To the Editor: With preterm infants often surviving into adulthood, prematurity has been identified as a risk factor for heart failure, leading to the recognition of a new disease entity: “heart failure of prematurity” (1). Preterm infants are susceptible to oxidative stress because of increased perinatal exposure and lack of antioxidant defenses (2). Oxidative stress also causes heart failure in adults and contributes to cardiovascular disease (3). Deranged oxidant signaling is a common pathway implicated in heart failure both in adults and in the perinatal period. We here report a heart failure model that permits dynamic regulation of reactive oxygen species both in adults and in the developing heart.
We utilized a chemogenetic approach exploiting a yeast d-amino acid oxidase (DAAO) that generates hydrogen peroxide (H2O2) upon provision of d-alanine (4). We previously used adeno-associated virus serotype 9 gene transfer to target DAAO expression to cardiomyocytes in vivo and showed that chronic generation of H2O2 causes heart failure (4–6). Here we generated a cardiomyocyte-specific transgenic mouse line (DAAO-TGCar) expressing DAAO as a fusion protein with the H2O2 biosensor HyPer allowing for simultaneous generation (DAAO) and detection (HyPer) of H2O2. We validated cardiomyocyte-specific DAAO expression and verified production of H2O2 upon addition of d-alanine (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/JCI178251DS1). Adult DAAO-TGCar mice developed heart failure after in vivo treatment with d-alanine. Cardiomyocytes from DAAO-TGCar mice showed higher baseline H2O2 levels after in vivo d-alanine treatment, indicating a higher intracellular oxidized state. Feeding with d-alanine increased mitochondrial superoxide (O2.–) in cardiomyocytes from adult DAAO-TGCar animals, decreased mitochondrial membrane potential, decreased respiratory capacity, and increased baseline glycolysis, providing evidence of mitochondrial dysregulation as a characteristic of cardiac dysfunction (Supplemental Figure 2, A–D).
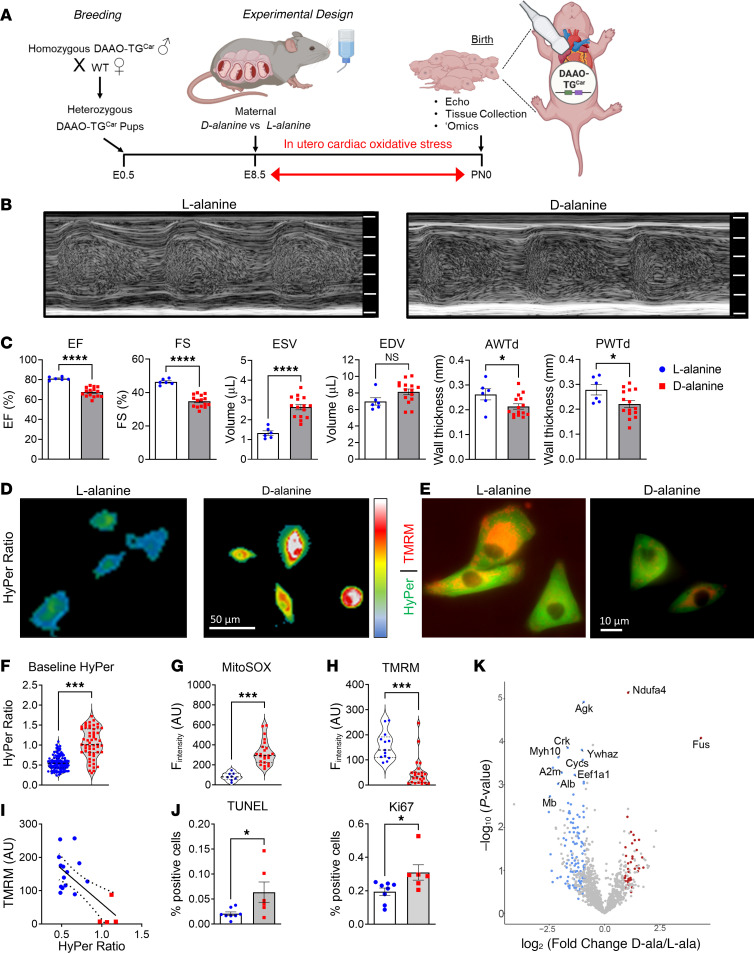
For the neonatal model, we generated heterozygous DAAO-TGCar pups by crossing homozygous male DAAO-TGCar sires with WT females so that all pups expressed DAAO. We induced in utero oxidative stress by providing d-alanine or l-alanine (control) in the mothers’ drinking water from embryonic day 8.5 until birth. Neonates exposed to d-alanine in utero had a decrease in cardiac function compared with controls, accompanied by decreased cardiac wall thickness and increased end-systolic volume. Neonatal cardiomyocytes isolated following in utero d-alanine showed deranged redox balance, elevated H2O2 and mitochondrial O2.– levels, along with mitochondrial dysfunction (Figure 1). Neonates exposed to d-alanine showed increased cardiomyocyte apoptosis and proliferation (Figure 1, D–F), decreased cardiomyocyte size, and disrupted cardiomyocyte architecture (Supplemental Figure 3, C–F).
