Abstract
Purpose
The summary presented herein covers recommendations on the early detection of prostate cancer and provides a framework to facilitate clinical decision-making in the implementation of prostate cancer screening, biopsy, and follow-up. This is Part I of a two-part series that focuses on prostate cancer screening. Please refer to Part II for discussion of initial and repeat biopsies as well as biopsy technique.
Materials and Methods
The systematic review utilized to inform this guideline was conducted by an independent methodological consultant. The systematic review was based on searches in Ovid MEDLINE and Embase and Cochrane Database of Systematic Reviews (January 1, 2000 – November 21, 2022). Searches were supplemented by reviewing reference lists of relevant articles.
The AUA employs a 3-tiered strength of evidence system to underpin evidence-based guideline statements (Table 1). The AUA nomenclature system explicitly links statement type to body of evidence strength, level of certainty, magnitude of benefit or risk/burdens, and the Panel’s judgment regarding the balance between benefits and risks/burdens (Table 2).
Results
The Early Detection of Prostate Cancer Panel developed evidence- and consensus-based guideline statements to provide guidance in prostate cancer screening, initial and repeat biopsy, and biopsy technique.
Conclusions
Prostate-specific antigen (PSA)-based prostate cancer screening in combination with shared decision-making (SDM) is recommended. Current data regarding risk from population-based cohorts provide a basis for longer screening intervals and tailored screening, and the use of available online risk calculators is encouraged.
Background
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy in American men. There will be an estimated 288,300 prostate cancer diagnoses and 34,700 deaths from prostate cancer in the United States in 2023.1, 2 Significant advances have been made in early detection, especially with the increasing availability and use of biomarkers as well as multi-parametric magnetic resonance imaging (mpMRI). This guideline addresses PSA-based screening and early detection, considerations for initial and repeat biopsy, and biopsy technique based on a systematic review of recently published literature, with the goal of identifying clinically significant prostate cancer while minimizing potential harms (e.g., anxiety, false positives, overdiagnosis of low-risk cancer, and side-effects from prostate biopsy).
This guideline provides recommendations and an algorithm (Figure 1) for prostate cancer screening in different groups based on their age range and risk criteria, with an emphasis on SDM. SDM is particularly necessary as there is no universally accepted standard definition of low versus elevated risk for prostate cancer detection. In practice, clinicians often resort to an elevated PSA level based on laboratory, prostate size, or age-based “norms” as a surrogate for an elevated prostate cancer risk, but such definitions, while easy to apply, are insufficient to describe all people and circumstances. Thus, clinicians may tailor the definitions of elevated risk and elevated PSA to the clinical situation at hand. Factors that may increase risk of clinically significant prostate cancer include, but are not limited to, Black ancestry, germline mutations, and strong family history of prostate cancer.
FIGURE 1:
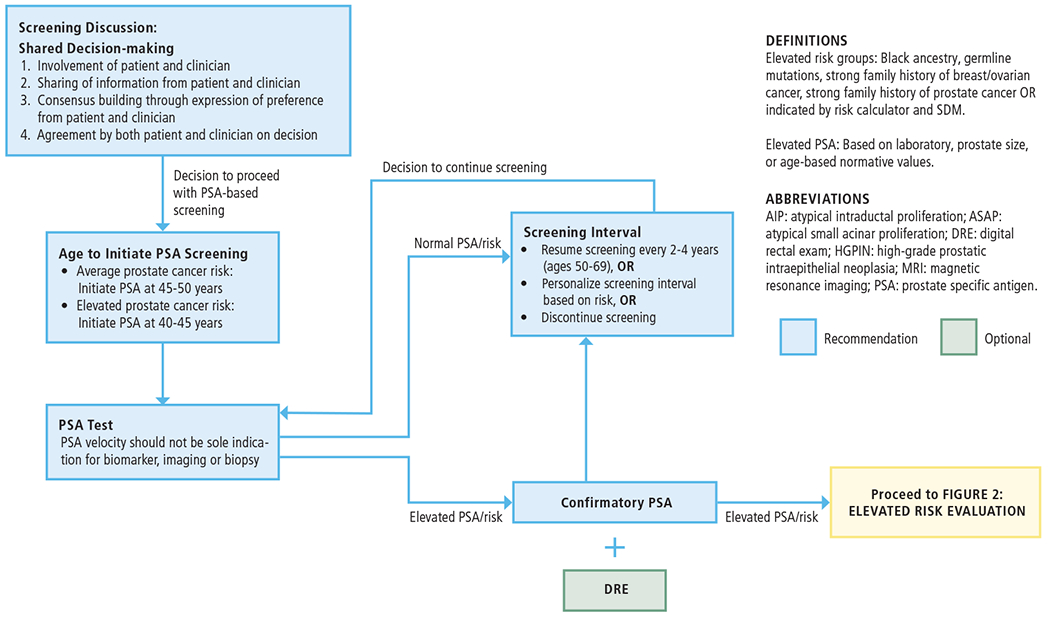
INITIAL SCREENING FOR PROSTATE CANCER
This guideline underscores the goal of detecting “clinically significant” prostate cancer for initial and repeat biopsy. The risk of prostate cancer mortality in patients with Grade Group (GG)1 prostate cancer is extremely low.3, 4 Thus, this guideline defines clinically significant prostate cancer as GG2 or higher (GG2+) prostate cancer and will use “clinically significant prostate cancer” and “GG2+” interchangeably throughout. However, the Panel acknowledges there are various definitions of “clinically significant” as not all “clinically significant” cancers are destined to impact quality or quantity of life.
