Abstract
Cough remains a serious unmet clinical problem, both as a symptom of a range of other conditions such as asthma, chronic obstructive pulmonary disease, gastroesophageal reflux, and as a problem in its own right in patients with chronic cough of unknown origin. This article reviews our current understanding of the pathogenesis of cough and the hypertussive state characterizing a number of diseases as well as reviewing the evidence for the different classes of antitussive drug currently in clinical use. For completeness, the review also discusses a number of major drug classes often clinically used to treat cough but that are not generally classified as antitussive drugs. We also reviewed a number of drug classes in various stages of development as antitussive drugs. Perhaps surprising for drugs used to treat such a common symptom, there is a paucity of well-controlled clinical studies documenting evidence for the use of many of the drug classes in use today, particularly those available over the counter. Nonetheless, there has been a considerable increase in our understanding of the cough reflex over the last decade that has led to a number of promising new targets for antitussive drugs being identified and thus giving some hope of new drugs being available in the not too distant future for the treatment of this often debilitating symptom.
I. Cough as an Unmet Clinical Problem
Cough is an important protective reflex and a universal symptom in health, but when persistent, it is the most common reason why patients seek medical attention. In epidemiologic studies, up to 40% of the population at any one time report cough (Janson et al., 2001). Upper respiratory tract infection (URTI) or the common cold is by far the most common cause of cough, but postinfectious cough, unexplained chronic cough, and cough due to pulmonary disorders such as asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, and lung cancer are also common. In children, the etiology of cough differs from adults; viral URTI, protracted bacterial bronchitis, and asthma are frequently the cause of cough (de Jongste and Shields, 2003). However, it is well recognized among clinicians that cough is refractory to specific therapy in a significant number of patients (Everett et al., 2007), even when an underlying cause has been identified (Birring et al., 2004). Cough is associated with significantly impaired health-related quality of life (French et al., 1998), regardless of whether it is acute or chronic (Birring et al., 2003a; Yousaf et al., 2011). Sleep disturbance, nausea, chest pains, and lethargy occur frequently, and patients with chronic cough often experience social embarrassment, urinary incontinence, and low mood (Brignall et al., 2008). There is a significant economic cost for the individual with cough and society when it leads to absence from work and lost productivity.
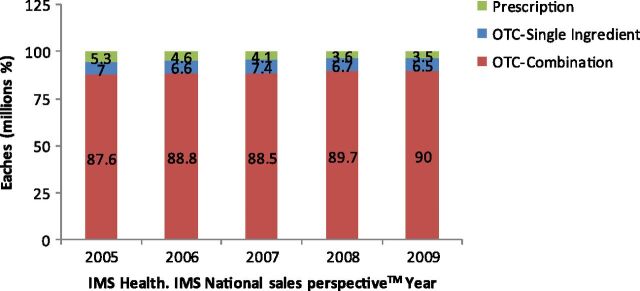
The clinical need for nonspecific antitussive drugs is reflected by the large sales of such medications: $3 billion/year in the United States alone and rising in recent years (Footitt and Johnston, 2009) (Fig. 1). A large number of people obtain over the counter (OTC) antitussive medications for themselves or their children. However, the efficacy of most antitussive drugs, particularly those for URTI, has been challenged recently; in fact, the American College of Chest Physicians (ACCP) advises against the use of antitussive drugs in URTI (Bolser, 2006). Dextromethorphan is the most widely sold antitussive drug and has been available OTC since 1958 in the United States. It was approved by the U.S. Food and Drug Adminstration (FDA) following a review of studies that included few clinical trials demonstrating modest benefit (Cass et al., 1954; Ralph, 1954). These studies were limited by the lack of placebo arms in the trials (Ralph, 1954), inclusion of hospitalized patients with respiratory disease (tuberculosis), and use of unvalidated outcome measures and questionable clinical benefit. A small placebo-controlled trial since then that addressed many of these limitations did not support earlier findings of clinical benefit (Jawad et al., 2000), although another clinical study (Parvez et al., 1996) did record a significant effect on cough (see below). Furthermore, the potential for accidental overdose and abuse of dextromethorphan has led some investigators to ask the FDA to review the efficacy and safety of OTC antitussive medications containing this drug, particularly in children where there are a paucity of studies (Sharfstein et al., 2007). As will be seen in this review, it is far from clear from the available evidence whether dextromethorphan and many other licensed antitussive drugs are effective, and therefore there is a need for further research to find better antitussive drugs (Birring, 2009). Nonetheless, there has been significant progress in our understanding of the pathogenesis of cough, and this has identified a number of potential targets for the development of new drug classes (Barnes, 2007). Cough reflex hypersensitivity is a key feature of most types of cough (Millqvist et al., 1998; Prudon et al., 2005; Morice, 2010) and an important challenge is to develop peripherally acting drugs that reset the cough reflex sensitivity to physiologic levels and avoid central nervous system (CNS) side effects that occur with many existing cough medicines. The recent development of validated health status tools and objective cough frequency monitors should also facilitate the evaluation of antitussive medications (Birring, 2011a). This article written by experts in the pharmacology, basic science, and clinical aspects of cough reviews currently available antitussive drugs and discusses potential new approaches to developing antitussives.
Fig. 1.
Total sales and market share percentage of the over-the-counter and prescription dextromethorphan products, years 2005–2009 in United States. It shows 19% increase in OTC combination products since 2005 to 2009. Eaches, the number of packets, bottles, and vials of a product shipped in a unit. Figure modified from www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/drugsafetyandriskmanagementadvisorycommittee/ucm226621.pdf
II. Basic Physiology of the Cough Reflex
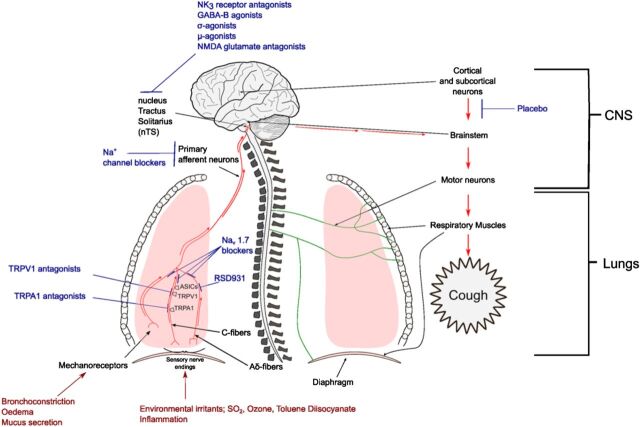
Cough is evoked by activation of vagal afferent nerves terminating in the larynx, trachea, and bronchi. Multiple vagal afferent nerve subtypes innervate the airways and lungs (Fig. 2). Differentiation of these subtypes is achieved through comparisons of their action potential conduction velocities, sites of termination, the location of their cell bodies, embryologic origin, neurochemistry, and their responses to chemical and mechanical stimuli. From these studies, conclusive evidence implicates subtypes of bronchopulmonary C-fibers and A-delta fibers in the initiation of cough (Canning and Chou, 2009). The A-delta fibers are characterized by being rapidly adapting and are responsive to punctate mechanical stimulation and acid environments (Undem and Carr, 2010). These A-delta fibers may provide a defensive mechanism for the airways from aspiration and can be activated to induce cough even in unconscious animals. The conduction velocity of A-delta fibers is approximately three to five times faster than C-fibers (reviewed in Undem and Carr, 2010). On the other hand, the C-fibers involved in the cough reflex are relatively insensitive to mechanical stimuli and lung stretch but are activated by bradykinin and by agonists of the ionotropic receptors, transient receptor potential vanilloid 1 (TRPV1) (capsaicin, resiniferatoxin, protons), and transient receptor potential A1 (TRPA1) (allyl isothiocyanate, acrolein, cinnamaldehyde). These chemical stimuli have been shown to evoke coughing in animals and in humans (Canning et al., 2004; Dicpinigaitis and Alva, 2005; Birrell et al., 2009; Grace et al., 2012). Single cell polymerase chain reaction analyses confirm the expression of TRPV1 and TRPA1 in the neurons projecting these bronchopulmonary C-fibers in animal lungs (Nassenstein et al., 2008; Brozmanova et al., 2012), and TRPV1 has been localized to nerve terminals in human airways (Groneberg et al., 2004). The A-delta fibers regulating cough are insensitive to agonists of either TRPV1 or TRPA1 but are exquisitely sensitive to protons and punctate mechanical stimulation (Canning et al., 2004; Mazzone et al., 2009). The proton-evoked activation of the A-delta fibers probably depends upon gating of acid-sensitive ion channels. However, unlike the C-fibers regulating cough, which terminate throughout the intrapulmonary and extrapulmonary airways, terminations of the A-delta fibers regulating cough are restricted to the extrapulmonary airways (Canning et al., 2004).
Fig. 2.
Multiple vagal afferent nerve subtypes innervate the airways and lungs.
Other vagal afferent nerves are also likely to modulate the cough reflex, and these pathways may either facilitate or inhibit coincidentally evoked coughing. Rapidly adapting receptors, for example, are exquisitely sensitive to mechanical stimuli, particularly stimuli that evoke bronchospasm. Although bronchospasm is typically a poor stimulus for cough, several experimental and clinical studies suggest that bronchospasm may enhance cough reflex sensitivity or evoke coughing in susceptible individuals such as subjects with asthma (House et al., 2004; Kamei and Takahashi, 2006; Ohkura et al., 2012). By contrast, a subtype of bronchopulmonary C-fibers innervating the intrapulmonary airways and lungs is acutely inhibitory for cough in animals (Tatar et al., 1988, 1994; Canning and Chou, 2009).
Cough is one of several unique reflexes that requires sustained high-frequency activation of afferent nerves for its initiation (Canning and Mori, 2011). This creates an urge to cough in patients that precedes the reflex. The need for sustained high-frequency sensory nerve activation in cough has important therapeutic implications. Drugs that limit vagal afferent nerve action potential peak frequencies or diminish the efficacy of synaptic transmission at the primary termination sites of these afferent nerves may have a profound effect on cough. Conversely, stimuli that increase the excitability of these vagal afferent nerves (e.g., airway inflammation) or facilitate synaptic transmission centrally (e.g., convergent afferent input from esophageal afferent nerves in patients with reflux disease) may enhance sensitivity to tussive stimuli.
Vagal afferent nerves terminate centrally in the nucleus tractus solitarius (nTS) (see Fig. 2). Afferent nerves innervating the nasal mucosa and esophagus can have direct or indirect inputs to the nTS, thus providing the anatomic basis required for the association of upper airways diseases and gastroesophageal reflux disease (GERD) with cough (Canning and Mori, 2010). Physiologic and pharmacological analyses reveal a unique and prominent role for N-methyl-d-aspartate (NMDA)-type glutamate receptors at the central synapses of the vagal afferent nerves regulating cough (Mutolo et al., 2007, 2010; Canning, 2009; Canning and Mori, 2011), an observation that may explain the antitussive effects of dextromethorphan in patients (see section III.B) and suggest that novel NMDA antagonists may provide useful antitussive drugs (Fig. 2).
III. Drugs in Current Use for the Treatment of Cough
A. H1-Receptor Antagonists
H1-Antihistamines, or histamine H1-receptor antagonists, can also in many instances act as inverse agonists that combine with and stabilize the inactive form of the H1-receptor, shifting the equilibrium toward the inactive state (Monczor et al., 2013). In addition, some H1-antihistamines can also inhibit muscarinic, α-adrenergic and serotonin receptors, as well as some ion channels. Traditionally, H1-antihistamines have been classified into six chemical types: ethanolamines, ethylenediamines, alkylamines, piperazines, piperidines, and phenothiazines. However, more recently, classification according to function of the first-generation H1-antihistamines, which are lipophilic, CNS-penetrating, and thus, sedating agents, compared with second-generation H1-antihistamines that are lipophobic, penetrate the CNS poorly, and thus, are relatively nonsedating, are more commonly used (Simons, 2004).
Numerous animal studies have demonstrated the ability of H1-antihistamines to be antitussive. In guinea pigs, both allergen and capsaicin-induced cough were inhibited by the first-generation agent chlorpheniramine, the second-generation H1-receptor antagonist loratadine, and by the muscarinic receptor antagonist ipratropium bromide, suggesting that the cough induced in this model was modulated by histamine H1-receptors, as well as cholinergic mechanisms (Bolser et al., 1995b). Of note, a subsequent series of experiments in conscious guinea pigs evaluating oral administration of an example of one of each of the six chemical classes (see above), concluded that the antitussive actions of H1-receptor antagonists are not directly related to histamine H1-receptor blockade, because several H1-receptor antagonists did not inhibit capsaicin-induced cough. Furthermore, the antitussive actions of the older H1-receptor antagonists were independent of their sedative effects and effects on minute ventilation (McLeod et al., 1998). Other studies have documented the antitussive effect of oxatomide in unanesthetized guinea pigs challenged with citric acid (Braga et al., 1993), the ability of epinastine to potentiate the antitussive effect of dihydrocodeine against capsaicin-induced cough in mice (Kamei et al., 1999), and the ability of azelastine to suppress capsaicin-induced cough in conscious guinea pigs through a mechanism perhaps partly due to inhibition of substance P release from sensory nerves (Ito et al., 2002).
A more recent study employing human HEK cells expressing TRPV1 demonstrated the inhibition of TRPV1 receptor activation by the first-generation H1-antihistamine dexbrompheniramine, thus suggesting another potential mechanism for the antitussive effect of H1-receptor antagonists (Sadofsky et al., 2008). This finding is of particular interest given the recent suggestion of the potential importance of TRPV1 receptors in human cough (Morice and Geppetti, 2004b; Lee et al., 2011).
Studies of induced cough in healthy human volunteers have yielded mixed results. Diphenhydramine administered as an oral, 25-mg dose was shown to inhibit citric acid-induced cough in a placebo-controlled, crossover study (Packman et al., 1991), whereas a 120-mg oral dose of terfenadine did not inhibit capsaicin-induced cough (Studham and Fuller, 1992). The second-generation agent fexofenadine (180-mg oral dose) was unable to inhibit capsaicin-induced cough in healthy volunteers or in subjects with acute viral upper respiratory tract infection (URTI) (Dicpinigaitis and Gayle, 2003a). However, another small study demonstrated the ability of the second-generation agent loratadine to inhibit ultrasonically nebulized distilled water (UNDW)-induced cough in nonasthmatic patients with chronic cough (Tanaka et al., 1996).
In terms of clinical management of cough in adults, the guidelines of the ACCP recommend the combination of a first-generation H1-antihistamine and a decongestant as the treatment of choice for chronic cough due to upper airway cough syndrome (formerly known as postnasal drip syndrome) and acute cough due to the common cold (Irwin et al., 2006). This recommendation is based largely on a vast body of clinical experience and expert opinion, in the absence of adequately powered, prospective, randomized, controlled clinical trials (Bjornsdottir et al., 2007). However, in support of this recommendation, a prospective evaluation of 45 adult patients presenting with chronic cough, treatment with a first-generation H1-antihistamine/decongestant combination as the first step of a therapeutic algorithm, demonstrated an improvement in cough in 39 patients, and this was the only therapy required by 16 patients (Pratter et al., 1993).
A number of small studies further support the antitussive efficacy of certain H1-antihistamines in a variety of types of pathologic cough. In a double-blind crossover study, diphenhydramine, administered in four 25-mg or 50-mg doses every 4 hours, induced a statistically and clinically significant reduction in cough frequency compared with placebo. Of note, although the most frequently reported side effect was drowsiness, especially with the 50-mg dose, there was little or no apparent correlation between the antitussive effect and the incidence of sedation (Lilienfield et al., 1976). In another randomized, double-blind study of patients with cough associated with allergic rhinoconjunctivitis, treatment with 10 mg daily doses of loratadine for 4 weeks resulted in significantly improved subjective ratings of cough frequency and cough intensity compared with placebo (Ciprandi et al., 1995). In a randomized trial of volunteers with experimental rhinovirus-induced colds, brompheniramine administered twice daily in a 12-mg oral dose for up to 4 days resulted in a significant decrease in cough counts after 1 day of therapy compared with a control group not receiving treatment (Gwaltney and Druce, 1997). Another small, prospective, randomized, open-design study demonstrated the combination of oxatomide and dextromethorphan to be more effective than dextromethorphan alone against chronic, postinfectious cough, as measured by subjective cough diaries (Fujimori et al., 1998). In an unblinded study of 22 asthmatic patients with chronic cough, a 4-week course of azelastine improved subjective cough scores as well as the cough threshold to inhaled capsaicin (Shioya et al., 1998). In a placebo-controlled study of subjects with atopic cough (eosinophilic bronchitis), a 4-week course of epinastine improved subjective cough scores and diminished cough reflex sensitivity to inhaled capsaicin, without altering bronchial responsiveness to methacholine (Shioya et al., 2004).
Studies of the antitussive effect of H1-receptor antagonists in the pediatric population are limited. One small, randomized, double-blind placebo-controlled study of children with cough due to pollen allergy demonstrated that 1 month of treatment with cetirizine significantly reduced subjective measures of cough intensity and cough frequency (Ciprandi et al., 1997). However, three studies evaluating nocturnal cough in children with URTI did not demonstrate a beneficial effect of diphenhydramine compared with placebo (Paul et al., 2004a; Yoder et al., 2006) or honey (Shadkam et al., 2010). A recent Cochrane Database Systematic Review concluded that, based on available data, H1-receptor antagonists cannot be recommended for the treatment of acute or chronic cough in children (Chang et al., 2008).
The fairly common usage of H1-antihistamines for the treatment of cough in adults, albeit based on decades of clinical experience, overall is not supported by adequately performed clinical trials clearly demonstrating their effectiveness. Furthermore, the mechanism by which certain H1-receptor antagonists affect cough (mainly first-generation drugs) remains unclear. One explanation for the widely recognized observation that first-generation H1-antihistamines, but not the second-generation, nonsedating agents, are effective antitussives, is that the former penetrate the CNS and also have anticholinergic activity. However, the rank order potency of these agents as muscarinic receptor antagonists does not support this hypothesis (Bolser, 2008) nor does a sedative effect offer an adequate explanation based on animal studies demonstrating a lack of correlation between the antitussive and sedating effects of H1-antihistamines (McLeod et al., 1998). Overall though, the evidence for certain older H1-receptor antagonists having an antitussive effect seems to be unrelated to H1-receptor antagonism. Thus, studies examining the mechanism by which certain H1-antihistamines exert an antitussive effect, as well as proper trials demonstrating clinical effectiveness, are still needed.
B. Dextromethorphan
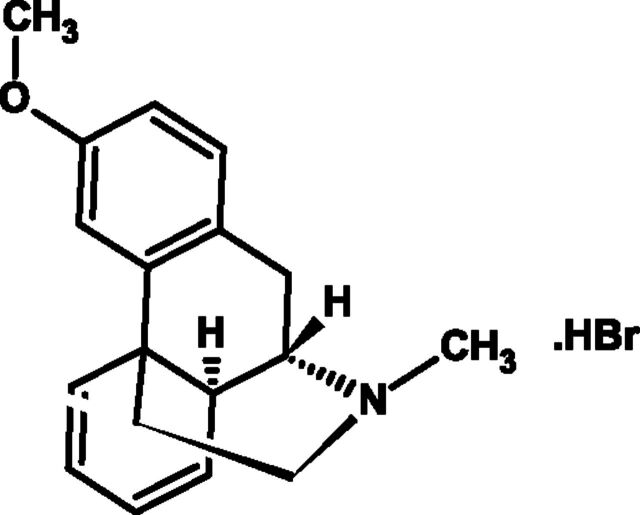
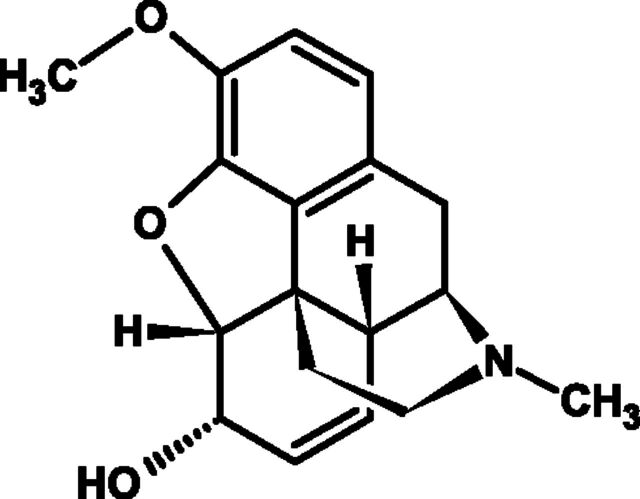
Dextromethorphan hydrobromide (dextromethorphan) (Fig. 3) was first reported in 1953 as a treatment of cough without the undesirable side effects of codeine, i.e., drowsiness, nausea, dependency, and constipation (Cass et al., 1954), and has since become the active ingredient in many OTC medicines.
Fig. 3.
Chemical structure of anhydrous dextromethorphan hydrobromide.
Dextromethorphan HBr [(+)-3-methoxy-17-methylmorphinan hydrobromide monohydrate] is the dextro-isomer (D-isomer) of levorphanol methylether, and it is thought to bind to high- and low-affinity sites in the brain that are distinct from opioid and other neurotransmitter binding sites (Grattan et al., 1995). A steric hindrance mechanism may exist where the (O) methylated (+) form of racemorphan (dextromethorphan) prevents binding to the analgesic/addictive receptors in the medulla to abate narcotic side effects (Delgado and Remers, 1998).
The pharmacology of dextromethorphan is not completely understood. It has been shown to bind to a series of receptors, including the N-methyl-d-aspartate (NMDA) glutamate receptors (Netzer et al., 1993, Chou et al., 1999), σ-1 receptors (Chou et al., 1999), nicotinic receptors (Glick et al., 2001), and serotonergic receptors (Meoni et al., 1997). This complex activity is believed to suppress cough by altering the threshold for cough initiation primarily via its effects as an NMDA antagonist at the level of antagonizing glutamate receptors in the nTS in the CNS (Ramsay et al., 2008).
Dextromethorphan is well absorbed from the gastrointestinal tract, with maximum dextromethorphan plasma concentrations occurring ~1–4 hours after oral administration in extensive metabolizers and 4–8 hours after oral administration in poor metabolizers. Dextromethorphan has a plasma elimination half-life of ~1–4 hours in extensive metabolizers and 17—42 hours in poor metabolizer subjects (Martindale, 2009). The plasma elimination half-life of the main metabolite dextromethorphan is approximately 1 to 3 hours in extensive metabolizers and 5 to 13 hours in poor metabolizers (Silvasti et al., 1987; Schadel et al., 1995; Capon et al., 1996).
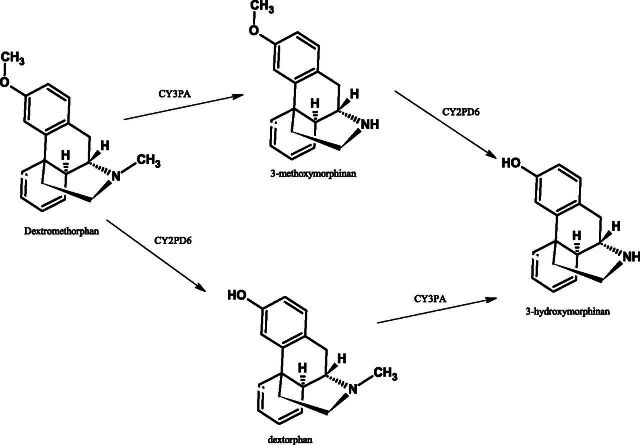
Cytochrome P450 metabolizes dextromethorphan to dextrorphan by O-demethylation and to a lesser extent to 3-methoxymorphinan by N-demethylation (Fig. 4). 3-Methoxymorphinan (via N-demethylation) is further metabolized to 3-hydroxymorphinan (Jacqz-Aigrain et al., 1993; Manap et al., 1999). Dextromethorphan has been further shown to be metabolized by the CYP2D6-mediated O-demethylation pathway by use of immunoinhibition and a known inhibitor of this pathway, quinidine.
Fig. 4.
Metabolism of dextromethorphan.
Long-term oral dosing of 120 mg daily, in divided doses, resulted in peak plasma dextromethorphan concentrations of 0.5–5.9 ng/ml (mean 2.4 ng/ml) in extensive metabolizers and 182–231 ng/ml (mean 207 ng/ml) in poor metabolizers (DeZeeuw and Johnkman, 1988). The main metabolite, dextorphan, is the only metabolite known to have an antitussive action and has been shown in animal studies to possess antitussive activity approximately three quarters of that of dextromethorphan at a dose of 2.0 mg/kg (Benson et al., 1953). Dextrorphan has been suggested to be responsible for the main pharmacological effect at therapeutic doses.
Studies demonstrating inhibition of the CYP2D6 enzyme pathway by quinidine have indicated that at expected therapeutic doses of 30 to 120 mg/day, dextromethorphan is unlikely to undergo significant interactions; however, differences in metabolic rates may become clinically important with high doses of specific concomitant medication for prolonged periods or in cases of abuse.
Six publications report the efficacy of dextromethorphan (30 mg) on cough induced by inhalation of aerosols of citric acid by healthy adult subjects. In all studies except one, oral administration of dextromethorphan was associated with a significant reduction in cough challenge when compared with placebo. Empey et al. (1979) studied 18 healthy volunteers to compare the antitussive effect of codeine (20 mg), dextromethorphan (30 mg), and noscapine (30 mg). Only codeine 20 mg had antitussive activity. This negative study was the smallest and it is likely to have insufficient power to produce a reliable negative result.
Of the positive studies, Packman and Ciccone (1983) reported a double-blind, three-period crossover study in 30 healthy volunteers of 30 mg of dextromethorphan and 7.5 mg of doxylamine with dextromethorphan and a placebo. Both treatments were significantly superior to placebo in reduction of overall cough frequency (P < 0.0001) for up to 8 hours posttreatment. Similarly, Karttunen et al. (1987) reported the antitussive effects of dextromethorphan (30 mg) plus salbutamol (2 mg), dextromethorphan (30 mg) alone, or placebo. Significant increases in cough threshold were shown after dextromethorphan and the dextromethorphan-salbutamol combination.
Grattan et al. (1995) investigated the effects of inhaled dextromethorphan with a single oral dose of dextromethorphan (30 mg) in 20 healthy subjects. Although oral dextromethorphan delivered significant (P < 0.002) reductions in induced cough frequency, it is noteworthy that the inhaled dextromethorphan (1, 3, and 30 mg) did not demonstrate an antitussive effect. Thus, a peripheral activity of dextromethorphan seems unlikely.
Hull et al. (2002) investigated a series of doses of dextromethorphan in a novel "pregastric" formulation designed to promote transepithelial absorption in the oral cavity and esophagus and thus provide a more rapid onset of activity. Dextromethorphan (50 mg p.o.), 22 mg of dextromethorphan free base (equivalent to 30 mg of dextromethorphan HBr) delivered pregastrically, codeine (60 mg p.o.), dextromethorphan (50 mg) plus codeine (60 mg p.o.), or placebo were compared. All doses of dextromethorphan delivered significant reductions in induced cough frequency from baseline, and when dosed pregastrically dextromethorphan reduced cough frequency compared with placebo at 15 minutes postadministration.
Finally, Ramsay et al. (2008) reported a placebo-controlled randomized, double-blind crossover study in subjects with smoking-related cough.
A single dose of dextromethorphan was administered pregastrically as 22 mg free base and was associated with a significant (P < 0.05) increase in the C2 at 1 and 2 hours postadministration. Overall these studies clearly demonstrate that dextromethorphan effectively diminished cough reflex sensitivity as revealed by citric acid challenge in humans. The question is whether this activity translates into clinical efficacy in pathologic cough.
The effect of dextromethorphan (30 mg) in adults suffering from acute cough has been reported in three clinical studies and one meta-analysis. Tukiainen et al. (1986) studied dextromethorphan (30 mg), dextromethorphan (30 mg), and salbutamol (2 mg) or placebo in 108 patients with cough associated with acute respiratory tract infection. Reported cough severity was reduced significantly in all groups, with dextromethorphan having no greater effect than placebo. Similarly, a study of 42 patients using objective methods to record count cough frequency failed to show significant effects (except in cough pressure levels) (Jawad et al., 2000). By contrast, Parvez et al. (1996) measured the effect of dextromethorphan on the objectively assessed cough frequency in a much larger study of 451 patients. Cough counts were significantly reduced compared with placebo (P < 0.05). Given the well-described placebo effects in cough due to acute URTI, only adequately powered studies involving hundreds of patients are likely to have sufficient power to demonstrate the relativity modest effects of dextromethorphan (30 mg). In support of this a meta-analysis of six randomized, double-blind placebo-controlled studies with dextromethorphan (30 mg) in URTI (Pavesi et al., 2001) demonstrated a significant peak effect on average reduction in cough frequency of 12–15% over 3 hours postdosing.
In other forms of cough, placebo-controlled studies are unfortunately rare, and without a placebo comparator, therapeutic efficacy is impossible to judge. Thus, Catena and Daffonchio (1997) compared levodropropizine syrup (60 mg three times a day for 5 days) with dextromethorphan syrup (15 mg three times a day for 5 days) in 209 adult patients. Both levodropropizine and dextromethorphan reduced cough intensity, and Equinozzi and Robuschi (2006) compared dextromethorphan and pholcodeine in patients with acute, frequent, nonproductive cough. Again unsurprisingly there was an equal reduction in the metrics of cough observed.
In five studies in patients with chronic cough, only two were placebo controlled. Dextromethorphan (15- or 20-mg doses) was shown to be of comparable efficacy to “therapeutic” doses of levodropropizine (Catena and Daffonchio, 1997), dihydrocodeine, noscapine (Matthys et al., 1983), or codeine (Matthys et al., 1983).
In early studies, Ralph (1954) compared three different doses of dextromethorphan for its ability to suppress chronic cough attributable to a range of conditions, tuberculosis, acute and chronic bronchitis, bronchiectasis, asthma, lung abscess, and bronchogenic carcinoma in 144 patients. A 15-mg dose of dextromethorphan was observed to be significantly better than 4 mg by patient report, although yet again this was not a placebo-controlled study.
Cass et al. (1954) reported cough suppressant activity of dextromethorphan compared with codeine in 69 patients with persistent cough. Dextromethorphan (6 mg) was significantly more effective than placebo, but significantly less effective than 12 mg of dextromethorphan. Knowing what we now know of the pharmacodynamics of dextromethorphan, such efficacy seems unlikely.
A number of small studies have been used to compare dextromethorphan with other putative antitussives. These are reported here for the sake of completeness, but methodological considerations make interpretation difficult. Matthys et al. (1983) studied chronic, stable cough due to pulmonary tuberculosis, bronchial carcinoma, or obstructive lung disease. Dextromethorphan (20 mg), 20 mg of codeine phosphate, or placebo was compared, and cough was measured by means of a pressure transducer attached over the trachea. Both drugs were significantly more effective than placebo (P < 0.0001). Ruhle et al. (1984) objectively compared glaucine, with dextromethorphan (30 mg) and placebo. In twenty-four patients affected by chronic cough, cough count frequency after dextromethorphan and glaucine was lower than after placebo, although only glaucine caused a significant reduction in cough frequency. A further study by Matthys et al. (1983) evaluated the antitussive effect of several drugs [noscapine (30 mg), dextromethorphan (20 mg), dihydrocodeine (30 mg), or codeine (20, 30, and 60 mg) administered twice daily] in patients with chronic stable cough due to bronchial carcinoma, pulmonary tuberculosis, or COPD. Patients received active antitussive drugs or placebo in a double-blind, randomized crossover design. Cough frequency and intensity were recorded for 8 hours. Noscapine, dextromethorphan, dihydrocodeine, and codeine (60 mg) all significantly reduced the cough frequency compared with placebo and produced a greater reduction of cough intensity than placebo, codeine (20 mg), or codeine (30 mg). Del Donno et al. (1994) compared moguisteine (3 doses of 200 mg, over 2 days) to dextromethorphan (3 doses of 30 mg, over 2 days) and found both drugs to be equally effective. In a final “comparison” study by Aylward et al. (1984), of eight patients with cough associated with "simple bronchitis," cough counts were statistically significantly different (P < 0.05) from placebo for both codeine (30 mg)- and dextromethorphan (60 mg)-treated patients.
Dextromethorphan is widely used in a number of pediatric antitussive preparations. However, data on the efficacy of dextromethorphan in pediatric populations are limited, and in each case the study sample size is judged to be too small to have detected efficacy differences compared with placebo (Pavesi et al., 2001). Four published studies (Korppi et al., 1991; Paul et al., 2004a,b; Yoder et al., 2006) examined the antitussive effect of dextromethorphan on acute cough in children with acute URTI, although none showed any significant antitussive effects.
Some additional data have been generated in work designed primarily to assess the value of treating children with honey for nocturnal cough wherein dextromethorphan was employed as a positive control (Paul et al., 2007, Shadkam et al., 2010). Paul et al. (2007) compared honey and dextromethorphan to no treatment in 35 patients per treatment arm and were unable to show a statistically significant effect of dextromethorphan or honey on subjective reports of cough severity or sleep quality. From the work of Shadkam et al. (2010) in a three-arm study of 138 patients receiving dextromethorphan, diphenhydramine, honey, or a control arm with "supportive treatment" (saline drops, water vapor, and acetaminophen), both dextromethorphan and diphenhydramine demonstrated an improvement in subjective parameters of cough compared with "supportive treatment," but these did not reach statistical significance.
Taken together these studies cannot be used to support the use of dextromethorphan in pediatric cough therapy. Clinical trials with objective assessments and of sufficient power to detect the 12–17% antitussive effect indicated by large adult trials are needed to establish whether dextromethorphan is a useful antitussive in children (Mabasa and Gerber, 2005).
