INTRODUCTION
Contrast-enhanced spectral mammography (CESM) has been increasingly described as a useful modality for the detection and evaluation of breast pathologies. It uses intravenous iodinated contrast during mammography acquisition to produce lesion enhancement, improving detection. This is based on tumoral angiogenesis, leading to increased permeability of the cellular basement membrane to contrast agents.
The technique involves the modification of standard full-field digital mammography (FFDM) equipment to acquire images of the breast at low and high X-ray energies. This dual-energy technique makes use of the difference in X-ray attenuation between the breast tissue and iodine. The low-energy images are similar to standard FFDM in terms of appearance and diagnostic quality.[1] Postprocessing of images from both energy sources involves recombination followed by subtracting the background breast tissue, thereby highlighting areas of contrast uptake. The radiologist is then presented with both recombined and low-energy images for interpretation.
Thus, CESM is able to provide physiological information similar to magnetic resonance imaging (MRI), and its diagnostic performance has been shown to be comparable to MRI in multiple published studies.[2,3,4] In addition, CESM is readily available, requires less time and is cheaper to perform. Patients also prefer CESM due to the reduced procedure time, greater comfort and lower noise levels as compared to MRI.[5]
TECHNIQUE
For this study, images were acquired on a Selenia Dimensions™ mammography system (Hologic Inc, Danbury, CT, USA) at our institute. Nonionic, low-osmolar, iodinated contrast material (Iohexol; GE Healthcare, Chicago, IL, USA) was administered intravenously, and in the initial 2 min after contrast injection, breast compression was avoided to prevent impediment to contrast flow into the breasts. Generally, image acquisition for CESM began at 2 min, starting with the side of concern in the view mediolateral oblique or craniocaudal [CC], which best showed the pathology. The subsequent imaging sequence was variable. Beyond 8 min, images were considered delayed. Studies were typically completed by 10 min, after which patients were observed for a short period for adverse effects before discharge. The imaging protocol followed at our institute is summarised in Box 1.
Box 1.
Technical parameters and acquisition protocols of contrast enhanced spectral mammography.
|
Intravenous contrast 1.5 mL/kg (maximum 80 mL), 2.5–3.0 mL via power injector with 20 mL of saline bolus push, with breasts uncompressed for up to 2 min |
|
|
|
Imaging protocol Affected site, most visible FFDM view first First time point at 2 min, successively followed by the remainder as below: |
| • Contralateral side: corresponding view |
| • Affected side: other view |
| • Contralateral side: corresponding view |
|
|
|
Delayed acquisitions At radiologist’s discretion |
FFDM: full field digital mammography
DIAGNOSTIC PERFORMANCE AND INDICATIONS
A published meta-analysis evaluating 13 studies showed comparable performance of CESM and MRI.[4] The overall findings showed high diagnostic performances in the area under the curve and high sensitivity of 0.97 for both modalities. By comparison, CESM was found to have slightly higher specificity than MRI (0.66 vs. 0.52). Similarly, a study by Sumkin et al.[3] showed comparable diagnostic performance of CESM and MRI in the detection and sizing of index malignancies, with CESM detecting fewer false-positive lesions.
Given its diagnostic performance and ready availability, CESM has a wide range of indications. In breast cancer screening, CESM has been found to be an excellent problem-solving tool in recalled patients, showing improvement in all diagnostic parameters over FFDM.[6,7] In one study, the mean sensitivity increased from 93.0% to 96.9% and the negative predictive value improved from 92.6% to 98.2%.[7] The high negative predictive value provides confidence in discharging patients with a negative study back to routine screening, potentially reducing the rate of unnecessary biopsies.
Dense breast tissue reduces the sensitivity of FFDM, obscuring underlying abnormalities, and is itself an independent risk factor for breast cancer. It has been shown that CESM has a higher sensitivity than FFDM (86.2% vs. 53.4%) in dense breasts.[8] This raises a potential role in breast screening, particularly in high-risk patients, who are often young and have denser breasts. A pilot study comparing CESM to MRI in high-risk screening reported comparable diagnostic performance of the two modalities.[9]
In a small study evaluating CESM in the assessment of architectural distortion, CESM showed a high sensitivity of 97% for malignancy, although with a low specificity of 58%.[10] The authors attributed the lower specificity to background parenchymal enhancement, which made it challenging to appreciate actual lesion enhancement.