Guideline Statements
PSA Screening
-
1
Clinicians should engage in SDM with people for whom prostate cancer screening would be appropriate and proceed based on a person’s values and preferences. (Clinical Principle)
Prostate cancer screening is a preference-sensitive decision. For this reason, the Panel recommends clinicians engage in SDM with people considering prostate cancer screening so they can make an informed choice. The Panel discourages the practice of ordering a PSA test without informing the patient upfront, and likewise discourages the practice of failing to inform the patient of the availability of PSA screening, as appropriate.
SDM is considered state-of-the art in patient counseling for preference-sensitive decisions.5 This practice can be facilitated using a decision aid. A 2019 systematic review and meta-analysis of 19 randomized controlled trials (RCTs) evaluating decision aids specifically designed for the prostate cancer screening decision versus conventional care, showed a small decrease in decisional conflict and a small increase in knowledge.6 The 2016 AUA White paper5 recommends that SDM include four elements: 1) Involvement of both the clinician and the patient in the decision-making process, 2) Sharing information by both the clinician and the patient, 3) Building consensus through the expression of preferences by both clinician and patient, and 4) Agreement by both the clinician and patient on the decision to implement. Although not explicitly stated for every guideline recommendation, it is implied that SDM should be utilized wherever there is a preference-sensitive decision that has any significant degree of uncertainty.
-
2
When screening for prostate cancer, clinicians should use PSA as the first screening test. (Strong Recommendation; Evidence Level: Grade A)
-
3
For people with a newly elevated PSA, clinicians should repeat the PSA prior to a secondary biomarker, imaging, or biopsy. (Expert Opinion)
The PSA blood test remains the first-line screening test of choice based on randomized trials of PSA-based screening showing reductions in metastasis and prostate cancer death.7, 8 At the time of this evidence review, very limited evidence has emerged regarding other candidates for first-line biomarkers or imaging.
The definition of an elevated PSA has changed over time. The commonly cited threshold of 4 ng/mL is based on very early studies that identify the highest levels typically observed among patients thought to be free of prostate cancer. Another cited threshold of 3 ng/mL is taken from the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial of prostate cancer screening that showed a significant reduction in prostate cancer deaths among patients who entered the trial between ages 55 to 69 years and were referred to biopsy based on that threshold. The knowledge that PSA generally increases with age in people without prostate cancer has led to the consensus that the threshold above which a PSA level should be considered elevated should increase with age. Most studies identifying age-varying thresholds specify threshold values of 2.5 ng/mL for people in their 40s, 3.5 ng/mL for people in their 50s, 4.5 ng/mL for people in their 60s, and 6.5 ng/mL for people in their 70s.9, 10
In people with a newly elevated PSA, it will return to a normal level in 25% to 40% upon retesting.11 Among 1,686 biopsied patients in the STHLM-3 study with a PSA of 3 to 10 ng/mL, and 2 PSA tests 8 weeks apart, 283 (17%) subsequently had a PSA < 3 ng/mL. Given the clear evidence that PSA tests may normalize, it is prudent to confirm a newly elevated PSA test before proceeding with further evaluation.12 A repeat PSA in a few months is recommended, though it can be shortened or lengthened depending on other clinical factors (e.g., recent bladder catheterization, prostate biopsy or cystoscopy, urinary retention).
-
4
Clinicians may begin prostate cancer screening and offer a baseline PSA test to people between ages 45 to 50 years. (Conditional Recommendation; Evidence Level: Grade B)
For people at average risk of developing prostate cancer, there is no randomized evidence showing a benefit to initiation of routine screening for prostate cancer before 45 years of age.