In conclusion, although dextromethorphan has been repeatedly shown to diminish cough sensitivity to tussive challenge, it has been more difficult to demonstrate clinically significant effects, particularly in acute URTI where many trials have failed to meet modern standards of design. Adequately powered studies with both objective and subjective measures of efficacy are required, particularly in acute cough in children.
C. Opiates: Codeine and Morphine
Codeine is a naturally occurring alkaloid found in extracts of the poppy, particularly Papaver bractreatum. Chemically codeine is morphine methylated in the 3 position (Fig. 5), and when used in preparations for the treatment of cough, it is usually synthesized from the parent molecule. Codeine itself is regarded as a weak opioid, and its major therapeutic action is through catabolism in the liver by cytochrome P450 2D6 to morphine. CYP3A4 also contributes to codeine’s metabolism to the active norcodeine. Codeine and its metabolites are then conjugated by UGT 2B7 to the 3 and 6 glucuronides that are thought to convey most of the central antitussive activity. Codeine is therefore best regarded as a prodrug and, because of this complicated metabolic pathway, liable to the well-described genetic variability and interactions with other drugs metabolized by the cytochrome system. Of particular relevance is the interaction with another widely used antitussive, dextromethorphan (see section III.B ), which competes for metabolism by cytochromes, particularly 2D6, leading to an alteration in the pharmacokinetic and pharmacodynamic profile of both agents.
Fig. 5.
Chemical structure of codeine.
The use of opium and its ethanolic extract laudanum in the suppression of cough has an ancient history (Mudge, 1778), and although such preparations are not used medicinally today, confusion still exists as to the relative contribution of the various exogenous or endogenous alkaloids to antitussive activity of opiates. Intravertebral artery injection of codeine inhibits brain stem electrically induced cough in the anesthetized cat, suggesting that codeine itself is active as an antitussive (Chou and Wang, 1975).
In a study by Adcock et al. (1988) in conscious guinea pigs, subcutaneous codeine was shown to be one-seventh as potent as morphine. The antagonism of the antitussive, but not the antinociceptive effects of these opiates by the quaternary antagonist N-methylnalorphine, was taken as indicating a possible peripheral activity (Adcock et al., 1988). Further evidence of a peripheral site of action of codeine was obtained by Callaway et al. (1991), again against citric acid-induced cough in guinea pigs. Aerosolized codeine at a dose of 72 μg/kg achieved a greater than 60% antitussive activity, whereas a dose of 3 mg/kg was required to achieve a similar effect via the intraperitoneal route. In the same study the aerosolized mu receptor agonist H-Tyr-D-Arg-Phe-Lys-NH2 caused significant cough inhibition, pointing to a possible mechanism of action. Inhaled codeine (30 mg/ml) was again shown to be an effective antitussive in the same animal model by Karlsson et al. (1990). The quaternary opioid antagonist levallorphan methyl iodide completely inhibited this peripheral antitussive effect, suggesting classic opioid activity at this site. However, in the cat, the central antitussive activity of codeine appears resistant to both naloxone and specific mu and kappa antagonists (Chau et al., 1983). This and the relative lack of stereoselectivity for the l-isomer, as opposed to the d-isomer of codeine (Chau and Harris, 1980), have led to the hypothesis that codeine’s main antitussive activity may be mediated through nonclassic opioid receptors.
However, codeine has not been demonstrated to suppress cough in all animal models. In guinea pigs, both cough induced by mechanical stimulation of the lower airways and sulfur dioxide-induced bronchitis are not inhibited by codeine (Takahama et al., 1997; Takahama and Shirasaki, 2007). Although inhibition of citric acid-induced cough by inhaled codeine has been demonstrated in the guinea pig by several authors, cough induced by capsaicin does not appear to be affected (Xiang et al., 1998). Conversely, codeine has been demonstrated to inhibit ozone-induced hypertussive responses in rabbits (Adcock et al., 2003). These conflicting results indicate the highly species and model dependency of codeine’s antitussive activity and question the use of this drug as a “gold standard” antitussive.
Although still one of the most widely used and prescribed antitussives, codeine has repeatedly been found to be poorly effective in clinical studies, leading some authors to question this widespread practice, particularly in children (Herbert and Brewster, 2000; Bolser and Davenport, 2007; Goldman, 2010; Chang et al., 2012; Paul, 2012). In cough evoked by capsaicin, codeine (30 and 60 mg p.o.) had no effect on the sensation of urge to cough or on cough number (Davenport et al., 2007), and there is no additional effect apparent when capsaicin cough is voluntarily suppressed (Hutchings and Eccles, 1994). As in animal studies, inhaled codeine (50 mg) also failed to inhibit capsaicin-induced cough in normal volunteers (Fuller et al., 1988). In contrast, Dicpinigaitis et al. (1997) observed significantly greater suppression of capsaicin-induced cough 2 hours after ingestion of 30 mg of codeine compared with placebo. In citric acid-induced cough, 20 mg of codeine was associated with significantly greater cough suppression than placebo (Empey et al., 1979)
In two exemplary studies investigating cough due to URTIs, Eccles et al. (1992) found that codeine at an initial dose of 30 mg, followed by 4 days of dosing at 30 mg four times a day, had no effect greater than placebo syrup, either on objective initial cough recording or on subsequent self-reported cough. In the second study by the same group, oral codeine (50 mg) was compared with placebo syrup in 82 subjects in a parallel group design using three measures of cough assessment (Freestone et al., 1996). Again, no effect greater than that of placebo was observed. In 21 coughing patients with COPD, Smith et al. (2006) measured both citric acid cough threshold and objectively counted ambulatory cough over 10 hours during the day and overnight. Codeine (60 mg) had no significant effect over placebo on either measure (Smith et al., 2006).
In support of codeine as an antitussive in humans, a small study in patients with chronic cough (Aylward et al., 1984) reported oral dosing with 60 mg of syrup to be more effective than placebo, with log plasma concentrations of codeine being related to cough suppression.
In conclusion, well-performed controlled studies in humans do not support codeine as an effective antitussive in man, and its frequent use as the “gold standard” comparator in equivalence studies of novel antitussives has led to much confusion. However, inhaled codeine has been repeatedly demonstrated to have efficacy in animal models, and it may be instructive to note that the original account of the effects of opioids on “catarrhous cough” over 200 years ago were via the first recorded inhaler device (Mudge, 1778). Such observations may suggest it may be more appropriate to use codeine locally in the airways to improve the therapeutic window of this drug.
Morphine has had a long history of use as an antitussive, particularly in the palliative care setting (Molassiotis et al., 2010). However the recognition that the major therapeutic effect of codeine was via its catabolism to morphine led to the investigation of the utility of morphine itself in the treatment of chronic cough. In a placebo-controlled, double-blind crossover study in 27 patients with chronic intractable cough, 5 mg of morphine sulfate twice daily for 1 month produced a significant reduction of greater than one-third in median diary record card recorded cough (Morice et al., 2007). Examination of the individual responses indicated a division into patients with an excellent response and those without benefit. Despite this clear clinical response, there was no effect on cough challenge with citric acid. In an open label extension to the study, dose escalation to 10 mg twice daily was permitted, and an additional six patients reported improvement. Constipation was the only adverse event of note.
The uptake of morphine as routine therapy in chronic cough has been hampered by its status as a controlled drug in some jurisdictions. Clinical experience suggests that, unlike pain, the ceiling of therapeutic response is reached at a daily dose of 20 mg and, given the rapid (within hours) effect, assessment of drug efficacy in individuals should be permissible. However, none of the opiate drugs should be viewed as the “gold standard” antitussive agent as a comparator in clinical studies given the marked individual variation in response, but because of its more reliable bioavailability, morphine is the preferred opioid of choice in the clinic.
D. Local Anesthetics
Local anesthetics have been reported to have antitussive effects and, given the involvement of sensory nerves in the cough reflex, it is perhaps not surprising that local anesthetics can inhibit both experimentally induced cough as well as cough in a variety of clinical circumstances. This antitussive activity is presumably due to the ability of local anesthetics to block NaV+ channels in sensory nerves (Undem and Carr, 2010). There is most information concerning the use of lignocaine/lidocaine (Fig. 6). For example, inhaled lignocaine (20 mg) has been shown to be able to suppress cough induced by inhaled capsaicin in nonsmoking volunteers (Hansson et al., 1994). Although local anesthetics are often used in combination with adrenaline to prolong their activity, Hansson and colleagues found no evidence that mixing adrenaline with lignocaine had any effect on either the antitussive effect of lignocaine or the plasma levels of this local anesthetic following inhalation. Intravenous lidocaine has also been used to suppress the coughing associated with tracheal intubation (Yukioka et al., 1985). Topical lidocaine has been widely used to suppress the coughing associated with bronchoscopy, and a number of studies have supported this use via randomized double-blind placebo-controlled studies (Gove et al., 1985; Berger et al., 1989; Jakobsen et al., 1993). A good example of this is the study by Antoniades and Worsnop (2009), who demonstrated significantly lower cough rates after administration of 2% lidocaine versus normal saline when applied through a flexible bronchoscope. These authors also found that use of lidocaine permitted less use of sedatives such as midazolam or fentanyl compared with placebo treatment (Antoniades and Worsnop, 2009). However, other investigators found a higher incidence of postoperative cough after the use of aerosolized lidocaine (Herlevsen et al., 1992; Soltani and Aghadavoudi, 2002) and after the use of lidocaine jelly (Selveraj and Dhanpal, 2002). However, a more recent study reported a reduction in postoperative coughing after the application of lidocaine jelly applied over the tracheal tube during elective surgery under general orotracheal anesthesia, albeit less than that produced by betamethasone gel (Sumathi et al., 2008). Nebulized lidocaine has also been shown to be of use in the treatment of a 52-year-old man with intractable cough (Trochtenberg, 1994), in four patients with chronic intractable cough (Howard et al., 1977), and in the treatment of cough near the end of life (Lingerfelt et al., 2007); mepivicaine aerosols have also been reported to be of value in the treatment of refractory cough (Almansa-Pastor, 1996). Nebulized lidocaine has also been reported to be of value in the treatment of cough in patients with COPD (Chong et al., 2005). Interestingly, oral mexilitine has been shown to reduce the cough response to tartaric acid and capsaicin (Fujimura et al., 2000).
Fig. 6.
Chemical structure of lidocaine or lignocaine.
It has been suggested that different types of local anesthetics differentially affect different airway reflexes, even when administered at doses producing the same degree of oropharyngeal anesthesia (Choudry et al., 1990). Thus, lignocaine and dyclonine both caused oral anesthesia, but only lignocaine inhibited cough induced by inhaled capsaicin in nonsmoking volunteers, and neither drug inhibited capsaicin-induced bronchospasm. This is of interest because another compound reported to be an analog of a local anesthetic, RSD 931, has experimentally been shown to inhibit capsaicin-induced cough but not bronchoconstriction in the rabbit (Adcock et al., 2003). This drug has been suggested from electrophysiological experiments to be a selective inhibitor of A-delta fibers in the lungs, rather than cause the inhibition of all sensory nerves fibers seen after nebulization of lidocaine. Such results suggest that the reflexes leading to bronchoconstriction are distinct from those that induce cough, raising the possibility of drugs such as RSD 931 to provide selective inhibition of airway reflexes, particularly A-delta fibers, and thus may be effective antitussive drugs without the side effects of local anesthetics. Such work also implies that choosing a drug that has local anesthetic activity as an antitussive cannot be based on local anesthetic potency at other anatomic sites (Choudry et al., 1990).
Benzonatate is a long-chain polyglycol derivative (Fig. 7) chemically related to the ester-linked class of local anesthetic drugs such as procaine and tetracaine. The rationale for developing benzonatate as a treatment of cough was based on a number of clinical and experimental observations at the time; firstly, local anesthetic agents were known to provide desirable antitussive effects when used in the preparation of the upper and lower airways prior to bronchoscopy (as described above). Secondly, it was well recognized that the pulmonary distention produced by inspiration stimulates vagal fibers that project and excite the expiratory center (Hering Breuer reflex) and Kroepfli (1950) showed that the intensity of an experimentally induced cough in cats was proportional to the depth of the preceding inspiration.
Fig. 7.
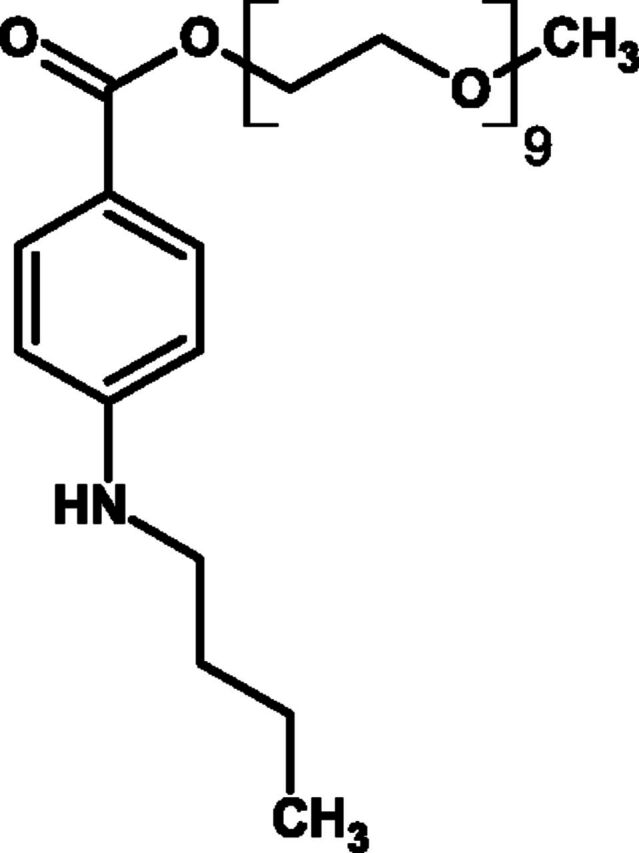
Chemical structure of benzonatate.
Consequently, Bucher and Jacot (1951) demonstrated that coughing could be attenuated if bronchodilatation was prevented during inhalation. Later, Bucher (1956) provided direct evidence for inhibition of pulmonary stretch receptors by benzonatate. As benzonatate is known to be an effective nerve conduction blocker (Thoren and Oberg, 1981), the presumed mechanism for its antitussive effect is the peripheral anesthesia and inhibition of afferent vagal fibers from pulmonary stretch receptors located in the bronchial tree. Evidence of the antitussive effect of benzonatate in humans was first reported in 1957. Healthy, noncoughing volunteers underwent citric acid cough challenge to determine their baseline cough response and then again on two further occasions (3 days apart) after pretreatment with either open label benzonatate (100 mg) or codeine (32 mg). Benzonatate was reported to be 2.5 times more effective as an antitussive than codeine (Shane et al., 1957). The following year Gregoire and colleagues (1958) demonstrated the efficacy of benzonatate (10 mg i.v.) in reducing cough induced by aerosolized acetylcholine. The same group conducted the first placebo-controlled clinical trial of benzonatate in patients with chronic cough. In 28 patients with treated pulmonary tuberculosis complaining of chronic cough that was resistant to other therapy, after a 1-week treatment with oral benzonatate (100 mg four times a day), there were lower cough scores compared with placebo-treated patients (Gregoire et al., 1958). Despite the limitations of the trial design in these clinical studies, benzonatate was marketed as Tessalon Pearls and approved by the U.S. FDA in 1958. It is quite remarkable that since then only one further study has been undertaken with benzonatate. In a double-blind, randomized placebo-controlled study, the effect of benzonatate has been investigated on capsaicin-induced cough in nonsmoking patients with acute upper respiratory infection. Benzonatate had no antitussive effect when used alone but was effective in combination with guafenasin (Dicpinigaitis et al., 2009). Benzonatate has since been adopted by oncologists for the treatment of refractory (opiate resistant) cough in palliative care settings (Doona and Walsh, 1998), and a recent consensus panel on the management of cough in cancer has recommended its use in this clinical setting (Molassiotis et al., 2010). Benzonatate is administered orally and absorbed systemically with an onset of action of 15–20 minutes and with a 3- to 8-hour duration of action (Sweetman, 2008). The recommended dose for adults and children over 10 years of age is 100 to 200 mg every 8 hours as required, with a maximum daily dose of 600 mg. Its side effect profile is relatively benign, although recent concerns have been raised as to the safety of benzonatate with case reports of seizures (Winter et al., 2010) and cardiac arrest (Cohen et al., 2011). Benzonatate remains a prescription drug for the relief of cough in patients over the age of 10 years, but the FDA recently issued a drug safety communication about this drug.
E. Caramiphen
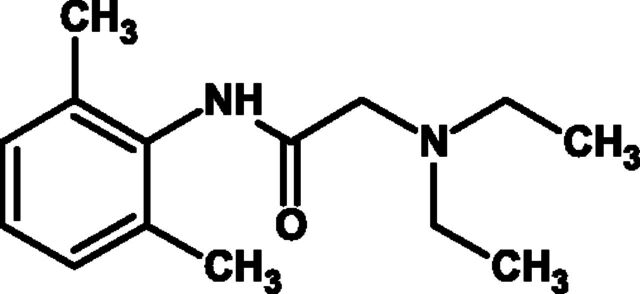
Caramiphen edisylate (Fig. 8) was originally developed as a muscle relaxant due to its anticholinergic activity. Evidence suggests that caramiphen exerts its antitussive effect centrally (Domino et al., 1985), although others have suggested it should be classified as a peripherally acting drug (Bolser et al., 1995a). Domenjoz (1952) found it equivalent to codeine in suppressing cough evoked by mechanical stimulation of rat trachea. Subsequent animal experiments confirmed this antitussive effect when caramiphen was administered intravenously (Toner and Macko, 1952; Chakravarty et al., 1956), although caramiphen was largely ineffective when administered orally (Stefko et al., 1961). However, in humans it does suppress cough when taken orally, although not consistently. A number of clinical trials have been conducted at doses typically between 10 and 20 mg daily and provided variable evidence for efficacy (Eddy et al., 1969). Only two of these were double-blind controlled trials; Abelmann and colleagues (1954) undertook a study in 20 patients with "chronic irritating cough" comparing 10 mg p.o. of caramiphen three times a day with 16.2 mg p.o. of codeine three times a day. They concluded that caramiphen was antitussive, but less effective than codeine. Glick (1963) reported similar efficacy to codeine in 12 patients with cough due to acute UTRIs. Bickerman and Itkin (1960) concluded that caramiphen was a less effective antitussive than codeine. Despite the lack of consistent evidence of efficacy, caramiphen was marketed by SmithKline Beecham as Tuss-Ornade, although in 2000, the FDA removed this drug from the United States market, citing a lack of substantial evidence that caramiphen edisylate is effective.
Fig. 8.
Chemical structure of caramiphen.
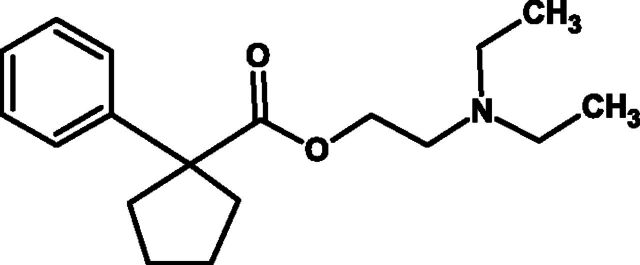
F. Carbetapentane (Also Known as Pentoxyverine)
Carbetapentane [2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentanecarboxylate] (Fig. 9) is a cough suppressant used to treat cough caused by the common cold, flu, bronchitis, or sinusitis. It is administered orally, often in combination with guaifenesin and H1-receptor antagonists. The exact antitussive mechanism is not known, although carbetapentane is thought to act mainly in the CNS where it has actions at sigma receptors (Hudkins and DeHaven-Hudkins 1991; Brown et al., 2004), kappa, and mu-opioid receptors (Kobayashi et al., 1996), which may be relevant, and additionally antimuscarinic and local anesthetic properties (at least in the skin) (Hung et al., 2012). Antitussive activity has been shown in anesthetized cats when cough was evoked by electrical stimulation of the trachea (Talbott et al., 1975) and against cough evoked by inhaled citric acid in conscious guinea pigs (Brown et al., 2004).
Fig. 9.
Chemical structure of carbetapentane.
In humans, maximum plasma concentrations are achieved 1.2 hours after oral dosing with carbetapentane and the half-life is 2.3 hours (Wen et al., 2010). However, there is very little published clinical data to suggest clinical efficacy with carbetapentane leading the FDA (2007) to conclude that this drug should not be made available as an OTC treatment of cough in the USA (2007).
G. Chlophedianol
Chlophedianol [1-phenyl-1-(o-chlorophenyl)-3-di-methylamino-propranol-1 hydrochloride] (Fig. 10) was first shown to have antitussive effects against experimentally induced cough in a variety of species (Gosswald, 1958; Boyd and Boyd, 1960; Chen et al., 1960). It was first introduced as an antitussive medicine in Germany in the 1950s (Boyd and Boyd, 1960) and has been available as an OTC oral medication (often in syrup form) and is frequently combined with H1-receptor antagonists and decongestants for treatment of URTIs. In addition to antitussive activity, clophedianol has also been reported to have local anesthetic and antihistamine activity. Chlophedianol is also thought to be a centrally acting cough suppressant, although the mechanism of action is not known. There is little information available on the pharmacokinetics of chlophedianol, and very few clinical studies have been published; one clinical study reported that chlophedianol had similar efficacy to isoaminilie citrate at suppressing experimentally induced cough in healthy subjects and in reducing cough counts in a double-blind randomized controlled trial in patients suffering from a variety of chest diseases (Diwan et al., 1982).
Fig. 10.
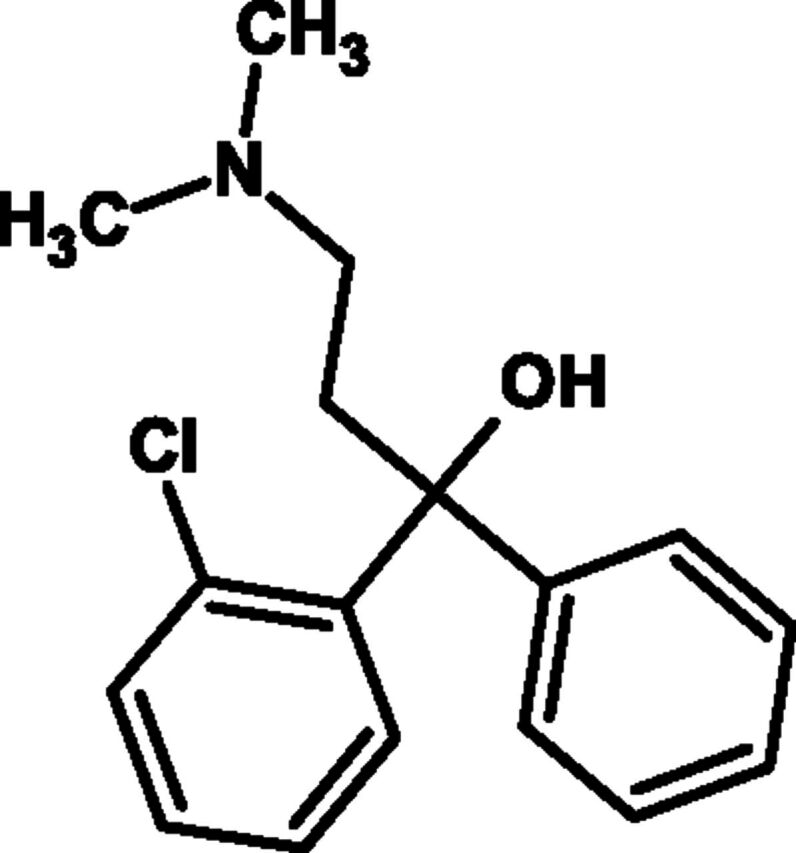
Chemical structure of chlophedianol.
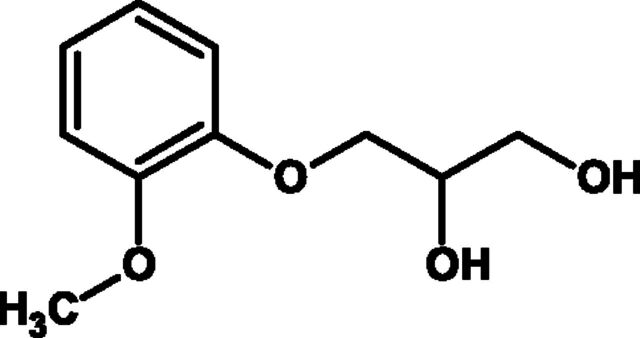
H. Levodropropizine
There have been a number of studies demonstrating the antitussive activities of levodropropizine in both adults and children. Levodropropizine is a nonopioid (Fig. 11), peripherally acting antitussive indicated for short-term symptomatic treatment of cough in adults and children older than 2 years. The mechanism of action of levodropropizine is not fully characterized, but it does not act as an antitussive secondary to bronchodilation or muscarinic receptor antagonism (Bossi et al., 1994), because at doses that reduce coughing induced by UNDW or allergen, it does not inhibit methacholine-induced bronchoconstriction in subjects with asthma (Bossi et al., 1994). One of the few studies to investigate the mechanism of action of levodropropizine has suggested that this drug can influence the firing of sensory C-fibers, at least in experimental animals (Lavezzo et al, 1992; Shams et al., 1996).
Fig. 11.
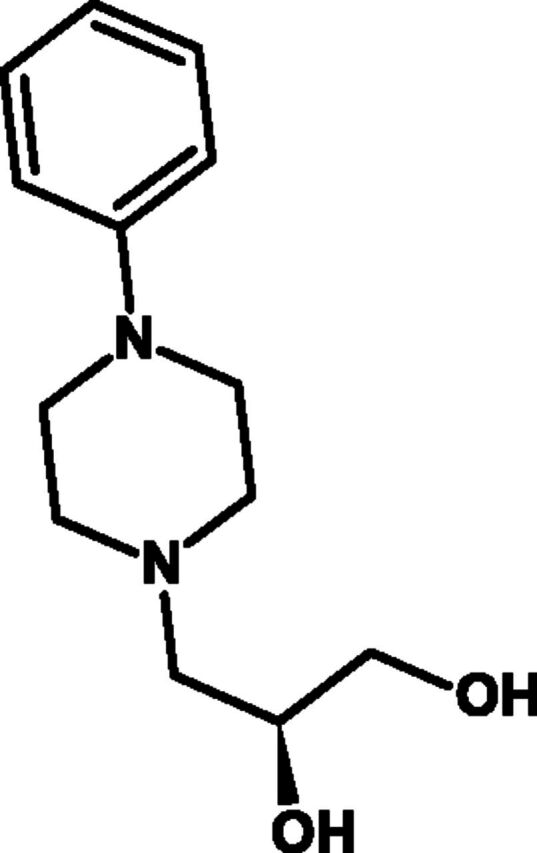
Chemical structure of levodropropizine.
Peak plasma levels were obtained between 40 and 60 minutes after administration of levodropropizine, indicating a rapid absorption from the gastrointestinal tract in normal adults (Zaratin et al., 1988). Additionally, the pharmacokinetics of levodropropizine were demonstrated to be linear over a dose range of 30–90 mg (Borsa et al., 1990), and levodropropizine dosing does not need to be adjusted in elderly patients (Lo Toro et al., 1990) or in children (Cordaro et al., 1990).
A number of studies have been performed with levododropropizine in subjects where cough was induced by mechanical or chemical stimulation. Bariffi et al. (1992) induced cough by inhalation of nebulized distilled water before and after 7 days of treatment with 60 mg of levodropropizine three times daily or placebo in a double-blind study in 10 people with allergic asthma or chronic bronchitis. After 7 days of treatment, cough was significantly reduced in the levodropropizine group but was not reduced in the placebo group. In a trial with crossover design, levodropropizine (30, 60, or 90 mg) was evaluated against citric acid-induced cough in 11 healthy volunteers. The frequency of cough was reduced significantly 2 hours after administration of 30 mg of levodropropizine and 1 hour after administration of either 60 or 90 mg of levodropropizine. In the same study, cough severity was tested in six patients with bronchitis before and after administration of 60 mg of levodropropizine. The cough severity was reduced overall after treatment with levodropropizine. An additional 22 patients with chronic bronchitis were administered 60 mg of levodropropizine three times daily for 1 week, and treatment with levodropropizine had no adverse effect on respiratory function or on airway clearance mechanisms, although drowsiness was observed in one patient after administration of 90 mg of levodropropizine (Bossi et al., 1988). In another double-blind trial involving eight healthy volunteers, levodropropizine again demonstrated a significant inhibition of citric acid induced cough at 1, 2, and 6 hours after administration (Fumagalli et al., 1992).
In another double-blind study, a single dose of 120 mg of levodropropizine or placebo was administered to 40 patients 1 hour before undergoing fiber optic bronchoscopy (Franco et al., 1992). Cough severity was significantly reduced in the levodropropizine group and also significantly fewer additional doses of local anesthetic were required. This study confirmed the results of an earlier trial involving 8 levodropropizine-treated patients and 8 placebo-treated patients. In this double-blind trial, 60 mg of levodropropizine was administered twice prior to fiber optic bronchoscopy, i.e., one dose the night before and one dose 40 minutes before the examination (Guarino et al., 1991). Mistretta et al. (1992) evaluated the efficacy of levodropropizine against capsaicin-induced cough. In a double-blind crossover study with 60 mg of levodropropizine three times a day and placebo, 12 patients with allergic rhinitis were treated for 8 days, with a wash-out period of at least 1 week. The capsaicin challenge test was performed before the treatment and on the last day of each treatment period. In the 10 evaluated patients, the total number of coughs induced by capsaicin was significantly reduced after levodropropizine treatment compared with baseline and placebo. Additionally, the threshold concentration of capsaicin required to induce 2 coughs and 5 coughs were significantly increased by levodropropizine but not with placebo. Schönffeldt et al. (2005) also used capsaicin-induced cough in a double-blind crossover study consisted of 3 days of treatment with 60 mg of levodropropizine three times a day and placebo and a wash-out period of 3 days. Twenty healthy subjects were included in the trial, but 2 subjects were excluded because of illness not related to the study. Cough threshold significantly increased after levodropropizine treatment but not with placebo. Allegra and Bossi (1988) performed 6 double-blind clinical trials with 60 mg of levodropropizine three times a day against placebo and two active controls. The subjects in the study were hospitalized patients with cough of various causes. The duration of the trial was 3 days. In the two trials against placebo, a total of 40 patients were treated with levodropropizine, and 40 were treated with placebo. In these trials, levodropropizine was significantly more effective than placebo. In two studies, a total of 28 patients were treated with levodropropizine and 29 with morclofone. The effect of levodropropizine was statistically significant, whereas morclofone did not show a significant improvement. The last two trials were against cloperastine and demonstrated a similar significant efficacy for both drugs. In these trials, 22 patients were treated with levodropropizine and 23 with cloperastine.
In most of the clinical studies with levodropropizine, there is no placebo control and they often lack validated objective end points. Thus, in a clinical trial by Catena and Daffonchio (1997), 60 mg of levodropropizine three times a day was compared with 15 mg of dextromethorphan three times a day for 5 days. In this double-blind study, 110 patients were treated with levodropropizine and 99 patients were treated with dextromethorphan. Statistically significant improvements were already seen after the second day of treatment with levodropropizine independent of the initial cough severity. The improvement associated with dextromethorphan treatment was significant only after 3 days. Night awakenings due to cough were significantly reduced for both drugs (92% for levodropropizine and 72% for dextromethorphan), but levodropropizine showed a significantly better improvement than dextromethorphan.
The effect of levodropropizine on cough associated with lung cancer was studied by Luporini et al. (1998). In a comparative double-blind trial, 75 mg of levodropropizine three times a day and 10 mg of dihydrocodeine three times a day were administered for 7 days in 66 and 69 patients, respectively. Both drugs produced a similar and significant decrease in cough score and night awakenings, but somnolence was reported in significantly more patients treated with dihydrocodeine (15 patients; 22%) than with levodropropizine (5 patients; 8%). In an open study investigating 25 patients with lung cancer, the efficacy of levodropropizine was judged according to both physician and patient satisfaction (Marchioni et al., 1990). After an average of 9 days of treatment (6–13 days) with 60 mg of levodropropizine three times a day, physicians rated the drug as sufficient in 12% of the cases, good in 48%, and optimal in 40%. The patients rated the efficacy as sufficient in 16%, good in 32%, and optimal in 48% of the cases, and the drug was well tolerated.
Efficacy and tolerability of levodropropizine and clobutinol (both 60 mg three times a day) were also compared in elderly patients of over 60 years, with a mean age of 71.4 years (Pontiroli and Daffonchio, 1997), in a double-blind study involving 95 patients treated with levodropropizine and 96 patients with clobutinol. An improvement in cough severity and frequency was already seen on the first day of treatment of both drugs, and cough was further reduced after 3 days of treatment. Occurrence of dyspnea was also investigated before and during the study, and improvement of dyspnea was significant for both drugs.
In 25 tuberculosis patients, 60 mg of levodropropizine three times a day was administered for an average of 7.5 days. The treatment was effective after 1 day, and a good or optimal result was seen at the end of the treatment of 96% of the cases (Di Pisa et al., 1989). In double-blind controlled study (Tao et al., 2005), 26 adults received levodropropizine three times a day orally, and 24 adults received dextromethorphan three times a day orally over 5 days. The overall efficacy was 70% for levodropropizine and 62% for dextromethorphan. In severe cough, the efficacy rate was significantly higher for levodropropizine (93%) than for dextromethorphan (70%) (P < 0.05).