Finally, with regards to local staging, CESM demonstrates good correlation with pathological tumour size, similar to MRI.[11] It also shows good performance in assessing residual malignancy following neoadjuvant therapy.[12] Overall, CESM can, therefore, be considered a promising alternative in the preoperative evaluation of breast cancer.
LIMITATIONS
Contrast-related: the use of iodinated contrast introduces the risk of contrast-induced nephropathy (CIN) and adverse contrast reactions. To mitigate these risks, our institution implemented preprocedural screening of CIN risk factors and a 30-min postprocedure observation period.
Radiation dose: due to the additional radiation exposure, the average glandular dose of CESM is higher than that of FFDM, although studies have shown it is still within the acceptable range for mammography.[13]
Technique-related: technological limitations include the inability to objectively evaluate enhancement kinetics and a lack of widely available biopsy functionality, which results in patients still having to undergo MRI if the lesion is occult on FFDM and ultrasonography (US).
Imaging pitfalls: increased background enhancement of the normal breast parenchyma can obscure pathological enhancement. Overlapping features can also be seen in enhancing benign lesions (atypical ductal hyperplasia, fibroadenoma, infection/inflammation and radial scar) and nonenhancing or poorly enhancing significant lesions (such as ductal carcinoma in situ [DCIS] and inflammatory breast cancer). These limitations are similar to MRI.[14,15]
INDICATIONS
Problem-solving of equivocal full-field digital mammography or ultrasound findings
Case 1
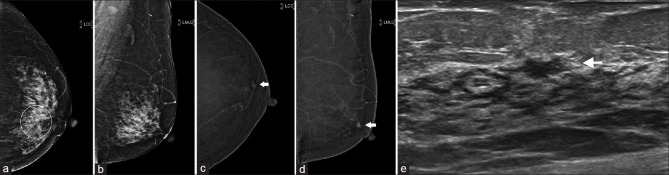
A 69-year-old woman was referred for evaluation of small nodular enhancing foci in the left breast incidentally detected on computed tomography (CT) of the chest. On both FFDM and US, no suspicious lesion was seen [Figure 1a]. Subsequent CESM revealed multiple enhancing foci in the left breast. This was further confirmed on MRI [Figure 1b and c]. The patient underwent MRI-guided vacuum-assisted biopsy (VAB), which showed low-grade DCIS. In this case, CESM was useful to guide further management on the same day of the patient’s visit to the breast clinic, despite the negative FFDM and US findings.
Figure 1.
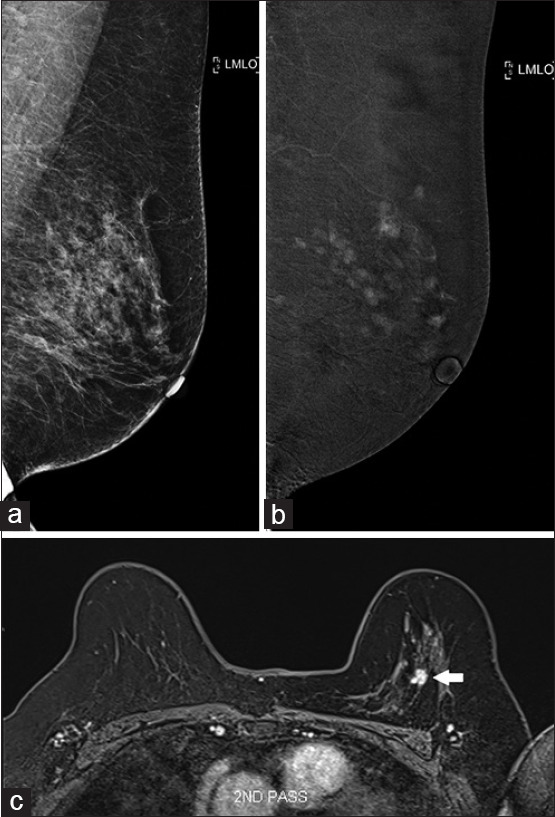
Case 1: A 69-year-old woman. (a) Full-field digital mammography shows no suspicious lesions in the left breast. (b) Contrast-enhanced spectral mammography shows multiple enhancing foci. (c) MR image confirms the presence of multiple enhancing lesions; the largest lesion (arrow) was targeted for MRI-guided vacuum-assisted biopsy, which showed low-grade ductal carcinoma in situ.
