Abstract
The year 2024 marks 70 years since the general outline of the carbon pathway in photosynthesis was published. Although several alternative pathways are now known, it is remarkable how many organisms use the reaction sequence described 70 yrs ago, which is now known as the Calvin–Benson cycle or variants such as the Calvin–Benson–Bassham cycle or Benson–Calvin cycle. However, once the carbon has entered the Calvin–Benson cycle and is converted to a 3-carbon sugar, it has many potential fates. This review will examine the last stages of photosynthetic metabolism in leaves. In land plants, this process mostly involves the production of sucrose provided by an endosymbiont (the chloroplast) to its host for use and transport to the rest of the plant. Photosynthetic metabolism also usually involves the synthesis of starch, which helps maintain respiration in the dark and enables the symbiont to supply sugars during both the day and night. Other end products made in the chloroplast are closely tied to photosynthetic CO2 assimilation. These include serine from photorespiration and various amino acids, fatty acids, isoprenoids, and shikimate pathway products. I also describe 2 pathways that can short circuit parts of the Calvin–Benson cycle. These final processes of photosynthetic metabolism play many important roles in plants.
Photosynthesis makes mostly starch and sucrose, but additional metabolic pathways involved in the last steps of photosynthesis are described here.
Advances box.
The last steps of photosynthetic carbon metabolism in the endosymbiont chloroplast provide important precursors for plants.
The last steps of photosynthetic carbon metabolism are essential in order to release phosphate from the organic phosphate pool for reuse in ATP.
Photorespiration can make serine as an end product of photosynthetic carbon metabolism although the estimates of 40% serine export seem high.
A stromal G6P shunt involving the oxidative pentose phosphate pathway consumes carbon from the Calvin–Benson cycle and consumes ATP. It is normally not functioning during the day.
A cytosolic G6P shunt reinjects free glucose, fructose, and sucrose into the Calvin–Benson cycle helping it refill very quickly after periods of low light.
Introduction
The phrase “path of carbon in photosynthesis” was used in the titles of 22 papers published in the 1940s and 1950s, plus 2 books, and Melvin Calvin's Nobel Lecture (Calvin 1964). The most consequential is the 21st such paper (Bassham et al. 1954), which will be 70 years old in 2024 and is referred to here as “paper 21.” This paper has been cited over 320 times (as of August 9, 2023) but laid the groundwork for many thousands of papers. The achievement of Bassham, Benson, and Calvin has been extensively documented (e.g. Nickelsen 2012; Sharkey 2019). Like the Krebs/tricarboxylic acid (TCA)/citric acid cycle, the scheme in paper 21 goes by various names, but here, I will refer to it as the Calvin–Benson cycle. Much current interest in this cycle focuses on sedoheptulose bisphosphatase (SBPase) (Lefebvre et al. 2005; Zhu et al. 2007; Rosenthal et al. 2011; Driever et al. 2017; Simkin et al. 2017). This enzyme is used in preference to transaldolase normally found in the nonoxidative pentose phosphate pathway, which has the effect of directing carbon from trioses to pentoses rather than from pentoses to hexoses and trioses, as is normally depicted for the transaldolase-dependent pentose phosphate pathway (Sharkey 2021)
In 1954, when paper 21 was published, almost nothing was known about how carbon enters the cycle, except that there are 3 sources of pentose and that a pentose (now called RuBP) is carboxylated to generate 3-phosphoglycerate (3-PGA). The carboxylase eventually became known as rubisco (Portis and Parry 2007; Sharkey 2022). A big question about photosynthetic carbon metabolism remained: what are the last steps as carbon leaves the cycle? While paper 21 did not track the phosphorylation status of the intermediates, it became clear that ATP drives the cycle forward. Therefore, the phosphate added to make RuBP must be released so that ATP can be regenerated. All the phosphate added by phosphoglycerate kinase is immediately released by glyceraldehyde 3-phosphate dehydrogenase. One third of the phosphate added by phosphoribulokinase is released by fructose bisphosphatase (FBPase) and one third by SBPase, but the last third must be released during end-product synthesis. This sets a reasonable boundary for photosynthetic carbon metabolism: it begins with CO2 and ends only when an equivalent amount of carbon in intermediates is dephosphorylated.
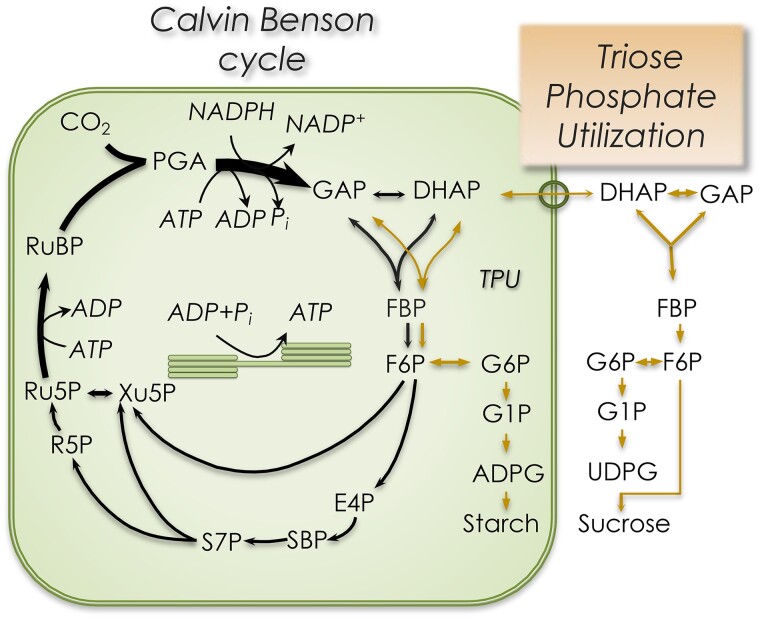
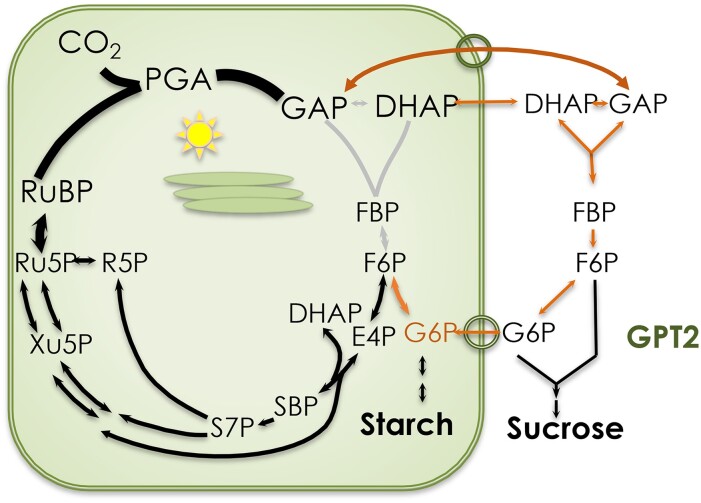
Glucose is often regarded as the ultimate product of photosynthetic carbon metabolism, but unphosphorylated glucose only occurs in part of the starch utilization pathway. The bulk of carbon fixed in photosynthesis ends up as either starch or sucrose (Fig. 1). If starch is made, all photosynthetic carbon metabolism is said to occur inside chloroplasts. However, as an endosymbiont, the chloroplast supplies the host cell with reduced carbon, and starch synthesis capacity is typically not sufficient to account for the high rates of CO2 fixation that could be observed using isolated intact chloroplasts (Walker and Herold 1977).
Figure 1.
The carbon dioxide that enters the Calvin–Benson cycle is metabolized primarily to starch and sucrose (lines on right side of the figure). Since both sucrose and starch synthesis can be readily traced back to the triose phosphates, this is often called triose phosphate utilization. PGA, 3-phosphoglycerate; TPI, triose phosphate isomerase; GAP, glyceraldehyde 3-phosphate (distinguished from glycerol 3-phosphate denoted G3P); DHAP, dihydroxyacetone phosphate; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; FBP, fructose 1,6-bisphosphate; E4P, erythrose 4-phosphate; glyco-TDP, glycoaldehyde thiamine diphosphate; SBP, sedoheptulose 1,7-bisphosphate; S7P, sedoheptulose 7-phosphate; R5P, ribose 5-phosphate; Xu5P; xylulose 5-phosphate; Ru5P, ribulose 5-phosphate.