Clinically, the efficacy and tolerability of levodropropizine is also similar in elderly patients and children compared with adults (Romandini and Rugarli, 1989; Pontiroli and Daffonchio, 1997). Fiocchi et al. (1989) studied 70 children with a mean age of 4.6 years (2 months–14 years) with acute URTI. Levodropropizine (2 mg/kg per day), subdivided into three dose administrations for 5 days or more, was reported as showing a clinical improvement in 69 of 70 patients, with three mild and transient adverse events related to levodropropizine were recorded.
In a larger clinical trial involving 180 patients between 0.5 and 12 years of age, with a mean age of 5.9 years treated for 1 week, efficacy was reported good or very good in 94% of patients. During treatment, eight (4.4%) adverse events occurred that were probably or definitely related to levodropropizine (Tamburrano and Romandini, 1989). In a double-blind, comparative clinical trial in children aged 2–14 years with a nonproductive cough due to various causes, 128 patients were treated with levodropropizine, and 122 patients were treated with dropropizine. Coughing frequency was significantly reduced on the first day for both drugs, as were the number of night awakenings due to cough. Improvement continued for the 2 days after treatment. Adverse events were reported in 11 patients treated with levodropropizine and in 16 patients treated with dropropizine (Banderali et al., 1995).
In one study, injected levodropropizine (Kim at al., 2002) was compared with dextromethorphan in 77 pediatric patients with acute or chronic bronchitis for 3 days. An improvement was already seen on the first day, and on days 2 and 3, levodropropizine was significantly more effective than dextromethorphan. The general tolerability was good.
De Blasio et al. (2012) performed an observational study on pediatricians’ routine clinical practice, including all children who presented to the offices of four family care pediatricians with acute cough (i.e., onset ≤3 weeks) associated with URTI. Of the 433 patients investigated having a mean age of 6.1 years, 80 received no treatment of cough, 101 children received levodropropizine, and 60 patients were treated with a centrally acting antitussive (51 with cloperastine and 9 with codeine). Cough resolution was significantly higher with levodropropizine than with centrally acting antitussives (47% versus 28%, respectively, P = 0.0012).
IV. Other Drugs Having an Effect on Cough
There are many other drug classes that have been shown to have a clinical benefit in reducing cough, often because they affect an underlying disease mechanism that leads to cough as a symptom (e.g., glucocorticosteroids and antibiotics) and thus probably are not strictly speaking antitussive drugs; nonetheless, we included them in this review for completeness because they are often clinically used for this indication.
A. Menthol and TRPM8 Agonists
Menthol is a monoterpine (Fig. 12) produced by the secretory gland of the peppermint plant Mentha x piperita, particularly Mentha avrensis from which most naturally occurring peppermint oil is extracted. The most common and most biologically active isomer is l-menthol, which is assumed to be the active antitussive component.
Fig. 12.
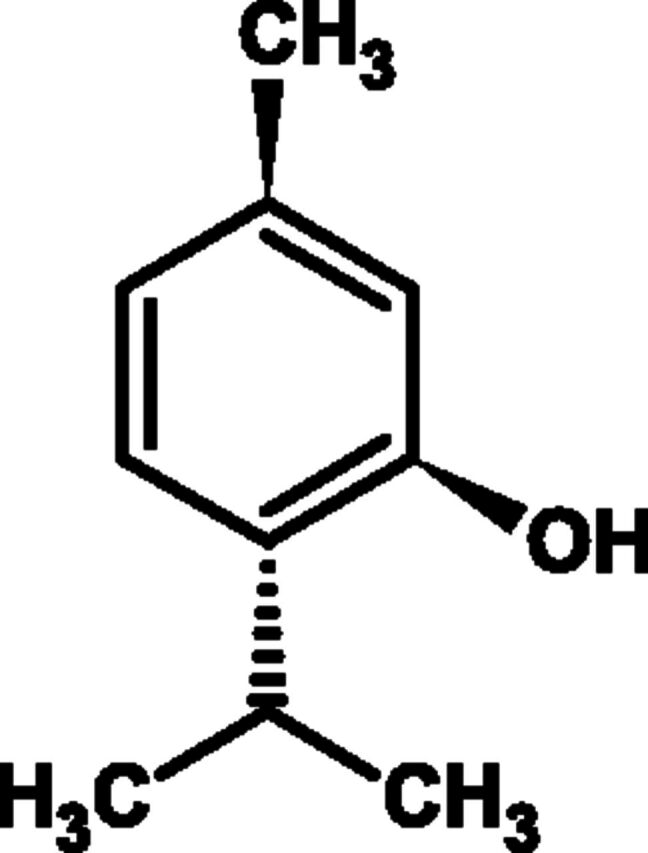
Chemical structure of menthol.
The pharmacological action of menthol was originally demonstrated by the specific binding to lung cell membranes (Wright et al., 1998). Subsequently, the molecular mechanism of menthol’s activity was revealed in a landmark paper from the David Julius laboratory (McKemy et al., 2002). They showed that menthol activates a member of the TRP family of nociceptors, which they named TRPM8. The menthol receptor is primarily located on afferent sensory neurons, and activation of this receptor gives rise to the cooling sensation experienced when inhaling menthol by allowing influx and intracellular release of calcium with subsequent depolarization. Menthol may also have a more central activity enhancing neurotransmission through a presynaptic augmentation of glutamate release (Tsuzuki et al., 2004). Another TRPM8-mediated menthol response is antinociception, which is suggested to be via blockade of neuronal voltage-gated sodium channels Nav1.8 and Nav1.9 (Gaudioso et al., 2012), although it is not yet known whether this action contributes to its antitussive activities. At higher concentrations menthol also has several other actions including activation of L-type calcium channels (Wright et al., 1997) and nicotinic acetylcholine receptors (Hans et al., 2012), but to what extent these pharmacological actions contribute to the antitussive activities of menthol remains unknown.
Menthol has an ancient history and has become a stock ingredient of many OTC preparations from dental care to food flavorings. The antitussive activity of menthol was commercialized by the development of a topical rub by Lumsford Richardson in 1890 (Al Aboud, 2010). The “vaporub” continues to be a multi-million dollar product and has been joined by numerous syrups, lozenges, and inhalers.
Animal studies have shown a greater than 50% reduction in citric acid-induced cough in conscious guinea pigs by inhalation of menthol vapor (Laude et al., 1994). In mice, irritancy induced by a wide range of protussive substances was inhibited by menthol, an effect that was antagonized by the TRPM8 antagonist AMTB (Willis et al., 2011). Recent evidence indicates that the antitussive activity of menthol may reside in the activation of nasal, as opposed to pulmonary sensory afferents (Plevkova et al., 2013), inferring that menthol inhibits cough by a central “gating” mechanism rather than any local antitussive activity.
Clinical evidence of menthol’s activity from controlled clinical studies is sparse. In a small and poorly controlled study, menthol vapor produced a short-lasting decrease in capsaicin-induced cough in normal subjects (Wise et al., 2012). Furthermore, cough induced by inhalation of citric acid was reduced in adults by inhalation of menthol vapor compared with air or pine oil control (Morice et al., 1994). In children, evoked cough was reduced compared with baseline challenge but failed to reach significance compared with placebo (Kenia et al., 2008). For such a widely used product, it is perhaps surprising that there are no published clinical studies on the effect of menthol or of the many products containing it in patients with either acute or chronic cough.
B. Erdosteine
Erdosteine is a homocysteine analog (Fig. 13) that is marketed in a number of countries as an expectorant and antioxidant in the treatment of bronchitis and COPD. Erdosteine has a wide range of interesting pharmacological properties of relevance to the treatment of respiratory diseases, including demonstrating some anti-inflammatory actions, antioxidant activity, and effects on the mucociliary escalator and properties of mucous. These have been reviewed elsewhere (Dal Negro, 2008). A recent meta-analysis demonstrated clear evidence from a number of controlled clinical trials of the effectiveness of erdosteine in the treatment of patients with COPD and bronchitis (Cazzola et al., 2010). The majority of the actions of erdosteine appear to be secondary to a metabolite called metabolite 1 (reviewed in Dal Negro, 2008). There is also some evidence that erdosteine can also have antitussive activity. Thus, in the guinea pig, erdosteine has been shown to inhibit citric acid-induced cough, although in the same study, these authors showed other effects in the airways, and therefore, it is not clear whether there is a direct antitussive effect of this drug or whether the antitussive effects are secondary to anti-inflammatory activity or effects of this drug on mucous (Hosoe et al., 1999). There is very limited, often anecdotal evidence for an antitussive effect of erdosteine clinically (Dal Negro, 2008), and there is a clear need to undertake well-controlled clinical trials to establish whether erdosteine does indeed possess clinically relevant antitussive activity.
Fig. 13.
Chemical structure of erdosteine.
C. Antibiotics
Antibiotics are used widely for the treatment of acute cough despite recommendations against their usage by international guidelines (Braman, 2006a). There are large cultural differences in the use of antibiotics, with the United Kingdom and the United States being among the highest users (Deschepper et al., 2008). Acute cough comprises a number of clinical conditions that include URTI or the common cold, acute bronchitis, and whooping cough. The distinction between them is often not possible with clinical assessment alone. However, viral infection is by far the most common cause of cough, for which antibiotics are not recommended. Antibiotics should, however, be used for severe bacterial infections of the respiratory tract and for treating the early phase of whooping cough but these conditions are often difficult to differentiate from viral illness; hence, clinical judgment in individual cases is required. There are a good number of placebo-controlled trials of antibiotics in the treatment of acute cough, most commonly investigating erythromycin (Fig. 14A), amoxicillin (Fig. 14B), or doxycycline (Fig. 14C) (Braman, 2006b). The focus of most clinical trials has been to assess resolution of the acute illness and general well-being. Cough outcomes are often not reported or are limited, and therefore, it is difficult to interpret their clinical importance. Cough reflex sensitivity has seldom been studied in placebo-controlled trials of antibiotics. In acute cough, cough reflex sensitivity is increased, although it is not clear whether the reduction that follows resolution of the illness is accelerated with antibiotics.
Fig. 14.

(A) Chemical structure of erythromycin. (B) Chemical structure of amoxicillin. (C) Chemical structure of doxycycline.
The impact of antibiotics on cough has largely been studied in patients with acute bronchitis. A recent meta-analysis reporting four studies that met the inclusion criteria and reported cough outcomes (3 acute bronchitis studies, 1 URTI, 1 doxycycline, 1 myrtol, and 2 erythromycin) concluded a modest benefit in favor of antibiotics (Smucny et al., 2004). At follow up, patients given antibiotics were less likely to have a cough (relative risk 0.64; number needed to treat for an additional beneficial outcome 6) and have a nocturnal cough (relative risk 0.67; number needed to treat for an additional beneficial outcome 7) (Smucny et al., 2004). However, the use of antibiotics was associated with side effects. The clinical benefit of antibiotics for cough is likely to be small for most patients, and one study suggested that patients aged greater than 55 years who coughed frequently at presentation benefited most from antibiotics (Verheij et al., 1994). Further studies are needed to confirm this and identify other clinical parameters that may help identify a subgroup of patients who benefit most. There are a few studies that have investigated delayed inhibition of antibiotics compared with immediate use of antibiotics for the treatment of acute cough and reported cough outcome measures, with cough not being worse in those who received delayed antibiotics (Arroll et al., 2002). Postinfectious (subacute) cough is another common clinical scenario in which antibiotics are often prescribed. However, there is a paucity of evidence to guide clinicians, and the ACCP does not recommend antibiotics in this group of patients (Braman, 2006b).
In chronic cough, two different paradigms have been applied to support the use of antibiotics. In children, the chronic colonization of the lower respiratory tract has led to the concept of “persistent bacterial bronchitis” (Fitch et al., 2000; Marchant et al., 2006). Chang, in a reanalysis of two studies of “wet” chronic cough in children, concluded that antibiotic usage may be of benefit (Marchant et al., 2005). In adults, the macrolide antibiotics are widely used to treat a number of respiratory conditions, including COPD (Albert et al., 2011), cystic fibrosis (Wolter et al., 2002), and bronchiectasis, with the major effect being on exacerbation rate. The suggestion has been made that this diverse activity resides in the antireflux (and hence antitussive) effects of macrolides through their motilin-like activity (Crooks et al., 2011). Unfortunately, a study by Yousaf et al. (2010) in patients with refractive chronic cough showed no effect of erythromycin on cough reflex sensitivity, despite a significant reduction in airway inflammation, although this trial was underpowered.
D. Glucocorticosteroids
The demonstration of airway inflammation in some patients with cough has provided important insights into a number of common causes of cough and some understanding of the peripheral mechanisms responsible for cough reflex sensitization (McGarvey et al., 2009). For example, the measurement of specific cellular and mediator profiles within the airway provides clinical value in distinguishing asthmatic from nonasthmatic cough and a means of identifying patients likely to respond to corticosteroid therapy (Pizzichini et al., 1999; Hahn et al., 2007; Prieto et al., 2009).
Corticosteroids have formed the mainstay of anti-inflammatory therapy in respiratory disease for almost 50 years. Corticosteroids exert their anti-inflammatory effect via diffusing across the cell membrane where they bind to and activate glucocorticoid receptors (GR) in the cytoplasm before moving across the nuclear membrane and binding with DNA sequences called glucocorticoid receptor elements (GRE). The resulting GR-GRE complex then regulates transcription to induce anti-inflammatory proteins [e.g., lipocortin-1, secretory leukocyte inhibitory protein, interleukin (IL)-10, IL-12] and repression of inflammatory proteins (e.g., IL-4, IL-5, IL-6, IL-13, and tumor necrosis factor α). Alternatively the GR-GRE complex may regulate other glucocorticoid responsive genes with the help of transcription factors such as nuclear factor-κB (Rhen and Cidlowski, 2005). Corticosteroids reduce the number and activity of inflammatory cells (eosinophils, mast cells, T-lymphocytes) in the airway most likely by inhibiting their recruitment and their survival in the airway. Anti-inflammatory effects are seen quite rapidly, with reduction in eosinophils within a few hours (Gibson et al., 2001; Ketchell et al., 2002), although other changes such as bronchial hyper-responsiveness (BHR) may take some months to improve (Juniper et al., 1990). Topically acting corticosteroids have been formulated so that they can be delivered by inhalation to the airway, so minimizing the systemic side effects commonly seen after oral or parenteral administration, exemplified by beclomethasone dipropionate (Fig. 15). All corticosteroids have similar efficacy, but different pharmacodynamic and pharmacokinetic profiles can account for differences in the therapeutic ratio seen among different members of this drug class. These include glucocorticoid receptor binding affinity, particle size, lung residence time, protein binding, and lipophilicity, all of which can influence lung and systemic bioavailability and ultimately efficacy and safety. Fluticasone propionate (FP), for example, has higher receptor binding affinity compared with budesonide or beclomethasone dipropionate (BDP) and also has a much greater volume of distribution.
Fig. 15.
Chemical structure of beclamethasone dipropionate.
Given that corticosteroid treatment is so widely used in the treatment of airway disease and corticosteroid responsive cough syndromes, it is surprising that stronger evidence in the form of large randomized controlled trials is still not available. Current practice has evolved mainly from anecdotal experience, consensus opinion [Morice et al., 2004, 2006; Irwin et al., 2006; Kohno et al., 2006; Gibson et al., 2010; Kardos et al., 2010; Asthma Workgroup, Chinese Society, Respiratory, Diseases (CSRD), Chinese Medical Association, 2011], and the extensive body of evidence showing the efficacy of corticosteroids in controlling symptoms (including cough) in more typical "classic" asthma. However, given the broad range in source and quality of evidence, there is no consistent recommendation on dose or duration of corticosteroid therapy for the treatment of cough.
Asthma accounts for cough in up to a third of patients referred for evaluation and who have an airway inflammatory profile characterized by eosinophilia, TH2 cytokines (Gibson et al., 1998), and airway remodeling (Niimi et al., 2005). However, distinct asthma phenotypes are now recognized, with asthma being a syndrome rather than a distinct disease entity, with cough sometimes being the sole respiratory symptom, and although spirometry is normal, BHR is present. This is referred to as cough variant asthma (CVA), and improvement in cough with bronchodilators and/or corticosteroids confirms the diagnosis of CVA (Corrao et al., 1979). Long-term follow up suggests that in some cases that CVA may be a precursor for a more typical asthma pattern of disease (Cheriyan et al., 1994), although inhaled corticosteroids (ICS) may help to prevent this.
The earliest studies of corticosteroid therapy for asthmatic cough comprised small prospective open label studies (Doan et al., 1992) or retrospective descriptions of empirical trials (Corrao et al., 1979). Daan and colleagues described the value of a trial of oral prednisone to diagnose CVA in a highly selected group of patients with cough. Nine of 10 patients responded, and control was maintained with inhaled therapy. This approach appears to be associated with good long-term control of symptoms (Corrao et al., 1979). A small number of randomized controlled trials have demonstrated the efficacy of ICS for asthmatic cough. In a small placebo-controlled study, treatment with high dose BDP (500 μg three times a day) administered for 1 month was associated with significant improvement of cough symptoms, cough reflex sensitivity, and BHR (Di Franco et al., 2001). Inhaled BDP was also effective in a placebo-controlled study of patients with cough, 60% of whom had an asthmatic cough (Irwin et al., 1997). The value of ICS therapy may go beyond symptom control, because evidence suggests that such treatment may prevent progression to "classic" asthma (Matsumoto et al., 2006).
The identification of a subgroup of patients manifesting as asthma with airway eosinophila and a corticosteroid responsive cough, but without evidence of airway motor dysfunction (specifically BHR), has led to the recognition of a new diagnostic etiology, nonasthmatic eosinophilic bronchitis (NAEB). Various reports suggest this may account for between 10 and 15% of cases of chronic cough (Brightling et al., 1999, 2000a). Trials of ICS therapy in NAEB are generally associated with improvement of symptoms and a reduction in airway eosinophils (Gibson et al., 1995; Brightling et al., 2000b). Oral corticosteroids are rarely required. Another cough syndrome, virtually identical to NAEB but presenting in atopic patients, has been described in the Japanese population (Fujimura et al., 2003).
Although most evidence for efficacy has been in asthmatic and NAEB patients, ICS appear effective in nonasthmatic patients presenting with cough. In a randomized, placebo-controlled 2-week trial of inhaled FP (500 μg twice a day) in patients with normal spirometry and cough for more than 1 year, significant improvement in cough symptoms and in the levels of airway inflammatory mediators was observed (Chaudhuri et al., 2004). However, a randomized, placebo-controlled crossover study of lower dose ICS (200 μg of BDP twice daily) given for a shorter period of time (2 weeks) did not appear to be effective (Evald et al., 1989). In contrast, a 4-week, placebo-controlled trial of another high-dose inhaled ICS (500 μg of FP twice a day) in primary care patients presenting with cough lasting more than 2 weeks appeared effective, but only in the subgroup of nonsmokers (Ponsioen et al., 2005). Given the inclusion of patients with recent onset cough in this study, a proportion is likely to have had post-viral cough. As respiratory viruses responsible for the common cold induce inflammation in both the upper and lower airways (Fraenkel et al., 1995; Trigg et al., 1996), the anti-inflammatory effects of ICS in reducing post-viral cough have also been studied. Gillissen et al. (2007) reported significantly faster and greater decline in cough frequency with extra-fine HFA-BDP compared with placebo in patients with cough lasting more than 3 days but less than 2 weeks. However, Pornsuriyasak et al. (2005) found no effect of inhaled budesonide (400 μg twice a day) given for 4 weeks in patients with cough lasting more than 3 weeks after a URTI.
When cough persists in a child for more than a few weeks after an URTI concerns are often raised as to whether there is underlying chest disease. There is little evidence that ICS are effective in the treatment of post-viral cough in children. Two randomized, placebo-controlled trials have been conducted. In one study, low-dose ICS (400 μg of BDP daily for 2 weeks) was ineffective compared with placebo (Chang et al., 1998). In a high-dose ICS study (2 mg of FP daily for 3 days followed by 1 mg daily for 11 days), there was a significant improvement in nocturnal cough with the ICS, although improvement was also seen in the placebo arm (Davies et al., 1999). The Cochrane Database of Systematic Review concluded that any clinical impact with very high dose ICS is unlikely to be beneficial (Tomerak et al., 2005).
Although cough is a common symptom in COPD, the focus of treatment has been on preventing progressive lung function decline, reducing exacerbation frequency and improving quality of life. Prior to current recommendations that ICS should not be used in isolation for the treatment of COPD, a large number of trials of ICS therapy were undertaken, although very few reported on any therapeutic impact on cough. Auffarth et al. (1991) reported no significant improvement in cough or cough reflex sensitivity after treatment with budesonide 1600 μg daily for 12 weeks compared with placebo, although a trial of inhaled FP (500 μg two times a day) for 6 months was reported as effective (Paggiaro et al., 1998).
Cough is a distressing symptom also suffered by patients with idiopathic pulmonary fibrosis (IPF) (Key et al., 2010) and an independent risk factor for disease progression (Ryerson et al., 2011). A small open label trial of oral prednisolone was associated with a reduction in the cough sensitivity to capsaicin and improvement in cough scores (Hope-Gill et al., 2003). Larger placebo-controlled trials to assess the efficacy of corticosteroid treatment of cough in this clinical setting are required.
E. β2-Agonists
Studies in both experimental animals and humans demonstrated the occurrence of cough suppression after administration of β2-agonists best exemplified by salbutamol (albuterol) (Fig. 16), but whether the antitussive effect of these drugs is due exclusively to their bronchodilator action or other mechanisms remains controversial (Freund-Michel et al., 2010; Ohkura et al., 2010). In guinea pigs, a variety of β2-agonists administered by various routes have been shown to inhibit cough induced by citric acid (Forsberg et al., 1992; Horiuchi et al., 1995), capsaicin (Nishitsuji et al., 2004), capsaicin and citric acid (Lewis et al., 2007; Freund-Michel et al., 2010), methacholine (Ohkura et al., 2009), and both allergic and capsaicin-induced cough (Bolser et al., 1995b). In one study, however, prior perfusion of the extrathoracic trachea with isoproterenol did not inhibit citric acid-induced cough in anesthetized animals (Canning et al., 2006).
Fig. 16.
Chemical structure of salbutamol (albuterol).
Studies of artificially induced cough in healthy human volunteers have yielded discordant results in terms of the antitussive effect of β2-agonists. Multiple studies have demonstrated the ability of these agents to inhibit cough, including the action of fenoterol against UNDW-induced cough (Lowry et al., 1987, 1988a), albuterol against citric acid-induced cough (Karttunen et al., 1987), and procaterol against substance P-induced cough in volunteers with acute URTIs (Katsumata et al., 1989). However, inhaled procaterol has been shown to have no effect on cough reflex sensitivity to inhaled capsaicin in normal volunteers or in subjects with asthma or chronic bronchitis, despite inducing significant bronchodilation (Fujimura et al., 1992, 1993). Furthermore, albuterol has been shown not to inhibit cough induced by capsaicin (Nichol et al., 1990; Smith et al., 1991) or citric acid (Pounsford et al., 1985) in normal volunteers. However, in smoking-related cough, albuterol afforded significant protection (Mulrennan et al., 2004).
In terms of pathologic cough, multiple studies have evaluated the effect of β2-agonists in patients with CVA. Clinical experience has shown that most patients with CVA will experience improvement in cough after 1 week of β2-agonist therapy, although full resolution of cough may require up to 8 weeks of treatment, including an ICS (Irwin et al., 1997). However, the leukotriene receptor antagonists (LTRAs) have been shown to be more effective than β2-agonists and ICS asthmatic cough in subjects with asthma (Dicpinigaitis et al., 2002; Tamaoki et al., 2010).
Available evidence does not support the use of β2-agonists in acute cough in children and adults without asthma or COPD (Smucny et al., 2001; Becker et al., 2011), nor for chronic, nonasthmatic cough in children (Chang et al., 1998; Tomerak et al., 2005). Furthermore, a recent review failed to reveal a beneficial effect of albuterol against whooping cough (pertussis) (Bettiol et al., 2010).
Although β2-agonists represent the standard of bronchodilator care in the treatment of airway obstruction associated with asthma or COPD, controversy persists regarding the mechanism(s) by which these agents alleviate cough. Additional studies are needed examining whether an antitussive effect of the β2-agonists is due solely to their action as bronchodilators or if other mechanisms are relevant. Further insight into this question may guide future research into a potential role for these agents in the treatment of other types of pathologic cough.
F. Muscarinic Receptor Antagonists
The parasympathetic nervous system regulates bronchomotor tone and release of mucus into the airways. These effects are mediated through muscarinic (M) receptors in the lung. Three types of M receptors (M1, M2, and M3) are present in the lungs, which are the targets of currently available muscarinic receptor antagonists (Flynn et al., 2009). Although a plethora of basic and clinical information exists regarding the effect of muscarinic receptor antagonists on airway tone and pulmonary function, relatively little attention has been devoted to the evaluation of the effect of these agents on various forms of pathologic cough.
Multiple animal studies have demonstrated an antitussive effect of muscarinic receptor antagonists. In awake guinea pigs, ipratropium bromide inhibited both allergic and capsaicin-induced cough (Bolser et al., 1995b), and atropine diminished cough reflex sensitivity to inhaled capsaicin (Jia et al., 1998; Li et al., 2002). In conscious dogs, atropine strongly inhibited electrically evoked cough (Tsubouchi et al., 2008). On the other hand, ipratropium bromide was shown to inhibit citric acid-induced bronchoconstriction, but not cough, in conscious guinea pigs (Forsberg et al., 1992), and atropine did not inhibit citric acid-induced cough in anesthetized guinea pigs (Canning et al., 2006). However, any antitussive effect of muscarinic receptor antagonists is unlikely to be a direct airway effect on the cough reflex, because there are no muscarinic receptors on airway afferent nerves (Birrell et al., 2014).
The ability of muscarinic receptor antagonists (exemplified by ipratropium bromide; Fig. 17) to inhibit experimentally induced cough in humans has been well documented. Ipratropium bromide was shown to inhibit cough induced by UNDW and hypotonic saline solution in healthy volunteers (Lowry et al., 1987), as well as in subjects with asthma (Lowry et al., 1988a). Additionally, ipratropium bromide, but not albuterol, inhibited citric acid-induced cough in subjects with asthma (Pounsford et al., 1985). Oxitropium bromide was shown to inhibit UNDW-induced cough in healthy volunteers as well as in subjects with asthma but was unable to do so in subjects with cough due to URTI (Lowry et al., 1994). Intravenously administered glycopyrrolate was also shown to diminish cough reflex sensitivity to inhaled capsaicin in healthy volunteers (van Wyk et al., 1994). However, four other studies were unable to demonstrate an effect of a muscarinic receptor antagonist. An aerosol of atropine diminished UNDW-induced bronchoconstriction, but not cough, in subjects with asthma (Sheppard et al., 1983; Fuller and Collier, 1984). In healthy volunteers, ipratropium bromide was unable to inhibit cough due to capsaicin (Smith et al., 1991), as well as cough due to capsaicin, prostaglandin F2α, and their combination (Nichol et al., 1990).
Fig. 17.
Chemical structure of ipratropium bromide.
Studies evaluating the clinical effect of muscarinic receptor antagonists in pathologic cough in adults are sparse and nonexistent in children (Chang et al., 2004). In one controlled, double-blind crossover trial evaluating 14 adult nonsmokers with persistent, postviral cough, inhaled ipratropium bromide significantly improved subjectively rated cough compared with placebo (Holmes et al., 1992), whereas oxitropium bromide did not demonstrate antitussive activity, based on subjective assessment, in a randomized, double-blind placebo-controlled study of 56 nonasthmatic volunteers with URTI (Lowry et al., 1994).
In another randomized, double-blind placebo-controlled study, tiotropium bromide was shown to inhibit cough reflex sensitivity to inhaled capsaicin in adult nonsmokers with acute viral URTI but had no effect in healthy subjects (Dicpinigaitis et al., 2008). Inhibition of cough reflex sensitivity was documented as early as 1 hour after tiotropium bromide administration, and there was no significant association between changes in cough reflex sensitivity and bronchodilation. Thus, the mechanism by which tiotropium bromide inhibited cough reflex sensitivity in this study remains unclear. Other potential mechanisms include an effect on airway mucus glands, inflammatory mediators, inflammatory cells, epithelial permeability, vascular blood flow, and clearance of substances applied to the airway lumen, all of which could induce an alteration in cough-receptor sensitivity (Bateman et al., 2009).
Despite a substantial body of evidence from studies in animals and in experimentally induced cough in humans supporting antitussive activity of muscarinic receptor antagonists, trials aimed at evaluating the clinical efficacy of these agents in pathologic cough have not yet been performed. Thus, properly executed clinical studies of currently available muscarinic receptor antagonists in various types of acute and chronic pathologic cough are required.
G. Mucolytics and Expectorants
Drugs that act upon the airway mucociliary apparatus, commonly known as expectorants and mucolytics are widely prescribed for the treatment of productive cough. Expectorants are thought to increase mucus volume and mucolytics reduce mucus viscosity, thereby facilitating clearance of airway secretions by cough and ciliary transport. The promotion of effective cough is thought to reduce cough frequency.
Guaifenesin (Fig. 18), formerly known as glyceryl guaicolate, is the only FDA-approved expectorant for children and adults. It is also used as a muscle relaxant in veterinary medicine. Guaifenisin has been extracted from the bark of the guaiac tree for many centuries for its expectorant qualities. Guaifenisin is a white crystalline powder that is soluble in water or alcohol and administered via the oral route. It has a short half-life of 1 hour and is excreted in urine. Modified release preparations given twice daily are available. The mechanism of action of guaifenisin is not clear, but it is thought to influence the cholinergic innervation of airway mucous glands (Rubin, 2007). Despite its widespread use, there is little evidence that guaifenesin alters ciliary motility or mucociliary clearance in vitro or in vivo (Sisson et al., 1995; Yeates et al., 1977). Furthermore, the clinical evidence for its expectorant and antitussive efficacy is conflicting. Kuhn et al. (1982) did not find a significant reduction in either subjectively reported cough severity or objective cough frequency in patients with URTI. However, patients with productive cough did report a reduction in sputum viscosity, although it is not clear if they found this to be clinically useful. In contrast, a larger study by Robinson et al. (1977) that administered a lower dose of guaifenesin reported a significant reduction in subjective cough severity; 75% of patients had reduced cough severity after therapy compared with 31% in the placebo group. A more recent study reported that guaifenesin inhibits cough reflex sensitivity in patients with URTI (Dicpinigaitis and Gayle, 2003b). However, a conclusive antitussive effect of guaifenesin or other expectorants cannot be established or dismissed based on the available data. Further studies are needed, ideally in patients with acute cough with adequate sample sizes and which include validated endpoints such as health status questionnaires and objective cough monitoring (Birring, 2011b). The interaction between guaifenesin and antitussive drugs such as dextromethorphan also needs investigating because this drug combination is widely sold, despite limited evidence for its efficacy.
Fig. 18.
Chemical structure of guaifenesin.
The evidence for antitussive activity of mucolytics is even more limited. Carbocisteine (S-carboxymethyl l-cysteine) is a derivative of the amino acid l-cysteine (Fig. 19). It is widely available in Europe and South America and is largely used in the treatment of patients with COPD, where it has been shown to reduce exacerbations (Poole and Black, 2010). Carbocisteine has a half-life of 1.3 hours and is excreted through urine. The evidence for mucolytic activity of this drug is conflicting: Goodman and colleagues (1978) and Thomson and colleagues (1976) found no effect of carbocisteine on mucus clearance, whereas Edwards and colleagues (1976) found a reduction in mucus viscosity. The mechanism of action is thought to be a resetting of the balance between sialomucins and fucomucins, increasing the former, an important component of mucus, and thus restoring viscoelastic properties (Hooper and Calvert, 2008). Carbocisteine may have a number of antitussive actions, including an ability to increase the concentration of chloride in airway secretions, reduce airway tachykinins, and reduce cough reflex hypersensitivity (Colombo et al., 1994; Katayama et al., 2001; Ishiura et al., 2003). However, overall the clinical evidence for an antitussive action of carbocisteine is limited because most studies focused on its mucolytic activity in patients with chronic bronchitis. When cough severity has been reported as an outcome measure in placebo-controlled trials, no effect has been demonstrated (Thomson et al., 1975; Edwards et al., 1976). Similarly for N-acetylcysteine, another widely used mucolytic drug, most studies reporting cough severity did not report a significant effect of this drug (Bolser, 2006).
Fig. 19.
Chemical structure of carbocisteine.
Bromhexine is a synthetic derivative of vasicine from the Indian shrub Adhatoda vasica (Acanthacae) first introduced in 1963. Vasicine has been used in India for centuries as an expectorant and antitussive. Bromhexine and its metabolite ambroxol are widely used as secretolytics, inducing hydrolytic depolymerization of mucoprotein fibers and for their ability to modulate the activity of mucus-secreting cells (Shimura et al., 1983). Hamilton et al. (1970) compared bromhexine and placebo in 22 hospitalized patients with bronchitis with mucoid sputum. Treatment with bromhexine resulted in a significant decrease in sputum viscosity and an increase in sputum volume, as well as a marked change in the rheological characteristics of the sputum.
Secretolytic treatment with bromhexine led to a reduction in expectoration and reduced the severity/frequency of coughing (Lal and Balla, 1975). Thirty-two patients suffering from acute-stage COPD were randomized to bromhexine (48 mg/day, n = 16) or to sobrerol (600 mg three times a day, n = 16) for 10 days treatment. The alleviation of cough and normalization of expectoration were significantly more pronounced with bromhexine (Iaia and Marenco, 1990).