Case 2
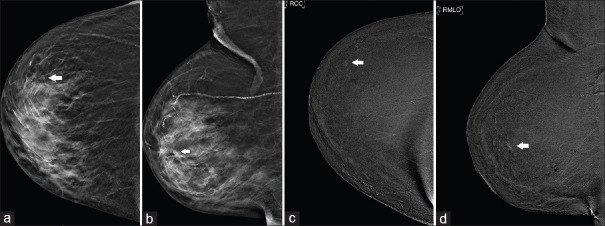
A 69-year-old woman who had previously undergone right mastectomy for breast cancer had an area of subtle architectural distortion detected in the central left breast during a routine follow-up mammogram [Figure 2a]. Further evaluation with CESM did not reveal any focal enhancement in this region, and it was deemed to be related to scarring related to previous surgery [Figure 2b]. However, a separate enhancing nodule was seen in the upper outer periareolar left breast [Figure 2b and d], which was previously obscured on FFDM due to heterogeneously dense fibroglandular tissue [Figure 2a and c]. Second-look US revealed an ill-defined, 8-mm hypoechoic lesion in the corresponding location [Figure 2e]. The patient underwent US-guided VAB, which showed low-grade intraductal carcinoma, for which she had a left mastectomy. In this case, CESM expedited the diagnostic evaluation; without it, the patient may have been erroneously discharged to routine surveillance.
Figure 2.
Case 2: A 69-year-old woman. (a & b) Full-field digital mammography (FFDM) shows architectural distortion in the central left breast (circle in a). Contrast-enhanced spectral mammography shows (c) no focal enhancement corresponding to the area of architectural distortion, but (b & c) a separate enhancing focus is detected in the upper outer periareolar left breast (arrows), which is not seen on FFDM. (e) US image shows an ill-defined, 8-mm hypoechoic lesion in the corresponding location (arrow); biopsy of the lesion showed intraductal carcinoma.
Case 3
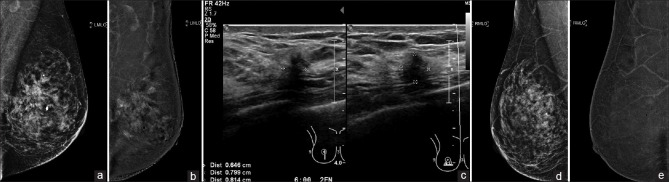
A 75-year-old woman presented with a palpable right breast lump. Initial evaluation revealed a tiny nodule in the upper outer quadrant of the right breast, faintly visible on tomosynthesis [Figure 3a and b]. No sonographic correlate was found. This was further evaluated with CESM, which showed an enhancing nodule at the site [Figure 3c and d]. Stereotactic-guided biopsy showed in situ lobular neoplasia. Subsequent excision biopsy showed low-grade DCIS and atypical lobular hyperplasia. In this case, CESM confirmed the presence of an equivocal finding on conventional imaging, thereby preventing a missed diagnosis of carcinoma in situ.
Figure 3.
Case 3: A 75-year-old woman. (a & b) Full-field digital mammography shows a tiny nodule in the upper outer quadrant of the right breast (arrows). (c & d) Contrast-enhanced spectral mammography confirms the diagnosis. Subsequent excision biopsy revealed low-grade ductal carcinoma in situ and atypical lobular hyperplasia.
Assessment of screening recalls
Case 4
An asymptomatic 62-year-old woman was recalled after screening for a nodular opacity in the right lower outer quadrant [Figure 4a]. On US, no sonographic correlate was found. However, CESM showed enhancement of the nodule [Figure 4b]. Biopsy was then performed on the same day under stereotactic guidance, and the nodule was proven to be fibroadenomatoid hyperplasia.
Figure 4.
