INTRODUCTION
Mycosis fungoides, a rare type of cutaneous T-cell lymphoma, has an annual incidence of approximately five per one million people1 and presents more commonly in males older than 50 years.2 No clear etiology of mycosis fungoides has been identified, but implicated causes include: genetic and epigenetic mutations, solvents and chemical irritants, and human T-lymphotropic virus type 1 (HTLV-1).3,4
The disease process occurs after a clonal expansion of CD4 T-cells, which accumulate in the skin’s dermis.5 Initially, these clusters of aberrant cells manifest as erythematous scaly patches on the skin’s surface, which may progress through stages of plaque formation and develop into tumors.6 As malignant cells accumulate, the clinical manifestation of mycosis fungoides becomes evident. When mycosis fungoides advances to include extracutaneous features (e.g., lymph node involvement), survival is typically less than 18 months.7 In this case, we present a patient with delayed diagnosis of stage IVa mycosis fungoides.
CASE REPORT
A 39-year-old male with a history of chronic methamphetamine and heavy tobacco use presented to an emergency department with a chief complaint of persistent skin plaques that had been present for more than two years prior to the visit. The patient described the lesions as pruritic and increasingly tender in the last two weeks. He reported weight loss of approximately 15 pounds in the last year, left leg swelling, “lumps” in his groin (which reportedly occurred in the past month), and mild fever and chills.
Multiple round, well-demarcated, erythematous, beefy plaques were noted on his trunk (Figure 1), back (Figure 2), and left lower extremity (12x14cm; Figure 3), as well as some smaller scaly lesions on his upper lip, left upper extremity, and anterior hair line. Plaques over the inguinal area and back were noted to have clear drainage. In addition, bilateral inguinal lymphadenopathy was noted, with a right inguinal node measuring 5.2x3.4cm and a left inguinal node measuring 6.6x3.5cm.
Figure 1.
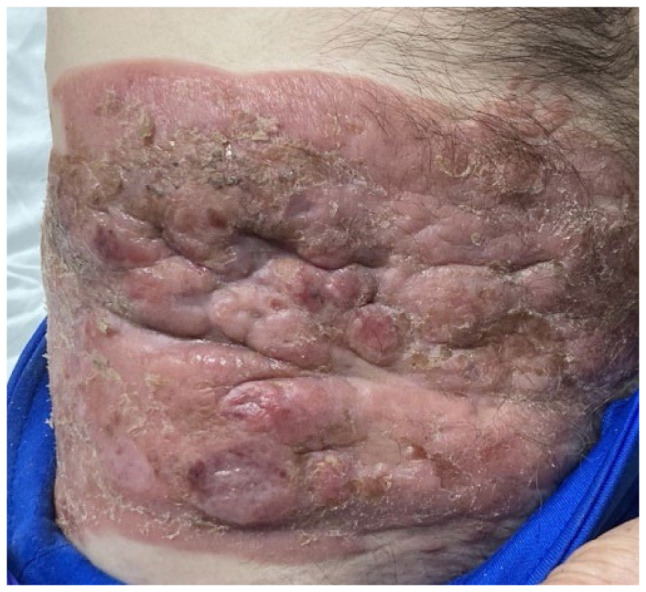
Right lower abdomen/flank lesion, tumor stage.
Figure 2.
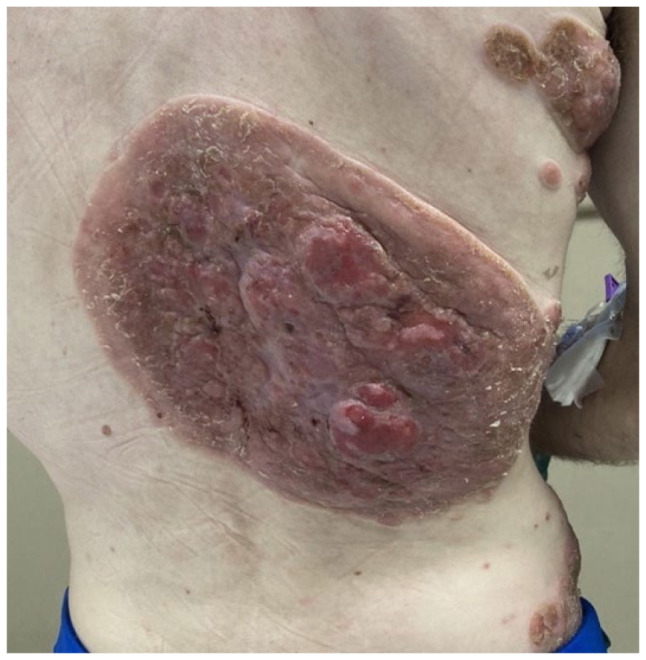
Right mid-back lesion, tumor stage.
