Abstract
Background.
Our aim was to evaluate the ease and utility of using indocyanine green fluorescence angiography for intraoperative localization of the parathyroid glands.
Methods.
Indocyanine green fluorescence angiography was performed during 60 parathyroidectomies for primary hyperparathyroidism during a 22-month period. Indocyanine green was administered intravenously to guide operative navigation using a commercially available fluorescence imaging system. Video files were graded by 3 independent surgeons for strength of enhancement using an adapted numeric scoring system.
Results.
There were 46 (77%) female patients and 14 (23%) male patients whose ages ranged from 17 to 87 (average 60) years old. Of the 60 patients, 43 (71.6%) showed strong enhancement, 13 (21.7%) demonstrated mild to moderate vascular enhancement, and 4 (6.7%) exhibited little or no vascular enhancement. Of the 54 patients who had a preoperative sestamibi scan, a parathyroid adenoma was identified in 36, while 18 failed to localize. Of the 18 patients who failed to localize, all 18 patients (100%) had an adenoma that fluoresced on indocyanine green imaging. The operations were performed safely with minimal blood loss and short operative times.
Conclusion.
Indocyanine green angiography has the potential to assist surgeons in identifying parathyroid glands rapidly with minimal risk.
Primary hyperparathyroidism (PHPT) is due to benign adenomatous proliferation of ≥1 parathyroid glands leading to excessive parathyroid hormone secretion. It is limited typically to a single adenoma, but multiglandular disease occurs in 10% to 15% of patients.1,2 Operative resection is the only definitive treatment, but successful preoperative and intraoperative localization of an aberrant parathyroid gland(s) can be challenging due to their variable location. Classic preoperative localization modalities are ultrasonography and nuclear scintigraphy. The fidelity of these techniques can vary by patient and institution, which has led to the development and utilization of computed tomography (CT), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) protocols for high-resolution axial images.3 These detailed images can provide valuable information in patients with negative sestamibi scans or in cases of reoperation for persistent hyperparathyroidism; however, their increased cost limits their widespread applicability. The sensitivity for detection of parathyroid glands with these studies is highly variable and can be institution dependent.
Successful localization allows for an efficient, focused dissection, avoids the need for routine 4-gland exploration, and decreases the risk of bilateral, recurrent laryngeal nerve injury. By contrast, there are very few methods for intraoperative localization of the parathyroid glands. Intraoperative sestamibi using a gamma probe has been attempted but is cumbersome, exposes the patients to further radiation, and is not used widely. Other fluorescent agents, such as aminolevulinic acid and methylene blue, also have been explored with variable success. With the current available techniques, the operating surgeon is reliant on intraoperative parathyroid hormone (PTH levels) and frozen sections to confirm that all abnormal parathyroid glands have been resected.
Indocyanine green (ICG) is an inert, nontoxic, organic dye that after intravenous injection circulates through the intravascular space bound to plasma proteins until it is cleared exclusively through the hepatobiliary system. With excitation with near infrared (NIR) light, the compound emits a fluorescent signal that can be detected with a number of Food & Drug Administration-approved, fluorescence imaging devices. Based on the biodistribution and excretion properties of the dye, ICG has taken the forefront in fluorescence-guided surgery for broad applications, such as fluorescence cholangiography, perfusion assessment of gastrointestinal anastomoses, real-time lymph node mapping, and adrenalectomy, all without the use of ionizing radiation.4 Because the parathyroid gland is a hypervascular endocrine organ, we hypothesize that ICG fluorescence angiography will assist with localization and resection of parathyroid adenomas.
Intravenous ICG administration for visualization of the parathyroid glands was first described in 2015 in a canine animal model.5 Later that year, we reported the use of ICG in a patient undergoing parathyroidectomy for PHPT6 and in another patient undergoing redo parathyroid surgery.7 Other groups have explored the utility of ICG in identification of parathyroid glands during parathyroidectomy and parathyroid preservation during total thyroidectomy.8–12 ICG is a nonselective agent that does not target specifically the parathyroid parenchyma; however, because the glands receive substantially more blood flow than the adjacent tissue, they emit a strong fluorescent signal that demarcates the borders of the gland. In this retrospective review of our early experience, we show the utility of ICG in the intraoperative detection of parathyroid adenomas during parathyroidectomy for PHPT.
