etc.21,36,37 Upon the basis of its low cost and current widespread industrial use among hydrofluorocarbon (HFCs) refrigerants, R134a was selected as the model fluorocarbon refrigerant. R134a is known to have a very low ozone depletion potential (ODP), but it has a high global warming potential (GWP). We acknowledge that such typical HFCs refrigerants are scheduled to be phased out/down as per the Kigali Amendment to the Montreal Protocol (UNEP, 2016), which requires the reduction of HFCs usage by 80–85% by the late 2040s.38 Although low-GWP hydrofluoroolefins (HFOs) are among the promising candidates proposed as replacements, their current high costs make them practically nonapplicable at this point of time. Here, our intention is to gain an in-depth understanding of the structure-dependence of the host−guest chemistry to further guide the design of sorbents in combination with fluorocarbon refrigerants; thus, the relatively inexpensive and readily available R134a refrigerant was utilized. We also note that since the refrigerant in chiller units is purified without open access to competing sorbates like water or other light gases, equilibrium and kinetic measurements on sorbents using pure R134a would be directly applicable in providing insight into potential performance. Only recently, initial studies on fluorocarbon adsorption in MOFs showed high adsorption capacity, excellent reversibility, and fast kinetics of adsorption/desorption cycles.21,39
Beyond the potential for excellent cooling efficiency with fluorocarbon/MOF working pairs, a fundamental understanding of the adsorption mechanism and sorbate/sorbent interaction is limited. The strategies to obtain such fundamental understanding between the host and guest require information from a combination of in situ/ex situ characterizations and molecular simulations.40–44 We aim to interpret molecular interactions during R134a adsorption on MOFs in addition to exploring these pore-engineered MOFs with fluorocarbon refrigerants as a potential, high-performing, viable option for adsorption cooling application. For the first time, the relationship between R134a and the pore environment of these analogs is explored via not only bulk phase equilibrium isotherms but also molecular level explorations using in-depth analysis based on a combination of molecular simulations, synchrotron X-ray powder diffraction, neutron powder diffraction, X-ray absorption spectroscopy, calorimetry, and infrared techniques. These insights are expected to benefit the design of future, tailored sorbent materials for chiller use.
EXPERIMENTAL SECTION
Synthesis and Experimental Methodology.
On the basis of the two isomers of the (dioxidobiphenyl-dicarboxylate)4− ligand which we refer to as the BPP (biphenyl-“para”) and BPM ligands (biphenyl-“meta”), as shown in Figure 1d, four analogs of Ni-MOF-74 in addition to the parent MOF were synthesized. The para and meta refer to the relative locations of the carboxylic acid group with respect to the phenyl linkages. Similarly, TPP (triphenyl-“para”) and TPM (triphenyl-“meta”) ligands were also synthesized to expand the theoretical linkage lengths. It should be noted that we report the first successful synthesis and corresponding resolved crystal structure of Ni-TPM.
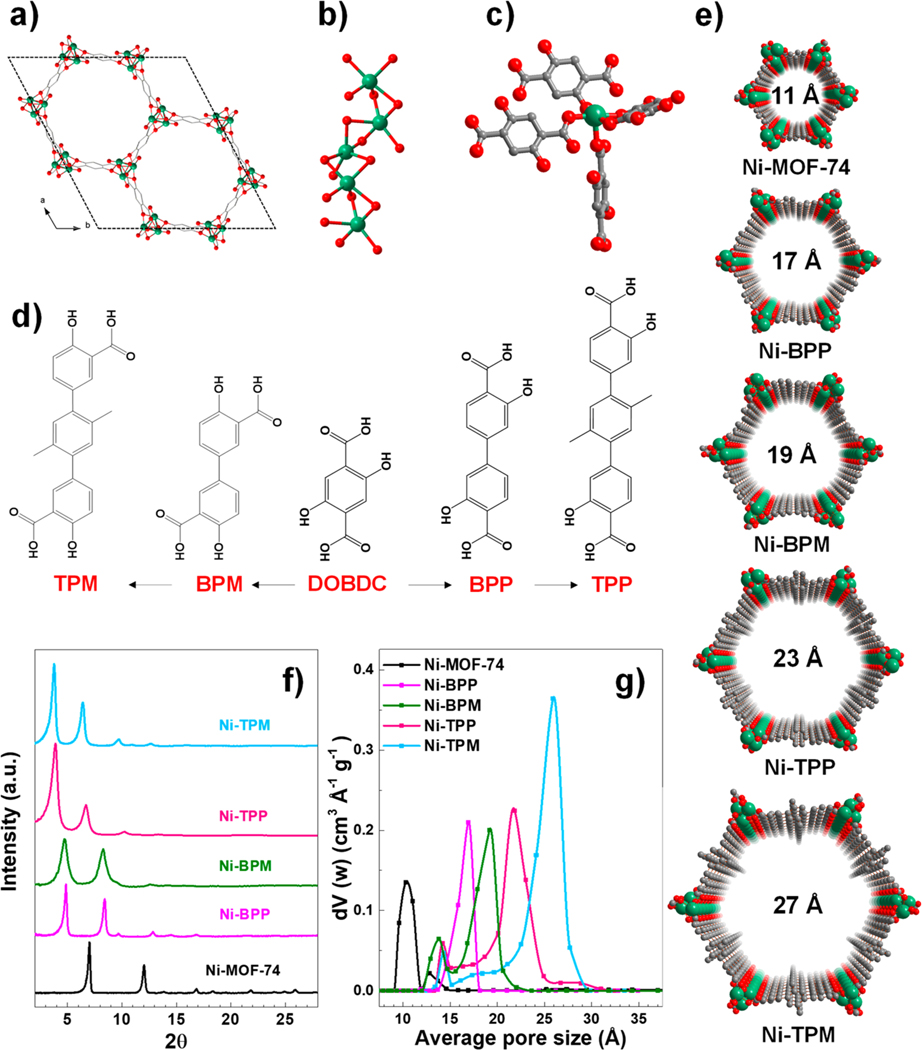
Figure 1.