Figure 3.
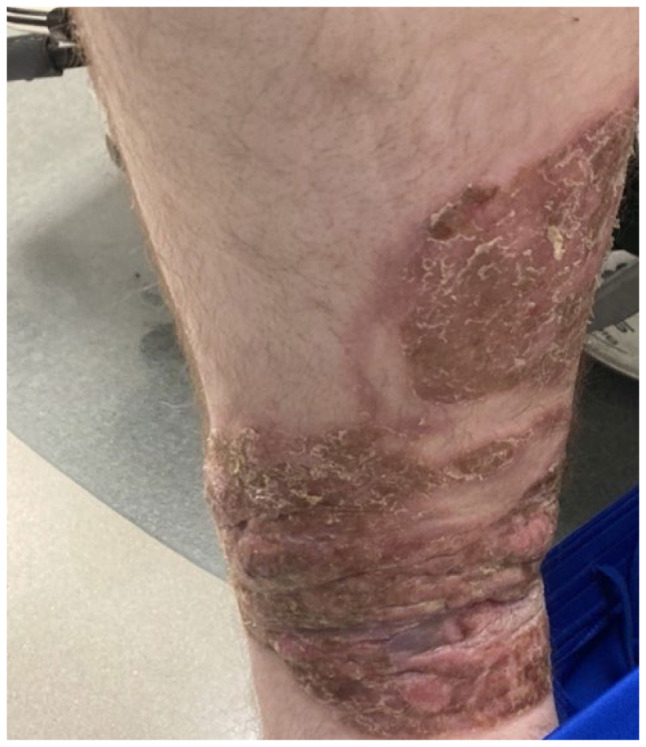
Left lower extremity lesion, tumor stage.
The patient’s labs were noteworthy for elevated levels of lactic acid (2.6mEq/L), serum glucose (155mg/dl), and C-reactive protein (CRP; 53.14mg/L). A lactate dehydrogenase (LDH) level was elevated at 265 U/L, and a beta 2 micro globulin was elevated at level greater than 3mg/L.
Peripheral smear for abnormal lymphocytes (Sézary cells), lymph node biopsy of the left inguinal lymph node, punch biopsy of the lower back lesion, and computerized tomography (CT) abdomen and chest were ordered for diagnostic workup of a suspected malignancy. The CT noted bilateral axillary lymphadenopathy and inguinal lymph node expansion as well as expansion in the lower iliac chain.
Histology of punch biopsies from the right flank and left lower extremity revealed a morphology and immunophenotype consistent with mycosis fungoides. No Sézary cells were detected in the peripheral smear. Additionally, next-generation sequencing of the skin biopsy specimen confirmed a clonal T-cell gene rearrangement. A core needle biopsy of the left inguinal lymph node revealed the presence of atypical lymphocytes that stained positive for cluster designation CD3 and CD5, with an increased abundance of CD4-positive T-cells compared to CD8-positive cells indicating lymphatic spread of disease. Further analysis with a bone marrow biopsy yielded no evidence of suspicious cellular invasion.
A diagnosis of mycosis fungoides was made. Considering the extracutaneous involvement observed with cancer cells noted in the lymph nodes and the description of the skin lesions, the cancer was classified as Stage IVa. At the time of this report, the patient is reportedly responding well to chemotherapy with lesion shrinkage at all sites.
DISCUSSION
This case report is unusual in that mycosis fungoides typically presents in older adults, but this patient was 39 years old. Mycosis fungoides is one of the most common types of cutaneous T-cell lymphoma, a type of non-Hodgkin lymphoma. Mycosis fungoides has four phases: the premycotic phase characterized by asymptomatic scaly, red rashes; the patch phase characterized by an eczema-like rash; the plaque phase characterized by papules or hardened lesions; and the tumor phase characterized by the development of skin tumors and ulcerations.8 Because the early stages can resemble eczema, diagnosis is sometimes delayed. In this case, the patient believed that the lesions were psoriasis, which did not worry him; as a result, he did not seek care.