Materials and Methods
The medical records of 60 consecutive patients with PHPT undergoing parathyroidectomy with ICG fluorescence angiography from April 2015 to January 2017 were retrospectively reviewed in an IRB-approved study. Informed consent for surgery was obtained from all patients. All operations were performed by a single surgeon (M.B.) at a single university hospital. All patients underwent intraoperative fluorescence imaging using ICG and the PINPOINT Endoscopic Fluorescence Imaging System (Novadaq Technologies Inc., Bonita Springs, FL), which was used in an extracorporeal fashion for the purposes of this study.
The parathyroid glands were accessed through a standard, transverse cervical incision. Patients received a focused unilateral neck dissection if the preoperative imaging modalities indicated laterality. If the preoperative imaging modalities did not indicate laterality, a 4 gland exploration was performed. The strap muscles were retracted laterally, and the thyroid gland was mobilized to identify a candidate parathyroid gland. After the suspected lesion was identified and exposed, 3 mL of ICG (2.5 mg/mL of ICG dissolved in sterile water) was administered as an intravenous bolus by the anesthesiologist, and the fluorescence-capable laparoscope was directed into the operative field. Exposed parathyroid adenomas exhibited fluorescent enhancement typically within 1 minute of injection. If a parathyroid adenoma was not localized, further dissection was performed, and the ICG fluorescence imaging was repeated. After confirming fluorescence with ICG, the vascular pedicle of the parathyroid gland was ligated, and the sample was sent for frozen section confirmation. No other intraoperative adjuncts were used for localization of the parathyroid glands in this study.
Video files were saved to a password-protected, external hard drive for further analysis. The video clips were evaluated by 2 independent surgeons based on a scoring system described by Fortuny et al, where 0 = no fluorescent signal, 1 = moderate fluorescent signal, and 2 = strong fluorescent signal.10 A third, senior endocrine surgeon was available to review the footage and to resolve any scoring discrepancies between the first 2 surgeons.
Results
There were 46 (77%) female patients and 14 (23%) male patients whose ages ranged from 17 to 87 (average 60) years old. Body mass index ranged from 19 to 56 (average 28). Of the 60 patients, 9 patients (15%) had a history of neck surgery in the immediate area of dissection, 4 (7%) had previous parathyroid operations at other institutions, 3 (5%) had a previous thyroidectomy, and one patient each had a previous cervical fusion and a previous Zenker’s diverticulectomy. Preoperative localization included ultrasonography in 35 patients (58.3%), sestamibi parathyroid nuclear scan in 54 (90%), neck CT in 25 (42%), and neck MRI in 4 (7%). Preoperative PTH levels ranged from 12 to 527 pg/mL, and postoperative PTH ranged from 7 to 80 pg/mL, with an average decrease in PTH of 124 pg/mL (range 5 to 510 pg/mL). Preoperative calcium (Ca) levels ranged from 8 to 15.1 mg/dL (average 11.1 mg/dL) and postoperative Ca levels ranged from 8.1–10.8 mg/dL (average 9.48 mg/dL) with an average decrease of 1.62 mg/dL. All specimens were sent to the pathology department for histologic analysis; 51 patients (85%) had a single adenoma, 4 patients (7%) had double adenomas, and 1 patient each had triple adenomas, a benign parathyroid cyst, a water clear cell parathyroid adenoma, non-necrotizing granulomatous inflammation, and 4 gland hyperplasia. The operations included 45 patients (75%) who had focused parathyroidectomies and 15 (25%) had bilateral explorations. Operative times ranged from 26 to 215 minutes with an average operative time of 54 minutes and a median of 46 minutes.
ICG was administered to all 60 patients undergoing parathyroidectomy. A typical single gland adenoma is shown in Fig 1. A patient with 4 gland hyperplasia is depicted in Fig 2. Of the 60 patients, 4 patients (7%) had parathyroid glands that exhibited little or no vascular enhancing, thereby receiving a score of 0, 13 patients (22%) demonstrated mild to moderate vascular enhancement receiving a score of 1, and 43 patients (72%) showed strong enhancement receiving a score of 2. Examples of the scoring system are shown in Fig 3. There were 3 discrepancies between the surgeons which were adjudicated by the operating surgeon (M.B.).
Fig. 1.