Bromhexine in combination with antibiotic treatment has been shown to have significant effects on sputum volume and viscosity. Thus, in 392 adult patients diagnosed clinically with acute bronchitis or pneumonia of bacterial etiology administered amoxicillin (250 mg) plus bromhexine (8 mg) or amoxicillin (250 mg) alone, those patients receiving the combined treatment had a significantly (P < 0.001) greater reduction of their symptom scores at day 3 for cough discomfort, cough frequency, ease of expectoration, and sputum volume (Roa and Dantes, 1995). Similarly, when ambroxol was given together with an antibiotic, cough was significantly reduced compared with placebo (Germouty and Jirou-Najou, 1987).
H. Antacids/Proton Pump Inhibitors and Gastrointestinal Motility Drugs
That drugs active on the gastrointestinal tract gut may be useful in the management of cough is, on first consideration, surprising. This proposition is based on the hypothesis that the mitigation of aspiration into the airway is a major and perhaps the primary physiologic function of the cough reflex. It follows that disorders of the upper gastrointestinal tract may precipitate cough either directly or by causing increased sensitivity of the afferent airway sensory nerves.
The aspiration of food has long been recognized as a precipitant of coughing, but it was not until 50 years ago that gastroesophageal disease related to reflux and aspiration was commonly associated with chronic cough (Belcher, 1949; Pellegrini et al., 1979). However, which form of gut disorder is associated with cough and the underlying mechanisms, particularly the precipitation of airway inflammation and afferent neuronal hypersensitivity, remain to this day a matter of considerable debate (Morice et al., 2012).
GERD with the classic symptoms of dyspepsia and heartburn was quickly recognized as a common association in some patients with chronic cough. GERD with esophagitis due to reflux of acid stomach contents thus became the first proposed therapeutic target of drugs acting on the gastrointestinal tract being used to treat cough. Indeed blockade of acid production by H2-receptor antagonists and proton pump inhibitors (PPI) has become standard therapy recommended in guideline documents from both sides of the Atlantic (Morice et al., 2004; Irwin et al., 2006). Initial open label and uncontrolled studies suggested a high response rate. However, subsequent randomized placebo-controlled studies indicate little effect of such treatment compared with placebo, suggesting that despite the epidemiologic evidence, either reflux was not an important cause of cough or that acid was not, as Congreve (1881) puts it, “the exciting cause.”
That non-acid reflux and aspiration may cause cough is suggested by the association of other, non-respiratory symptoms such as rhinitis and dysphonia. Indeed the loss of voice and lack of classic peptic symptoms led to use of the term “silent reflux” (Kennedy, 1962)—hardly an appropriate epithet for a condition causing cough. These observations coupled with the realization that dysmotility of the upper gastrointestinal tract may precipitate cough (Fouad et al., 1999; Kastelik et al., 2003) led to the use of promotility agents as antitussives. More recently a wide variety of agents in addition to those with licensed indications for gastrointestinal motility disorders have been advocated for the treatment of cough, reflecting the paucity of effective drugs for this common symptom.
A single, double-blind crossover study has compared the H2-receptor antagonist 150 mg of ranitidine two times a day for 2 weeks with placebo on reported cough scores in 25 patients (Ing et al., 1992). Treatment response was seen in both groups but was significantly greater in the ranitidine arm. The addition of ranitidine at night to proton pump inhibitor therapy has been advocated on the basis that nocturnal acid secretion is under vagal control via a histaminergic mechanism and that the typical morning cough may be reduced by an increase in gastric pH (Khoury et al., 1999).
A number of uncontrolled studies reported a high and occasionally complete success with “aggressive” antacid therapy with PPIs in patients with chronic cough (Irwin et al., 1989; Waring et al., 1995). These reports led to the adoption of twice daily PPIs as standard therapy for reflux cough in many centers and into local and international guidelines. Placebo-controlled studies have, however, been much less positive, leading a recent Cochrane review (Chang et al., 2011) to conclude “there is insufficient evidence to conclude that in adults treatment with PPI for cough associated with GERD is beneficial.”
Two of the most widely quoted studies claiming to have positive outcomes with PPI therapy have serious flaws in their analysis as discussed below. In a randomized, placebo-controlled, double-blind crossover trial, Kiljander et al. (2000) studied the effect of 8 weeks 40 mg of omeprazole once daily in 21 completing patients with cough and pH-metry proven GERD. Not suprisingly, there was a highly significant improvement in classic peptic symptoms. Diary record card day and night-time cough scores, however, were not significantly improved, except in a subgroup analysis of the 12 patients who received placebo first.
Ours et al. (1999) randomized 23 patients in a placebo-controlled parallel group study of omeprazole (40 mg) twice daily for 12 weeks. Patients were stratified depending on whether there was GERD present on baseline pH-metry. The six subjects without acid reflux had no response, whereas the authors report a striking improvement in cough in 6 of the 17 pH-positive subjects. However, this observation was made in the open label termination part of the study.
Two randomized placebo controlled studies have been published subsequent to the Cochrane analysis. Both clearly demonstrate no additional effect of PPIs on cough, reinforcing the negative conclusions to the efficacy of acid suppression in chronic reflux-related cough. Faruqi et al. (2011) compared twice daily esomeprazole (40 mg) in 50 patients in a parallel group study. Reduction in cough scores was similar in both treatment and placebo arms, with a trend to greater effect in those patients with prominent peptic symptoms. Shaheen et al. (2011) similarly found no additional effect over placebo of esomeprazole (40 mg) twice daily for 12 weeks in patients with isolated chronic cough without dyspepsia.
A number of studies of upper airways symptom management by PPIs have included cough as a secondary measure. Eherer et al. (2003) studied the effect of pantoprazole (40 mg) twice daily on reflux-induced laryngitis and again observed no greater improvement in symptoms with active treatment over placebo. Vaezi et al. (2006) in a study of patients with chronic posterior laryngitis showed a slightly greater effect of placebo over esomeprazole (40 mg) twice daily for 16 weeks.
Thus, recent studies add to the considerable body of evidence that antacid therapy should not be considered in the treatment of reflux cough, except in those patients with marked peptic symptoms. Indeed given the evidence for an increased risk of pneumonia in patients on PPIs there is reason to suppose that their injudicious use may even be harmful (Laheij et al., 2004).
With the increasing evidence that esophageal dysmotility rather than simple GERD underlies the genesis of gastrointestinal-related cough, promotility agents such as the peripheral dopamine agonists metoclopramide and domperidone are increasingly used. However, there is a dearth of high-quality studies showing benefit. Poe and Kallay (2003) describe the addition of metoclopramide (or cisapride) to PPI therapy in chronic reflux cough with a doubling of response rate, whereas Gupta (2007) suggests a dramatic response in patients with paroxysmal cough, although a central mechanism is proposed.
Baclofen and the novel GABA agonist lesogaberan increase lower esophageal tone and inhibit transient opening of the lower esophageal sphincter in both normal subjects (Lidums et al., 2000) and in patients with acid and nonacid reflux (Vela et al., 2003). Baclofen has been shown to reduce capsaicin-induced cough (Dicpinigaitis et al., 1998) by a suggested central mechanism. In angiotensin-converting enzyme (ACE) inhibitor-induced cough (Dicpinigaitis, 1996) and in patients with reflux cough in the general cough clinic (Menon et al., 2005), open label studies have demonstrated clinical efficacy. Unfortunately, the high side effect profile and narrow therapeutic window of baclofen make it a very difficult agent to use in routine clinical practice, although the novel GABA agonist lesogaberan has a much improved safety profile and is also effective in inhibiting sphincter opening (Shaheen et al., 2013) and in a randomized controlled developmental study (AZ D9120C00011) days with a cough were recorded as a secondary end point. The results were encouraging in that significantly more patients reported improvement in daytime cough in the last week of therapy. Any positive result is remarkable because patients were selected for PPI-resistant dyspepsia rather than chronic cough. However, information available in the public domain is currently scant and is insufficient to estimate the actual effect of this novel drug in patients with cough.
The macrolide antibiotics, particularly erythromycin and azithromycin, have long been used as promotility agents through their activity as agonists of the gut hormone motilin (Moshiree et al., 2010). In patients with cough, erythromycin (250 mg once a day) has been studied in one randomized placebo-controlled trial (Yousaf et al., 2010). However, only 15 patients were recruited in each parallel arm, falling short of even the authors' own optimistic power calculations based on a 50% reduction in objective cough counts. Not surprisingly, there was no effect of therapy. No cough-specific study of azithromycin has been performed to date, but in a long-term study of COPD exacerbations by Albert et al. (2011), the greatest effect compared with placebo in the quality of life score was seen on symptoms, and it has been suggested that this effect is due to a reduction in airway reflux (Crooks et al., 2011).
I. Xanthines
Theophylline is a drug commonly used as a bronchodilator that has also been shown to have clearly demonstrable anti-inflammatory activity in patients with asthma (Sullivan et al., 1994) or COPD (Ford et al., 2010). However, theophylline has also been shown to exhibit antitussive activity. For example, theophylline has been reported to inhibit cough associated with the ingestion of ACE inhibitors by some patients (Cazzola et al., 1993), and both theophylline and theobromine can inhibit citric acid-induced cough in both healthy and allergic guinea pigs (Mokry et al., 2009). More recently, theobromine (Fig. 20) has been demonstrated to inhibit sensory nerve activation and cough in humans (Usmani et al., 2005), and a large multicenter trial is ongoing evaluating the effects of theobromine on chronic cough clinically (Clancy et al., 2013). Theophylline has recently been demonstrated to inhibit the cough reflex via an effect on BK K+ channels (Dubuis et al., 2014).
Fig. 20.
Chemical structure of theobromine.
J. Cromones
Disodium cromoglycate (DSCG) (Fig. 21) and the related drug, nedecromil sodium, have long been used as antiallergic drugs; while the mechanism of action of these drugs has been widely reported as stabilization of mast cells (Mackay and Pearce, 1996), since the original discovery of DSCG, it has been appreciated that the cromones also affect sensory nerve function (Dixon et al., 1980a,b). Thus, both DSCG (Dixon et al., 1980a,b) and nedecromil sodium (Jackson et al., 1992) have been reported to influence sensory nerve fibers. Nedecromil sodium has been reported to induce a long-lasting chloride-dependent depolarization (Jackson et al., 1992), and this latter property may explain the ability of DSCG to inhibit cough both experimentally and clinically. For example, nedecromil has been reported to inhibit the cough induced by citric acid in dogs (Jackson et al., 1992), and DSCG has been demonstrated to inhibit the cough associated in some patients with the ingestion of ACE inhibitors (Hargreaves and Benson, 1995). In addition, both DSCG and nedecromil sodium have been shown to inhibit the cough associated with asthma (Barnes, 1993; Boulet et al., 1994). The mechanism of action of cromones on sensory nerves is not completely understood, although in addition to their action on Cl− channels (Alton and Norris, 1996), both DSCG and nedecromil sodium were recently suggested to act as agonists at G-coupled receptor 35 (Deng and Fang, 2012), whereas DSCG has also been suggested to be a tachykinin antagonist (Page, 1994).
Fig. 21.
Chemical structure of disodium cromoglycate.
V. New Approaches to Treating Cough
A. Levocloperastine
Levocloperastine [1-[2-[(4-chlorophenyl)-phenylmethoxy]ethyl]piperidine] (Fig. 22) is the levorotatory isomer of dl-cloperastine (Catania and Cuzzocrea, 2011) and is described as having antitussive effects both centrally on the bulbar cough center and peripherally on the cough receptors in the tracheobronchial tree, but limited mechanistic data have been published. Cloperastine [1-[2-(p-chloro-α-phenylbenzyloxy)ethyl] piperidine] is also said to have central antitussive effects along with antihistamine and papaverine-like activity (Catania and Cuzzocrea, 2011).
Fig. 22.
Chemical structure of levocloperastine.
A single publication describes six open clinical trials enrolling patients of all ages with cough associated with various respiratory disorders including bronchitis, asthma, pneumonia, and COPD (Aliprandi et al., 2004). Levocloperastine significantly improved cough symptoms (reported intensity and frequency of cough) in all trials with similar efficacy to codeine, levodropropizine, and dl-cloperastine.
B. Amitriptyline
Amitriptyline (Fig. 23) is the prototypical tricyclic antidepressant, with a broad range of pharmacological actions including effects on the adrenergic, serotonergic, muscarinic, and histaminergic systems (Garattini and Samanin, 1988). Low-dose amitriptyline is also used in chronic neuropathic pain states such as migraine, postherpetic neuralgia, and painful diabetic neuropathy and has recently been suggested to be useful in the treatment of cough. Although the antidepressant effects of amitriptyline are clearly central effects, the site of the antitussive effect and relevant pharmacological actions contributing to this effect remain unknown.
Fig. 23.
Chemical structure of amitriptyline.
Low-dose amitriptyline has been used in the treatment of suspected "postviral vagal neuropathy" (Bastian et al., 2006), a condition described clinically by ear-nose-throat specialists as being characterized by a dry cough for more than 6 months, precipitated by a throat tickle, dry sensation, laughter, or speaking following the exclusion of other causes of chronic cough (Lee and Woo, 2005). Therefore, patients closely resemble those generally described as having unexplained chronic cough in respiratory medicine. An initial open-label uncontrolled series of 12 patients were treated with amitriptyline (10 mg) and 11 of 12 patients reported some reduction in cough, with an average rating of 75% improvement. No adverse effects were reported. In a subsequent randomized, but unblinded trial, 28 subjects were treated for 10 days with either 10 mg of amitriptyline or codeine/guaifenesin (20 mg/200 mg) (Jeyakumar et al., 2006). Significantly more subjects reported improved cough specific quality of life in the amitriptyline group and a median 75% improvement in ratings of cough frequency and severity compared with 0% for codeine/guaifenesin. Clearly, these pilot studies are of interest, and blinded placebo-controlled clinical trials are now required.
C. Novel N-Methyl-d-aspartate Receptor Antagonists
In addition to dextromethorphan, the classic NMDA receptor antagonist extensively used as an antitussive, recent evidence has suggested that other drugs acting at this receptor may have therapeutic benefit in treating cough. NMDA receptors are widely expressed in the CNS and play important roles in synaptic transmission and plasticity. In the somatic nervous system, modulation of NMDA receptor function has been associated with central sensitization, a key mechanism in neuropathic pain (Woolf, 2011). It is noteworthy that a study assessing the effects of low-dose ketamine, a common tool for assessing central sensitization (Chizh, 2007), had no effect on cough reflex sensitivity or objective cough counts in either patients with unexplained chronic cough or healthy control subjects (Young et al., 2010). This implies that not all NMDA receptor antagonists are equivalent in their antitussive effects, perhaps as a consequence of the involvement of receptor subtypes.
Memantine (Fig. 24) is an established treatment of Alzheimer’s disease that has recently been suggested as a potential antitussive treatment (Canning 2009). Similar to dextromethorphan, memantine is a low-affinity uncompetitive NMDA receptor blocker but has the additional characteristic of binding preferentially to open channels (Chen and Lipton 2006), reducing interference with physiologic nerve transmission, which may explain its clinical tolerability. In a guinea pig study (Smith et al., 2012), memantine significantly inhibited experimentally induced cough. Because NMDA receptors are present in both central and peripheral tissues, the site of action of the antitussive effect of memantine is yet to be determined and translation of these findings into clinical studies is awaited.
Fig. 24.
Chemical structure of memantine.
However, another novel NMDA receptor antagonist, V3381 (Young et al., 2012), has recently undergone preliminary testing in patients with unexplained chronic cough following initial development for the treatment of neuropathic pain. V3381 is a low-affinity, noncompetitive use-dependent NMDA antagonist, which also has competitive, reversible inhibition of monoamine oxidase type A (MAOA). An open label pilot study recruited nine patients with chronic cough treated for 8 weeks. There was a median reduction in objective cough frequency of 37.2% after 8 weeks therapy, suggesting some therapeutic benefit. However, 83% of subjects reported dizziness, and most patients could not tolerate the maximum dose (J.A. Smith, personal communication). It is impossible to be certain to what degree the beneficial and adverse effects were NMDA or MAOA mediated, but previous work has suggested that MAOA inhibition modulates bronchoconstriction but not cough (Choudry et al., 1993). Therefore, other specific NMDA receptor antagonists without MAO-related side effects may have a role in treating patients with chronic cough.
D. Glaucine
Initial studies evaluating the alkaloid agent glaucine (Fig. 25) demonstrated a central mechanism for its antitussive action in rodents (Petkov et al., 1979; Jagiełło-Wójtowicz et al., 1994), as well as in nonanesthetized cats, where the antitussive effect of the drug was approximately equal to an equivalent dose of codeine (Nosal’ova et al., 1989). Also in cats, dl-glaucine phosphate (DL-832) was demonstrated to exert a central antitussive activity (Kase et al., 1983). A central mechanism of action in humans is supported by a study in healthy volunteers in which the highest dose (60 mg) of (±)-glaucine phosphate, administered as a syrup, caused respiratory depression associated with sedation and decreased performance in the digit symbol substitution test (Redpath and Pleuvry, 1982).
Fig. 25.
Chemical structure of glaucine.
More recent studies support a peripheral, anti-inflammatory and bronchodilator action of glaucine, through a mechanism of phosphodiesterase (PDE) 4 inhibition (Pons et al., 2000). Thus, in ovalbumin sensitized guinea pigs, inhaled glaucine inhibited the acute bronchoconstriction produced by aerosolized antigen. Furthermore, pretreatment with inhaled glaucine markedly reduced BHR to histamine, reduced eosinophil lung accumulation, increased eosinophil peroxidase activity in bronchoalveolar lavage fluid 24 hour after exposure of conscious guinea pigs to aerosol antigen and inhibited microvascular leakage produced after inhaled antigen (Pons et al., 2000). Furthermore, in a study using mouse peritoneal macrophages, glaucine demonstrated Toll-like receptor-mediated anti-inflammatory activity, including reduction of pro-inflammatory cytokines and increased the levels of the anti-inflammatory cytokine IL-10 (Remichkova et al., 2009). Whether these pharmacological actions of glaucine contribute to its antitussive activity has not yet been shown.
Three studies have evaluated the effect of glaucine in pathologic cough. In a double-blind comparative trial of glaucine and codeine (both administered as a syrup in a dosage of 30 mg three times daily for 7 days), involving 90 patients reporting subjective symptoms using a 4-point scale, as well as a visual analog scale, glaucine was deemed more effective and better tolerated than codeine, with no patient reporting adverse effects from glaucine (Gastpar et al., 1984). A second study evaluated 24 hospitalized patients affected by chronic cough of various etiologies in a single-dose, double-blind, crossover study of placebo, glaucine (30 mg) and dextromethorphan (30 mg). Coughs were recorded, using a writing cough recorder during an 8-hour overnight period with the patient alone in a hospital room. Although coughs were decreased after both dextromethorphan and glaucine, only glaucine achieved a statistically significant decrement in cough relative to placebo, but only if the 1–6 hour measurement interval was considered. Glaucine was better tolerated than dextromethorphan, with only one patient reporting a side effect (vomiting) compared with eight patients reporting adverse effects after dextromethorphan (Ruhle et al., 1984). A third study of 38 hospitalized patients with chronic cough compared single, 30-mg oral doses of glaucine and codeine, and placebo, in a double-blind crossover fashion. Coughs were recorded by a tape recorder for 8 hour after study drug administration. Mean cough counts after placebo, glaucine, and codeine were 269.3, 241.8 and 201.9, respectively. However, patients were unable to discern any difference between treatment arms (Dierckx et al., 1981). Finally, one small study of 8 healthy adult volunteers was unable to demonstrate an effect of a 60 mg of liquid dose of glaucine on cough reflex sensitivity to inhaled citric acid (Rees and Clark, 1983).
Although preclinical studies support an antitussive mechanism of action, currently available clinical data do not support the efficacy of glaucine as a cough suppressant in humans, although clinical experience does suggest that the agent is well tolerated at the doses studied. Adequately powered, properly performed clinical trials with appropriate objective and subjective end points are still needed to evaluate the therapeutic potential of glaucine.
E. Moguisteine
Moguisteine [(R,G)-2-(2-methoxyphenoxy)-methyl-3-ethoxycarbonyl-acetyl-1,3 thiazolidine] (Fig. 26) is a nonnarcotic antitussive drug that has been shown to inhibit cough in a variety of species of experimental animal induced by citric acid, capsaicin and mechanical or electrical stimulation of the trachea (Gallico et al., 1994). Moguisteine has also been found to be effective in patients with cough resulting from lung disease (Aversa et al., 1993; Del Donno et al., 1994; Fasciolo et al., 1994; Barnabe et al., 1995). Moguisteine does not inhibit cough when administered directly into the cerebral ventricles, suggesting a peripheral mechanism of action (Gallico et al., 1994). In anesthetized guinea pigs, a peripheral site of action of moguisteine was confirmed by the observations showing that this drug was antitussive by suppressing RARs in anesthetized guinea-pigs (Morikawa et al., 1997).
Fig. 26.
Chemical structure of moguisteine.
F. Phosphodiesterase Inhibitors
A recent study in guinea-pigs has reported that a range of selective phosphodiesterase (PDE) inhibitors can inhibit citric acid-induced cough. Thus, a PDE1, PDE3, and PDE4 inhibitor have all been reported to inhibit citric acid-induced cough in both healthy and allergic guinea pigs (Mokry and Nosolova, 2011), and the selective PDE4 inhibitor cilomilast (Fig. 27) has been reported to inhibit allergen-induced hypertussive responses in allergic guinea pigs (Lü et al., 2004). However, although the selective PDE4 inhibitor roflumilast has recently been approved for the treatment of severe COPD, there are no clinical data thus far demonstrating the antitussive activities of this drug class in the clinic. However, the PDE3 inhibitor cilostazol has been shown to have modest effects on capsaicin-induced cough in humans (Ishiura et al., 2005).
Fig. 27.
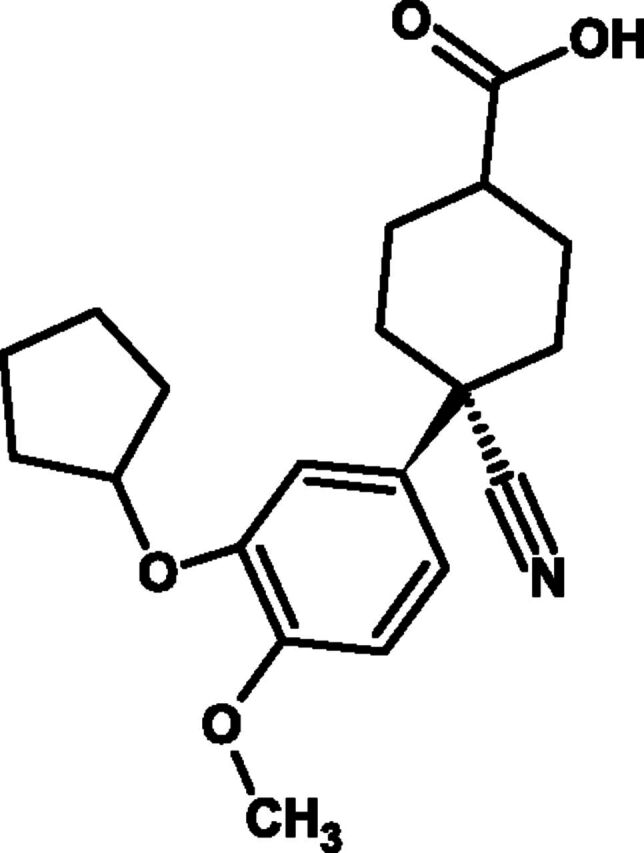
Chemical structure of cilomilast.
G. K+ Channel Openers
Several categories of potassium ion channels are responsible for maintaining the resting membrane potential in nerves. A role for potassium channel openers in the modulation of cough responses was first suggested based upon the hypothesis that the inhibitory effect of substances such as opioids and GABAB agonists on airway nerves may be mediated by a common mechanism, which involved the opening of large conductance calcium-activated potassium channels. In support of this hypothesis, a benzimidazolone compound and calcium-activated potassium channel opener, NS1619 (Fig. 28), was shown to inhibit single fiber nerve firing from the guinea pig trachea and citric acid-evoked cough in conscious guinea pigs (Fox et al., 1997). More recent publications have replicated the inhibitory effect of NS1619 on cough in conscious guinea pigs and described similar effects for pinacidil and cromakalin, both openers of K+ATP (ATP sensitive) channels (Poggioli et al., 1999; Sutovska et al., 2007) and suggested that moguisteine (see section V.E) may exert some of its antitussive action via K+ATP channels (Morita and Kamei, 2000). Despite this preclinical evidence, clinical studies evaluating K+ channel openers as antitussives are absent from the current literature.
Fig. 28.
Chemical structure of NS1619.
H. Cl− Pump Inhibitors
Cough in animals and humans can be induced by inhalation of aerosols with low chloride ion (Cl−) concentration that reduce the Cl− content of airway surface lining fluid (Higenbottam, 1984; Lowry et al., 1988b). In multiple species of animals, such experimentally induced cough has been blocked by prior administration of inhaled diuretics. In guinea pigs, both the loop diuretic furosemide (Fig. 29) and the thiazide diuretic hydrochlorothiazide inhibited citric acid-induced cough (Karlsson et al., 1992) as did furosemide and the CL− inhibitors DIDS and niflumic acid in a more recent study (Mazzone and McGovern, 2006). In anesthetized dogs spontaneously breathing through a tracheostomy, furosemide diminished the activity of laryngeal irritant receptors stimulated by administration of isotonic dextrose aerosol (Sant’Ambrogio et al., 1993). In nonanesthetized cats, inhaled furosemide diminished the enhanced cough reflex sensitivity induced by pretreatment with the ACE-inhibitor enalapril (Franova, 2001). Mechanisms proposed to explain the antitussive effect of inhaled furosemide include inhibition of irritant receptor stimulation and regulation of airway afferent neuronal activity, perhaps by modulation of the activity of the furosemide-sensitive Na+-K+-2Cl-cotransporter (Mazzone and McGovern, 2006).
Fig. 29.
Chemical structure of furosemide.
In human volunteers, inhaled furosemide has also been demonstrated to inhibit cough induced by aerosols of low chloride content solutions but not induced by capsaicin, thus suggesting that furosemide may act by inhibiting the cough reflex indirectly, perhaps by changing local chloride ion concentrations in the vicinity of airway epithelial cough receptors (Ventresca et al., 1990, 1992; Karlsson et al., 1992). Furosemide was also shown to inhibit PGF2α-induced cough (Ventresca et al., 1992). In a randomized, double-blind study of healthy volunteers challenged with low chloride ion-concentration aerosols, the carbonic-anhydrase inhibitor acetazolamide demonstrated a significantly better antitussive effect than furosemide, suggesting that inhibition of carbonic anhydrase activity may be involved in modulating the effects of low chloride ion concentrations within the airway microenvironment (Foresi et al., 1996).
In a randomized, double-blind, placebo-controlled study of children with asthma, both inhaled furosemide as well as amiloride, a diuretic having no effect on the Na+-K+-2Cl-cotransporter, demonstrated a significant protective effect against acetic acid-induced cough (Mochizuki et al., 1995a). Inhaled furosemide also inhibited bronchoconstriction induced by UNDW in children with asthma (Mochizuki et al., 1995b). In a study of adults with mild asthma, however, inhaled furosemide did not inhibit cough due to an aerosol of a Cl-deficient solution, although it was protective in nonasthmatic volunteers (Stone et al., 1993).
Despite a significant body of evidence supporting the antitussive effect of inhaled diuretics in humans, clinical trials evaluating the potential value of these agents in pathologic cough are lacking. Of interest would be well-designed studies assessing the clinical value of currently available inhaled diuretics in various types of pathologic cough.
I. Ouabain-Sensitive Na+-K+ ATPase Inhibitors
Cough is unique among visceral reflexes in that it is threshold-dependent and requires sustained high-frequency sensory nerve activation for initiation (Bolser et al., 2006; Shannon et al., 2004; Canning and Mori, 2011). Therapeutically, a drug that prevents the repetitive sensory nerve action potential patterning associated with cough initiation should have a profound effect on the cough reflex. Recent studies have revealed that vagal afferents regulating the cough reflex uniquely express isozymes of Na+-K+ ATPase in their peripheral terminals (Mazzone et al., 2009). These isozymes use α3 subunits of the sodium pump, found primarily in neurons (Blanco and Mercer, 1998; Dobretsov et al., 1999; Dobretsov et al., 2003). The α3 subunits have a high affinity for ATP but a comparatively low affinity for Na+, perfectly suited to active cells requiring rapid re-establishment of membrane potential and Na+ gradients (Geering, 2008; Jewell and Lingrel, 1991; Crambert et al., 2000). Sodium pumps are critical to all cells, especially excitable cells that require Na+ gradients to initiate excitation. Given the need for sustained, high-frequency action potential patterning, the vagal afferents that regulate cough are especially dependent upon the re-establishment of Na+ gradients during continued activation. Electrophysiological analysis confirmed that inhibition of Na+-K+ ATPase selectively inhibits high-frequency action potential patterning in airway sensory neurons. In subsequent studies, it was found that acid-evoked coughing in guinea pigs could be prevented following Na+-K+ ATPase inhibition with ouabain (Fig. 30), an endogenous cardiotonic steroid hormone, and digoxin, used clinically to treat heart disease. These steroid inhibitors of the sodium pump were effective at inhibiting cough when administered topically to the airway mucosa. When given intravenously, ouabain markedly inhibited citric acid-evoked coughing in guinea pigs at doses that are without effect on blood pressure, heart rate, or respiratory rate (Mazzone et al., 2009).
Fig. 30.
Chemical structure of ouabain.
Clinical studies provide circumstantial support for the hypothesis that sodium pump inhibition will be effective in treating cough. Ouabain, which markedly inhibited coughing in guinea pigs (Mazzone et al., 2009), was used with apparently great success in a more than 100-year-old study of whooping cough (Gemmell, 1890). The Japanese make a cough remedy from extracts of Ophiopogon japonicus, rich in glycosides chemically similar to ouabain and digoxin (Wang et al., 2011a). In more recent clinical literature, amitriptyline has been found to be an effective treatment in chronic, intractable cough (Chung, 2009). Among the many pharmacological properties of amitryptyline, the inhibition of Na+-K+ ATPase is of interest (Carfagna and Muhoberac, 1993). The tricyclic H1-receptor antagonists promethazine and chlorpromazine also inhibit the sodium pump and are used clinically as cough medicines (Wirth, 1958; Hewlett et al., 1983; Carfagna and Muhoberac, 1993; McLeod et al., 1998; Horvat et al., 2006). Multiple studies and a meta-analysis reveal that Zn2+, which also inhibits sodium pump function (Ribeiro et al., 2002), is especially effective at relieving the cough associated with respiratory tract infections (Mossad et al., 1996; Singh and Das, 2011).
Proton pump inhibitors, typified by omeprazole, can also inhibit Na+-K+ ATPase and are somewhat selective for the neuronal isoforms of the sodium pump (Keeling et al., 1985; Iwata et al., 1988). As discussed above, the results of studies evaluating the antitussive effects of PPIs have been disappointing (Chang et al., 2006, 2011; Faruqi et al., 2011; Shaheen et al., 2011). There have, however, also been several remarkable results with PPIs in patients with cough (Benini et al., 2000; Oribe et al., 2005; Ebihara et al., 2007). Thus, it is noteworthy that PPIs are pro-drugs that require acid activation for inhibition of not only proton pumps but also sodium pumps. The mechanism of inhibition of both of these enzymes requires covalent interactions between the electrophilic sulfonamides formed upon PPI interactions with acid and the electrophilic cysteines on the proton and sodium pumps. Thus, it seems reasonable to assert that PPIs such as omeprazole will provide no cough relief unless they are acid activated within the airways, thereby facilitating their electrophilic interactions with neuronal sodium pumps. Interestingly, acid activation can occur at weakly acidic pH values such as those present in inflamed airways.
J. Leukotriene Receptor Antagonists
Asthma and NAEB are among the most common causes of chronic cough in adults, accounting for ~25% and 10% of cases, respectively (Dicpinigaitis, 2006; Brightling, 2010). Along with the standard therapeutic regimen of inhaled bronchodilators and ICS, the orally administered leukotriene receptor antagonists (LTRAs) (Fig. 31) have attained common usage in the treatment of asthma. Although there is some evidence that LTRAs can have modest anti-inflammatory effects and that their ability to improve subjective and objective parameters in asthmatic patients is well established, the mechanism by which these agents may function as antitussives remains poorly understood. A reasonable assumption is that the LTRAs inhibit cough in asthmatics through their inhibition of eosinophilic airway inflammation (Takemura et al., 2012). However, a recent study in severe asthmatics demonstrating that anti-IL-5 therapy had significant beneficial effect on the frequency of severe exacerbations without an effect on cough calls into question a causal relationship between eosinophilic inflammation and cough in patients with asthma (Haldar et al., 2009). An alternative mechanism proposed is that LTRAs may inhibit cough by modulating leukotriene-induced activation of airway mast cells (Kawai et al., 2008).
Fig. 31.
Chemical structure of montelukast.
Two animal studies have demonstrated the antitussive effect of the LTRA montelukast. In awake guinea pigs with allergen-induced rhinitis, montelukast inhibited citric acid-induced cough (Brozmanova et al., 2005), and in a guinea pig model of CVA montelukast suppressed cough induced by inhaled antigen (Nishitsuji et al., 2008).