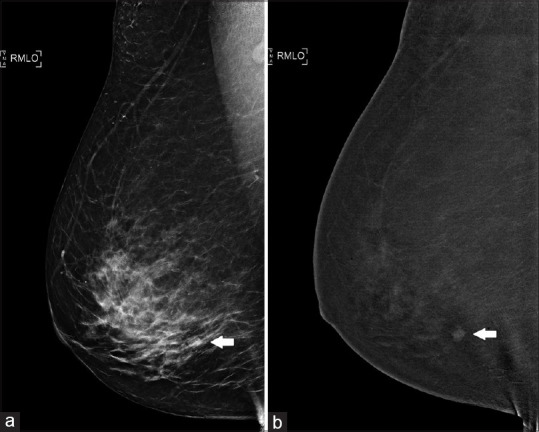
Case 4: A 62-year-old woman. (a) Full-field digital mammography shows a nodular opacity in the right lower outer quadrant (arrow). However, no sonographic correlate was found. (b) Contrast-enhanced spectral mammography shows the presence of a nodule in the right lower outer quadrant (arrow), which was proven to be fibroadenomatoid hyperplasia on stereotactic-guided biopsy.
Case 5
A 59-year-old woman was recalled after screening for focal asymmetry in the outer half of the right breast on the CC view [Figure 5a]. No sonographic correlate was seen, and CESM confirmed the presence of a small enhancing nodule corresponding to the area of focal asymmetry in the right upper outer quadrant [Figure 5b]. Subsequent MRI showed type I enhancement kinetics of the nodule suggestive of a benign lesion [Figure 5c]. The patient was discharged to routine mammographic screening.
Figure 5.
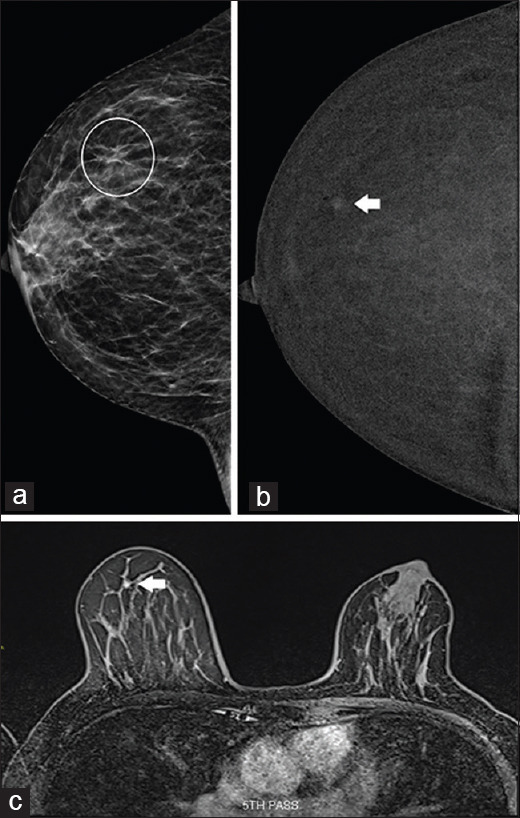
Case 5: A 59-year-old woman. (a) Full-field digital mammography shows an asymmetry in the outer right breast (circled), visible only on craniocaudal view. (b) Contrast-enhanced spectral mammography (CESM) shows that the lesion was localised to the right upper outer quadrant (arrow). (c) Subsequent MR image shows type I enhancement kinetics, suggestive of a benign lesion (arrow), which confirmed the CESM finding.
Cases 4 and 5 demonstrate the increased sensitivity of CESM when compared to FFDM in detecting small lesions, and its inferiority to MRI in assessing enhancement kinetics.
Preoperative local staging of breast cancer
Case 6
A 62-year-old woman presented with a left breast lump of 1-month duration. On FFDM, a corresponding spiculated mass was seen in the left upper outer quadrant. Subsequently, CESM was performed for local staging, confirming heterogeneous enhancement of only the mass [Figure 6a and b], which was found to be invasive ductal carcinoma (IDC) on core biopsy. The patient eventually opted for a mastectomy, which confirmed the presence of unifocal grade 3 IDC. This case illustrates the possible option of using CESM as an adjunct to MRI for preoperative staging if breast conservation surgery is being considered.
Figure 6.
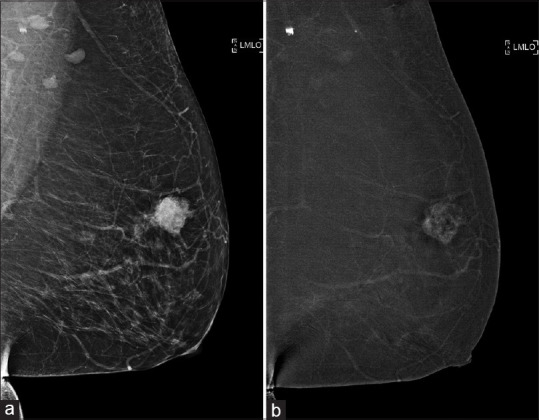
Case 6: A 62-year-old woman. (a) Full field digital mammography shows a spiculated mass seen in the left upper outer quadrant. (b) Contrast-enhanced spectral mammography for local staging shows enhancement of the mass, with no additional enhancing foci, confirming unifocal disease.