Structural features of pore-engineered MOFs. (a) Bidimensional view (c axis) of the hexagonal pore of basic Ni-MOF-74. (b) Inorganic Ni2+ nodes. (c) Coordination sphere of Ni2+ nodes. (d) Chemical structures of ligands used for constructing para and meta pore-engineered Ni-MOF-74 series. (e) Crystal structures of the pore-engineered MOFs. Green, red, and gray spheres represent Ni, O, and C atoms, respectively. (f) PXRD spectra and (g) pore size distributions of pore-engineered MOFs.
R134a equilibrium sorption experiments for each MOF were performed using gravimetric analysis.13 To interpret the pore filling mechanism and corroborate the full range of experimental isotherms, Grand canonical Monte Carlo (GCMC) simulations were performed for each MOF-R134a system. The force field parameters and partial charges employed for both guests and Ni-MOFs are fully detailed in the Supporting Information (SI). Complementary Density Functional Theory (DFT) calculations were considered to accurately describe the interactions between R134a and the Ni-open sites present in the investigated Ni-MOFs at the very preliminary stage of adsorption. The host−guest interactions observed from these simulations guided the need for high resolution Synchrotron X-ray powder diffraction (sXPD) data (Beamline 17-BM, Advanced Photon Source (APS)), which was collected using λ = 0.45212 Å for bare structures and λ = 0.45236 Å for gas loaded experiments. Neutron powder diffraction (NPD) data (Beamline BT-1, NIST Center for Neutron Diffraction) was additionally collected to better observe low-Z adsorbed gas molecules using λ = 2.0775 Å. Further, to support the observed coordination of fluorocarbon to MOF, Ni K-edge X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine-structure (EXAFS) spectra were also collected (Beamline 20-BM, APS). Before the synchrotron experiments, the MOF samples were preadsorbed with one R134a molecule on each Ni2+ ion and sealed in thin wall quartz capillaries. Additionally, infrared spectra during the gas adsorption of fluorocarbons with varying functional moieties confirmed the coordination behavior of R134a. Finally, promising adsorption uptakes were translated to cooling performance via working capacity calculations and by modeling a two-bed refrigeration cycle using fitted equilibrium and kinetic data. Complete experimental procedures and simulation parameters are detailed in the SI.
RESULTS AND DISCUSSION
Structural Integrity and Porosimetry Analysis.
The five framework structures showing the accessible Ni2+ nodes and their connections with the oxygen atoms of the carboxylate-containing organic linkers are depicted in Figure 1b,c. The varying pore sizes that can be engineered with this approach range from a diameter of ca. 11 Å, as derived from the native Ni-MOF-74, up to ca. 27 Å with Ni-TPM (Table S1). The structural integrity of these synthesized MOFs was initially characterized using powder X-ray diffraction (PXRD) (Cu Kα, λ = 1.5406 Å) as shown in Figure 1f. The most intense diffraction peaks are indexed based on reflections from the ab-plane of the MOF-74 rhombohedral structure and shift to smaller diffraction angles (2θ°) for the larger, pore-engineered analogues. This shift of the Bragg peaks to lower angles is consistent with the increase in the a lattice parameter, indicative of increasing pore sizes. Further confirmation of the variation in pore sizes were quantified for each analogue using non localized DFT simulations from nitrogen isotherms measured at 77 K (Figure 1g). The observed trend of increasing pore sizes is as follows: Ni-MOF-74 (11 Å) < Ni-BPP (17 Å) < Ni-BPM (19 Å) < Ni-TPP (23 Å) < Ni-TPM (27 Å) as shown in Figure 1e. Brunauer−Emmett−Teller (BET) areas and pore volumes derived from nitrogen isotherms collected at 77 K are shown in Table 1. As expected, the pore volumes for Ni-TPP and Ni-TPM are larger than those of Ni-BPP and Ni-BPM, attributed to the increase in mesoporosity.45 Moreover, the meta-analogs show higher total pore volumes and pore sizes than the para-analogs. The measurement of BET areas, known to be inconsistent for microporous materials, show no particular trend given their strong dependence on the range of points selected. These values fall in the range of 1900 to 2400 m2/g, similar to the previously reported Zn (typically lower BET areas) and Mg (typically higher BET areas) analogs.30,32 The successful synthesis of these frameworks with increasing porosity indicates the capability of engineering targeted pore sizes for specific applications.
Table 1.
Summary of Physicochemical Properties,a Coordination Environment,b and Adsorption Cooling Performance for the Whole Series of Ni-MOFsc
| Sorbent | BET area (m2/g) |
Pore volume (cm3/g) |
Ni−F distance (Å) |
COPce | Cooling Capacity (kW) | ||
|---|---|---|---|---|---|---|---|
| Expt. | Sim. | Expt. | Sim. | PDd | |||
| Ni-MOF-74 | 1150 | 1229.7 | 0.51 | 0.55 | 2.19 | 0.35 (0.22) | 2.52 |
| Ni-BPP | 2040 | 1831.6 | 0.89 | 1.01 | 2.15 | 0.41 (0.24) | 2.66 |
| Ni-BPM | 2340 | 1869.0 | 1.01 | 1.07 | 2.02 | 0.44 (0.24) | 2.91 |
| Ni-TPP | 1980 | 2246.9 | 1.14 | 1.19 | 2.26 | 0.55 (0.20) | 3.01 |
| Ni-TPM | 2420 | 2514.1 | 1.49 | 1.52 | 2.20 (2.29) | 0.56 (0.22) | 3.56 |
Detailed physicochemical properties given in Tables S1 and S10. The error in these measurements between different sample batches is less than 8%.
The coordination environment determined by X-ray absorption spectroscopy data and the molecular interaction distances based on refinements from powder diffraction experiments (details in Tables S2 and S3).
The adsorption cooling performance was examined with a two-bed chiller model (see Figure 5c). The COPc and average cooling capacities were obtained with varying cycle times and a sorbent quantity of 20 kg (see Tables S11 and S12 for detailed parameters used in the numerical model).
The number in parentheses corresponds to the Ni−F distance obtained from X-ray absorption spectroscopy (XAS).
The reported COPc values correspond to a cycle time of 33.33 min while the numbers in parentheses corresponds to a COPc value reported for a cycle time of 5 min (detailed performance summary given in Table S13).
Equilibrium Adsorption of R134a via Experiments and Simulations.
