Abstract
Background
Preoperative carbohydrate treatments have been widely adopted as part of enhanced recovery after surgery (ERAS) or fast‐track surgery protocols. Although fast‐track surgery protocols have been widely investigated and have been shown to be associated with improved postoperative outcomes, some individual constituents of these protocols, including preoperative carbohydrate treatment, have not been subject to such robust analysis.
Objectives
To assess the effects of preoperative carbohydrate treatment, compared with placebo or preoperative fasting, on postoperative recovery and insulin resistance in adult patients undergoing elective surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 3), MEDLINE (January 1946 to March 2014), EMBASE (January 1947 to March 2014), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 1980 to March 2014) and Web of Science (January 1900 to March 2014) databases. We did not apply language restrictions in the literature search. We searched reference lists of relevant articles and contacted known authors in the field to identify unpublished data.
Selection criteria
We included all randomized controlled trials of preoperative carbohydrate treatment compared with placebo or traditional preoperative fasting in adult study participants undergoing elective surgery. Treatment groups needed to receive at least 45 g of carbohydrates within four hours before surgery or anaesthesia start time.
Data collection and analysis
Data were abstracted independently by at least two review authors, with discrepancies resolved by consensus. Data were abstracted and documented pro forma and were entered into RevMan 5.2 for analysis. Quality assessment was performed independently by two review authors according to the standard methodological procedures expected by The Cochrane Collaboration. When available data were insufficient for quality assessment or data analysis, trial authors were contacted to request needed information. We collected trial data on complication rates and aspiration pneumonitis.
Main results
We included 27 trials involving 1976 participants Trials were conducted in Europe, China, Brazil, Canada and New Zealand and involved patients undergoing elective abdominal surgery (18), orthopaedic surgery (4), cardiac surgery (4) and thyroidectomy (1). Twelve studies were limited to participants with an American Society of Anaesthesiologists grade of I‐II or I‐III.
A total of 17 trials contained at least one domain judged to be at high risk of bias, and only two studies were judged to be at low risk of bias across all domains. Of greatest concern was the risk of bias associated with inadequate blinding, as most of the outcomes assessed by this review were subjective. Only six trials were judged to be at low risk of bias because of blinding.
In 19 trials including 1351 participants, preoperative carbohydrate treatment was associated with shortened length of hospital stay compared with placebo or fasting (by 0.30 days; 95% confidence interval (CI) 0.56 to 0.04; very low‐quality evidence). No significant effect on length of stay was noted when preoperative carbohydrate treatment was compared with placebo (14 trials including 867 participants; mean difference ‐0.13 days; 95% CI ‐0.38 to 0.12). Based on two trials including 86 participants, preoperative carbohydrate treatment was also associated with shortened time to passage of flatus when compared with placebo or fasting (by 0.39 days; 95% CI 0.70 to 0.07), as well as increased postoperative peripheral insulin sensitivity (three trials including 41 participants; mean increase in glucose infusion rate measured by hyperinsulinaemic euglycaemic clamp of 0.76 mg/kg/min; 95% CI 0.24 to 1.29; high‐quality evidence).
As reported by 14 trials involving 913 participants, preoperative carbohydrate treatment was not associated with an increase or a decrease in the risk of postoperative complications compared with placebo or fasting (risk ratio of complications 0.98, 95% CI 0.86 to 1.11; low‐quality evidence). Aspiration pneumonitis was not reported in any patients, regardless of treatment group allocation.
Authors' conclusions
Preoperative carbohydrate treatment was associated with a small reduction in length of hospital stay when compared with placebo or fasting in adult patients undergoing elective surgery. It was found that preoperative carbohydrate treatment did not increase or decrease postoperative complication rates when compared with placebo or fasting. Lack of adequate blinding in many studies may have contributed to observed treatment effects for these subjective outcomes, which are subject to possible biases.
Keywords: Adult, Humans, Elective Surgical Procedures, Length of Stay, Beverages, Carbohydrates, Carbohydrates/administration & dosage, Fatigue, Fatigue/prevention & control, Flatulence, Insulin Resistance, Postoperative Complications, Postoperative Complications/prevention & control, Postoperative Nausea and Vomiting, Postoperative Nausea and Vomiting/prevention & control, Preoperative Care, Preoperative Care/methods, Randomized Controlled Trials as Topic
Plain language summary
Does giving patients carbohydrate supplements before planned surgery lead to improved recovery?
Review question
We reviewed the evidence on effects of carbohydrate supplements on the recovery of people undergoing planned surgical procedures. We found 27 studies investigating this question.
Background
Carbohydrate (sugar‐containing) nutritional supplements have become a routine part of the package of care for people undergoing planned surgical procedures. We wanted to discover whether carbohydrate supplements are a useful part of care packages used by doctors to improve recovery after planned surgical procedures.
Study characteristics
The evidence is current up to March 2014. We identified 27 studies and included the outcomes of 1976 participants. Studies investigated the outcomes of patients undergoing planned surgical procedures on the abdomen (18), the bones or joints (4), the heart (4) or the thyroid gland (1).
Eighteen studies compared carbohydrate supplements versus an identical appearing placebo drink that did not contain carbohydrates; in six of these studies, an additional group of patients had nothing to eat or drink for at least six hours before surgery. In nine studies, taking carbohydrate supplements was compared with having nothing to eat or drink for six hours before surgery.
The primary outcomes of length of hospital stay and complication rate were reported by 19 and 14 studies, respectively.
Key results
Patients given carbohydrates before planned surgical procedures went home between 0.04 and 0.56 days sooner than those receiving a placebo drink or having nothing to eat or drink before surgery. Carbohydrate supplements had little or no effect on complication rate or on how people feel in‐hospital during recovery from surgery.
Quality of the evidence
The overall quality of the evidence varied from very low to high. The quality of evidence in support of carbohydrate supplements resulting in a shorter hospital stay was very low because the included studies had important flaws in their design, a very wide range of results was described and evidence revealed that studies showing no differences in length of hospital stay may not have been published. When we looked only at well‐conducted studies, we found that carbohydrate supplements had little or no effect on length of hospital stay.
The quality of evidence to support the effects of carbohydrate supplements on complication rate was low because issues with study design were identified and results were not similar across studies.
Summary of findings
for the main comparison.
| Preoperative carbohydrates compared with placebo or fasting for people undergoing elective surgery | ||||||
|
Patient or population: adult patients undergoing elective surgery Settings: hospitals providing elective surgery Intervention: preoperative carbohydrate supplementationa Comparison: placebo drink or traditional preoperative fasting | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fasting or placebo | Preoperative carbohydrates | |||||
| Complication rate | Low‐risk populationb | RR 0.98 (0.86 to 1.11) | 913 (14 studies) | ⊕⊕⊝⊝ lowc | Evidence is insufficient to support the hypothesis that preoperative carbohydrate drinks reduce postoperative complication rates | |
| 4 per 100 | 4 per 100 (3 to 4) | |||||
| Medium‐risk populationb | ||||||
| 18 per 100 | 17 per 100 (15 to 19) | |||||
| High‐risk populationb | ||||||
| 39 per 100 | 38 per 100 (34 to 43) | |||||
|
Length of hospital stay (days) |
Mean length of hospital stay ranged across control groups from 1 to 16 days | Mean length of hospital stay in the intervention groups was 0.30 days lower (0.56 days lower to 0.04 days lower) | 1351 (19 studies) | ⊕⊝⊝⊝ very lowd | High degree of heterogeneity across all studies and all subgroups. Evidence of publication bias noted on sensitivity analysisd | |
|
Postoperative well‐being by visual analogue scale or by standardized questionnaire |
Mean well‐being score ranged across control groups from 25 to 63 mm | Mean well‐being score in the intervention groups was 0 mm different (5.18 mm lower to 5.41 mm higher)e | 310 (4 studies) | ⊕⊕⊕⊝ moderatef | As the confidence intervals include no effect, evidence is insufficient to show whether perioperative carbohydrate drinks increase or decrease postoperative well‐being | |
|
Postoperative nausea by visual analogue scale at 24 hours postop |
Mean nausea score ranged across control groups from 10 to 16 mm | Mean nausea score in the intervention groups was 1.69 mm lower (4.12 mm lower to 0.74 mm higher) | 292 (2 studies) | ⊕⊕⊕⊝ moderateg | As the confidence intervals include no effect, evidence is insufficient to show whether perioperative carbohydrate drinks increase or decrease postoperative nausea | |
| Postoperative vomiting | Medium‐risk populationh | RR 1.25 (0.77 to 2.04) | 407 (4 studies) | ⊕⊕⊝⊝ lowi | As the confidence intervals include no effect, evidence is insufficient to show whether perioperative carbohydrate drinks increase or decrease postoperative vomiting | |
| 12 per 100 | 15 per 100 (9 to 24) | |||||
|
Postoperative fatigue by visual analogue scale or by 10‐point ordinal scale |
Mean fatigue score ranged across control groups from 28 to 30.6 mm | Mean fatigue score in the intervention groups was 1.77 mm higher (6.77 mm lower to 10.31 mm higher)j | 576 (6 studies) | ⊕⊕⊕⊝ moderatek | As the confidence intervals include no effect, evidence is insufficient to show whether perioperative carbohydrate drinks increase or decrease postoperative fatigue | |
|
Postop insulin sensitivity (clamp) measured as standardized glucose infusion rate (mg/kg/min)l |
Mean glucose infusion rate ranged across control groups from 1.4 to 2.41 mg/kg/min | Mean glucose infusion rate in the intervention groups was 0.76 mg/kg/min higher (0.24 mg/kg/min higher to 1.29 mg/kg/min higher) | 41 (3 studies) | ⊕⊕⊕⊕ high | Glucose infusion rate is a measure of total body glucose utilization during the hyperinsulinaemic euglycaemic clamp | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = confidence interval; RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAll studies included in this review examined a preoperative dose of at least 45 g of carbohydrate administered within 4 hours of induction of anaesthesia or surgery start time. Most studies administered a dose of 50 g of carbohydrate in the form of an oral beverage, but 2 included studies administered preoperative carbohydrates in intravenous form.
bLow‐, intermediate‐ and high‐risk populations were taken as first quartile, median and third quartile, respectively, of reported complication rates in the control groups of included studies.
cThe quality of evidence was graded as low because of limitations of primary studies and imprecision of effect estimates. Note that the summary effect estimate crosses the line of no effect, meaning that a small increase or reduction in the complication rate cannot be excluded.
dThe quality of evidence was graded as very low because of the quality limitations of the primary studies, imprecision of effect estimates, heterogeneity and evidence of publication bias. It is important to note that both sensitivity analyses restricting analysis to well‐blinded studies and 'trim and fill' analysis to explore the effects of publication bias reduced the magnitude of the effect of carbohydrate treatment to the point where the 95% confidence intervals crossed the line of no effect.
eThe standardized mean difference was converted back to millimetres of a visual analogue scale by using the median of the standard deviation from the control groups of 3 studies reporting data from a visual analogue scale.
fThe quality of evidence was graded as moderate because of the imprecision of effect estimates from included studies. Note that the summary effect estimate includes the line of no effect; however a small increase or decrease in postoperative well‐being cannot be excluded.
gThe quality of evidence was graded as moderate because of the imprecision of effect estimates from included studies. Note that the summary effect estimate includes the line of no effect; however a small increase or decrease in postoperative nausea cannot be excluded.
hThe medium‐risk population was taken as the median of the reported postoperative vomiting rates in the control groups of included studies. As only 4 studies reported these data, no attempt was made to define low‐ and high‐risk populations.
iThe quality of evidence was graded as low because of the limitations of the primary studies and the imprecision of the effect estimates. Note that the summary effect estimate includes the line of no effect; however a small increase or decrease in postoperative vomiting cannot be excluded.
jThe standardized mean difference was converted back to mm of visual analogue scale by using the median of the standard deviation from the control groups of 3 studies reporting data from a visual analogue scale.
kThe quality of evidence was graded as moderate because of the heterogeneity of the primary studies. Note that the summary effect estimate includes the line of no effect; however a small increase or decrease in postoperative fatigue cannot be excluded.
lGlucose infusion rate is a standardized measure of total body glucose utilization during the hyperinsulinaemic euglycaemic clamp. A higher value corresponds with greater insulin sensitivity.
Background
Description of the condition
Humans and other mammals respond to surgery and trauma with multiple neuroendocrine changes leading to catabolism of stored body fuels and retention of salt and water (Desborough 2000; Kehlet 1997). This surgical stress response was first described by Cuthbertson (Wilmore 2002) in the late 1920s, as noted among patients admitted to hospital with long bone fractures. Cuthbertson discovered dramatic increases in nitrogen, potassium, phosphorus, sulphur and creatine urinary losses and concluded that these represented a systemic breakdown in skeletal muscle. Later experimental studies showed increased levels of adrenal cortical hormones in response to injury. Furthermore, severing afferent nerve pathways from the site of injury diminished this response.
Modern understanding of the surgical stress response is that it involves activation of the sympathetic nervous system, secretion of catabolic hormones and local cytokine responses to tissue injury (Desborough 2000). This response is usually proportional to the degree of surgical trauma or injury incurred (Kehlet 1997). The endocrine component includes activation of the hypothalamic‐pituitary‐adrenal axis with increased cortisol secretion, increased secretion of vasopressin and increased pancreatic secretion of glucagon (Desborough 2000); this response leads to a net increase in peripheral insulin resistance and catabolism of skeletal muscle. The degree of peripheral insulin resistance has been linked to the magnitude of the catabolic response (Nygren 2006).
The stress response to surgery has likely developed as an evolutionary response, allowing injured animals to survive without food and with healing of their wounds (Desborough 2000). However, in the current highly controlled surgical environment, this response is associated with several deleterious effects (Kehlet 1997), including organ dysfunction, hypercoagulation, immunosuppression, catabolism and impaired wound healing. Peripheral insulin resistance in particular is associated with hyperglycaemia—a possible cause of postoperative complications and an independent predictor of length of hospital stay (Nygren 2006).
Description of the intervention
In an attempt to improve surgical outcomes, excessive and undesirable features of the surgical stress response are now routinely targeted by multi‐modal therapies, known as 'fast‐track surgery' or 'enhanced recovery after surgery' (ERAS).
A common feature of fast‐track surgical protocols is that interventions are aimed at reducing the degree of postoperative insulin resistance. These interventions include preoperative administration of oral or intravenous carbohydrates up to two hours before surgery, in contrast to a traditional preoperative fast (Ljungqvist 2003; Nygren 2006).
Reported studies have mainly investigated a clear liquid beverage containing 12.5 g of carbohydrates per 100 mL (Nutricia preOp®, Numico, Zoetermeer, The Netherlands) (Bisgaard 2004; Mathur 2010; Wang 2010). This drink contains polymers of carbohydrates that reduce osmotic load and do not delay gastric emptying. It contains 50 kcal per 100 mL, 290 mOsm/kg, and has a pH of 5.0. Gastric emptying studies have shown that when up to 400 mLis consumed by patients at least two hours before they are given opiate‐containing analgesia, residual gastric volume is equivalent to overnight fasting (Ljungqvist 2003). This beverage is indistinguishable in appearance and taste from a placebo beverage containing flavoured sweetened water (0 kcal per 100 mL, 107 mOsm/kg) (Bisgaard 2004).
How the intervention might work
Preoperative carbohydrate treatment aims to replicate normal metabolic responses to eating breakfast (Ljungqvist 2003). This treatment stimulates an endogenous insulin release, which switches off the overnight fasting metabolic state and is given to decrease the extent of peripheral insulin resistance while ameliorating the surgical stress response.
Studies in rodents demonstrate that fasted animals respond to trauma with increased catabolism, poorer muscle strength and greater bacterial translocation than do fed animals (Ljungqvist 2003). Animals in the metabolic 'fed' state fared better than fasted animals.
Animal studies have been followed by studies in patients undergoing elective surgery (Ljungqvist 2003; Nygren 2006). Intravenous glucose infusion has been compared with overnight fasting in participants undergoing upper abdominal surgery or arthroplasty. Both studies showed a reduction in postoperative insulin resistance among participants given intravenous glucose. Preoperative oral carbohydrate treatment was also shown to reduce insulin resistance compared with overnight fasting in participants undergoing colorectal surgery or arthroplasty.
Why it is important to do this review
Because of its effect in reducing the postoperative development of insulin resistance, preoperative carbohydrate treatment is commonly advocated as part of multi‐modal fast‐track surgery or ERAS pathways. These pathways frequently include routine neuraxial blockade, reduced use of nasogastric tubes and surgical drains and early postoperative ambulation and enteral feeding (Kehlet 1997). Fast‐track surgery protocols have been widely studied, and reduced hospital stays and decreased rates of complication have been demonstrated (Desborough 2000; Gouvas 2009).
In contrast to the traditional preoperative fast, administration of preoperative oral carbohydrate drinks has been shown to improve patient comfort before surgery. A randomized trial comparing oral carbohydrates with placebo or overnight fasting showed that oral carbohydrate treatment was associated with reduced anxiety and thirst before surgery (Hausel 2001). Carbohydrate beverages were as effective in reducing preoperative thirst as placebo beverages when compared with fasting in this study. A systematic review of the effects of preoperative fasting on perioperative complications (Brady 2003) noted that study participants given an oral carbohydrate beverage reported reduced anxiety compared with those who followed traditional fasting procedures.
On the other hand, evidence to support improvement in postoperative outcomes following preoperative administration of carbohydrates is less robust. A randomized trial of preoperative intravenous carbohydrates in patients undergoing cholecystectomy showed decreased insulin resistance in the treatment group but no difference in clinical outcomes (Ljungqvist 1994). A 2004 study on oral carbohydrate treatment in participants undergoing laparoscopic cholecystectomy found no meaningful differences in a variety of clinical outcomes, including pain, nausea and vomiting, fatigue and general well‐being (Bisgaard 2004). Another study on participants undergoing laparoscopic cholecystectomy showed a reduction in postoperative nausea and vomiting but no difference in mean hospital stay or non‐discharge at 24 hours (Hausel 2005). A larger, single‐centre, randomized trial on elective colorectal and liver resections found that oral carbohydrate drinks offered no improvement in postoperative fatigue and no reduction in hospital stay (Mathur 2010). The study authors were not able to identify any systematic reviews addressing the independent effects of preoperative carbohydrate treatment among patients undergoing elective surgery at the time of writing of the protocol for this review.
Objectives
To assess the effects of preoperative carbohydrate treatment, compared with placebo or preoperative fasting, on postoperative recovery and insulin resistance in adult patients undergoing elective surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that compared the effects on postoperative recovery and well‐being when preoperative carbohydrate treatment was used versus placebo or preoperative fasting.
We included studies irrespective of language and publication status.
We excluded non‐randomized studies such as cohort studies because of the increased potential for bias. We also excluded cross‐over trials, as this methodology is not suitable for evaluating an intervention that must be given at a specific time point.
We included a single trial known to be quasi‐randomized (Ljungqvist 1994), but this was subject to a sensitivity analysis.
Types of participants
We included adult patients (18 years of age or older) undergoing any type of elective surgical procedure while under general, spinal or epidural anaesthesia. We included patients who underwent spinal or epidural blockade in addition to general anaesthesia.
We excluded patients who required urgent or emergency surgery (cases in which surgery is required within 24 hours after the first physician contact for a potentially life‐threatening condition).
Types of interventions
The intervention group included all participants who were given at least 45 g of carbohydrate by oral beverage or by the intravenous route. To be included, studies must have planned to administer the carbohydrates within four hours of surgery start time, or induction of anaesthesia. Co‐intervention with other oral substances in the four hours before surgery was permitted so long as the dose of carbohydrate was at least 45 g.
The intervention group was compared with a control group consisting of participants who received less than 45 g of carbohydrate in the four hours before anaesthesia. Control participants may have received a placebo drink containing less than 45 g of carbohydrate, clear liquids or nothing by mouth during this time. The control group may have received intravenous fluid therapy during the four hours before surgery start time, so long as the total combined dose of carbohydrates given by oral and intravenous routes remained less than 45 g.
Types of outcome measures
Primary outcomes
Length of hospital stay: measured in days.
Postoperative complication rate: as defined by trial authors.
We included all trials reporting length of hospital stay or complication rate following elective surgery.
Secondary outcomes
We included all trials with the following secondary outcomes, measured postoperatively.
Aspiration pneumonitis rate: defined as observed regurgitation or vomiting in association with abnormal chest radiography or gas exchange.
Insulin resistance or sensitivity: measured by hyperinsulinaemic euglycaemic clamp or Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR). Because of differences in estimates made by these two instruments, we combined the data from each separately in the meta‐analysis.
Fatigue: measured by such instruments as ordinal or visual analogue scales.
General well‐being: measured by such instruments as ordinal, visual analogue or composite scales.
Nausea 24 hours postoperatively: measured by such instruments as ordinal, visual analogue or composite scales.
Vomiting within 24 hours postoperatively: measured as an incidence rate.
Return of intestinal function: defined as time in days from operation to first passage of flatus, and to first bowel movement.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 3), MEDLINE (January 1946 to March 2014), EMBASE (January 1947 to March 2014), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 1980 to March 2014) and Web of Science (January 1900 to March 2014).
We applied no language restrictions.
We used the sensitivity maximizing search strategies described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search MEDLINE and EMBASE for RCTs. We also used the free‐text and associated exploded medical subject heading (MeSH) terms found in Appendix 1, in combination with sensitivity maximizing RCT search strategies.
We searched CENTRAL using the search terms provided in Appendix 2. We modified our MEDLINE search strategy (Appendix 3) to reflect subject headings found in the thesauri used by EMBASE (Appendix 4), CINAHL (Appendix 5) and Web of Science (Appendix 6).
Searching other resources
For ongoing trials, we searched the WHO international clinical trials registry platform. This includes clinicaltrials.gov, the metaRegister of Controlled Trials (mRCT) and other national trial registries.
We used free‐text terms in all databases and subject headings in combination when thesauri were components of a database.
We reviewed the related articles feature of PubMed to look for eligible trials and reviews and screened the reference lists of those identified.
We contacted experts in this field in an effort to identify unpublished research and trials still under way.
Data collection and analysis
Selection of studies
The results of the searches described above were combined, and duplicate records were excluded. Two review authors (MDS and JM) independently screened all titles and abstracts for eligibility. We were not blinded to any details of the published trials. Review authors independently recorded the reason for exclusion for each excluded trial. (See Appendix 7 for a copy of the study selection form.)
We first sought to resolve disagreements between review authors on trial selection by discussion. If consensus could not be reached, we consulted with a third review author (PH), who arbitrated on trial inclusion. If further information was required before a decision could be made about trial inclusion, we (MDS) contacted the first author of the relevant trial.
We compiled a list of all eligible trials. (See Appendix 8 for a copy of the form for eligible trials.)
Data extraction and management
Two review authors (MDS and LP) independently extracted and collected data on a paper data extraction form. (A copy of this form is provided in Appendix 9.) We resolved discrepancies between data extracted by discussion. If we were unable to reach a consensus, we consulted with a third review author (PH). If further information from the trial authors was needed, MDS contacted the first author of the relevant trial.
Assessment of risk of bias in included studies
Two review authors (MDS and LP) independently assessed the methodological quality of eligible trials. We resolved disagreements by discussion, and if we could not reach consensus, a third review author (PH) arbitrated.
We performed risk of bias assessment using the 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). (A copy of the form we used to do this is provided in Appendix 10.)
We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential threats to validity.
We considered a trial as having low risk of bias if all domains were assessed as adequate. We considered a trial as having high risk of bias if one or more domains were assessed as inadequate or unclear. We conducted sensitivity analyses to determine whether excluding studies at high risk of bias might have affected the results of the meta‐analysis.
We provided the 'Risk of bias' table under Characteristics of included studies and presented a 'Risk of bias summary,' which details all judgements made for all studies included in the review (Figure 1).
1.