Sucrose metabolism
Carbon export from chloroplasts during CO2 fixation depends on the obligatory export of triose phosphate and the import of inorganic phosphate (Heldt et al. 1977; Fliege et al. 1978). Phosphate and triose phosphate exchange occurs at a triose phosphate/phosphate antiporter (TPT), an abundant protein on the inner envelope of the chloroplast and a member of a family of related phosphate antiporters (Bockwoldt et al. 2019). The evolution of these antiporters is thought to have been an essential step to establishing the endosymbiotic nature of chloroplasts (Weber et al. 2006; Linka and Weber 2010). The export of triose phosphate supports the synthesis of sucrose in the cytosol of plant cells (Bird et al. 1974). As sucrose is a phosphate-free molecule, its production marks the end of photosynthetic carbon metabolism.
Sucrose synthesis involves the formation of sucrose 6-phosphate from fructose 6-phosphate (F6P) and uridine diphosphate (UDP)-glucose by the enzyme sucrose-phosphate synthase (SPS). The sucrose phosphate is readily broken down to sucrose by SPS. Sucrose-phosphate synthase is highly regulated, especially by phosphorylation (Huber and Huber 1996; Hardin et al. 2003). Several sites can be phosphorylated with different effects, and it is dephosphorylated by SPS protein phosphatase, a type 2A protein phosphatase (Huber and Huber 1996). This regulation also involves a 14-3-3 protein; such proteins are often involved in regulating phosphorylation (Bachmann et al. 1996). While SPS activity is lower and more sensitive to metabolite concentrations at night, it is not turned off completely (Jones and Ort 1997), allowing sucrose synthesis to occur during both day and night.
Sucrose is a disaccharide that plays several central roles in plants (Salerno and Curatti 2003). A similar disaccharide, trehalose, is more widespread phylogenetically. The answer to the question “why sucrose?” is unclear (Salerno and Curatti 2003). In plants, trehalose metabolism appears to be specialized for carbohydrate status signaling, especially trehalose 6-phosphate (Lastdrager et al. 2014; Fichtner and Lunn 2021; Peixoto et al. 2021) and its interactions with sucrose nonfermenting-related kinase (SnRK) and target of rapamycin (TOR) regulatory mechanisms (Jamsheer et al. 2019; Ryabova et al. 2019; Sharma et al. 2022).
Starch metabolism
The identification of the TPT explained carbon export from the chloroplast during the day, but what about carbon export at night? Starch accumulates throughout the day in most plants. At low rates of photosynthesis, most carbon is partitioned to sucrose, but as the photosynthetic rate increases, due to either increased light or increased CO2 availability, more and more carbon is partitioned to starch (Sharkey et al. 1985; Mullen and Koller 1988). The ratio of starch to sucrose is higher in plants growing in short days (Sulpice et al. 2014; Xu et al. 2023) and can be low when leaves are incubated at low temperature (Pollock and Lloyd 1987). The ratio of partitioning to starch versus sucrose can be regulated by activity of SPS (Galtier et al. 1993). Increasing SPS activity by transforming tomato (Solanum lycopersicum) with an SPS gene from maize (Zea mays) increased the proportion of carbon going to sucrose and decreased the proportion going to starch (Fig. 2, redrawn from Laporte (1997)). The starch/sucrose ratio declined with increasing temperature in both the transformed line and the controls.
Figure 2.
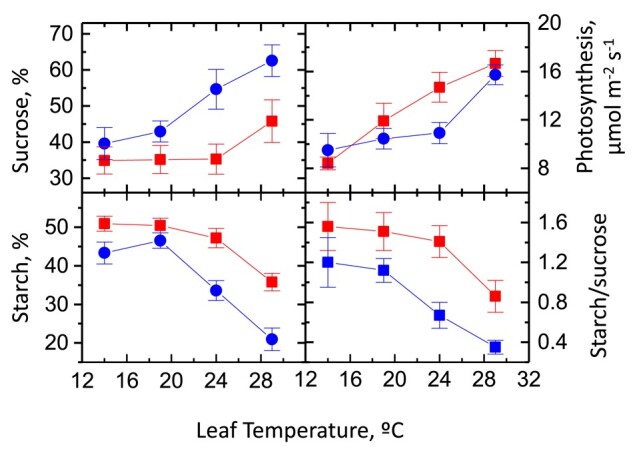
Sucrose and starch synthesis as a function of temperature in tomato with and without a SPS gene from maize. Squares are data from transformed plants, and circles are from untransformed plants. The proportion of sucrose synthesis from CO2 fixation increases with temperature, while starch synthesis declines. The SPS gene had little effect on the rate of photosynthesis, which increased over a temperature range of 14 °C to 29 °C. Data are from Laporte (1997).
Another critical control point for sucrose synthesis is the ratio of FBPase activity in the stroma to that in the cytosol. In the stroma, this regulation involves light activation, among other mechanisms. In the cytosol, FBPase activity is regulated by fructose 2,6-bisphosphate (Stitt 1990). F6P is phosphorylated and dephosphorylated at the 2 position, a process that integrates many different signals. The regulation of FBPase, and its mirror image, i.e. the regulation of phosphofructokinase, prevents a futile cycle in which ATP is used to convert F6P to F1,6BP, and FBPase removes that phosphate, consuming ATP. Regulation involving fructose 2,6-bisphosphate ensures that these reactions are occurring in only 1 direction, toward sucrose during photosynthesis and consuming glucose only when photosynthesis is not occurring. The regulation at FBPase is more complete than the regulation of SPS so that at night, sucrose cannot be produced from triose phosphates but it can be produced from hexose phosphates.
Starch breakdown then supplies the plant with sugars at night. Starch is broken down at a constant rate at night; more than 90% of the carbon present in starch at the end of the day is released from starch at night (Lu et al. 2005; Pokhilko et al. 2014; Flis et al. 2019). As a result, the chloroplast supplies reduced carbon to the host at an almost constant rate day and night (Sulpice et al. 2014).
It was assumed that at night, the sugars in starch broke down to triose phosphates, which were then exported on the TPT. However, deuterium labeling showed that when starch breaks down and is converted to sucrose, the carbon skeletons are never broken down to triose phosphates (Schleucher et al. 1998). Instead, maltose, the product of β-amylase, is the primary export sugar resulting from starch breakdown (Weise et al. 2004). Initial attempts to show a gradient for maltose export were unsuccessful, leading to the finding that β-maltose is the primary exported sugar. β-maltose, but not total maltose, shows a significant gradient in favor of export from chloroplasts (Weise et al. 2005).
β-amylase cleaves 2 glucose residues from α-1,4 maltodextrins (glucose chains) to generate β-maltose. However, this enzyme cannot work on maltotriose, only longer maltodextrins. To finish breaking down starch remnants, an enzyme called disproportionating enzyme 1 (DPE1) (1 for the plastidial form) uses maltotriose in this reaction:
where Glu3 is maltotriose and Glu5 is maltopentaose. Other reengagements are possible, but the net effect is that maltodextrins can be broken down primarily to produce maltose but some glucose as well. Glucose in the chloroplast can exit through a glucose transporter, which was first reported in 2000 (Weber et al. 2000). A maltose transporter was discovered soon after (Niittylä et al., 2004). Once in the cytosol, the maltose is acted on by DPE2 (Chia et al. 2004; Lu and Sharkey 2004) (Fig. 3). DPE2 is similar to DPE1 but contains a 150 amino acid insert (Steichen et al. 2008). However, unlike DPE1, DPE2 transfers the nonreducing glucose to a soluble heteroglycan (SHG) (Fettke et al. 2006). This large-molecular-weight polysaccharide primarily comprises arabinose, galactose, ribose, and some glucose (Yang and Steup 1990). The glucose transferred to the heteroglycan can be liberated by phosphorolysis. In this way, the energy in the glucose–glucose bond of maltose can be conserved in glucose 1-phosphate (G1P), which can be isomerized to glucose 6-phosphate (G6P) or used to make UDP-glucose.
Figure 3.
