Abstract
The large family of human rhinoviruses, the main causative agents of the common cold, is divided into the major and the minor group based on receptor specificity. Major group viruses attach to intercellular adhesion molecule 1 (ICAM-1), a member of the immunoglobulin superfamily, whereas minor group viruses use low-density lipoprotein receptors (LDLR) for cell entry. During early attempts aimed at isolating the minor group receptor, we discovered that a protein with virus binding activity was released from HeLa cells upon incubation with buffer at 37°C (F. Hofer, B. Berger, M. Gruenberger, H. Machat, R. Dernick, U. Tessmer, E. Kuechler, and D. Blaas, J. Gen. Virol. 73:627–632, 1992). In light of the recent discovery of several new members of the LDLR family, we reinvestigated the nature of this protein and present evidence for its being derived from the human very-low density lipoprotein receptor (VLDLR). A soluble VLDLR fragment encompassing the eight complement type repeats and representing the N-terminal part of the receptor was then expressed in the baculovirus system; both the shed protein and the recombinant soluble VLDLR bind minor group viruses and inhibit viral infection of HeLa cells in a concentration-dependent manner.
Since the determination of the primary structure of the bovine low-density lipoprotein receptor (LDLR) (37), the number of discovered membrane receptors with similar structures has been constantly growing. The prototype, LDLR, the very-low density lipoprotein receptor (VLDLR), the LDLR-related protein (LRP), and megalin are currently being considered the most-prominent members of the LDLR gene family (for reviews, see for example, references 20, 29, and 33). A structural feature common to all of these membrane proteins includes various numbers of imperfect direct repeats of about 40 amino acids each, which are located at the extracellular N terminus and exhibit similarity with the C9 component of complement. These complement type repeats are involved in the attachment of a number of different ligands belonging to functionally and structurally unrelated protein classes. Next to the complement type repeats (or interspersed with them) are several copies of elements with similarity to epidermal growth factor precursor. In addition to asparagine-linked oligosaccharides present within these domains, most of the receptors also contain a highly O-glycosylated region close to the plasma membrane. The transmembrane region is followed by a cytoplasmic tail with amino acid sequence motives characteristic of localization to coated pits.
The function of the LDLR is the maintenance of cholesterol homeostasis by internalizing LDL upon binding to apolipoprotein-E and B-100. LRP is a multiligand receptor which binds ligands as diverse as chylomicron remnants and various proteinase-proteinase inhibitor complexes (among other unrelated ligands) and mediates their transport to lysosomes for degradation. Megalin, which is also termed gp330, is a multiligand receptor as well and was originally characterized as the Heymann nephritis antigen in experimental membranous glomerulonephritis in rats (6, 14). However, the normal physiological function of this large membrane receptor is still unknown. VLDLR was discovered in rabbit heart by homology screening (34), shortly followed by the isolation of its human (28) and mouse homologues (24). The tissue distribution of this receptor suggested a function in uptake of triglycerides as energy source (22).
Human rhinoviruses (HRVs) are small, icosahedral, nonenveloped viruses with an RNA genome of positive (messenger sense) polarity (for a review, see, for example, reference 27). The large number of viral serotypes was divided into a major receptor group (91 serotypes) and a minor receptor group (10 serotypes), based on specific binding to intercellular adhesion molecule 1 (ICAM-1) or to members of the LDL receptor family. There is one exception; HRV87 binds to an uncharacterized glycoprotein (35). In attempts to isolate and characterize proteins which bind to minor receptor group HRVs, we had previously found that a protein with virus-binding activity was shed from HeLa cells in a soluble form upon incubation with buffer at 37°C (12). We have reinvestigated the nature of the shed protein and show that it is an N-terminal fragment of VLDLR. A recombinant soluble receptor encompassing only the ligand-binding domain of human VLDLR was then expressed in the baculovirus system. In the present communication, we demonstrate that the shed material as well as the recombinant fragment inhibit viral infection in vitro.
A protein shed from HeLa cells binds HRV2.