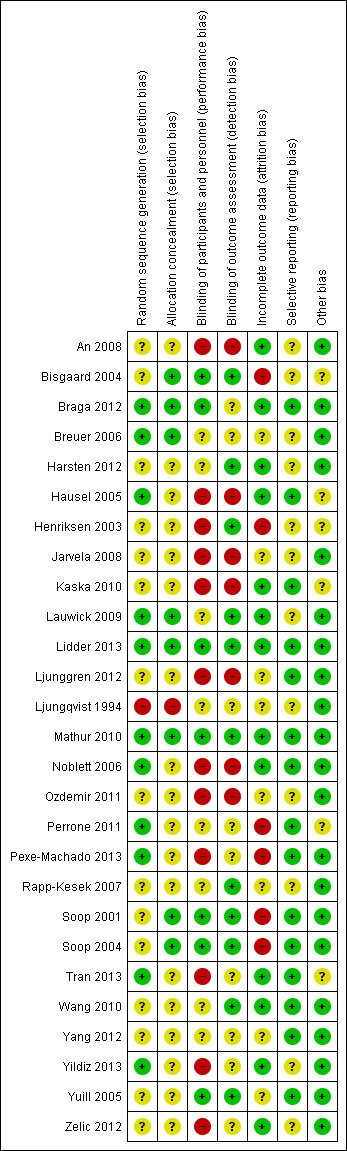
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We present categorical data as risk ratios (RRs). We present continuous data as mean differences (MDs) or as standardized mean differences (SMDs), as appropriate.
Unit of analysis issues
We combined control groups of placebo drink and preoperative fasting when trials compared carbohydrate drink versus placebo drink and preoperative fasting. For one study conducted as a 2 × 2 factorial design (Lidder 2013), we combined data from the two carbohydrate groups and the two placebo groups according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We (LP) contacted the first author of included trials to obtain missing data necessary for meta‐analysis. We calculated missing standard deviations from standard errors or confidence intervals, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), or from ranges or interquartile ranges, as provided by Hozo et al (Hozo 2005). When standard deviations could not be calculated, we imputed these using the median of reported standard deviations from other similar trials.
We address the impact of missing data in the discussion section of the review.
Assessment of heterogeneity
We assessed the clinical heterogeneity of the included studies according to their clinical diversity (e.g. different surgical procedures, different participant characteristics, different doses, timing of preoperative carbohydrate) and methodological diversity (risk of bias assessment).
We addressed clinical heterogeneity by performing subgroup and sensitivity analyses.
We assessed statistical heterogeneity by performing visual inspection of the forest plot, the I2 statistic (Higgins 2011) and the Chi2 test. We considered an I2 statistic greater than 50% along with a P value less than 0.10 in the Chi2 test to be indicative of the need for further examination of heterogeneity.
Assessment of reporting biases
We assessed publication bias and other small‐study effects in a qualitative manner using a funnel plot. We tested for funnel plot asymmetry by using weighted linear regression of effect estimates on their standard error (Egger 1997) for comparisons and outcomes in which more than 10 trials were included.
Data synthesis
If the degree of clinical heterogeneity was not excessive, we generated a quantitative summary by meta‐analysis. We performed the meta‐analysis using Review Manager software (RevMan 5.1). We performed both fixed‐effect model and random‐effects model meta‐analyses and explored differences between these two estimates.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for subgroups of participants and interventions. We compared subgroups by using an interaction term when appropriate.
Subgroups of participants
Subgroup analysis according to type of surgery focused on the following.
Participants undergoing major abdominal surgery.
Participants undergoing minor abdominal surgery.
Participants undergoing cardiac surgery.
Participants undergoing orthopaedic surgery.
Subgroups of interventions
These analyses examined the following.
Preoperative carbohydrate drink versus preoperative fasting.
Preoperative carbohydrate drink versus placebo drink.
Preoperative carbohydrate administered by intravenous route versus preoperative fasting or placebo drink.
Sensitivity analysis
We performed sensitivity analyses to exclude trials at high risk of bias, such as known quasi‐randomized trials. We compared random‐effects and fixed‐effect estimates of each outcome variable. If publication bias was suspected, we performed a 'trim and fill' sensitivity analysis of the primary outcomes. To assess trial influence, we performed sensitivity analyses by sequentially excluding each trial. We used R 2.13.2 (R 2.13.2) using package meta to perform sensitivity analyses not available in RevMan.
Summary of findings
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) in our review to assess the quality of the body of evidence associated with specific outcomes such as length of hospital stay, complication rate, insulin resistance (hyperinsulinaemic euglycaemic clamp), fatigue, well‐being and nausea and vomiting and constructed a 'Summary of findings' (SoF) table.
The GRADE approach is used to assess the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers study methodological quality, directness of the evidence, heterogeneity of the data, precision of the effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
Electronic searches began on 27 October 2011 and were repeated on 10 July 2013 and 14 March 2014. Electronic searches identified a total of 7074 articles across four databases (Figure 2). An additional 51 studies were identified through handsearching of reference lists of included papers and through contact with experts in the field. After duplicate papers had been excluded, the titles and abstracts of 5890 studies were reviewed by JM and MS. This process led to the exclusion of 5011 studies on review of titles and a further 768 studies on review of abstracts that were obviously not relevant to this review, leaving 111 papers for full retrieval.
2.
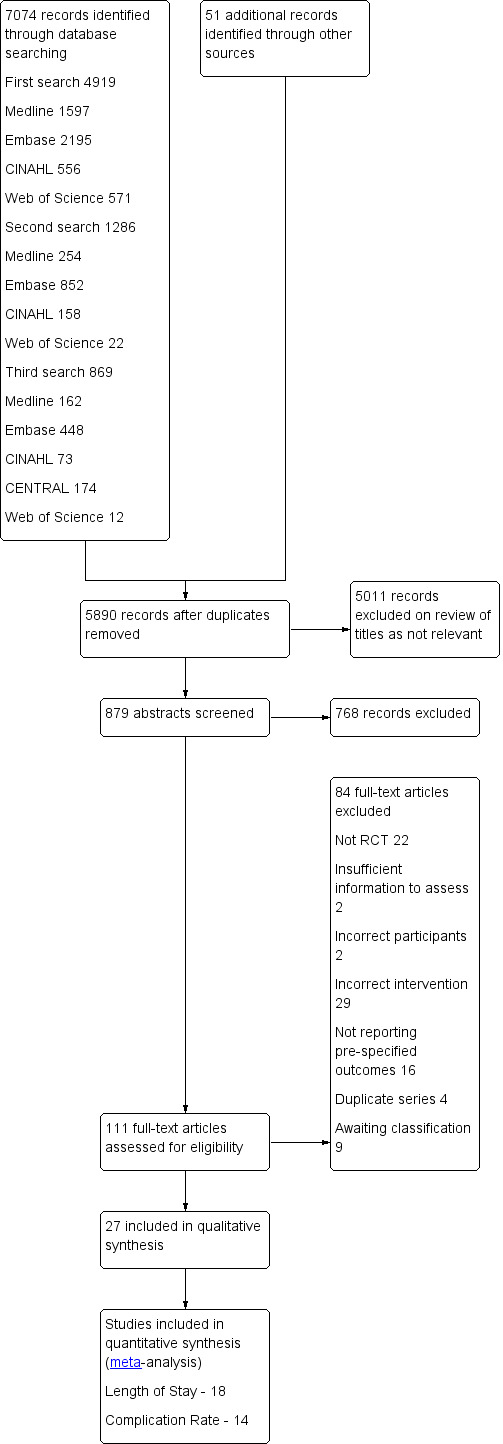
Study flow diagram.
Of the papers reviewed in full, 27 were included in the review and 75 were excluded for various reasons.
We contacted nine study authors (Braga 2012; Breuer 2006; Kaska 2010; Lidder 2013; Mathur 2010; Perrone 2011; Soop 2001; Soop 2004; Yang 2012) to obtain further information about their papers; five responded (Breuer 2006; Lidder 2013; Mathur 2010; Soop 2001; Soop 2004).
Included studies
We included 27 studies in this review. These studies are described in detail under Characteristics of included studies. In total, these studies included 1976 participants, of whom 935 received carbohydrate, 595 received placebo and 446 were fasted preoperatively. Studies were published between 1994 and 2013. Seven studies were conducted in Sweden (Harsten 2012; Hausel 2005; Ljunggren 2012; Ljungqvist 1994; Rapp‐Kesek 2007; Soop 2001; Soop 2004), and 13 were conducted elsewhere in Europe (Bisgaard 2004; Braga 2012; Breuer 2006; Henriksen 2003; Jarvela 2008; Kaska 2010; Lauwick 2009; Lidder 2013; Noblett 2006; Ozdemir 2011; Yildiz 2013; Yuill 2005; Zelic 2012), three in China (An 2008; Wang 2010; Yang 2012), two in Brazil (Perrone 2011; Pexe‐Machado 2013) and one each in Canada (Tran 2013) and New Zealand (Mathur 2010).
Most of the identified studies involved study participants undergoing elective abdominal surgery (18) (An 2008; Bisgaard 2004; Braga 2012; Hausel 2005; Henriksen 2003; Kaska 2010; Lidder 2013; Ljungqvist 1994; Mathur 2010; Noblett 2006; Ozdemir 2011; Perrone 2011; Pexe‐Machado 2013; Wang 2010; Yang 2012; Yildiz 2013; Yuill 2005; Zelic 2012), with four examining orthopaedic surgery (Harsten 2012; Ljunggren 2012; Soop 2001; Soop 2004), three cardiac surgery (Breuer 2006; Jarvela 2008; Rapp‐Kesek 2007), one cardiac or spinal surgery (Tran 2013) and one thyroidectomy (Lauwick 2009). Specific inclusion criteria for American Society of Anaesthesiologists (ASA) grading were reported in 13 studies, with eight limiting participants to those with an ASA of I‐II (Bisgaard 2004; Hausel 2005; Kaska 2010; Lauwick 2009; Ozdemir 2011; Perrone 2011; Soop 2004; Zelic 2012), four to those with an ASA of I‐III (Harsten 2012; Ljunggren 2012; Mathur 2010; Pexe‐Machado 2013) and only one study specifically including only ASA III‐IV participants (Breuer 2006).
Twenty‐five studies (An 2008; Bisgaard 2004; Braga 2012; Breuer 2006; Harsten 2012; Hausel 2005; Henriksen 2003; Jarvela 2008; Lauwick 2009; Lidder 2013; Ljunggren 2012; Mathur 2010; Noblett 2006; Ozdemir 2011; Perrone 2011; Pexe‐Machado 2013; Rapp‐Kesek 2007; Soop 2001; Soop 2004; Tran 2013; Wang 2010; Yang 2012; Yildiz 2013; Yuill 2005; Zelic 2012) administered the preoperative carbohydrates as an oral beverage, one via the intravenous route (Ljungqvist 1994) and one by both oral and intravenous routes (Kaska 2010). Eighteen studies used a placebo as a control (Bisgaard 2004; Braga 2012; Breuer 2006; Harsten 2012; Hausel 2005; Lauwick 2009; Lidder 2013; Ljunggren 2012; Mathur 2010; Noblett 2006; Ozdemir 2011; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004; Wang 2010; Yang 2012; Yuill 2005), with six of these including an additional fasting participant group (Breuer 2006; Hausel 2005; Ljunggren 2012; Noblett 2006; Ozdemir 2011; Wang 2010) and nine studies comparing carbohydrates in an unblinded fashion versus fasting alone (An 2008; Henriksen 2003; Jarvela 2008; Kaska 2010; Ljungqvist 1994; Rapp‐Kesek 2007; Tran 2013; Yildiz 2013; Zelic 2012).
The primary outcomes of length of hospital stay and complication rate were reported by 19 (An 2008; Braga 2012; Breuer 2006; Harsten 2012; Hausel 2005; Kaska 2010; Lidder 2013; Ljunggren 2012; Mathur 2010; Noblett 2006; Ozdemir 2011; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004; Tran 2013; Yang 2012; Yildiz 2013; Yuill 2005) and 14 studies (Braga 2012; Hausel 2005; Kaska 2010; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004; Tran 2013; Yang 2012; Yuill 2005; Zelic 2012), respectively. Mean and standard deviation were reported (or provided by study authors) in nine studies (An 2008; Harsten 2012; Hausel 2005; Mathur 2010; Ozdemir 2011; Soop 2001; Soop 2004; Yang 2012; Yildiz 2013) and were calculated from the median and range or interquartile range in nine studies (Breuer 2006; Kaska 2010; Lidder 2013; Ljunggren 2012; Noblett 2006; Perrone 2011; Pexe‐Machado 2013; Tran 2013; Yuill 2005) by using the techniques described by Hozo 2005; standard deviation was imputed from similar studies in two instances (Braga 2012; Yildiz 2013). Sensitivity analyses excluding these two trials with imputed data did not significantly change analysis outcomes in any case. A single study (Lidder 2013), which was conducted in a 2 × 2 factorial design, required that data from the two carbohydrate groups and the two placebo groups be combined according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Insulin resistance was reported with the HOMA‐IR in seven studies (Mathur 2010; Perrone 2011; Pexe‐Machado 2013; Rapp‐Kesek 2007; Tran 2013; Wang 2010; Yang 2012), and insulin sensitivity by a hyperinsulinaemic euglycaemic clamp in three studies (Ljungqvist 1994; Soop 2001; Soop 2004). A further three studies (Breuer 2006; Kaska 2010; Ljunggren 2012) reported other measures of insulin sensitivity or resistance. Because of the high degree of heterogeneity observed, these additional measures did not contribute to the quantitative analysis.
Excluded studies
It was determined that 74 studies did not meet the inclusion criteria for this review for various reasons, which are summarized in Figure 2 and are detailed in full under Characteristics of excluded studies and Characteristics of studies awaiting classification. Twenty‐one studies were not randomized controlled trials (ASAC 2011; Awad 2011; Bisgaard 2006; Brady 2009; Burden 2012; Goodwin 1991; Jones 2011; Lassen 2010; Lin 1997; Ljungqvist 1991; Ljungqvist 2000; Ljungqvist 2001; Ljungqvist 2010; Longarela 2005; Maltby 1991; Maltby 2006; Nygren 1998; Power 2004; Smith 2011; Soop 2000; Stuart 2006), in two studies participants did not undergo surgery (Awad 2011a; Awad 2011b) and in 26 studies the participants did not receive at least 45 g of carbohydrates within four hours of surgery (Adanir 2008; Aronsson 2009; Bopp 2011; Breitman 2011; Dock‐Nascimento 2011; Dock‐Nascimento 2012; Faria 2009; Helminen 2009; Hendry 2010; Hubner 2010; Itou 2012; Maltby 2004; McCaul 2003; Meisner 2008; Muehling 2009; Phillips 1993; Protic 2010; Protic 2010a; Serclova 2009; Tanabe 1996; Taniguchi 2009; Vincent 1991; Wendel 2013a; Wilson 1999; Zargar‐Shoshtari 2009; Zhang 2010). In 16 studies the interventions were correct, but none of the prespecified outcomes of this review were reported (Awad 2010; Awad 2012; Crowe 1984; Enoki 1992; Hausel 2001; Hutchinson 1988; Korusic 2009; Maltby 1988; Melis 2006; Nygren 1995; Okabayashi 2010; Okabayashi 2011; Schricker 2008; Svanfeldt 2007; Thorell 1996; Yagci 2008). An additional four duplicate patient series were identified (Kaska 2006; Ljungqvist 1998; Noblett 2004; Nygren 1999), in which only the most recent and complete data were retained; in two studies information was insufficient to permit assessment of whether review criteria were met (Hausel 1999; Jones 2012).
Studies awaiting classification
A further eight studies, recently published in abstract form, are awaiting sufficient information to allow classification (Aguilar‐Nascimento 2012; Asakura 2013; Forde 2012; Jodlowski 2011; Ozer 2013; Tsutsumi 2011; Zelic 2013; Zhao 2013); one trial was identified during the peer review process and will be incorporated into the next version of this review (Yilmaz 2013).
Risk of bias in included studies
Risk of bias of the included studies is detailed under Characteristics of included studies and is summarized in Figure 1 and Figure 3 Most studies were assessed as having unclear or high risk of bias across at least some of the seven domains. Only two studies were assessed as having low risk of bias across all seven domains (Lidder 2013; Mathur 2010).
3.
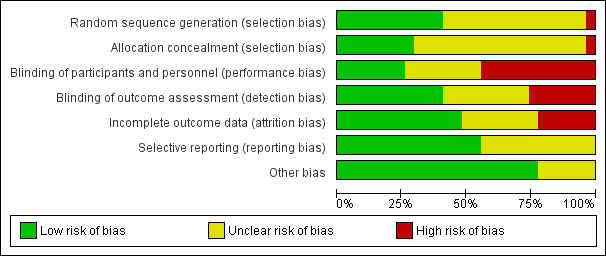
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The method of sequence generation was assessed as being at low risk of bias in 11 studies (Braga 2012; Breuer 2006; Hausel 2005; Lauwick 2009; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Pexe‐Machado 2013; Tran 2013; Yildiz 2013). Low‐risk methods of sequence generation included computer random number generation in nine studies, a random number table in another study and random number allocation in another study. In one study, quasi‐randomization by date of birth was used for allocation (Ljungqvist 1994), and in the remainder, the methods of sequence generation were not adequately reported.
Allocation concealment was assessed as being at low risk of bias in eight studies (Bisgaard 2004; Braga 2012; Breuer 2006; Lauwick 2009; Lidder 2013; Mathur 2010; Soop 2001; Soop 2004). In seven of these, central randomization was used and the study centres were supplied with identical (coded) packages of carbohydrate and placebo drinks (Bisgaard 2004; Braga 2012; Breuer 2006; Lauwick 2009; Mathur 2010; Soop 2001; Soop 2004). One study specifically described using opaque, sealed envelopes for allocation concealment (Lidder 2013). In the quasi‐randomized study, participant allocation could not have been concealed from the investigators (Ljungqvist 1994), and in the remaining studies, details of allocation concealment were not reported.
Blinding
Overall, details of blinding were poorly reported by the included studies. By definition, only studies in which a placebo drink was used were capable of adequate blinding; however only six studies were assessed as being at low risk of both performance and detection bias (Bisgaard 2004; Lidder 2013; Mathur 2010; Soop 2001; Soop 2004; Yuill 2005). Adequate blinding has a much greater effect on the results of subjective outcomes than of objective outcomes; however both of the primary outcome measures for this review were subjective. In unblinded studies, participants or treating clinicians may have assessed intervention group participants as being ready for discharge sooner (performance bias). Also, complications may have been recorded at a lower threshold in the control group. Bias associated with incomplete blinding may have affected assessment of well‐being, time to passage of flatus and bowel movements, fatigue and nausea. Insulin sensitivity is unlikely to be affected by performance or detection bias.
Incomplete outcome data
In 13 studies, no withdrawals were reported post randomization or all withdrawals were described, balanced between groups and deemed unlikely to affect reported outcomes (An 2008; Braga 2012; Harsten 2012; Hausel 2005; Kaska 2010; Lauwick 2009; Lidder 2013; Mathur 2010; Noblett 2006; Tran 2013; Wang 2010; Yildiz 2013; Zelic 2012). In six studies, the numbers of participants excluded post randomization could have been sufficient to affect reported outcomes (Bisgaard 2004; Henriksen 2003; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004).
Selective reporting
Fifteen studies were assessed as being at low risk of selective reporting bias (Braga 2012; Hausel 2005; Kaska 2010; Lidder 2013; Ljunggren 2012; Mathur 2010; Noblett 2006; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004; Tran 2013; Wang 2010; Yang 2012; Yuill 2005). In six of these, the trial protocol was obtained and all registered outcomes were reported in the final publication (Braga 2012; Mathur 2010; Perrone 2011; Pexe‐Machado 2013; Tran 2013; Wang 2010). In the remaining eight studies, no protocol could be identified; however all end points that were likely to have been measured were reported by the study authors (Hausel 2005; Kaska 2010; Lidder 2013; Noblett 2006; Soop 2001; Soop 2004; Yang 2012; Yuill 2005). In one study (Ljunggren 2012), additional outcomes were reported in the final publication, as compared with the registered protocol; however again it is likely that all measured outcomes were reported.
Other potential sources of bias
In two studies participants with postoperative complications or mortality were excluded from the analysis, potentially biasing the reported length of hospital stay, as well as complication rate (Bisgaard 2004; Henriksen 2003). Another two studies did not report baseline characteristics of participants (Kaska 2010) or described a significant difference between groups (Perrone 2011). This may potentially lead to differences in measured outcomes for reasons other than the intervention. It may also reflect bias in the randomization or allocation process. In Hausel 2005 only a small proportion of potentially eligible patients participated in this study, raising questions about the generalizability of its results. Finally, in Tran 2013, both patients undergoing spinal surgery and those undergoing cardiac surgery were included. It is unclear what effect on outcome resulted from combining the heterogeneous groups of participants.
None of the above potential sources of bias were judged to place the studies at high risk of bias.
Effects of interventions
See: Table 1
1 Carbohydrates (CHO) versus placebo
The first comparison analysed CHO versus placebo and included 18 studies and 1191 participants.
Length of hospital stay (Analysis 1.1)
1.1. Analysis.
Comparison 1 CHO versus placebo, Outcome 1 Length of hospital stay.
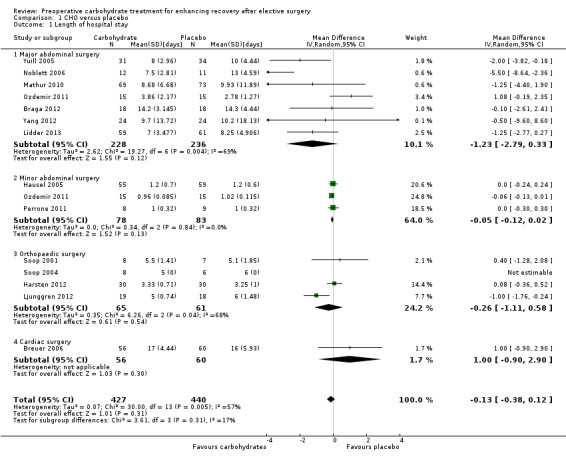
The outcome 'length of hospital stay' included 14 studies (867 participants) of four subgroups: major abdominal surgery (mean length of stay greater than two days) (Braga 2012; Lidder 2013; Mathur 2010; Noblett 2006; Ozdemir 2011; Yang 2012; Yuill 2005), minor abdominal surgery (mean length of stay less than two days) (Hausel 2005; Ozdemir 2011; Perrone 2011), orthopaedic surgery (Harsten 2012; Ljunggren 2012; Soop 2001; Soop 2004) and cardiac surgery (Breuer 2006). Evidence of heterogeneity was high in all subgroups, except for those undergoing minor abdominal surgery, as was heterogeneity for the overall treatment effect (I2 = 57%). No evidence showed of an effect of preoperative carbohydrates on length of hospital stay (overall MD ‐0.13 days, 95% confidence interval (CI) ‐0.38 to 0.12). Nor was any significant evidence of treatment effect observed in any of the four subgroups.
Postoperative complication rate (Analysis 1.2)
1.2. Analysis.
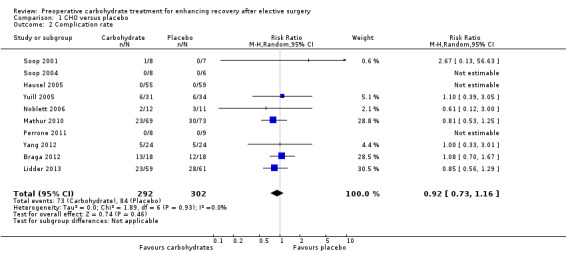
Comparison 1 CHO versus placebo, Outcome 2 Complication rate.