Case 7
A 46-year-old woman was referred for a right breast lump, which was proven to represent IDC. Incidentally, an ill-defined area of architectural distortion was noted in the left breast upper outer quadrant [Figure 7a], which had no sonographic correlate. On CESM of the left breast, clustered non-mass enhancement that was closely related to the area of architectural distortion in the left breast [Figure 7b] was demonstrated. Stereotactic-guided VAB in this region yielded histology of the radial scar. Subsequent MRI also showed no appreciable abnormality in this location. Findings in the left breast were concluded to be benign, and the patient underwent right mastectomy for the established cancer.
Figure 7.
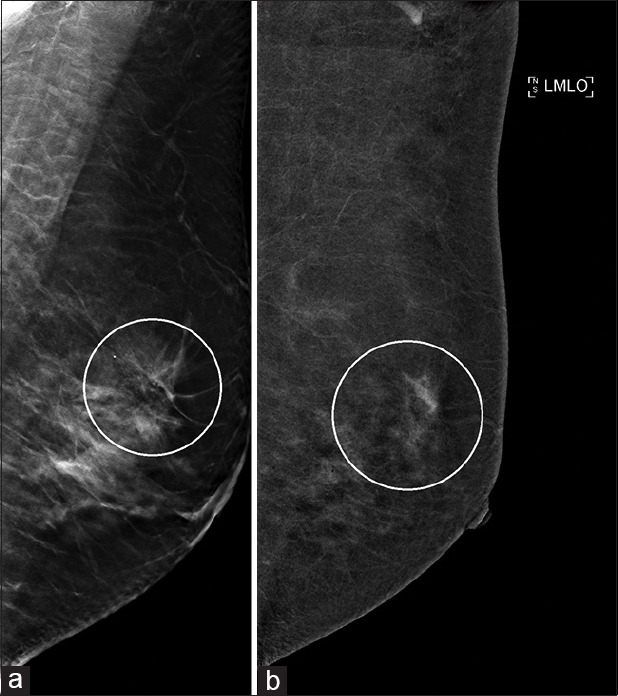
Case 7: Local staging for a 46-year-old woman with biopsy-proven right breast invasive ductal carcinoma. (a) Full-field digital mammography shows an area of architectural distortion in the left breast upper outer quadrant (circle). (b) Contrast-enhanced spectral mammography shows closely related clustered non-mass enhancement (circle). On biopsy, this was found to be a radial scar, and subsequent MRI also showed no suspicious abnormality.
Case 8
A 57-year-old-woman presented with a left breast lump of 1-month duration. A spiculated mass in the left upper central breast [Figure 8a] was seen on FFDM, while US revealed an incidental suspicious hypoechoic lesion in a nonparallel orientation in the right breast [Figure 8c]. Further evaluation with CESM showed extensive regional clustered non-mass enhancement of the left upper breast with extension to the nipple, indicating a much larger tumour than what was appreciable on FFDM [Figure 8b]. In the right breast, no enhancing lesion corresponding to the US abnormality was seen [Figure 8d and e]. Vacuum-assisted excision biopsy of the right breast lesion was performed in view of its suspicious morphology, and it yielded benign histology of fibrocystic change. This was deemed concordant in view of complete lesion removal. Left mastectomy showed grade 2 invasive lobular carcinoma and extensive lobular carcinoma in situ measuring 60 mm, concordant with CESM findings.
Figure 8.
Case 8: A 57-year-old woman. (a) Full-field digital mammography (FFDM) of the left breast shows a spiculated mass in the left upper central breast, whereas (b) contrast-enhanced spectral mammography (CESM) for local staging shows extensive non-mass enhancement extending to the nipple, indicating more extensive disease than that seen on FFDM. (c) US image of the contralateral right breast shows a suspicious, taller-than-wide hypoechoic lesion; however (d & e) FFDM and CESM show no corresponding abnormality in the right breast. On complete excision, the right breast lesion showed fibrocystic change.