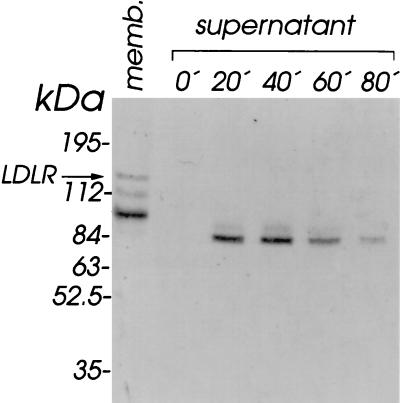
Rhino HeLa cells (Flow Laboratories) grown in T flasks (150 cm2) to a confluency of 80% were washed twice with phosphate-buffered saline (PBS) and incubated at 37°C in 5 ml of PBS for the times indicated in Fig. 1. Detached cells were pelleted by a low-speed centrifugation, and the supernatant was clarified in a type 65Ti rotor (Beckman) at 50,000 rpm for 60 min (S80) and concentrated to a final volume of 50 μl with a Centricon concentrator (10-kDa cutoff; Amicon). Aliquots corresponding to 107 cells were then run under nonreducing conditions on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, and the separated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore) in 20 mM Tris-HCl–150 mM glycine (pH 8.8) at 40 mA for 16 h in the cold. The membranes were blocked with 20 mM Tris-HCl (pH 7.5)–150 mM NaCl–2 mM CaCl2 (1× TBS/Ca) containing 2% Tween 20 (blocking buffer) for 1 h and incubated with 35S-labeled HRV2 (20,000 cpm/ml [23]) as described previously (16, 19). The membranes were dried and autoradiographed for 24 h on Kodak Biomax MR films (Fig. 1). In accordance with earlier experiments (12), HRV2 bound to a protein with a molecular mass of approximately 84 kDa which appeared in the cell supernatant upon incubation with PBS for 20 min; the binding activity peaked at 40 min of incubation time, whereupon it declined and was barely detectable after 80 min of incubation. This points to inactivation of the protein either by denaturation or by proteolytic degradation. HeLa cell membranes prepared as described elsewhere (17) were also analyzed for control purposes. HRV2 binding to LDLR (120 kDa) was clearly detectable in the cell membranes. Two additional bands at positions corresponding to approximate Mrs of 110 and 95 were also apparent. Previously, these had been tentatively assigned to degradation products of LDLR (13).
FIG. 1.
Virus-binding activity is shed from HeLa cells upon incubation with PBS. HeLa cells grown in T flasks were incubated with PBS at 37°C. At the times indicated, cell supernatants from individual flasks were saved and an S80 was prepared and the supernatant was concentrated to 50 μl. Samples were adjusted to 1× Laemmli sample buffer without β-mercaptoethanol and run on an 8% polyacrylamide–SDS gel, and the separated proteins were electrophoretically transferred onto a PVDF membrane. The membrane was blocked, incubated with 2 × 105 cpm of [35S]methionine-labeled HRV2 (19, 23), and exposed to X-ray film. As a control, a HeLa cell membrane (memb.) preparation corresponding to 5 × 106 cells was also used. For the position of LDLR (120 kDa), compare also with Fig. 2. Positions of marker proteins run on the same gel are indicated.
The shed protein is derived neither from LDLR nor from LRP.
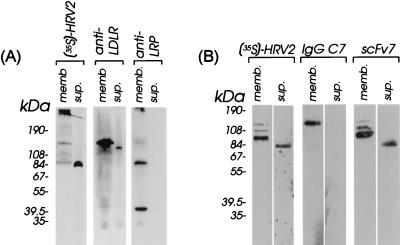
Rabbit antisera against bovine LDLR (diluted 1:10,000), against rat LRP (diluted 1:5,000), and monoclonal antibodies IgG-C7 (0.2 μg/ml [2]) and scFv7 (0.2 μg/ml [11]) were then used to further characterize the nature of the 84-kDa protein by Western blotting. In order to resolve the 515-kDa α-chain of LRP, a polyacrylamide gradient gel was used (Fig. 2A), whereas analysis of smaller proteins was carried out on a linear 10% polyacrylamide gel (Fig. 2B). To allow for easy comparison, HeLa cell membranes were always analyzed in parallel to the material released from the cells into the PBS supernatant. HRV2 attachment was also monitored and revealed binding to the same proteins shown in Fig. 1. Note, however, that the migration behavior of the virus-binding proteins somewhat varied in the different gel systems (compare Fig. 2A and B to Fig. 1). In addition to these virus-binding proteins, the gradient gel also allowed detection of binding of HRV2 to the large subunit (515 kDa) of LRP (13).
FIG. 2.