The outcome of complication rate involved analysis of 10 studies (Braga 2012; Hausel 2005; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Soop 2001; Soop 2004; Yang 2012; Yuill 2005) and 594 participants. Heterogeneity was low (I2 = 0%), but three studies in which no complications were reported could not contribute to the meta‐analysis (Hausel 2005; Perrone 2011; Soop 2001). No evidence was found of effects of preoperative carbohydrates on postoperative complication rate(RR 0.92, 95% CI 0.73 to 1.16).
Aspiration pneumonitis
The secondary outcome of aspiration pneumonitis was reported in 10 studies (Bisgaard 2004; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Soop 2001; Soop 2004; Wang 2010; Yang 2012; Yuill 2005), including 562 participants. However, no study reported any events of aspiration pneumonitis, so no meta‐analysis could be performed.
Insulin resistance (HOMA‐IR) (Analysis 1.3)
1.3. Analysis.
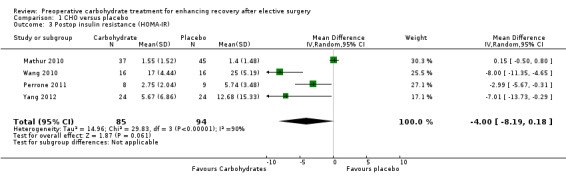
Comparison 1 CHO versus placebo, Outcome 3 Postop insulin resistance (HOMA‐IR).
Postoperative insulin resistance using the HOMA‐IR measure was reported in four studies (Mathur 2010; Perrone 2011; Wang 2010; Yang 2012), including 179 participants. A high degree of heterogeneity was observed (I2 = 90%), and no evidence of treatment effect was found (MD ‐4.00, 95% CI ‐8.19 to 0.18).
Insulin sensitivity (clamp) (Analysis 1.4)
1.4. Analysis.
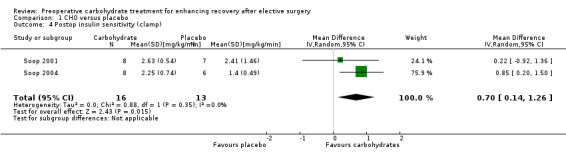
Comparison 1 CHO versus placebo, Outcome 4 Postop insulin sensitivity (clamp).
An alternative approach was taken in two studies (Soop 2001; Soop 2004) (29 participants) that measured postoperative insulin sensitivity using a hyperinsulinaemic euglycaemic clamp technique. This approach reports insulin sensitivity by the standardized steady state glucose infusion rate—a measure of whole body glucose utilization. In these studies, preoperative carbohydrates were associated with increased postoperative insulin sensitivity (MD 0.70, 95% CI 0.14 to 1.26).
Postoperative fatigue (Analysis 1.5)
1.5. Analysis.
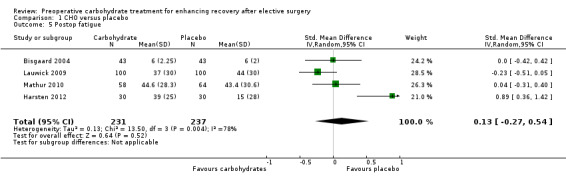
Comparison 1 CHO versus placebo, Outcome 5 Postop fatigue.
Postoperative fatigue was reported by four studies including 468 participants. In three studies it was measured on a visual analogue scale (Harsten 2012; Lauwick 2009; Mathur 2010), and in one study it was measured on a 10‐point ordinal scale (Bisgaard 2004). A high degree of heterogeneity was noted between studies (I2 = 78%), and no evidence of treatment effect was found (SMD 0.13, 95% CI ‐0.27 to 0.54).
Postoperative well‐being (Analysis 1.6)
1.6. Analysis.
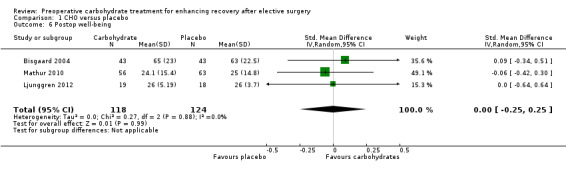
Comparison 1 CHO versus placebo, Outcome 6 Postop well‐being.
Postoperative well‐being was reported using different measures of effect, with two studies using a visual analogue scale (VAS) (Bisgaard 2004; Mathur 2010) and one (Ljunggren 2012) using the well‐being questionnaire (W‐BQ12) (Pouwer 2000). In total, these studies included 242 participants, and no evidence of treatment effect was found (SMD 0.00, 95% CI ‐0.25 to 0.25).
Postoperative nausea at 24 hours (Analysis 1.7)
1.7. Analysis.
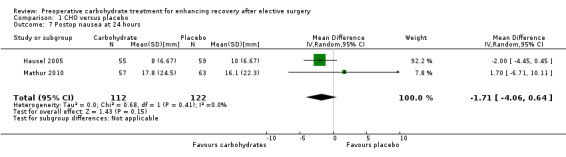
Comparison 1 CHO versus placebo, Outcome 7 Postop nausea at 24 hours.
Postoperative nausea at 24 hours (by 100 mm VAS) was reported in two studies (Hausel 2005; Mathur 2010) (234 participants) with no heterogeneity (I2 = 0%) and no evidence of treatment effect (MD ‐1.71, 95% CI ‐4.06 to 0.64).
Postoperative vomiting (Analysis 1.8)
1.8. Analysis.
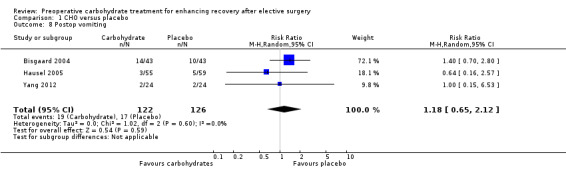
Comparison 1 CHO versus placebo, Outcome 8 Postop vomiting.
Postoperative vomiting was reported as an event rate by three studies (248 participants) (Bisgaard 2004; Hausel 2005; Yang 2012). No evidence of a treatment effect from preoperative carbohydrates was found (RR 1.18, 95% CI 0.65 to 2.12).
Return of intestinal function (Analysis 1.9)
1.9. Analysis.
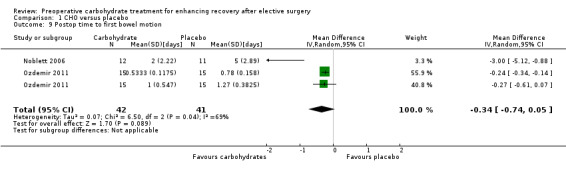
Comparison 1 CHO versus placebo, Outcome 9 Postop time to first bowel motion.
Return of intestinal function was reported as time to passage of flatus by a single study (Noblett 2006) including 33 participants, and no evidence showed of a treatment effect of preoperative carbohydrates. Time to first bowel movement in days was reported in two studies (Noblett 2006; Ozdemir 2011) (83 participants). A high degree of heterogeneity was noted between studies (I2 = 69%), and no evidence of a treatment effect was found (MD ‐0.34, 95% CI ‐0.74 to 0.05).
2 CHO versus fasting
The second comparison looked at CHO versus fasting and included 15 studies and 973 participants. All of the same outcomes were analysed for this comparison as for comparison one.
Length of hospital stay (Analysis 2.1)
2.1. Analysis.
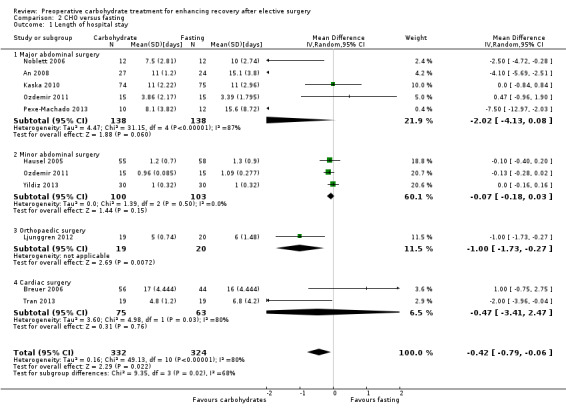
Comparison 2 CHO versus fasting, Outcome 1 Length of hospital stay.
The primary outcome of length of hospital stay was reported by 10 studies (656 participants) and was analysed as four subgroups (major abdominal surgery (An 2008; Kaska 2010; Noblett 2006; Ozdemir 2011; Pexe‐Machado 2013), minor abdominal surgery (Hausel 2005; Ozdemir 2011; Yildiz 2013), orthopaedic surgery (Ljunggren 2012) and cardiac surgery (Breuer 2006)). Although heterogeneity remained high (I2 = 80%), preoperative carbohydrate treatment was associated with a reduced mean length of stay of 0.42 days (95% CI ‐0.79 to ‐0.06 days) in the overall analysis. Reduced length of stay was also found in the subgroup of orthopaedic surgery (MD ‐1.00, 95% CI ‐1.73 to ‐0.27), although this contained only a single study (Ljunggren 2012). No evidence of effect of carbohydrates was seen in any of the other subgroups—major abdominal surgery (MD ‐2.02, 95% CI ‐4.13 to 0.08), minor abdominal surgery (MD ‐0.07, 95% CI ‐0.18 to 0.03) and cardiac surgery (MD 1.00, 95% CI ‐0.90 to 2.90).
Postoperative complication rate (Analysis 2.2)
2.2. Analysis.
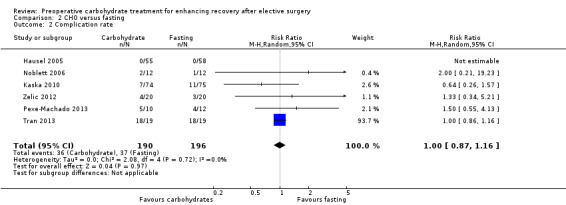
Comparison 2 CHO versus fasting, Outcome 2 Complication rate.
Postoperative complication rates were reported by six studies (Hausel 2005; Kaska 2010; Noblett 2006; Pexe‐Machado 2013; Tran 2013; Zelic 2012) (386 participants). Heterogeneity was low (I2 = 0%), and no effect of preoperative carbohydrate drinks on postoperative complication rate was found (RR 1.00, 95% CI 0.87 to 1.16). Notably, the study by Tran 2013 was heavily weighted in this analysis because of the high event rate in both groups. Excluding this study from the analysis did not change the overall effect however (RR 1.05, 95% CI 0.59 to 1.87).
Aspiration pneumonitis.
Again, although aspiration pneumonitis was mentioned by five studies (Jarvela 2008; Noblett 2006; Tran 2013; Wang 2010; Yildiz 2013) (255 participants), no events were reported, precluding meta‐analysis.
Insulin resistance (HOMA‐IR) (Analysis 2.3)
2.3. Analysis.
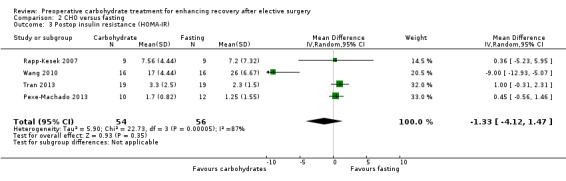
Comparison 2 CHO versus fasting, Outcome 3 Postop insulin resistance (HOMA‐IR).
Insulin resistance was reported with the use of HOMA‐IR in four studies (Pexe‐Machado 2013; Rapp‐Kesek 2007; Tran 2013; Wang 2010) (110 participants) with high heterogeneity (I2 = 87%); no treatment effect was identified (MD ‐1.33, 95% CI ‐4.12 to 1.47).
Insulin sensitivity (clamp)
The alternative of determining insulin sensitivity according to a hyperinsulinaemic euglycaemic clamp was reported by only a single study (Ljungqvist 1994), which found no evidence of effect of preoperative carbohydrate treatment.
Postoperative fatigue (Analysis 2.4)
2.4. Analysis.
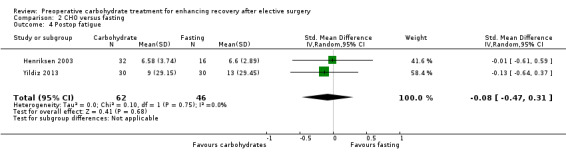
Comparison 2 CHO versus fasting, Outcome 4 Postop fatigue.
Postoperative fatigue was reported by two trials (Henriksen 2003; Yildiz 2013) with low heterogeneity (I2 = 0%). No evidence of a treatment effect of preoperative carbohydrate administration was identified (SMD ‐0.08, 95% CI ‐0.47 to 0.31).
Postoperative well‐being (Analysis 2.5)
2.5. Analysis.
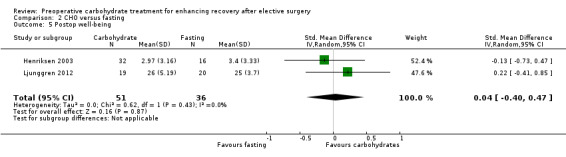
Comparison 2 CHO versus fasting, Outcome 5 Postop well‐being.
Postoperative well‐being was reported by two studies (Henriksen 2003; Ljunggren 2012), which used different instruments (100‐mm VAS and W‐BQ12). These studies included 87 participants, but no evidence of a treatment effect was found (SMD 0.04, 95% CI ‐0.40 to 0.47).
Postoperative nausea at 24 hours
Postoperative nausea at 24 hours (by 100‐mm VAS) was also reported by only a single study (Hausel 2005), which showed no evidence of a treatment effect (MD ‐2.00 mm, 95% CI ‐5.52 to 1.52).
Postoperative vomiting (Analysis 2.6)
2.6. Analysis.
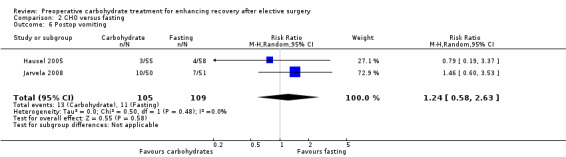
Comparison 2 CHO versus fasting, Outcome 6 Postop vomiting.
Two trials (Hausel 2005; Jarvela 2008) including 214 participants reported postoperative vomiting as count data. Heterogeneity was low (I2 = 0%), but no effect of preoperative carbohydrate treatment was shown (RR 1.24, 95% CI 0.58 to 2.63).
Return of intestinal function (Analysis 2.7; Analysis 2.8)
2.7. Analysis.
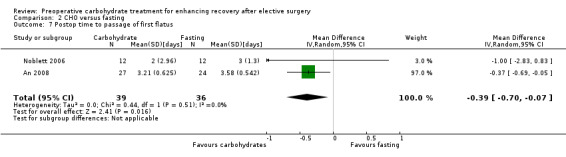
Comparison 2 CHO versus fasting, Outcome 7 Postop time to passage of first flatus.
2.8. Analysis.
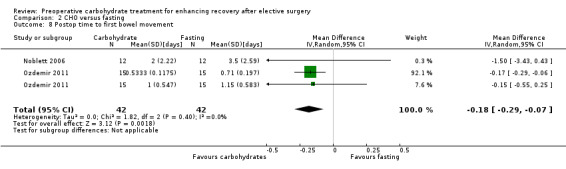
Comparison 2 CHO versus fasting, Outcome 8 Postop time to first bowel movement.
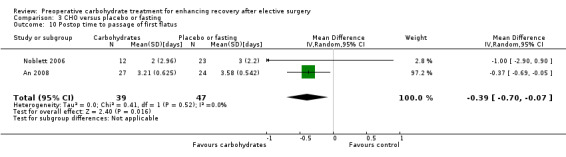
Postoperative time to passage of flatus was reported by two studies (An 2008; Noblett 2006), which included 75 participants. In this analysis, participants receiving preoperative carbohydrate treatment reported passage of flatus a mean of 0.39 days earlier (95% CI ‐0.70 to ‐0.07) than those fasted preoperatively. Heterogeneity was low (I2 = 0%), although the results of this analysis were strongly influenced by An 2008, which was weighted at 97%.
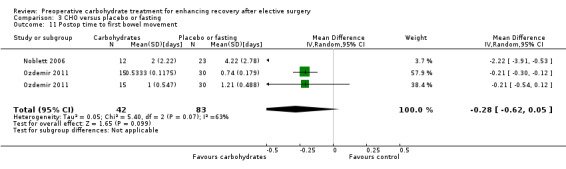
Postoperative time to first bowel movement was reported in two studies (Noblett 2006; Ozdemir 2011) incorporating a total of 84 participants. One of these (Ozdemir 2011) reported separately on the outcomes of participants undergoing major and minor abdominal surgery. When the outcomes of all three participant groups were combined, reduced time to first bowel movement was found (MD ‐0.18 days, 95% CI ‐0.29 to ‐0.07); however this analysis heavily weighted the outcomes of the group receiving minor abdominal surgery at 92.1% because of the reduced variability of this outcome. When sensitivity analysis was undertaken, restricting this analysis to only those undergoing major abdominal surgery, the precision of this effect was reduced, leaving the confidence intervals crossing the line of no effect (MD ‐0.48 days, 95% CI ‐1.62 to 0.66).
3 CHO versus placebo or fasting
For the final comparison, all studies were included, regardless of whether preoperative carbohydrates were compared with placebo or fasting. When studies contained both placebo and fasting arms, these were combined as described in Table 7.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Overall this comparison included 27 studies and 1976 participants. Because of the larger numbers of studies and participants, this comparison was used to conduct sensitivity analyses. The Table 1 was also constructed with the outcomes of this comparison.
Length of hospital stay (Analysis 3.1)
3.1. Analysis.
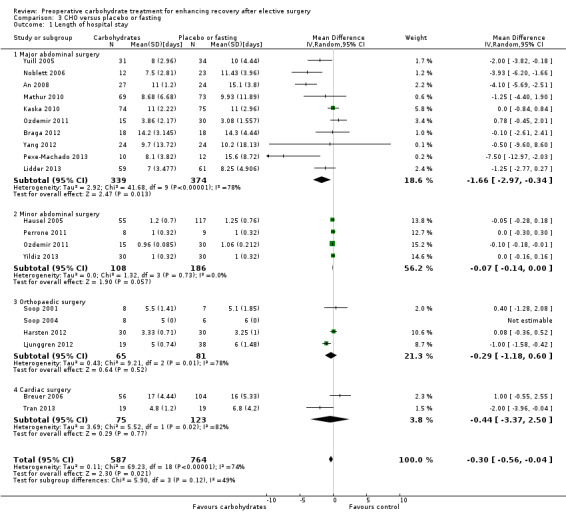
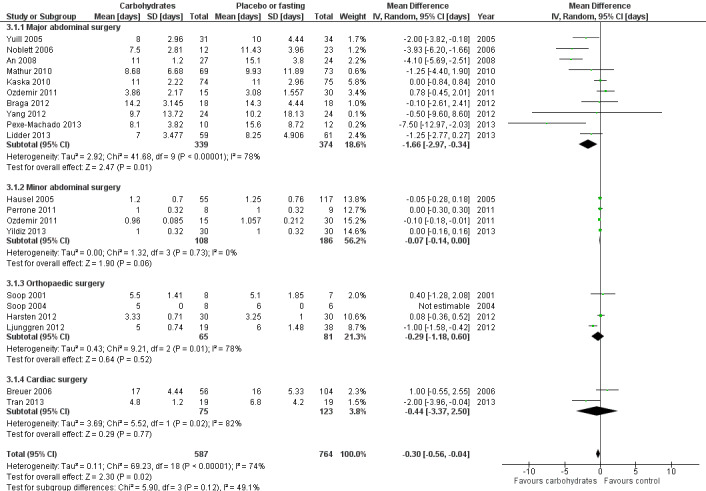
Comparison 3 CHO versus placebo or fasting, Outcome 1 Length of hospital stay.
This analysis includes 19 studies and 1351 participants. Four subgroups were constructed: major abdominal surgery (with mean length of stay greater than two days) (An 2008; Braga 2012; Kaska 2010; Lidder 2013; Mathur 2010; Noblett 2006; Ozdemir 2011; Pexe‐Machado 2013; Yang 2012; Yuill 2005), minor abdominal surgery (mean length of stay less than two days) (Hausel 2005; Ozdemir 2011; Perrone 2011; Yildiz 2013), orthopaedic surgery (Harsten 2012; Ljunggren 2012; Soop 2001; Soop 2004) and cardiac surgery (Breuer 2006; Tran 2013). One study that included participants undergoing both cardiac surgery and spinal surgery (Tran 2013) was placed into the cardiac surgery subgroup, as this is the procedure that most of the participants underwent. Sensitivity analysis performed by moving this study to the orthopaedic subgroup did not affect subgroup outcomes.
Overall administration of preoperative carbohydrate was associated with a modest reduction in hospital stay (MD ‐0.30 days, 95% CI ‐0.56 to ‐0.04) compared with the placebo or fasting group. This analysis is illustrated in Figure 4.
4.
Forest plot of comparison: 3 CHO versus placebo or fasting, outcome: 3.1 Length of hospital stay [days].
It should also be noted that overall heterogeneity is high (I2 = 74%), as is heterogeneity in all subgroups except that of minor abdominal surgery. The low heterogeneity in the minor abdominal surgery subgroup is to be expected, as all participants undergoing these operations would be expected to stay in hospital for no longer than 24 hours.
No difference was seen in the relative effects of carbohydrate across subgroups (P value 0.12). Patients undergoing major abdominal surgery have a longer average length of stay, hence a larger absolute decrease in average length of stay was seen in this subgroup (MD ‐1.66 days, 95% CI ‐2.97 to ‐0.34). Caution is needed in interpreting these findings because of the degree of heterogeneity observed in average lengths of stay in the major abdominal surgery subgroup, and because of variation in study quality.
A funnel plot of this analysis is presented in Figure 5. Weighted linear regression of effect estimates on their standard error (Egger 1997) revealed evidence of publication bias or other small‐study effects in this analysis (t = ‐2.19, degrees of freedom (df) = 17, P value 0.04). A sensitivity analysis was then conducted using the 'trim and fill' method, resulting in a reduced and non‐significant effect estimate (MD ‐0.13 days, 95% CI ‐0.43 to 0.18).
5.
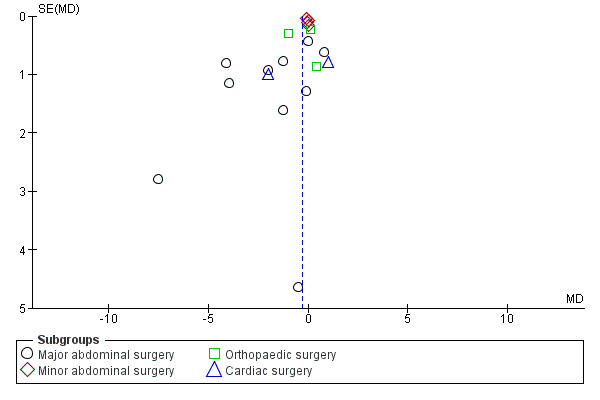
Funnel plot of comparison: 3 CHO versus placebo or fasting, outcome: 3.1 Length of hospital stay [days].
Through a sensitivity analysis, the outcome of length of hospital stay was reanalysed with subgroups of adequate blinding versus unclear or inadequate blinding (Analysis 3.2). Among the four studies judged to have adequate blinding of participants, treating clinicians and outcome assessors (Lidder 2013; Mathur 2010; Soop 2001; Soop 2004), no evidence of treatment effect of preoperative carbohydrates was found (MD ‐0.59, 95% CI ‐1.73 to 0.55) and heterogeneity was low (I2 = 10%).
3.2. Analysis.
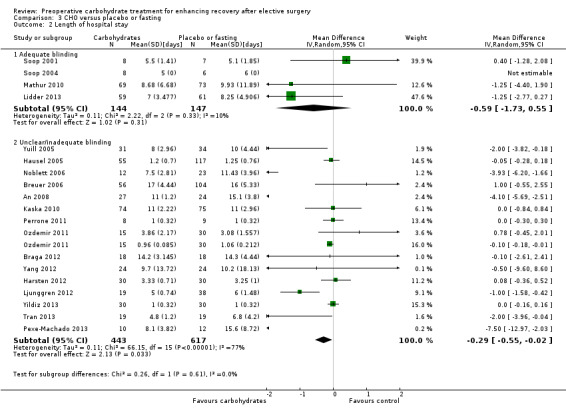
Comparison 3 CHO versus placebo or fasting, Outcome 2 Length of hospital stay.