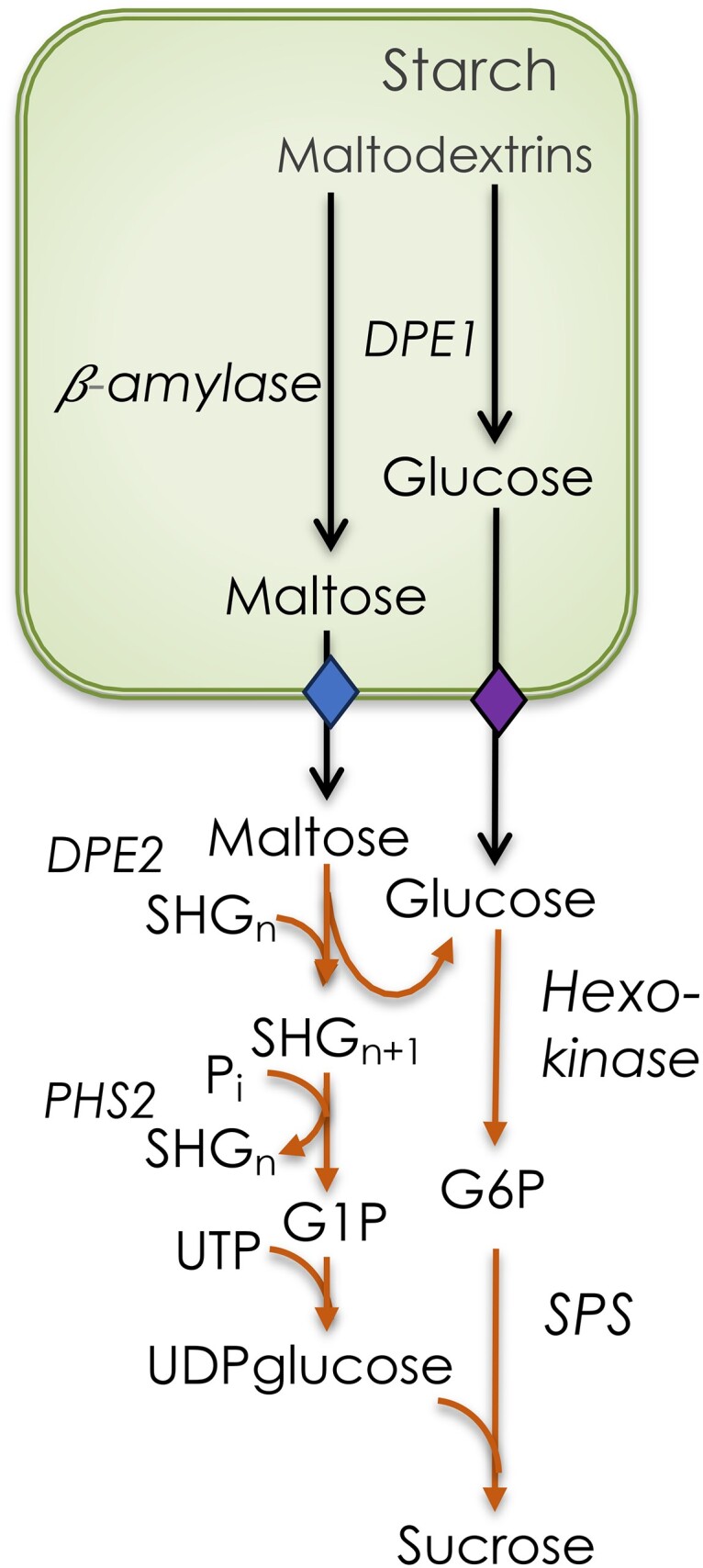
Conversion of starch to sucrose at night. Various starch-degrading enzymes break the starch down to linear maltodextrins, which are hydrolyzed by β-amylase to generate β-maltose. Once the maltodextrin reaches a degree of polymerization of 3, it is acted on by DPE1. Mostly maltose, but some glucose too, leaves the chloroplast through specific transporters. Maltose is acted on by disproportionating enzyme 2 (DPE2) to add 1 glucose to a SHG and glucose. Starch phosphorylase 2 (PHS2) removes a glucose from the SHG to produce G1P. The glucose is acted on by hexokinase to make G6P. SPS generates sucrose phosphate, which is then dephosphorylated to sucrose.
Currently, there is much interest in how starch breakdown is regulated. The remarkably constant rate of starch breakdown at night has attracted much attention (Lu et al. 2005; Graf et al. 2010; Scialdone et al. 2013; Fernandez et al. 2017; Mengin et al. 2017). Transitory starch is phosphorylated by a glucan water dikinase (Ritte et al. 2002) and phosphoglucan water dikinase (Kötting et al. 2005). Without this phosphorylation, starch becomes more difficult to break down. Some plants accumulate nontransitory starch as they age (Chu et al. 2022), possibly due to the accumulation of unphosphorylated starch, which is no longer accessible to the plant, over wks or mos.
Photorespiration
It was recently hypothesized that photorespiration can provide some capacity for end-product synthesis in the form of glycine and serine (Harley and Sharkey 1991; Busch et al. 2018; Fu et al. 2023). It has long been recognized that O2 inhibits photosynthesis. This is sometimes called the (green) Warburg effect (Kutschera et al. 2020); the red effect is related to cancer metabolism (Liberti and Locasale 2016). Rabinowitch (1945) used the term “photorespiration” to describe metabolism that begins upon illumination and ends upon darkness. Decker (1955) described a postillumination burst of CO2 release from leaves believed to result from the light-dependent process that releases CO2. Wilson and Calvin (1955) found that glycolate was produced in their reactions and that this could be suppressed by using 1% CO2. Bassham and Kirk (1962) established that the production of glycolate (and glycine) could be affected by O2. However, the major advance in the field of photorespiration was the discovery that the CO2-fixing enzyme rubisco also fixes O2 (Bowes et al. 1971; Ogren and Bowes 1971). The 2-phosphoglycolate formed in this reaction is a potent inhibitor of triose phosphate isomerase (Anderson 1971; Flügel et al. 2017; Li et al. 2019). Phosphoglycolate also inhibits phosphofructokinase (Kelly and Latzko 1976), but at higher concentrations. Phosphofructokinase inside chloroplasts is not operational during photosynthesis when FBPase is functioning. NADPH provides 1 mechanism for ensuring that phosphofructokinase is not active in the light (Cséke et al. 1982). The pathway for metabolizing 2-phosphoglycolate formed by the oxygenation of RuBP was proposed by Tolbert (1971).
In the view of photorespiration presented above, this process is not a method for end-product synthesis, since all the carbon that enters the pathway is either released as CO2 or rephosphorylated to reenter the Calvin–Benson cycle. However, for many years, it appeared as though photorespiration could add capacity for end-product synthesis. An early example was reported by Jolliffe and Tregunna (1973), who showed that under high CO2 and low temperature conditions, the photosynthetic rate under low O2 conditions (low photorespiration) was lower than the rate in 21% O2. Sharkey (1985) proposed that when end-product synthesis limits photosynthesis, CO2 assimilation becomes insensitive to CO2 and O2 (in other words, photorespiration does not decrease the rate of CO2 assimilation). We now know that photorespiration in fact increases the capacity for photosynthesis when starch and sucrose synthesis rates are maximal. This may be explained by the hypothesis that glycine, and more likely serine, leaves photorespiratory metabolism, providing both reduced nitrogen and carbon for use in leaf metabolism (Busch et al. 2018). If this process occurs at a rate proportional to the rate of photorespiration, and if end-product synthesis sets the upper bound of CO2 assimilation, then photorespiration increases the upper bound. As a result, as CO2 levels increase and the rate of photorespiration decreases, the overall rate of CO2 assimilation will also decrease.
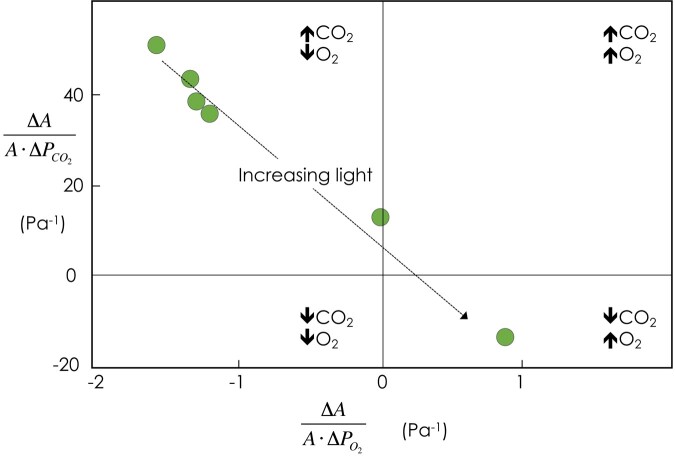
Under low light conditions, increasing CO2 or decreasing O2 levels stimulate photosynthesis, as would be expected if the rate of photorespiration is reduced (Fig. 4). However, as light levels increase, the upper bound of the rate of CO2 assimilation falls under the control of end-product synthesis, and increased CO2 or decreased O2 levels reduce photorespiration, thereby reducing the overall rate of CO2 assimilation. The value of 30% to 40% of carbon diverted from 2-phosphoglycolate to serine found from curve fitting (Busch et al. 2018), and isotopically non–steady-state mass flux analysis (Fu et al. 2023) seems high. It is possible that other metabolisms occur that have the same effect as serine export for nitrogen metabolism.
Figure 4.
Assimilation-weighted sensitivity to CO2 (vertical axis) and O2 (horizontal axis) as affected by increasing light levels. At limiting light levels, the energy cost of photorespiration results in a reduction in CO2 assimilation capacity. As light levels increase, the upper bound becomes sensitive to end-product synthesis. Photorespiration stimulates CO2 assimilation by adding end-product synthesis capacity in the form of serine export. Redrawn from Sharkey (1990). Arrows indicate the response of photosynthesis to the indicated gases.