Characterization of the material shed from HeLa cells by Western analysis with specific antibodies. Material released from HeLa cells upon incubation with PBS for 40 min at 37°C was cleared and concentrated, and proteins were separated on a 4 to 12% polyacrylamide gradient gel (A) or on a 10% gel (B) under nonreducing conditions and electrophoretically transferred onto PVDF membranes. The membranes were incubated with 35S-labeled HRV2, antisera, and monoclonal antibodies as indicated at the top of the panels. Virus was detected by autoradiography. Bound antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Promega) diluted 1:5,000; detection of scFv7 was with the anti-myc antibody 9E10 (Stratagene), followed by HRP-conjugated rabbit anti-mouse IgG (Southern Biotechnology) diluted 1:5,000. IgG-C7 was detected with HRP-conjugated rabbit anti-mouse IgG (Southern Biotechnology) diluted 1:5,000. HRP activity was revealed with the chemiluminescence substrate Supersignal (Pierce). Control HeLa cell membranes were prepared as described previously (17). The positions of marker proteins run on the same gels are indicated. memb., HeLa membranes; sup., material shed from HeLa cells.
Rabbit antiserum against bovine LDLR, which cross-reacts with the human homologue, strongly labeled a protein migrating with an apparent molecular mass of 120 kDa in HeLa cell membranes; a minor band of approximately 110 kDa was also found to be recognized by the LDLR-specific antiserum identifying it as a degradation product or as an incompletely glycosylated form of LDLR. In the cell supernatant, a faint band at the same position was found to be labeled by anti-LDLR antibody; this LDLR-related product is thus clearly different from the 84-kDa virus-binding protein. As expected, antiserum raised against rat LRP and cross-reacting with the human homologue labeled the 515- and the 85-kDa chain of LRP, together with receptor-associated protein (RAP), which is strongly associated with this receptor (9, 32). However, no band was seen in the cell supernatant, suggesting that the 84-kDa protein was not a degradation product of LRP.
The monoclonal antibody IgG-C7, which is directed against the N-terminal complement type repeat of LDLR (2), was then used for further identification. As seen in Fig. 2B, this antibody recognized LDLR present in HeLa cell membranes but failed to bind to the material in the cell supernatant. The absence of any reaction with the protein in the cell supernatant identified as derived from LDLR (with rabbit antiserum; compare to Fig. 2A) might be explained by IgG-C7 binding requiring a native conformation for recognition, whereas the antiserum also binds to denatured LDLR (data not shown).
The human single-chain antibody scFv7 (11, 25) binds with high affinity to chicken ovarian VLDLR, cross-reacts with human LRP and, to some extent, with human LDLR, and inhibits attachment of various ligands including HRV2. As seen in Fig. 2B, scFv7 strongly labeled a protein present in HeLa cell membranes which migrates on the polyacrylamide gel with an apparent molecular mass of 95 kDa and which is clearly different from LDLR (compare also with Fig. 2A). Two other bands with slightly lower mobilities were also visible. However, none of them could be unequivocally assigned to LDLR, as revealed with anti-LDLR antiserum. In the cell supernatant, a strong band at 84 kDa became apparent upon incubation of the blot with scFv7.
The protein shed from HeLa cells is a fragment of VLDLR.
The data from the Western blot experiments made it highly unlikely that the shed protein was a fragment of LDLR or of LRP. We thus asked whether it might be related to VLDLR. Plasma membrane preparations from HeLa cells and, for control purposes, from small oocytes from chicken ovaries (31) and from CHO cells transformed with a plasmid encoding the human VLDLR splicing variant which lacks the O-linked sugar domain (28) were subjected to Western blotting with a rabbit antiserum raised against recombinant human VLDLR. As seen in Fig. 3, all three membrane preparations contained a protein which exhibited an apparent molecular mass of 95 kDa under nonreducing conditions. This makes it clear that the short splicing variant of VLDLR is expressed in HeLa cells. Moreover, the cross-reaction of the VLDLR antiserum with the chicken homologue emphasizes the high similarity between these two proteins. The absence of any additional bands demonstrates the high specificity of the antiserum and thus its suitability for identification of the soluble 84-kDa protein in the HeLa cell supernatant as obtained upon incubation with PBS.
FIG. 3.