In the Malmö Preventive Project, the risk of prostate cancer metastases by 15 years’ follow-up was low (0.6%) for patients with PSA in the highest percentile (≥ 1.3 ng/mL) at 40 years of age. For patients aged 45 to 49 years with PSA below the median (0.68 ng/mL), the risk of prostate cancer metastasis within 25 years was 0.85%. Patients with PSA in the highest decile (≥ 1.6 ng/mL) at ages 45 to 49 years contributed to nearly half of prostate cancer deaths over the next 25 to 30 years.13 A randomized trial of risk-adapted screening for prostate cancer comparing patients starting at age 45 versus 50 years (the PROBASE trial) is currently ongoing, with 23,301 patients having participated in screening in the first round of the trial.14
-
5
Clinicians should offer prostate cancer screening beginning at age 40 to 45 years for people at increased risk of developing prostate cancer based on the following factors: Black ancestry, germline mutations, strong family history of prostate cancer. (Strong Recommendation; Evidence Level: Grade B)
If a person has risk factors associated with increased risk of developing prostate cancer (e.g., Black ancestry, germline mutations, strong family history of prostate cancer), particularly if they have an increased risk of metastatic disease, an earlier age to begin screening may be appropriate in addition to a shorter re-screening interval.15
Black individuals have a disproportionate cancer burden and a two-fold higher risk of death from prostate cancer compared to white individuals.16 A study using three models discovered that patients who self-identify as Black have an earlier age of onset and increased risk of metastases before clinical diagnosis.17 This study found the risk of a Black patient developing fatal prostate cancer, if not diagnosed, reached the same level as that of the general population three to nine years earlier, informing the proposal that Black patients initiate screening approximately five to ten years prior to the recommendation for average-risk individuals.17 This increased risk may be addressed by screening Black patients more frequently (e.g., annually), but the risk of overdiagnosis among older Black patients is considerably higher than the average-risk population, making SDM and personalized screening particularly important.
Although there is no standard definition of strong family history, several guidelines and consensus statements propose common criteria that include: 1) people with one brother or father or two or more male relatives with one of the following: a) diagnosed with prostate cancer at age < 60 years; b) any of whom died of prostate cancer; c) any of whom had metastatic prostate cancer. 2) family history of other cancers with two or more cancers in hereditary breast and ovarian cancer syndrome or Lynch syndrome spectrum.18, 19
Studies have consistently found elevated risk of prostate cancer in patients with a family history of prostate cancer20, 21 and also in patients with a family history of prostate and breast cancer.22, 23 Patients with a strong family history (e.g., two or more first-degree relatives) have a four-fold relative risk compared to those without a family history24 and should ideally be genotyped to ascertain whether this is associated with a pathogenic variant (e.g., BRCA1/2, Lynch Syndrome, ATM, CHEK2) or one or more of a growing set of identified germline DNA damage-repair mutations found in patients with metastatic prostate cancer diagnoses.25
Empirical studies have shown patients with germline BRCA1 and BRCA2 variants have increased risks of both disease onset and progression.26 The IMPACT study revealed a high positive predictive value (PPV) of PSA screening (with biopsy referral threshold 3 ng/mL) in these patients and a high frequency of clinically significant cancers,27 particularly among BRCA2 carriers.28 The IMPACT study showed a stronger relationship (eight-fold increased risk) between BRCA2 carriers and aggressive cancer for whom systematic PSA screening is indicated, while further study is needed to determine the role of screening among BRCA1 mutation carriers.28 Similarly, mutations in ATM, MLH1, MSH2, MSH6, PMS2, HOXB13, NBS1, and CHEK2 need further study. In the IMPACT study, after one screening round, carriers of pathogenic variants in mismatch-repair genes MSH2 and MSH6 had a higher risk of prostate cancer compared with age-matched non-carrier controls, potentially supporting screening of these patients.26
-
6
Clinicians should offer regular prostate cancer screening every 2 to 4 years to people aged 50 to 69 years. (Strong Recommendation; Evidence Level: Grade A)
Two RCTs, ERSPC7 and the Goteborg population-based prostate cancer screening trial (Goteborg-1),29 provide evidence that regular PSA screening every 2 to 4 years in patients aged 50 to 69 years reduces the risk of metastatic prostate cancer and prostate cancer mortality at 16 to 22 years, compared to no or opportunistic screening.
The number needed to be screened (NNS, the inverse of the absolute risk reduction in prostate cancer mortality) and number needed to be diagnosed (NND, additional cases diagnosed) to prevent one death from prostate cancer depends on the screening protocol (including screening ages) and follow-up time.