While serine export can explain many observations of reversed sensitivity, some observations are too extreme to be accounted for by serine export from photorespiration. Sharkey and Vassey (1989) determined that the rate of CO2 assimilation decreased by 20% upon switching from 200 to 20 kPa O2. This decline was almost entirely the result of a reduced rate of starch synthesis. The amount of stromal G6P fell by 73%, likely due to the inhibition of phosphoglucoisomerase (PGI). This enzyme is known to be out of equilibrium in the stroma (Gerhardt et al. 1987; Schleucher et al. 1999) and can be inhibited by some Calvin–Benson cycle intermediates, especially erythrose 4-phosphate (Preiser et al. 2020). In this case, the reversed sensitivity to O2 is not a function of increased end-product synthesis capacity associated with photorespiration. Instead, it is a function of a metabolic “traffic jam” in which increases in the levels of some Calvin–Benson cycle intermediates reduce the capacity for end-product (in this case starch) synthesis.
There is currently substantial interest in devising alternative pathways for metabolizing 2-phosphoglycerate, the metabolite that initiates photorespiration (Peterhänsel and Maurino 2011; Peterhänsel et al. 2013; Dalal et al. 2015; Xin et al. 2015; Engqvist and Maurino 2017; South et al. 2019). On the other hand, some researchers believe that photorespiration plays an important role in nitrogen metabolism (Rachmilevitch et al. 2004; Bloom 2015; Busch et al. 2018) and that plants experience evolutionary pressure to maintain photorespiration.
The lower glycolysis metabolism of chloroplasts
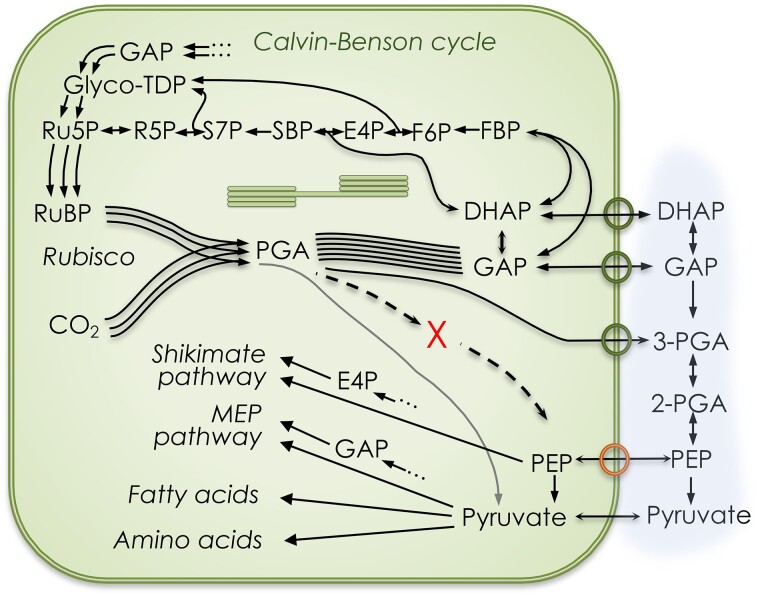
The reduction step and several steps in the regeneration portion of the Calvin–Benson cycle are gluconeogenic, that is, the reverse of glycolysis, from 3-PGA to F6P and G6P. The lower branch of glycolysis from PGA to phosphoenolpyruvate (PEP) appears to be absent from photosynthesizing chloroplasts. Phosphoglyceromutase and enolase activities were found to be <1% of the activities in the cytosol (Stitt and ap Rees 1979; Schulze-Siebert et al. 1987; Hoppe et al. 1993) (X in Fig. 5). The TPT (circles on the chloroplast membrane in Fig. 5) exchanges phosphate, dihydroxyacetone phosphate (DHAP), glyceraldehyde 3-phosphate (GAP), and PGA. The metabolism in the stroma and cytosol determines the concentration gradients of these 4 molecules, and they are exchanged based on their concentration gradients. A triose phosphate isomerase can isomerize DHAP and GAP, but whole-leaf triose phosphate concentrations are not in isomerase equilibrium (Li et al. 2019). A nonphosphorylating glyceraldehyde 3-phosphate dehydrogenase (GAPN) that converts GAP to PGA is present and can provide NADPH in the cytosol. This reaction is irreversible. There are also phosphorylating GAPD enzymes, i.e. GAPC1 and GAPC2 (Guo et al. 2014). The amount of PGA that can be converted to GAP depends on the energetic status of the cytosol.
Figure 5.
Chloroplast metabolism emphasizing the lower glycolytic pathway. The TPT (circles on the chloroplast membrane) exchanges DHAP, GAP, and PGA. The PEP/phosphate antiporter exchanges PEP for phosphate. The production of PEP inside the chloroplast is restricted by very low phosphoglyceromutase and enolase activities (denoted with X). Pyruvate is generated at a rate of ∼0.5% of total carboxylations, directly providing pyruvate for chloroplast reactions (line connecting rubisco to pyruvate). Additional products of the Calvin–Benson cycle include shikimate pathway products (e.g. aromatic amino acids), isoprenoids, and pentoses. See Raines (2011, 2022) (Fig. 1) for a more complete depiction of export sites from the Calvin–Benson cycle.
Several biosynthetic pathways require PEP (e.g. the shikimate pathway and its many products). PEP is imported from the cytosol by a PEP/Pi antiporter (bottom ring on the chloroplast membrane in Fig. 5). When the antiporter is absent, as in the Arabidopsis (Arabidopsis thaliana) cue1 (chlorophyll a/b binding protein [CAB] underexpressors) mutants, the plants do not grow well (Voll et al. 2003). Voll et al. (2003) found that the cue1 mutant could be rescued by either expressing a gene encoding a PEP/Pi antiporter (PPT) or by expressing a pyruvate phosphate dikinase (PPDK) gene in the stroma. The finding that expressing a PPT gene in the mutant background rescued this mutant indicates that the chloroplast does not have sufficient phosphoglycerate mutase to support the requirement for PEP in the chloroplast.
The reaction from PEP to pyruvate catalyzed by pyruvate kinase is not easily reversed, but the PPDK reaction is energetically favorable because it, in essence, uses the energy of 2 ATPs. The rescue of the cue1 mutant by PPDK means that there is sufficient pyruvate to satisfy the need for PEP inside the chloroplast. Thus, either pyruvate is transported into the chloroplast or the small amount of pyruvate made by rubisco (Andrews and Kane 1991) is sufficient for the plant's needs.
Other chloroplast-localized metabolic pathways require pyruvate, e.g. the methylerythritol 4-phosphate (MEP) pathway (the source of carotenoids among other classes of molecules), fatty acid synthesis, and the synthesis of branched chain amino acids. There are 4 possible sources of pyruvate in the chloroplast stroma: PEP that enters through the PPT could be converted to pyruvate by pyruvate kinase inside the chloroplast, pyruvate could be imported from the cytosol by passive permeation, pyruvate could be transported by the BASS2 importer (Furumoto et al. 2011), or pyruvate could be supplied as a side reaction of rubisco (Andrews and Kane 1991). One source of cytosolic pyruvate is malic enzyme acting on malate from the vacuole.
The pyruvate paradox
When 13CO2 is fed to leaves, intermediates of the Calvin–Benson cycle are labeled very quickly at first, but the rate of labeling slows considerably when 80% to 90% of the intermediates are labeled. All the intermediates of the Calvin–Benson cycle are labeled with very similar kinetics (Szecowka et al. 2013; Ma et al. 2014; Xu et al. 2021; Xu et al. 2022). This includes 2-PGA (Szecowka et al. 2013), which should only be present in the cytosol, not the chloroplast, and PEP (Hasunuma et al. 2010; Ma et al. 2014; Xu et al. 2022), which should mix with any PEP in the cytosol. However, pyruvate, which should be produced from PEP, is labeled much more slowly (Szecowka et al. 2013; Xu et al. 2022) than all the other molecules shown in Fig. 5. Although whole-leaf pyruvate is relatively unlabeled after 20 min, isoprene, 40% of which is derived from pyruvate, is labeled to the same degree as Calvin–Benson cycle intermediates (Delwiche and Sharkey 1993; Sharkey et al. 2020), indicating that the metabolically active pyruvate pool is labeled to the same degree as Calvin–Benson cycle intermediates. Perhaps a very large amount of pyruvate in the vacuole is labeled very slowly, masking the very rapid labeling of a small, metabolically active pyruvate pool in the chloroplast (and presumably also in the cytosol). It is possible that pyruvate is generated in the chloroplast by rubisco (Andrews and Kane 1991) and that this rubisco-derived pyruvate is heavily labeled and is used preferentially in the MEP pathway and presumably for fatty acid synthesis and other stromal reactions requiring pyruvate.