The histopathology of mycosis fungoides found on biopsy can vary according to disease stage. Epidermotropism, a cardinal feature of mycosis fungoides, can be seen in the early stages of mycosis fungoides and is defined by lymphocytic infiltrate into the epidermis.9 Patch stage can be defined on histology as an infiltrate in a band-like distribution along the basal cell layer.10 Plaque stage can be defined by lymphocyte penetration into the dermis itself and collections of lymphocytes in the epidermis known as Pautrier’s abscesses. Tumor stage is often defined by a loss of epidermotropism, as well as a concentrated dermal lymphocytic infiltrate that indicates deeper penetration of lymphocytes into the skin itself.11 Our patient’s punch biopsies revealed abnormal findings for tumor stage pathology with epidermotropic infiltrates found in both specimens. Tumor stage was confirmed however, given the dense dermal infiltration shown throughout both specimens.
Prognosis is dependent on the cancer stage. A retrospective cohort study by the Cutaneous Lymphoma International Consortium reported four independent prognostic markers indicative of a worse survival: stage IV disease, age > 60 years, large-cell transformation, and increased lactate dehydrogenase.12 Five-year survival is 91% to 97% if only localized, non-infiltrating patches or plaques are present. If there is lymph node involvement, five-year survival is 20% to 30%.13
Treatment of mycosis fungoides is palliative, not curative. Remission can be achieved among patients who undergo allogeneic stem cell transplantation. 14 In advanced stage III/IV disease, treatments may include: photodynamic therapy (such as ultraviolet radiation or extracorporeal photopheresis and electron-beam radiation), biologics, chemotherapy, topical corticosteroids, bexarotene, targeted therapy with brentuximab vedotin, or checkpoint inhibiters such as pembrolizumab.15
Further research needs to be done to develop global treatment regimens and curative forms of therapy for cutaneous T-cell lymphomas. Additionally, programs or interventions to enhance early detection of mycosis fungoides and encourage patients to seek regular appointments with primary care providers for skin checks should be investigated and implemented.
REFERENCES
- 1.Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: A SEER population data analysis. JAMA Oncol. 2022;8(11):1690–1692. doi: 10.1001/jamaoncol.2022.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorim GM, Niemeyer-Corbellini JP, Quintella DC, Cuzzi T, Ramos-E-Silva M. Clinical and epidemiological profile of patients with early stage mycosis fungoides. An Bras Dermatol. 2018;93(4):546–552. doi: 10.1590/abd1806-4841.20187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126(4):508–519. doi: 10.1182/blood-2014-11-611194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonin S, Tothova SM, Barbazza R, Brunetti D, Stanta G, Trevisan G. Evidence of multiple infectious agents in mycosis fungoides lesions. Exp Mol Pathol. 2010;89(1):46–50. doi: 10.1016/j.yexmp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 5.García-Díaz N, Piris MÁ, Ortiz-Romero PL, Vaqué JP. Mycosis fungoides and Sézary syndrome: An integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers (Basel) 2021;13(8):1931. doi: 10.3390/cancers13081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonak C, Tittes J, Brunner PM, Guenova E. Mycosis fungoides and Sézary syndrome. J Dtsch Dermatol Ges. 2021;19(9):1307–1334. doi: 10.1111/ddg.14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome: An update. Hematol Oncol Clin North Am. 2019;33(1):103–120. doi: 10.1016/j.hoc.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gawkrodger DJ, Ardern-Jones MR. Dermatology: An Illustrated Colour Text. 6th edition 2017. [Google Scholar]
- 9.Vaidya T, Badri T. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Jan, Mycosis fungoides. [PubMed] [Google Scholar]
- 10.Muñoz-González H, Molina-Ruiz AM, Requena L. Clinicopathologic variants of mycosis fungoides. Actas Dermosifiliogr. 2017;108(3):192–208. doi: 10.1016/j.ad.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):2–10. doi: 10.12788/j.sder.2018.002. [DOI] [PubMed] [Google Scholar]
- 12.Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: Effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33(32):3766–3773. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyashiro D, Sanches JA. Mycosis fungoides and Sézary syndrome: Clinical presentation, diagnosis, staging, and therapeutic management. Front Oncol. 2023;13:1141108. doi: 10.3389/fonc.2023.1141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal A, O’Leary D, Foss F. Allogeneic stem cell transplant for treatment of mycosis fungoides and Sezary syndrome: A systematic review and meta-analysis. Bone Marrow Transplant. 2024;59(1):41–51. doi: 10.1038/s41409-023-02122-0. [DOI] [PubMed] [Google Scholar]
- 15.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries [Internet] Bethesda (MD): National Cancer Institute (US); 2002. Mycosis fungoides (including Sézary syndrome) treatment (PDQ®): Patient version. [PubMed] [Google Scholar]


