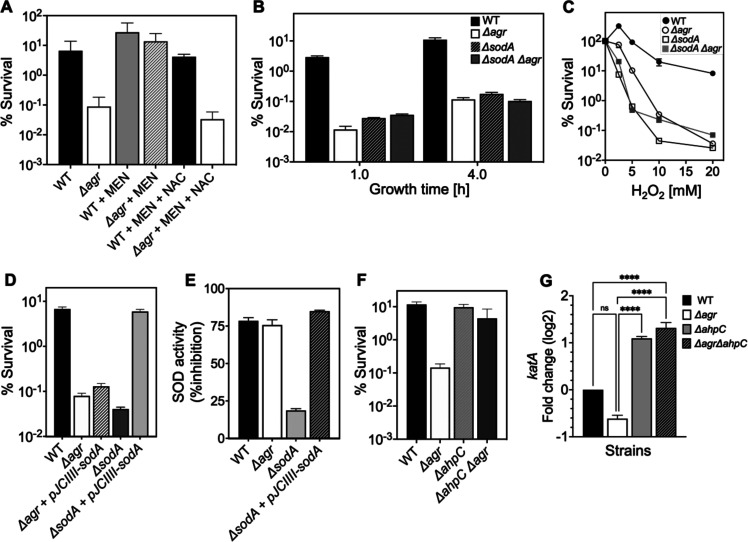
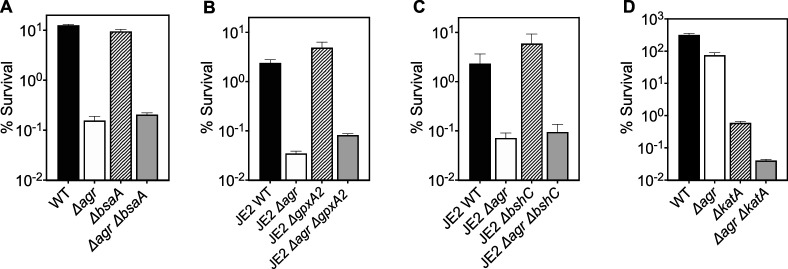
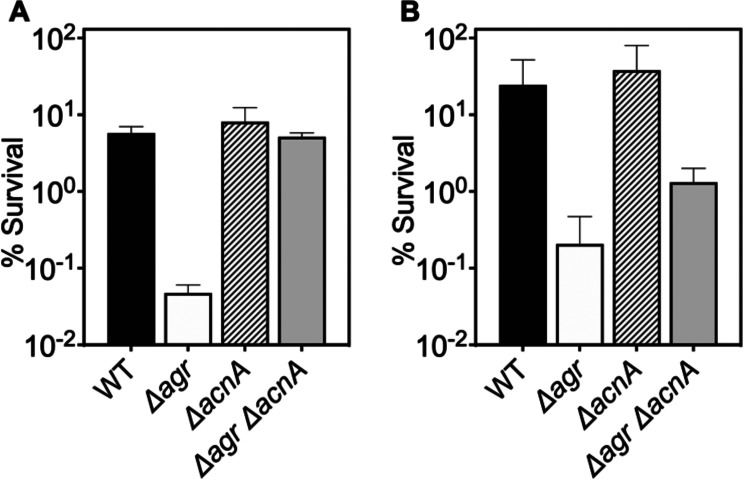
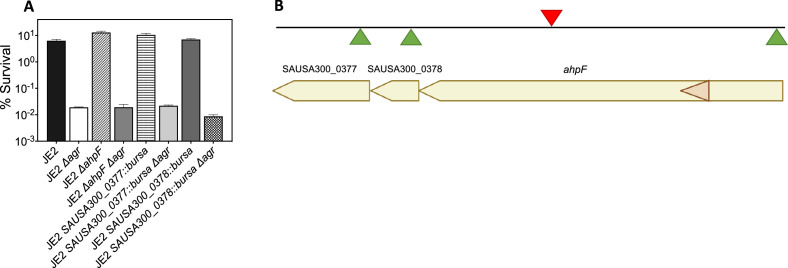
Figure 8. Involvement of endogenous reactive oxygen species (ROS) in agr-mediated protection from lethal H2O2 stress.
(A) Protective effect of menadione on survival. S. aureus LAC wild type (BS819) and Δagr mutant (BS1348) cultures were grown to late exponential phase (4 hr after dilution of overnight cultures), exposed to 80 μM menadione (MD) with or without 4 mM N-acetyl cysteine (NAC) for 30 min prior to treatment with H2O2 (20 mM for 1 hr) and measurement of survival. (B) Effect of sodA deletion on survival. Cultures of wild-type (BS819), Δagr (BS1348), a sodA::tetM (BS1422), and sodA::tetM-agr double mutant (BS1423) were grown to early (1 hr after dilution, OD600~0.15) or late log (4 hr after dilution, OD600~4.0) prior to treatment with 20 mM H2O2 for 60 min. (C) Effect of H2O2 concentration on survival. Late log (4 hr, OD600~4.0) cultures of the wild-type and Δagr mutant strains were treated with indicated concentrations of H2O2 for 60 min. (D) Complementation of sodA deletion mutation. A plasmid-borne wild-type sodA gene was expressed under control of the sarA constitutive promoter (pJC1111-sodA) in late log-phase (4 hr, OD600~4.0) cells treated with 20 mM H2O2 for 60 min. (E) SodA activity. Wild-type or the indicated mutants were grown to late-exponential phase (OD600~4.0); Sod activity was measured as in Methods. (F) Effect of ahpC deletion on survival. Late log-phase cultures of wild-type (BS819), Δagr (BS1348), ahpC::bursa (BS1486), and ΔahpC::bursa-agr double-mutant (BS1487) cells were treated with 20 mM H2O2 for 60 min. (G) Effect of ahpC deletion on expression of katA in the indicated mutants. Total cellular RNA was extracted from late exponential-phase cultures (OD600~4.0), followed by reverse transcription and PCR amplification of the indicated genes, using rpoB as an internal standard. mRNA levels were normalized to those of each gene to wild-type control. Data represent the mean ± SD. from (n=3) biological replicates. One-way ANOVA was used to determine statistical differences between samples (****p<0.0001).