The R134a adsorption isotherms measured at 298 K for each of the five MOF materials are shown in Figure 2a,b. Type I isotherms are evident for Ni-MOF-74, Ni-BPP, and Ni-BPM with saturation uptakes between 0.5 and 0.7 g/g while Ni-TPP and Ni-TPM show a Type IV isotherm. Ni-TPM in particular shows immense uptake at higher pressures with a saturation capacity higher than 1.4 g/g (~300% increase compared to Ni-MOF-74). These experimentally obtained R134a isotherm profiles show a similar trend for nitrogen adsorption as shown in Figure S47. Adsorption kinetic profiles also collected during isotherm measurements between each equilibration point show that for all MOF analogs, R134a uptake quickly reaches steady state concentration within 5 min of exposure (Figures S52−S54). To capture the mechanism of adsorption for the full isotherm range, R134a sorption on pore-engineered MOFs was calculated via GCMC simulations. Importantly, the simulated R134a adsorption isotherms were in good agreement with the corresponding experimental data for the whole series of Ni-MOFs (Figure S47). Moreover, the experimentally observed trend in nitrogen adsorption as well as the free pore volume for each MOF are consistent with those estimated from simulations (Figure S46 and Table S10).
Figure 2.
Comparison of experimentally collected equilibrium adsorption (solid) and desorption (open) R134a isotherms for two different series of pore-engineered MOFs at 298 K: (a) para-series and (b) meta-series. Snapshots from GCMC simulations showing pore filling with fluorocarbon as a function of the pressure in (c) Ni-TPM and (d) Ni-MOF-74. (e) DFT optimized geometry of R134a interaction with Ni-sites for Ni-TPM.
A careful analysis of the adsorption mechanism from pore filling snapshots of Ni-TPM and Ni-TPP obtained from GCMC simulations show that R134a initially sits in the vicinity of the nickel node of the framework (Figure 2 and Figure S50). As pressure increases, the fluorocarbon forms a monolayer in the vicinity of the pore wall interacting with both the inorganic node and the organic linker of the MOF. This is followed by a pore filling at higher pressure. In contrast, while the initial stage of adsorption is similar for the smaller pore frameworks Ni-MOF-74, Ni-BPP, and Ni-BPM, the filling of their porosity occurs at much lower pressures as observed by the snapshot at P = 0.02 bar in Figure 2d and Figure S48. Interestingly, both experimental and simulated data revealed that Ni-TPM achieves the highest R134a saturation uptake of ca. 1.4 g/g even outperforming Ni-TPP. This observation is consistent with Ni-TPM having the highest pore volume (Table S10).
Intermolecular Sorbate/Sorbent Interactions.
While equilibrium isotherms and GCMC simulations characterize the gradual pore filling of fluorocarbon R134a, an accurate assessment of atomic level interactions between fluorocarbon molecules and the MOFs is equally important. As a preliminary step, the analysis of the DFT-optimized geometry reveals that the very initial stage of adsorption involves an interaction between the fluorine of R134a and the Ni-open metal sites present in the MOF surface (Figure 2e and Figure S50). Interestingly, this optimized geometry evidences a favorable fluorine interaction. R134a has two unique fluorine-containing branches, one with CF3 and the other with CH2F (i.e., CF3−CH2F). DFT optimization suggests that the CH2F moiety of R134a rather than the CF3 moiety interacts with the metal node of the MOF. This prediction encouraged us to further probe this fluorine−nickel interaction using advanced characterization tools. Experimental techniques to interpret this sorption mechanism involve direct measurement to probe the sorbate/sorbent interactions. Therefore, X-ray absorption spectroscopy (XANES and EXAFS) was used to explore the structural changes to the Ni2+ nodes in the MOFs upon fluorocarbon adsorption. Due to the similarity in the Ni2+ nodes contained in each MOF independent of linkers, we noticed negligible differences in the Ni2+ first shell coordination environment for all three meta-series MOFs (Figures S9−S11). With specific focus on Ni-TPM, which possesses the highest R134a uptake, the XANES spectra before activation, after activation, and after R134a uptake are shown in Figure 3a. For all three curves, the position of the peak at ~8349 eV and a pre-edge feature at 8332−8333 eV, correspond to the 1s-4p and 1s-3d electronic transitions, respectively, evident for Ni2+ after different treatments.46 The decrease in the peak intensity upon activation and slight increase following R134a loading is consistent with the removal of terminal H2O and binding of the fluorocarbon to the Ni2+ sites. It should also be noted that the intensity of the pre-edge peak increases after activation (inset, Figure 3a). This suggests an increased distortion of the Ni2+ structure, i.e., from 6-coordinated pseudo-octahedral (NiO6) to 5-coordinated square-pyramidal (NiO5) structure due to the removal of one H2O molecule (Figure S5).47 With the loading of R134a, a small decrease in the pre-edge feature is observed, although this intensity is still higher than that of the fresh sample. This might be due to a longer/weaker Ni−F bond than the Ni−O (H2O) of the fresh sample representing a distorted 6-coordinated octahedral (NiO5F) structure.
Figure 3.
(a) Normalized Ni−K edge XANES (inset shows the pre-edge feature) and (b) k2-weighted Ni−K edge EXAFS (insets show the respective x(k) plots) spectra of Ni-TPM before activation (i.e., fresh), after activation (i.e., activated), and after R134a uptake (i.e., adsorbed). (c) Enthalpy of adsorption as a function of R134a uptake at low loadings in the meta-pore-engineered MOFs as measured by calorimetric techniques. (d) In situ FTIR spectra at 5 mbar, 298 K of R134a adsorbed in the meta-pore-engineered MOFs. IR spectrum of free R134a is shown as a reference.
Figure 3b and Figure S33 show the EXAFS spectra for Ni-TPM under the same treatment condition as the XANES spectra in Figure 3a. A decrease of the amplitude of the peak at ~1.5 Å (phase uncorrected) assigned to the Ni−O scattering after heating the sample in vacuum indicates a lower number of first shell coordination (Ni−O). Upon exposure to R134a, this feature slightly increases and it is accompanied by an additional peak at ~1.95 Å, suggesting again that the R134a is adsorbed via a longer Ni−F bond length than Ni−O. The R134a uptake also affects the Ni−Ni scattering signal at ~2.6 Å, similar to the observation previously reported for N2 adsorption in Ni-MOF-74.48 Yet another peak alteration is observed at the scattering feature at ~3.4 Å. As with the other peaks, this similar trend is attributed to the increased distortion of octahedral NiO5F, which agrees well with the XANES spectra.47 The Ni-EXAFS fits derived from the FEFF9 code are shown in Figures S30−S32, with fit parameters listed in Table S2. Given the similarity in atomic numbers between F and O as well as the backscattering from Ni−F and Ni−O, it is typically difficult to differentiate these two features with EXAFS. However, we found that the overall fitting quality was improved when an additional Ni−F path was included during the FEFF fitting. The resulting interatomic Ni−F distance for the gas-loaded sample was observed to be ~2.3 Å (Table S2).