Figure 1. A chemogenetic/transgenic model of neonatal heart failure.
(A) Breeding strategy for heterozygous DAAO-TGCar pups treated in utero. Created with BioRender.com. E, embryonic day; PN, postnatal day. (B) M-mode images of the left ventricle and (C) echocardiographic parameters of ejection fraction (EF), fractional shortening (FS), end-systolic (ESV) and end-diastolic (EDV) volume, and anterior (AWTd) and posterior (PWTd) wall thickness in neonates exposed to d-alanine (0.4 M) (red squares) or l-alanine (0.4 M) (blue circles) from E8.5 to PN0. (D) Baseline HyPer ratio and (E) HyPer and tetramethylrhodamine methyl ester perchlorate (TMRM) images of cardiomyocytes from neonates (PN0) exposed to d-alanine or l-alanine. Quantification of (F) HyPer ratio, (G) mitoSOX, and (H) TMRM fluorescence in arbitrary units (a.U.). (I) Correlation between TMRM and HyPer (r = 0.857, P = 0.0004); dashed lines represent 95% CI. (J) Quantification of TUNEL staining (apoptosis) and Ki67 (proliferation). *P < 0.05, ***P < 0.001, ****P < 0.0001 by unpaired t test. Values shown as mean ± SEM. (K) Volcano plot showing protein abundances in d- versus l-alanine–exposed neonatal hearts; fold-changes and P values are log-transformed. Differentially expressed proteins (P = 0.05; FDR = 0.1) displayed as upregulated (red) and downregulated (blue). Proteins not significantly changed indicated in gray.
Proteomic analyses of heart tissues from animals exposed to oxidative stress as adults or after in utero exposure revealed marked alterations in the cardiac proteome. There were 594 and 441 differentially expressed proteins in the neonates and adults exposed to cardiac H2O2 compared with controls, respectively (Figure 1K and Supplemental Figure 2, E and F). In both adult and neonatal hearts exposed to H2O2, highly enriched terms related to energy metabolism, mitochondrial organization, cardiac function, cell death, and oxidative stress. In neonatal hearts exposed to H2O2, uniquely enriched terms included proteins involved in cardiac development (TNNI1, SGCD, TNNC1), protein translation (SARS, TARS1, EARS2), and mitochondrial biogenesis (TOMM70, OPA1, TIMM13). The adult cardiac proteome was uniquely enriched for terms related to inflammation (PDIA3, STIP1, HSPD1), cardiomyocyte death (ATP2A2, HSPB6, EIF5A), and glucose metabolism (GPI, PGAM2, ENO3), demonstrating both striking similarities and marked differences in the response to oxidative stress between adult and neonatal myocardium (Supplemental Figure 4).