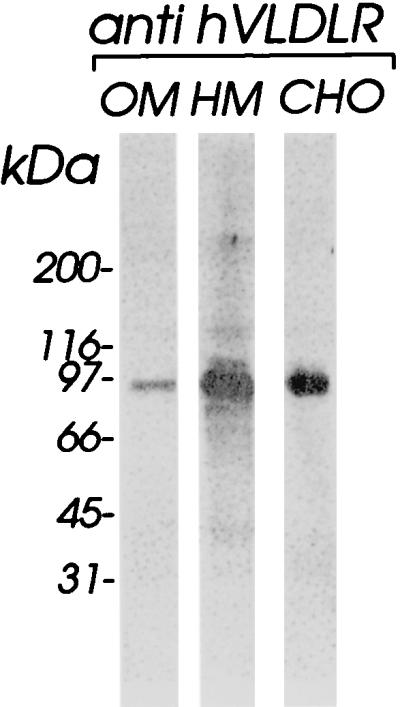
VLDLR is present in HeLa cells. Chicken oocyte membranes (OM) (30), HeLa cell membrane extract (HM), and membranes from CHO cells expressing the short splicing variant of human VLDLR (CHO [28]) were analyzed for the presence of VLDLR by Western blotting with antiserum raised against recombinant human VLDLR. Approximately 25 μg of total protein per lane was loaded. Note the cross-reaction of the VLDLR antiserum with chicken OVR.
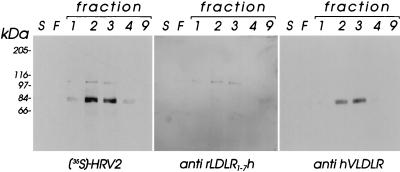
RAP, which was originally found in preparations of LRP (10), is considered a specific chaperone (3, 36) and was shown to compete with all ligands for binding to members of the LDLR family (1, 9, 18). Proteins released from HeLa cells upon incubation in PBS were thus subjected to affinity chromatography on a glutathione S-transferase (GST)-RAP column (15). HeLa suspension cells (8 × 108) were washed twice with PBS and gently stirred at 37°C for 40 min in 20 ml of PBS. The cell supernatant was centrifuged at 50,000 rpm in a Beckman 65Ti rotor for 60 min and applied to a GST-RAP column (0.5 ml) which had been equilibrated with PBS. GST-RAP Superose was prepared by coupling 30 mg of GST-RAP (9) to 8 ml of CNBr-activated Sepharose (Pharmacia) as described by the manufacturer. The column was washed with 1× Tris-buffered saline (TBS)-Ca, and bound proteins were eluted in 0.5-ml fractions with 1 M NH3 in 0.3× TBS-Ca. NH3 was removed, and fractions were concentrated to a final volume of 150 μl in a SpeedVac concentrator. Aliquots (20 μl) from the cell supernatant, from the flowthrough, and from the eluted fractions were applied onto polyacrylamide gels, and the proteins were separated under nonreducing conditions and were electrophoretically transferred onto PVDF membranes. The membranes were incubated with [35S]methionine-labeled HRV2, an IgY fraction prepared from eggs of a chicken immunized with rLDLR1–7h, a recombinant LDL receptor fragment comprising the ligand binding domain (17), at a final concentration of 2 μg/ml and with an antiserum against recombinant human VLDLR (diluted 1:10,000). The respective antibodies were detected with alkaline phosphatase (AP)-conjugated goat anti-IgY (1:5,000) and nitroblue tetrazolium salt (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) as a substrate; horseradish peroxidase (HRP)-conjugated anti-rabbit IgG was revealed with chemiluminescence substrate. As seen in Fig. 4 (left panel), the 84-kDa protein binding HRV2 was strongly enriched in fractions 2 and 3. A faint band migrating with an apparent molecular mass of 110 kDa which also bound HRV2 appeared in the same fractions. Development of the blot with the IgY fraction reacting with LDLR showed that this latter band was clearly related to LDLR (middle panel). Final proof of the identity of the 84-kDa virus-binding protein with a fragment of VLDLR was then obtained by its reaction with the antiserum raised against human recombinant VLDLR (right panel). Coomassie staining of gels identical to those used for Western blotting revealed only several faint bands (data not shown) which did not correspond to any of the proteins revealed with virus or antisera upon Western blotting (Fig. 4). The amount of protein shed from approximately 5 × 107 HeLa cells is thus certainly less than 100 ng, the approximate detection limit of Coomassie blue staining.
FIG. 4.
The virus-binding activity shed from HeLa cells is a VLDLR fragment. HeLa suspension cells (8 × 108) were incubated with 20 ml of PBS for 40 min at 37°C. The cells were pelleted, and the S80 supernatant was applied onto a GST-RAP affinity column. Fractions (0.5 ml) obtained upon elution with 1 M ammonia were collected and concentrated to 150 μl, and aliquots of 20 μl (corresponding to approximately 5 × 107 cells) were analyzed by polyacrylamide gel electrophoresis on 10% gels, followed by ligand blotting with [35S]methionine-labeled HRV2, with LDLR-specific IgY, and with rabbit antiserum against VLDLR, respectively. A 20-μl aliquot of the original unconcentrated sample (lanes S) and 20 μl of the flowthrough (lanes F) were also analyzed. The positions of marker proteins run on the same gels are also indicated. hVLDLR, human VLDLR.