In summary, the intermediates of the Calvin–Benson cycle, starch synthesis precursors (e.g. ADP-glucose) and both PEP and pyruvate, constitute a large metabolically active pool of carbon that is labeled with similar kinetics. Of all these molecules, only pyruvate is also present in a metabolically inactive pool.
Shunts that bypass Calvin–Benson cycle reactions
The radioactive isotope 14C (Ruben and Kamen 1940) played a critical role in elucidating the carbon pathway in photosynthesis. However, when 99+% labeled CO2 is fed to photosynthesizing leaves, Calvin–Benson cycle intermediates do not become fully labeled (Mahon et al. 1974; Hasunuma et al. 2010; Szecowka et al. 2013; Ma et al. 2014), indicating that either nonmetabolic pools of most Calvin–Benson cycle intermediates are present or a source of unlabeled carbon enters the cycle.
A model of photosynthetic carbon metabolism has been used in metabolic flux analysis that includes oxidative pentose phosphate pathway reactions forming a shunt that bypasses a substantial portion of the Calvin–Benson cycle (Xu et al. 2022). This model (Fig. 6) can account for the lack of complete labeling and a nonphotorespiratory CO2 release known as day respiration (Rd) or respiration in the light (RL).
Figure 6.
Model of photosynthetic carbon metabolism including sources of unlabeled carbon that is imported into the Calvin–Benson cycle. The import of unlabeled carbon that enters as hexose slows the rate of labeling of Calvin–Benson cycle intermediates. The G6P shunt also releases moderately labeled CO2 that can be detected as 12CO2 emitted into a 99+% 13CO2 atmosphere. Abbreviations as in Fig. 1 plus 6PGL, 6-phosphoglucanolactone; 6PG, 6-phosphogluconate; XPT, xylulose 5-phosphate/phosphate antiporter (also transports Ru5P). A detailed flux map based on this model is available in Xu et al. (2022).
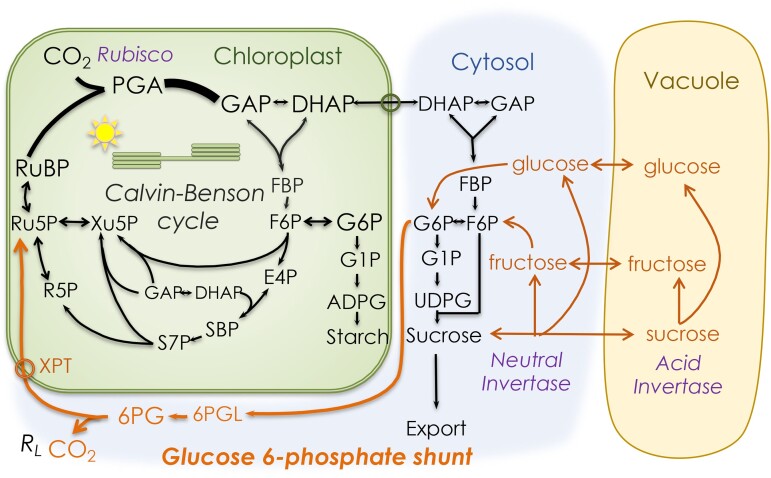
The G6P shunt occurring in the cytosol appears to operate continuously and at a reasonably constant rate, as assessed by measuring RL (Tcherkez et al. 2017; Schmiege et al. 2023). A similar shunt can operate in the stroma, but this appears to occur only in response to stress (Sharkey et al. 2020), although a recent report describes a very large stromal shunt that operates under either low or high CO2 levels (Wieloch et al. 2022). A different shunt can be induced to bypass missing enzymes in the Calvin–Benson cycle. This shunt involves GAP leaving the chloroplast via the TPT, its conversion to G6P, and its reimport through an inducible G6P transporter called GPT2 (Kammerer et al. 1998).
Ordinarily, there is no exchange of G6P across the chloroplast envelope, and large gradients of G6P between the stroma and cytosol have been reported (Gerhardt et al. 1987; Sharkey and Vassey 1989; Schleucher et al. 1998; Schleucher et al. 1999). Keeping the G6P level low in the chloroplast has the advantage of preventing stimulation of the stromal G6P dehydrogenase (G6PD), which would lead to a futile cycle. The stromal G6PD is normally inactive during the day due to the thioredoxin-dependent reduction of a disulfide bridge (Wenderoth et al. 1997; Née et al. 2009; Née et al. 2014; Cardi et al. 2016; Yoshida et al. 2019). However, the deactivation is mostly the result of an increase in Km rather than a reduction in overall capacity (Scheibe et al. 1989; Née et al. 2014). In addition, G6P can overcome the redox regulation of G6PD (Cossar et al. 1984; Preiser et al. 2019). Therefore, G6PD activity in the stroma can be very sensitive to G6P concentration. This G6P sensitivity likely explains why regulatory mechanisms are in place to limit the G6P concentration in the stroma, including the lack of G6P exchange with the cytosol and the limited capacity at the stromal PGI. However, plants lacking otherwise essential enzymes such as stromal triose phosphate isomerase, aldolase, and FBPase can survive by making use of the cytosolic versions of these enzymes and letting G6P back into the stroma through the inducible GPT2 (Fig. 7). The low capacity of PGI will cause G6P to build up, stimulating the futile G6P shunt in the stroma. This leads to cyclic electron flow to make up for the ATP lost in the futile cycle. Therefore, although there is evidence for the cytosolic bypass mechanism described here (Gotoh et al. 2010; Livingston et al. 2010; Li et al. 2019), plants that depend on this bypass mechanism are generally compromised.
Figure 7.
Mechanism for the cytosolic bypass of critical enzymes of the Calvin–Benson cycle. Triose phosphate isomerase, aldolase, and FBPase can be bypassed when the G6P transporter GPT2 is induced. However, the low capacity of PGI causes G6P to build up in the stroma, ultimately activating stromal G6PD, causing a futile cycle that consumes ATP. The ATP is replaced by cyclic electron flow, which has been observed in plants carrying out cytosolic bypass.
Conclusion
The core of photosynthetic carbon metabolism is the Calvin–Benson cycle. To keep this cycle going, end products must be generated that result in the release of phosphate for reuse in ATP. Chloroplasts produce many end products. The rates of sucrose, starch, and serine synthesis (from photorespiration) can affect the gas exchange behavior of leaves. These processes, plus some bypass mechanisms, are the end game of photosynthetic CO2 fixation, but they mark the beginning of the next phase, such as using the resources for maintenance, to build the plant, to flower, and to reproduce. The production of end products of photosynthesis also represents a starting point for designing ways to modify photosynthesis to enhance food, fuel, and fiber production for human use. There has been substantial progress in understanding the end-product metabolism of photosynthesis, but regulation of G6P in the stroma and the pyruvate paradox remain important areas for research (Outstanding questions box).
Outstanding questions box.
The apparent large flux of serine out of photorespiration requires additional study. What is the fate of all of that serine?
G6P in the chloroplast appears to be strongly controlled by regulation of G6P isomerase. How this regulation occurs and whether it can be modified to improve how photosynthesis meets human interests is an important area for research.
Solving the pyruvate paradox also is an outstanding concern that should be investigated. The ability of rubisco to make pyruvate has been reported once but needs to be independently verified, and then, the consequences of this pyruvate production need additional study.
Author contributions
T.D.S. conceived the idea and wrote the paper.
Funding
The ideas presented here draw on my photosynthesis research, which is supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, of the U.S. Department of Energy (grant DE-FG02-91ER20021), and my isoprene research, which is supported by U.S. National Science Foundation, award number IOS-2022495. I also received partial salary support from Michigan AgBioResearch.