A study comparing patients 60 years of age who have been screened every 2 years in the Goteborg-1 trial, compared to unscreened patients 60 years of age in the Malmö Preventive Project, showed that continuing to screen patients with PSA ≥ 2 ng/mL at 60 years of age had a favorable net-benefit in terms of reducing risk of prostate cancer metastasis and mortality at 15 years. At 15 years, the NNS to prevent 1 death from prostate cancer was 23 and NND was 6.30
-
7
Clinicians may personalize the re-screening interval, or decide to discontinue screening, based on patient preference, age, PSA, prostate cancer risk, life expectancy, and general health following SDM. (Conditional Recommendation; Evidence Level: Grade B)
The randomized trials (PLCO, Goteborg-1, ERSPC) screened patients aged 50 to 69 years every 1 to 4 years and demonstrated a reduction in prostate cancer mortality. However, increasing evidence from additional analyses of the randomized trials, observational studies, and modeling studies show the balance between benefits and harms of screening can be modulated through personalized risk-stratified screening approaches.9, 13, 30–36
The re-screening interval can be 1 to 4 years for patients with PSA levels of 1 to 3 ng/mL between the ages of 45 to 70 years, while the re-screening interval can be prolonged for patients aged 45 to 70 years with a PSA < 1 ng/mL or those with a PSA below the age-specific median.31, 37, 38 Studies have shown that patients in the age range of 40 to 59 years with a PSA below the age-specific median, without a strong family history of prostate cancer, and no known pathogenic germline mutation, have a very low risk of metastatic cancer or long-term prostate cancer mortality.
In a case-control study conducted in Sweden (Malmö Preventive Project cohort),13 among patients aged 40 to 55 years, the 15-year risk of metastasis for patients with PSA below the median at ages 45 to 49 years was 0.09%, and below the median at ages 51 to 55 years was 0.23%. In a U.S. case-control study (Physicians’ Health Study cohort)35 among patients aged 40 to 59 years, 82%, 71%, and 86% of lethal cases occurred in patients with PSA above the median at ages 40 to 49 years (median PSA 0.68 ng/mL), 50 to 54 years (median PSA 0.88 ng/mL), and 55 to 59 years (median PSA 0.96 ng/mL), respectively. Both studies suggest risk-stratified screening based on midlife PSA and should be considered in patients aged 45 to 59 years.
Amongst patients 60 years of age with a PSA < 1 ng/mL (age-specific median), the 25-year risk of metastases or death from prostate cancer in a largely unscreened population (Malmö Preventive Project) is extremely low (0.5% and 0.2%, respectively).32 These empiric findings are supported by modeling data that suggest a higher likelihood of death from prostate cancer if screening were discontinued in these patients (5% to 13.1% fewer lives saved compared with continuing screening to 69 years of age);39 therefore, it is reasonable to significantly lengthen the re-screening interval or discontinue screening based on SDM provided there are no other risk factors, such as strong family history of prostate cancer.30, 32, 39
The decision to screen patients should be an SDM conversation predicated upon a person’s prior PSA levels and general health, and a flexible age to discontinue screening may be based on individualized decision-making to balance detection of aggressive cancers and overdiagnosis. This is particularly important in people between the ages of 70 to 80 years where there is a higher risk of competing mortality.40, 41 Clinicians may discontinue or substantially lengthen the re-screening interval for patients 75 years of age or older if PSA is < 3 ng/mL. In the Baltimore Longitudinal Study of Aging, patients 75 years or older with a PSA < 3 ng/mL were unlikely to be diagnosed with aggressive prostate cancer, and no patients between the ages of 75 to 80 years with PSA < 3 ng/mL died of prostate cancer.42
In select patients who are very healthy with an estimated life expectancy of at least ten years, ongoing screening every two to four years is reasonable following SDM as these patients are more likely to benefit from therapeutic interventions, if indicated. However, for patients with less than a ten-year estimated life expectancy, screening is not likely to provide a benefit in terms of disease-specific or overall mortality. The 95% confidence interval around the relative risk of prostate cancer mortality between the screening and control groups in ERSPC for patients aged 70 to 74 years excluded any benefit (RR: 1.18; 95% CI: 0.81 to 1.7).43 Furthermore, the evidence from randomized treatment trials comparing surgery, radiation, and monitoring has shown to have less benefit and more risk from curative treatment with increasing age.44–46 Additionally, the risk in overdiagnosis of prostate cancer increases with increasing age.41, 47
Risk calculators have been developed to estimate a patient’s life expectancy and can be informative during SDM. While several methods have been applied to estimate life expectancy, a simple approach is to use the social security life tables (https://www.ssa.gov/oact/STATS/table4c6.html). Based on current Social Security Administration (SSA) data, American men older than 77 years of age have less than a 10-year life expectancy. Insurance companies are known to be particularly astute at estimating life expectancy and many have online calculators that include, but are not limited to, the use of tobacco, alcohol, physical activities, and comorbidities. For the purpose of estimating life expectancy, the use of these tools is likely more reliable than individual clinician judgment.48
-
8
Clinicians may use digital rectal exam (DRE) alongside PSA to establish risk of clinically significant prostate cancer. (Conditional Recommendation; Evidence Level: Grade C)
The primary screening modality recommended for the early detection of prostate cancer is a PSA blood test. Clinicians should not use DRE as the sole screening method.