The 15 remaining studies, in which blinding was at unclear or high risk of bias, showed evidence of treatment effect (MD ‐0.29, 95% CI ‐0.55 to ‐0.02) and increased heterogeneity (I2 = 77%).
Postoperative complication rate (Analysis 3.3)
3.3. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 3 Complication rate.
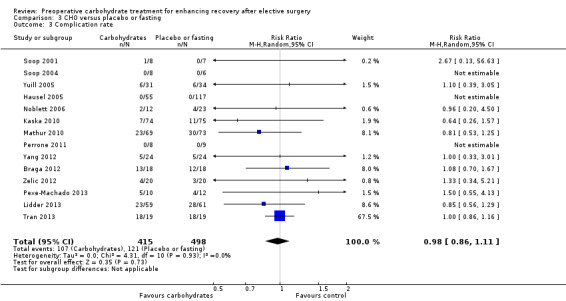
This analysis includes 14 studies (Braga 2012; Hausel 2005; Kaska 2010; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Pexe‐Machado 2013; Soop 2001; Soop 2004; Tran 2013; Yang 2012; Yuill 2005; Zelic 2012) and 913 participants, although three studies in which no complications were reported did not contribute to the meta‐analysis (Hausel 2005; Perrone 2011; Soop 2004). Heterogenity was low (I2 = 0%), and no evidence of effect of preoperative carbohydrate treatment on postoperative complication rate was found (RR 0.98, 95% CI 0.86 to 1.11). One study with a high event rate (Tran 2013) contributed 67.5% of the weighting of this analysis; however exclusion of this study, or any of the other studies sequentially, did not alter the results of this analysis. This analysis is presented as a funnel plot for investigation of small‐study effects in Figure 6. No evidence of publication bias was identified using weighted linear regression (t = 0.3217, df = 9, P value 0.76).
6.
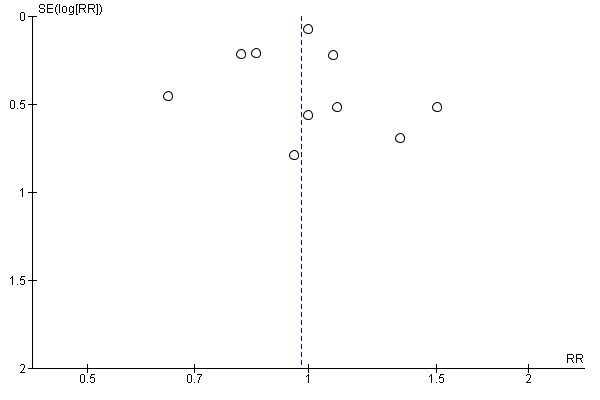
Funnel plot of comparison: 3 CHO versus placebo or fasting, outcome: 3.3 Complication rate.
Aspiration pneumonitis
Thirteen studies (Bisgaard 2004; Jarvela 2008; Lidder 2013; Mathur 2010; Noblett 2006; Perrone 2011; Soop 2001; Soop 2004; Tran 2013; Wang 2010; Yang 2012; Yildiz 2013; Yuill 2005) involving 789 participants reported on the outcome of aspiration pneumonitis; however no cases of this were described. Therefore it was not possible to undertake meta‐analysis of this outcome.
Insulin resistance (HOMA‐IR) (Analysis 3.4)
3.4. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 4 Postop insulin resistance (HOMA‐IR).
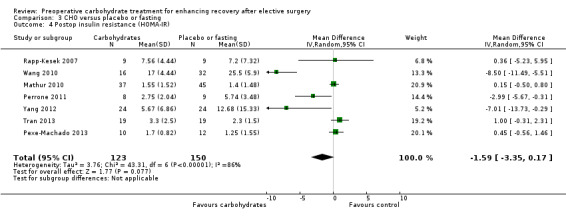
This outcome was reported in seven studies (Mathur 2010; Perrone 2011; Pexe‐Machado 2013; Rapp‐Kesek 2007; Tran 2013; Wang 2010; Zelic 2012) that included 273 participants. Overall heterogeneity was again high (I2 = 86%), and no evidence of treatment effect was found (MD ‐1.59, 95% CI ‐3.35 to 0.17). Influential analysis performed by excluding studies in turn found that exclusion of Wang 2010 reduced heterogeneity to an I2 of 57%, as well as reducing the magnitude of the effect estimate (MD ‐0.05, 95% CI ‐1.06 to 0.97). No obvious explanation for this could be identified. Excluding other studies had no significant effect on the results, and in no case did exclusion of a single study lead to evidence of treatment effect within 95% confidence intervals.
Insulin sensitivity (clamp) (Analysis 3.5)
3.5. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 5 Postop insulin sensitivity (clamp).
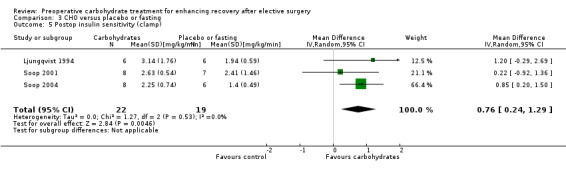
Postoperative insulin sensitivity was determined by hyperinsulinaemic euglycaemic clamp in three studies (Ljungqvist 1994; Soop 2001; Soop 2004) incorporating 41 participants. Results are presented as a standardized glucose infusion rate in mg/kg/min, with higher figures showing increased peripheral glucose utilization and therefore increased insulin sensitivity. Meta‐analysis showed that preoperative carbohydrate treatment was associated with postoperative increased insulin sensitivity (MD 0.76 mL/kg/min, 95% CI 0.24 to 1.29). Heterogeneity was low (I2 = 0%), although influential analysis found that by excluding Soop 2004, the precision of the effect estimate was reduced and the 95% confidence intervals crossed the line of no effect (MD 0.59 mL/kg/min, 95% CI ‐0.34 to 1.52).
Postoperative fatigue (Analysis 3.6)
3.6. Analysis.
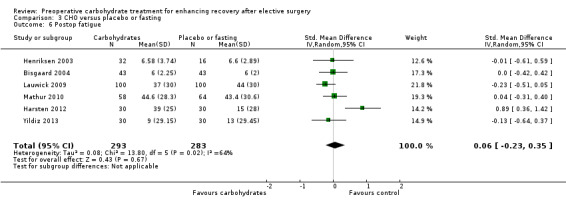
Comparison 3 CHO versus placebo or fasting, Outcome 6 Postop fatigue.
Postoperative fatigue was reported by six studies incorporating 576 participants. Five studies measured fatigue using a VAS (Harsten 2012; Henriksen 2003; Lauwick 2009; Mathur 2010; Yildiz 2013); one measured fatigue using a 10‐point ordinal scale (Bisgaard 2004). Combining this outcome by using standardized mean differences yielded high heterogeneity (I2 = 64%) and no evidence of treatment effect (SMD 0.06, 95% CI ‐0.23 to 0.35). Influential analysis found that exclusion of any study did not result in a significant treatment effect; however exclusion of Harsten 2012 did reduce heterogeneity (I2 = 0%).
Postoperative well‐being (Analysis 3.7)
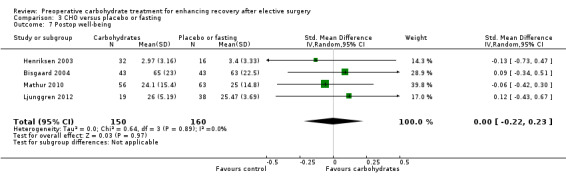
3.7. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 7 Postop well‐being.
Postoperative well‐being was reported by four studies, which included 310 participants. Three studies measured well‐being on a VAS (Bisgaard 2004; Henriksen 2003; Mathur 2010), and one used a 12‐question W‐BQ12 (Ljunggren 2012). Heterogenity between studies was low (I2 = 0%), and no evidence of a treatment effect was found (SMD 0.00, 95% CI ‐0.22 to 0.23). Influential analysis found that exclusion of any study did not yield evidence of a treatment effect.
Postoperative nausea at 24 hours (Analysis 3.8)
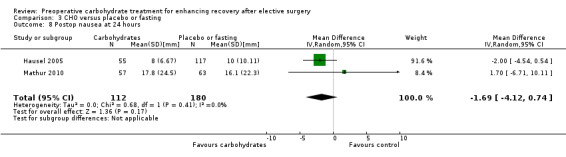
3.8. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 8 Postop nausea at 24 hours.
This outcome was reported by only two studies (Hausel 2005; Mathur 2010), which included in total 292 participants. Both studies measured nausea using a 100‐mm VAS. Combining these studies did not reveal evidence of a treatment effect (MD ‐1.69, 95% CI ‐4.12 to 0.74).
Postoperative vomiting (Analysis 3.9)
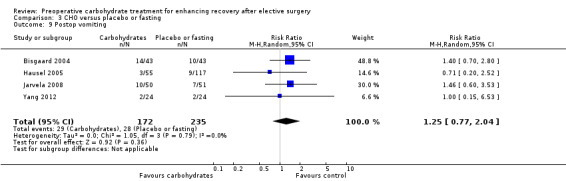
3.9. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 9 Postop vomiting.
Postoperative vomiting was reported by four studies (Bisgaard 2004; Hausel 2005; Jarvela 2008; Yang 2012), which included 407 participants. Meta‐analysis yielded no evidence of a treatment effect from preoperative carbohydrates (RR 1.25, 95% CI 0.77 to 2.04). Heterogeneity was low (I2 = 0%), and influential analysis found that exclusion of any individual study did not alter findings of the meta‐analysis.
Return of intestinal function (Analysis 3.10; Analysis 3.11)
3.10. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 10 Postop time to passage of first flatus.
3.11. Analysis.
Comparison 3 CHO versus placebo or fasting, Outcome 11 Postop time to first bowel movement.
Time to passage of first flatus was reported by two studies including 86 participants. Overall, preoperative carbohydrate treatment was associated with a reduction in mean time to passage of flatus of 0.39 days (95% CI ‐0.70 to ‐0.07). This analysis was heavily weighted (97.2%) toward the study by An 2008, which reported a statistically significant reduction in time to passage of flatus on its own. The remaining study (Noblett 2006) found no evidence of a treatment effect with preoperative carbohydrates.
Time to first bowel motion after surgery was reported by two studies (Noblett 2006; Ozdemir 2011) including 125 participants. One study (Ozdemir 2011) separately reported the outcomes of subgroups of participants undergoing major or minor abdominal surgery. Overall comparison revealed moderate heterogeneity (I2 = 63%) and no evidence of treatment effect (MD ‐0.28 days, 95% CI ‐0.62 to 0.05). Influential analysis was not performed, as only two studies were included in this analysis.
Discussion
Summary of main results
This review reports that patients undergoing elective surgery who receive preoperative carbohydrate treatment have a small overall reduction in length of hospital stay (0.30 days shorter, 95% CI ‐0.56 to ‐0.04) compared with those receiving placebo or subject to traditional preoperative fasting. However, no difference in length of stay was seen in studies that compared preoperative carbohydrate treatment with placebo (MD ‐0.13 days, 95% CI ‐0.38 to 0.12).
Preoperative carbohydrate treatment was not associated with any decrease or increase in postoperative complication rate when compared with placebo or fasting.
When secondary outcomes were considered, no evidence of effect was found for preoperative carbohydrate treatment when compared with placebo or fasting for the postoperative outcome of insulin resistance (HOMA‐IR), fatigue, well‐being, nausea, vomiting or time to first bowel movement. Preoperative carbohydrate treatment when compared with placebo or fasting was associated with a small increase in the return of intestinal function when measured as time to passage of flatus (MD ‐0.39 days, 95% CI ‐0.70 to ‐0.07). Preoperative carbohydrate treatment was also associated with an increase in postoperative insulin sensitivity when measured by a hyperinsulinaemic euglycaemic clamp (MD 0.76 mg/kg/min, 95% CI 0.24 to 1.29).
These main findings of the review are presented in Table 1.
Overall completeness and applicability of evidence
This review examined data from 27 studies including 1976 participants undergoing a range of elective surgical procedures. Most participants received an oral carbohydrate drink, with only two studies examining the effect of intravenously administered carbohydrates. The limited number of included studies examining carbohydrates administered via the intravenous route precluded separate analysis of this subgroup and limited the ability of this review to draw conclusions regarding this method of administration.
Overall the range of elective operations studied in this review was broad; however little evidence was found that specifically addressed patients undergoing minimally invasive surgery. The only minimally invasive surgical technique for which outcomes were specifically reported was laparoscopic cholecystectomy, with all other studies specifically excluding participants undergoing minimally invasive surgery or including their results with those of participants undergoing open surgical techniques.
It is important to note that many studies limited participation to patients with an ASA rating of I‐II or I‐III. Only one study was specifically limited to participants with an ASA of III or IV undergoing cardiac surgery (Breuer 2006). Even studies that included ASA III and IV participants generally reported smaller numbers, as these patients are less likely to undergo elective surgical procedures. This limits generalizability of the findings of this review to higher‐risk patients undergoing elective surgery.
Length of hospital stay was the most commonly reported outcome, followed by complication rate. Secondary outcomes were reported much more infrequently, with insulin resistance (HOMA‐IR), sensitivity (clamp), fatigue, well‐being, nausea, vomiting and return of intestinal function reported by six, three, five, four, two, four and three studies, respectively. This limited the ability of this review to perform subgroup analyses of these outcomes across participants undergoing different operations and, consequently, hinders the generalizability of these findings.
Although aspiration pneumonitis as a specific complication was mentioned by 13 studies (Soop 2001; Soop 2004; Bisgaard 2004; Yuill 2005; Noblett 2006; Jarvela 2008; Wang 2010; Mathur 2010; Perrone 2011; Yang 2012; Tran 2013; Yildiz 2013; Lidder 2013), no incidents of this admittedly rare complication were reported. This review is unable to present any conclusions with respect to this outcome.
It is important to note that all three studies (Ljungqvist 1994; Soop 2001; Soop 2004) reporting insulin sensitivity using the hyperinsulinaemic euglycaemic clamp technique were conducted in Sweden—two by the same author and the other by a colleague at the same institution. Two of these studies (Soop 2001; Soop 2004) included participants undergoing total hip joint replacement, and the other, open cholecystectomy (Ljungqvist 1994). This may limit the generalizability of this outcome to other populations and contexts.
It is also important to consider the outcome of insulin resistance when measured by HOMA‐IR, for which no significant effects of preoperative carbohydrate were found. This fact may be due to differences between these two measures, that is, HOMA‐IR measures basal (essentially hepatic) insulin resistance, and the hyperinsulinaemic euglycaemic clamp measures peripheral insulin sensitivity at active levels of insulin production (Muniyappa 2008).
Geographically, although overall reasonable variability was noted, seven (Harsten 2012; Hausel 2005; Ljunggren 2012; Ljungqvist 1994; Rapp‐Kesek 2007; Soop 2001; Soop 2004) of the 27 included studies were conducted in Sweden and only one in North America (Canada) (Tran 2013). This may limit the applicability of these results to other countries with different models of health care delivery.
Quality of the evidence
The 27 studies and 1976 participants that form the basis of this review certainly provide a considerable body of evidence. The overall methodological quality of the included studies was moderate, with most studies assessed as having one or more domains of unclear or high risk of bias. In light of the subjective nature of most of the outcomes of this review, perhaps of greatest concern is the lack of adequate blinding resulting from lack of identical placebo, or other potential risks to adequate blinding.
The main significant clinical effect of preoperative carbohydrate treatment compared with control was a reduction in hospital stay. That this reduction in stay was limited to comparisons of preoperative carbohydrate versus fasting and preoperative carbohydrate versus placebo or fasting, but was not found in the meta‐analysis of preoperative carbohydrate versus placebo, raises concerns that performance bias may be contributing to this finding. The subgroup analysis comparing studies with low risk of performance bias versus those with unclear or high risk would also support this assumption, given that reduced length of stay was not found in the low risk of performance bias subgroup.
Potential biases in the review process
This review was conducted according to procedures specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We rigorously searched multiple electronic databases, personal libraries and reference lists without language restriction with the goal of identifying studies. This review group consists of several authors with expertise in this field, who have published some of the studies that make up this review (JM, JN, LP, MS) and are in contact with those continuing to undertake research in this field. Titles, abstracts and papers were reviewed by at least two review authors independently. Thus we can be confident that all relevant studies have been included.
Data abstraction and quality assessment were also performed by at least two review authors, reducing the chances of error. Unfortunately, the published report did not always provide enough information to allow review authors to abstract the data or to be certain about the quality assessment. When possible, we contacted study authors, but some information is still outstanding as of the publication date of this review. In particular, four studies published in abstract form only, which were identified at the time of the second literature search, have provided insufficient information to allow review authors to determine their eligibility for this review (Aguilar‐Nascimento 2012; Forde 2012; Jodlowski 2011; Tsutsumi 2011).
We also note that 16 studies were excluded because they did not report any of the prespecified outcomes. It is possible that some of these trials did examine our prespecified outcomes but did not report the findings; this is known as reporting bias and is difficult to identify because to do so requires cross‐referencing of published trial protocols or making contact with the authors of excluded trials. We did not attempt to contact the authors of these trials and could identify protocols for only four (Awad 2010; Awad 2012; Okabayashi 2011; Schricker 2008).
It is significant to note that when the data for evidence of publication bias were examined, weighted linear regression of effect estimates on their standard error (Egger 1997) revealed evidence of publication bias or another small‐study effect. Reporting bias might have contributed to this finding. Sensitivity 'trim and fill' analysis resulted in attenuation and reduction in precision of the observed treatment effect. No other evidence of publication bias was identified in this review.
For several studies, the central tendency of the data was reported as a median rather than as a mean, and the spread was reported as a range or interquartile range. These data were approximated to mean and standard deviation by using the techniques described in Hozo 2005; however it is important to note that these approximations may differ from the reported mean and standard deviation statistics. Two studies (Braga 2012; Yildiz 2013) also required standard deviations for length of hospital stay and postoperative fatigue to be imputed from the median of the reported standard deviations of other similar studies. Sensitivity analysis performed to exclude these studies did not significantly alter any of the outcomes.
Agreements and disagreements with other studies or reviews
The authors of this review could not identify any other systematic reviews addressing this question at the time of submission of our protocol (Smith 2011), although three were subsequently published. In 2011 Jones 2011 published a narrative review that was based on a systematic literature search of three electronic databases. The search was limited to articles published in the English language over the previous 10 years. These review authors specifically restricted their search to papers relevant to colorectal surgery, and it is unclear whether the search was limited to randomized controlled trials.
These review authors retrieved a total of 20 papers and included 11 in their review (Jones 2011). They did not undertake a quantitative synthesis of the results by meta‐analysis but concluded that preoperative carbohydrate treatment was both safe and effective. Lack of formal study quality assessment, lack of quantitative synthesis and more limited study inclusion criteria by Jones et al make it difficult to compare their results with those of the present review.
The next review was published by Li et al in 2012 (Li 2012) and consisted of a systematic literature search of multiple electronic databases without language restrictions. Inclusion criteria were randomized controlled trials evaluating preoperative carbohydrate administration in surgical patients. Data were abstracted by multiple reviewers and quality was graded according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This review identified 22 studies and measured a variety of outcomes, including multiple other measures of insulin sensitivity and resistance. The main findings of this study were that preoperative carbohydrate administration was associated with greater insulin sensitivity when measured by various instruments. This study did not find an association between preoperative carbohydrate treatment and reduced length of hospital stay, potentially because of the fewer included studies.
Finally, another systematic review published in 2013 addressed the effects of preoperative carbohydrate treatment on length of hospital stay, insulin resistance, complication rate and nausea and vomiting (Awad 2013). This review was conducted in a systematic manner and specifically stated that standard methods recommended by The Cochrane Collaboration were used. A comprehensive search was conducted of multiple electronic databases between January 1980 and April 2012. Inclusion criteria included prospective randomized trials of adult patients undergoing elective surgery for whom preoperative carbohydrate treatment of at least 50 g was administered before surgery, and participants were compared with a control arm.
Awad et al identified 21 studies for inclusion in the qualitative synthesis, and length of hospital stay was determined on the basis of 12 studies and 1198 participants (Awad 2013). Similar to the present review, Awad et al found that preoperative carbohydrate treatment was associated with a reduction in length of stay after major abdominal surgery, but not after minor surgery or orthopaedic surgery. Awad et al also found no evidence of a treatment effect of preoperative carbohydrates on complication rate and an increase in insulin sensitivity when measured by hyperinsulinaemic euglycaemic clamp.
It is not surprising that the authors of Awad 2013 reported findings similar to those of the present review, given that the search strategy and the methodology used were almost identical. However the more up‐to‐date search and the greater number of included papers in the present review have improved its precision and external validity in comparison.
Authors' conclusions
Implications for practice.
Preoperative carbohydrate loading already forms an integral part of many enhanced recovery or fast‐track surgery protocols. Results of the present review provide very low‐quality evidence on the efficacy of preoperative oral carbohydrate drinks as part of fast‐track surgical protocols in reducing hospital stay, possibly mediated in part through reduced postoperative insulin resistance and faster return of intestinal function. However overall reduction in hospital stay was modest and of uncertain clinical significance (0.30 days, 95% CI ‐0.56 to ‐0.04 days), and no evidence of effect of preoperative carbohydrate treatment was demonstrated for complication rate or other important clinical outcomes. With this in mind, the potential benefits with respect to hospital stay need to be balanced against the costs of this intervention, as well as patient preferences, when fast‐track protocols are designed.
Implications for research.
The strength of the evidence found to support preoperative carbohydrate treatment in reducing hospital stay could be improved if a treatment effect could be demonstrated by well‐designed, blinded, placebo‐controlled trials, and across different types of surgery. Additional well‐conducted studies in major abdominal surgery, minimally invasive surgery, orthopaedic surgery and cardiac surgery could contribute usefully to the literature and to future meta‐analyses. Studies need to be well blinded and should aim to measure as many patient‐relevant outcomes as possible by using standardized instruments such as visual analogues scales. Further studies reporting insulin sensitivity by hyperinsulinaemic euglycaemic clamp and studies conducted in North America would be particularly valuable.
What's new
| Date | Event | Description |
|---|---|---|
| 15 August 2014 | Amended | Typo corrected, footnote D, Summary of findings table. |
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)), Karen Hovhannisyan (Trial Search Co‐ordinator, CARG) and Maggi Paki and Frances Harrington (Librarians, Southland Hospital) for assistance provided in the preparation of this review. We would also like to thank Anna Lee (Content Editor), Nathan Pace (Statistical Editor), Richard L Nelson and Krishna K Varadhan (Peer Reviewers) and Robert Wyllie (Consumer Referee) for help and editorial advice provided during preparation of this systematic review; and Jonas Nygren, Rick Nelson, Olle Ljungqvist (Peer Reviewers) and Ann Fonfa (Cochrane Consumer Network) for help and editorial advice given during preparation of the protocol (Smith 2011).
Dr Jonas Nygren commented on the first draft of this protocol. He was then asked to collaborate with us and became an author of the protocol and of the subsequent review. Dr Nygren's contribution to the review is described in the Contributions of authors section.