Soluble recombinant human VLDLR inhibits HRV infection.
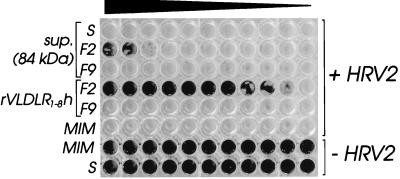
We have previously shown that the chicken ovarian VLDL receptor (OVR) binds HRV2 on ligand blots and inhibits HRV2 infection of HeLa cells in its detergent solubilized form (8). However, HRVs fail to replicate in birds; therefore, the function of OVR as a viral receptor is questionable. We thus asked whether human VLDLR would bind to HRV2 in solution and thereby inhibit viral infection. Since further purification of the 84-kDa protein appeared tedious and greater amounts were difficult to obtain, soluble human VLDLR encompassing only the eight complement type repeats (rVLDLR1–8h) was expressed in insect cells with a C-terminal hexahistidine tag by using the baculovirus system. A DNA fragment encoding the eight complement type repeats of human VLDLR (28) was PCR amplified with pfu polymerase (Stratagene) by using the two synthetic oligonucleotides (forward, 5′-ATGCGGATCCAGGGAGAAAAGCCAAATGTG-3′; reverse, 5′-GCTGCCCGGGACACTCTTTCAGGGGCTCAT-3′) hybridizing at positions 82 to 100 and 1046 to 1066, respectively, of the coding region of human VLDLR cDNA. Nucleotides shown in boldface were added to create restriction sites for BamHI and XmaI (underlined). DNA was denatured at 94°C for 3 min; amplification consisted of 30 cycles at 94 (1 min), 57 (45 s), and 72°C (2 min). The fragment was digested with BamHI and XmaI, purified by agarose gel electrophoresis, and ligated into the baculovirus transposition vector pTM1 (16). Recombinant bacmid DNA was obtained by transposition in DH10Bac cells according to the manufacturer’s protocol (Life Technologies) and was used for lipofection of Sf9 insect cells. Recombinant baculovirus (vVLDLR1–8h) was used to infect Sf9 cells at a multiplicity of infection of 5 for production of recombinant VLDL receptor fragment rVLDLR1–8h. Cells were harvested at 80 h postinfection and the secreted protein was purified from the cell culture supernatant on a Ni-nitriloacetic acid (NTA) column as described previously (17). The Ni-NTA eluate was then further purified on a GST-RAP column (data not shown) as described above, and matched fractions were compared with those of the 84-kDa protein with respect to protection of HeLa cells against infection with HRV2 essentially as described elsewhere (17). Briefly, 1.4 μg of Ni-NTA and GST-RAP affinity-purified rVLDLR1–8h (20 μl) or 20 μl of the 84-kDa protein (fraction 2 from the GST-RAP column [Fig. 4]) was mixed with 80 μl of infection medium (minimal essential medium containing 2% fetal calf serum and 30 mM MgCl2), and serial twofold dilutions were made in the same medium. To each dilution (50 μl), 100 50% tissue culture infectious doses (TCID50) of HRV2 (in 50 μl of infection medium) were added, and the mixtures were incubated for 1.5 h at 34°C. They were then transferred onto monolayers of HeLa cells in 96-well plates (containing 100 μl of infection medium), and the plates were incubated for 3 days at 34°C. Remaining cells attached to the plastic were stained with amido black (0.1% in acetic acid, methanol, water; 10/40/50 [vol/vol]). Noninfected cells and cells infected in the absence of receptor were used as positive and negative controls, respectively. As seen in Fig. 5, both the 84-kDa protein and rVLDLR1–8h inhibited infection of the cells in a dose-dependent manner. Whereas the concentration of the 84-kDa protein in the crude cell supernatant was not sufficient for cell protection, significant inhibition of cytopathic effect was observed upon partial purification of the material on the RAP column (Fig. 4, fraction 2). In contrast, fraction 9, which was used as a negative control, failed to protect the cells against infection. Under the same conditions, the purified recombinant soluble receptor present in fraction 2 from the GST-RAP column (data not shown) strongly inhibited viral infection, whereas fraction 9 was again negative. Quantitative comparison of the cell protective effect with the enriched 84-kDa protein was, however, not possible due to its small amount and the presence of contaminating proteins.