There is insufficient evidence to support adding DRE to PSA-based prostate cancer screening. The PPV of DRE as a screening method to detect prostate cancer is low. In the PROBASE trial, DRE was not effective for early detection; the PPV of a suspicious DRE at 50 years of age was 0.87% (as compared to 4.9% among patients aged 55 to 59 years in PLCO); of the 57 participants with suspicious DRE, 37 were biopsied and only 2 had prostate cancer (both GG1).14
In contrast to a screening application, use of DRE after the screening encounter may be of value. The greatest utility of DRE in randomized trials is demonstrated in the workup of patients with an elevated PSA. For this reason, among patients with PSA ≥ 2 ng/mL, clinicians should strongly consider supplementary DRE to establish risk of clinically significant prostate cancer.
-
9
For people undergoing prostate cancer screening, clinicians should not use PSA velocity as the sole indication for a secondary biomarker, imaging, or biopsy. (Strong Recommendation; Evidence Level: Grade B)
With knowledge of a patient’s age, PSA, DRE, percent free PSA, family history of prostate cancer, and history of a previous biopsy, large-scale studies in Europe and the U.S. have shown the addition of PSA velocity at various thresholds does not add value in predicting the presence of clinically significant prostate cancer.49, 50 Therefore, PSA velocity should not be used as sole indication for secondary biomarker, imaging, or a biopsy.
-
10
Clinicians and patients may use validated risk calculators to inform the SDM process regarding prostate biopsy. (Conditional Recommendation; Evidence Level: Grade B)
Contemporary evaluations of prostate cancer risk now typically include patient demographic factors, medical history, family history of prostate cancer, biomarkers, and imaging findings. Simple nomograms in tabular format are suboptimal in presenting risk for more than a few such factors; therefore, several groups have developed risk calculators based on actual patient data that allow patients and clinicians to simultaneously incorporate a larger number of these risk factors (Table 3).51, 52 While these risk calculators provide estimates that facilitate clinician-patient discussion of detection risk, it should be kept in mind that these are population averages with potentially wide intervals in some subsets. Moreover, the data for a number of these, while extensive, may be based on historic screening and detection approaches (e.g., prior to widespread prostate MRI adoption). Furthermore, calibration of risk calculators may differ by subgroups.
Table 3:
Select Risk Calculators with Risk Factors and Risk Factors Evaluated
| PCPT V2 (https://riskcalc.org/PCPTRC/) | Chun (There is no publicly available online calculator for Chun) | ERSPC (https://www.prostatecancer-riskcalculator.com) | PBCG (https://riskcalc.org/PBCG/) | |
|---|---|---|---|---|
| Race | x | |||
| Family history of prostate cancer | x | x | ||
| Age | x | x | x | x |
| PSA | x | x | x | x |
| Free PSA % | x | x | ||
| DRE | x | x | x | x |
| Prior biopsy | x | x | x | |
| Urinary PCA3 | x | x | ||
| TMPRSS2:ERG fusion | x | |||
| Prostate volume | x | x | ||
| Sampling density | x | |||
| MRI – PI-RADS score | x |
-
11
When the risk of clinically significant prostate cancer is sufficiently low based on available clinical, laboratory, and imaging data, clinicians and patients may forgo near-term prostate biopsy. (Clinical Principle)
When assessing a patient’s risk for prostate cancer, the estimated risk for clinically significant prostate cancer may be considered low as perceived by both the clinician and patient. Therefore, it would be reasonable to forgo a prostate biopsy in such instances following SDM, even where there may be some clinical features that indicate a risk for prostate cancer existing (e.g., mildly elevated PSA). If a decision is made after SDM to forgo a biopsy or additional testing, patients should be informed of their risk for underdiagnosing clinically significant prostate cancer and the need for future follow up screening, as appropriate.