Data availability
No new data were created for this work.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Anderson LE. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971:235(1):237–244. 10.1016/0005-2744(71)90051-9 [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Kane HJ. Pyruvate is a by-product of catalysis by ribulosebisphosphate carboxylase/oxygenase. J Biol Chem. 1991:266(15):9447–9452. 10.1016/S0021-9258(18)92841-3 [DOI] [PubMed] [Google Scholar]
- Bachmann M, Huber JL, Athwal GS, Wu K, Ferl RJ, Huber SC. 14-3-3 proteins associate with the regulatory phosphorylation site of spinach leaf nitrate reductase in an isoform-specific manner and reduce dephosphorylation of Ser-543 by endogenous protein phosphatases. FEBS Lett. 1996:398(1):26–30. 10.1016/S0014-5793(96)01188-X [DOI] [PubMed] [Google Scholar]
- Bassham JA, Benson AA, Kay LD, Harris AZ, Wilson AT, Calvin M. The path of carbon in photosynthesis. XXI. The cyclic regeneration of carbon dioxide acceptor. J Am Chem Soc. 1954:76(7):1760–1770. 10.1021/ja01636a012 [DOI] [Google Scholar]
- Bassham JA, Kirk M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962:9(5):376–380. 10.1016/0006-291X(62)90019-0 [DOI] [PubMed] [Google Scholar]
- Bird IF, Cornelius MJ, Keys AJ, Whittingham CP. Intracellular site of sucrose synthesis in leaves. Phytochemistry 1974:13(1):59–64. 10.1016/S0031-9422(00)91267-6 [DOI] [Google Scholar]
- Bloom AJ. Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynth Res. 2015:123(2):117–128. 10.1007/s11120-014-0056-y [DOI] [PubMed] [Google Scholar]
- Bockwoldt M, Heiland I, Fischer K. The evolution of the plastid phosphate translocator family. Planta 2019:250(1):245–261. 10.1007/s00425-019-03161-y [DOI] [PubMed] [Google Scholar]
- Bowes G, Ogren WL, Hageman RH. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971:45(3):716–722. 10.1016/0006-291X(71)90475-X [DOI] [PubMed] [Google Scholar]
- Busch FA, Sage RF, Farquhar GD. Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nat Plants. 2018:4(1):46–54. 10.1038/s41477-017-0065-x [DOI] [PubMed] [Google Scholar]
- Calvin M. The path of carbon in photosynthesis. In nobel lectures, chemistry 1942–1962. Amsterdam: Elsivier Publishing Company; 1964. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1961/calvin-lecture.html [Google Scholar]
- Cardi M, Zaffagnini M, De Lillo A, Castiglia D, Chibani K, Gualberto JM, Rouhier N, Jacquot J-P, Esposito S. Plastidic P2 glucose-6P dehydrogenase from poplar is modulated by thioredoxin m-type: distinct roles of cysteine residues in redox regulation and NADPH inhibition. Plant Sci. 2016:252:257–266. 10.1016/j.plantsci.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004:37(6):853–863. 10.1111/j.1365-313X.2003.02012.x [DOI] [PubMed] [Google Scholar]
- Chu KL, Koley S, Jenkins LM, Bailey SR, Kambhampati S, Foley K, Arp JJ, Morley SA, Czymmek KJ, Bates PD, et al. . Metabolic flux analysis of the non-transitory starch tradeoff for lipid production in mature tobacco leaves. Metab Eng. 2022:69:231–248. 10.1016/j.ymben.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossar JD, Rowell P, Stewart WDP. Thioredoxin as a modulator of glucose-6-phosphate dehydrogenase in a N2-fixing cyanobacterium. J Gen Microbiol. 1984:130:991–998. 10.1099/00221287-130-4-991 [DOI] [Google Scholar]
- Cséke C, Nishizawa AN, Buchanan BB. Modulation of chloroplast phosphofructokinase by NADPH: a mechanism for linking light to the regulation of glycolysis. Plant Physiol. 1982:70(3):658–661. 10.1104/pp.70.3.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal J, Lopez H, Vasani NB, Hu Z, Swift JE, Yalamanchili R, Dvora M, Lin X, Xie D, Qu R, et al. . A photorespiratory bypass increases plant growth and seed yield in biofuel crop Camelina sativa. Biotechnol Biofuels. 2015:8(1):175. 10.1186/s13068-015-0357-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JP. A rapid, postillumination deceleration of respiration in green leaves. Plant Physiol. 1955:30(1):82–84. 10.1104/pp.30.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CF, Sharkey TD. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993:16(5):587–591. 10.1111/j.1365-3040.1993.tb00907.x [DOI] [Google Scholar]
- Driever SM, Simkin AJ, Alotaibi S, Fisk SJ, Madgwick PJ, Sparks CA, Jones HD, Lawson T, Parry MAJ, Raines CA. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos Trans R Soc Lond B Biol Sci. 2017:372(1730):20160384. 10.1098/rstb.2016.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist MKM, Maurino VG. Metabolic engineering of photorespiration. In: Fernie AR, Bauwe H, Weber APM, editors. Photorespiration: methods and protocols. New York (NY): Springer New York; 2017. p. 137–155. [DOI] [PubMed] [Google Scholar]
- Fernandez O, Ishihara H, George GM, Mengin V, Flis A, Sumner D, Arrivault S, Feil R, Lunn JE, Zeeman SC, et al. . Leaf starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 2017:174(4):2199–2212. 10.1104/pp.17.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettke J, Chia T, Eckermann N, Smith A, Steup M. A transglucosidase necessary for starch degradation and maltose metabolism in leaves at night acts on cytosolic heteroglycans (SHG). Plant J. 2006:46(4):668–684. 10.1111/j.1365-313X.2006.02732.x [DOI] [PubMed] [Google Scholar]
- Fichtner F, Lunn JE. The role of trehalose 6-phosphate (Tre6P) in plant metabolism and development. Annu Rev Plant Biol. 2021:72(1):737–760. 10.1146/annurev-arplant-050718-095929 [DOI] [PubMed] [Google Scholar]
- Fliege R, Flügge U-I, Werdan K, Heldt HW. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978:502(2):232–247. 10.1016/0005-2728(78)90045-2 [DOI] [PubMed] [Google Scholar]
- Flis A, Mengin V, Ivakov AA, Mugford ST, Hubberten H-M, Encke B, Krohn N, Höhne M, Feil R, Hoefgen R, et al. . Multiple circadian clock outputs regulate diel turnover of carbon and nitrogen reserves. Plant Cell Environ. 2019:42(2):549–573. 10.1111/pce.13440 [DOI] [PubMed] [Google Scholar]
- Flügel F, Timm S, Arrivault S, Florian A, Stitt M, Fernie AR, Bauwe H. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. Plant Cell. 2017:29(10):2537–2551. 10.1105/tpc.17.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Gregory LM, Weise SE, Walker BJ. Integrated flux and pool size analysis in plant central metabolism reveals unique roles of glycine and serine during photorespiration. Nat Plants. 2023:9(1):169–178. 10.1038/s41477-022-01294-9 [DOI] [PubMed] [Google Scholar]
- Furumoto T, Yamaguchi T, Ohshima-Ichie Y, Nakamura M, Tsuchida-Iwata Y, Shimamura M, Ohnishi J, Hata S, Gowik U, Westhoff P, et al. . A plastidial sodium-dependent pyruvate transporter. Nature 2011:476(7361):472–475. 10.1038/nature10250 [DOI] [PubMed] [Google Scholar]
- Galtier N, Foyer CH, Huber J, Voelker TA, Huber SC. Effects of elevated sucrose-phosphate synthase activity on photosynthesis, assimilate partitioning, and growth in tomato (Lycopersicon esculentum var UC82B). Plant Physiol. 1993:101(2):535–543. 10.1104/pp.101.2.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. Subcellular metabolite levels in spinach leaves: regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol. 1987:83(2):399–407. 10.1104/pp.83.2.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh E, Matsumoto M, Ogawa K, Kobayashi Y, Tsuyama M. A qualitative analysis of the regulation of cyclic electron flow around photosystem I from the post-illumination chlorophyll fluorescence transient in Arabidopsis: a new platform for the in vivo investigation of the chloroplast redox state. Photosynth Res. 2010:103(2):111–123. 10.1007/s11120-009-9525-0 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci U S A. 2010:107(20):9458–9463. 10.1073/pnas.0914299107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Ma F, Wei F, Fanella B, Allen DK, Wang X. Cytosolic phosphorylating glyceraldehyde-3-phosphate dehydrogenases affect Arabidopsis cellular metabolism and promote seed oil accumulation. Plant Cell. 2014:26(7):3023–3035. 10.1105/tpc.114.126946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin SC, Tang GQ, Scholz A, Holtgraewe D, Winter H, Huber SC. Phosphorylation of sucrose synthase at serine 170: occurrence and possible role as a signal for proteolysis. Plant J. 2003:35(5):588–603. 10.1046/j.1365-313X.2003.01831.x [DOI] [PubMed] [Google Scholar]
- Harley PC, Sharkey TD. An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth Res. 1991:27(3):169–178. 10.1007/BF00035838 [DOI] [PubMed] [Google Scholar]