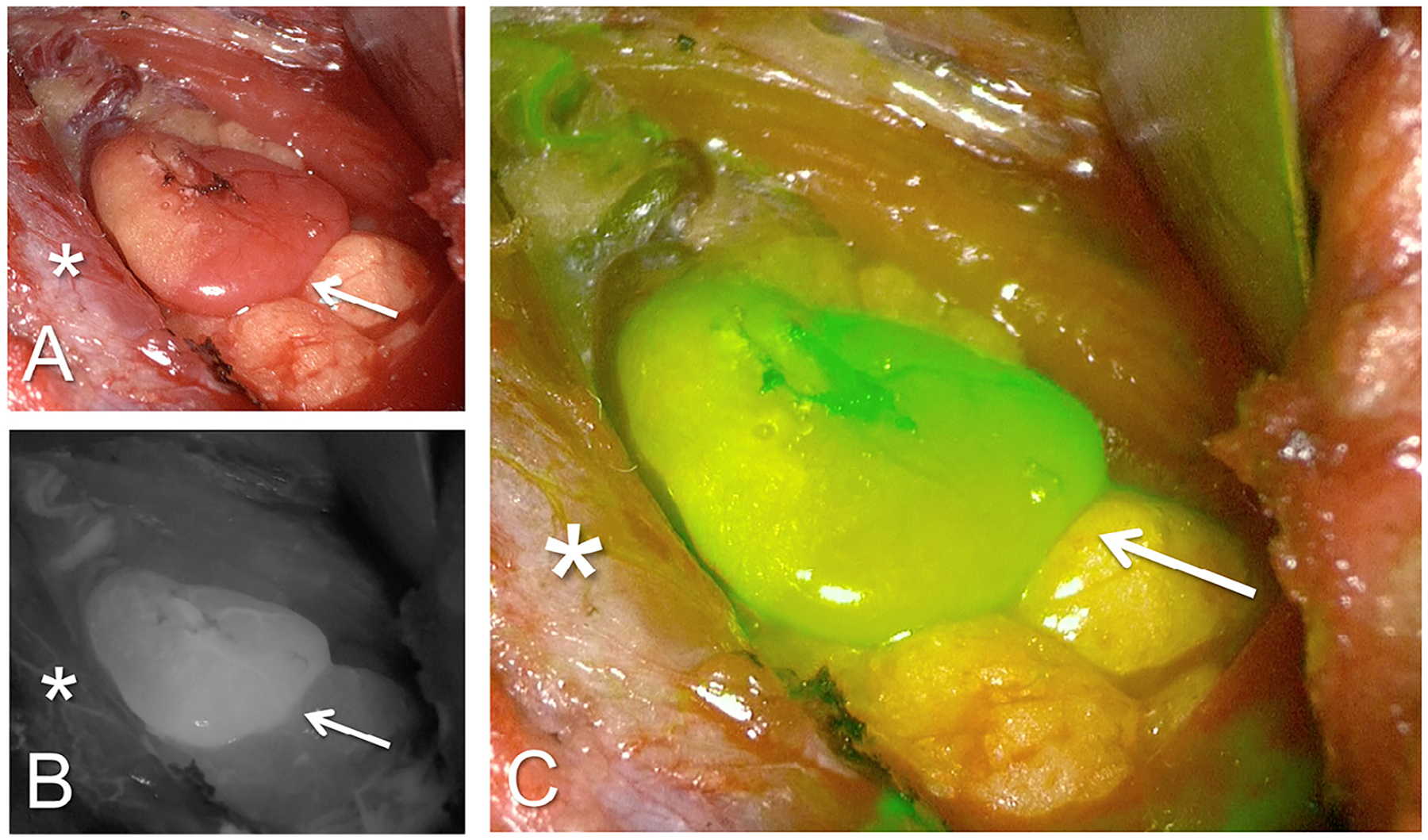
ICG fluorescence angiography in a patient with a single parathyroid adenoma. (A) The bright light image of the operating field. Arrows indicate parathyroid gland and asterisk indicates the thyroid gland in all. (B) Same field in the greyscale fluorescent mode. (C) Fluorescent mode with computer-generated overlay where green color saturation is proportional to the intensity of the fluorescent signal.
Fig. 2.