Appendices
Appendix 1. Free‐text terms and associated exploded MeSH terms
Surcial Procedures, Operative
Surgical Procedures, Elective
Surgical
Surgery
Elective surgery
Abdominal surgery
Carbohydrates
Carbohydrate
CHO
Nutricia
Maltodextrin
Oral
Drink
Placebo
Fasting
Preoperative
Postoperative Care
Postoperative Period
Postoperative Complications
Insulin resistance
Pain, Postoperative
Recovery
Nausea
Vomiting
Fatigue
Clinical trials
Controlled‐clinical trials
Randomized‐controlled trials
Appendix 2. CENTRAL search
#1 MeSH descriptor Carbohydrates explode all trees #2 ((carbohydrat* or CHO) near (oral or load* or treatment or drink* or fluid* or administrat* or rich)) #3 (oral fluid* or CHO or fasting):ti,ab #4 nutricia* or maltodextrin #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Postoperative Care, this term only #7 MeSH descriptor Postoperative Period, this term only #8 MeSH descriptor Insulin Resistance, this term only #9 MeSH descriptor Surgical Procedures, Elective, this term only #10 MeSH descriptor Postoperative Complications, this term only #11 MeSH descriptor Cholecystectomy, Laparoscopic, this term only #12 MeSH descriptor Pain, Postoperative, this term only #13 pre?op*:ti,ab #14 (postoperative near (recovery or pain or nausea or vomiting or fatigue)):ti,ab #15 (insulin near resistance):ti,ab #16 (surgery near (elective or abdominal)):ti,ab #17 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#5 AND #17)
Appendix 3. MEDLINE search
((carbohydrat* or CHO) adj3 (oral or load* or treatment or drink* or fluid* or administrat* or rich)).mp. or (oral fluid* or CHO or fasting).ti,ab. or (nutricia* or maltodextrin).mp. or Carbohydrates/
Postoperative Care/ or Postoperative Period/ or Insulin Resistance/ or Surgical Procedures, Elective/ or Postoperative Complications/ or Cholecystectomy, Laparoscopic/ or Pain, Postoperative/ or pre?op*.ti,ab. or (post?operative adj3 (recovery or pain or nausea or vomiting or fatigue)).ti,ab. or (insulin adj3 resistance).ti,ab. or (surgery adj3 (elective or abdominal)).ti,ab.
1 and 2
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
3 and 4
Appendix 4. EMBASE search
((carbohydrat* or CHO) adj3 (oral or load* or treatment or drink* or fluid* or administrat* or rich)).mp. or (oral fluid* or CHO or fasting).ti,ab. or (nutricia* or maltodextrin).mp. or carbohydrate/
pre?op*.ti,ab. or (post?operative adj3 (recovery or pain or nausea or vomiting or fatigue)).ti,ab. or (insulin adj3 resistance).ti,ab. or (surgery adj3 (elective or abdominal)).ti,ab. or postoperative care/ or postoperative period/ or postoperative pain/ or insulin resistance/ or elective surgery/ or postoperative complication/ or cholecystectomy/
(placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals.sh not (humans.sh and animals.sh))
3 and 2 and 1
Appendix 5. CINAHL search
S1 TX ( carbohydrat* or CHO ) and AB ( oral or load* or treatment or drink* or fluid* or administrat* or rich ) S2 AB oral fluid* or CHO or fasting S3 TX nutricia* or maltodextrin S4 (MM "Carbohydrates") S5 S1 or S2 or S3 or S4 S6 (MH "Postoperative Pain") OR (MH "Postoperative Period") OR (MH "Postoperative Care") OR (MH "Postoperative Complications") OR (MH "Insulin Resistance") OR (MH "Surgery, Elective") OR (MH "Cholecystectomy, Laparoscopic") S7 TI pre?op* or AB pre?op* S8 AB postoperative and AB ( recovery or pain or nausea or vomiting or fatigue ) S9 AB insulin and AB resistance S10 AB surgery and AB ( elective or abdominal ) S11 S6 or S7 or S8 or S9 or S10 S12 S5 and S11 S13 ( random* or placebo or trial* ) or ( ((single or double or triple or treble) and (mask* or blind*)) ) or ( multicenter* or prospective ) S14 (MH "Random Assignment") OR (MH "Clinical Trials") OR (MH "Placebos") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") S15 S13 or S14 S16 S12 and S15
Appendix 6. Web of Science search
TS=((carbohydrat* or CHO) same (oral or load or treatment or drink* or fluid* or administrat* or rich)) or TS=(oral fluid* or CHO or fasting) or TS=(nutricia* or maltodextrin)
TS=(post$op* same (care or period or complications or pain or recovery or nausea or vomiting or fatigue)) or TS=(pre$op*) or TS=((cholecystectomy or surgery or surgical) same (elective or abdominal or laparoscopic)) or TS=(insulin same resistance)
#1 AND #2
Appendix 7. Study selection form
Preoperative oral carbohydrate loading for enhancing recovery after elective surgery
Study selection form
| ISDN | First author | Journal/Conference, etc. | Year | Reviewer | Title reviewed | Abstract reviewed | Full text reviewed |
| Yes/No | Yes/No | Yes/No |
Study selection guidelines
| RCT |
Relevant participants (elective surgical procedure) (aged 18 years or older) |
Relevant interventions (preop administration of greater than 45 g of carbohydrate between 2 and 4 hours before induction of anaesthesia) (control group of fasting for 4 hours before induction of anaesthesia or placebo containing less than 45 g of carbohydrate) |
Relevant outcomes (length of hospital stay, complication rate) (postoperative insulin resistance, aspiration, fatigue, well‐being, return of intestinal function, nausea and vomiting) |
Duplicate patient series |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Do not proceed if any of the above answers is 'No.' If study is to be included in the 'Excluded studies' section of the review, record the reason for exclusion below |
| Outcome: Include/Exclude |
|
If excluded: Record in 'Excluded studies'—Yes/No |
| Reason for exclusion |
|
If included: Unique ID |
Appendix 8. Eligible trials form
Preoperative oral carbohydrate loading for enhancing recovery after elective surgery
Eligible trials form
| Unique ID | ISDN | Author(s) | Journal/Conference proceedings, etc. | Year |
| 1 | ||||
| 2 | ||||
| 3 | ||||
| ... |
Appendix 9. Data extraction form
Preoperative oral carbohydrate loading for enhancing recovery after elective surgery
Data extraction form
| Unique ID | ISDN | First author | Year | Reviewer | Date reviewed |
Notes
Study methods and details
| Trial characteristics | Details |
| Study site(s) | |
| Country/countries | |
| How was participant eligibility defined? | |
| Mean or median age of participants | |
| Risk breakdown of participants (ASA where defined) | |
| Surgery type/number | |
| Number receiving: * Epidural anaesthesia * Spinal anaesthesia * General anaesthesia |
|
| Number undergoing laparoscopic/minimally invasive surgery | |
| How many participants were randomized? | |
| Number of participants allocated to: * Carbohydrate * Placebo * Fasting |
|
| Number of participants analysed: * Carbohydrate * Placebo * Fasting |
|
| Carbohydrate group: * Preparation * Time administered * Route administered * Volume of drink * Carbohydrate dose * Co‐intervention |
|
| Placebo group: * Details of placebo * Time administered * Amount of drink * Carbohydrate dose |
|
| Fasting group: * Duration of preop fast—solids * Duration of preop fast—liquids * Duration of preop fast—carbohydrate |
References to other trials
| Did this report include any references to published or unpublished trials potentially eligible for this review? | Yes/No | |||
| First author | Journal/Conference | Title | Year of publication/presentation | Contact details |
Outcomes—Complete a separate copy for each relevant subgroup
| Subgroup | n |
For continuous data
| Outcome | Unit of measurement | Carbohydrate group | Placebo group | Fasting group | Details | ||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| Primary outcome—Length of hospital stay | |||||||||||
| Insulin resistance (HOMA‐IR) | |||||||||||
| Insulin resistance (clamp) | |||||||||||
| Postop fatigue | |||||||||||
| Postop well‐being | |||||||||||
| Postop nausea at 24 hours | |||||||||||
| Postop vomiting in the first 24 hours | |||||||||||
| Postop time to passage of flatus | |||||||||||
| Postop time to first bowel movement | |||||||||||
For dichotomous data
| Outcome | Carbohydrate group | Placebo group | Fasting group | Details | |||
| Number with event | Number without event | Number with event | Number without event | Number with event | Number without event | ||
| Primary outcome—Total complications | |||||||
| Aspiration pneumonitis | |||||||
| Postop vomiting 1 or more episodes | |||||||
Appendix 10. Quality assessment of eligible trials form
Preoperative oral carbohydrate loading for enhancing recovery after elective surgery
Quality assessment form
| Unique ID | ISDN | First author | Journal/Conference, etc. | Year | Reviewer |
| Domain | Describe | Reviewer's judgement—Risk of bias |
| 1. Adequate sequence generation | Low/Unclear/High | |
| 2. Allocation concealment | Low/Unclear/High | |
| 3. Blinding—Subjective | Low/Unclear/High/NA | |
| Blinding—Objective | Low/Unclear/High/NA | |
| 4. Incomplete outcome data—Length of stay | Low/Unclear/High/NA | |
| Incomplete outcome data—Complication rate | Low/Unclear/High/NA | |
| Incomplete outcome data—Secondary end points | Low/Unclear/High/NA | |
| 5. Selective outcome reporting | Low/Unclear/High | |
| 6. Other potential threats to validity | Low/Unclear/High |
Data and analyses
Comparison 1. CHO versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 14 | 867 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.12] |
| 1.1 Major abdominal surgery | 7 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.79, 0.33] |
| 1.2 Minor abdominal surgery | 3 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 1.3 Orthopaedic surgery | 4 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.11, 0.58] |
| 1.4 Cardiac surgery | 1 | 116 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.90, 2.90] |
| 2 Complication rate | 10 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 3 Postop insulin resistance (HOMA‐IR) | 4 | 179 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐8.19, 0.18] |
| 4 Postop insulin sensitivity (clamp) | 2 | 29 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.14, 1.26] |
| 5 Postop fatigue | 4 | 468 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.27, 0.54] |
| 6 Postop well‐being | 3 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.25, 0.25] |
| 7 Postop nausea at 24 hours | 2 | 234 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐4.06, 0.64] |
| 8 Postop vomiting | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.65, 2.12] |
| 9 Postop time to first bowel motion | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.74, 0.05] |
Comparison 2. CHO versus fasting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 10 | 656 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.79, ‐0.06] |
| 1.1 Major abdominal surgery | 5 | 276 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐4.13, 0.08] |
| 1.2 Minor abdominal surgery | 3 | 203 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.03] |
| 1.3 Orthopaedic surgery | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.73, ‐0.27] |
| 1.4 Cardiac surgery | 2 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.41, 2.47] |
| 2 Complication rate | 6 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.87, 1.16] |
| 3 Postop insulin resistance (HOMA‐IR) | 4 | 110 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐4.12, 1.47] |
| 4 Postop fatigue | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.47, 0.31] |
| 5 Postop well‐being | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.40, 0.47] |
| 6 Postop vomiting | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.58, 2.63] |
| 7 Postop time to passage of first flatus | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.70, ‐0.07] |
| 8 Postop time to first bowel movement | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.29, ‐0.07] |
Comparison 3. CHO versus placebo or fasting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 19 | 1351 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.56, ‐0.04] |
| 1.1 Major abdominal surgery | 10 | 713 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.97, ‐0.34] |
| 1.2 Minor abdominal surgery | 4 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.14, 0.00] |
| 1.3 Orthopaedic surgery | 4 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.18, 0.60] |
| 1.4 Cardiac surgery | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐3.37, 2.50] |
| 2 Length of hospital stay | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Adequate blinding | 4 | 291 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.73, 0.55] |
| 2.2 Unclear/inadequate blinding | 15 | 1060 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.55, ‐0.02] |
| 3 Complication rate | 14 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.11] |
| 4 Postop insulin resistance (HOMA‐IR) | 7 | 273 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐3.35, 0.17] |
| 5 Postop insulin sensitivity (clamp) | 3 | 41 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.24, 1.29] |
| 6 Postop fatigue | 6 | 576 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.23, 0.35] |
| 7 Postop well‐being | 4 | 310 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.22, 0.23] |
| 8 Postop nausea at 24 hours | 2 | 292 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐4.12, 0.74] |
| 9 Postop vomiting | 4 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.77, 2.04] |
| 10 Postop time to passage of first flatus | 2 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.70, ‐0.07] |
| 11 Postop time to first bowel movement | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.62, 0.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2008.
| Methods | Randomized controlled trial January 2006 to January 2007 |
|
| Participants | Patients with histologically confirmed colon cancer undergoing elective resection Participants randomized: 51 (27 CHO, 24 fasting) Participants analysed: 51 (27 CHO, 24 fasting) Exclusions: diabetes mellitus, medication use that might affect insulin sensitivity, renal or hepatic insufficiency, inflammation |
|
| Interventions | Carbohydrates: oral beverage containing 50 g of carbohydrates in 200 mL administered 3 hours before anaesthesia Fasting: nil by mouth for 6 hours for solids and 2 hours for liquids before surgery |
|
| Outcomes | Length of hospital stay, postop time to passage of flatus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded; this may affect subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and personnel were not blinded; this may affect subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomization withdrawals reported in this study |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and not all important outcomes were reported |
| Other bias | Low risk | No other important threats to validity have been identified in this study; however limited information is available in the text |
Bisgaard 2004.
| Methods | Double‐blind randomized controlled trial October 1999 to March 2001 |
|
| Participants | Patients undergoing elective laparoscopic cholecystectomy. Participants randomized: 94 Participants analysed: 86 (43 CHO:43 placebo) Age, years: inclusion range 18‐75, median age 42 CHO and 44 placebo ASA: I‐II only Exclusions: previous endoscopic retrograde cholangiopancreatography with papillotomy within 1 month before surgery, diabetes, gastric disease or previous gastric surgery, chronic pain, expected poor compliance, receiving opioids or tranquillizers for longer than 1 week before surgery, protocol violations, postoperative complications |
|
| Interventions | Carbohydrate: oral carbohydrate drink (containing 50 g carbohydrates in 400 mL administered 2 hours before surgery) Placebo: placebo drink of 400 mL given 2 hours before surgery |
|
| Outcomes | Postop fatigue by visual analogue scale, postop well‐being by visual analogue scale, postop vomiting | |
| Notes | Participants suffering postop complications were specifically excluded from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomization by product manufacturer; method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Central randomization with supply of identically coded tetra packs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinical team and study observers all blinded to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, clinical team and study observers all blinded to intervention group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants with postoperative complications were excluded post randomization; numbers excluded post randomization were not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not prepublished, and not all potentially relevant outcomes were reported |
| Other bias | Unclear risk | Exclusion of participants with postop complications |
Braga 2012.
| Methods | Double‐blind randomized controlled trial September 2007 to May 2008 |
|
| Participants | Patients undergoing elective pancreaticoduodenectomy for pancreatic cancer or periampullary cancer Patients randomized: 36 (18 CHO, 18 placebo) Participants analysed: 36 (18 CHO, 18 placebo) Age, years: Inclusion range 18‐80, mean 64.1 Exclusions: severe malnutrition, impaired gastric emptying, uncontrolled diabetes mellitus, renal failure, cardiovascular dysfunction, ongoing infection, low plasma neutrophil level, psychiatric disease, epilepsy, suspicion of drug abuse, severe alcohol abuse, pregnancy, breast‐feeding or fertile women refusing to use contraceptives, allergy to any component of the investigational product, inability to co‐operate adequately, enrolment in other studies |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g carbohydrate, glutamine and antioxidants in 250 mL given 3 hours before induction of anaesthesia Placebo: low‐energy product containing orange juice concentrate, starch, sodium saccharin and colours in 250 mL given 3 hours before induction of anaesthesia |
|
| Outcomes | Length of hospital stay, complication rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed random number generation by computer |
| Allocation concealment (selection bias) | Low risk | Concealed random number generation by computer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identically packaged intervention and control drinks, described as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as double‐blind; however it is not reported whether assessors and data collectors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals post randomization were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol was registered online, and all protocol end points were reported |
| Other bias | Low risk | No other deficiencies were identified |
Breuer 2006.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Adults undergoing elective coronary artery bypass grafting or valve replacement Patients randomized: 188 Participants analysed: 160 (56 CHO, 44 placebo, 60 fasting) Age, years: patients 18 or older, median 64 ASA: III‐IV only Exclusions: conditions likely to impair gastrointestinal motility or to enhance gastro‐oesophageal reflux, potentially difficult airway management, non‐elective surgery, presence of infection, pregnancy, maltose or fructose intolerance, type 1 diabetes |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g carbohydrates in 400 mL administered 2 hours before induction of anaesthesia Placebo: oral 400 mL placebo drink administered 2 hours before induction of anaesthesia Fasting: from midnight before surgery |
|
| Outcomes | Length of hospital stay, insulin resistance by postoperative insulin requirement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Computer‐based central randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Uniform bottles of carbohydrate versus placebo drink; however fasting group not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Uniform bottles of carbohydrate versus placebo drink; however fasting group not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Distribution of exclusions and withdrawals post randomization not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and not all relevant end points were reported (such as complication rate) |
| Other bias | Low risk | No other risks of bias were identified |
Harsten 2012.
| Methods | Double‐blind randomized controlled trial September 2009 to April 2011 |
|
| Participants | Patients undergoing elective total hip replacement under spinal anaesthesia Patients randomized: 60 (30 CHO, 30 placebo) Participants analysed: 60 (30 CHO, 30 placebo) Age, years: inclusion range 50‐80, mean 69 treatment, 71 placebo ASA: I‐III BMI: less than or equal to 35 kg/m2 Exclusions: diabetes mellitus, prior hip surgery on same hip, ongoing infection, immunological deficiency |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g CHO in 400 mL administered 90 minutes before induction and again 2 hours post surgery Placebo: oral flavoured water 400 mL administered 90 minutes before induction and again 2 hours post surgery |
|
| Outcomes | Length of hospital stay, postop fatigue | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not given |
| Allocation concealment (selection bias) | Unclear risk | Allocation by sealed envelopes, not known whether these were opaque or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nurse administering intervention drink was not blinded as to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All other care providers were blinded as to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts post randomization |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not registered, and not all relevant outcomes were reported (such as complication rate) |
| Other bias | Low risk | No other potential sources of significant bias were identified |
Hausel 2005.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Patients undergoing elective laparoscopic cholecystectomy Participants randomized: 174 Participants analysed: 172 (55 CHO, 59 placebo, 58 fasting) Age, years: "adults," mean 48.3 CHO, 48.0 fasting, 46.8 placebo ASA: I‐II only Exclusions: conditions that may impair gastrointestinal motility, gastro‐oesophageal reflux, potentially difficult airways, diabetes mellitus, suspected or documented choledocholithiasis, participants scheduled for afternoon surgery |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g in 400 mL delivered at least 2 hours before premedication Placebo: oral 400 mL placebo drink at least 2 hours before premedication Fasting: from midnight before surgery |
|
| Outcomes | Length of hospital stay, total complications, postoperative vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Fasting from midnight group was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fasting from midnight group was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two withdrawals post randomization out of 174 participants |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not published, but all relevant and likely end points were reported |
| Other bias | Unclear risk | Only 22% of available laparoscopic cholecystectomy patients participated in this study, thus limiting generalizability of its findings |
Henriksen 2003.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Patients undergoing elective bowel resection Participants randomized: 58 Participants analysed: 48 (17 CHO, 15 CHO + protein, 16 fasting) Age, years: mean 64 for CHO, 63 for CHO + protein, 64 for fasting Exclusions: inflammatory bowel disease, disseminated malignant disease, previous treatment for intra‐abdominal cancer, serious cardiovascular disease (NYHA angina class III and IV), diabetes mellitus, disabling mental disease, dementia; history of alcohol, medicine or drug abuse |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g CHO in 400 mL administered 3 hours before surgery Carbohydrate + protein: oral carbohydrate + protein drink containing 50 g CHO and 3.5% soy protein in 400 mL delivered 3 hours before surgery Fasting: preoperative fasting for 3 hours before surgery |
|
| Outcomes | Postoperative fatigue and well‐being | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization described by "closed envelope technique"; however method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | 1 study author was aware of allocation but did not have input into data collection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to fasting versus active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinded investigator was not involved in data collection |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Distribution and effect of participants excluded post randomization was not clear |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and not all important end points were reported (length of stay, complication rate) |
| Other bias | Unclear risk | One postoperative death excluded from analysis |
Jarvela 2008.
| Methods | 2‐Arm parallel‐group randomized controlled trial November 2004 to June 2005 |
|
| Participants | Patients undergoing elective coronary artery bypass grafting Participants randomized: 101 (CHO 50, fasting 51) Participants analysed: 101 (CHO 50, fasting 51) Age, years: inclusion criteria not stated; mean 64 CHO, 66.8 placebo Exclusions: diagnosed diabetes, any cause of delayed gastric emptying |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g in 400 mL administered 2 hours before surgery Fasting: overnight fasting the night before surgery |
|
| Outcomes | Postoperative aspiration pneumonitis, postoperative vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization with consecutively sealed envelopes, method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether consecutively sealed envelopes were opaque or numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operating room staff were blinded to treatment group; however participants were not blinded because of trial design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded because of trial design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions post randomization were not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and not all important end points were reported |
| Other bias | Low risk | No other risks of bias were identified |
Kaska 2010.
| Methods | Randomized trial involving 3 parallel treatment groups | |
| Participants | 221 patients undergoing colorectal surgery Participants randomized: 221 (oral CHO 74, IV CHO 72, fasting 75) Participants analysed: 221 (oral CHO 74, IV CHO 72, fasting 75) Age, years: inclusion range 35‐75, mean 60.4 ASA: I‐II BMI: 20‐30 kg/m2 as inclusion criterion Exclusions: metabolic disease, systolic cardiac dysfunction, atrial fibrillation, moderate to severe valvular disease |
|
| Interventions | Oral carbohydrate: Nutricia preop (50 g CHO/400 mL) up to 2 hours before surgery IV carbohydrate: 500 mL 10% glucose delivered between 6 and 2 hours before surgery Fasting: from midnight the night before surgery |
|
| Outcomes | Length of hospital stay, total complications, insulin resistance by quantitative insulin sensitivity check index (QUICKI) | |
| Notes | IV CHO group only received 25 g of carbohydrate during the 4 hours directly before surgery and therefore were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment by "envelope method," details not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as "blinded," but no placebo was used for interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study described as "blinded," but no placebo was used for interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions post randomization were reported |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not published, but all relevant end points were likely reported |
| Other bias | Unclear risk | Baseline clinical characteristics of participants were not reported |
Lauwick 2009.
| Methods | Double‐blind 2‐parallel‐arm randomized controlled trial January 2004 to July 2006 |
|
| Participants | Adult women undergoing elective thyroidectomy Participants randomized: 208 (105 CHO, 103 placebo) Participants analysed: 200 (100 CHO, 100 placebo) Age, years: inclusion range 19‐70, mean 45 CHO and 46 placebo ASA: I‐II only BMI: less than 35 kg/m2 as inclusion criterion Exclusions: men, diabetes mellitus, gastro‐oesophageal reflux, preoperative vomiting and antiemetic therapy, steroid treatment, history of motion sickness or postoperative nausea and vomiting |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g of carbohydrates in 400 mL administered 2 hours before transfer to the operating room Placebo: drink of 100 mL water administered 2 hours before transfer to the operating room |
|
| Outcomes | Postop fatigue | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization online |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomization online |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeons and clinical personnel were blinded, but intervention drinks were of different volumes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Surgeons and clinical personnel were blinded, but intervention drinks were of different volumes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions post randomization were reported and were unlikely to affect outcome |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and many clinically significant end points were not reported |
| Other bias | Low risk | No other potential sources of bias were identified |
Lidder 2013.