An interpretation of the differences in the heat generated by adsorption allows us to distinguish between the affinities of R134a toward each of the pore-engineered MOFs. The measured differential enthalpies of adsorption at 298 K are shown in Figure 3c as derived from calorimetric measurements (Figures S24−S26). The enthalpy of R134a adsorption on Ni-MOF-74 is ~50 kJ/mol at near-zero coverage which is in excellent agreement with the binding energy simulated by DFT calculations (−48.7 kJ/mol) at low coverage (see Supporting Information Section 4 for more details of the calculations). As pore size increases, the magnitude of the adsorption enthalpies slightly decreases, consistent with a lowering of the confinement; thus, Ni-BPM and Ni-TPM are observed to have enthalpies of adsorption of ~48.0 and ~45 kJ/mol, respectively. We also note that with more gas uptake, as the open Ni2+ sites are saturated, the enthalpy decreases, which is due to less favorable sorbate/sorbent interactions that play a greater role at higher loadings (Figure S27). These adsorption enthalpies are lower than values representing chemisorption (>80 kJ/mol) but are, however, higher than the values previously reported for R134a adsorbed in other porous materials (<35 kJ/mol) that do not have open metal sites.21,49
Figure 3d shows the in situ IR spectra of gas-phase and adsorbed R134a in meta-pore-engineered MOFs at ~5 mbar. In comparison to the IR spectrum of free R134a, we note the appearance of a new band centered at ca. 1034 cm−1 for the adsorbed gas, which can be assigned to the C−F stretching frequency in the R134a molecule. In order to determine which of the two branches this C−F stretch originates from during R134a adsorption, other fluorocarbons, namely R116 (hexafluoroethane, CF3−CF3) and R161 (fluoroethane, CH3−CH2F), were also examined. The IR spectra of gas-phase and adsorbed R116 and R161 (in Ni-BPM) are given in Figures S20−S23. In a comparison of the spectra of all three probe gases, we observed the same absorption band at 1034 cm−1 with R161 adsorption (Figure S23) as with R134a adsorption. Although a similar absorption band with a slight upward shift to ~1050 cm−1 is observed during R116 adsorption (Figure S21), which did not appear in the IR spectra of free R116, this can be attributed to the C−F stretching frequency of the −CF3 moiety. These results suggest, in agreement with the DFT optimized geometry (Figure 2e), that this C−F stretching mode at 1034 cm−1 can be assigned to the CH2F moiety that is perturbed by the interaction of fluorine with the open Ni2+ node. Further comparison of the intensity of the band at ~1034 cm−1 among the meta-type engineered MOFs is shown in Figure S17−S19. It is observed that the overall IR band intensities are in the order of Ni-MOF-74 > Ni-BPM > Ni-TPM, consistent with the calorimetric results (Figure 3c). This signature can be associated with the F−Ni interactions as predicted by DFT calculations, similar to prior observation in the para-type engineered MOF.35 In addition, we also note a slight red-shift of −CH2 rocking band of free R134a at ~975 to ~966 cm−1 of adsorbed gas, which further confirms that the CH2F moiety of R134a is perturbed by the interaction of the fluorine with Ni2+ node.
Preferred Molecular Orientation of Adsorbed R134a.
To further confirm the EXAFS and in situ IR results, crystallographic analysis was performed using in situ neutron and synchrotron X-ray powder diffraction to ultimately resolve crystal structures of our pore-engineered MOFs with and without R134a. These atomistic-level observations are summarized in Figure 4. The diffraction data were fitted with a model that represents not only the location of the R134a molecules in the unit cell but also the orientation and bond distances of the fluorocarbon with the metal node and the MOF framework. To the best of our knowledge, in combination with in situ IR analysis, this is the first direct experimental evidence that R134a coordinates toward the metal node of the MOF framework and even more importantly, there is a strong favorable interaction between the fluorine atoms of the fluorocarbon and the Ni2+-containing backbone of these MOFs. Table S3 gives a summary of the notable R134a interaction distances, while Figures S12−S14 and Figures S34−S39 show the refinements to the diffraction data. The most notable interaction is the Ni−F distance, with an average distance of 2.16(9) Å. This is shorter than typical metal site adsorbed species. However, such a short distance is consistent with the high electronegativity of fluorine as well as the relatively short Ni−F distance measured using EXAFS and the resulting high enthalpy of adsorption. More surprising is the secondary hydrogen bonding distance of 2.34(15) Å between the H from R134a and O from the linker. However, this interaction makes chemical sense and highlights why the R134a linker preferentially adsorbs from the H2CF side, rather than the CF3 side, where a highly electronegative F would unfavorably interact with an electronegative O. This validation helps to further fortify the elucidated molecular mechanism of adsorption at the initial stage of adsorption and supports the conclusion regarding R134a orientation in these MOFs. Lastly, a tertiary interaction between H from the linker and F from the CF3 side of the R134a molecule can be seen, with a weaker interaction distance of 3.0(3) Å. Once again, this is chemically favorable, as F from the R134a molecule avoids the electronegative π-orbitals from the phenyl ring and instead interacts with the slightly electropositive H. Thus, the so-obtained geometry reflecting the orientation of R134a in the pore and resulting interactions is consistent with the geometry of DFT-optimized structure mentioned earlier (Figure 2e). In addition, for the larger pore Ni-TPM and Ni-TPP MOFs, the R134a molecule rotates slightly into the pore due to the presence of the bulky methyl group on the central phenyl ring, consistent with the R134a enthalpy of adsorption decreasing as pore size increases. The larger pore MOFs would be expected to adsorb a larger amount of fluorocarbon; however, given the lack of sensitivity to low-Z elements in the X-ray data, their positions were not resolved in the current experiments. More details on the structural characterization are provided in SI.
Figure 4.