- Hasunuma T, Harada K, Miyazawa S-I, Kondo A, Fukusaki E, Miyake C. Metabolic turnover analysis by a combination of in vivo 13C-labelling from 13CO2 and metabolic profiling with CE-MS/MS reveals rate-limiting steps of the C3 photosynthetic pathway in Nicotiana tabacum leaves. J Exp Bot. 2010:61(4):1041–1051. 10.1093/jxb/erp374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Chon CJ, Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977:59(6):1146–1155. 10.1104/pp.59.6.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe P, Heintze A, Riedel A, Creuzer C, Schultz G. The plastidic 3-phosphoglycerate → acetyl-CoA pathway in barley leaves and its involvement in the synthesis of amino acids, plastidic isoprenoids and fatty acids during chloroplast development. Planta 1993:190(2):253–262. 10.1007/BF00196619 [DOI] [Google Scholar]
- Huber SC, Huber JL. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996:47(1):431–444. 10.1146/annurev.arplant.47.1.431 [DOI] [PubMed] [Google Scholar]
- Jamsheer KM, Jindal S, Laxmi A. Evolution of TOR-SnRK dynamics in green plants and its integration with phytohormone signaling networks. J Exp Bot. 2019:70(8):2239–2259. 10.1093/jxb/erz107 [DOI] [PubMed] [Google Scholar]
- Jolliffe PA, Tregunna EB. Environmental regulation of the oxygen effect on apparent photosynthesis in wheat. Can J Bot. 1973:51(5):841–853. 10.1139/b73-107 [DOI] [Google Scholar]
- Jones TL, Ort DR. Circadian regulation of sucrose phosphate synthase activity in tomato by protein phosphatase activity. Plant Physiol. 1997:113(4):1167–1175. 10.1104/pp.113.4.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge UI. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell. 1998:10(1):105–117. 10.1105/tpc.10.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GJ, Latzko E. Inhibition of spinach-leaf phosphofructokinase by 2-phosphoglycollate. FEBS Lett. 1976:68(1):55–58. 10.1016/0014-5793(76)80403-6 [DOI] [PubMed] [Google Scholar]
- Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 2005:137(1):242–252. 10.1104/pp.104.055954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U, Pieruschka R, Farmer S, Berry JA. The Warburg-effects: basic metabolic processes with reference to cancer development and global photosynthesis. Plant Signal Behav. 2020:15(7):1776477. 10.1080/15592324.2020.1776477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte MM. The role of sucrose synthesis in determining patterns of growth, development, and allocation to reproduction in plants. Madison (WI): University of Wisconsin-Madison; 1997. [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development. J Exp Bot. 2014:65(3):799–807. 10.1093/jxb/ert474 [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005:138(1):451. 10.1104/pp.104.055046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Weraduwage SM, Preiser AL, Tietz S, Weise SE, Strand DD, Froehlich JE, Kramer DM, Hu J, Sharkey TD. A cytosolic bypass and G6P shunt in plants lacking peroxisomal hydroxypyruvate reductase. Plant Physiol. 2019:180(2):783–792. 10.1104/pp.19.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016:41(3):211–218. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka N, Weber AP. Intracellular metabolite transporters in plants. Mol Plant. 2010:3(1):21–53. 10.1093/mp/ssp108 [DOI] [PubMed] [Google Scholar]
- Livingston AK, Cruz JA, Kohzuma K, Dhingra A, Kramer DM. An Arabidopsis mutant with high cyclic electron flow around photosystem I (hcef) involving the NADPH dehydrogenase complex. Plant Cell. 2010:22(1):221. 10.1105/tpc.109.071084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Gehan JP, Sharkey TD. Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 2005:138(4):2280–2291. 10.1104/pp.105.061903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sharkey TD. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 2004:218(3):466–473. 10.1007/s00425-003-1127-z [DOI] [PubMed] [Google Scholar]
- Ma F, Jazmin LJ, Young JD, Allen DK. Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proc Natl Acad Sci U S A. 2014:111(47):16967–16972. 10.1073/pnas.1319485111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon JD, Fock H, Canvin DT. Changes in specific radioactivities of sunflower leaf metabolites during photosynthesis in 14CO2 and 12C02 at normal and low oxygen. Planta 1974:120(2):125–134. 10.1007/BF00384922 [DOI] [PubMed] [Google Scholar]
- Mengin V, Pyl E-T, Alexandre Moraes T, Sulpice R, Krohn N, Encke B, Stitt M. Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant Cell Environ. 2017:40(11):2608–2627. 10.1111/pce.13000 [DOI] [PubMed] [Google Scholar]
- Mullen JA, Koller HR. Daytime and nighttime carbon balance and assimilate export in soybean leaves at different photon flux densities. Plant Physiol. 1988:86(3):880–884. 10.1104/pp.86.3.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Née G, Aumont-Nicaise M, Zaffagnini M, Nessler S, Valerio-Lepiniec M, Issakidis-Bourguet E. Redox regulation of chloroplastic G6PDH activity by thioredoxin occurs through structural changes modifying substrate accessibility and cofactor binding. Biochem J. 2014:457(1):117–125. 10.1042/BJ20130337 [DOI] [PubMed] [Google Scholar]
- Née G, Zaffagnini M, Trost P, Issakidis-Bourguet E. Redox regulation of chloroplastic glucose-6-phosphate dehydrogenase: a new role for f-type thioredoxin. FEBS Lett. 2009:583(17):2827–2832. 10.1016/j.febslet.2009.07.035 [DOI] [PubMed] [Google Scholar]
- Nickelsen K. The path of carbon in photosynthesis: how to discover a biochemical pathway. Ambix 2012:59(3):266–293. 10.1179/174582312X13457672281867 [DOI] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unknown maltose transporter essential for starch degradation in leaves. Science 2004:303(5654):87–89. 10.1126/science.1091811 [DOI] [PubMed] [Google Scholar]
- Ogren WL, Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971:230(13):159–160. 10.1038/newbio230159a0 [DOI] [PubMed] [Google Scholar]
- Peixoto B, Moraes TA, Mengin V, Margalha L, Vicente R, Feil R, Höhne M, Sousa AGG, Lilue J, Stitt M, et al. . Impact of the SnRK1 protein kinase on sucrose homeostasis and the transcriptome during the diel cycle. Plant Physiol. 2021:187(3):1357–1373. 10.1093/plphys/kiab350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhänsel C, Krause K, Braun HP, Espie GS, Fernie AR, Hanson DT, Keech O, Maurino VG, Mielewczik M, Sage RF. Engineering photorespiration: current state and future possibilities. Plant Biol (Stuttg). 2013:15(4):754–758. 10.1111/j.1438-8677.2012.00681.x [DOI] [PubMed] [Google Scholar]
- Peterhänsel C, Maurino VG. Photorespiration redesigned. Plant Physiol. 2011:155(1):49–55. 10.1104/pp.110.165019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhilko A, Flis A, Sulpice R, Stitt M, Ebenhoh O. Adjustment of carbon fluxes to light conditions regulates the daily turnover of starch in plants: a computational model. Mol Biosyst. 2014:10(3):613–627. 10.1039/C3MB70459A [DOI] [PubMed] [Google Scholar]
- Pollock CJ, Lloyd EJ. The effect of low temperature upon starch, sucrose and fructan synthesis in leaves. Ann Bot. 1987:60(2):231–235. 10.1093/oxfordjournals.aob.a087441 [DOI] [Google Scholar]
- Portis AR Jr, Parry MA. Discoveries in Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase): a historical perspective. Photosyn Res. 2007:94(1):121–143. 10.1007/s11120-007-9225-6 [DOI] [PubMed] [Google Scholar]
- Preiser AL, Banerjee A, Weise SE, Renna L, Brandizzi F, Sharkey TD. Phosphoglucoisomerase is an important regulatory enzyme in partitioning carbon out of the Calvin-Benson cycle. Front Plant Sci. 2020:11:580726. 10.3389/fpls.2020.580726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser AL, Fisher N, Banerjee A, Sharkey TD. Plastidic glucose-6-phosphate dehydrogenases are regulated to maintain activity in the light. Biochem J. 2019:476(10):1539–1551. 10.1042/BCJ20190234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch EI. Photosynthesis and related processes. New York: Interscience Publishers Inc.; 1945. [Google Scholar]
- Rachmilevitch S, Cousins AB, Bloom AJ. Nitrate assimilation in plant shoots depends on photorespiration. Proc Natl Acad Sci U S A. 2004:101(31):11506–11510. 10.1073/pnas.0404388101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines CA. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 2011:155(1):36–42. 10.1104/pp.110.168559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines CA. Improving plant productivity by re-tuning the regeneration of RuBP in the Calvin–Benson–Bassham cycle. New Phytol. 2022:236(2):350–356. 10.1111/nph.18394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M. The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci U S A. 2002:99(10):7166–7171. 10.1073/pnas.062053099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 2011:11(1):123. 10.1186/1471-2229-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S, Kamen MD. Radioactive carbon of long half-life. Phys Rev. 1940:57(6):549–549. 10.1103/PhysRev.57.549 [DOI] [Google Scholar]