Future Directions
Screening and diagnosis of prostate cancer remain intensely debated topics with major implications for individual and population health. There continue to be many unanswered questions that can prompt future research, preferably in the form of clinical trials and modeling studies to enhance and optimize patient care. Future trials will hopefully prioritize inclusion of historically underrepresented populations.
Table 1:
Strength of Evidence Definitions
| AUA Strength of Evidence Category | GRADE Certainty Rating | Definition |
|---|---|---|
| A | High | • Very confident that the true effect lies close to that of the estimate of the effect |
| B | Moderate | • Moderately confident in the effect estimate • The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| C | Low Very Low |
• Confidence in the effect estimate is limited • The true effect may be substantially different from the estimate of the effect • Very little confidence in the effect estimate • The true effect is likely to be substantially different from the estimate of effect |
Table 2:
AUA Nomenclature Linking Statement Type to Level of Certainty, Magnitude of Benefit or Risk/Burden, and Body of Evidence Strength
| Evidence Grade | Evidence Strength A (High Certainty) | Evidence Strength B (Moderate Certainty) | Evidence Strength C (Low Certainty) |
|---|---|---|---|
| Strong Recommendation (Net benefit or harm substantial) | -Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) is substantial -Applies to most patients in most circumstances and future research is unlikely to change confidence |
-Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) is substantial -Applies to most patients in most circumstances but better evidence could change confidence |
-Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) appears substantial -Applies to most patients in most circumstances but better evidence is likely to change confidence (rarely used to support a Strong Recommendation) |
| Moderate Recommendation (Net benefit or harm moderate) | -Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) is moderate -Applies to most patients in most circumstances and future research is unlikely to change confidence |
-Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) is moderate -Applies to most patients in most circumstances but better evidence could change confidence |
-Benefits > Risks/Burdens (or vice versa) -Net benefit (or net harm) appears moderate -Applies to most patients in most circumstances but better evidence is likely to change confidence |
| Conditional Recommendation (Net benefit or harm comparable to other options) | -Benefits=Risks/Burdens -Best action depends on individual patient circumstances -Future Research is unlikely to change confidence |
-Benefits= Risks/Burdens -Best action appears to depend on individual patient circumstances -Better evidence could change confidence |
-Balance between Benefits & Risks/Burdens unclear -Net benefit (or net harm) comparable to other options -Alternative strategies may be equally reasonable -Better evidence likely to change confidence |
| Clinical Principle | a statement about a component of clinical care that is widely agreed upon by urologists or other clinicians for which there may or may not be evidence in the medical literature | ||
| Expert Opinion | a statement, achieved by consensus of the Panel, that is based on members’ clinical training, experience, knowledge, and judgment for which there may or may not be evidence in the medical literature | ||
Abbreviations
- 95% CI
95% confidence interval
- AUA
American Urological Association
- DRE
Digital rectal exam
- ERSPC
European randomized study of screening for prostate cancer
- GG
Grade group
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- mpMRI
Multi-parametric magnetic resonance imaging
- MRI
Magnetic resonance imaging
- NND
Number needed to diagnose
- NNS
Number needed to screen
- PBCG
Prostate biopsy collaborative group
- PCPT
Prostate cancer prevention trial
- PI-RADS
Prostate Imaging Reporting & Data System
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
- PPV
Positive predictive value
- PSA
Prostate-specific antigen
- RCT
Randomized control trial
- RR
Relative risk
- SDM
Shared decision-making
- SSA
Social Security Administration
- STHLM-3
Stockholm-3
- SUO
Society of Urologic Oncology
Contributor Information
John T. Wei, University of Michigan.
Daniel Barocas, Vanderbilt University.
Sigrid Carlsson, Memorial Sloan Kettering Cancer Center.
Fergus Coakley, Oregon Health & Science University.
Scott Eggener, University of Chicago.
Ruth Etzioni, Fred Hutchinson Cancer Center.
Samson W. Fine, Memorial Sloan Kettering Cancer Center.
Misop Han, Johns Hopkins University.
Sennett K. Kim, American Urological Association
Erin Kirkby, American Urological Association.
Badrinath R. Konety, Allina Health.
Martin Miner, Brown University.
Kelvin Moses, Vanderbilt University.
Merel G. Nissenberg, National Alliance of State Prostate Cancer Coalitions.
Peter A. Pinto, National Institutes of Health.
Simpa S. Salami, University of Michigan.
Lesley Souter, Nomadic E.B.M Methodology.
Ian M. Thompson, CHRISTUS Health.
Daniel W. Lin, University of Washington.