R134a gas loaded in (a) Ni-BPM and (b) Ni-TPM, highlighting the orientation of R134a. Molecular interactions are shown (c) down the c-axis and (d) in the ab plane, displaying a primary Ni−F (from H2CF) bond length ~2.2 Å, a secondary H−O (from H2CF) bond length ~2.3 Å, and a tertiary F−H (from CF3) bond length ~3.0 Å.
Performance and Stability toward Adsorption Cooling.
Given the equilibrium behavior of R134a on these MOFs, it is necessary to interpret differences between each material in the context of the targeted application. To do this, the applicability of these materials for adsorption cooling systems is compared in terms of their potential for high fluorocarbon throughput in the refrigeration cycle. In short, the optimization of the sorbate/sorbent pair will result in a high working capacity combined with fast loading kinetics that yield higher fluorocarbon throughput resulting in more cooling output. Therefore, the working capacity has been evaluated for each MOF-R134a system as shown in Figure 5a. This capacity is defined as the theoretical limit of adsorption and desorption for a given pair of thermodynamic state points. Since the optimization of operating conditions is not the focus of this work, predetermined state point conditions from a prior study have been chosen for the working capacity calculation.50 This relation is derived from equilibrium uptakes between the adsorption and desorption temperature/pressure state points. From this chart and the calculations shown in Figure S51, it is observed that the working capacity of Ni-TPM is more than 60 wt %, a value significantly higher than that of Ni-TPP (160% increase) as well as that of Ni-MOF-74 (400% increase). Moreover, the robustness of this promising high-performance material must be tested for applicability. To simulate this, the stability was probed by cycling a constant partial pressure of R134a in an enclosed thermogravimetric balance between fixed adsorption and desorption temperatures. From this experiment, as shown in Figure 5b, it is evident that Ni-TPM maintains a high-level of performance even after more than 50 cycles with no decay in adsorption amount.
Figure 5.
(a) Comparison of working capacity calculations for each MOF derived from the uptake difference, ΔUpt*, between given state points from adsorption and desorption equilibrium isotherms. State points for ΔUpt* represent adsorption at 300 kPa and 303 K, or P/P0 = 0.39 and desorption at 700 kPa and 358 K, or P/P0 = 0.24 as modeled from a prior simulated adsorption chiller system.50 (b) 50 cycles of R134a adsorption/desorption in Ni-TPM via heating to 373 K and cooling to 298 K at constant partial pressure of R134a (c.a.100 kPa). (c) Schematic diagram of a two bed sorption cooling system. (d) COPc and average cooling capacity calculations for Ni-TPM vs Ni-MOF-74 as a function of cycle time.
Ultimately, regardless of working capacity benefits, the potential applicability of these pore-engineered MOFs for adsorption cooling relies on their performance in a chiller system. The performance of any sorbate/sorbent pair is based on working capacity, thermophysical properties, and the associated mass transfer rate. To understand these effects, we developed and validated a lumped numerical model for a refrigeration cycle (Figure 5c) with a two-bed adsorption/desorption configuration as per previously reported methods (details given in SI section 5.3).50,51 The parameters specific to the MOF systems utilized here are tabulated in Tables S11 and S12. It should be noted that a reported lumped numerical model that combines the thermophysical properties such as packing density along with the heat and mass transfer rates into lumped parameters was utilized in the development of this model. The performance of the refrigeration cycle is typically represented by the coefficient of performance (COPc), a ratio of the cooling effect to the heat input, as well as the average cooling capacity (CC) assuming a fixed amount of adsorbent utilized. In this model, we utilized the same operating conditions as those previously reported to demonstrate the superior performance of Ni-TPM over other analogs. Figure 5d and Figure S55 show the comparison of COPc and CC between the parent Ni-MOF-74 and the pore-engineered Ni-TPM as a function of cycle time. Although the performance at very low cycle times is comparable between the two MOFs, it is clearly evident that with increasing cycle times, the performance of the chiller using Ni-TPM is significantly better (Figure S55 and Table S13). The maximum COPc values for wider pore Ni-TPM were 110% larger, and the maximum cooling capacity value was ~30% larger than that of Ni-MOF-74. Per unit mass of sorbent, an optimal cycle time on the order of 3−5 min exists that generates both maximum cooling power and maximum corresponding COPc. At a cycle time of 5 min, the average cooling capacity increased with an expansion in pore size from Ni-MOF-74 to Ni-TPM. Our pore-engineered MOF Ni-TPM shows a cooling capacity of more than 3.5 kW (Table 1). This value is ~40% larger than the parent Ni-MOF-74 at the same condition, further emphasizing the importance of engineering larger pores.
On the basis of conventional intuition, we pursued this pore-engineering concept to increase the pore geometry of the MOF framework under the primary assumption that larger pore volume and BET area would lead to better adsorption cooling performance. To this end, the application and subsequent analysis of Ni-TPM, a pore-engineered MOF that has favorable kinetics and strong sorption sites, was probed in a modeled adsorption chiller to reveal promising cooling potential compared to its parent analog. This analysis conducted in conjunction with the fundamental characterizations of the sorbent/sorbate interactions provides confidence in our approach for the design of higher performing sorbents for adsorption cooling applications. Further research in optimizing this model and corresponding parameters such as adsorption/desorption pressures and temperatures as well as heat/mass transfer coefficients for these and other MOF materials is ongoing.
CONCLUSIONS
Pore-engineered MOF analogs containing ligands of expanding lengths and alternate carboxylate/hydroxyl linker locations were explored in this contribution for the adsorption of the R134a fluorocarbon refrigerant. The influence of pore geometries on bulk phase adsorption properties, potential adsorption cooling performance, and proposed molecular interactions of R134a with each Ni-MOF framework was thoroughly interpreted. From our findings, it is observed that the combination of high pore volume and accessibility to a high density of open metal centers contributes to nearly a 300% increase in saturation uptake for Ni-TPM compared with Ni-MOF-74 as determined via equilibrium isotherm measurements. By increasing these saturation limits, we allow more fluorocarbon to be taken up by the sorbent per cycle of adsorption and desorption based on specified chiller process conditions. This increased throughput translates to a 400% increase in the theoretical working capacity to cycle between adsorption/desorption state points. Ultimately, correlation of its bulk sorption performance under realistic chiller conditions results in modeled cooling capacities for Ni-TPM that are at least 40% larger than that of the parent MOF. These advancements in cooling potential and energy transfer represent implications at an engineered scale that have been designed from the molecular level.