- Ryabova LA, Robaglia C, Meyer C. Target of rapamycin kinase: central regulatory hub for plant growth and metabolism. J Exp Bot. 2019:70(8):2211–2216. 10.1093/jxb/erz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno GL, Curatti L. Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci. 2003:8(2):63–69. 10.1016/S1360-1385(02)00029-8 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Geissler A, Fickenscher K. Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch Biochem Biophys. 1989:274(1):290–297. 10.1016/0003-9861(89)90441-4 [DOI] [PubMed] [Google Scholar]
- Schleucher J, Vanderveer P, Markley JL, Sharkey TD. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant Cell Environ. 1999:22(5):525–533. 10.1046/j.1365-3040.1999.00440.x [DOI] [Google Scholar]
- Schleucher J, Vanderveer PJ, Sharkey TD. Export of carbon from chloroplasts at night. Plant Physiol. 1998:118(4):1439–1445. 10.1104/pp.118.4.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiege SC, Sharkey TD, Walker B, Hammer J, Way DA. Laisk measurements in the nonsteady state: tests in plants exposed to warming and variable CO2 concentrations. Plant Physiol. 2023:193(2):1045–1057. 10.1093/plphys/kiad305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Siebert D, Heintze A, Schultz G. Substrate flow from photosynthetic carbon metabolism to chloroplast isoprenoid synthesis in spinach evidence for a plastidic phosphoglycerate mutase. Z Naturforsch C. 1987:42(5):570–580. 10.1515/znc-1987-0513 [DOI] [Google Scholar]
- Scialdone A, Mugford ST, Feike D, Skeffington A, Borrill P, Graf A, Smith AM, Howard M. Arabidopsis plants perform arithmetic division to prevent starvation at night. eLife. 2013:2:e00669. 10.7554/eLife.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. O2-insensitive photosynthesis in C3 plants: its occurrence and a possible explanation. Plant Physiol. 1985:78(1):71–75. 10.1104/pp.78.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. Feedback limitation of photosynthesis and the physiological role of ribulose bisphosphate carboxylase carbamylation. Botanical Magazine. Tokyo special issue. 1990:2:87–105. [Google Scholar]
- Sharkey TD. Discovery of the canonical Calvin–Benson cycle. Photosynth Res. 2019:140(2):235–252. 10.1007/s11120-018-0600-2 [DOI] [PubMed] [Google Scholar]
- Sharkey TD. Pentose phosphate pathway reactions in photosynthesizing cells. Cells 2021:10(6):1547. 10.3390/cells10061547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. The discovery of Rubisco. J Exp Bot. 2022:74(2):510–519. 10.1093/jxb/erac254 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Berry JA, Raschke K. Starch and sucrose synthesis in Phaseolus vulgaris as affected by light, CO2 and abscisic acid. Plant Physiol. 1985:77(3):617–620. 10.1104/pp.77.3.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Preiser AL, Weraduwage SM, Gog L. Source of 12C in Calvin-Benson cycle intermediates and isoprene emitted from plant leaves fed with 13CO2. Biochem J. 2020:477(17):3237–3252. 10.1042/BCJ20200480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Vassey TL. Low oxygen inhibition of photosynthesis is caused by inhibition of starch synthesis. Plant Physiol. 1989:90(2):385–387. 10.1104/pp.90.2.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Sharma M, Jamsheer K M, Laxmi A. Jasmonic acid coordinates with light, glucose and auxin signalling in regulating branching angle of Arabidopsis lateral roots. Plant Cell Environ. 2022:45(5):1554–1572. 10.1111/pce.14290 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Lopez-Calcagno PE, Davey PA, Headland LR, Lawson T, Timm S, Bauwe H, Raines CA. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO(2) assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol J. 2017:15(7):805–816. 10.1111/pbi.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South PF, Cavanagh AP, Liu HW, Ort DR. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019:363(6422):eaat9077. 10.1126/science.aat9077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen JM, Petty RV, Sharkey TD. Domain characterization of a 4-α-glucanotransferase essential for maltose metabolism in photosynthetic leaves. J Biol Chem. 2008:283(30):20797–20804. 10.1074/jbc.M803051200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol. 1990:41(1):153–185. 10.1146/annurev.pp.41.060190.001101 [DOI] [Google Scholar]
- Stitt M, ap Rees T. Capacities of pea chloroplasts to catalyse the oxidative pentose phosphate pathway and glycolysis. Phytochemistry 1979:18(12):1905–1911. 10.1016/S0031-9422(00)82700-4 [DOI] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant. 2014:7(1):137–155. 10.1093/mp/sst127 [DOI] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, Nunes-Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M, et al. . Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell. 2013:25(2):694–714. 10.1105/tpc.112.106989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Gauthier P, Buckley TN, Busch FA, Barbour MM, Bruhn D, Heskel MA, Gong XY, Crous KY, Griffin K, et al. . Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 2017:216(4):986–1001. 10.1111/nph.14816 [DOI] [PubMed] [Google Scholar]
- Tolbert N. Microbodies-peroxisomes and glyoxysomes. Annu Rev Plant Physiol. 1971:22(1):45–74. 10.1146/annurev.pp.22.060171.000401 [DOI] [Google Scholar]
- Voll L, Hausler RE, Hecker R, Weber A, Weissenbock G, Fiene G, Waffenschmidt S, Flugge U-I. The phenotype of the Arabidopsis cue1 mutant is not simply caused by a general restriction of the shikimate pathway. Plant J. 2003:36(3):301–317. 10.1046/j.1365-313X.2003.01889.x [DOI] [PubMed] [Google Scholar]
- Walker DA, Herold A. Can the chloroplast support photosynthesis unaided. Plant Cell Physiol. 1977:SI:295–310. [Google Scholar]
- Weber AP, Linka M, Bhattacharya D. Single, ancient origin of a plastid metabolite translocator family in Plantae from an endomembrane-derived ancestor. Eukaryot Cell. 2006:5(3):609–612. 10.1128/EC.5.3.609-612.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge UI. Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell. 2000:12(5):787–801. 10.1105/tpc.12.5.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Kim KS, Stewart RP, Sharkey TD. β-maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiol. 2005:137(2):756–761. 10.1104/pp.104.055996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Weber AP, Sharkey TD. Maltose is the major form of carbon exported from the chloroplast at night. Planta 2004:218(3):474–482. 10.1007/s00425-003-1128-y [DOI] [PubMed] [Google Scholar]
- Wenderoth I, Scheibe R, von Schaewen A. Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem. 1997:272(43):26985–26990. 10.1074/jbc.272.43.26985 [DOI] [PubMed] [Google Scholar]
- Wieloch T, Augusti A, Schleucher J. Anaplerotic flux into the Calvin–Benson cycle: hydrogen isotope evidence for in vivo occurrence in C3 metabolism. New Phytol. 2022:234(2):405–411. 10.1111/nph.17957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AT, Calvin M. The photosynthetic cycle. CO2 dependent transients. J Am Chem Soc. 1955:77(22):5948–5957. 10.1021/ja01627a050 [DOI] [Google Scholar]
- Xin C-P, Tholen D, Devloo V, Zhu X-G. The benefits of photorespiratory bypasses: how can they work? Plant Physiol. 2015:167(2):574–585. 10.1104/pp.114.248013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fu X, Sharkey TD, Shachar-Hill Y, Walker ABJ. The metabolic origins of non-photorespiratory CO2 release during photosynthesis: a metabolic flux analysis. Plant Physiol. 2021:186(1):297–314. 10.1093/plphys/kiab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Koroma AA, Weise SE, Fu X, Sharkey TD, Shachar-Hill Y. Daylength variation affects growth, photosynthesis, leaf metabolism, partitioning, and metabolic fluxes. Plant Physiol.2024:194(1):475–490. 10.1093/plphys/kiad507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wieloch T, Kaste JAM, Shachar-Hill Y, Sharkey TD. Reimport of carbon from cytosolic and vacuolar sugar pools into the Calvin-Benson cycle explains photosynthesis labeling anomalies. Proc Natl Acad Sci U S A. 2022:119(11):e2121531119. 10.1073/pnas.2121531119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Steup M. Polysaccharide fraction from higher plants which strongly interacts with the cytosolic phosphorylase isozyme. Plant Physiol. 1990:94(3):960–969. 10.1104/pp.94.3.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Uchikoshi E, Hara S, Hisabori T. Thioredoxin-like2/2-Cys peroxiredoxin redox cascade acts as oxidative activator of glucose-6-phosphate dehydrogenase in chloroplasts. Biochem J 2019:476(12):1781–1790. 10.1042/BCJ20190336 [DOI] [PubMed] [Google Scholar]
- Zhu X-G, de Sturler E, Long SP. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 2007:145(2):513–526. 10.1104/pp.107.103713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created for this work.