References
- 1.Siegel RL, Miller KD, Wagle NS et al. : Cancer statistics, 2023. CA Cancer J Clin 2023; 73: 17. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P: Epidemiology of prostate cancer. World J Oncol 2019; 10: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggener SE, Scardino PT, Walsh PC et al. : Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011; 185: 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahal BA, Berman RA, Taplin ME et al. : Prostate cancer-specific mortality across gleason scores in black vs nonblack men. Jama 2018; 320: 2479. [DOI] [PubMed] [Google Scholar]
- 5.Makarov DV, Chrouser K, Gore JL et al. : Aua white paper on implementation of shared decision making into urological practice. Urology Practice 2016; 3: 355. [DOI] [PubMed] [Google Scholar]
- 6.Riikonen JM, Guyatt GH, Kilpelainen TP et al. : Decision aids for prostate cancer screening choice: A systematic review and meta-analysis. JAMA Intern Med 2019; 179: 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugosson J, Roobol MJ, Mansson M et al. : A 16-yr follow-up of the european randomized study of screening for prostate cancer. Eur Urol 2019; 76: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugosson J, Godtman RA, Carlsson SV et al. : Eighteen-year follow-up of the goteborg randomized population-based prostate cancer screening trial: Effect of sociodemographic variables on participation, prostate cancer incidence and mortality. Scand J Urol 2018; 52: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati R, Gore JL and Etzioni R: Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: Model estimates of potential benefits and harms. Annals of Internal Medicine 2013; 158: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partin AW, Criley SR, Subong EN et al. : Standard versus age-specific prostate specific antigen reference ranges among men with clinically localized prostate cancer: A pathological analysis. J Urol 1996; 155: 1336. [PubMed] [Google Scholar]
- 11.Eastham JA, Riedel E, Scardino PT et al. : Variation of serum prostate-specific antigen levels: An evaluation of year-to-year fluctuations. JAMA 2003; 289: 2695. [DOI] [PubMed] [Google Scholar]
- 12.Nordström T, Adolfsson J, Grönberg H et al. : Repeat prostate-specific antigen tests before prostate biopsy decisions. J Natl Cancer Inst 2016; 108 [DOI] [PubMed] [Google Scholar]
- 13.Vickers AJ, Ulmert D, Sjoberg DD et al. : Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: Case-control study. BMJ 2013; 346: f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsov C, Albers P, Herkommer K et al. : A randomized trial of risk-adapted screening for prostate cancer in young men-results of the first screening round of the probase trial. Int J Cancer 2022; 150: 1861. [DOI] [PubMed] [Google Scholar]
- 15.Gulati R, Cheng HH, Lange PH et al. : Screening men at increased risk for prostate cancer diagnosis: Model estimates of benefits and harms. Cancer Epidemiology, Biomarkers & Prevention 2017; 26: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giaquinto AN, Miller KD, Tossas KY et al. : Cancer statistics for african american/black people 2022. CA Cancer J Clin 2022; 72: 202. [DOI] [PubMed] [Google Scholar]
- 17.Tsodikov A, Gulati R, de Carvalho TM et al. : Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 2017; 123: 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaeffer EM, Srinivas S, Adra N et al. : Nccn guidelines® insights: Prostate cancer, version 1.2023. J Natl Compr Canc Netw 2022; 20: 1288. [DOI] [PubMed] [Google Scholar]
- 19.Giri VN, Knudsen KE, Kelly WK et al. : Role of genetic testing for inherited prostate cancer risk: Philadelphia prostate cancer consensus conference 2017. J Clin Oncol 2018; 36: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements MB, Vertosick EA, Guerrios-Rivera L et al. : Defining the impact of family history on detection of high-grade prostate cancer in a large multi-institutional cohort. Eur Urol 2022; 82: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bratt O, Drevin L, Akre O et al. : Family history and probability of prostate cancer, differentiated by risk category: A nationwide population-based study. J Natl Cancer Inst 2016; 108 [DOI] [PubMed] [Google Scholar]
- 22.Barber L, Gerke T, Markt SC et al. : Family history of breast or prostate cancer and prostate cancer risk. Clin Cancer Res 2018; 24: 5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JA 2nd, Gerber L, Moreira DM et al. : Prostate cancer risk in men with prostate and breast cancer family history: Results from the reduce study (r1). J Intern Med 2012; 272: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albright F, Stephenson RA, Agarwal N et al. : Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015; 75: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard CC, Mateo J, Walsh MF et al. : Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016; 375: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bancroft EK, Page EC, Brook MN et al. : A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (impact): Initial results from an international prospective study. Lancet Oncol 2021; 22: 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra AV, Bancroft EK, Barbachano Y et al. : Targeted prostate cancer screening in men with mutations in brca1 and brca2 detects aggressive prostate cancer: Preliminary analysis of the results of the impact study. BJU International 2011; 