Such atomic level manipulations to framework precursors that allow for significant differences in bulk sorption performance inspired probe sorbate−sorbent interactions to gain insight into how adsorption fundamentals can be further tuned for future improvements. Accordingly, we observed experimentally the favorable interactions between the fluorine of the CH2F moiety of R134a and the Ni2+ backbone of the MOFs as per gas-loaded sXRD and X-ray absorption spectra, which are consistent with conclusions drawn from DFT calculations and GCMC simulations. These insights regarding the host−guest chemistry can be advantageous in elucidating effective sorbent/sorbate pairs for potential use with even lower GWP hydrofluorocarbons. More importantly, the ability to tailor porous frameworks, as shown in this joint experimental/theoretical study, allows us to reveal a favorable mechanism of refrigerant adsorption that is a building block for the design of future sorbent materials for such applications.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the U.S. Department of Energy (DOE), Energy Efficiency and Renewable Energy’s Geothermal Technologies Office (GTO) for financial support. PNNL is operated by Battelle for the U.S. DOE under Contract DE-AC05-76RL01830. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory under Contract No. DE-AC0206CH11357. We thank the staff of 17-BM and 20-BM for help with synchrotron X-ray data collection. We also acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce, in providing neutron facilities used in this work.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b11963.
Additional experimental details, structure models, nitrogen sorption data, Powder XRD, synchrotron XRD, TGA, XANES, EXAFS, kinetics measurement, calorimetric tests, in situ FTIR, COP calculation, and molecular simulation details (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.9b11963
The authors declare no competing financial interest.
Contributor Information
Jian Zheng, Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
Dushyant Barpaga, Energy and Environment Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
Benjamin A. Trump, Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, Maryland 20899, United States
Manish Shetty, Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
Yanzhong Fan, Institut Charles Gerhardt, Montpellier UMR 5253 CNRS ENSCM UM, Université Montpellier, 34095 Montpellier, CEDEX 05, France; MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China.
Papri Bhattacharya, Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
Jeromy J. Jenks, Energy and Environment Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States
Cheng-Yong Su, MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China.
Craig M. Brown, Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, Maryland 20899, United States; Department of Chemical and Biochemical Engineering, University of Delaware, Newark, Delaware 19716, United States
Guillaume Maurin, Institut Charles Gerhardt, Montpellier UMR 5253 CNRS ENSCM UM, UniversitéMontpellier, 34095 Montpellier, CEDEX 05, France.
B. Peter McGrail, Energy and Environment Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
Radha Kishan Motkuri, Energy and Environment Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.
REFERENCES
- (1).IEA The Future of Cooling Opportunities for energy-efficient air conditioning. https://www.iea.org/publications/freepublications/publication/The_Future_of_Cooling.pdf.
- (2).Deng J; Wang RZ; Han GY A Review of Thermally Activated Cooling Technologies for Combined Cooling, Heating and Power Systems. Prog. Energy Combust. Sci. 2011, 37 (2), 172–203. [Google Scholar]
- (3).Sah RP; Choudhury B; Das RK A Review on Adsorption Cooling Systems with Silica Gel and Carbon as Adsorbents. Renewable Sustainable Energy Rev. 2015, 45, 123–134. [Google Scholar]
- (4).Choudhury B; Saha BB; Chatterjee PK; Sarkar JP An Overview of Developments in Adsorption Refrigeration Systems Towards a Sustainable Way of Cooling. Appl. Energy 2013, 104, 554–567. [Google Scholar]
- (5).Agyenim F; Knight I; Rhodes M. Design and Experimental Testing of the Performance of an Outdoor LiBr/H2O Solar Thermal Absorption Cooling System with a Cold Store. Sol. Energy 2010, 84 (5), 735–744. [Google Scholar]
- (6).Yokozeki A; Shiflett MB Water Solubility in Ionic Liquids and Application to Absorption Cycles. Ind. Eng. Chem. Res. 2010, 49 (19), 9496–9503. [Google Scholar]
- (7).Li H; Eddaoudi M; O’Keeffe M; Yaghi OM Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402 (6759), 276–279. [Google Scholar]
- (8).Eddaoudi M; Kim J; Rosi N; Vodak D; Wachter J; O’Keeffe M; Yaghi OM Systematic Design of Pore Size and Functionality in Isoreticular MOFs and their Application in Methane Storage. Science 2002, 295 (5554), 469–472. [DOI] [PubMed] [Google Scholar]
- (9).Rieth AJ; Yang S; Wang EN; Dinca M. Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit. ACS Cent. Sci. 2017, 3 (6), 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Henninger SK; Habib HA; Janiak C. MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J. Am. Chem. Soc. 2009, 131 (8), 2776. [DOI] [PubMed] [Google Scholar]
- (11).de Lange MF; van Velzen BL; Ottevanger CP; Verouden KJFM; Lin LC; Vlugt TJH; Gascon J; Kapteijn F. Metal-Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31 (46), 12783–12796. [DOI] [PubMed] [Google Scholar]