Multiple studies have demonstrated the antitussive effects of orally administered LTRAs in adults with CVA, a form of asthma in which cough is the sole or predominant symptom. In a randomized, double-blind, placebo-controlled, crossover study of eight subjects with CVA, a 14-day course of zafirlukast significantly improved subjective cough scores and diminished cough reflex sensitivity to inhaled capsaicin (Dicpinigaitis et al., 2002). Of note, zafirlukast significantly improved cough in five subjects whose cough had been refractory to long-term therapy with ICS. In a randomized, controlled, parallel-group, multicenter trial of 49 patients with newly diagnosed CVA, the LTRA pranlukast improved cough symptom scores to a greater degree than did the long-acting inhaled β2-agonist salmeterol. Furthermore, unlike with salmeterol, a 4-week course of therapy with pranlukast significantly decreased eosinophil counts and eosinophil cationic protein (ECP) contents in peripheral blood and induced sputum (Tamaoki et al., 2010). Three studies have demonstrated the antitussive effect of montelukast (10 mg daily). In a small, randomized, double-blind, placebo-controlled trial, a 4-week course of montelukast significantly improved subjectively rated cough frequency, with beneficial effects noted by the second week of therapy (Spector and Tan, 2004). In a prospective, observational study of 23 adult nonsmokers with CVA, a 4-week course of montelukast significantly improved subjective cough scores, and diminished cough reflex sensitivity to capsaicin, as well as sputum eosinophil counts, whereas not affecting pulmonary function, airway responsiveness to methacholine, or sputum mediator levels (Takemura et al., 2012). Another study of adults with CVA demonstrated the efficacy of a 2-week course of montelukast against subjectively rated cough, but the agent was ineffective against cough in atopic subjects (Kita et al., 2010).
In terms of pediatric cough, one large multinational study of asthmatic children aged 2 to 5 years demonstrated a significant improvement in the cough symptom score (P = 0.003) after a 12-week treatment period with montelukast (n = 461; 4-mg chewable tablet) versus placebo (Knorr et al., 2001). In an observational study of 22 children with chronic cough, 68% experienced resolution of cough by the third week of treatment. Those children responding to therapy had higher baseline blood levels of ECP and IgE and higher absolute eosinophil blood counts compared with nonresponders (Kopriva et al., 2004). One randomized, double-blind, placebo-controlled crossover study evaluated the effect of an 8-week course of treatment with montelukast in 23 children (ages 6–18) with cystic fibrosis. Montelukast significantly decreased cough and wheezing scale scores (P < 0.001), as well as serum and sputum levels of ECP and IL-8, without affecting pulmonary physiology. Montelukast therapy also resulted in decreased sputum levels of myeloperoxidase and increased serum and sputum levels of IL-10 (Stelmach et al., 2005).
Given the significant body of evidence documenting the antitussive efficacy of the LTRAs in cough due to asthma, further work should be done to evaluate the potential efficacy of these agents in non-asthmatic cough. Other clinically important questions that remain unanswered are the mechanism(s) through which LTRAs achieve their antitussive effect, and whether monotherapy with LTRAs for asthmatic cough is sufficient to prevent the sequelae of chronic airway inflammation. However, a recent study has reported that leukotrienes may not play an important role in other forms of cough as a randomized, placebo-controlled trial of montelukast had no effect in adult patients with postinfectious cough (Wang et al, 2014).
K. Interferon-α
Interferons are naturally occurring proteins produced by eukaryotic cells in response to viral infection and other stimuli. They are cytokines that have antiviral, antiproliferative, and immunoregulatory properties. There are three major classes of interferons: α, β, and γ. Interferon-αs are prescribed for chronic viral diseases such as hepatitis B and C, malignancies such as leukemia, and autoimmune disorders such as inflammatory bowel disease. Interferon-α may be derived from leukocytes or lymphoblasts or produced by recombinant DNA technology. The alpha gene can produce protein variants such as interferon-α2. The attachment of interferon to large inert macrogel molecules, termed pegylation, substantially reduces the rate of absorption and excretion of interferon and increases the plasma concentration. Interferon-α is most commonly administered subcutaneously. The elimination half-life is 2–7 hours. Cough has been reported as a side effect of interferon therapy (Kartal et al., 2007), whereas interstitial pneumonitis and a sarcoid-like granulomatous reaction in the lung have been associated with interferon-α treatment (Kartal et al., 2007). Interferon is often administered in combination with another antiviral drug ribavirin for patients with hepatitis C. Cough and cough reflex hypersensitivity is thought to associate with ribavirin rather than interferon-α (Dicpinigaitis and Weiner, 2011).
The ability of interferons to inhibit viral replication led to its evaluation in patients with URTI. Biologic activity of low-dose interferon-α by oromucosal administration has been reported in several species including humans, despite rapid inactivation by digestive enzymes (Cummins et al., 2005). Early studies of intranasal interferon-α2 reported a modest relief of symptoms (Hayden and Gwaltney, 1984). This led to studies of combined interferon-α2, H1-receptor antagonists, and nonsteroidal, anti-inflammatory drugs. One study suggested an additive antitussive effect of interferon-α when combined with H1-receptor antagonists (Gwaltney et al., 2002).
A recent uncontrolled study reported an improvement in cough-specific health status with low-dose oral interferon-α in patients with IPF (Lutherer et al., 2011), although this needs confirming in a controlled clinical trial. Further studies should evaluate the antitussive activities of this and other interferons for their ability to inhibit cough, because airway cells expressing interferon-γ have been reported to be reduced in patients with idiopathic chronic cough (Birring et al., 2003b).
L. Tropan Derivatives with High Affinity for the Nociceptin Opioid Receptor (Nociceptin)
The nociceptin opioid receptor (NOP) is a recently identified receptor that shares significant homology with classic opioid receptors but is not activated by opioids but by the endogenous peptide nociceptin (orphanin FQ). Nociceptin was found to attenuate capsaicin- and citric acid-induced coughing in guinea pigs (McLeod et al., 2001; Lee et al., 2006) and mechanically induced cough in cats (Bolser et al., 2001).
SCH486757 [8-[bis(2-chlorophenyl) methyl]-3-(2-pyrimidinyl)-8-azabicyclo[3.2.1.]octan-3-ol] (Fig. 32), was identified as an orally absorbed highly selective NOP receptor agonist with no major safety issues. SCH486757 inhibited capsaicin-evoked cough in guinea pigs with similar efficacy to codeine and hydrocodone and mechanically induced cough in cats (McLeod et al., 2010). After these positive results in preclinical animal models, two phase II clinical trials were performed in patients with postviral and chronic cough (J.A. Smith, personal communication). A double-blind, randomized, controlled parallel group study (~30 subjects per arm) compared SCH486757 to 30 mg of codeine two times a day and placebo in patients suffering from cough, persisting 2 weeks after a viral upper respiratory tract infection, i.e., postviral cough. Cough scores improved to a similar degree with codeine and SCH486757 but were not statistically significantly different from placebo. Objective cough frequency measured using the Lifeshirt (Vivometrica, Ventura, CA) also showed no significant improvement over placebo for either drug (Woodcock et al., 2010). Unfortunately, the maximum clinical dose for SCH486757 is limited by its tendency to produce somnolence, which was seen in 7 of 27 subjects randomized to this treatment. The therapeutic ratio of NOP1 agonists would need to be improved for these drugs to be viable as effective antitussives.
Fig. 32.
Chemical structure of tropan.
M. Selective Cannabinoid 2 Receptor Agonists
Nonselective cannabinoids such as anandamide (Fig. 33) have been shown to suppress the cough reflex experimentally (Gordon et al., 1976; Calignano et al., 2000), probably via the ability of this substance to inhibit sensory nerve function (Tucker et al., 2001). In the guinea pig, this cough reflex appears to be mediated via activation of the CB2 receptor for cannabinoids because the nonselective cannabinoid agonist CP55940 and the selective CB2 agonist JWH133 inhibit sensory nerve depletion of the vagus nerve. Similar results were obtained on human vagus nerve preparations stimulated with capsaicin, and such effects of the cannabinoid agonists could be inhibited by the CB2 receptor antagonist S8144528 (Patel et al., 2003). Similar results have been reported with the novel CB2 receptor agonist GW833972A (Belvisi et al., 2008). Additionally, the cannabinoid receptor agonist WIN5512-2 has also been demonstrated to be antitussive in mice (Morita and Kamei, 2003). It is noteworthy that an anandamide transporter inhibitor VDM12 [N-arachidonyl-(2-methyl-4-hydroxyphenyl)] has also been shown to inhibit capsaicin-induced cough in mice (Kamei et al., 2006).
Fig. 33.
Chemical structure of anandamide.
N. Neurokinin Receptor Antagonists
Sensory neuropeptides have been suggested to be involved in a range of airway diseases and have been implicated in cough (Chung, 2009) and cough variant asthma (Nishitsuji et al., 2004). In addition to having local effects in the airways such as vasodilation, mucous secretion, edema, and bronchoconstriction (Fahy et al., 1995), substance P has been shown to be important for the cough reflex at the level of the commissural region of the nTS (Mutolo et al., 2008), at least in the rabbit. Sensory neuropeptides can exert their effects via NK1, NK2, or NK3 receptors and the NK1 receptor antagonist CP99,994 administered to the caudal aspect of the nTS markedly reduced the cough reflex, whereas the NK2 antagonist MEN10376 did not (Mutolo et al., 2008). However, to date there is no evidence that either an NK1 or an NK2 receptor antagonist have any clinical benefit in the treatment of cough (Fahy et al., 1995), and indeed in other disease areas involving sensory nerves in the periphery such as pain, such drugs have been very disappointing (Hill, 2000). However, it has been suggested that there is a mechanism analogous to the “wind up” mechanism implicated in hyperalgesia and pain involving plasticity of nerves in the CNS (Bonham et al., 2004), and thus, it may be necessary to have a centrally acting NK receptor antagonist or an NK3 receptor antagonist. Therefore, it is noteworthy that a recently reported triple NK receptor antagonist CS-003 has been reported to inhibit cough in the guinea pig (Tsuchida et al., 2008), although it has been shown that an NK3 receptor antagonist is ineffective against citric acid-induced cough in healthy volunteers (Khalid et al., 2010).
O. Transient Receptor Potential Vanilloid 1 Receptor Antagonists
It is very well established that inhaled aerosols of capsaicin, the extract of hot chili peppers, reliably provoke coughing in multiple species including humans, and it has now been appreciated that this effect is mediated by agonism at the transient receptor potential vanilloid 1 (TRPV1) receptor (Caterina et al., 1997), which is also activated by low pH, temperatures above 43°C, and several proinflammatory mediators. Furthermore, certain inflammatory stimuli such as activation of protease activated receptor-2 can exaggerate TRPV1-mediated cough, at least in guinea pigs (Gatti et al., 2006). A role for the TRPV1 receptor in patients with chronic dry cough has been suggested on the basis of a number of studies consistently reporting heightened cough responses to inhaled capsaicin compared with healthy volunteers (Choudry and Fuller, 1992; Nieto et al., 2003; Groneberg et al., 2004; Prudon et al., 2005; Ternesten-Hasseus et al., 2006). This may also be the case in IPF (Doherty et al., 2000; Hope-Gill et al., 2003), ACE inhibitor-induced cough (Morice et al., 1987), and cough due to URTI (O'Connell et al., 1996; Dicpinigaitis et al., 2011), but findings in smokers and those with COPD have been less consistent. However, cough responses induced by capsaicin represent activation of the whole neural pathway and, therefore, cannot discriminate between sensitization of peripheral and/or central pathways when responses are heightened. One study has suggested that patients with chronic cough may have increased expression of TRPV1 receptors in the airways (Groneberg et al., 2004) and increased immunohistochemical staining weakly correlated with capsaicin cough responses, suggesting a possible peripheral component. This body of evidence would suggest that the TRPV1 receptor is a logical target for antitussive therapy, but studies investigating several TRPV1 antagonists as analgesics found significant hyperthermia, implying that this receptor also plays a part in temperature regulation (Gavva et al., 2007, 2008). These safety concerns have prevented the development of some compounds, although others seem not to be associated with significant temperature disturbances for reasons that are not well understood (Chizh et al., 2007; Round et al., 2011).
There is very limited information on TRPV1 antagonists in humans, and the results from tests with only one TRPV1 antagonist (SB-705498) (Fig. 34) has been reported in patients with cough. SB-705498 is a highly selective competitive antagonist at the TRPV1 receptor (Rami et al., 2006), previously found to have a significant inhibitory effect on experimentally induced inflammatory hyperalgesia in healthy volunteers without temperature regulation disturbance. However, a recent study in patients with chronic idiopathic cough suggested that despite a significant (4-fold) reduction in cough reflex sensitivity to capsaicin with SB-705498 compared with placebo (Khalid et al., 2013), there was no difference in 24 hour objective cough frequency. This study questions the validity of at least capsaicin-evoked cough as a predictive end point for clinical activity of drugs as antitussives, and the discordance between capsaicin C5 responses and cough frequency suggests caution should be exercised in the extrapolation of improvements in capsaicin responses to clinically relevant antitussive effects in pathologic cough. Further investigations are required to understand whether more potent TRPV1 antagonists producing even larger reductions in capsaicin responses can have antitussive effects and furthermore whether this effect can be separated from hyperthermia.
Fig. 34.
Chemical structure of SB-705498.
P. Transient Receptor Potential A1 Receptor Antagonists
The nociceptor TRPA1 has been suggested as one of the major mediators of peripheral irritant sensation because of its ability to bind and be activated by a wide range of pungent natural compounds known to cause pain and inflammation (Bandell et al., 2004; MacPherson et al., 2005; Bautista et al., 2013). For example, in the respiratory tract, irritation by tear gas and isocyanates is mediated by activation of TRPA1 and is absent in TRPA1 knockout animals or in the presence of TRPA1 antagonists (Bessac et al., 2009). Cigarette smoke and acreolin (a substance found in tobacco smoke) similarly cause TRPA1-mediated neurogenic and nonneurogenic airway inflammation that is inhibited by specific TRPA1 antagonists (Nassini et al., 2012).
The molecular mechanism of TRPA1 activation has been shown to be by electrophilic covalent binding of the agonist by direct addition (Sadofsky et al., 2011) to reactive cysteines within the channel (Hinman et al., 2006), allowing such agents as cinnamaldehyde, acreolin, and mustard oil to activate the receptor. It is, however, less clear how oxidants, gasotransmitters, weak acids, and Cl− cause TRPA1 channel opening (Wang et al., 2011b; Andersson et al., 2012).
A dose-related tussive response is produced by the inhalation of TRPA1 agonists in both animals and humans (Birrell et al., 2009). In conscious guinea pigs, a range of electrophiles has been used to stimulate cough that has been partially inhibited by TRPA1 antagonists of varying specificity. Allyl-isothiocyanate-induced cough was inhibited by AP-18 (Brozmanova et al., 2012) and by the selective (McNamara et al., 2007) TRPA1 antagonist HC-030031 (Fig. 35) (Daller et al., 2012). Both prostaglandin- and bradykinin induced-cough, although not directly mediated through TRPA1, were also partially blocked by HC-030031 (Grace et al., 2012). Complete abolition of tussive responses in these studies required simultaneous blockade of TRPA1 and TRPV1, inferring that although clearly an important candidate as a cough receptor in vivo, TRPA1 is unlikely to be the sole mediator of cough sensitivity. Studies in clinical cough with potent TRPA1 antagonists such as GRC17536 (Mukhopadhyay et al., 2011) are eagerly awaited.
Fig. 35.
Chemical structure of HC-030031.
Q. VRP700
VRP700 has recently been reported to reduce the frequency of cough in patients with idiopathic lung disease (www.veronapharma.com). This drug is being developed as an inhaled treatment, although very little information is currently available in the public domain.
R. GABA Receptor Agonists
GABA (Fig. 36) is a central inhibitory neurotransmitter that also exists in peripheral tissues, including the lung (Ong and Kerr, 1990). Baclofen, an agonist at the GABAB receptor, has been shown to inhibit capsaicin-induced cough in guinea pigs as well as mechanically stimulated cough in cats in a dose-dependent manner (Bolser et al., 1993). The antitussive effect of baclofen occurs through a central site of action, whereas the GABAB agonists 3-APPi (3-amino-propylphophine) and lesogaberan have been demonstrated to inhibit cough through a peripheral site of action (Bolser et al, 1994; Canning et al, 2012). More recently, microinjections of baclofen into the caudal ventral respiratory group region of pentobarbital-anesthetized, spontaneously breathing rabbits were shown to inhibit mechanically stimulated cough (Mutolo et al., 2010). In a study of citric acid-induced cough in conscious guinea pigs, however, baclofen did not demonstrate an antitussive effect (Callaway and King, 1992).
Fig. 36.
Chemical structure of baclofen.
In healthy human volunteers, baclofen has been demonstrated to inhibit capsaicin-induced cough when administered orally, 10 mg three times daily for 14 days (Dicpinigaitis and Dobkin, 1997), and following administration of 20 mg once daily for 14–28 days (Dicpinigaitis et al., 1998). In subjects with cervical spinal cord injury maintained on oral baclofen therapy long term, cough reflex sensitivity was diminished relative to a matched group of subjects not treated with baclofen (Dicpinigaitis et al., 2000).
The demonstration of the effect of baclofen against pathologic cough is limited to two small case series. An open-label study evaluating a 4-week course of low-dose, oral baclofen confirmed an antitussive effect against ACE inhibitor-induced cough (Dicpinigaitis, 1996). It is noteworthy that subjects reported prolonged cough suppression (25–74 days) beyond the 28-day treatment period. In a double-blind, crossover trial in two subjects with chronic, refractory cough, a 14-day course of oral baclofen, 10 mg three times daily, suppressed cough reflex sensitivity to inhaled capsaicin and improved subjectively graded cough frequency and severity. Improvement in cough persisted for approximately 2 weeks beyond the 14-day course of treatment (Dicpinigaitis and Rauf, 1998).
Given the encouraging evidence demonstrating the antitussive effect of baclofen and other GABAB agonists in animals and humans, randomized, placebo-controlled trials are necessary to properly evaluate the efficacy of these agents in various forms of pathologic cough. However, in light of the sedating effect of baclofen, other GABAB agonists, ideally with a greater antitussive action and less sedative effects, should be sought. In addition, further investigation of the antitussive mechanism of action of GABAB agonists in humans is required. For example, in addition to their putative actions centrally, and perhaps peripherally within the lungs, these agents may act through an effect on lower esophageal sphincter pressure and hence, gastroesophageal reflux (discussed in section V.H) (Sifrim et al., 2007).
S. E121
E121 is an enaminone (Fig. 37) that belongs to the class 1 anticonvulsants that have been shown to have effects in the CNS (Edafiogho et al., 1992) and to be anti-inflammatory (El-Hashim et al., 2010). More recently, E121 has been reported to have antitussive activity in guinea pigs where it is able to cause a dose-dependent suppression of citric acid-induced cough when administered by aerosol but not when administered via ICV administration (El-Hashim et al., 2011). This antitussive effect was inhibited by the K+ ATP antagonist glibenclamide, suggesting that this drug is antitussive via activation of K+ ATP channels.
Fig. 37.
Chemical structure of E121.
T. Gabapentin
A recent study has reported that gabapentin (Fig. 38) is effective in patients with chronic cough (Ryan et al., 2012). Gabapentin was approved by the FDA in 1994 for use an anticonvulsant for partial seizures. It has since been widely used for the treatment of neuropathic pain such as postherpetic neuralgia and restless legs syndrome. The concentration of gabapentin in plasma reaches a steady state within 1–2 days and peaks 2–3 hours after each dose. Gabapentin is not appreciably metabolized and is excreted largely unchanged in urine. The elimination half-life is 5–7 hours. Gabapentin is also available as its prodrug gabapentin enacarbil in a modified release preparation. Although gabapentin is an analog of GABA, it is neither a GABA agonist nor an antagonist and its mechanism of action is unknown. One possible mechanism of gabapentin is inhibition of central sensitization by binding selectively to the α2δ subunit of voltage-gated calcium channels and possibly NMDA receptors (Kimos et al., 2007; Hendrich et al., 2008).
Fig. 38.
Chemical structure of gabapentin.
Patients with chronic cough frequently report symptoms suggestive of central sensitization, similar to those with chronic pain. An abnormal sensation in the throat such as a tickle may represent laryngeal paresthesia (abnormal sensation in the absence of a stimulus). Hypertussia (increased cough sensitivity in response to known tussigens) is thought to be similar to hyperalgesia (pain triggered by a low exposure to a known painful stimulus). Allotussia (cough triggered by nontussive stimuli such as talking) is thought to be similar to allodynia (pain triggered by nonpainful stimulus) (Chung et al., 2013). The similarities between the mechanisms of neuropathic pain and cough led to two small studies of gabapentin in patients with cough (Lee and Woo, 2005; Mintz and Lee, 2006). Both reported an antitussive effect. Gabapentin has recently been further evaluated by Ryan et al. (2012) in a double-blind, randomized placebo-controlled trial in patients with refractory chronic cough. The subjects had a severe persistent cough, despite having undergone extensive evaluation and treatment trials prior to inclusion in the study. The target dose was 1800 mg per day for 10 weeks, but a lower dose was allowed if cough resolved or if subjects experienced side effects. There was a clinically significant improvement in health-related, cough-specific quality of life assessed with the Leicester Cough Questionnaire in patients receiving gabapentin compared with placebo (>minimal important difference: 74% versus 46%) (Birring et al., 2003a; Lee and Woo, 2005). This was supported by a reduction in cough severity scores (visual analogs scale) and objective cough frequency assessed with an automated cough monitor (Leicester Cough Monitor) (Birring and Fleming, 2008). The cough worsened when gabapentin was discontinued. There was no change in peripheral cough reflex sensitivity assessed with capsaicin, suggesting a central mechanism of action. The antitussive effect of gabapentin was larger in patients with symptoms of central sensitization and heightened cough reflex sensitivity, suggesting it may be possible to predict patients that respond favorably. Gabapentin was reasonably well tolerated, although side effects were more common in patients receiving gabapentin (24%) (largely CNS side effects), but they were manageable by reducing the dose.
Although the findings of Ryan et al. (2012) need to be confirmed in a larger study, it is important for a number of reasons. Gabapentin was beneficial in patients with a refractory chronic cough, a condition that is common in pulmonary outpatient clinics and for which there is currently no effective pharmacological therapy (Birring, 2011a,b). Gabapentin was reasonably well tolerated and cost effective because gabapentin therapy costs less than £100 per year in the United Kingdom. Ryan et al. (2012) used a validated quality of life questionnaire and automated cough monitor that assesses cough frequency objectively. This should encourage future studies to adopt validated outcome measures (Birring, 2009). This study should also encourage development of other drugs that suppress the sensitization of the cough reflex or those targeting afferent nerves that mediate cough.
U. Thalidomide
An open label clinical trial evaluating the immunomodulatory drug thalidomide (Fig. 39) in patients with IPF suggested that this drug may reduce cough in this population (Horton et al., 2008). More recently this has been followed up by a 24-week, double-blind, two-treatment, two-period crossover trial with thalidomide administered to 20 patients with IPF (Horton et al., 2012). In this study, thalidomide significantly improved cough quality of life questionnaire scores versus placebo treatment, as well as respiratory quality of life in the patient population, despite 74% of the thalidomide-treated population reporting adverse events. Further controlled studies are required to evaluate the effects of thalidomide on objective cough measures and in other forms of pathologic cough.
Fig. 39.
Chemical structure of thalidomide.
V. Botulinum A Toxin
A couple of small pilot clinical studies have reported an antitussive effect of botulinum toxin A; thus, in four patients diagnosed with laryngeal spasm and chronic cough, electromyography guided injections of botulinum toxin A into the thyroaryteroid muscles caused significant relief of the cough, with complete resolution after seven injections, which persisted for around 25 months (Chu et al., 2010). Furthermore vocal fold injection with botulinum toxin A has been claimed to be of value in the treatment of habit cough in three children (Sipp et al., 2007).
W. NaV Channel Blockers
Given the important role of vagal afferent nerves in initiating the cough reflex (Clancy et al., 2013) and the fact that local anesthetics that are known to block all Na+ channels are effective antitussive agents (see section III.D), it is perhaps not surprising that attention has been given to trying to understand which subtype(s) of NaV channel are involved in the cough reflex (Carr, 2013). Although local anesthetics are effective antitussive agents, their nonselectivity precludes their regular use in patients with intractable cough because there is the potential for serious side effects in the CNS and in the heart (Undem and Carr, 2010). There are now known to be nine subtypes of NaV channel (NaV1.1–1.9) and NaV1.7, 1.8, and 1.9 have been shown to be expressed in sensory nerves in the airways and are not really expressed in the CNS, skeletal muscle, or cardiac tissue, raising the possibility of finding inhibitors that preferentially inhibit NaV in sensory nerves (Kwong et al., 2008; Undem and Carr, 2010). From studies in NaV1.9(−/−) mice in comparison with their wild-type littermates, it is clear that the NaV1.9(−/−) mice have altered responses to irritants such as bradykinin, providing evidence of the importance of this NaV channel in the vagal responses to noxious stimuli (Carr, 2013), although because mice do not cough, it is not yet known whether these results translates into an important role of this channel in C-fiber-mediated cough reflexes. In contrast, NaV1.7, which is abundantly expressed in vagal ganglia (Kwong et al., 2008), does appear to be important in the cough reflex because the use of an siRNA to selectively inhibit NaV1.7 expression in the vagal sensory nerves in guinea pigs reduced citric acid-induced cough (Muroi et al., 2011). Recently, a pan-NaV channel inhibitor, GSK 2339345, has been described that can inhibit nerve impulses in the vagus and cough in several species, whereas having limited local anesthetic activity (Hunsberger et al., 2013; Kwong et al., 2013).
X. P2X2/3 Antagonists
P2X3 receptors have been identified on small diameter sensory C-fibers found in a number of tissues in the periphery, although to date, these receptors have not been identified in the CNS. P2X3 receptors are activated by ATP and can lead to sensitization of vagal afferents (North and Jarvis, 2013). The availability of low molecular weight antagonists of the P2X3 receptors has allowed the investigation of the role these receptors play in a number of diseases involving heightened sensory nerve reflexes. In particular, AF 219, an orally active P2X3 receptor antagonist has been shown to reduce coughing in patients with chronic refractory cough of unknown origin (Abdulqawi et al., 2013). This compound was administered twice daily for two weeks in a placebo controlled trial and showed significant improvement in all end points measured compared with placebo (Abdulqawi et al., 2013). However, a significant number of patients in the study complained of a loss of taste when taking AF 219, suggesting that although sensory nerves expressing P2X receptors are involved in the cough reflex in such patients, P2X receptors are also involved in the physiologic processes of taste perception which may limit their use.
VI. Clinical Trial Design for Evaluation of Antitussive Drugs
When conducting a trial, patient selection is of paramount importance. Patients with unexplained chronic cough provide a useful model for studying antitussive agents as cough frequency is high and stable over time making such studies powerful for demonstrating treatment effects (Birring et al., 2006; Decalmer et al., 2007). However, it is important to appreciate that mechanisms underlying chronic cough are not understood, and therefore, failure of an agent in this patient group may not exclude significant activity in other conditions. Acute cough has similarly high cough frequency, and although cough rapidly improves, cough measures are highly repeatable; thus, such studies are powerful for detecting effects of drugs with expected rapid onset of action (Sunger et al., 2013). Sub-acute cough due to viral URTIs has also been suggested, but the one study to target this group found subjects challenging to identify and recruit (Woodcock et al., 2010).
Randomized, placebo-controlled, double-blind clinical trials are obviously the gold standard. Crossover and parallel group designs are both possible for patients with chronic cough, and the particular choice of design should be dictated by the characteristics/mechanism of action of the agent to be assessed. The choice of comparator, whether inert placebo or active, depends on the incidence and severity of drug side-effects. For proof of concept studies, the primary outcome measure should be objective, and cough frequency monitors are the ideal tool (Birring et al., 2008; Smith, 2010a; Barton et al., 2012). However, given the large placebo response reported in many clinical trials evaluating cough (Eccles, 2006), a placebo arm would seem to be vital in determining the likely antitussive action of any drug—new or old. Subjective outcome measures are also useful and capture the patients’ perception of symptoms e.g., visual analog scales and health related quality of life (French et al., 2002; Birring et al., 2003a), important in later phase studies to determine whether reductions in cough frequency result in significant clinical benefit. Cough reflex sensitivity measurements may provide useful pharmacodynamic evidence of target engagement when the tussive agent activates a relevant pathway e.g., capsaicin challenge in TRPV1 antagonist testing (Khalid et al., 2014). However, whether changes in cough reflex sensitivity necessarily predict improvements in cough frequency remains to be established. Sample size, minimal important differences of outcome tools and further information about clinical trial design has been described in more detail elsewhere (Birring, 2009; Smith, 2010b). Carefully designed clinical trials utilizing validated endpoints are needed to properly evaluate currently available therapies and those in development.
VII. Conclusions—What’s Needed
It is evident from this review that cough remains a significant unmet medical need. While our understanding of the cough reflex and the factors that lead to a hypertussive state have increased, particularly over the last decade, this information has yet to translate into the appearance of approved new medicines. It is clear that there is some hope, but the current reality is that for the more severe end of the cough scale, the desperate need for a drug that works is leading to evaluation of drugs used in other therapeutic areas as possible treatments for cough as evident in the recent studies with thalidomide, gabapentin, botulinum A toxin, and baclofen. This is obviously not ideal and there are a number of major challenges to overcome before significant progress can be made in the identification and development of new drugs for such a common, often debilitating, symptom. These include the need for better preclinical models of the hypertussive state (Adcock et al., 2003; Venkatasamy et al., 2010), a better understanding of the changes in airway sensory nerves that underpin the hypertussive state, and very importantly, a clear development pathway accepted by the regulatory authorities of the clinical end points and type of clinical trials required to show acceptable clinical efficacy of any new treatment of cough. We hope this review will provide a suitable stimulus to encourage more laboratories to recognize cough as a significant medical problem and to therefore investigate this problem. It is anticipated that the current decade will see some of these challenges overcome and hopefully the arrival of new medicines for the treatment of cough.
Abbreviations
- 3-APPi
3-aminopropylphosphine
- ACCP
American College of Chest Physicians
- ACE
angiotensin-converting enzyme
- BDP
beclomethasone dipropionate
- BHR
bronchial hyper-responsiveness
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CVA
cough variant asthma
- DSCG
disodium cromoglycate
- ECP
eosinophil cationic protein
- FDA
Food and Drug Administration
- FP
fluticasone propionate
- GERD
gastroesophageal reflux disease
- GR
glucocorticoid receptors
- GRE
glucocorticoid receptor elements
- ICS
inhaled corticosteroids
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- IPF
idiopathic pulmonary fibrosis
- LTRA
leukotriene receptor antagonist
- M
muscarinic
- MAO
monoamine oxidase
- NAEB
nonasthmatic eosinophilic bronchitis
- NMDA
N-methyl-d-aspartate
- NOP
nociceptin opioid receptor
- nTS
nucleus tractus solitarius
- OTC
over the counter
- PDE
phosphodiesterase
- RSD 931
carcainium chloride
- SCH486757
8-[bis(2-chlorophenyl) methyl]-3-(2-pyrimidinyl)-8-azabicyclo[3.2.1.]octan-3-ol
- TRPA1
transient receptor potential A1
- TRPV1
transient receptor potential vanilloid 1
- UNDW
ultrasonically nebulized distilled water
- URTI
upper respiratory tract infection
- VDM12
N-arachidonyl-(2-methyl-4-hydroxyphenyl)
Footnotes
P.V.D., A.H.M., S.S.B., L.M., J.A.S., B.C., and C.P.P. contributed equally to this work.