FIG. 5.
VLDLR fragments protect HeLa cells against infection with HRV2. HRV2 (100 TCID50) was incubated for 90 min at 34°C with serial twofold dilutions (left to right) of HeLa cell supernatant obtained upon incubation with PBS (S), of fraction 2 (F2) and of fraction 9 (F9) as eluted from the GST-RAP column (see Fig. 4), and of corresponding F2 and F9 of the GST-RAP column purification of rVLDLR1–8h. Cells infected with virus preincubated with plain infection medium (MIM) were used as the negative control. HeLa cell monolayers were challenged with the mixtures and stained with amido black after incubation for 3 days at 34°C. MIM and supernatant from the PBS incubation (S) were also compared in the absence of virus to exclude any influence on HeLa cell viability.
Conclusions.
In an attempt to isolate the rhinovirus minor group receptor, we discovered that a protein with virus binding activity was released upon incubation of the cells with PBS at 37°C (12). This was not entirely unexpected, since several other receptors have been shown to be shed from the cell surface by the action of membrane-bound specific proteinases (5, 21). We thus argued that shedding might also occur upon cultivation of the cells and finally succeeded in purifying a virus-binding activity from large amounts of spent tissue culture supernatant and unambiguously identified it as human LDLR (13). However, during the purification process, detergent was used and the protein which was finally isolated migrated slightly more slowly than that found upon incubation of HeLa cells with PBS. In the light of the discovery of novel members of the LDLR family during the last few years, we decided to reexamine the nature of the shed protein and thus to clarify the discrepancy between its apparent molecular weight (12) with that of LDLR or degradation products and precursors thereof (13).
Using various specific antisera, we here demonstrate that HeLa cells express a protein which specifically reacts with antiserum against VLDLR. It migrates with an apparent molecular weight corresponding to the smaller form of VLDLR lacking the O-linked sugar domain (28). In addition to membrane-bound receptor, a soluble fragment of this protein is released from the cells upon incubation with PBS. However, since identification of this protein as VLDLR relies only on immunological reagents in the absence of any amino acid sequence data, it cannot be excluded with absolute certainty that the protein has high similarity to VLDLR but is not the VLDLR proper.
Based on the results from Western blots with various antisera (Fig. 2), only very minor amounts of soluble LDLR were present in the HeLa cell supernatant. LDLR isolated from the spent tissue culture supernatant (13) must thus have originated from membrane fragments. Shedding of plasma membrane fragments is a widespread feature of viable cells and has previously been reported for HeLa cells (4).
Evidence for the presence of soluble LRP α-chain in normal human serum was recently obtained by affinity chromatography on methylamine-activated α2-macroglobulin (26). The physiological significance of the soluble LRP is not clear at the present time. VLDLR does not bind to α2 macroglobulin, and, when present in human serum, it might have escaped detection by this procedure. Attempts to isolate soluble VLDLR from spent tissue culture media by affinity chromatography on a GST-RAP column indeed revealed an HRV2-binding protein migrating with an apparent molecular mass of 84 kDa; however, large amounts of immunoglobulins were unspecifically retained, preventing efficient purification of VLDLR by this method (data not shown).
The 84-kDa protein released from HeLa cells upon incubation in PBS was efficiently enriched by affinity chromatography on a GST-RAP column (Fig. 4). The eluted material was then assayed for its capacity to inhibit the cytopathic effect of HRV2 infection of HeLa cells. For control purposes, we also expressed a soluble N-terminal fragment of VLDLR encompassing the eight complement type repeats in Sf9 insect cells. The shed 84-kDa protein and the recombinant soluble receptor, both of which were enriched on a GST-RAP column, efficiently protected HeLa cells from infection. Quantitative comparison was not attempted because of the difficulties in obtaining a homogenous preparation of the 84-kDa protein.
LDLR fragments composed of various numbers of the ligand binding repeats have been shown to exhibit antiviral activity toward HRVs (16, 17). An antiviral helper factor, a 28-kDa N-terminal fragment from LDLR, whose release from various cell types into the medium was induced with gamma interferon, has been described previously. Interestingly, it was active against vesicular stomatitis virus (VSV) infection (7), but its activity was not exerted by interference with virus attachment but probably by an unknown effect on virus assembly or budding. Whether the release of the VLDLR fragment can also be stimulated by interferon has not been studied so far. Further work is required to investigate whether shedding of the VLDLR fragment also occurs under normal conditions in the healthy organism.