| Methods | Prospective 4‐arm (2 × 2 factorial) double‐blind randomized trial | |
| Participants | Patients having a planned curative resection with primary anastomosis of histologically confirmed colorectal cancer Participants randomized: 120 Participants analysed: 120 Age, years: 18 or older Exclusions: inability to consent, frailty, pregnancy, diabetes, fasting plasma glucose greater than 7 mmol/L, use of steroids or immunosuppressants, abnormal gastric emptying, intestinal obstruction, use of enteral or parenteral nutrition |
|
| Interventions | Group A: oral placebo drink (400 mL) administered 2 hours before surgery, and oral placebo drink 600 mL per day on each postoperative day in hospital Group B: oral carbohydrate drink (50 g in 400 mL) administered 2 hours before surgery, and oral placebo drink 600 mL per day on each postoperative day in hospital Group C: oral placebo drink (400 mL) administered 2 hours before surgery, and oral polymeric nutritional supplement drink 900 kcal and 600 mL per day on each postoperative day in hospital Group D: oral carbohydrate drink (50 g in 400 mL) administered 2 hours before surgery, and oral polymeric nutritional supplement drink 900 kcal and 600 mL per day on each postoperative day in hospital |
|
| Outcomes | Length of hospital stay, total complications, aspiration pneumonitis, insulin resistance (HOMA‐IR) | |
| Notes | 4‐Arm randomized trial (2 independent binary random treatment allocations) involving postoperative nutritional supplementation, as well as preoperative carbohydrate supplementation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identically packaged intervention and placebo products produced centrally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Codes to unblind interventions not released until database was locked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomization withdrawals |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not published, but all relevant end points were likely reported |
| Other bias | Low risk | No other potential sources of significant bias were identified |
Ljunggren 2012.
| Methods | Open‐label randomized clinical trial with 3 parallel treatment groups May 2008 to September 2009 |
|
| Participants | Patients undergoing elective total hip joint replacement under spinal anaesthesia Participants randomized: 60 (20 CHO, 20 placebo, 20 fasting) Participants analysed: 57 (19 CHO, 18 placebo, 20 fasting) Age, years: range 44‐89, mean 69 ASA: I 9, II 37, III 11 Exclusion: endocrine disorders including diabetes and treatment with cortisone |
|
| Interventions | Carbohydrate: oral carbohydrate drink (50 g CHO/400 mL) administered 2 hours before entry into OR Placebo: oral tap water 800 mL administered 2 hours before entry into OR Fasting: from midnight the night before surgery |
|
| Outcomes | Length of hospital stay, insulin resistance (IV glucose tolerance test and quantitative insulin sensitivity check index (QUICKI)), postop well‐being | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope method mixed in large batches. Unclear whether envelopes were opaque or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study with no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how postrandomization withdrawals might affect results, although numbers of exclusions post randomization were small |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published, but some reported outcomes were not prespecified in the protocol; however it is likely that all relevant outcomes were reported |
| Other bias | Low risk | No other significant risks of bias were identified |
Ljungqvist 1994.
| Methods | Quasi‐randomized controlled trial | |
| Participants | Patients undergoing elective open cholecystectomy Participants analysed: 12 (6 CHO, 6 fasting) Age, years: mean 45 CHO and 42 fasting Exclusions: taking regular medications, history of metabolic disease or history of metabolic disease in first‐degree relatives, fasting blood glucose greater than 6 mmol/L, signs of previous unknown disease or alcohol abuse |
|
| Interventions | Carbohydrate: intravenous glucose infusion (5 mg/kg/min) from 1 hour after the last extensive meal on the day before operation until 30‐60 minutes before the beginning of anaesthesia Fasting: preoperative fasting from 6 pm the night before surgery, without intravenous infusions |
|
| Outcomes | Insulin sensitivity by hyperinsulinaemic euglycaemic clamp | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomization by date of birth |
| Allocation concealment (selection bias) | High risk | Quasi‐randomization by date of birth |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intervention was not blinded; however it is unclear how this might affect the objective outcome of insulin sensitivity |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Intervention was not blinded; however it is unclear how this might affect the objective outcome of insulin sensitivity |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions and dropouts were not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not published, and not all relevant outcomes were reported |
| Other bias | Low risk | No other sources of potential bias were identified |
Mathur 2010.
| Methods | Double‐blind randomized controlled trial July 2004 to December 2005 |
|
| Participants | Patients undergoing major elective colorectal (n = 97) or liver resection (n = 45) Participants randomized: 162 (80 CHO, 82 placebo) Participants analysed: 142 (69 CHO, 73 placebo) Age, years: inclusion range 18‐80, median 60 CHO and 65 placebo ASA: inclusion range I‐III (n = 19:93:30) Exclusions: pregnancy, inability to consumer clear fluids, gastrointestinal obstruction, diabetes mellitus, liver cirrhosis, corticosteroid treatment exceeding 5 mg/d |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g CHO in 400 mL administered 2 hours before anaesthesia Placebo: oral 400 mL placebo drink administered 2 hours before anaesthesia |
|
| Outcomes | Length of hospital stay, insulin resistance by HOMA‐IR, postoperative fatigue | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated variable block size central randomization, stratified by type of surgery |
| Allocation concealment (selection bias) | Low risk | Central randomization using opaque sealed envelopes. Placebo and active drinks identically packaged |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical coded tetra packs provided by study sponsor |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical coded tetra packs provided by study sponsor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were described and balanced between groups, unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published, and all planned outcomes were reported |
| Other bias | Low risk | No other source of potential bias was identified |
Noblett 2006.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Patients undergoing elective colorectal surgery Participants randomized: 36 (12 CHO, 12 placebo, 12 fasting) Participants analysed: 35 (CHO 12, placebo 11, fasting 12) Age, years: mean 58 CHO, 59 placebo, 55 fasting ASA: median II in all groups Exclusions: diabetes mellitus, gastro‐oesophageal reflux, disorders of gastric emptying |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 47.5 g CHO in 400 mL administered 3 hours before surgery Placebo: oral 400 mL water administered 3 hours before surgery Fasting: from midnight before surgery |
|
| Outcomes | Length of hospital stay, time to passage of flatus and bowel movement, postop complications, postop aspiration pneumonitis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation is described as by "random number allocation" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes opened by researcher, not clear whether these were opaque or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Taste of intervention drinks different, and fasting group not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Taste of intervention drinks different, and fasting group not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion post randomization only because of cancellation of surgery |
| Selective reporting (reporting bias) | Low risk | Study protocol was not published, but all likely important outcomes were reported in this study |
| Other bias | Low risk | No other threats to validity were identified |
Ozdemir 2011.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Patients undergoing elective major (abdominal hysterectomy) or minor (inguinal hernia) surgery Participants randomized: 90 (45 major, 45 minor; each group randomly assigned 15:15:15 to CHO, placebo and fasting) Age, years: inclusion criteria 30‐70, mean major: 45.66 CHO, 51.53 placebo, 47.66 fasting; minor: 45.80 CHO, 48.06 placebo, 45.40 fasting ASA: inclusion range I‐II only |
|
| Interventions | Carbohydrates: oral carbohydrate beverage (50 g/400 mL) given 2 hours before surgery Placebo: oral water (400 mL) given 2 hours before surgery Fasting: from the night before surgery |
|
| Outcomes | Length of hospital stay, time to first bowel movement | |
| Notes | Postop nausea + vomiting and fatigue were described as categorical outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo drink of water was not indistinguishable from carbohydrate drink; therefore blinding was unlikely to be complete |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was unlikely to be complete, and subjective outcomes were included |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Presence or absence of dropouts or withdrawals was not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published in advance, and not all relevant outcomes were reported |
| Other bias | Low risk | No other sources of potential bias was identified |
Perrone 2011.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Patients undergoing elective cholecystectomy (open or laparoscopic) or unilateral inguinal hernia repair Participants randomized: 26 (14 treatment, 12 placebo) Participants analysed: 17 (8 treatment, 9 placebo) Age, years: inclusion range 18‐65, mean 35 treatment, 41 placebo ASA: I‐II BMI: less than or equal to 35 kg/m2 Exclusions: acute cholecystitis, type 2 diabetes mellitus, chronic liver or kidney disease, gastro‐oesophageal reflux, gastroparesis, intestinal obstruction, prolonged operations, "significant intraoperative occurrences" |
|
| Interventions | Carbohydrate + protein: oral carbohydrate + protein drink (54 g CHO, 9 g protein, 237 mL) administered 3 hours before surgery Placebo: oral 237 mL water drink 3 hours before surgery |
|
| Outcomes | Length of hospital stay, insulin resistance (HOMA‐IR), total complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as blinded but details not reported. Water may not be suitable as placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportionally high numbers of postrandomization withdrawals, reasons not reported |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published with primary outcomes reported as per protocol |
| Other bias | Unclear risk | Significant difference in preoperative ASA distribution between treatment groups |
Pexe‐Machado 2013.
| Methods | Single‐blind randomized controlled trial | |
| Participants | Patients undergoing elective laparotomy for gastrointestinal malignancy (subtotal gastrectomy, hemicolectomy, anterior resection of the rectum) Participants randomized: 30 (15 CHO, 15 control) Participants analysed: 22 (10 CHO, 12 control) Age, years: inclusion range 18‐65, mean 48 CHO and 49 control ASA: inclusion range I‐III (I 6, II 13, III 3) Exclusions: diabetes mellitus, chronic kidney failure, chronic liver disease, serum bilirubin > 2 mg/dL, BMI > 35 kg/m2, gastro‐oesophageal reflux, gastroparesis, intestinal obstruction, non‐compliance with study protocol, severe intraoperative complications, operation time longer than 6 hours |
|
| Interventions | CHO: oral carbohydrate drink containing 67 g of carbohydrate and 8 g of protein in 200 mL administered 3 hours before surgery Control: fasting 6‐8 hours before surgery |
|
| Outcomes | Length of hospital stay, Insulin resistance (HOMA‐IR), total complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded, and treating clinicians might have been blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study was described as single‐blind; however it is not reported whether treating clinicians or data collection personnel were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 out of 30 participants were excluded post randomization, including 2 for intraoperative complications; this may have affected study outcomes |
| Selective reporting (reporting bias) | Low risk | Study protocol was registered and published. No selective outcome reporting was identified |
| Other bias | Low risk | No other specific risks were identified |
Rapp‐Kesek 2007.
| Methods | 2‐Arm parallel‐group randomized controlled trial | |
| Participants | Patients over 65 years of age scheduled to undergo elective coronary artery bypass grafting Participants randomized: 18 (9 CHO, 9 fasting) Participants analysed: 18 (9 CHO, 9 fasting) Age, years: older than 65. Mean age, 71 CHO and 73 fasting Exclusions: diabetes mellitus, other metabolic disease; severely impaired respiratory, circulatory or renal function |
|
| Interventions | CHO: oral carbohydrate drink containing 50 g of carbohydrate in 400 mL administered 3‐5 hours before induction of anaesthesia Fasting: from 8 PM the evening before surgery |
|
| Outcomes | Insulin resistance (HOMA‐IR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study is not blinded, but it is unclear how this would affect an objective measurement such as insulin resistance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This study is not blinded, but it is unlikely that lack of blinding will affect measurement of insulin resistance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts and withdrawals were not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not published, and not all likely relevant outcomes were reported |
| Other bias | Low risk | None were identified |
Soop 2001.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Patients undergoing elective total hip joint replacement surgery under regional anaesthesia Participants randomized: 19 Participants analysed: 15 (CHO 8, placebo 7) Age, years: inclusion range 18‐80, mean 66 CHO and 58 placebo BMI: 18‐28 kg/m2 Exclusions: medications known to affect gastric emptying or intermediary metabolism; signs or symptoms of metabolic, renal, hepatic or gastric disease; abnormal fasting glucose, creatinine, C‐reactive protein or liver function tests |
|
| Interventions | Carbohydrate: oral drink containing 50 g of carbohydrates in 400 mL administered 2 hours before epidural anaesthesia Placebo: 400 mL placebo drink administered 2 hours before epidural anaesthesia |
|
| Outcomes | Length of hospital stay, insulin resistance by hyperinsulinaemic euglycaemic clamp, total complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomization but method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Central randomization with identical coded tetra packs supplied by manufacturer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical coded tetra packs supplied by manufacturer |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical coded tetra packs supplied by manufacturer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 (out of 19) participants were excluded post randomization; treatment assignment of excluded participants was not reported |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not published, but all likely relevant outcomes were reported |
| Other bias | Low risk | |
Soop 2004.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Patients undergoing elective total hip joint replacement under epidural anaesthesia Participants randomized: 15 (8 CHO, 7 placebo) Participants analysed: 14 (8 CHO, 6 placebo) Age, years: inclusion range 18‐80, mean 59 CHO versus 66 placebo ASA: I‐II only BMI: 18‐28 kg/m2 Exclusions: conditions or medications known to affect insulin sensitivity, upper gastrointestinal disease, fasting glucose, C‐reactive protein, liver function tests or creatinine outside reference range, intolerance to non‐steroidal anti‐inflammatory drugs or epidural anaesthesia, major complications that could affect metabolic or clinical recovery |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g CHO in 400 mL delivered 150 minutes before surgery Placebo: 400 mL oral placebo drink delivered 150 minutes before surgery |
|
| Outcomes | Length of hospital stay, insulin resistance by hyperinsulinaemic euglycaemic clamp, complication rate | |
| Notes | Participants with major complications were excluded from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized and double‐blinded," details not given |
| Allocation concealment (selection bias) | Low risk | Central allocation of intervention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded and centrally allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded and centrally allocated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant receiving placebo was excluded post randomization |
| Selective reporting (reporting bias) | Low risk | Published protocol was not available, but all likely relevant outcomes were reported |
| Other bias | Low risk | No other potential source of bias was identified |
Tran 2013.
| Methods | Open‐label randomized controlled trial April 2008 to February 2009 |
|
| Participants | Adult patients undergoing elective coronary artery bypass grafting or spinal surgery with fusion Participants randomized: 38 (26 CABG (13 CHO, 13 fasting), 12 spine (6 CHO, 6 fasting)) Participants analysed: 38 (19 CHO, 19 fasting) Age, years: median 59 (CHO and fasting) BMI: only included participants with BMI < 40 kg/m2, median 26.9 kg/m2 CHO and 25.6 kg/m2 fasting Exclusions: gastrointestinal motility or reflux issues, existing type 1 or type 2 diabetes, inability to speak English, urgent or emergency surgery, entry into surgery more than 5 hours after ingestion of morning drink |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g carbohydrates in 400 mL administered 2 hours before surgery Fasting: from 8 PM the night before surgery |
|
| Outcomes | Length of hospital stay, insulin resistance (HOMA‐IR), postoperative complication rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was described as being conducted in permuted blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants was undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of participants was undertaken, and blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants are accounted for |
| Selective reporting (reporting bias) | Low risk | Protocol was published, and all proposed outcomes were reported |
| Other bias | Unclear risk | Mix of included operations was heterogeneous and unusual; unclear how this may affect results |
Wang 2010.
| Methods | 3‐Arm parallel‐group randomized trial | |
| Participants | Adult patients undergoing open surgery for colorectal cancer Participants randomized: 52 (18 CHO, 17 placebo, 17 fasting) Participants analysed: 48 (16 CHO, 16 placebo, 16 fasting) Age, years: inclusion range 25‐75, median 66 CHO, 62 placebo, 63 fasting Exclusions: diabetes or impaired glucose tolerance, medication affecting insulin sensitivity, greater than 10% body weight loss during previous 6 months, presence of distant metastases, renal or hepatic insufficiency, gastro‐oesophageal reflux, gastrointestinal obstruction or conditions known to affect gastric emptying rate |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g in 400 mL administered between 3 hours and 1 hour before induction of anaesthesia Placebo: oral placebo beverage of 400 mL volume administered between 3 hours and 1 hour before induction of anaesthesia Fasting: overnight the night before surgery |
|
| Outcomes | Insulin resistance (HOMA‐IR), aspiration pneumonitis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomly assigned, but details not given |
| Allocation concealment (selection bias) | Unclear risk | Participants described as randomly assigned, but details not given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants assigned to fasting were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded; blinding unlikely to affect HOMA‐IR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions post randomization balanced and unlikely to affect outcome |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published, and all relevant outcomes were reported |
| Other bias | Low risk | No other sources of potential bias were identified |
Yang 2012.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Patients undergoing open radical distal gastrectomy for gastric cancer Participants randomized: 60 Participants analysed: 48 (CHO 24, placebo 24) Age, years: mean 63.4 CHO versus 62.6 placebo Exclusions: emergency or laparoscopic surgery; pre‐existing nausea, vomiting, pyloric obstruction or delayed gastric emptying; metabolic disease including diabetes mellitus or impaired glucose tolerance; chemotherapy or radiotherapy before surgery; presence of distal metastases on CT; weight loss greater than 10% in 6 months; medications affecting insulin sensitivity; renal or hepatic insufficiency |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g of carbohydrates in 500 mL administered 2‐3 hours before induction of anaesthesia Placebo: 500 mL fluid containing (carbohydrate‐free) sweetener administered 2‐3 hours before induction of anaesthesia |
|
| Outcomes | Length of hospital stay, Insulin resistance (HOMA‐IR), total complications, aspiration pneumonitis, postoperative vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described in text |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as double‐blind, but method of blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as double‐blind, but method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Postrandomization withdrawal of 12 participants, balanced between groups, but reasons for withdrawals incompletely described |
| Selective reporting (reporting bias) | Low risk | Study protocol was not registered or published online, but all likely relevant outcomes were reported |
| Other bias | Low risk | No other potential threats to validity were identified |
Yildiz 2013.
| Methods | Single‐blind randomized controlled trial | |
| Participants | Patients undergoing elective laparoscopic cholecystectomy under general anaesthesia Participants randomized: 60 (30 CHO, 30 fasting) Age, years: inclusion criteria 25‐65, mean 47.63 CHO and 43.56 fasting ASA: I‐II only by inclusion criteria Exclusions: gastro‐oesophageal reflux, gastrointestinal motility disorders, diabetes mellitus, cardiac disease, mental retardation or dementia, allergy history, use of sedating or antidepressive medications, use of alcohol, anticipated difficult airways, those who could not understand pain scoring system |
|
| Interventions | Carbohydrate: oral carbohydrate drink (50 g/400 mL) given 2‐3 hours preoperatively Fasting: for 8 hours preop |
|
| Outcomes | Length of hospital stay, aspiration pneumonitis, postop nausea, postop fatigue | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used for generation of randomization sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described, other than "Randomized in a single blind fashion" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of intervention was undertaken with participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "Randomized in a single blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals were reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not published, and not all potentially relevant outcomes were reported (such as total complication rate) |
| Other bias | Low risk | No other source of potential bias was identified |
Yuill 2005.
| Methods | Double‐blind randomized placebo‐controlled trial August 1999 to March 2001 |
|
| Participants | Patients undergoing elective major abdominal surgery via open approach Participants randomized: 72 Participants analysed: 65 (31 CHO, 34 placebo) Age, years: mean 52.8 CHO and 52.1 placebo BMI: mean 25.2 kg/m2 CHO and 25.1 kg/m2 placebo Exclusions: impaired renal function, liver cirrhosis, diabetes, metabolic abnormalities, gastric stasis or obstruction, emergency or laparoscopic procedures |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50.4 g carbohydrates in 400 mL administered 2‐3 hours before anaesthesia Placebo: 400 mL oral placebo electrolyte drink administered 2‐3 hours before anaesthesia |
|
| Outcomes | Length of hospital stay, total complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as "Double‐blind, randomized, placebo controlled trial," details not reported |
| Allocation concealment (selection bias) | Unclear risk | Trial described as "Double‐blind, randomized, placebo controlled trial," details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double‐blind; placebo drink was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial described as double;blind; placebo drink was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawal or exclusion were not reported. Split between intervention and control groups was not described |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not published, but all likely relevant outcomes were reported |
| Other bias | Low risk | No other risks were identified |
Zelic 2012.
| Methods | Observer‐blinded randomized controlled trial | |
| Participants | Patients undergoing surgery for colon, upper rectal or rectosigmoid cancer Participants randomized: 40 Participants analysed: 40 (20 CHO, 20 fasting) Age, years: inclusion range not specified, mean 70.2 for treatment and 68.6 for fasting ASA: I‐II BMI: less than or equal to 30 kg/m2 Exclusions: previous operations, metastatic disease, diabetes mellitus, conditions that might impair gastrointestinal motility, gastro‐oesophageal reflux, potential for a difficult airway |
|
| Interventions | Carbohydrate: oral carbohydrate drink containing 50 g CHO in 400 mL given 2 hours before induction of anaesthesia Fasting: from the evening before surgery |
|
| Outcomes | Time to first passage of flatus, time to first bowel movement, total complications | |
| Notes | Trial was described as "double blind"; however no placebo was given, and 2 investigators were aware of participant allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence allocation not described |
| Allocation concealment (selection bias) | Unclear risk | Closed envelope technique, unclear whether envelopes were opaque or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Observers were blinded as to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomization withdrawals were reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not published, and not all relevant outcomes were reported |
| Other bias | Low risk | No other significant sources of potential bias were identified |
Abbreviations: ASA = American Society of Anesthesiologists physical classification score. BMI = body mass index. CABG = coronary artery bypass graft. CHO = carbohydrates. CT = computed tomography. HOMA‐IR = Homeostatic Model Assessment of Insulin Resistance. IV = intravenous. OR = operating room. QUICKI = quantitative insulin sensitivity check index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adanir 2008 | Intervention group did not receive carbohydrates |
| Aronsson 2009 | Insufficient dose of carbohydrate administered in intervention group |
| ASAC 2011 | Review article, not randomized controlled trial |
| Awad 2010 | None of the prespecified outcomes were reported in this study |
| Awad 2011 | Review article, not a randomized controlled trial |
| Awad 2011a | Participants did not undergo surgery |
| Awad 2011b | Participants did not undergo surgery |
| Awad 2012 | None of the prespecified end points for this review were reported |
| Bisgaard 2006 | Letter to the editor, not a randomized controlled trial |
| Bopp 2011 | Intervention group received only 25 g of carbohydrates preoperatively |
| Brady 2009 | Review article, not a randomized controlled trial |
| Breitman 2011 | Participants received an insufficient dose of carbohydrate, which was administered postoperatively |
| Burden 2012 | Review article, not a randomized controlled trial |
| Crowe 1984 | None of the prespecified outcomes were reported by this trial |
| Dock‐Nascimento 2011 | Insufficient dose of carbohydrate was administered preoperatively |
| Dock‐Nascimento 2012 | Only 25 g of carbohydrates was administered within 2 hours of surgery |
| Enoki 1992 | None of the prespecified outcomes were reported by this trial |
| Faria 2009 | Insufficient dose of carbohydrate was administered preoperatively |
| Goodwin 1991 | Non‐randomized assignment to treatment groups; unclear as to whether the intervention group (orange juice) received sufficient carbohydrates |
| Hausel 1999 | Number of participants randomly assigned to each group was not reported |
| Hausel 2001 | None of the prespecified outcomes were reported by this trial |
| Helminen 2009 | Intervention group was not planned to receive carbohydrates within 4 hours of induction of anaesthesia |
| Hendry 2010 | This was a study of a multi‐modal enhanced recovery protocol with multiple co‐interventions |
| Hubner 2010 | This was a study of a multi‐modal enhanced recovery protocol with multiple co‐interventions |
| Hutchinson 1988 | None of the prespecified outcomes were reported by this trial |
| Itou 2012 | Only 25 g of carbohydrates was administered within 2 hours of surgery |
| Jones 2011 | Review article, not a randomized controlled trial |
| Jones 2012 | Conference proceedings with insufficient information to include in the review |
| Kaska 2006 | Duplicate patient series |
| Korusic 2009 | None of the prespecified outcomes were reported by this trial |
| Lassen 2010 | Letter to the editor, not a randomized controlled trial |
| Lin 1997 | Non‐randomized assignment to treatment and control groups |
| Ljungqvist 1991 | Non‐randomized assignment to treatment and control groups |
| Ljungqvist 1998 | Duplicate patient series |
| Ljungqvist 2000 | Review article, not a randomized controlled trial |
| Ljungqvist 2001 | Review article, not a randomized controlled trial |
| Ljungqvist 2010 | Letter to the editor, not a randomized controlled trial |
| Longarela 2005 | Letter to the editor, not a randomized controlled trial |
| Maltby 1988 | None of the prespecified outcomes were reported by this trial |
| Maltby 1991 | Non‐randomized assignment to treatment and control groups |
| Maltby 2004 | Non‐standardized administration of carbohydrate intervention |
| Maltby 2006 | Review article, not a randomized controlled trial |
| McCaul 2003 | Insufficient dose of carbohydrates in the intervention group |
| Meisner 2008 | Insufficient dose of carbohydrates administered to the intervention group |
| Melis 2006 | None of the prespecified outcomes were reported by this trial |
| Muehling 2009 | Studied intervention was multi‐modal enhanced recovery protocol; unclear whether this included any carbohydrate intervention |
| Noblett 2004 | Duplicate patient series |
| Nygren 1995 | None of the prespecified outcomes were reported by this trial |
| Nygren 1998 | Non‐randomized assignment to intervention and control groups |
| Nygren 1999 | Duplicate patient series |
| Okabayashi 2010 | None of the prespecified outcomes were reported by this trial |
| Okabayashi 2011 | None of the prespecified outcomes were reported by this trial |
| Phillips 1993 | Non‐standardized drink as intervention |
| Power 2004 | Review article, not a randomized controlled trial |
| Protic 2010 | Insufficient dose of carbohydrates was administered to intervention group |
| Protic 2010a | Insufficient dose of carbohydrates was administered to intervention group |
| Schricker 2008 | None of the prespecified outcomes were reported by this trial |
| Serclova 2009 | This was a study of a multi‐modal enhanced recovery protocol with multiple co‐interventions |
| Smith 2011 | Review article, not a randomized controlled trial |
| Soop 2000 | Review article, not a randomized controlled trial |
| Stuart 2006 | Review article, not a randomized controlled trial |
| Svanfeldt 2007 | None of the prespecified outcomes were reported by this trial |
| Tanabe 1996 | Intervention group received an insufficient dose of carbohydrates |
| Taniguchi 2009 | Intervention group received an insufficient dose of carbohydrates |
| Thorell 1996 | None of the prespecified outcomes were reported by this trial |
| Vincent 1991 | Intervention group received an insufficient dose of carbohydrates |
| Wendel 2013 | Intervention group received an insufficient dose of carbohydrates |
| Wilson 1999 | Intervention group received an insufficient dose of carbohydrates |
| Yagci 2008 | None of the prespecified outcomes were reported by this trial |
| Zargar‐Shoshtari 2009 | This was a study of a multi‐modal enhanced recovery protocol with multiple co‐interventions |
| Zhang 2010 | Intervention group received an insufficient dose of carbohydrates |
Characteristics of studies awaiting assessment [ordered by study ID]
Aguilar‐Nascimento 2012.