- (12).Lin RB; Li TY; Zhou HL; He CT; Zhang JP; Chen XM Tuning Fluorocarbon Adsorption in New Isoreticular Porous Coordination Frameworks for Heat Transformation Applications. Chem. Sci. 2015, 6 (4), 2516–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Motkuri RK; Annapureddy HVR; Vijaykumar M; Schaef HT; Martin PF; McGrail BP; Dang LX; Krishna R; Thallapally PK Fluorocarbon Adsorption in Hierarchical Porous Frameworks. Nat. Commun. 2014, 5, 5. [DOI] [PubMed] [Google Scholar]
- (14).Wang S; Lee JS; Wahiduzzaman M; Park J; Muschi M; Martineau-Corcos C; Tissot A; Cho KH; Marrot J; Shepard W; Maurin G; Chang JS; Serre C. A Robust Large-Pore Zirconium Carboxylate Metal-Organic Framework for Energy-Efficient Water-Sorption-Driven Refrigeration. Nat. Energy 2018, 3 (11), 985–993. [Google Scholar]
- (15).Rieth AJ; Wright AM; Rao S; Kim H; LaPotin AD; Wang EN; Dinca M. Tunable Metal-Organic Frameworks Enable High-Efficiency Cascaded Adsorption Heat Pumps. J. Am. Chem. Soc. 2018, 140 (50), 17591–17596. [DOI] [PubMed] [Google Scholar]
- (16).Lenzen D; Zhao J; Ernst S-J; Wahiduzzaman M; Ken Inge A; Fröhlich D; Xu H; Bart H-J; Janiak C; Henninger S; Maurin G; Zou X; Stock N. A Metal−Organic Framework for Efficient Water-Based Ultra-Low-Temperature-Driven Cooling. Nat. Commun. 2019, 10 (1), 3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chen ZJ; Li PH; Zhang X; Li P; Wasson MC; Islamoglu T; Stoddart JF; Farha OK Reticular Access to Highly Porous acs-MOFs with Rigid Trigonal Prismatic Linkers for Water Sorption. J. Am. Chem. Soc. 2019, 141 (7), 2900–2905. [DOI] [PubMed] [Google Scholar]
- (18).Chen HY; Chen ZJ; Zhang L; Li P; Liu J; Redfern LR; Moribe S; Cui Q; Snurr RQ; Farha OK Toward Design Rules of Metal-Organic Frameworks for Adsorption Cooling: Effect of Topology on the Ethanol Working Capacity. Chem. Mater. 2019, 31 (8), 2702–2706. [Google Scholar]
- (19).Barpaga D; Nguyen V; Medasani BK; Chatterjee S; McGrail BP; Motkuri RK; Dang LX Insight into Fluorocarbon Adsorption in Metal-Organic Frameworks via Experiments and Molecular Simulations. Sci. Rep. 2019, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lenzen D; Bendix P; Reinsch H; Fröhlich D; Kummer H; Möllers M; Hügenell PPC; Glas̈er, R.; Henninger, S.; Stock, N. Scalable Green Synthesis and Full-Scale Test of the Metal−Organic Framework CAU-10-H for Use in Adsorption-Driven Chillers. Adv. Mater. 2018, 30 (6), 1705869 [DOI] [PubMed] [Google Scholar]
- (21).Zheng J; Barpaga D; Gutiérrez OY; Browning ND; Mehdi BL; Farha OK; Lercher JA; McGrail BP; Motkuri RK Exceptional Fluorocarbon Uptake with Mesoporous Metal−Organic Frameworks for Adsorption-Based Cooling Systems. ACS Applied Energy Materials 2018, 1 (11), 5853–5858. [Google Scholar]
- (22).Britt D; Furukawa H; Wang B; Glover TG; Yaghi OM Highly Efficient Separation of Carbon Dioxide by a Metal-Organic Framework replete with Open Metal Sites. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (49), 20637–20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zhou W; Wu H; Yildirim T. Enhanced H2 Adsorption in Isostructural Metal-Organic Frameworks with Open Metal Sites: Strong Dependence of the Binding Strength on Metal Ions. J. Am. Chem. Soc. 2008, 130 (46), 15268–15269. [DOI] [PubMed] [Google Scholar]
- (24).Rieth AJ; Hunter KM; Dinca M; Paesani F. Hydrogen Bonding Structure of Confined Water Templated by a Metal-Organic Framework with Open Metal Sites. Nat. Commun. 2019, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).An J; Rosi NL Tuning MOF CO2 Adsorption Properties via Cation Exchange. J. Am. Chem. Soc. 2010, 132 (16), 5578. [DOI] [PubMed] [Google Scholar]
- (26).Couck S; Denayer JFM; Baron GV; Remy T; Gascon J; Kapteijn F. An Amine-Functionalized MIL-53 Metal-Organic Framework with Large Separation Power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131 (18), 6326. [DOI] [PubMed] [Google Scholar]
- (27).Furukawa H; Ko N; Go YB; Aratani N; Choi SB; Choi E; Yazaydin AO; Snurr RQ; O’Keeffe M; Kim J; Yaghi OM Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329 (5990), 424–428. [DOI] [PubMed] [Google Scholar]
- (28).Yuan SA; Zou LF; Qin JS; Li JL; Huang L; Feng LA; Wang XA; Bosch M; Alsalme A; Cagin T; Zhou HC Construction of Hierarchically Porous Metal-Organic Frameworks Through Linker Labilization. Nat. Commun. 2017, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Deng HX; Grunder S; Cordova KE; Valente C; Furukawa H; Hmadeh M; Gandara F; Whalley AC; Liu Z; Asahina S; Kazumori H; O’Keeffe M; Terasaki O; Stoddart JF; Yaghi OM Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336 (6084), 1018–1023. [DOI] [PubMed] [Google Scholar]
- (30).McDonald TM; Lee WR; Mason JA; Wiers BM; Hong CS; Long JR Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal-Organic Framework mmen-Mg-2(dobpdc). J. Am. Chem. Soc. 2012, 134 (16), 7056–7065. [DOI] [PubMed] [Google Scholar]
- (31).McDonald TM; Mason JA; Kong XQ; Bloch ED; Gygi D; Dani A; Crocella V; Giordanino F; Odoh SO; Drisdell WS; Vlaisavljevich B; Dzubak AL; Poloni R; Schnell SK; Planas N; Lee K; Pascal T; Wan LWF; Prendergast D; Neaton JB; Smit B; Kortright JB; Gagliardi L; Bordiga S; Reimer JA; Long JR Cooperative Insertion of CO2 in Diamine-Appended Metal-Organic Frameworks. Nature 2015, 519 (7543), 303. [DOI] [PubMed] [Google Scholar]