References
- Abelmann WH, Gaensler EA, Badger TL. (1954) Clinical evaluation of toryn, a new synthetic cough depressant. Dis Chest 25:532–541. [DOI] [PubMed] [Google Scholar]
- Abdulqwai R, McCarthy B, Layton G, Woodcock A, Ford A, Smith JA. (2013) Inhibition of ATP-gated P2X3 channels by AF 219: an effective antitussive mechanisms in chronic cough. Eur Resp J Abstract 1965. [Google Scholar]
- Adcock JJ, Schneider C, Smith TW. (1988) Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea-pig. Br J Pharmacol 93:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock JJ, Douglas GJ, Garabette M, Gascoigne M, Beatch G, Walker M, Page CP. (2003) RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol 138:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Aboud K. (2010) The founder of Vicks: Lunsford Richardson (1854-1919). Skinmed 8:100–101. [PubMed] [Google Scholar]
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JAD, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC.et al. COPD Clinical Research Network (2011) Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliprandi P, Castelli C, Bernorio S, Dell’Abate E, Carrara M. (2004) Levocloperastine in the treatment of chronic nonproductive cough: comparative efficacy versus standard antitussive agents. Drugs Exp Clin Res 30:133–141. [PubMed] [Google Scholar]
- Allegra L, Bossi R. (1988) Clinical trials with the new antitussive levodropropizine in adult bronchitic patients. Arzneimittelforschung 38: 1163–1166. [PubMed] [Google Scholar]
- Almansa-Pastor A. (1996) Treating refractory cough with aerosols of mepivacaine. Chest 110:1374–1375 letter. [DOI] [PubMed] [Google Scholar]
- Alton EW, Norris AA. (1996) Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J Allergy Clin Immunol 98:S102–S105, discussion S105–S106. [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Bevan S. (2012) TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide. PLoS ONE 7:e46917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades N, Worsnop C. (2009) Topical lidocaine through the bronchoscope reduces cough rate during bronchoscopy. Respirology 14:873–876. [DOI] [PubMed] [Google Scholar]
- Arroll B, Kenealy T, Kerse N. (2002) Do delayed prescriptions reduce the use of antibiotics for the common cold? A single-blind controlled trial. J Fam Pract 51:324–328. [PubMed] [Google Scholar]
- Asthma Workgroup, Chinese Society, Respiratory, Diseases (CSRD), Chinese Medical, Association (2011) The Chinese national guidelines on diagnosis and management of cough (December 2010). Chin Med J (Engl) 124:3207–3219. [PubMed] [Google Scholar]
- Auffarth B, Postma DS, de Monchy JG, van der Mark TW, Boorsma M, Koëter GH. (1991) Effects of inhaled budesonide on spirometric values, reversibility, airway responsiveness, and cough threshold in smokers with chronic obstructive lung disease. Thorax 46:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa C, Cazzola M, Clini V, Dal Negro R, Maiorano V, Tana F, Allegra L. (1993) Clinical trial of the efficacy and safety of moguisteine in patients with cough associated with chronic respiratory diseases. Drugs Exp Clin Res 19:273–279. [PubMed] [Google Scholar]
- Aylward M, Maddock J, Davies DE, Protheroe DA, Leideman T. (1984) Dextromethorphan and codeine: comparison of plasma kinetics and antitussive effects. Eur J Respir Dis 65:283–291. [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857. [DOI] [PubMed] [Google Scholar]
- Banderali G, Riva E, Fiocchi A, Cordaro CI, Giovannini M. (1995) Efficacy and tolerability of levodropropizine and dropropizine in children with non-productive cough. J Int Med Res 23:175–183. [DOI] [PubMed] [Google Scholar]
- Bariffi F, Tranfa C, Vatrella A, Ponticiello A. (1992) Protective effect of levodropropizine against cough induced by inhalation of nebulized distilled water in patients with obstructive lung disease. Drugs Exp Clin Res 18:113–118. [PubMed] [Google Scholar]
- Barnabe R, Berni F, Clini V, Pirrelli M, Pisani Ceretti A, Rouschi M, Rossi M, Sestini P, Tana F, Varghi A. (1995) The efficacy and safety of mogguisteine in comparison with codeine phosphate in patients with chronic cough. Monaldi Arch Chest Dis 50:93–97. [PubMed] [Google Scholar]
- Barnes PJ. (1993) Effect of nedocromil sodium on airway sensory nerves. J Allergy Clin Immunol 92:182–186. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. (2007) The problem of cough and development of novel antitussives. Pulm Pharmacol Ther 20:416–422. [DOI] [PubMed] [Google Scholar]
- Barton A, Gaydecki P, Holt K, Smith JA. (2012) Data minimisation in the detection of cough from sound recordings. Cough 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian RW, Vaidya AM, Delsupehe KG. (2006) Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg 135:17–21. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Rennard S, Barnes PJ, Dicpinigaitis PV, Gosens R, Gross NJ, Nadel JA, Pfeifer M, Racké K, Rabe KF, et al. (2009) Alternative mechanisms for tiotropium. Pulm Pharmacol Ther 22:533–542. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Pellegrino M, Tsunozaki M. (2013) TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 75:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LA, Hom J, Villasis-Keever M, van der Wouden JC. (2011) Beta2-agonists for acute bronchitis. Cochrane Database Syst Rev (7):CD001726. [DOI] [PubMed] [Google Scholar]
- Belcher JR. (1949) The pulmonary complications of dysphagia. Thorax 4:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi MG, Patel HJ, Freund-Michel V, Hele DJ, Crispino N, Birrell MA. (2008) Inhibitory activity of the novel CB2 receptor agonist, GW833972A, on guinea-pig and human sensory nerve function in the airways. Br J Pharmacol 155:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini L, Ferrari M, Sembenini C, Olivieri M, Micciolo R, Zuccali V, Bulighin GM, Fiorino F, Ederle A, Cascio VL, et al. (2000) Cough threshold in reflux oesophagitis: influence of acid and of laryngeal and oesophageal damage. Gut 46:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson WM, Stefko PL, Randall LO. (1953) Comparative pharmacology of levorphan, racemorphan and dextrorphan and related methyl ethers. J Pharmacol Exp Ther 109:189–200. [PubMed] [Google Scholar]
- Berger R, McConnell JW, Phillips B, Overman TL. (1989) Safety and efficacy of using high-dose topical and nebulized anesthesia to obtain endobronchial cultures. Chest 95:299–303. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. (2009) Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23:1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettiol S, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, Harnden A. (2010) Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev (1):CD003257. [DOI] [PubMed] [Google Scholar]
- Bickerman HA, Itkin SE. (1960) Further studies on the evaluation of antitussive agents employing experimentally induced cough in human subjects. Clin Pharmacol Ther 1:180–191. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. (2009) TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 180:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell MA, Bonvini SJ, Dubois E, Maler SA, Grace MS, Raemdonck K, Adcock JJ, Belvisi MG. (2014) Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: a beneficial off target effect? J Allergy Clin Immunol 133:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birring SS. (2009) Developing antitussives: the ideal clinical trial. Pulm Pharmacol Ther 22:155–158. [DOI] [PubMed] [Google Scholar]
- Birring SS. (2011a) New concepts in the management of chronic cough. Pulm Pharmacol Ther 24:334–338. [DOI] [PubMed] [Google Scholar]
- Birring SS. (2011b) Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med 183:708–715. [DOI] [PubMed] [Google Scholar]
- Birring SS, Brightling CE, Symon FA, Barlow SG, Wardlaw AJ, Pavord ID. (2003b) Idiopathic chronic cough: association with organ specific autoimmune disease and bronchoalveolar lymphocytosis. Thorax 58:1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birring SS, Fleming T, Matos S, Raj AA, Evans DH, Pavord ID. (2008) The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 31:1013–1018. [DOI] [PubMed] [Google Scholar]
- Birring SS, Matos S, Patel RB, Prudon B, Evans DH, Pavord ID. (2006) Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med 100:1105–1109. [DOI] [PubMed] [Google Scholar]
- Birring SS, Murphy AC, Scullion JE, Brightling CE, Browning M, Pavord ID. (2004) Idiopathic chronic cough and organ-specific autoimmune diseases: a case-control study. Respir Med 98:242–246. [DOI] [PubMed] [Google Scholar]
- Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. (2003a) Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 58:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdóttir I, Einarson TR, Gudmundsson LS, Einarsdóttir RA. (2007) Efficacy of diphenhydramine against cough in humans: a review. Pharm World Sci 29:577–583. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275:F633–F650. [DOI] [PubMed] [Google Scholar]
- Bolser DC. (2006) Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest 129(1, Suppl)238S–249S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC. (2008) Older-generation antihistamines and cough due to upper airway cough syndrome (UACS): efficacy and mechanism. Lung 186 (Suppl 1):S74–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Aziz SM, DeGennaro FC, Kreutner W, Egan RW, Siegel MI, Chapman RW. (1993) Antitussive effects of GABAB agonists in the cat and guinea-pig. Br J Pharmacol 110:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. (2007) Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol 7:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser D, DeGennaro FC, Chapman RW. (1995a) Central and peripheral sites of action of antitussive drugs in the cat, in Ventral Brainstem Mechanisms and Control of Respiration and Blood Pressure (Trouth C, Millis RM, eds) pp 95–102, Marcel Dekker, New York, NY. [Google Scholar]
- Bolser DC, DeGennaro FC, O’Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA. (1994) Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol 113:1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O’Reilly S, Hey JA, Chapman RW. (1995b) Pharmacological studies of allergic cough in the guinea pig. Eur J Pharmacol 277:159–164. [DOI] [PubMed] [Google Scholar]
- Bolser DC, McLeod RL, Tulshian DB, Hey JA. (2001) Antitussive action of nociceptin in the cat. Eur J Pharmacol 430:107–111. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. (2006) Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir Physiol Neurobiol 152:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Sekizawa SI, Joad JP. (2004) Plasticity of central mechanisms for cough. Pulm Pharmacol Ther 17:453–457, discussion 469–470. [DOI] [PubMed] [Google Scholar]
- Bossi R, Banfi P, Filipazzi V, Castelli C, Braga PC. (1994) Levodropropizine (LD) activity in allergic asthmatic patients, challenged with ultrasonically nebulized distilled water, metacholine and allergen-induced bronchospasm. Clin Trials Metaanal 29:9–20. [PubMed] [Google Scholar]
- Bossi R, Braga PC, Centanni S, Legnani D, Moavero NE, Allegra L. (1988) Antitussive activity and respiratory system effects of levodropropizine in man. Arzneimittelforschung 38:1159–1162. [PubMed] [Google Scholar]
- Boulet LP, Milot J, Boutet M, St Georges F, Laviolette M. (1994) Airway inflammation in nonasthmatic subjects with chronic cough. Am J Respir Crit Care Med 149:482–489. [DOI] [PubMed] [Google Scholar]
- Boyd EM, Boyd CE. (1960) Chlophedianol hydrochloride: a new antitussive agent. Can Med Assoc J 83:1298–1301. [PMC free article] [PubMed] [Google Scholar]
- Braga PC, Bossi R, Piatti G, Dal Sasso M. (1993) Antitussive effect of oxatomide on citric acid-induced cough in conscious guinea pig. Preliminary communication. Arzneimittelforschung 43:550–553. [PubMed] [Google Scholar]
- Braman SS. (2006a) Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest 129(1, Suppl)138S–146S. [DOI] [PubMed] [Google Scholar]
- Braman SS. (2006b) Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest 129(1, Suppl)95S–103S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling CE. (2010) Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung 188 (Suppl 1):S13–S17. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. (1999) Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med 160:406–410. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Ward R, Wardlaw AJ, Pavord ID. (2000b) Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J 15:682–686. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Ward R, Woltmann G, Bradding P, Sheller JR, Dworski R, Pavord ID. (2000a) Induced sputum inflammatory mediator concentrations in eosinophilic bronchitis and asthma. Am J Respir Crit Care Med 162:878–882. [DOI] [PubMed] [Google Scholar]
- Brignall K, Jayaraman B, Birring SS. (2008) Quality of life and psychosocial aspects of cough. Lung 186 (Suppl 1):S55–S58. [DOI] [PubMed] [Google Scholar]
- Brown C, Fezoui M, Selig WM, Schwartz CE, Ellis JL. (2004) Antitussive activity of sigma-1 receptor agonists in the guinea-pig. Br J Pharmacol 141:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozmanova M, Plevkova J, Bartos V, Plank L, Tatar M. (2005) Antileukotriene treatment and allergic rhinitis-related cough in guinea pigs. J Physiol Pharmacol 56 (Suppl 4):21–30. [PubMed] [Google Scholar]
- Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M. (2012) Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs. Eur J Pharmacol 689:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher K, Jacot C. (1951) [Mechanism of coughing]. Helv Physiol Pharmacol Acta 9:454–462. [PubMed] [Google Scholar]
- Bucher K. (1956) [New effect mechanism of the antitussive drug tessalon]. Schweiz Med Wochenschr 86:94–96. [PubMed] [Google Scholar]
- Calignano A, Kátona I, Désarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D. (2000) Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 408:96–101. [DOI] [PubMed] [Google Scholar]
- Callaway JK, King RG. (1992) Effects of inhaled α 2-adrenoceptor and GABAB receptor agonists on citric acid-induced cough and tidal volume changes in guinea pigs. Eur J Pharmacol 220:187–195. [DOI] [PubMed] [Google Scholar]
- Callaway JK, King RG, Boura AL. (1991) Evidence for peripheral mechanisms mediating the antitussive actions of opioids in the guinea pig. Gen Pharmacol 22:1103–1108. [DOI] [PubMed] [Google Scholar]
- Canning BJ. (2009) Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther 22:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chou YL. (2009) Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol 187:23–47. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Farmer DG, Mori N. (2006) Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291:R454–R463. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N. (2010) An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24:3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N. (2011) Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 300:R369–R377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N, Lehmann A. (2012) Antitussive effects of the peripherally restricted GABA-B receptor agonist lesogaberan in guinea-pigs: comparison to baclofen and other GABA-B receptor-selective agonists. Cough 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfagna MA, Muhoberac BB. (1993) Interaction of tricyclic drug analogs with synaptic plasma membranes: structure-mechanism relationships in inhibition of neuronal Na+/K(+)-ATPase activity. Mol Pharmacol 44:129–141. [PubMed] [Google Scholar]
- Carr MJ. (2013) Regulation of cough and action potentials by voltage-gated Na channels. Pulm Pharmacol Ther 26:508–509. [DOI] [PubMed] [Google Scholar]
- Cass LJ, Frederik WS, Andosca JB. (1954) Quantitative comparison of dextromethorphan hydrobromide and codeine. Am J Med Sci 227:291–296. [PubMed] [Google Scholar]
- Catania MA, Cuzzocrea S. (2011) Pharmacological and clinical overview of cloperastine in treatment of cough. Ther Clin Risk Manag 7:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena E, Daffonchio L. (1997) Efficacy and tolerability of levodropropizine in adult patients with non-productive cough. Comparison with dextromethorphan. Pulm Pharmacol Ther 10:89–96. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Floriani I, Page CP. (2010) The therapeutic efficacy of erdosteine in the treatment of chronic obstructive bronchitis: a meta-analysis of individual patient data. Pulm Pharmacol Ther 23:135–144. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Matera MG, Liccardi G, De Prisco F, D’Amato G, Rossi F. (1993) Theophylline in the inhibition of angiotensin-converting enzyme inhibitor-induced cough. Respiration 60:212–215. [DOI] [PubMed] [Google Scholar]
- Chakravarty NK, Matallana A, Jensen R, Borison HL. (1956) Central effects of antitussive drugs on cough and respiration. J Pharmacol Exp Ther 117:127–135. [PubMed] [Google Scholar]
- Chang AB, McKean M, Morris P. (2004) Inhaled anti-cholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev (1):CD004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. (2011) Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev (1):CD004823. [DOI] [PubMed] [Google Scholar]
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. (2006) Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 332:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Peake J, McElrea MS. (2008) Anti-histamines for prolonged non-specific cough in children. Cochrane Database Syst Rev 2:CD005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. (1998) A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child 79:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Cheng AC, Chang AB. (2012) Over-the-counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults. Cochrane Database Syst Rev 2:CD006088. [DOI] [PubMed] [Google Scholar]
- Chau TT, Carter FE, Harris LS. (1983) Antitussive effect of the optical isomers of mu, kappa and sigma opiate agonists/antagonists in the cat. J Pharmacol Exp Ther 226:108–113. [PubMed] [Google Scholar]
- Chau TT, Harris LS. (1980) Comparative studies of the pharmacological effects of the d- and l-isomers of codeine. J Pharmacol Exp Ther 215:668–672. [PubMed] [Google Scholar]
- Chaudhuri R, McMahon AD, Thomson LJ, MacLeod KJ, McSharry CP, Livingston E, McKay A, Thomson NC. (2004) Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. J Allergy Clin Immunol 113:1063–1070. [DOI] [PubMed] [Google Scholar]
- Chen JY, Biller HF, Montgomery EG., Jr (1960) Pharmacologic studies of a new antitussive, alpha (dimethylaminoethyl)-ortho-chlorobenzhydrol hydrochloride (SL-501, Bayer B-186). J Pharmacol Exp Ther 128:384–391. [PubMed] [Google Scholar]
- Chen HS, Lipton SA. (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97:1611–1626. [DOI] [PubMed] [Google Scholar]
- Cheriyan S, Greenberger PA, Patterson R. (1994) Outcome of cough variant asthma treated with inhaled steroids. Ann Allergy 73:478–480. [PubMed] [Google Scholar]
- Chizh BA. (2007) Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol 21:259–271. [DOI] [PubMed] [Google Scholar]
- Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, et al. (2007) The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132:132–141. [DOI] [PubMed] [Google Scholar]
- Chong CF, Chen CC, Ma HP, Wu YC, Chen YC, Wang TL. (2005) Comparison of lidocaine and bronchodilator inhalation treatments for cough suppression in patients with chronic obstructive pulmonary disease. Emerg Med J 22:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DT, Wang SC. (1975) Studies on the localization of central cough mechanism; site of action of antitussive drugs. J Pharmacol Exp Ther 194:499–505. [PubMed] [Google Scholar]
- Chou YC, Liao JF, Chang WY, Lin MF, Chen CF. (1999) Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan. Brain Res 821:516–519. [DOI] [PubMed] [Google Scholar]
- Choudry NB, Fuller RW. (1992) Sensitivity of the cough reflex in patients with chronic cough. Eur Respir J 5:296–300. [PubMed] [Google Scholar]
- Choudry NB, Fuller RW, Anderson N, Karlsson JA. (1990) Separation of cough and reflex bronchoconstriction by inhaled local anaesthetics. Eur Respir J 3:579–583. [PubMed] [Google Scholar]
- Choudry NB, Studham J, Harland D, Fuller RW. (1993) Modulation of capsaicin induced airway reflexes in humans: effect of monoamine oxidase inhibition. Br J Clin Pharmacol 35:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu MW, Lieser JD, Sinacori JT. (2010) Use of botulinum toxin type A for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg 136:447–452. [DOI] [PubMed] [Google Scholar]
- Chung KF. (2009) Clinical cough VI: the need for new therapies for cough: disease-specific and symptom-related antitussives. Handb Exp Pharmacol 187:343–368. [DOI] [PubMed] [Google Scholar]
- Chung KF, McGarvey L, Mazzone SB. (2013) Chronic cough as a neuropathic disorder. Lancet Respiratory Medicine 1:414–422. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Buscaglia S, Catrullo A, Marchesi E, Bianchi B, Canonica GW. (1995) Loratadine in the treatment of cough associated with allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol 75:115–120. [PubMed] [Google Scholar]
- Ciprandi G, Tosca M, Ricca V, Passalacqua G, Fregonese L, Fasce L, Canonica GW. (1997) Cetirizine treatment of allergic cough in children with pollen allergy. Allergy 52:752–754. [DOI] [PubMed] [Google Scholar]
- Clancy JA, Deuchars SA, Deuchars J. (2013) The wonders of the Wanderer. Exp Physiol 98:38–45. [DOI] [PubMed] [Google Scholar]
- Cohen V, Jellinek SP, Stansfield L, Truong H, Baseluos C, Marshall JP. (2011) Cardiac arrest with residual blindness after overdose of Tessalon® (benzonatate) perles. J Emerg Med 41:166–171. [DOI] [PubMed] [Google Scholar]
- Colombo B, Turconi P, Daffonchio L, Fedele G, Omini C, Cremaschi D. (1994) Stimulation of Cl- secretion by the mucoactive drug S-carboxymethylcysteine-lysine salt in the isolated rabbit trachea. Eur Respir J 7:1622–1628. [DOI] [PubMed] [Google Scholar]
- Congreve GT. (1881) On Consumption of the Lungs, or, Decline and Its Successful Treatment: Showing That Formidable Disease to Be Curable in All Its Stages: With Observations on Coughs, Colds, Asthma, Chronic Bronchitis Etc, Author and Eliot Stock, London. [Google Scholar]
- Corrao WM, Braman SS, Irwin RS. (1979) Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 300:633–637. [DOI] [PubMed] [Google Scholar]
- Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelièvre L, Geering K. (2000) Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem 275:1976–1986. [DOI] [PubMed] [Google Scholar]
- Crooks MG, Hart SP, Morice AH. (2011) Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365:2234–2235, author reply 2236. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Krakowka GS, Thompson CG. (2005) Systemic effects of interferons after oral administration in animals and humans. Am J Vet Res 66:164–176. [DOI] [PubMed] [Google Scholar]
- Daller JR, Wong J, Brooks BD, McKee JS. (2012) An inexpensive system for evaluating the tussive and anti-tussive properties of chemicals in conscious, unrestrained guinea pigs. J Pharmacol Toxicol Methods 66:232–237. [DOI] [PubMed] [Google Scholar]
- Dal Negro RW. (2008) Erdosteine: antitussive and anti-inflammatory effects. Lung 186(Suppl 1):S70–S73. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, Danzig M. (2007) The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther 20:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Fuller P, Picciotto A, McKenzie SA. (1999) Persistent nocturnal cough: randomised controlled trial of high dose inhaled corticosteroid. Arch Dis Child 81:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasio F, Dicpinigaitis PV, Rubin BK, De Danieli G, Lanata L, Zanasi A. (2012) An observational study on cough in children: epidemiology, impact on quality of sleep and treatment outcome. Cough 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decalmer SC, Webster D, Kelsall AA, McGuinness K, Woodcock AA, Smith JA. (2007) Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 62:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongste JC, Shields MD. (2003) Cough. 2: Chronic cough in children. Thorax 58:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Donno M, Averso C, Corsico R. (1994) Efficacy and safety of moguistine in comparison with dextromethorphan in patients with persistent cough. Drug Invest 7:93–100. [Google Scholar]
- Delgado J, Remers W. (1998) Wilson and Gisvold’s textbook of organic and medicinal pharmaceutical chemistry, Ed. 10th. Raven Publishers, Philadelphia, PA. [Google Scholar]
- Deng H, Fang Y. (2012) Anti-inflammatory gallic acid and wedelolactone are G protein-coupled receptor-35 agonists. Pharmacology 89:211–219. [DOI] [PubMed] [Google Scholar]
- Deschepper R, Grigoryan L, Lundborg CS, Hofstede G, Cohen J, Kelen GV, Deliens L, Haaijer-Ruskamp FM. (2008) Are cultural dimensions relevant for explaining cross-national differences in antibiotic use in Europe? BMC Health Serv Res 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicpinigaitis PV. (1996) Use of baclofen to suppress cough induced by angiotensin-converting enzyme inhibitors. Ann Pharmacother 30:1242–1245. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. (2006) Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest 129(1, Suppl)75S–79S. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Alva RV. (2005) Safety of capsaicin cough challenge testing. Chest 128:196–202. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Bhat R, Rhoton WA, Tibb AS, Negassa A. (2011) Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 105:615–618. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB. (1997) Antitussive effect of the GABA-agonist baclofen. Chest 111:996–999. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB, Rauf K, Aldrich TK. (1998) Inhibition of capsaicin-induced cough by the gamma-aminobutyric acid agonist baclofen. J Clin Pharmacol 38:364–367. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Rauf K. (1998) Treatment of chronic, refractory cough with baclofen. Respiration 65:86–88. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Grimm DR, Lesser M. (2000) Baclofen-induced cough suppression in cervical spinal cord injury. Arch Phys Med Rehabil 81:921–923. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB, Reichel J. (2002) Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma 39:291–297. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Gayle YE. (2003a) Effect of the second-generation antihistamine, fexofenadine, on cough reflex sensitivity and pulmonary function. Br J Clin Pharmacol 56:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Gayle YE. (2003b) Effect of guaifenesin on cough reflex sensitivity. Chest 124:2178–2181. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Gayle YE, Solomon G, Gilbert RD. (2009) Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med 103:902–906. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Spinner L, Santhyadka G, Negassa A. (2008) Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung 186:369–374. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Weiner FR. (2011) Chronic cough associated with interferon/ribavirin therapy for hepatitis C. J Clin Pharm Ther 36:416–418. [DOI] [PubMed] [Google Scholar]
- Dierckx P, Leblanc G, Decoster A, Criscuolo D. (1981) Double-blind study of glaucine in chronic cough. Int J Clin Pharmacol Ther Toxicol 19:396–399. [PubMed] [Google Scholar]
- Di Franco A, Dente FL, Giannini D, Vagaggini B, Conti I, Macchioni P, Scuotri L, Taccola M, Bacci E, Paggiaro PL. (2001) Effects of inhaled corticosteroids on cough threshold in patients with bronchial asthma. Pulm Pharmacol Ther 14:35–40. [DOI] [PubMed] [Google Scholar]
- Di Pisa G, Catanzaro A, Beungarbe D, and Cosentina R (1989) Controllo della tosse nella tubercolosi con levodropropizine. Hospital Management Anno X 76:104–105.
- Diwan JR, Dhand R, Jindal SK, Malik SK, Sharma PL. (1982) A comparative randomized double-blind clinical trial of isoaminile citrate and chlophedianol hydrochloride as antitussive agents. Int J Clin Pharmacol Ther Toxicol 20:373–375. [PubMed] [Google Scholar]
- Dixon M, Jackson DM, Richards IM. (1980a) The action of sodium cromoglycate on ‘C’ fibre endings in the dog lung. Br J Pharmacol 70:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M, Jackson DM, Richards IM. (1980b) A study of the afferent and efferent nerve distribution to the lungs of dogs. Respiration 39:144–149. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Hastings SL, Stimers JR. (1999) Functional Na+/K+ pump in rat dorsal root ganglia neurons. Neuroscience 93:723–729. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Hastings SL, Sims TJ, Stimers JR, Romanovsky D. (2003) Stretch receptor-associated expression of alpha 3 isoform of the Na+, K+-ATPase in rat peripheral nervous system. Neuroscience 116:1069–1080. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Mister R, Pearson MG, Calverley PM. (2000) Capsaicin induced cough in cryptogenic fibrosing alveolitis. Thorax 55:1028–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenjoz R. (1952) [Evaluation of cough remedies]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 215:19–24. [PubMed] [Google Scholar]
- Domino EF, Krutak-Krol H, Lal J. (1985) Evidence for a central site of action for the antitussive effects of caramiphen. J Pharmacol Exp Ther 233:249–253. [PubMed] [Google Scholar]
- Doona M, Walsh D. (1998) Benzonatate for opioid-resistant cough in advanced cancer. Palliat Med 12:55–58. [DOI] [PubMed] [Google Scholar]
- Dubuis E, Wortley MA, Grace MS, Maher SA, Adcock JJ, Birrell MA, Belvisi MG. (2014) Theophylline inhibits the cough reflex through a novel mechanism of action. J Allergy Clin Immunol pii: S0091-6749:01781–01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Ebihara T, Yamasaki M, Asada M, Yamanda S, Niu K, Sasaki H, Arai H. (2007) Contribution of gastric acid in elderly nursing home patients with cough reflex hypersensitivity. J Am Geriatr Soc 55:1686–1688. [DOI] [PubMed] [Google Scholar]
- Eccles R. (2006) Mechanisms of the placebo effect of sweet cough syrups. Respir Physiol Neurobiol 152:340–348. [DOI] [PubMed] [Google Scholar]
- Eccles R, Morris S, Jawad M. (1992) Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 17:175–180. [DOI] [PubMed] [Google Scholar]
- Edafiogho IO, Hinko CN, Chang H, Moore JA, Mulzac D, Nicholson JM, Scott KR. (1992) Synthesis and anticonvulsant activity of enaminones. J Med Chem 35:2798–2805. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Friebel H, Hahn KJ, Halbach H. (1969) Codeine and its alternates for pain and cough relief. 4. Potential alternates for cough relief. Bull World Health Organ 40:639–719. [PMC free article] [PubMed] [Google Scholar]
- Edwards GF, Steel AE, Scott JK, Jordan JW. (1976) S-carboxymethylcysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest 70:506–513. [DOI] [PubMed] [Google Scholar]
- Eherer AJ, Habermann W, Hammer HF, Kiesler K, Friedrich G, Krejs GJ. (2003) Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 38:462–467. [DOI] [PubMed] [Google Scholar]
- El-Hashim A, Yousefi S, Edafiogho I, Raghupathy R, Yousif M, Simon HU. (2010) Anti-inflammatory and immunosuppressive effects of the enaminone E121. Eur J Pharmacol 632:73–78. [DOI] [PubMed] [Google Scholar]
- El-Hashim AZ, Edafiogho IO, Jaffal SM, Yousif MH, Ezeamuzie CI, Kombian SB. (2011) Anti-tussive and bronchodilator mechanisms of action for the enaminone E121. Life Sci 89:378–387. [DOI] [PubMed] [Google Scholar]
- Empey DW, Laitinen LA, Young GA, Bye CE, Hughes DTD. (1979) Comparison of the antitussive effects of codeine phosphate 20mg, dextromethorphan 30 mg and noscapine 30mg using citric acid induced cough in normal subjects. Eur J Clin Pharmacol 16:393–397. [DOI] [PubMed] [Google Scholar]
- Equinozzi R, Robuschi M, Italian Investigational Study Group on Pholcodine in Acute Cough (2006) Comparative efficacy and tolerability of pholcodine and dextromethorphan in the management of patients with acute, non-productive cough: a randomized, double-blind, multicenter study. Treat Respir Med 5:509–513. [DOI] [PubMed] [Google Scholar]
- Evald T, Munch EP, Kok-Jensen A. (1989) Chronic non-asthmatic cough is not affected by inhaled beclomethasone dipropionate. A controlled double blind clinical trial. Allergy 44:510–514. [DOI] [PubMed] [Google Scholar]
- Everett CF, Kastelik JA, Thompson RH, Morice AH. (2007) Chronic persistent cough in the community: a questionnaire survey. Cough 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Wong HH, Geppetti P, Reis JM, Harris SC, Maclean DB, Nadel JA, Boushey HA. (1995) Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 152:879–884. [DOI] [PubMed] [Google Scholar]
- Faruqi S, Molyneux ID, Fathi H, Wright C, Thompson R, Morice AH. (2011) Chronic cough and esomeprazole: a double-blind placebo-controlled parallel study. Respirology 16:1150–1156. [DOI] [PubMed] [Google Scholar]
- Fasciolo G, Nicolini A, Vacca N, Viglierchio P. (1994) Efficacy and safety of moguisteine in comparison with levodropenzine in patients with cough associated with COPD, lung cancer or pulmonary fibrosis. Curr Ther Res 55:251–261. [Google Scholar]
- Food and Drug Administration, HHS (2007) Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the-counter human use; final rule for over-the-counter antitussive drug products; technical amendment. Final rule, technical amendment. Fed Regist 72:67639–67640. [PubMed] [Google Scholar]
- Fiocchi A, Zuccotti GV, Vignati B, Pogliani L, Sala M, Riva E. (1989) Valutazione del trattamento con Levodropropizina nelle affezioni respiratorie del bambino. Pediatr Med Chir 11:519–522. [PubMed] [Google Scholar]
- Fitch PS, Brown V, Schock BC, Taylor R, Ennis M, Shields MD. (2000) Chronic cough in children: bronchoalveolar lavage findings. Eur Respir J 16:1109–1114. [DOI] [PubMed] [Google Scholar]
- Flynn RA, Glynn DA, Kennedy MP. (2009) Anticholinergic treatment in airways diseases. Adv Ther 26:908–919. [DOI] [PubMed] [Google Scholar]
- Footitt J, Johnston SL. (2009) Cough and viruses in airways disease: mechanisms. Pulm Pharmacol Ther 22:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford PA, Durham AL, Russell RE, Gordon F, Adcock IM, Barnes PJ. (2010) Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest 137:1338–1344. [DOI] [PubMed] [Google Scholar]
- Foresi A, Cavigioli G, Pelucchi A, Mastropasqua B, Marazzini L. (1996) Effect of acetazolamide on cough induced by low-chloride-ion solutions in normal subjects: comparison with furosemide. J Allergy Clin Immunol 97:1093–1099. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Karlsson JA, Zackrisson C, Persson CG. (1992) Selective inhibition of cough and bronchoconstriction in conscious guinea pigs. Respiration 59:72–76. [DOI] [PubMed] [Google Scholar]
- Fouad YM, Katz PO, Hatlebakk JG, Castell DO. (1999) Ineffective esophageal motility: the most common motility abnormality in patients with GERD-associated respiratory symptoms. Comment Am J Gastroenterol 94:1464–1467. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. (1997) Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea pig. J Clin Invest 99:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. (1995) Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med 151:879–886. [DOI] [PubMed] [Google Scholar]
- Franco C, Paoletti A, Barbadori S, Zullo R, and Palma S (1992) Valutazione dell’azione antitossse della levodropropizine in pazienti sottoposti a fibrobroncoscopia. Giornale Italiano di Recerche Cliniche Terapeutiche 13:25–28.