| Methods | Parallel‐group randomized controlled trial |
| Participants | Adult patients undergoing major operations for gastrointestinal cancer Participants analysed: 22 (10 CHO, 12 fasting) |
| Interventions | Carbohydrate: oral beverage 200 mL containing 11% protein and 89% carbohydrate administered 3 hours before surgery Fasting: for 6 to 8 hours before surgery |
| Outcomes | Length of hospital stay |
| Notes | Published as recent conference proceedings with insufficient information to determine whether inclusion criteria for this review are met |
Asakura 2013.
| Methods | 3‐Arm parallel‐group randomized controlled trial |
| Participants | Patients undergoing surgery of the body surface Age, years: inclusion range 20‐79 ASA: I‐II only |
| Interventions | Carbohydrate: OS‐1 1000 mL from 20:00 the evening before surgery until 2 hours before anaesthesia Arginaid water: 250 mL of oral arginaid water at 0600 the morning of surgery Fasting: from midnight the night before surgery |
| Outcomes | Quality of recovery score |
| Notes | Although unlikely to meet the inclusion criteria for this review, this trial is published in abstract form as conference proceedings, and information is insufficient at present to show whether inclusion criteria for this review are met |
Forde 2012.
| Methods | Double‐blind parallel‐group randomized controlled trial |
| Participants | Patients undergoing curative colorectal cancer surgery |
| Interventions | Carbohydrate: oral beverage containing 48 g of carbohydrates in 400 mL administered 3 hours before anaesthesia Placebo: details not reported in conference proceedings |
| Outcomes | Length of hospital stay, postoperative well‐being |
| Notes | Published as recent conference proceedings with insufficient information to show whether inclusion criteria for this review are met. |
Jodlowski 2011.
| Methods | Parallel‐group randomized controlled trial |
| Participants | Patients undergoing elective colorectal surgery Participants randomized: 48 |
| Interventions | Carbohydrate: details not reported in conference proceedings Fasting: details not reported in conference proceedings |
| Outcomes | Length of hospital stay, complication rate, insulin resistance, postoperative return of gut function, postoperative well‐being |
| Notes | Published as recent conference proceedings with insufficient information to show whether inclusion criteria for this review are met |
Ozer 2013.
| Methods | 2‐Arm parallel‐group randomized controlled trial |
| Participants | Female patients undergoing general anaesthesia |
| Interventions | Carbohydrate: 400 mL oral carbohydrate beverage administered 2 hours before surgery Fasting: for 8 hours before surgery |
| Outcomes | Postoperative body temperature and vasoconstriction threshold |
| Notes | Although unlikely to meet the inclusion criteria for this review, this trial is published in abstract form as conference proceedings, and information is insufficient at present to show whether inclusion criteria for this review are met |
Tsutsumi 2011.
| Methods | 3‐Arm parallel‐group randomized controlled trial |
| Participants | Patients receiving surgery Participants randomized: 60 Other details not reported in conference proceedings |
| Interventions | High carbohydrate: 50 g carbohydrates given between 19:00 hours the day before surgery and 2 hours before surgery Low carbohydrate: 25 g carbohydrates given between 19:00 hours the day before surgery and 2 hours before surgery No carbohydrate: 0 g carbohydrates given between 19:00 hours the day before surgery and 2 hours before surgery |
| Outcomes | Oxygen consumption, carbon dioxide production and respiratory quotient |
| Notes | Published as recent conference proceedings with insufficient information to show whether inclusion criteria for this review are met |
Yilmaz 2013.
| Methods | 2‐Arm parallel‐group randomized controlled trial December 2008 to March 2009 |
| Participants | Adult patients scheduled to undergo elective laparoscopic cholecystectomy Participants randomized: 40 (20 CHO, 20 fasting) Age, years: inclusion range 18‐60, mean 42.6 for carbohydrate group and 45.7 for fasting group ASA: I‐II BMI: less than 30 kg/m2 Exclusions: conditions that may impair gastrointestinal motility including gastro‐oesophageal reflux, potential for a difficult airway, history of motion sickness, diabetes mellitus, severe hepatic or renal failure, any endocrine disorder, allergy to study medications, pregnancy |
| Interventions | Carbohydrate: oral beverage containing 50 g of carbohydrates in 400 mL administered 2 hours before surgery Fasting: for 8 hours before surgery |
| Outcomes | Complication rate, postoperative nausea and vomiting |
| Notes | This study likely meets the inclusion criteria for this review; however it was identified at the time of the third and final literature search and will be incorporated into the next version of this review. Given that no participants in this study were reported as suffering from complications, inclusion will not affect either of the primary outcomes reported in this review |
Zelic 2013.
| Methods | 2‐Arm parallel‐group randomized controlled trial |
| Participants | Patients undergoing elective laparoscopic cholecystectomy |
| Interventions | Carbohydrate: carbohydrate‐rich beverage given before surgery Fasting: from the evening before surgery |
| Outcomes | Stress response following surgery measured by serum cortisol and C‐reactive protein |
| Notes | Although unlikely to meet the inclusion criteria for this review, information is insufficient at present to show whether inclusion criteria for this review are met |
Zhao 2013.
| Methods | 2‐Arm parallel‐group randomized controlled trial November 2009 to March 2011 |
| Participants | Oesophageal cancer patients receiving radical operation Participants randomized: 68 (34 fast‐track surgery, 34 control) |
| Interventions | Interventions were not described in the abstract of the conference proceedings |
| Outcomes | Length of hospital stay, complication rate, return of gastrointestinal function, insulin resistance |
| Notes | Although unlikely to meet the inclusion criteria for this review, this trial is published in abstract form as conference proceedings, and information is insufficient at present to show whether inclusion criteria for this review are met |
ASA: American Society of Anesthesiologists physical classification score.
BMI: body mass index.
CHO: carbohydrate.
Differences between protocol and review
The subgroup analyses conducted differ between the protocol (Smith 2011) and this review. With regards to subgroup analysis according to type of surgery, we planned to split the subgroup of participants undergoing abdominal surgery into those undergoing open surgery and those undergoing laparoscopic surgery. The only laparoscopic surgery for which outcome data were reported separately was laparoscopic cholecystectomy; other outcome data reported for laparoscopic or minimally invasive techniques were scant, as were data that were reported in combination with those of open surgery.
Instead we reported the outcomes of major versus minor abdominal surgery separately, both to ensure consistency with the published review by Awad et al (Awad 2013) and to explore the differential effects of preoperative carbohydrate treatment on study participants undergoing abdominal operations with a short expected hospital stay versus those with a more prolonged hospital stay.
Subgroup analysis was not performed according to type of anaesthesia, again because this detail was not well described in the included trials, and because outcome data often were not reported separately for participants undergoing neuraxial anaesthesia.
For subgroups of the intervention, only two trials were identified in which carbohydrate was administered via the intravenous route, precluding meaningful subgroup analysis. Instead outcomes of participants receiving a placebo drink were combined with those of participants undergoing traditional fasting in a separate analysis, to increase statistical power and increase precision.
Sensitivity analysis to exclude trials at high risk of bias was confined to trials with unclear or high risk of bias across the two domains of blinding. As only two trials were judged to be at low risk of bias across all domains, this was considered to preclude meaningful meta‐analysis.
Finally, sensitivity analyses were conducted by using the software package 'R' (R 2.13.2) instead of Stata because of the primary review author's (MDS) familiarity with this software package. Also, the open source licence of this software makes it easier for other researchers to confirm the analyses contained in this review.
Contributions of authors
Mark D Smith (MDS), John McCall (JM), Lindsay Plank (LP), G Peter Herbison (PH), Mattias Soop (MS), Jonas Nygren (JN)
Conceiving of the review: JM, LP, MS, JN.
Designing the review: MDS, JM.
Co‐ordinating the review: MDS.
Undertaking manual searches: MDS.
Screening search results: MDS.
Organizing retrieval of papers: MDS, MS.
Screening retrieved papers against inclusion criteria: MDS, JM, PH.
Appraising quality of papers: MDS, LP, PH.
Abstracting data from papers: MDS, LP, PH.
Writing to authors of papers for additional information: MDS, LP.
Providing additional data about papers: LP, JN.
Obtaining and screening data on unpublished studies: MDS, MS, JN.
Managing data for the review: MDS, PH.
Entering data into Review Manager (RevMan 5.1): MDS, LP.
Analysing RevMan statistical data: MDS, PH.
Performing other statistical analysis not using RevMan: MDS.
Completing double entry of data: (data entered by person one: MDS; data entered by person two: LP).
Interpreting data: MDS, PH, JM, LP, MS, JN.
Making statistical inferences: MDS, PH.
Writing the review: MDS, JM, LP, PH, MS, JN.
Providing guidance on the review: JM, PH, JN.
Securing funding for the review: N/A.
Performing previous work that served as the foundation of the present study: JM, LP, JN, MS.
Serving as guarantor for the review (one review author): MDS.
Taking responsibility for reading and checking the review before submission: MDS.
Declarations of interest
Mark D Smith (MDS), John McCall (JM), Lindsay Plank (LP), G Peter Herbison (PH), Mattias Soop (MS), Jonas Nygren (JN)
JM and LP were involved in the design, conduct and publication of a study that was included in this review (Mathur 2010). Funding support for this study was provided by Nutricia (NZ) Ltd. They have no pecuniary interest in the product used in any of the studies.
MDS and PH have no known conflicts of interest to declare.
MS and JN have conducted and published studies on preoperative oral carbohydrate treatment. They are not receiving funding in relation to this review.
To avoid the risk of bias, initial study selection and quality appraisal were performed by at least one review author without a declared interest (MDS), as was arbitration in cases of disagreement between review authors (PH).
Edited (no change to conclusions)
References
References to studies included in this review
An 2008 {published data only}
- An G‐Q, Zhao X‐L, Gao Y‐C, Wang G‐Y, Yu Y‐M. Effects of preoperative carbohydrate loading on the changes in serum tumor necrosis factor receptors 1 and 2 and insulin resistance in patients of colon carcinoma. [Chinese]. National Medical Journal of China 2008;88(29):2041‐4. [PubMed] [Google Scholar]
Bisgaard 2004 {published data only}
- Bisgaard T, Kristiansen VB, Hjortso NC, Jacobsen LS, Rosenberg J, Kehlet H. Randomized clinical trial comparing an oral carbohydrate beverage with placebo before laparoscopic cholecystectomy. The British Journal of Surgery 2004;91(2):151‐8. [PUBMED: 14760661] [DOI] [PubMed] [Google Scholar]
Braga 2012 {published data only (unpublished sought but not used)}
- Braga M, Bissolati M, Rocchetti S, Beneduce A, Pecorelli N, Carlo V. Oral preoperative antioxidants in pancreatic surgery: a double‐blind, randomized, clinical trial. Nutrition 2012;28:160‐4. [DOI: 10.1016/j.nut.2011.05.014; PUBMED: 21890323] [DOI] [PubMed] [Google Scholar]
Breuer 2006 {published and unpublished data}
- Breuer JP, Dossow V, Heymann C, Griesbach M, Schickfus M, Mackh E, et al. Preoperative oral carbohydrate administration to ASA III‐IV patients undergoing elective cardiac surgery. Anesthesia and Analgesia 2006;103(5):1099‐108. [DOI] [PubMed] [Google Scholar]
Harsten 2012 {published data only}
- Harsten A, Hjartarson H, Toksvig‐Larsen S. Total hip arthroplasty and perioperative oral carbohydrate treatment: a randomised, double‐blind, controlled trial. European Journal of Anaesthesiology 2012;29:271‐4. [DOI] [PubMed] [Google Scholar]
Hausel 2005 {published data only}
- Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. The British Journal of Surgery 2005;92(4):415‐21. [PUBMED: 15739210 ] [DOI] [PubMed] [Google Scholar]
Henriksen 2003 {published data only}
- Henriksen MG, Hessov I, Dela F, Hansen HV, Haraldsted V, Rodt SA. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiologica Scandinavica 2003;47(2):191‐9. [DOI] [PubMed] [Google Scholar]
Jarvela 2008 {published data only}
- Jarvela K, Maaranen P, Sisto T. Pre‐operative oral carbohydrate treatment before coronary artery bypass surgery. Acta Anaesthesiologica Scandinavica 2008;52(6):793‐7. [DOI] [PubMed] [Google Scholar]
Kaska 2010 {published data only (unpublished sought but not used)}
- Kaska M, Grosmanov T, Havel E, Hyspler R, Petrov Z, Brtko M, et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery—a randomized controlled trial. Wiener Klinische Wochenschrift 2010;122(1‐2):23‐30. [DOI] [PubMed] [Google Scholar]
Lauwick 2009 {published data only}
- Lauwick SM, Kaba A, Maweja S, Hamoir EE, Joris JL. Effects of oral preoperative carbohydrate on early postoperative outcome after thyroidectomy. Acta Anaesthesiologica Belgica 2009;60(2):67‐73. [PubMed] [Google Scholar]
Lidder 2013 {published and unpublished data}
- Lidder P, Thomas S, Fleming S, Hoise K, Shaw S, Lewis S. A randomized trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Disease 2013;15:737‐46. [DOI: 10.1111/codi.12130] [DOI] [PubMed] [Google Scholar]
Ljunggren 2012 {published data only}
- Ljunggren S, Hahn RG. Oral nutrition or water loading before hip replacement surgery: a randomized clinical trial. Trials 2012;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ljungqvist 1994 {published data only}
- Ljungqvist O, Thorell A, Gutniak M, Haggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. Journal of the American College of Surgeons 1994;178(4):329‐36. [PUBMED: 8149032] [PubMed] [Google Scholar]
Mathur 2010 {published and unpublished data}
- Mathur S, Plank LD, McCall JL, Shapkov P, McIlroy K, Gillanders LK, et al. Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery. The British Journal of Surgery 2010;97(4):485‐94. [PUBMED: 20205227] [DOI] [PubMed] [Google Scholar]
Noblett 2006 {published data only}
- Noblett SE, Watson DS, Huong H, Davison B, Hainsworth PJ, Horgan AF. Pre‐operative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Colorectal Disease 2006 Sep;8(7):563‐9. [DOI] [PubMed] [Google Scholar]
Ozdemir 2011 {published data only}
- Ozdemir F, Eti Z, Dincer P, Gogus FY, Bekiroglu N. The effect of preoperative oral carbohydrate loading on stress response in patients undergoing major or minor surgery. Turkiye Klinikleri Journal of Medical Science 2011;31(6):1392‐400. [Google Scholar]
Perrone 2011 {published data only (unpublished sought but not used)}
- Perrone F, Da‐Silva‐Filho AC, Adorno IF, Anabuki NT, Leal FS, Colombo T, et al. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutrition Journal 2011;10:66. [PUBMED: 21668975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pexe‐Machado 2013 {published data only}
- Pexe‐Machado PA, Oliveira BD, Dock‐Nascimento DB, Aguilar‐Nascimento JE. Shrinking preoperative fast time with maltodextrin and protein hydrolysate in gastrointestinal resections due to cancer. Nutrition 2013;29:1054‐9. [PUBMED: 23759267] [DOI] [PubMed] [Google Scholar]
Rapp‐Kesek 2007 {published data only}
- Rapp‐Kesek D, Stridsberg M, Andersson L, Berne C, Karlsson T. Insulin resistance after cardiopulmonary bypass in the elderly patient. Scandanavian Cardiovascular Journal 2007;41:102‐8. [DOI] [PubMed] [Google Scholar]
Soop 2001 {published data only}
- Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. American Journal of Physiology, Endocrinology and Metabolism 2001;280(4):E576‐83. [DOI] [PubMed] [Google Scholar]
Soop 2004 {published data only}
- Soop M, Nygren J, Thorell A, Weidenhielm L, Lundberg M, Hammarqvist F, et al. Preoperative oral carbohydrate treatment attenuates endogenous glucose release 3 days after surgery. Clinical Nutrition 2004;23:733‐41. [PUBMED: 15297112] [DOI] [PubMed] [Google Scholar]
Tran 2013 {published data only}
- Tran S, Wolever TM, Errett LE, Ahn H, Mazer CD, Keith M. Preoperative carbohydrate loading in patients undergoing coronary artery bypass or spinal surgery. Anesthesia and Analgesia 2013;117(2):305‐13. [PUBMED: 23757474] [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. The British Journal of Surgery 2010;97(3):317‐27. [PUBMED: 20101593] [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only (unpublished sought but not used)}
- Yang Y, Yan‐Bing Z, Xue‐Long J, Dong C, Zhi‐Hao W. Effects and safety of preoperative oral carbohydrate in radical distal gastrectomy ‐ a randomized clinical trial. Journal of Cancer Science and Therapy 2012;4(5):116‐9. [Google Scholar]
Yildiz 2013 {published data only}
- Yildiz H, Gunal SE, Yilmaz G, Yucel S. Oral carbohydrate supplementation reduces preoperative discomfort in laparoscopic cholecystectomy. Journal of Investigative Surgery 2013;26:89‐95. [DOI] [PubMed] [Google Scholar]
Yuill 2005 {published data only}
- Yuill KA, Richardson RA, Davidson HI, Garden OJ, Parks RW. The administration of an oral carbohydrate‐containing fluid prior to major elective upper‐gastrointestinal surgery preserves skeletal muscle mass postoperatively—a randomised clinical trial. Clinical Nutrition 2005 Feb;24(1):32‐7. [DOI] [PubMed] [Google Scholar]
Zelic 2012 {published data only}
- Zelic M, Stimac D, Mendrila D, Tokmadzic VS, Fisic E, Uravic M, et al. Influence of preoperative oral feeding on stress response after resection for colon cancer. Hepato‐Gastroenterology 2012;59:1385‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adanir 2008 {published data only}
- Adanir T, Aksun M, Ozgurbuz U, Altin F, Sencan A. Does preoperative hydration affect postoperative nausea and vomiting? A randomized, controlled trial. Journal of Laparoendoscopic & Advanced Surgical Techniques Part A 2008;18(1):1‐4. [PUBMED: 18266566] [DOI] [PubMed] [Google Scholar]
Aronsson 2009 {published data only}
- Aronsson A, Al‐Ani NA, Brismar K, Hedstrom M. A carbohydrate‐rich drink shortly before surgery affected IGF‐I bioavailability after a total hip replacement. A double‐blind placebo controlled study on 29 patients. Aging Clinical and Experimental Research 2009;21(2):97‐101. [PUBMED: 19448380] [DOI] [PubMed] [Google Scholar]
ASAC 2011 {published data only}
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2011;114(3):495‐511. [PUBMED: 21307770] [DOI] [PubMed] [Google Scholar]
Awad 2010 {published data only}
- Awad S, Constantin‐Teodosiu D, Constantin D, Rowlands BJ, Fearon KC, Macdonald IA, et al. Cellular mechanisms underlying the protective effects of preoperative feeding: a randomized study investigating muscle and liver glycogen content, mitochondrial function, gene and protein expression. Annals of Surgery 2010;252(2):247‐53. [PUBMED: 20622656] [DOI] [PubMed] [Google Scholar]
Awad 2011 {published data only}
- Awad S, Lobo DN. What's new in perioperative nutritional support?. Current Opinion in Anaesthesiology 2011;24(3):339‐48. [PUBMED: 21451404] [DOI] [PubMed] [Google Scholar]
Awad 2011a {published data only}
- Awad S, Fearon KC, Macdonald IA, Lobo DN. A randomized cross‐over study of the metabolic and hormonal responses following two preoperative conditioning drinks. Nutrition (Burbank, Los Angeles County, Calif.) 2011;27(9):938‐42. [PUBMED: 21126861] [DOI] [PubMed] [Google Scholar]
Awad 2011b {published data only}
- Awad S, Blackshaw PE, Wright JW, Macdonald IA, Perkins AC, Lobo DN. A randomized crossover study of the effects of glutamine and lipid on the gastric emptying time of a preoperative carbohydrate drink. Clinical Nutrition (Edinburgh, Scotland) 2011;30(2):165‐71. [PUBMED: 20971535] [DOI] [PubMed] [Google Scholar]
Awad 2012 {published data only}
- Awad S, Stephens F, Shannon C, Lobo DN. Perioperative perturbations in carnitine metabolism are attenuated by preoperative carbohydrate treatment: another mechanism by which preoperative feeding may attenuate development of postoperative insulin resistance. Clinical Nutrition (Edinburgh, Scotland) 2012;31(5):717‐20. [PUBMED: 22444237] [DOI] [PubMed] [Google Scholar]
Bisgaard 2006 {published data only}
- Bisgaard T, Kehlet H. Letter. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy (The British Journal of Surgery 2005;92:415‐21). The British Journal of Surgery 2006;93:120. [DOI] [PubMed] [Google Scholar]
Bopp 2011 {published data only}
- Bopp C, Hofer S, Klein A, Weigand MA, Martin E, Gust R. A liberal preoperative fasting regimen improves patient comfort and satisfaction with anesthesia care in day‐stay minor surgery. Minerva Anestesiologica 2011;77(7):680‐6. [PUBMED: 19190563] [PubMed] [Google Scholar]
Brady 2009 {published data only}
- Brady M, Kinn S, Ness V, O’Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005285.pub2] [DOI] [PubMed] [Google Scholar]
Breitman 2011 {published data only}
- Breitman I, Saraf N, Kakade M, Yellumahanthi K, White M, Hackett JA, et al. The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. Journal of the American College of Surgeons 2011;212(4):617‐25; discussion 625‐7. [PUBMED: 21463799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burden 2012 {published data only}