- (32).Gygi D; Bloch ED; Mason JA; Hudson MR; Gonzalez MI; Siegelman RL; Darwish TA; Queen WL; Brown CM; Long JR Hydrogen Storage in the Expanded Pore Metal-Organic Frameworks M-2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 28 (4), 1128–1138. [Google Scholar]
- (33).Cavka JH; Jakobsen S; Olsbye U; Guillou N; Lamberti C; Bordiga S; Lillerud KP A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130 (42), 13850–13851. [DOI] [PubMed] [Google Scholar]
- (34).Guillerm V; Ragon F; Dan-Hardi M; Devic T; Vishnuvarthan M; Campo B; Vimont A; Clet G; Yang Q; Maurin G; Ferey G; Vittadini A; Gross S; Serre C. A Series of Isoreticular, Highly Stable, Porous Zirconium Oxide Based Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2012, 51 (37), 9267–9271. [DOI] [PubMed] [Google Scholar]
- (35).Zheng J; Vemuri RS; Estevez L; Koech PK; Vargas T; Camaioni DM; Blake TA; McGrail BP; Motkuri RK Pore-Engineered Metal-Organic Frameworks with Excellent Adsorption of Water and Fluorocarbon Refrigerant for Cooling Applications. J. Am. Chem. Soc. 2017, 139 (31), 10601–10604. [DOI] [PubMed] [Google Scholar]
- (36).Chen TH; Popov I; Kaveevivitchai W; Chuang YC; Chen YS; Jacobson AJ; Miljanic OS Mesoporous Fluorinated Metal-Organic Frameworks with Exceptional Adsorption of Fluorocarbons and CFCs. Angew. Chem., Int. Ed. 2015, 54 (47), 13902–13906. [DOI] [PubMed] [Google Scholar]
- (37).Mo ZW; Zhou HL; Zhou DD; Lin RB; Liao PQ; He CT; Zhang WX; Chen XM; Zhang JP Mesoporous Metal-Organic Frameworks with Exceptionally High Working Capacities for Adsorption Heat Transformation. Adv. Mater. 2018, 30 (4), 1704350. [DOI] [PubMed] [Google Scholar]
- (38).Hurwitz MM; Fleming EL; Newman PA; Li F; Liang Q. Early Action on HFCs Mitigates Future Atmospheric Change. Environ. Res. Lett. 2016, 11 (11), 114019. [Google Scholar]
- (39).Jenks JJ; Motkuri RK; TeGrotenhuis W; Paul BK; McGrail BP Simulation and Experimental Study of Metal Organic Frameworks Used in Adsorption Cooling. Heat Transfer Eng. 2017, 38 (14−15), 1305–1315. [Google Scholar]
- (40).Rowland CA; Lorzing GR; Gosselin EJ; Trump BA; Yap GPA; Brown CM; Bloch ED Methane Storage in Paddlewheel-Based Porous Coordination Cages. J. Am. Chem. Soc. 2018, 140 (36), 11153–11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Borges DD; Semino R; Devautour-Vinott S; Jobic H; Paesani F; Maurin G. Computational Exploration of the Water Concentration Dependence of the Proton Transport in the Porous UiO-66(Zr)-(CO2H)(2) Metal-Organic Framework. Chem. Mater. 2017, 29 (4), 1569–1576. [Google Scholar]
- (42).Kim IS; Li ZY; Zheng J; Platero-Prats AE; Mavrandonakis A; Pellizzeri S; Ferrandon M; Vjunov A; Gallington LC; Webber TE; Vermeulen NA; Penn RL; Getman RB; Cramer CJ; Chapman KW; Camaioni DM; Fulton JL; Lercher JA; Farha OK; Hupp JT; Martinson ABF Sinter-Resistant Platinum Catalyst Supported by Metal-Organic Framework. Angew. Chem., Int. Ed. 2018, 57 (4), 909–913. [DOI] [PubMed] [Google Scholar]
- (43).Mukherjee S; Desai AV; Ghosh SK Potential of Metal-Organic Frameworks for Adsorptive Separation of Industrially and Environmentally Relevant Liquid Mixtures. Coord. Chem. Rev. 2018, 367, 82–126. [Google Scholar]
- (44).Zheng J; Ye JY; Ortuno MA; Fulton JL; Gutierrez OY; Camaioni DM; Motkuri RK; Li ZY; Webber TE; Mehdi BL; Browning ND; Penn RL; Farha OK; Hupp JT; Truhlar DG; Cramer CJ; Lercher JA Selective Methane Oxidation to Methanol on Cu-Oxo Dimers Stabilized by Zirconia Nodes of an NU-1000 Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141 (23), 9292–9304. [DOI] [PubMed] [Google Scholar]
- (45).Walton KS; Snurr RQ Applicability of the BET Method for Determining Surface Areas of Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2007, 129 (27), 8552–8556. [DOI] [PubMed] [Google Scholar]
- (46).Bonino F; Chavan S; Vitillo JG; Groppo E; Agostini G; Lamberti C; Dietzel PD; Prestipino C; Bordiga S. Local Structure of CPO-27-Ni Metallorganic Framework upon Dehydration and Coordination of NO. Chem. Mater. 2008, 20 (15), 4957–4968. [Google Scholar]
- (47).Platero-Prats AE; League AB; Bernales V; Ye JY; Gallington LC; Vjunov A; Schweitzer NM; Li ZY; Zheng J; Mehdi BL; Stevens AJ; Dohnalkova A; Balasubramanian M; Farha OK; Hupp JT; Browning ND; Fulton JL; Camaioni DM; Lercher JA; Truhlar DG; Gagliardi L; Cramer CJ; Chapman KW Bridging Zirconia Nodes within a Metal-Organic Framework via Catalytic Ni-Hydroxo Clusters to Form Heterobimetallic Nanowires. J. Am. Chem. Soc. 2017, 139 (30), 10410–10418. [DOI] [PubMed] [Google Scholar]
- (48).Chavan S; Bonino F; Vitillo JG; Groppo E; Lamberti C; Dietzel PDC; Zecchina A; Bordiga S. Response of CPO-27-Ni towards CO, N-2 and C2H4. Phys. Chem. Chem. Phys. 2009, 11 (42), 9811–9822. [DOI] [PubMed] [Google Scholar]
- (49).Saha BB; Habib K; El-Sharkawy II; Koyama S. Adsorption Characteristics and Heat of Adsorption Measurements of R-134a on Activated Carbon. Int. J. Refrig. 2009, 32 (7), 1563–1569. [Google Scholar]
- (50).Jribi S; Saha BB; Koyama S; Chakraborty A; Ng KC Study on Activated Carbon/HFO-1234ze(E) based Adsorption Cooling Cycle. Appl. Therm. Eng. 2013, 50 (2), 1570–1575. [Google Scholar]
- (51).Al-Mousawi FN; Al-Dadah R; Mahmoud S. Low Grade Heat Driven Adsorption System for Cooling and Power Generation with Small-Scale Radial Inflow Turbine. Appl. Energy 2016, 183, 1302–1316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.