In cases 7 and 8, CESM was able to provide preoperative assessment on the same day of the initial clinic visit, expediting the diagnostic process and guiding management.
Response to neoadjuvant chemotherapy
Case 9
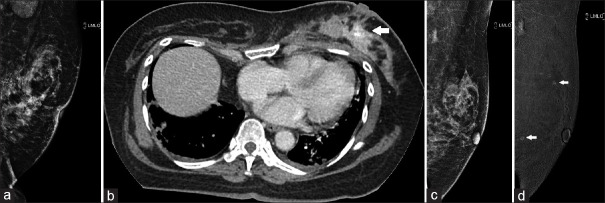
A 58-year-old woman with biopsy-proven high-grade DCIS was planned for neoadjuvant chemotherapy. Pretreatment FFDM showed an asymmetric density in the left central breast with architectural distortion and extensive pleomorphic calcifications [Figure 9a]. Staging CT showed multiple enhancing foci in the left breast, particularly in the left retroareolar region, corresponding to the FFDM findings [Figure 9b]. Post-NAC response was evaluated with CESM, which showed a marked reduction in the number and size of enhancing foci since the staging CT [Figure 9c and d]. The patient then underwent mastectomy. Histopathology supported the CESM findings of residual 4 mm of invasive carcinoma in the left breast on a background of high-grade DCIS.
Figure 9.
Case 9: A 59-year-old woman. (a) Full-field digital mammography (FFDM) of the left breast shows architectural distortion and extensive pleomorphic calcifications. High-grade ductal carcinoma in situ was found on core biopsy. (b) Staging CT image shows multiple enhancing foci in the left breast (arrow). (c) Post-neoadjuvant chemotherapy FFDM and (d) contrast-enhanced spectral mammography show improvement in the architectural distortion and a marked reduction in enhancing foci (arrows).
Comparison of tumour burden using CT and CESM in this case was unconventional due to the differences in resolution and timing of the CT acquisition. This was, however, mitigated as the lesion was large. Case 9 illustrates the benefit of pre- and posttreatment CESM, which allows for easier monitoring of treatment, and is less costly and more readily available as compared to MRI.
CONCLUSION
Contrast-enhanced spectral mammography provides additional physiological information to FFDM using iodinated contrast and a dual-energy X-ray technique. Studies have shown comparable diagnostic performance of CESM and MRI, with the former being less costly, and easier and quicker to perform. As demonstrated through the cases presented, CESM is a useful modality in problem-solving, expediting the diagnostic process and guiding management of both benign and malignant lesions. While CESM does have its limitations, we believe that patients will benefit from the increased use of CESM in the diagnostic workflow.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SMC CATEGORY 3B CME PROGRAMME
Online Quiz: https://www.sma.org.sg/cme-programme
Deadline for submission: 6 pm, 22 April 2024
| Question: Answer True or False |
|---|
| 1. Contrast-enhanced spectral mammography (CESM) uses high- and low-energy X-rays, taking advantage of the difference in X-ray attenuation between the breast tissue and iodine, to highlight the areas of contrast uptake. |
|
|
| 2. Malignant lesions tend to enhance due to tumoral angiogenesis, which increases the permeability of the basement membrane to contrast agents. |
|
|
| 3. The low-energy images from CESM are superior to those of standard full-field digital mammography (FFDM) in terms of diagnostic quality. |
|
|
| 4. Interpretation of CESM consists of reading the high- and low-energy images. |
|
|
| 5. The breasts should be compressed before administration of the intravenous contrast. |
|
|
| 6. The average time needed for image acquisition in CESM is 1 h. |
|
|
| 7. The imaging sequence in CESM is variable, depending on the site of the lesion. |
|
|
| 8. Multiple studies have shown that CESM is significantly inferior to magnetic resonance imaging (MRI). |
|
|
| 9. Studies have shown that patients prefer the reduced procedure time, greater comfort and lower noise levels of CESM as compared to MRI. |
|
|
| 10. CESM shows improvement over FFDM in all diagnostic parameters. |
|
|
| 11. The high negative predictive value of CESM provides confidence in discharging patients back to routine mammographic screening. |
|
|
| 12. In the evaluation of dense breasts, CESM is more sensitive compared to FFDM and has potential use in screening of high-risk patients. |