Acknowledgments
This work was supported by grant no. P12189-MOB from the Austrian Science Foundation.
We thank J. Nimpf, M. Huettinger, and H. Hobbs for the generous gifts of antisera against LDLR, LRP, and VLDLR, respectively; J. Nimpf for the VLDLR clone; I. Goesler for excellent tissue culture work; and R. Wandl for the Western blot shown in Fig. 4.
REFERENCES
- 1.Battey F D, Gafvels M E, Fitzgerald D J, Argraves W S, Chappell D A, Strauss J F, Strickland D K. The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J Biol Chem. 1994;269:23268–23273. [PubMed] [Google Scholar]
- 2.Beisiegel U, Schneider W J, Goldstein J L, Anderson R G, Brown M S. Monoclonal antibodies to the low density lipoprotein receptor as probes for study of receptor-mediated endocytosis and the genetics of familial hypercholesterolemia. J Biol Chem. 1981;256:11923–11931. [PubMed] [Google Scholar]
- 3.Bu G J, Geuze H J, Strous G J, Schwartz A L. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Broe M E, Wieme R J, Logghe G N, Roels F. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81:237–245. doi: 10.1016/0009-8981(77)90054-7. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers M R W, Riordan J F. Membrane proteins with soluble counterparts—role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991;30:10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- 6.Farquhar M G, Saito A, Kerjaschki D, Orlando R A. The Heymann nephritis antigenic complex: Megalin (gp330) and RAP. J Am Soc Nephrol. 1995;6:35–47. doi: 10.1681/ASN.V6135. [DOI] [PubMed] [Google Scholar]
- 7.Fischer D G, Tal N, Novick D, Barak S, Rubinstein M. An antiviral soluble form of the LDL receptor induced by interferon. Science. 1993;262:250–253. doi: 10.1126/science.8211145. [DOI] [PubMed] [Google Scholar]
- 8.Gruenberger M, Wandl R, Nimpf J, Hiesberger T, Schneider W J, Kuechler E, Blaas D. Avian homologs of the mammalian low-density lipoprotein receptor family bind minor receptor group human rhinovirus. J Virol. 1995;69:7244–7247. doi: 10.1128/jvi.69.11.7244-7247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herz J, Goldstein J L, Strickland D K, Ho Y K, Brown M S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- 10.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley K K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodits R A, Nimpf J, Pfistermueller D M, Hiesberger T, Schneider W J, Vaughan T J, Johnson K S, Haumer M, Kuechler E, Winter G, Blaas D. An antibody fragment from a phage display library competes for ligand binding to the low density lipoprotein receptor family and inhibits rhinovirus infection. J Biol Chem. 1995;270:24078–24085. doi: 10.1074/jbc.270.41.24078. [DOI] [PubMed] [Google Scholar]
- 12.Hofer F, Berger B, Gruenberger M, Machat H, Dernick R, Tessmer U, Kuechler E, Blaas D. Shedding of a rhinovirus minor group binding protein—evidence for a Ca2+-dependent process. J Gen Virol. 1992;73:627–632. doi: 10.1099/0022-1317-73-3-627. [DOI] [PubMed] [Google Scholar]
- 13.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, Blaas D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci USA. 1994;91:1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerjaschki D, Horvat R, Binder S, Susani M, Dekan G, Ojha P P, Hillemanns P, Ulrich W, Donini U. Identification of a 400-kd protein in the brush borders of human kidney tubules that is similar to gp330, the nephritogenic antigen of rat Heymann nephritis. Am J Pathol. 1987;129:183–191. [PMC free article] [PubMed] [Google Scholar]
- 15.Kounnas M Z, Argraves W S, Strickland D K. The 39-kDa receptor-associated protein interacts with 2 members of the low density lipoprotein receptor family, alpha2-macroglobulin receptor and glycoprotein-330. J Biol Chem. 1992;267:21162–21166. [PubMed] [Google Scholar]
- 16.Marlovits, T. C., C. Abrahamsberg, and D. Blaas. Soluble LDL-minireceptors: minimal structure requirements for recognition of minor group human rhinovirus. J. Biol. Chem., in press. [DOI] [PubMed]
- 17.Marlovits T C, Zechmeister T, Gruenberger M, Ronacher B, Schwihla H, Blaas D. Recombinant soluble low density lipoprotein receptor fragment inhibits minor group rhinovirus infection in vitro. FASEB J. 1998;12:695–703. doi: 10.1096/fasebj.12.9.695. [DOI] [PubMed] [Google Scholar]