- Franova S. (2001) The influence of inhaled furosemide on adverse effects of ACE-inhibitors in airways. Bratisl Lek Listy (Tlacene Vyd) 102:309–313. [PubMed] [Google Scholar]
- Freestone C, Eccles R, Morris S, Jawad MSM. (1996) Assessment of the antitussive efficacy of codeine using cough sound pressure levels as a means of measuring cough. Pulm Pharmacol 9:365. [DOI] [PubMed] [Google Scholar]
- French CL, Irwin RS, Curley FJ, Krikorian CJ. (1998) Impact of chronic cough on quality of life. Arch Intern Med 158:1657–1661. [DOI] [PubMed] [Google Scholar]
- French CT, Irwin RS, Fletcher KE, Adams TM. (2002) Evaluation of a cough-specific quality-of-life questionnaire. Chest 121:1123–1131. [DOI] [PubMed] [Google Scholar]
- Freund-Michel VC, Birrell MA, Giembycz MA, Hele DJ, Haj-Yahia S, Belvisi MG. (2010) Beta(2)-agonists block tussive responses in guinea pigs via an atypical cAMP-dependent pathway. Eur Respir J 35:647–654. [DOI] [PubMed] [Google Scholar]
- Fujimori K, Suzuki E, Arakawa M. (1998) [Effects of oxatomide, H1-antagonist, on postinfectious chronic cough; a comparison of oxatomide combined with dextromethorphan versus dextromethorphan alone]. Arerugi 47:48–53. [PubMed] [Google Scholar]
- Fujimura M, Kamio Y, Myou S, Hashimoto T. (2000) Effect of oral mexiletine on the cough response to capsaicin and tartaric acid. Thorax 55:126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Sakamoto S, Kamio Y, Bando T, Kurashima K, Matsuda T. (1993) Effect of inhaled procaterol on cough receptor sensitivity to capsaicin in patients with asthma or chronic bronchitis and in normal subjects. Thorax 48:615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. (1992) Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax 47:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Ogawa H, Nishizawa Y, Nishi K. (2003) Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax 58:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Collier JG. (1984) Sodium cromoglycate and atropine block the fall in FEV1 but not the cough induced by hypotonic mist. Thorax 39:766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Karlsson JA, Choudry NB, Pride NB. (1988) Effect of inhaled and systemic opiates on responses to inhaled capsaicin in humans. J Appl Physiol (1985) 65:1125–1130. [DOI] [PubMed] [Google Scholar]
- Fumagalli G, Cordaro CI, Vanasia M, Balzarotti C, Camusso L, Caiazzo G, Maghini L, Mazzocchi M, Zennaro M. (1992) A comparative study of the antitussive activity of levodropropizine and dropropizine in the citric acid-induced cough model in normal subjects. Drugs Exp Clin Res 18:303–309. [PubMed] [Google Scholar]
- Gallico L, Borghi A, Dalla Rosa C, Ceserani R, Tognella S. (1994) Moguisteine: a novel peripheral non-narcotic antitussive drug. Br J Pharmacol 112:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini S, Samanin R. (1988) Biochemical hypotheses on antidepressant drugs: a guide for clinicians or a toy for pharmacologists? Psychol Med 18:287–304. [DOI] [PubMed] [Google Scholar]
- Gastpar H, Criscuolo D, Dieterich HA. (1984) Efficacy and tolerability of glaucine as an antitussive agent. Curr Med Res Opin 9:21–27. [DOI] [PubMed] [Google Scholar]
- Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. (2006) Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol (1985) 101:506–511. [DOI] [PubMed] [Google Scholar]
- Gaudioso C, Hao J, Martin-Eauclaire MF, Gabriac M, Delmas P. (2012) Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain 153:473–484. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Hovland DN, Jr, Lehto SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, et al. (2007) Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther 323:128–137. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210. [DOI] [PubMed] [Google Scholar]
- Geering K. (2008) Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17:526–532. [DOI] [PubMed] [Google Scholar]
- Gemmell W. (1890) On ouabain in whooping cough. BMJ 1:950–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germouty J, Jirou-Najou JL. (1987) Clinical efficacy of ambroxol in the treatment of bronchial stasis. Clinical trial in 120 patients at two different doses. Respiration 51 (Suppl 1):37–41. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Chang AB, Glasgow NJ, Holmes PW, Katelaris P, Kemp AS, Landau LI, Mazzone S, Newcombe P, Van Asperen P, et al. CICADA (2010) CICADA: Cough in Children and Adults: Diagnosis and Assessment. Australian cough guidelines summary statement. Med J Aust 192:265–271. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Hargreave FE, Girgis-Gabardo A, Morris M, Denburg JA, Dolovich J. (1995) Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy 25:127–132. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Saltos N, Fakes K. (2001) Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am J Respir Crit Care Med 163:32–36. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Zlatic K, Scott J, Sewell W, Woolley K, Saltos N. (1998) Chronic cough resembles asthma with IL-5 and granulocyte-macrophage colony-stimulating factor gene expression in bronchoalveolar cells. J Allergy Clin Immunol 101:320–326. [DOI] [PubMed] [Google Scholar]
- Gillissen A, Richter A, Oster H. (2007) Clinical efficacy of short-term treatment with extra-fine HFA beclomethasone dipropionate in patients with post-infectious persistent cough. J Physiol Pharmacol 58(Suppl 5)223–232. [PubMed] [Google Scholar]
- Glick J. (1963) Codeine vs. caramiphen in cough control. Del Med J 35:180. [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. (2001) Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur J Pharmacol 422:87–90. [DOI] [PubMed] [Google Scholar]
- Goldman RD. (2010) Codeine for acute cough in children. Can Fam Physician 56:1293–1294. [PMC free article] [PubMed] [Google Scholar]
- Goodman RM, Yergin BM, Sackner MA. (1978) Effects of S-carboxymethylcysteine on tracheal mucus velocity. Chest 74:615–618. [DOI] [PubMed] [Google Scholar]
- Gordon R, Gordon RJ, Sofia D. (1976) Antitussive activity of some naturally occurring cannabinoids in anesthetized cats. Eur J Pharmacol 35:309–313. [DOI] [PubMed] [Google Scholar]
- Gosswald R. (1958) [1-Phenyl-1-(o-chlorophenyl)-3-dimethylaminopropanol, a new anti-tussive agent]. Arzneimittelforschung 8:550–553. [PubMed] [Google Scholar]
- Gove RI, Wiggins J, Stableforth DE. (1985) A study of the use of ultrasonically nebulized lignocaine for local anaesthesia during fibreoptic bronchoscopy. Br J Dis Chest 79:49–59. [DOI] [PubMed] [Google Scholar]
- Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. (2012) Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan TJ, Marshall AE, Higgins KS, Morice AH. (1995) The effect of inhaled and oral dextromethorphan on citric acid induced cough in man. Br J Clin Pharmacol 39:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire F, Thibaudeau Y, Comeau M. (1958) The treatment of cough by a non-narcotic antitussive. Can Med Assoc J 79:180–184. [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. (2004) Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170:1276–1280. [DOI] [PubMed] [Google Scholar]
- Guarino C, Cautiero V, Cordaro C, Catena E. (1991) Levodropropizine in the premedication to fibrebronchoscopy. Drugs Exp Clin Res 17:237–241. [PubMed] [Google Scholar]
- Gupta VK. (2007) Metoclopramide aborts cough-induced headache and ameliorates cough—a pilot study. Int J Clin Pract 61:345–348. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Jr, Druce HM. (1997) Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis 25:1188–1194. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Jr, Winther B, Patrie JT, Hendley JO. (2002) Combined antiviral-antimediator treatment for the common cold. J Infect Dis 186:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PY, Morgenthaler TY, Lim KG. (2007) Use of exhaled nitric oxide in predicting response to inhaled corticosteroids for chronic cough. Mayo Clin Proc 82:1350–1355. [DOI] [PubMed] [Google Scholar]
- Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. (2009) Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 360:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WFD, Palmer KNV, Gent M. (1970) Expectorant action of bromhexine in chronic obstructive bronchitis. BMJ 3:260–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M, Wilhelm M, Swandulla D. (2012) Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses 37:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Midgren B, Karlsson JA. (1994) Effects of inhaled lignocaine and adrenaline on capsaicin-induced cough in humans. Thorax 49:1166–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves MR, Benson MK. (1995) Inhaled sodium cromoglycate in angiotensin-converting enzyme inhibitor cough. Lancet 345:13–16. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Gwaltney JM., Jr (1984) Intranasal interferon-alpha 2 treatment of experimental rhinoviral colds. J Infect Dis 150:174–180. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. (2008) Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA 105:3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert ME, Brewster GS. (2000) Myth: codeine is an effective cough suppressant for upper respiratory tract infections. West J Med 173:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlevsen P, Bredahl C, Hindsholm K, Kruhøffer PK. (1992) Prophylactic laryngo-tracheal aerosolized lidocaine against postoperative sore throat. Acta Anaesthesiol Scand 36:505–507. [DOI] [PubMed] [Google Scholar]
- Hewlett EL, Myers GA, Pearson RD. (1983) Susceptibility of Bordetella species to growth inhibition and killing by chlorpromazine. Antimicrob Agents Chemother 23:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higenbottam T. (1984) Cough induced by changes of ionic composition of airway surface liquid. Bull Eur Physiopathol Respir 20:553–562. [PubMed] [Google Scholar]
- Hill R. (2000) NK1 (substance P) receptor antagonists—why are they not analgesic in humans? Trends Pharmacol Sci 21:244–246. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103:19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes PW, Barter CE, Pierce RJ. (1992) Chronic persistent cough: use of ipratropium bromide in undiagnosed cases following upper respiratory tract infection. Respir Med 86:425–429. [DOI] [PubMed] [Google Scholar]
- Hooper C, Calvert J. (2008) The role for S-carboxymethylcysteine (carbocisteine) in the management of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 3:659–669. [PMC free article] [PubMed] [Google Scholar]
- Hope-Gill BD, Hilldrup S, Davies C, Newton RP, Harrison NK. (2003) A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 168:995–1002. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Nonaka T, Ito M, Kaniwa T, Kishimoto T, Fuchikami J, Kohjimoto Y, Kiyoki M. (1995) Suppressive effect of clenbuterol on citric acid-induced cough reflex in guinea pigs. Res Commun Mol Pathol Pharmacol 88:293–301. [PubMed] [Google Scholar]
- Horton MR, Danoff SK, Lechtzin N. (2008) Thalidomide inhibits the intractable cough of idiopathic pulmonary fibrosis. Thorax 63:749. [DOI] [PubMed] [Google Scholar]
- Horton MR, Santopietro V, Mathew L, Horton KM, Polito AJ, Liu MC, Danoff SK, Lechtzin N. (2012) Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med 157:398–406. [DOI] [PubMed] [Google Scholar]
- Horvat A, Momić T, Petrović S, Nikezić G, Demajo M. (2006) Selective inhibition of brain Na,K-ATPase by drugs. Physiol Res 55:325–338. [DOI] [PubMed] [Google Scholar]
- Hosoe H, Kaise T, Ohmori K, Isohama Y, Kai H, Takahama K, Miyata T. (1999) Mucolytic and antitussive effects of erdosteine. J Pharm Pharmacol 51:959–966. [DOI] [PubMed] [Google Scholar]
- House A, Celly C, Skeans S, Lamca J, Egan RW, Hey JA, Chapman RW. (2004) Cough reflex in allergic dogs. Eur J Pharmacol 492:251–258. [DOI] [PubMed] [Google Scholar]
- Howard P, Cayton RM, Brennan SR, Anderson PB. (1977) Lignocaine aerosol and persistent cough. Br J Dis Chest 71:19–24. [PubMed] [Google Scholar]
- Hudkins RL, DeHaven-Hudkins DL. (1991) M1 muscarinic antagonists interact with sigma recognition sites. Life Sci 49:1229–1235. [DOI] [PubMed] [Google Scholar]
- Hull D, Wright C, Cronin J, Dobroszi D, Hoke S, Noronha A, et al. (2002) Optimised delivery of an antitussive agent improves effectiveness. Eur Resp Rev 12:85 270–274. [Google Scholar]
- Hung CH, Chu CC, Chen YC, Liu KS, Chen YW, Wang JJ. (2012) Cutaneous analgesia and systemic toxicity of carbetapentane and caramiphen in rats. Reg Anesth Pain Med 37:34–39. [DOI] [PubMed] [Google Scholar]
- Hunsberger GE, Ingleby LJ, Brown BS, Ghatta S, Kerns JA, Rumsey W, Kwong K. (2013) In vitro characterisation of GSK 2339345: a novel voltage gated sodium channel blocker for the treatment of chronic cough. Am J Respir Crit Care Med 187:A4937. [Google Scholar]
- Hutchings HA, Eccles R. (1994) The opioid agonist codeine and antagonist naltrexone do not affect voluntary suppression of capsaicin induced cough in healthy subjects. Eur Respir J 7:715–719. [DOI] [PubMed] [Google Scholar]
- Iaia E, Marenco G. (1990) Efficacy and tolerability of a new bromhexine granulate formulation versus sobrerol granulate in patients with chronic obstructive bronchopneumopathies. Med Thorac 12:215–218. [Google Scholar]
- Ing AJ, Ngu MC, Breslin AB. (1992) A randomised double-blind placebo controlled crossover study of ranitidine in patients with chronic persistent cough associated with gastroesophageal reflux. Am Rev Respir Dis 145:A11. [Google Scholar]
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, et al. American College of Chest Physicians (ACCP) (2006) Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 129(Suppl 1)1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RS, French CT, Smyrnios NA, Curley FJ. (1997) Interpretation of positive results of a methacholine inhalation challenge and 1 week of inhaled bronchodilator use in diagnosing and treating cough-variant asthma. Arch Intern Med 157:1981–1987. [PubMed] [Google Scholar]
- Irwin RS, Zawacki JK, Curley FJ, French CL, Hoffman PJ. (1989) Chronic cough as the sole presenting manifestation of gastroesophageal reflux. Am Rev Respir Dis 140:1294–1300. [DOI] [PubMed] [Google Scholar]
- Ishiura Y, Fujimura M, Nobata K, Abo M, Oribe T, Myou S, Nakamura H. (2005) Phosphodiesterase 3 inhibition and cough in elderly asthmatics. Cough 1:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura Y, Fujimura M, Yamamori C, Nobata K, Myou S, Kurashima K, Michishita Y, Takegoshi T. (2003) Effect of carbocysteine on cough reflex to capsaicin in asthmatic patients. Br J Clin Pharmacol 55:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Shioya T, Watanabe A, Sano M, Sasaki M, Miura M. (2002) Mechanism of the antitussive effect of azelastine in guinea pigs. Arzneimittelforschung 52:441–447. [DOI] [PubMed] [Google Scholar]
- Iwata H, Iwata C, Matsuda T. (1988) Difference between two isozymes of (Na2+ K+)-ATPase in the interaction with omeprazole. Jpn J Pharmacol 46:35–42. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Pollard CE, Roberts SM. (1992) The effect of nedocromil sodium on the isolated rabbit vagus nerve. Eur J Pharmacol 221:175–177. [DOI] [PubMed] [Google Scholar]
- Jacqz-Aigrain E, Funck-Brentano C, Cresteil T. (1993) CYP2D6- and CYP3A-dependent metabolism of dextromethorphan in humans. Pharmacogenetics 3:197–204. [DOI] [PubMed] [Google Scholar]
- Jakobesen CJ, Ahlburg P, Holdgord HO, Olsen KH, Thomsen A. (1993) Comparison of intravenous and topical lidocaine as a suppressant of cough after bronchoscopy during general anaesthesia. Acta Anaesthesiol Scand 35:238–241. [DOI] [PubMed] [Google Scholar]
- Jagiełło-Wójtowicz E, Kleinrok Z, Jusiak L, Matuszek B, Daniłoś J, Chodakowska A. (1994) Central action of glaucine in rodents. Acta Pol Pharm 51:179–184. [PubMed] [Google Scholar]
- Janson C, Chinn S, Jarvis D, Burney P. (2001) Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J 18:647–654. [DOI] [PubMed] [Google Scholar]
- Jawad MS, Eccles R, Eccles R, Lee PCL (2000) Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory tract infection. J Pharm Pharmacol 52:1137–1142. [DOI] [PubMed] [Google Scholar]
- Jewell EA, Lingrel JB. (1991) Comparison of the substrate dependence properties of the rat Na,K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J Biol Chem 266:16925–16930. [PubMed] [Google Scholar]
- Jeyakumar A, Brickman TM, Haben M. (2006) Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope 116:2108–2112. [DOI] [PubMed] [Google Scholar]
- Jia YX, Sekizawa K, Sasaki H. (1998) Cholinergic influence on the sensitivity of cough reflex in awake guinea-pigs. J Auton Pharmacol 18:257–261. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O’Byrne PM, Hargreave FE. (1990) Long-term effects of budesonide on airway responsiveness and clinical asthma severity in inhaled steroid-dependent asthmatics. Eur Respir J 3:1122–1127. [PubMed] [Google Scholar]
- Kamei J, Morita K, Ohsawa M, Onodera K. (1999) Effects of epinastine on the antitussive and rewarding effects of dihydrocodeine in mice. Methods Find Exp Clin Pharmacol 21:663–668. [DOI] [PubMed] [Google Scholar]
- Kamei J, Takahashi Y. (2006) Involvement of ionotropic purinergic receptors in the histamine-induced enhancement of the cough reflex sensitivity in guinea pigs. Eur J Pharmacol 547:160–164. [DOI] [PubMed] [Google Scholar]
- Kamei J, Yoshikawa Y, Saitoh A. (2006) Effect of the N-arachidonoyl-(2-methyl-4-hydroxyphenyl) amine (VDM11), an anandamide transporter inhibitor, on capsaicin induced cough in mice. Cough 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos P, Berck H, Fuchs KH, Gillissen A, Klimek L, Morr H, Pfeiffer-Kascha D, Schultze-Werninghaus G, Sitter H, Voshaar T, et al. German Respiratory Society for diagnosis and treatment of adults suffering from acute or chronic cough (2010) Guidelines of the German Respiratory Society for diagnosis and treatment of adults suffering from acute or chronic cough. Pneumologie 64:701–711. [DOI] [PubMed] [Google Scholar]
- Karlsson JA, Choudry NB, Zackrisson C, Fuller RW. (1992) A comparison of the effect of inhaled diuretics on airway reflexes in humans and guinea pigs. J Appl Physiol (1985) 72:434–438. [DOI] [PubMed] [Google Scholar]
- Karlsson JA, Lanner AS, Persson CG. (1990) Airway opioid receptors mediate inhibition of cough and reflex bronchoconstriction in guinea pigs. J Pharmacol Exp Ther 252:863–868. [PubMed] [Google Scholar]
- Kartal ED, Alpat SN, Ozgunes I, Usluer G. (2007) Adverse effects of high-dose interferon-alpha-2a treatment for chronic hepatitis B. Adv Ther 24:963–971. [DOI] [PubMed] [Google Scholar]
- Karttunen P, Tukiainen H, Silvasti M, Kolonen S. (1987) Antitussive effect of dextromethorphan and dextromethorphan-salbutamol combination in healthy volunteers with artificially induced cough. Respiration 52:49–53. [DOI] [PubMed] [Google Scholar]
- Kasé Y, Matsumoto Y, Takahama K, Miyata T, Hitoshi T, Hirotsu I, Okano Y. (1983) On the sites of antitussive action of dl-glaucine phosphate. Arzneimittelforschung 33:947–952. [PubMed] [Google Scholar]
- Kastelik JA, Redington AE, Aziz I, Buckton GK, Smith CM, Dakkak M, Morice AH. (2003) Abnormal oesophageal motility in patients with chronic cough. Thorax 58:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama N, Fujimura M, Ueda A, Kita T, Abo M, Tachibana H, Myou S, Kurashima K. (2001) Effects of carbocysteine on antigen-induced increases in cough sensitivity and bronchial responsiveness in guinea pigs. J Pharmacol Exp Ther 297:975–980. [PubMed] [Google Scholar]
- Katsumata U, Sekizawa K, Inoue H, Sasaki H, Takishima T. (1989) Inhibitory actions of procaterol, a beta-2 stimulant, on substance P-induced cough in normal subjects during upper respiratory tract infection. Tohoku J Exp Med 158:105–106. [DOI] [PubMed] [Google Scholar]
- Kawai S, Baba K, Matsubara A, Shiono H, Okada T, Yamaguchi E. (2008) The efficacy of montelukast and airway mast cell profiles in patients with cough variant asthma. J Asthma 45:243–250. [DOI] [PubMed] [Google Scholar]
- Keeling DJ, Fallowfield C, Milliner KJ, Tingley SK, Ife RJ, Underwood AH. (1985) Studies on the mechanism of action of omeprazole. Biochem Pharmacol 34:2967–2973. [DOI] [PubMed] [Google Scholar]
- Kenia P, Houghton T, Beardsmore C. (2008) Does inhaling menthol affect nasal patency or cough? Pediatr Pulmonol 43:532–537. [DOI] [PubMed] [Google Scholar]
- Kennedy JH. (1962) Silent gastroesophageal reflux; an important but little known cause of pulmonary complications. Dis Chest 42:42–45. [Google Scholar]
- Ketchell RI, Jensen MW, Lumley P, Wright AM, Allenby MI, O’connor BJ. (2002) Rapid effect of inhaled fluticasone propionate on airway responsiveness to adenosine 5′-monophosphate in mild asthma. J Allergy Clin Immunol 110:603–606. [DOI] [PubMed] [Google Scholar]
- Key AL, Holt K, Hamilton A, Smith JA, Earis JE. (2010) Objective cough frequency in idiopathic pulmonary fibrosis. Cough 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S, Woodcock A, Haumann B, Ventresca P, Langley SJ, Smith JA. (2010) A double blind, placebo controlled, randomised study to assess the effect of placebo, codeine and Talenant on citric acid threshold in healthy subjects. Thorax 65:A252–A253. [Google Scholar]
- Khalid S, Murdoch R, Newlands A, Smart K, Kelsall A, Holt K, Dockray R, Woolcock A, Smith JA. (2014) TRPV1 antagonism in refractory chronic cough: A double blind randomised controlled trial. J Allergy Clin Immunol, in press. [DOI] [PubMed] [Google Scholar]
- Khoury RM, Katz PO, Hammod R, Castell DO. (1999) Bedtime ranitidine does not eliminate the need for a second daily dose of omeprazole to suppress nocturnal gastric pH. Aliment Pharmacol Ther 13:675–678. [DOI] [PubMed] [Google Scholar]
- Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. (2000) Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. Eur Respir J 16:633–638. [DOI] [PubMed] [Google Scholar]
- Kim DS, Sohn MH, Jang GC. (2002) The effect of levodropropizine in children with bronchitis. Diagnosis Treatment 9:1029–1034. [Google Scholar]
- Kimos P, Biggs C, Mah J, Heo G, Rashiq S, Thie NM, Major PW. (2007) Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain 127:151–160. [DOI] [PubMed] [Google Scholar]
- Kita T, Fujimura M, Ogawa H, Nakatsumi Y, Nomura S, Ishiura Y, Myou S, Nakao S. (2010) Antitussive effects of the leukotriene receptor antagonist montelukast in patients with cough variant asthma and atopic cough. Allergol Int 59:185–192. [DOI] [PubMed] [Google Scholar]
- Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, Michele TM, Reiss TF, Nguyen HH, Bratton DL. (2001) Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 108:E48. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Ichikawa T, Togashi S, Kumanishi T. (1996) Effects of sigma ligands on the cloned mu-, delta- and kappa-opioid receptors co-expressed with G-protein-activated K+ (GIRK) channel in Xenopus oocytes. Br J Pharmacol 119:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Ishida T, Uchida Y, Kishimoto H, Sasaki H, Shioya T, Tokuyama K, Niimi A, Nishi K, Fujimura M, et al. Committee for the Japanese Respiratory Society Guidelines for Management of Cough (2006) The Japanese Respiratory Society guidelines for management of cough. Respirology 11 (Suppl 4):S135–S186. [DOI] [PubMed] [Google Scholar]
- Kopriva F, Sobolová L, Szotkowská J, Zápalka M. (2004) Treatment of chronic cough in children with montelukast,a leukotriene receptor antagonist. J Asthma 41:715–720. [DOI] [PubMed] [Google Scholar]
- Korppi M, Laurikainen K, Pietikäinen M, Silvasti M. (1991) Antitussives in the treatment of acute transient cough in children. Acta Paediatr Scand 80:969–971. [DOI] [PubMed] [Google Scholar]
- Kroepfli P. (1950) Uber das Verhalten einiger Atmungsgrossen beim husten. Helv Physiol Pharmacol Acta 8:33–42. [Google Scholar]
- Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM., Jr (1982) Antitussive effect of guaifenesin in young adults with natural colds. Objective and subjective assessment. Chest 82:713–718. [DOI] [PubMed] [Google Scholar]
- Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. (2008) Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurones innervating guinea-pig lungs. J Physiol 586:1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Webb EF, Hunsberger GE, Osborn RR, Ghatta S, Ingleby L, Carr MJ, Kerns JK, Rumsey W. (2013) Pharmacological characterisation of GSK 2339345, a novel voltage gated sodium channel blocker for the treatment of symptomatic cough. Am J Respir Crit Care Med 187:A4936. [Google Scholar]
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. (2004) Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 292:1955–1960. [DOI] [PubMed] [Google Scholar]
- Lal S, Bhalla KK. (1975) A controlled trial of bromhexine (‘Bisolvon’) in out-patients with chronic bronchitis. Curr Med Res Opin 3:63–67. [DOI] [PubMed] [Google Scholar]
- Laude EA, Morice AH, Grattan TJ. (1994) The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm Pharmacol 7:179–184. [DOI] [PubMed] [Google Scholar]
- Lavezzo A, Melillo G, Clavenna G, Omini C. (1992) Peripheral site of action of levodropropizine in experimentally-induced cough: role of sensory neuropeptides. Pulm Pharmacol 5:143–147. [DOI] [PubMed] [Google Scholar]
- Lee B, Woo P. (2005) Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol 114:253–257. [DOI] [PubMed] [Google Scholar]
- Lee LY, Ni D, Hayes D, Jr, Lin RL. (2011) TRPV1 as a cough sensor and its temperature-sensitive properties. Pulm Pharmacol Ther 24:280–285. [DOI] [PubMed] [Google Scholar]
- Lee MG, Undem BJ, Brown C, Carr MJ. (2006) Effect of nociceptin in acid-evoked cough and airway sensory nerve activation in guinea pigs. Am J Respir Crit Care Med 173:271–275. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Ambrose C, Banner K, Battram C, Butler K, Giddings J, Mok J, Nasra J, Winny C, Poll C. (2007) Animal models of cough: literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm Pharmacol Ther 20:325–333. [DOI] [PubMed] [Google Scholar]
- Li JQ, Jia YX, Yamaya M, Arai H, Ohrui T, Sekizawa K, Sasaki H. (2002) Neurochemical regulation of cough response to capsaicin in guinea-pigs. Auton Autacoid Pharmacol 22:57–63. [DOI] [PubMed] [Google Scholar]
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. (2000) Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology 118:7–13. [DOI] [PubMed] [Google Scholar]
- Lilienfield LS, Rose JC, Princiotto JV. (1976) Antitussive activity of diphenhydramine in chronic cough. Clin Pharmacol Ther 19:421–425. [DOI] [PubMed] [Google Scholar]
- Lingerfelt BM, Swainey CW, Smith TJ, Coyne PJ. (2007) Nebulized lidocaine for intractable cough near the end of life. J Support Oncol 5:301–302. [PubMed] [Google Scholar]
- Lo Toro P, Raffaghelli G, Caselli GF, Vanasia M, Ferrari MP, De Nicola P. (1990) Farmacocinetica di una dose songola di 60 mg di levo dropropizina sciroppo in pazienti volontari anziani. Acta Gerontol (Milano) 1990. [Google Scholar]
- Lowry R, Higenbottam T, Johnson T, Godden D. (1987) Inhibition of artificially induced cough in man by bronchodilators. Br J Clin Pharmacol 24:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R, Wood A, Johnson T, Higenbottam T. (1988a) Antitussive properties of inhaled bronchodilators on induced cough. Chest 93:1186–1189. [DOI] [PubMed] [Google Scholar]
- Lowry R, Wood A, Higenbottam T. (1994) The effect of anticholinergic bronchodilator therapy on cough during upper respiratory tract infections. Br J Clin Pharmacol 37:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry RH, Wood AM, Higenbottam TW. (1988b) Effects of pH and osmolarity on aerosol-induced cough in normal volunteers. Clin Sci (Lond) 74:373–376. [DOI] [PubMed] [Google Scholar]
- Lü HJ, Qiu ZM, Wei WL, Yu L, Liu RL, Zhang M. (2004) Effects of phosphodiesterase 4 inhibitor on cough response in guinea pigs sensitized and challenged with ovalbumin. Chin Med J (Engl) 117:1620–1624. [PubMed] [Google Scholar]
- Luporini G, Barni S, Marchi E, Daffonchio L. (1998) Efficacy and safety of levodropropizine and dihydrocodeine on nonproductive cough in primary and metastatic lung cancer. Eur Respir J 12:97–101. [DOI] [PubMed] [Google Scholar]
- Lutherer LO, Nugent KM, Schoettle BW, Cummins MJ, Raj R, Birring SS, Jumper CA. (2011) Low-dose oral interferon α possibly retards the progression of idiopathic pulmonary fibrosis and alleviates associated cough in some patients. Thorax 66:446–447. [DOI] [PubMed] [Google Scholar]
- Mabasa V and Gerber P (2005) Dose-response relationship with increasing doses of dextromethorphan for children with cough. Clin Ther 27:1980–1981; author reply 1981–1982. [DOI] [PubMed]
- Mackay GA, Pearce FL. (1996) Extracellular guanosine 3′,5′-cyclic monophosphate and disodium cromoglycate share a similar spectrum of activity in the inhibition of histamine release from isolated mast cells and basophils. Int Arch Allergy Immunol 109:258–265. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934. [DOI] [PubMed] [Google Scholar]
- Manap RA, Wright CE, Gregory A, Rostami-Hodjegan A, Meller ST, Kelm GR, Lennard MS, Tucker GT, Morice AH. (1999) The antitussive effect of dextromethorphan in relation to CYP2D6 activity. Br J Clin Pharmacol 48:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JM, Masters IB, Taylor SM, Cox NC, Seymour GJ, Chang AB. (2006) Evaluation and outcome of young children with chronic cough. Chest 129:1132–1141. [DOI] [PubMed] [Google Scholar]
- Marchant JM, Morris P, Gaffney JT, Chang AB. (2005) Antibiotics for prolonged moist cough in children. Cochrane Database Syst Rev 4:CD004822. [DOI] [PubMed] [Google Scholar]
- Marchioni CF, Livi E, Corona M, Baldini EV, Rapisardi MI, Vanasia M. (1990) L’impiego dell’antitosse levodropropizina in pazienti affetti da neoplasia broncopolmonare. Giornale Italiano di Ricerche Cliniche e Terapeutiche 11:105–109. [Google Scholar]
- Martindale (2009) The complete drug reference (Sweetman S, ed). Pharmaceutical Press, London.