- Burden S, Todd C, Hill J, Lal S. Pre‐operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008879.pub2; PUBMED: 23152265] [DOI] [PubMed] [Google Scholar]
Crowe 1984 {published data only}
- Crowe PJ, Dennison A, Royle GT. The effect of pre‐operative glucose loading on postoperative nitrogen metabolism. The British Journal of Surgery 1984;71(8):635‐7. [PUBMED: 6430379] [DOI] [PubMed] [Google Scholar]
Dock‐Nascimento 2011 {published data only}
- Dock‐Nascimento BD, Aguilar‐Nascimento JE, Caporossi C, Sepulveda Magalhaes Faria M, Bragagnolo R, Caporossi FS, et al. Safety of oral glutamine in the abbreviation of preoperative fasting: a double‐blind, controlled, randomized clinical trial. Nutricion Hospitalaria 2011;26(1):86‐90. [PUBMED: 21519733] [PubMed] [Google Scholar]
Dock‐Nascimento 2012 {published data only}
- Dock‐Nascimento DB, Aguilar‐Nascimento JE, Magalhaes Faria MS, Caporossi C, Slhessarenko N, Waitzberg DL. Evaluation of the effects of a preoperative 2‐hour fast with maltodextrine and glutamine on insulin resistance, acute‐phase response, nitrogen balance, and serum glutathione after laparoscopic cholecystectomy: a controlled randomized trial. JPEN Journal of Parenteral & Enteral Nutrition 2012;36(1):43‐52. [PUBMED: 22235107] [DOI] [PubMed] [Google Scholar]
Enoki 1992 {published data only}
- Enoki T, Hatano Y, Tsujimura Y, Nomura R. Attenuation of gastric effects of famotidine by preoperative administration of intravenous fluids. Anesthesia and Analgesia 1992;74(1):68‐71. [PUBMED: 1734801] [DOI] [PubMed] [Google Scholar]
Faria 2009 {published data only}
- Faria MS, Aguilar‐Nascimento JE, Pimenta OS, Alvarenga LC Jr, Dock‐Nascimento DB, Slhessarenko N. Preoperative fasting of 2 hours minimizes insulin resistance and organic response to trauma after video‐cholecystectomy: a randomized, controlled, clinical trial. World Journal of Surgery 2009;33(6):1158‐64. [PUBMED: 19363695] [DOI] [PubMed] [Google Scholar]
Goodwin 1991 {published data only}
- Goodwin AP, Rowe WL, Ogg TW, Samaan A. Oral fluids prior to day surgery. The effect of shortening the pre‐operative fluid fast on postoperative morbidity. Anaesthesia 1991;46(12):1066‐8. [PUBMED: 1781536] [DOI] [PubMed] [Google Scholar]
Hausel 1999 {published data only}
- Hausel J, Nygren J, Almstrom C, Thorell A, Ljungqvist O. Preoperative oral carbohydrates improve well‐being after elective colorectal surgery [abstract]. Clinical Nutrition 1999;18(Suppl 1):21. [Google Scholar]
Hausel 2001 {published data only}
- Hausel J, Nygren J, Lagerkranser M, Hellstrom PM, Hammarqvist F, Almstrom C, et al. A carbohydrate‐rich drink reduces preoperative discomfort in elective surgery patients. Anesthesia and Analgesia 2001;93(5):1344‐50. [PUBMED: 11682427] [DOI] [PubMed] [Google Scholar]
Helminen 2009 {published data only}
- Helminen H, Viitanen H, Sajanti J. Effect of preoperative intravenous carbohydrate loading on preoperative discomfort in elective surgery patients. European Journal of Anaesthesiology 2009;26(2):123‐7. [PUBMED: 19142085] [DOI] [PubMed] [Google Scholar]
Hendry 2010 {published data only}
- Hendry PO, Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. The British Journal of Surgery 2010;97(8):1198‐206. [PUBMED: 20602497] [DOI] [PubMed] [Google Scholar]
Hubner 2010 {published data only}
- Hubner M, Muller S, Schafer M, Clavien PA, Demartines N. Impact of the nutritional risk score in fast‐track colon surgery. Digestive Surgery 2010;27(5):436‐9. [PUBMED: 21051894] [DOI] [PubMed] [Google Scholar]
Hutchinson 1988 {published data only}
- Hutchinson A, Maltby JR, Reid CR. Gastric fluid volume and pH in elective inpatients. Part I: Coffee or orange juice versus overnight fast. Canadian Journal of Anaesthesia 1988;35(1):12‐5. [PUBMED: 3349549] [DOI] [PubMed] [Google Scholar]
Itou 2012 {published data only}
- Itou K, Fukuyama T, Suzuki N, Taniguchi H, Iwao Y, Hinenoya H, et al. Safety and efficacy of oral rehydration therapy until 2 h before surgery: a multicenter randomized controlled trial. Journal of Anesthesia 2012;26(1):20‐7. [PUBMED: 22041970] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones C, Badger SA, Hannon R. The role of carbohydrate drinks in pre‐operative nutrition for elective colorectal surgery. Annals of the Royal College of Surgeons of England 2011;93(7):504‐7. [PUBMED: 22004631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2012 {published data only}
- Jones C, Day A, Kelliher L, Levy B, Smith R, Fawcett W, et al. Safety of pre‐operative oral carbohydrate loading in laparoscopic colorectal surgery. Colorectal Disease 2012:19. [Google Scholar]
Kaska 2006 {published data only}
- Kaska M, Grosmanova T, Havel E, Hyspler R. [Preparation of patients for operation with per‐oral intake on the day of the planned surgery] [Priprava k operaci s peroralnim prijmem v den planovane operace.]. Rozhledy v Chirurgii : Mesicnik Ceskoslovenske Chirurgicke Spolecnosti 2006;85(11):554‐9. [PUBMED: 17323547] [PubMed] [Google Scholar]
Korusic 2009 {published data only}
- Korusic A, Hauptman A, Brundula A, Duzel V, Husedzinovic I, Horic M, et al. Perioperative management with glucose solution and insulin. Collegium Antropologicum 2009;33(2):653‐7. [PUBMED: 19662793] [PubMed] [Google Scholar]
Lassen 2010 {published data only}
- Lassen K, Lobo DN. Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery (The British Journal of Surgery 2010;97:485‐94). The British Journal of Surgery 2010;97(4):494‐5. [PUBMED: 20205225] [DOI] [PubMed] [Google Scholar]
Lin 1997 {published data only}
- Lin MT, Saito H, Fukushima R, Inaba T, Fukatsu K, Inoue T, et al. Preoperative total parenteral nutrition influences postoperative systemic cytokine responses after colorectal surgery. Nutrition (Burbank, Los Angeles County, Calif.) 1997;13(1):8‐12. [PUBMED: 9058440] [DOI] [PubMed] [Google Scholar]
Ljungqvist 1991 {published data only}
- Ljungqvist O, Efendic S, Gutniak M, Haggmark T, Thorell A. Glucose infusion during preoperative fasting reduces postoperative development of insulin resistance [abstract]. Clinical Nutrition 1991;10(Spec Suppl 2):27. [Google Scholar]
Ljungqvist 1998 {published data only}
- Ljungqvist O, Nygren J, Thorell A. Preoperative carbohydrates instead of overnight fasting reduces hospital stay following elective surgery [abstract]. Clinical Nutrition 1998;17(Suppl 1):3. [Google Scholar]
Ljungqvist 2000 {published data only}
- Ljungqvist O, Nygren J, Hausel J, Thorell A. Preoperative nutrition therapy ‐ Novel developments. Scandinavian Journal of Nutrition 2000;44(1):3‐7. [Google Scholar]
Ljungqvist 2001 {published data only}
- Ljungqvist O, Nygren J, Thorell A, Brodin U, Efendic S. Preoperative nutrition ‐ Elective surgery in the fed or the overnight fasted state. Clinical Nutrition 2001;20(1):167‐71. [Google Scholar]
Ljungqvist 2010 {published data only}
- Ljungqvist O. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery (The British Journal of Surgery 2010;97:317‐27). The British Journal of Surgery 2010;97(3):327. [PUBMED: 20140949] [DOI] [PubMed] [Google Scholar]
Longarela 2005 {published data only}
Maltby 1988 {published data only}
- Maltby JR, Reid CR, Hutchinson A. Gastric fluid volume and pH in elective inpatients. Part II: Coffee or orange juice with ranitidine. Canadian Journal of Anaesthesia 1988;35(1):16‐9. [PUBMED: 3349550] [DOI] [PubMed] [Google Scholar]
Maltby 1991 {published data only}
- Maltby JR, Lewis P, Martin A, Sutheriand LR. Gastric fluid volume and pH in elective patients following unrestricted oral fluid until three hours before surgery. Canadian Journal of Anaesthesia 1991;38(4 Pt 1):425‐9. [PUBMED: 2065409] [DOI] [PubMed] [Google Scholar]
Maltby 2004 {published data only}
- Maltby JR, Pytka S, Watson NC, Cowan RA, Fick GH. Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non‐fasting obese patients. Canadian Journal of Anaesthesia 2004;51(2):111‐5. [PUBMED: 14766684] [DOI] [PubMed] [Google Scholar]
Maltby 2006 {published data only}
- Maltby JR. Preoperative fasting guidelines. Canadian Journal of Surgery 2006; Vol. 49, issue 2:138‐9; author reply 139. [PUBMED: 16630428] [PMC free article] [PubMed]
McCaul 2003 {published data only}
- McCaul C, Moran C, O'Cronin D, Naughton F, Geary M, Carton E, et al. Intravenous fluid loading with or without supplementary dextrose does not prevent nausea, vomiting and pain after laparoscopy. Canadian Journal of Anaesthesia 2003;50(5):440‐4. [PUBMED: 12734150] [DOI] [PubMed] [Google Scholar]
Meisner 2008 {published data only}
- Meisner M, Ernhofer U, Schmidt J. [Liberalisation of preoperative fasting guidelines: effects on patient comfort and clinical practicability during elective laparoscopic surgery of the lower abdomen] [Liberalisierte praoperative Flussigkeitskarenz: Patientenbefinden und klinische Praktikabilitat bei elektiven laparoskopischen Eingriffen im Unterbauch.]. Zentralblatt fur Chirurgie 2008;133(5):479‐85. [PUBMED: 18924048] [DOI] [PubMed] [Google Scholar]
Melis 2006 {published data only}
- Melis GC, Leeuwen PA, Blomberg‐van der Flier BM, Goedhart‐Hiddinga AC, Uitdehaag BM, Strack van Schijndel RJ, et al. A carbohydrate‐rich beverage prior to surgery prevents surgery‐induced immunodepression: a randomized, controlled, clinical trial. JPEN. Journal of Parenteral and Enteral Nutrition 2006;30(1):21‐6. [PUBMED: 16387895] [DOI] [PubMed] [Google Scholar]
Muehling 2009 {published data only}
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder‐Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast‐track patient care in elective open infrarenal aneurysm repair. World Journal of Surgery 2009;33(3):577‐85. [PUBMED: 19137363] [DOI] [PubMed] [Google Scholar]
Noblett 2004 {published data only}
Nygren 1995 {published data only}
- Nygren J, Thorell A, Jacobsson H, Larsson S, Schnell PO, Hylen L, et al. Preoperative gastric emptying. Effects of anxiety and oral carbohydrate administration. Annals of Surgery 1995;222(6):728‐34. [PUBMED: 8526579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nygren 1998 {published data only}
- Nygren J, Soop M, Thorell A, Efendic S, Nair KS, Ljungqvist O. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clinical Nutrition (Edinburgh, Scotland) 1998;17(2):65‐71. [PUBMED: 10205319] [DOI] [PubMed] [Google Scholar]
Nygren 1999 {published data only}
- Nygren J, Soop M, Thorell A, Sree Nair K, Ljungqvist O. Preoperative oral carbohydrates and postoperative insulin resistance. Clinical Nutrition (Edinburgh, Scotland) 1999;18(2):117‐20. [PUBMED: 10459075] [DOI] [PubMed] [Google Scholar]
Okabayashi 2010 {published data only}
- Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Namikawa T, Maeda H, et al. Preoperative oral supplementation with carbohydrate and branched‐chain amino acid‐enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids 2010;38(3):901‐7. [PUBMED: 19399583] [DOI] [PubMed] [Google Scholar]
Okabayashi 2011 {published data only}
- Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K. Oral supplementation with carbohydrate‐ and branched‐chain amino acid‐enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids 2011;40(4):1213‐20. [PUBMED: 20852905] [DOI] [PubMed] [Google Scholar]
Phillips 1993 {published data only}
- Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. British Journal of Anaesthesia 1993;70(1):6‐9. [PUBMED: 8431336] [DOI] [PubMed] [Google Scholar]
Power 2004 {published data only}
- Power H. Review: evidence is lacking that adults given fluids 1.5 to 3 hours preoperatively have greater risks of aspiration or regurgitation than those given a standard fast. Evidence‐Based Nursing 2004;7(2):44. [PUBMED: 15106594] [DOI] [PubMed] [Google Scholar]
Protic 2010 {published data only}
- Protic A, Bobinac M, Ivancic A, Zuvic‐Butorac M, Sustis A, Jakljevic T. Effect of preoperative feeding on gallbladder size and peristaltic of the small bowel following spinal anesthesia for the hip surgery. Collegium Antropologicum 2010;34(Suppl 2):195‐8. [PUBMED: 21305734] [PubMed] [Google Scholar]
Protic 2010a {published data only}
- Protic A, Turina D, Matanic D, Spanjol J, Zuvic‐Butorac M, Sustic A. Effect of preoperative feeding on gastric emptying following spinal anesthesia: a randomized controlled trial. Wiener Klinische Wochenschrift 2010;122(1‐2):50‐3. [PUBMED: 20177860] [DOI] [PubMed] [Google Scholar]
Schricker 2008 {published data only}
- Schricker T, Meterissian S, Lattermann R, Adegoke OA, Marliss EB, Mazza L, et al. Anticatabolic effects of avoiding preoperative fasting by intravenous hypocaloric nutrition: a randomized clinical trial. Annals of Surgery 2008;248(6):1051‐9. [PUBMED: 19092350] [DOI] [PubMed] [Google Scholar]
Serclova 2009 {published data only}
- Serclova Z, Dytrych P, Marvan J, Nova K, Hankeova Z, Ryska O, et al. [Tolerance of accelerated postoperative rehabilitation following intestinal resections] [Tolerance akcelerovane pooperacni rehabilitace po strevnich resekcnich vykonech]. Rozhledy v Chirurgii: Mesicnik Ceskoslovenske Chirurgicke Spolecnosti 2009;88(4):178‐84. [PUBMED: 19645142] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. European Journal of Anaesthesiology 2011;28(8):556‐69. [PUBMED: 21712716] [DOI] [PubMed] [Google Scholar]
Soop 2000 {published data only}
- Soop M, Carlson G. Perioperative nutritional support. CME Journal Gastroenterology, Hepatology and Nutrition 2000;3(3):99‐103. [Google Scholar]
Stuart 2006 {published data only}
- Stuart PC. The evidence base behind modern fasting guidelines. Best Practice & Research. Clinical Anaesthesiology 2006;20(3):457‐69. [PUBMED: 17080696] [DOI] [PubMed] [Google Scholar]
Svanfeldt 2007 {published data only}
- Svanfeldt M, Thorell A, Hausel J, Soop M, Rooyackers O, Nygren J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole‐body protein and glucose kinetics. The British Journal of Surgery 2007;94(11):1342‐50. [PUBMED: 17902094] [DOI] [PubMed] [Google Scholar]
Tanabe 1996 {published data only}
- Tanabe T, Hashimoto Y, Sugihara K, Miyata A, Maeda A, Ishihara H, et al. [The effect of preoperative oral fluid intake on the volume and pH of gastric contents in elective surgical patients—a comparison of tea with apple juice]. Masui. The Japanese Journal of Anesthesiology 1996;45(8):967‐70. [PUBMED: 8818093] [PubMed] [Google Scholar]
Taniguchi 2009 {published data only}
- Taniguchi H, Sasaki T, Fujita H, Takamori M, Kawasaki R, Momiyama Y, et al. Preoperative fluid and electrolyte management with oral rehydration therapy. Journal of Anesthesia 2009;23(2):222‐9. [DOI] [PubMed] [Google Scholar]
Thorell 1996 {published data only}
- Thorell A, Alston‐Smith J, Ljungqvist O. The effect of preoperative carbohydrate loading on hormonal changes, hepatic glycogen, and glucoregulatory enzymes during abdominal surgery. Nutrition (Burbank, Los Angeles County, Calif.) 1996;12(10):690‐5. [PUBMED: 8936492] [DOI] [PubMed] [Google Scholar]
Vincent 1991 {published data only}
- Vincent RD Jr, McNeil TJ, Spaid CL, MacMahon FR, Maxwell SJ, Brenner JS, et al. Does 360 ml of apple juice ingested before elective surgery worsen gastric volume and acidity in patients given acid aspiration prophylaxis?. Journal of Clinical Anesthesia 1991;3(4):285‐9. [PUBMED: 1910795] [DOI] [PubMed] [Google Scholar]
Wendel 2013 {published data only}
- Wendel K, Karlsson A, Polits S, Vilstrup C, Hedenbro J. Preoperative carbohydrate intake is coupled to increased nausea after gastric bypass. Obesity Surgery 2013;23(8):1228. [Google Scholar]
Wilson 1999 {published data only}
- Wilson D, Douglas J, Heid R, Rurak D. Preoperative dextrose does not affect spinal‐induced hypotension in elective Cesarean section. Canadian Journal of Anaesthesia 1999;46(11):1024‐9. [PUBMED: 10566921] [DOI] [PubMed] [Google Scholar]
Yagci 2008 {published data only}
- Yagci G, Can MF, Ozturk E, Dag B, Ozgurtas T, Cosar A, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 2008;24(3):212‐6. [PUBMED: 18096368] [DOI] [PubMed] [Google Scholar]
Zargar‐Shoshtari 2009 {published data only}
- Zargar‐Shoshtari K, Paddison JS, Booth RJ, Hill AG. A prospective study on the influence of a fast‐track program on postoperative fatigue and functional recovery after major colonic surgery. The Journal of Surgical Research 2009;154(2):330‐5. [PUBMED: 19118844] [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang H, Cui M‐M, Liu D‐S, Feng Y, Chen C‐S. Effect of fast track surgery on serum levels of CRP, IL‐6 and insulin resistance in patients with colorectal cancer. [Chinese]. World Chinese Journal of Digestology 2010;18(35):3813‐7. [Google Scholar]
References to studies awaiting assessment
Aguilar‐Nascimento 2012 {published data only}
- Aguilar‐Nascimento JE, Pexe‐Machado PA, Dock‐Nascimento DB, Oliveira BD. Abbreviation of preoperative fast with a beverage containing maltodextrin and pea protein hydrolysate to improve recovery from laparotomies for gastrointestinal malignancies. Clinical Nutrition Supplements 2012;7:163‐4. [Google Scholar]
Asakura 2013 {published data only}
- Asakura A, Goto T. Does quality of recovery improve by preoperative oral carbohydrate?. European Journal of Anaesthesiology 2013;30:29. [Google Scholar]
Forde 2012 {published data only}
- Forde CL, Farrer K, Meskell R, Anderson I, Slade D, Lees N, et al. Long term metabolic and clinical effects of preoperative carbohydrate loading. A prospective randomised clinical trial. Clinical Nutrition Supplements 2012;7:155‐6. [Google Scholar]
Jodlowski 2011 {published data only}
- Jodlowski T, Dobosz M, Noga M. Preoperative oral carbohydrate load in colorectal surgery reduces insulin resistance and may improve outcomes preliminary results of prospective randomized study. Clinical Nutrition Supplements 2011;6:134. [Google Scholar]
Ozer 2013 {published data only}
- Ozer Ayse B, Demirel I, Kavak Burcin S, Gurbuz O, Unlu S, Bayar Mustafa K, et al. Effects of preoperative oral carbohydrate solution intake on thermoregulation. Medical Science Monitor 2013;19:625‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsutsumi 2011 {published data only}
- Tsutsumi YM, Horikawa YT, Tsutsumi R, Tanaka K, Ohshita S. Effect of preoperative oral carbohydrate intake on respiratory metabolism during surgery. Clinical Nutrition Supplements 2011;6:188‐9. [Google Scholar]
Yilmaz 2013 {published data only}
- Yilmaz N, Cekmen N, Bilgin F, Erten E, Odie, Zhan MO, et al. Preoperative carbohydrate nutrition reduces postoperative nausea and vomiting compared to preoperative fasting. Journal of Research in Medical Sciences 2013;18(10):827‐32. [PMC free article] [PubMed] [Google Scholar]
Zelic 2013 {published data only}
- Zelic M, Stimac D, Mendrila D, Tokmadzic VS, Fisic E, Uravic M, et al. Preoperative oral feeding reduces stress response after laparoscopic cholecystectomy. Hepato‐Gastroenterology 2013;60(127):1602‐6. [PubMed] [Google Scholar]
Zhao 2013 {published data only}
- Zhao G, Cao S, Cui J. Effect of fast track surgery on insulin resistance indexes of esophageal cancer patients. Supportive Care in Cancer 2013;21:S62‐3. [Google Scholar]
Additional references
Awad 2013
- Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta‐analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clinical Nutrition (Edinburgh, Scotland) 2013;32(1):34‐44. [PUBMED: 23200124] [DOI] [PubMed] [Google Scholar]
Brady 2003
- Brady MC, Kinn S, Stuart P, Ness V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004423; PUBMED: 14584013] [DOI] [PubMed] [Google Scholar]
Desborough 2000
- Desborough JP. The stress response to trauma and surgery. British Journal of Anaesthesia 2000;85(1):109‐17. [PUBMED: 10927999] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gouvas 2009
- Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast‐track vs standard care in colorectal surgery: a meta‐analysis update. International Journal of Colorectal Disease 2009;24(10):1119‐31. [PUBMED: 19415308] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 . www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 1997
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia 1997;78(5):606‐17. [PUBMED: 9175983] [DOI] [PubMed] [Google Scholar]
Li 2012
- Li L, Wang Z, Ying X, Tian J, Sun T, Yi K, et al. Preoperative carbohydrate loading for elective surgery: a systematic review and meta‐analysis. Surgery Today 2012;42(7):613‐24. [PUBMED: 22581289] [DOI] [PubMed] [Google Scholar]
Ljungqvist 2003
- Ljungqvist O, Soreide E. Preoperative fasting. The British Journal of Surgery 2003;90(4):400‐6. [PUBMED: 12673740] [DOI] [PubMed] [Google Scholar]
meta [Computer program]
- Guido Schwarzer. meta: Meta‐Analysis with R. Version 1.6‐1. R Foundation for Statistical Computing, 2010.
Muniyappa 2008
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. American Journal of Physiology ‐ Endocrinology and Metabolism 2008;294(1):E15‐26. [DOI] [PubMed] [Google Scholar]
Nygren 2006
- Nygren J. The metabolic effects of fasting and surgery. Best Practice & Research. Clinical Anaesthesiology 2006;20(3):429‐38. [PUBMED: 17080694] [DOI] [PubMed] [Google Scholar]
Pouwer 2000
- Pouwer F, Snoek FJ, Ploeg HM, Ader HJ, Heine RJ. The well‐being questionnaire: evidence for a three‐factor structure with 12 items (W‐BQ12). Psychological Medicine 2000;30(2):455‐62. [PUBMED: 10824665] [DOI] [PubMed] [Google Scholar]
R 2.13.2 [Computer program]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2011.
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 20011.
Wilmore 2002
- Wilmore DW. From Cuthbertson to fast‐track surgery: 70 years of progress in reducing stress in surgical patients. Annals of Surgery 2002;236(5):643‐8. [PUBMED: 12409671] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Smith 2011
- Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD009161] [DOI] [PMC free article] [PubMed] [Google Scholar]