|
|
| 13. CESM shows good correlation with pathological tumour size, aiding in local staging and assessing response to neoadjuvant therapy. |
|
|
| 14. The use of iodinated contrast in CESM poses no significant risks to patients. |
|
|
| 15. Patients may be discharged immediately following CESM. |
|
|
| 16. The average glandular dose of CESM is greater than that of FFDM, but within the acceptable limits for mammography. |
|
|
| 17. CESM is able to objectively evaluate enhancement kinetics of lesions. |
|
|
| 18. Background parenchymal enhancement is not an issue in CESM. |
|
|
| 19. Enhancing benign lesions include fibroadenoma and infection/inflammation. |
|
|
| 20. Malignant lesions that may be nonenhancing or poorly enhancing include ductal carcinoma in situ and inflammatory breast cancer. |
REFERENCES
- 1.Francescone MA, Jochelson MS, Dershaw DD, Sung JS, Hughes MC, Zheng J, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM) Eur J Radiol. 2014;83:1350–5. doi: 10.1016/j.ejrad.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Fallenberg EM, Schmitzberger FF, Amer H, Ingold-Heppner B, Balleyguier C, Diekmann F, et al. Contrast-enhanced spectral mammography vs. mammography and MRI – clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27:2752–64. doi: 10.1007/s00330-016-4650-6. [DOI] [PubMed] [Google Scholar]
- 3.Sumkin JH, Berg WA, Carter GJ, Bandos AI, Chough DM, Ganott MA, et al. Diagnostic performance of MRI, molecular breast imaging, and contrast-enhanced mammography in women with newly diagnosed breast cancer. Radiology. 2019;293:531–40. doi: 10.1148/radiol.2019190887. [DOI] [PubMed] [Google Scholar]
- 4.Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer. 2020;11:1423–32. doi: 10.1111/1759-7714.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J Med Imaging Radiat Oncol. 2015;59:300–5. doi: 10.1111/1754-9485.12296. [DOI] [PubMed] [Google Scholar]
- 6.Lobbes MB, Lalji U, Houwers J, Nijssen EC, Nelemans PJ, van Roozendaal L, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol. 2014;24:1668–76. doi: 10.1007/s00330-014-3154-5. [DOI] [PubMed] [Google Scholar]
- 7.Lalji UC, Houben IP, Prevos R, Gommers S, van Goethem M, Vanwetswinkel S, et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: Validation of results in a large multireader, multicase study. Eur Radiol. 2016;26:4371–9. doi: 10.1007/s00330-016-4336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori M, Akashi-Tanaka S, Suzuki S, Daniels MI, Watanabe C, Hirose M, et al. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer. 2017;24:104–10. doi: 10.1007/s12282-016-0681-8. [DOI] [PubMed] [Google Scholar]
- 9.Jochelson MS, Pinker K, Dershaw DD, Hughes M, Gibbons GF, Rahbar K, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: A pilot study. Eur J Radiol. 2017;97:37–43. doi: 10.1016/j.ejrad.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Patel BK, Naylor ME, Kosiorek HE, Lopez-Alvarez YM, Miller AM, Pizzitola VJ, et al. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin Imaging. 2017;46:44–52. doi: 10.1016/j.clinimag.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Lobbes MBI, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer. 2015;6:144–50. doi: 10.7150/jca.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE, Lobbes M, et al. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy. Ann Surg Oncol. 2018;25:1350–6. doi: 10.1245/s10434-018-6413-x. [DOI] [PubMed] [Google Scholar]
- 13.Jeukens CR, Lalji UC, Meijer E, Bakija B, Theunissen R, Wildberger JE, et al. Radiation exposure of contrast-enhanced spectral mammography compared with full-field digital mammography. Invest Radiol. 2014;49:659–65. doi: 10.1097/RLI.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 14.Wurdinger S, Kamprath S, Eschrich D, Schneider A, Kaiser WA. False-negative findings of malignant breast lesions on preoperative magnetic resonance mammography. Breast. 2001;10:131–9. doi: 10.1054/brst.2000.0232. [DOI] [PubMed] [Google Scholar]
- 15.Ghai S, Muradali D, Bukhanov K, Kulkarni S. Nonenhancing breast malignancies on MRI: Sonographic and pathologic correlation. Am J Roentgenol. 2005;185:481–7. doi: 10.2214/ajr.185.2.01850481. [DOI] [PubMed] [Google Scholar]