- Matsumoto H, Niimi A, Takemura M, Ueda T, Tabuena R, Yamaguchi M, Matsuoka H, Hirai T, Muro S, Ito Y, et al. (2006) Prognosis of cough variant asthma: a retrospective analysis. J Asthma 43:131–135. [DOI] [PubMed] [Google Scholar]
- Matthys H, Bleicher B, Bleicher U. (1983) Dextromethorphan and codeine: objective assessment of antitussive activity in patients with chronic cough. J Int Med Res 11:92–100. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE. (2006) Na+-K+-2Cl− cotransporters and Cl− channels regulate citric acid cough in guinea pigs. J Appl Physiol (1985) 101:635–643. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ. (2009) Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 29:13662–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey L, McKeagney P, Polley L, MacMahon J, Costello RW. (2009) Are there clinical features of a sensitized cough reflex? Pulm Pharmacol Ther 22:59–64. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Mingo G, O’Reilly S, Ruck LA, Bolser DC, Hey JA. (1998) Antitussive action of antihistamines is independent of sedative and ventilation activity in the guinea pig. Pharmacology 57:57–64. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Parra LE, Mutter JC, Erickson CH, Carey GJ, Tulshian DB, Fawzi AB, Smith-Torhan A, Egan RW, Cuss FM, et al. (2001) Nociceptin inhibits cough in the guinea-pig by activation of ORL(1) receptors. Br J Pharmacol 132:1175–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod RL, Tulshian DB, Bolser DC, Varty GB, Baptista M, Fernandez X, Parra LE, Zimmer JC, Erickson CH, Ho GD, et al. (2010) Pharmacological profile of the NOP agonist and cough suppressing agent SCH 486757 (8-[bis(2-chlorophenyl)methyl]-3-(2-pyrimidinyl)-8-azabicyclo[3.2.1]octan-3-Ol) in preclinical models. Eur J Pharmacol 630:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MS, Mulrennan SA, Everett CF, Thompson R, Morice AH. (2005) Experience with baclofen in cough secondary to gastro-oesophageal reflux disease. Am Rev Respir Dis A323. [Google Scholar]
- Meoni P, Tortella FC, Bowery NG. (1997) An autoradiographic study of dextromethorphan high-affinity binding sites in rat brain: sodium-dependency and colocalization with paroxetine. Br J Pharmacol 120:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millqvist E, Bende M, Löwhagen O. (1998) Sensory hyperreactivity—a possible mechanism underlying cough and asthma-like symptoms. Allergy 53:1208–1212. [DOI] [PubMed] [Google Scholar]
- Mistretta A, Privitera S, Pulvirenti G, Oliveri R, Palermo B, Palermo F, Vancheri C, Crimi N. (1992) Protective effect of levodropropizine against capsaicin-induced cough in allergic patients: a double-blind, placebo-controlled study. Adv Ther 2:98–106. [Google Scholar]
- Mintz S, Lee JK. (2006) Gabapentin in the treatment of intractable idiopathic chronic cough: case reports. Am J Med 119:e13–e15. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Shimizu T, Maeda S, Tokuyama K, Morikawa A, Kuroume T. (1995b) Relationship between ultrasonically nebulized distilled water-induced bronchoconstriction and acetic acid-induced cough in asthmatic children. J Allergy Clin Immunol 96:193–199. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Shimizu T, Morikawa A, Kuroume T. (1995a) Inhaled diuretics attenuate acid-induced cough in children with asthma. Chest 107:413–417. [DOI] [PubMed] [Google Scholar]
- Mokry J, Nosolova G. (2011) The influence of the PDE inhibitors on cough reflex in guinea pig. Bratisl. Lek. Listying 112:131–135. [PubMed] [Google Scholar]
- Mokry J, Nosalova G, Mokra D. (2009) Influence of xanthine derivatives on cough and airway reactivity in guinea pigs. J Physiol Pharmacol 60 (Suppl 5):87–91. [PubMed] [Google Scholar]
- Molassiotis A, Smith JA, Bennett MI, Blackhall F, Taylor D, Zavery B, Harle A, Booton R, Rankin EM, Lloyd-Williams M, et al. (2010) Clinical expert guidelines for the management of cough in lung cancer: report of a UK task group on cough. Cough 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monczor F, Fernandez N, Fitzsimons CP, Shayo C, Davio C. (2013) Antihistaminergics and inverse agonism: potential therapeutic applications. Eur J Pharmacol 715:26–32. [DOI] [PubMed] [Google Scholar]
- Morice AH. (2010) The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 188 (Suppl 1):S87–S90. [DOI] [PubMed] [Google Scholar]
- Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O’Connell F, Geppetti P, Gronke L, De Jongste J, et al. ERS Task Force (2004) The diagnosis and management of chronic cough. Eur Respir J 24:481–492. [DOI] [PubMed] [Google Scholar]
- Morice AH, Geppetti P. (2004b) Cough. 5: the type 1 vanilloid receptor: a sensory receptor for cough. Thorax 59:257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice AH, Lowry R, Brown MJ, Higenbottam T. (1987) Angiotensin-converting enzyme and the cough reflex. Lancet 2:1116–1118. [DOI] [PubMed] [Google Scholar]
- Morice AH, Marshall AE, Higgins KS, Grattan TJ. (1994) Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 49:1024–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice AH, McGarvey L, Pavord I, British Thoracic Society Cough Guideline Group (2006) Recommendations for the management of cough in adults. Thorax 61 (Suppl 1):i1–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice AH, McGarvey LP, Dicpinigaitis PV. (2012) Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung 190:3–9. [DOI] [PubMed] [Google Scholar]
- Morice AH, Menon MS, Mulrennan SA, Everett CF, Wright C, Jackson J, Thompson R. (2007) Opiate therapy in chronic cough. Am J Respir Crit Care Med 175:312–315. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Gallico L, Widdicombe J. (1997) Action of moguisteine on cough and pulmonary respiratory adapting receptor acitivty in the guinea-pig. Pharm Res 35:113–118. [DOI] [PubMed] [Google Scholar]
- Morita K, Kamei J. (2000) Involvement of ATP-sensitive K(+) channels in the anti-tussive effect of moguisteine. Eur J Pharmacol 395:161–164. [DOI] [PubMed] [Google Scholar]
- Morita K, Kamei J. (2003) Antitussive effect of WIN 55212-2, a cannabinoid receptor agonist. Eur J Pharmacol 474:269–272. [DOI] [PubMed] [Google Scholar]
- Moshiree B, McDonald R, Hou W, Toskes PP. (2010) Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis. Dig Dis Sci 55:675–683. [DOI] [PubMed] [Google Scholar]
- Mossad SB, Macknin ML, Medendorp SV, Mason P. (1996) Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med 125:81–88. [DOI] [PubMed] [Google Scholar]
- Mudge J. (1778) A Radical and Expeditious Cure for a Recent Catarrhous Cough, Allen, London. [Google Scholar]
- Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, Kuruganti S, Thorat S, Khairatkar-Joshi N. (2011) Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res 31:350–358. [DOI] [PubMed] [Google Scholar]
- Mulrennan S, Wright C, Thompson R, Goustas P, Morice A. (2004) Effect of salbutamol on smoking related cough. Pulm Pharmacol Ther 17:127–131. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Ru F, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ. (2011) Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol 589:5663–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. (2008) Modulation of the cough reflex by anti-tussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol 295:R243–R251 [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Pantaleo T. (2010) Depression of cough reflex by microinjections of antitussive agents into caudal ventral respiratory group of the rabbit. J Appl Physiol (1985) 109:1002–1010. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Fontana GA, Pantaleo T. (2007) The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull 74:284–293. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. (2008) Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, et al. (2012) Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 7:e42454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer R, Pflimlin P, Trube G. (1993) Dextromethorphan blocks N-methyl-d-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol 238:209–216. [DOI] [PubMed] [Google Scholar]
- Nichol G, Nix A, Barnes PJ, Chung KF. (1990) Prostaglandin F2 alpha enhancement of capsaicin induced cough in man: modulation by beta 2 adrenergic and anticholinergic drugs. Thorax 45:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi A, Torrego A, Nicholson AG, Cosio BG, Oates TB, Chung KF. (2005) Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol 116:565–570. [DOI] [PubMed] [Google Scholar]
- Nieto L, de Diego A, Perpiñá M, Compte L, Garrigues V, Martínez E, Ponce J. (2003) Cough reflex testing with inhaled capsaicin in the study of chronic cough. Respir Med 97:393–400. [DOI] [PubMed] [Google Scholar]
- Nishitsuji M, Fujimura M, Oribe Y, Nakao S. (2004) A guinea pig model for cough variant asthma and role of tachykinins. Exp Lung Res 30:723–737. [DOI] [PubMed] [Google Scholar]
- Nishitsuji M, Fujimura M, Oribe Y, Nakao S. (2008) Effect of montelukast in a guinea pig model of cough variant asthma. Pulm Pharmacol Ther 21:142–145. [DOI] [PubMed] [Google Scholar]
- North RA, Jarvis MF. (2013) P2X receptors as drug targets. Mol Pharmacol 83:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosál’ová G, Strapková A, Korpás J, Criscuolo D. (1989) Objective assessment of cough suppressants under normal and pathological experimental conditions. Drugs Exp Clin Res 15:77–81. [PubMed] [Google Scholar]
- O’Connell F, Thomas VE, Studham JM, Pride NB, Fuller RW. (1996) Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 90:279–286. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Fujimura M, Hara J, Ohsawa M, Kamei J, Nakao S. (2009) Bronchoconstriction-triggered cough in conscious guinea pigs. Exp Lung Res 35:296–306. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Fujimura M, Nakade Y, Okazaki A, Katayama N. (2012) Heightened cough response to bronchoconstriction in cough variant asthma. Respirology 17:964–968. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Fujimura M, Tokuda A, Katayama N. (2010) Do β2-agonists inhibit capsaicin-induced cough? correspondence Eur Respir J 36:459–460, author reply 460–461. [DOI] [PubMed] [Google Scholar]
- Ong J, Kerr DIB. (1990) GABA-receptors in peripheral tissues. Life Sci 46:1489–1501. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Fujimura M, Kita T, Katayama N, Nishitsuji M, Hara J, Myou S, Nakao S. (2005) Attenuating effect of H+K+ATPase inhibitors on airway cough hypersensitivity induced by allergic airway inflammation in guinea-pigs. Clin Exp Allergy 35:262–267. [DOI] [PubMed] [Google Scholar]
- Ours TM, Kavuru MS, Schilz RJ, Richter JE. (1999) A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Comment Am J Gastroenterol 94:3131–3138. [DOI] [PubMed] [Google Scholar]
- Packman EW, Ciccone PE. (1983) The utility of artificially induced cough as a clinical model for evaluating long-lasting antitussive drug combinations. Am J Pharm 155:42–48. [Google Scholar]
- Packman EW, Ciccone PE, Wilson J, Masurat T. (1991) Antitussive effects of diphenhydramine on the citric acid aerosol-induced cough response in humans. Int J Clin Pharmacol Ther Toxicol 29:218–222. [PubMed] [Google Scholar]
- Page CP. (1994) Sodium cromoglycate, a tachykinin antagonist? Lancet 343:70. [DOI] [PubMed] [Google Scholar]
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J, International COPD Study Group (1998) Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet 351:773–780. [DOI] [PubMed] [Google Scholar]
- Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. (1996) Evaluation of antitussive agents in man. Pulm Pharmacol 9:299–308. [DOI] [PubMed] [Google Scholar]
- Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ, Yacoub MH, Belvisi MG. (2003) Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol 140:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IM. (2012) Therapeutic options for acute cough due to upper respiratory infections in children. Lung 190:41–44. [DOI] [PubMed] [Google Scholar]
- Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr (2007) Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med 161:1140–1146. [DOI] [PubMed] [Google Scholar]
- Paul IM, Shaffer ML, Yoder KE, Sturgis SA, Baker MS, Berlin CM., Jr (2004b) Dose-response relationship with increasing doses of dextromethorphan for children with cough. Clin Ther 26:1508–1514. [DOI] [PubMed] [Google Scholar]
- Paul IM, Yoder KE, Crowell KR, Shaffer ML, McMillan HS, Carlson LC, Dilworth DA, Berlin CM., Jr (2004a) Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics 114:e85–e90. [DOI] [PubMed] [Google Scholar]
- Pavesi L, Subburaj S, Porter-Shaw K. (2001) Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest 120:1121–1128. [DOI] [PubMed] [Google Scholar]
- Pellegrini CA, DeMeester TR, Johnson LF, Skinner DB. (1979) Gastroesophageal reflux and pulmonary aspiration: incidence, functional abnormality, and results of surgical therapy. Surgery 86:110–119. [PubMed] [Google Scholar]
- Petkov V, Todorov S, Georgiev V, Petkova B, Donev N. (1979) Pharmacological studies of a group of semi-synthetic structural analogues of glaucine. Acta Physiol Pharmacol Bulg 5:3–12. [PubMed] [Google Scholar]
- Pizzichini MM, Pizzichini E, Parameswaran K, Clelland L, Efthimiadis A, Dolovich J, Hargreave FE. (1999) Nonasthmatic chronic cough: No effect of treatment with an inhaled corticosteroid in patients without sputum eosinophilia. Can Respir J 6:323–330. [DOI] [PubMed] [Google Scholar]
- Plevkova J, Antosiewicz J, Varechova S, Poliacek I, Jakus J, Tatar M, Pokorski M. (2009) Convergence of nasal and tracheal neural pathways in modulating the cough response in guinea pigs. J Physiol Pharmacol 60:89–93. [PubMed] [Google Scholar]
- Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, Mori N, Canning BJ. (2013) The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol 115:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe RH, Kallay MC. (2003) Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment. Chest 123:679–684. [DOI] [PubMed] [Google Scholar]
- Poggioli RA, Benelli A, Arletti R, Cavazzuti E, Bertolini A. (1999) Antitussive effect of K+ channel openers. Eur J Pharmacol 371:39–42. [DOI] [PubMed] [Google Scholar]
- Pons R, Santamaría P, Suchankova J, Cortijo J, Morcillo EJ. (2000) Effects of inhaled glaucine on pulmonary responses to antigen in sensitized guinea pigs. Eur J Pharmacol 397:187–195. [DOI] [PubMed] [Google Scholar]
- Ponsioen BP, Hop WC, Vermue NA, Dekhuijzen PN, Bohnen AM. (2005) Efficacy of fluticasone on cough: a randomised controlled trial. Eur Respir J 25:147–152. [DOI] [PubMed] [Google Scholar]
- Pontiroli AE, Daffonchio L. (1997) Efficacy and tolerability of levodropropizine and clobutinol in elderly patients with non-productive cough. Clin Drug Investig 14:175–182. [Google Scholar]
- Poole P, Black PN. (2010) Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev (2):CD001287. [DOI] [PubMed] [Google Scholar]
- Pornsuriyasak P, Charoenpan P, Vongvivat K, Thakkinstian A. (2005) Inhaled corticosteroid for persistent cough following upper respiratory tract infection. Respirology 10:520–524. [DOI] [PubMed] [Google Scholar]
- Pounsford JC, Birch MJ, Saunders KB. (1985) Effect of bronchodilators on the cough response to inhaled citric acid in normal and asthmatic subjects. Thorax 40:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratter MR, Bartter T, Akers S, DuBois J. (1993) An algorithmic approach to chronic cough. Ann Intern Med 119:977–983. [DOI] [PubMed] [Google Scholar]
- Prieto L, Ferrer A, Ponce S, Palop J, Marín J. (2009) Exhaled nitric oxide measurement is not useful for predicting the response to inhaled corticosteroids in subjects with chronic cough. Chest 136:816–822. [DOI] [PubMed] [Google Scholar]
- Prudon B, Birring SS, Vara DD, Hall AP, Thompson JP, Pavord ID. (2005) Cough and glottic-stop reflex sensitivity in health and disease. Chest 127:550–557. [DOI] [PubMed] [Google Scholar]
- Raj AA, Pavord DI, Birring SS. (2009) Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handbook Exp Pharmacol 187:311–320. [DOI] [PubMed] [Google Scholar]
- Ralph N. (1954) Evaluation of a new cough suppressant. Am J Med Sci 227:297–303. [PubMed] [Google Scholar]
- Rami HK, Thompson M, Stemp G, Fell S, Jerman JC, Stevens AJ, Smart D, Sargent B, Sanderson D, Randall AD, et al. (2006) Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett 16:3287–3291. [DOI] [PubMed] [Google Scholar]
- Ramsay J, Wright C, Thompson R, Hull D, Morice AH. (2008) Assessment of antitussive efficacy of dextromethorphan in smoking related cough: objective vs. subjective measures. Br J Clin Pharmacol 65:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath JB, Pleuvry BJ. (1982) Double-blind comparison of the respiratory and sedative effects of codeine phosphate and (±)-glaucine phosphate in human volunteers. Br J Clin Pharmacol 14:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees PJ, Clark TJH. (1983) Assessment of antitussive effects by citric acid threshold. Br J Dis Chest 77:94–97. [PubMed] [Google Scholar]
- Remichkova M, Dimitrova P, Philipov S, Ivanovska N. (2009) Toll-like receptor-mediated anti-inflammatory action of glaucine and oxoglaucine. Fitoterapia 80:411–414. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353:1711–1723. [DOI] [PubMed] [Google Scholar]
- Ribeiro MG, Pedrenho AR, Hassón-Voloch A. (2002) Electrocyte (Na(+),K(+))ATPase inhibition induced by zinc is reverted by dithiothreitol. Int J Biochem Cell Biol 34:516–524. [DOI] [PubMed] [Google Scholar]
- Roa CC, Jr, Dantes RB. (1995) Clinical effectiveness of a combination of bromhexine and amoxicillin in lower respiratory tract infection. A randomized controlled trial. Arzneimittelforschung 45:267–272. [PubMed] [Google Scholar]
- Robinson RE, Cummings WB, Deffenbaugh ER. (1977) Effectiveness of guaifenesin as an expectorant:a cooperative double-blind study. Curr Ther Res 22:284–296. [Google Scholar]
- Romandini S, Rugarli PL. (1989) Post marketing surveillance di Levo dropropizina un nuovo agente antitussivo. Post Marketing Surveillance 3:150–155. [Google Scholar]
- Round P, Priestley A, Robinson J. (2011) An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol 72:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BK. (2007) Mucolytics, expectorants, and mucokinetic medications. Respir Care 52:859–865. [PubMed] [Google Scholar]
- Rühle KH, Criscuolo D, Dieterich HA, Köhler D, Riedel G. (1984) Objective evaluation of dextromethorphan and glaucine as antitussive agents. Br J Clin Pharmacol 17:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NM, Birring SS, Gibson PG. (2012) Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 380:1583–1589. [DOI] [PubMed] [Google Scholar]
- Ryerson CJ, Abbritti M, Ley B, Elicker BM, Jones KD, Collard HR. (2011) Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 16:969–975. [DOI] [PubMed] [Google Scholar]
- Sadofsky LR, Boa AN, Maher SA, Birrell MA, Belvisi MG, Morice AH. (2011) TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids. Pharmacol Res 63:30–36. [DOI] [PubMed] [Google Scholar]
- Sadofsky LR, Campi B, Trevisani M, Compton SJ, Morice AH. (2008) Transient receptor potential vanilloid-1-mediated calcium responses are inhibited by the alkylamine antihistamines dexbrompheniramine and chlorpheniramine. Exp Lung Res 34:681–693. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio FB, Sant’Ambrogio G, Anderson JW. (1993) Effect of furosemide on the response of laryngeal receptors to low-chloride solutions. Eur Respir J 6:1151–1155. [PubMed] [Google Scholar]
- Schadel M, Wu D, Otton SV, Kalow W, Sellers EM. (1995) Pharmacokinetics of dextromethorphan and metabolites in humans: influence of the CYP2D6 phenotype and quinidine inhibition. J Clin Psychopharmacol 15:263–269. [DOI] [PubMed] [Google Scholar]
- Selveraj T, Dhanpal R. (2002) Evaluation of the applications of topical steroids on the endotracheal tube in decreasing past operative sore throat. J.Anaesthical Clin.Pharmacol. 18:167–170. [Google Scholar]
- Shadkam MN, Mozaffari-Khosravi H, Mozayan MR. (2010) A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Altern Complement Med 16:787–793. [DOI] [PubMed] [Google Scholar]
- Shaheen NJ, Crockett SD, Bright SD, Madanick RD, Buckmire R, Couch M, Dellon ES, Galanko JA, Sharpless G, Morgan DR, et al. (2011) Randomised clinical trial: high-dose acid suppression for chronic cough—a double-blind, placebo-controlled study. Aliment Pharmacol Ther 33:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen NJ, Denison H, Bjorck K, Karlsson M, Silberg DG. (2013) Efficacy and safety of lesogaberan in gastro-oesophageal reflux disease: a randomised controlled trial. Gut 62:1248–1255. [DOI] [PubMed] [Google Scholar]
- Shane SJ, Krzyski TK, Copp SE. (1957) Clinical evaluation of a new antitussive agent. Can Med Assoc J 77:600–602. [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. (2004) Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther 17:369–376. [DOI] [PubMed] [Google Scholar]
- Sharfstein JM, North M, Serwint JR. (2007) Over the counter but no longer under the radar—pediatric cough and cold medications. N Engl J Med 357:2321–2324. [DOI] [PubMed] [Google Scholar]
- Sheppard D, Rizk NW, Boushey HA, Bethel RA. (1983) Mechanism of cough and bronchoconstriction induced by distilled water aerosol. Am Rev Respir Dis 127:691–694. [DOI] [PubMed] [Google Scholar]
- Shioya T, Ito N, Watanabe A, Kagaya M, Sano M, Shindo T, Miura S, Kimura K, Miura M. (1998) Antitussive effect of azelastine hydrochloride in patients with bronchial asthma. Arzneimittelforschung 48:149–153. [PubMed] [Google Scholar]
- Shioya T, Satake M, Kagaya M, Sano MA, Watanabe A, Fukui S, Sasaki M. (2004) Antitussive effects of the H1-receptor antagonist epinastine in patients with atopic cough (eosinophilic bronchitis). Arzneimittelforschung 54:207–212. [DOI] [PubMed] [Google Scholar]
- Sifrim D, Mittal R, Fass R, Smout A, Castell D, Tack J, Gregersen H. (2007) Review article: acidity and volume of the refluxate in the genesis of gastro-oesophageal reflux disease symptoms. Aliment Pharmacol Ther 25:1003–1017. [DOI] [PubMed] [Google Scholar]
- Shams H, Daffonchio L, Scheid P. (1996) Effects of levodropropizine on vagal afferent C-fibres in the cat. Br J Pharmacol 117:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura S, Okubo T, Maeda S, Aoki T, Tomioka M, Shindo Y, Takishima T, Umeya K. (1983) Effect of expectorants on relaxation behaviour of sputum viscoelasticity in vivo. Biorheology 20:677–683. [DOI] [PubMed] [Google Scholar]
- Silvasti M, Karttunen P, Tukiainen H, Kokkonen P, Hanninen U, Nykanen S. (1987) Pharmacokinetics of dextromethorphan: a single dose comparison of three preparations in human volunteers. Int J Clin Pharmacol Ther Toxicol 25:493–497. [PubMed] [Google Scholar]
- Simons FER. (2004) Advances in H1-antihistamines. N Engl J Med 351:2203–2217. [DOI] [PubMed] [Google Scholar]
- Singh M, Das RR. (2011) Zinc for the common cold. Cochrane Database Syst Rev 2:CD001364. [DOI] [PubMed] [Google Scholar]
- Sipp JA, Haver KE, Masek BJ, Hartnick CJ. (2007) Botulinum toxin A: a novel adjunct treatment for debilitating habit cough in children. Ear Nose Throat J 86:570–572. [PubMed] [Google Scholar]
- Sisson JH, Yonkers AJ, Waldman RH. (1995) Effects of guaifenesin on nasal mucociliary clearance and ciliary beat frequency in healthy volunteers. Chest 107:747–751. [DOI] [PubMed] [Google Scholar]
- Smith CA, Adamson DL, Choudry NB, Fuller RW. (1991) The effect of altering airway tone on the sensitivity of the cough reflex in normal volunteers. Eur Respir J 4:1078–1079. [PubMed] [Google Scholar]
- Smith J. (2010a) Monitoring chronic cough: current and future techniques. Expert Rev Respir Med 4:673–683. [DOI] [PubMed] [Google Scholar]
- Smith JA. (2010b) Assessing efficacy of therapy for cough. Otolaryngol Clin North Am 43:157–166, xi xi. [DOI] [PubMed] [Google Scholar]
- Smith J, Owen E, Earis J, Woodcock A. (2006) Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol 117:831–835. [DOI] [PubMed] [Google Scholar]
- Smith JA, Hilton EC, Saulsberry L, Canning BJ. (2012) Antitussive effects of memantine in guinea pigs. Chest 141:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny JJ, Flynn CA, Becker LA, Glazier RH. (2001) Are beta2-agonists effective treatment for acute bronchitis or acute cough in patients without underlying pulmonary disease? A systematic review. J Fam Pract 50:945–951. [PubMed] [Google Scholar]
- Smucny J, Fahey T, Becker L, Glazier R. (2004) Antibiotics for acute bronchitis. Cochrane Database Syst Rev (4):CD000245. [DOI] [PubMed] [Google Scholar]
- Soltani HA, Aghadavoudi O. (2002) The effect of different lidocaine application methods on postoperative cough and sore throat. J Clin Anesth 14:15–18. [DOI] [PubMed] [Google Scholar]
- Spector SL, Tan RA. (2004) Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol 93:232–236. [DOI] [PubMed] [Google Scholar]
- Stefko PL, Denzel J, Hickey M. (1961) Experimental investigation of nine antitussive drugs. J Pharm Sci 50:216–221. [Google Scholar]
- Stelmach I, Korzeniewska A, Stelmach W, Majak P, Grzelewski T, Jerzynska J. (2005) Effects of montelukast treatment on clinical and inflammatory variables in patients with cystic fibrosis. Ann Allergy Asthma Immunol 95:372–380. [DOI] [PubMed] [Google Scholar]
- Stone RA, Barnes PJ, Chung KF. (1993) Effect of frusemide on cough responses to chloride-deficient solution in normal and mild asthmatic subjects. Eur Respir J 6:862–867. [PubMed] [Google Scholar]
- Studham J, Fuller RW. (1992) The effect of oral terfenadine on the sensitivity of the cough reflex in normal volunteers. Pulm Pharmacol 5:51–52. [DOI] [PubMed] [Google Scholar]
- Sullivan P, Bekir S, Jaffar Z, Page CP, Jeffery P, Costello JF. (1994) Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet 343:1006–1008. [DOI] [PubMed] [Google Scholar]
- Sumathi PA, Shenoy T, Ambareesha M, Krishna HM. (2008) Controlled comparison between betamethasone gel and lidocaine jelly applied over tracheal tube to reduce postoperative sore throat, cough, and hoarseness of voice. Br J Anaesth 100:215–218. [DOI] [PubMed] [Google Scholar]
- Sunger K, Powley W, Kelsall A, Summer H, Murdoch R, Smith JA. (2013) Objective measurement of cough in otherwise healthy volunteers with acute cough. Eur Respir J 41:277–284. [DOI] [PubMed] [Google Scholar]
- Sutovska M, Nosalova G, Franova S. (2007) The role of potassium ion channels in cough and other reflexes of the airways. J Physiol Pharmacol 58(Pt 2, Suppl 5)673–683. [PubMed] [Google Scholar]
- Sweetman S. (ed) (2008) Martindale: The Complete Drug Reference, 36th edition. Pharmaceutical Press: London. [Google Scholar]
- Talbott MW, Barch GK, Gabel LP. (1975) A new method for evaluating antitussives in cats using an electrode-cannula. Eur J Pharmacol 34:59–63. [DOI] [PubMed] [Google Scholar]
- Takahama K, Shirasaki T. (2007) Central and peripheral mechanisms of narcotic antitussives: codeine-sensitive and -resistant coughs. Cough 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama K, Wakuda I, Fukushima H, Isohama Y, Kai H, Miyata T. (1997) Differential effect of codeine on coughs caused by mechanical stimulation of two different sites in the airway of guinea pigs. Eur J Pharmacol 329:93–97. [DOI] [PubMed] [Google Scholar]
- Takemura M, Niimi A, Matsumoto H, Ueda T, Matsuoka H, Yamaguchi M, Jinnai M, Chin K, Mishima M. (2012) Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration 83:308–315. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Yokohori N, Tagaya E, Kirishi S, Miyamoto Y, Ochiai K, Kondo M, Nagai A. (2010) Comparable effect of a leukotriene receptor antagonist and long-acting beta₂-adrenergic agonist in cough variant asthma. Allergy Asthma Proc 31:78–84. [DOI] [PubMed] [Google Scholar]
- Tamburrano D, Romandini S. (1989) Studio multicentrico sulla tollerabilità ed efficacia della levodropropizina, un nuovo farmaco anti-tosse, in una ampia casistica pediatrica. Terapie Essenziali in Clinica 4:3–7. [Google Scholar]
- Tanaka S, Hirata K, Kurihara N, Yoshikawa J, Takeda T. (1996) Effect of loratadine, an H1 antihistamine, on induced cough in non-asthmatic patients with chronic cough. Thorax 51:810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao XN, Xin JB, Zhang XJ, Li YM, Xiong XZ, Bai M. (2005) Clinical Efficacy of Levodropropizine in Cough Treatment. Herald of Medicine J 24:23–24. [Google Scholar]
- Tatar M, Sant’Ambrogio G, Sant’Ambrogio FB. (1994) Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol (1985) 76:2672–2679. [DOI] [PubMed] [Google Scholar]
- Tatar M, Webber SE, Widdicombe JG. (1988) Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternesten-Hasséus E, Johansson K, Löwhagen O, Millqvist E. (2006) Inhalation method determines outcome of capsaicin inhalation in patients with chronic cough due to sensory hyperreactivity. Pulm Pharmacol Ther 19:172–178. [DOI] [PubMed] [Google Scholar]
- Thomson ML, Pavia D, Jones CJ, McQuiston TA. (1975) No demonstrable effect of S-carboxymethylcysteine on clearance of secretions from the human lung. Thorax 30:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorén P, Oberg B. (1981) Studies on the endoanesthetic effects of lidocaine and benzonatate on non-medullated nerve endings in the left ventricle. Acta Physiol Scand 111:51–58. [DOI] [PubMed] [Google Scholar]
- Tomerak AA, McGlashan JJ, Vyas HH, McKean MC. (2005) Inhaled corticosteroids for non-specific chronic cough in children. Cochrane Database Syst Rev 4:CD004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner JJ, Macko E. (1952) Pharmacology studies on bis-(1-carbo-beta-diethylaminoethoxy)-1-phenylcyclopentane)-ethane disulfonate. J Pharmacol Exp Ther 106:246–251. [PubMed] [Google Scholar]
- Trigg CJ, Nicholson KG, Wang JH, Ireland DC, Jordan S, Duddle JM, Hamilton S, Davies RJ. (1996) Bronchial inflammation and the common cold: a comparison of atopic and non-atopic individuals. Clin Exp Allergy 26:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochtenberg S. (1994) Nebulized lidocaine in the treatment of refractory cough. Chest 105:1592–1593. [DOI] [PubMed] [Google Scholar]
- Tsubouchi T, Tsujimoto S, Sugimoto S, Katsura Y, Mino T, Seki T. (2008) Swallowing disorder and inhibition of cough reflex induced by atropine sulfate in conscious dogs. J Pharmacol Sci 106:452–459. [DOI] [PubMed] [Google Scholar]
- Tsuchida H, Takahashi S, Nosaka E, Kuraya T, Yamashita M, Morimoto K. (2008) Novel triple neurokinin receptor antagonist CS-003 inhibits respiratory disease models in guinea pigs. Eur J Pharmacol 596:153–159. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Xing H, Ling J, Gu JG. (2004) Menthol-induced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J Neurosci 24:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RC, Kagaya M, Page CP, Spina D. (2001) The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br J Pharmacol 132:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukiainen H, Karttunen P, Silvasti M, Flygare U, Korhonen R, Korhonen T, Majander R, Seuri M. (1986) The treatment of acute transient cough: a placebo-controlled comparison of dextromethorphan and dextromethorphan-beta 2-sympathomimetic combination. Eur J Respir Dis 69:95–99. [PubMed] [Google Scholar]
- Usmani OS, Belvisi MG, Patel HJ, Crispino N, Birrell MA, Korbonits M, Korbonits D, Barnes PJ. (2005) Theobromine inhibits sensory nerve activation and cough. FASEB J 19:231–233. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Carr MJ. (2010) Targeting primary afferent nerves for novel antitussive therapy. Chest 137:177–184. [DOI] [PubMed] [Google Scholar]
- Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, Hwang C, Sostek MB, Shaker R. (2006) Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 116:254–260. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Sommers DK, Snyman JR. (1994) Effects of glycopyrrolate on capsaicin-induced cough in normal volunteers treated with captopril. Eur J Clin Pharmacol 46:437–439. [DOI] [PubMed] [Google Scholar]
- Vela MF, Tutuian R, Katz PO, Castell DO. (2003) Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther 17:243–251. [DOI] [PubMed] [Google Scholar]
- Venkatasamy R, McKenzie A, Page CP, Walker MJ, Spina D. (2010) Use of within-group designs to test anti-tussive drugs in conscious guinea-pigs. J Pharmacol Toxicol Methods 61:157–162. [DOI] [PubMed] [Google Scholar]
- Ventresca PG, Nichol GM, Barnes PJ, Chung KF. (1990) Inhaled furosemide inhibits cough induced by low chloride content solutions but not by capsaicin. Am Rev Respir Dis 142:143–146. [DOI] [PubMed] [Google Scholar]
- Ventresca PG, Nichol GM, Barnes PJ, Chung KF. (1992) Effect of frusemide on the induction and potentiation of cough induced by prostaglandin F2 α. Br J Clin Pharmacol 33:514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij TJ, Hermans J, Mulder JD. (1994) Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. Br J Gen Pract 44:400–404. [PMC free article] [PubMed] [Google Scholar]
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. (2002) Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Birring SS, Taylor K, Fry NK, Hay AD, Moore M, Jin J, Perera R, Farmer A, Little P, et al. (2014) Montelukast for postinfectious cough in adults: a double blind randomized placebo-controlled trial. Lancet Respir Med 2:35–43. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu J, Zhang L, Qu H. (2011a) A new steroidal glycoside from the Ophiopogon japonicus Ker-Gawler (Liliaceae). Nat Prod Res 25:31–35. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. (2011b) A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol 137:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring JP, Lacayo L, Hunter J, Katz E, Suwak B. (1995) Chronic cough and hoarseness in patients with severe gastroesophageal reflux disease. Diagnosis and response to therapy. Dig Dis Sci 40:1093–1097. [DOI] [PubMed] [Google Scholar]
- Wen J, Zhang H, Xia C, Hu X, Xu W, Cheng X, Gao J, Xiong Y. (2010) A sensitive liquid chromatography-electrospray ionization-mass spectrometry method for the simultaneous determination of pentoxyverine citrate and guaifenesin in human plasma---application to pharmacokinetic and bioequivalence studies. Biomed Chromatogr 24:351–357. [DOI] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. (2011) Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J 25:4434–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter ML, Spiller HA, Griffith JR. (2010) Benzonatate ingestion reported to the national poison center database system (NPDS). J Med Toxicol 6:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth L. (1958) Use of chlorpromazine in cough, with particular reference to whooping cough. Mil Med 122:195–196. [PubMed] [Google Scholar]
- Wise PM, Breslin PA, Dalton P. (2012) Sweet taste and menthol increase cough reflex thresholds. Pulm Pharmacol Ther 25:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock ARL, McLeod RL, Sadeh J, Smith JA. (2010) The efficacy of a NOP1 agonist (SCH486757) in subacute cough. Lung 188 (Suppl 1):S47–S52. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(Suppl. 3):S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Bowen WP, Grattan TJ, Morice AH. (1998) Identification of the L-menthol binding site in guinea-pig lung membranes. Br J Pharmacol 123:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Laude EA, Grattan TJ, Morice AH. (1997) Capsaicin- and neurokinin A-induced bronchoconstriction in the anaesthetised guinea-pig: Evidence for a bronchodilator effect of L-menthol. Br J Pharmacol 121:1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang A, Uchida Y, Nomura A, Iijima H, Dong F, Zhang MJ, Hasegawa S. (1998) Effects of airway inflammation on cough response in the guinea pig. J Appl Physiol (1985) 85:1847–1854. [DOI] [PubMed] [Google Scholar]
- Yeates DB, Cohen VR, Davis AL, Albert RE, Waillant J, Lippman M. (1977) Effect of glyceryl guaiacolate on bronchial clearance in patients with chronic bronchitis. Am Rev Respir Dis 115 (Suppl):182. [Google Scholar]
- Yoder KE, Shaffer ML, La Tournous SJ, Paul IM. (2006) Child assessment of dextromethorphan, diphenhydramine, and placebo for nocturnal cough due to upper respiratory infection. Clin Pediatr (Phila) 45:633–640. [DOI] [PubMed] [Google Scholar]
- Young EC, Sumner H, Declamer L, Houghton A, Woodcock A, Smith JA. (2010) Does central up-regulation of the N-methyl-D-aspartate receptor contribute to cough reflex hypersensitivity. Am J Respir Crit Care Med 181:A5906. [Google Scholar]
- Young EC, Pawsey S, Woodcock A, Smith JA. (2012) An open label study of V3381, a novel N-methyl-d-aspartate receptor (NMDA-R) antagonist in chronic cough (abstract). Lung 19:63. [Google Scholar]
- Yousaf N, Monteiro W, Parker D, Matos S, Birring S, Pavord ID. (2010) Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax 65:1107–1110. [DOI] [PubMed] [Google Scholar]
- Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. (2011) The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukioka H, Yoshimoto N, Nishimura K, Fujimori M. (1985) Intravenous lidocaine as a suppressant of coughing during tracheal intubation. Anesth Analg 64:1189–1192. [PubMed] [Google Scholar]
- Zaratin P, De Angelis L, Cattabeni F. (1988) Gas chromatographic-mass spectrometric determination of levodropropizine plasma levels in healthy volunteers. Arzneimittelforschung 8:1156–1158. [PubMed]