107: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page EC, Bancroft EK, Brook MN et al. : Interim results from the impact study: Evidence for prostate-specific antigen screening in brca2 mutation carriers. European Urology 2019; 76: 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franlund M, Mansson M, Godtman RA et al. : Results from 22 years of followup in the goteborg randomized population-based prostate cancer screening trial. J Urol 2022; 208: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson S, Assel M, Sjoberg D et al. : Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: Population based cohort study. BMJ 2014; 348: g2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roobol MJ, Roobol DW and Schroder FH: Is additional testing necessary in men with prostate-specific antigen levels of 1.0 ng/ml or less in a population-based screening setting? (erspc, section rotterdam). Urology 2005; 65: 343. [DOI] [PubMed] [Google Scholar]
- 32.Vickers AJ, Cronin AM, Bjork T et al. : Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: Case-control study. BMJ 2010; 341: c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vertosick EA, Haggstrom C, Sjoberg DD et al. : Prespecified 4-kallikrein marker model at age 50 or 60 for early detection of lethal prostate cancer in a large population based cohort of asymptomatic men followed for 20 years. J Urol 2020; 204: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston MA, Gerke T, Carlsson SV et al. : Baseline prostate-specific antigen level in midlife and aggressive prostate cancer in black men. Eur Urol 2019; 75: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston MA, Batista JL, Wilson KM et al. : Baseline prostate-specific antigen levels in midlife predict lethal prostate cancer. J Clin Oncol 2016; 34: 2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovac E, Carlsson SV, Lilja H et al. : Association of baseline prostate-specific antigen level with long-term diagnosis of clinically significant prostate cancer among patients aged 55 to 60 years: A secondary analysis of a cohort in the prostate, lung, colorectal, and ovarian (plco) cancer screening trial. JAMA Netw Open 2020; 3: e1919284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heijnsdijk EA, Wever EM, Auvinen A et al. : Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012; 367: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross KS, Carter HB, Pearson JD et al. : Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA 2000; 284: 1399. [DOI] [PubMed] [Google Scholar]
- 39.Heijnsdijk EAM, Gulati R, Tsodikov A et al. : Lifetime benefits and harms of prostate-specific antigen-based risk-stratified screening for prostate cancer. Journal of the National Cancer Institute 2020; 112: 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grenabo Bergdahl A, Holmberg E, Moss S et al. : Incidence of prostate cancer after termination of screening in a population-based randomised screening trial. European Urology 2013; 64: 703. [DOI] [PubMed] [Google Scholar]
- 41.Godtman RA, Kollberg KS, Pihl CG et al. : The association between age, prostate cancer risk, and higher gleason score in a long-term screening program: Results from the goteborg-1 prostate cancer screening trial. Eur Urol 2022; 82: 311. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer EM, Carter HB, Kettermann A et al. : Prostate specific antigen testing among the elderly--when to stop? Journal of Urology 2009; 181: 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder FH, Hugosson J, Roobol MJ et al. : Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine 2012; 366: 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bill-Axelson A, Holmberg L, Garmo H et al. : Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370: 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamdy FC, Donovan JL, Lane JA et al. : 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415. [DOI] [PubMed] [Google Scholar]
- 46.Wilt TJ, Vo TN, Langsetmo L et al. : Radical prostatectomy or observation for clinically localized prostate cancer: Extended follow-up of the prostate cancer intervention versus observation trial (pivot). Eur Urol 2020; 77: 713. [DOI] [PubMed] [Google Scholar]
- 47.Vickers AJ, Sjoberg DD, Ulmert D et al. : Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med 2014; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson JR, Clarke MG, Ewings P et al. : The assessment of patient life-expectancy: How accurate are urologists and oncologists? BJU Int 2005; 95: 794. [DOI] [PubMed] [Google Scholar]
- 49.Vickers AJ, Till C, Tangen CM et al. : An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. Journal of the National Cancer Institute 2011; 103: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vickers AJ, Wolters T, Savage CJ et al. : Prostate-specific antigen velocity for early detection of prostate cancer: Result from a large, representative, population-based cohort. European Urology 2009; 56: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carbunaru S, Nettey OS, Gogana P et al. : A comparative effectiveness analysis of the pbcg vs. Pcpt risks calculators in a multi-ethnic cohort. BMC Urol 2019; 19: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breza J, Subin F, Bernadic M et al. : The use of european randomized study of screening for prostate cancer calculator as a diagnostic tool for prostate biopsy indication. Bratisl Lek Listy 2019; 120: 331. [DOI] [PubMed] [Google Scholar]