- 18.Medh J D, Fry G L, Bowen S L, Pladet M W, Strickland D K, Chappell D A. The 39-kDa receptor-associated protein modulates lipoprotein catabolism by binding to LDL receptors. J Biol Chem. 1995;270:536–540. doi: 10.1074/jbc.270.2.536. [DOI] [PubMed] [Google Scholar]
- 19.Mischak H, Neubauer C, Berger B, Kuechler E, Blaas D. Detection of the human rhinovirus minor group receptor on renaturing Western blots. J Gen Virol. 1988;69:2653–2656. doi: 10.1099/0022-1317-69-10-2653. [DOI] [PubMed] [Google Scholar]
- 20.Moestrup S K. The alpha 2-macroglobulin receptor and epithelial glycoprotein-330: two giant receptors mediating endocytosis of multiple ligands. Biochim Biophys Acta. 1994;1197:197–213. doi: 10.1016/0304-4157(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Mullberg J, Rauch C T, Wolfson M F, Castner B, Fitzner J N, Otten Evans C, Mohler K M, Cosman D, Black R A. Further evidence for a common mechanism for shedding of cell surface proteins. FEBS Lett. 1997;401:235–238. doi: 10.1016/s0014-5793(96)01480-9. [DOI] [PubMed] [Google Scholar]
- 22.Multhaupt H A B, Gafvels M E, Kariko K, Jin H, Arenas Elliott C, Goldman B I, Strauss J F, Angelin B, Warhol M J, McCrae K R. Expression of very low density lipoprotein receptor in the vascular wall: analysis of human tissues by in situ hybridization and immunohistochemistry. Am J Pathol. 1996;148:1985–1997. [PMC free article] [PubMed] [Google Scholar]
- 23.Neubauer C, Frasel L, Kuechler E, Blaas D. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology. 1987;158:255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 24.Oka K, Ishimuraoka K, Chu M J, Sullivan M, Krushkal J, Li W H, Chan L. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur J Biochem. 1994;224:975–982. doi: 10.1111/j.1432-1033.1994.00975.x. [DOI] [PubMed] [Google Scholar]
- 25.Pfistermueller D M, Blaas D, Hodits R A. Preferential recognition of the very low-density lipoprotein receptor ligand binding site by antibodies from phage display libraries. FEBS Lett. 1996;396:14–20. doi: 10.1016/0014-5793(96)00964-7. [DOI] [PubMed] [Google Scholar]
- 26.Quinn K A, Grimsley P G, Dai Y P, Tapner M, Chesterman C N, Owensby D A. Soluble low density lipoprotein receptor related protein (Lrp) circulates in human plasma. J Biol Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- 27.Rueckert R R. Picornaviridae. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 609–654. [Google Scholar]
- 28.Sakai J, Hoshino A, Takahashi S, Miura Y, Ishii H, Suzuki H, Kawarabayasi Y, Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem. 1994;269:2173–2182. [PubMed] [Google Scholar]
- 29.Schneider W J, Nimpf J, Bujo H. Novel members of the low density lipoprotein receptor superfamily and their potential roles in lipid metabolism. Curr Opin Lipidol. 1997;8:315–319. doi: 10.1097/00041433-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Stifani S, Barber D L, Aebersold R, Steyrer E, Shen X, Nimpf J, Schneider W J. The laying hen expresses two different low density lipoprotein receptor-related proteins. J Biol Chem. 1991;266:19079–19087. [PubMed] [Google Scholar]
- 31.Stifani S, Barber D L, Nimpf J, Schneider W J. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc Natl Acad Sci USA. 1990;87:1955–1959. doi: 10.1073/pnas.87.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strickland D K, Ashcom J D, Williams S, Burgess W H, Migliorini M, Argraves W S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- 33.Strickland D K, Kounnas M Z, Argraves W S. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci USA. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uncapher C R, Dewitt C M, Colonno R J. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology. 1991;180:814–817. doi: 10.1016/0042-6822(91)90098-v. [DOI] [PubMed] [Google Scholar]
- 36.Willnow T E, Rohlmann A, Horton J, Otani H, Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996;15:2632–2639. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Davis C G, Brown M S, Schneider W J, Casey M L, Goldstein J L, Russell D W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984;39:27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]