This study reports the development and characterization of a DAAO-TGCar mouse model that permits manipulation of H2O2 in the heart. We induced cardiac oxidative stress in both adult and neonatal mice and demonstrated substantial impairment in cardiac function, associated with striking changes in cardiomyocyte redox balance and mitochondrial function, along with alterations in the proteome, suggesting a shared pathogenic mechanism. We found marked differences after in vivo oxidative stress across these 2 developmental stages. In vivo cardiac oxidative stress in adult DAAO-TGCar mice recapitulated pathological findings we reported in adult rats (4–6), showing cardiac dysfunction over a period of several weeks in response to d-alanine feeding. In contrast, in utero cardiac oxidative stress markedly affected developing hearts following only days of exposure, underscoring a potential predisposition for cardiac complications later in life. Oxidative stress impairs cardiac regenerative capacity (7), and this model can be utilized to examine effects of in utero versus postnatal oxidative stress on cardiomyocyte maturation. Our neonatal model represents an informative platform to deepen our understanding of the implications of prematurity for the developing myocardium. We propose this chemogenetic approach as a comprehensive model for examining oxidative stress–induced cardiac injury across developmental stages, from in utero to adult exposures. Our model provides insights into the differential effects of oxidative stress on adult and neonatal hearts, establishing a foundation for understanding divergent pathophysiological pathways affected by redox stress across different developmental states.
Further information can be found in Supplemental Methods.
Supplementary Material
Version 1. 03/14/2024
In-Press Preview
Version 2. 05/01/2024
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2024, Spyropoulos et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Submitted: December 7, 2023; Accepted: March 5, 2024; Published: March 14, 2024.
Reference information: J Clin Invest. 2024;134(9):e178251.
Contributor Information
Fotios Spyropoulos, Email: fspyropoulos@bwh.harvard.edu.
Apabrita Ayan Das, Email: adas7@bwh.harvard.edu.
Markus Waldeck-Weiermair, Email: mwaldeck-weiermair@bwh.harvard.edu.
Shambhu Yadav, Email: shambhu.iiser@gmail.com.
Arvind K. Pandey, Email: apandey5@bwh.harvard.edu.
Ruby Guo, Email: rubyguo@hms.harvard.edu.
Taylor A. Covington, Email: tcovington@bwh.harvard.edu.
Venkata Thulabandu, Email: vxt77@case.edu.
Kosmas Kosmas, Email: kkosmas@bwh.harvard.edu.
Benjamin Steinhorn, Email: BSTEINHORN@MGH.HARVARD.EDU.
Xiaoli Liu, Email: xliu1@bwh.harvard.edu.
Helen Christou, Email: hchristou@partners.org.
Thomas Michel, Email: thomas_michel@hms.harvard.edu.
References
- 1.Crump C, et al. Association of preterm birth with long-term risk of heart failure into adulthood. JAMA Pediatr. 2021;175(7):689–697. doi: 10.1001/jamapediatrics.2021.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pena-Bautista C, et al. Non-invasive assessment of oxidative stress in preterm infants. Free Radic Biol Med. 2019;142:73–81. doi: 10.1016/j.freeradbiomed.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Steinhorn B, et al. Chemogenetic approaches to probe redox pathways: implications for cardiovascular pharmacology and toxicology. Annu Rev Pharmacol Toxicol. 2022;62:551–571. doi: 10.1146/annurev-pharmtox-012221-082339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinhorn B, et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat Commun. 2018;9(1):4044. doi: 10.1038/s41467-018-06533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorrentino A, et al. Reversal of heart failure in a chemogenetic model of persistent cardiac redox stress. Am J Physiol Heart Circ Physiol. 2019;317(3):617–626. doi: 10.1152/ajpheart.00177.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spyropoulos F, et al. Metabolomic and transcriptomic signatures of chemogenetic heart failure. Am J Physiol Heart Circ Physiol. 2022;322(3):451–465. doi: 10.1152/ajpheart.00628.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puente BN, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.