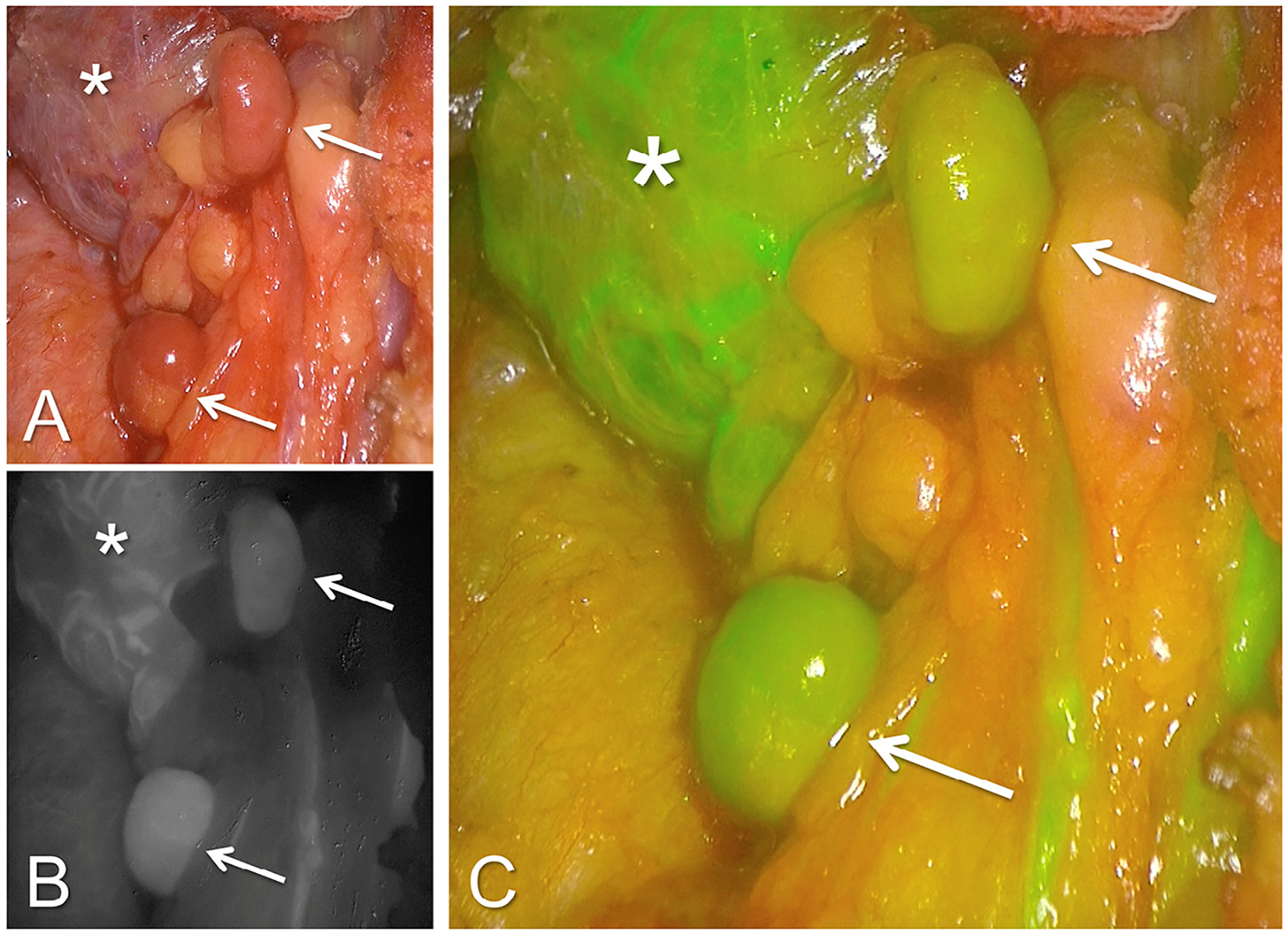
ICG fluorescence angiography in a patient with 4 gland hyperplasia. (A) The bright light image of the operating field. Arrows indicate 2 parathyroid glands and asterisk indicates the thyroid gland in all. (B) Same field in the greyscale fluorescent mode. (C) Fluorescent mode with computer-generated overlay where green color saturation is proportional to the intensity of the fluorescent signal.
Fig. 3.
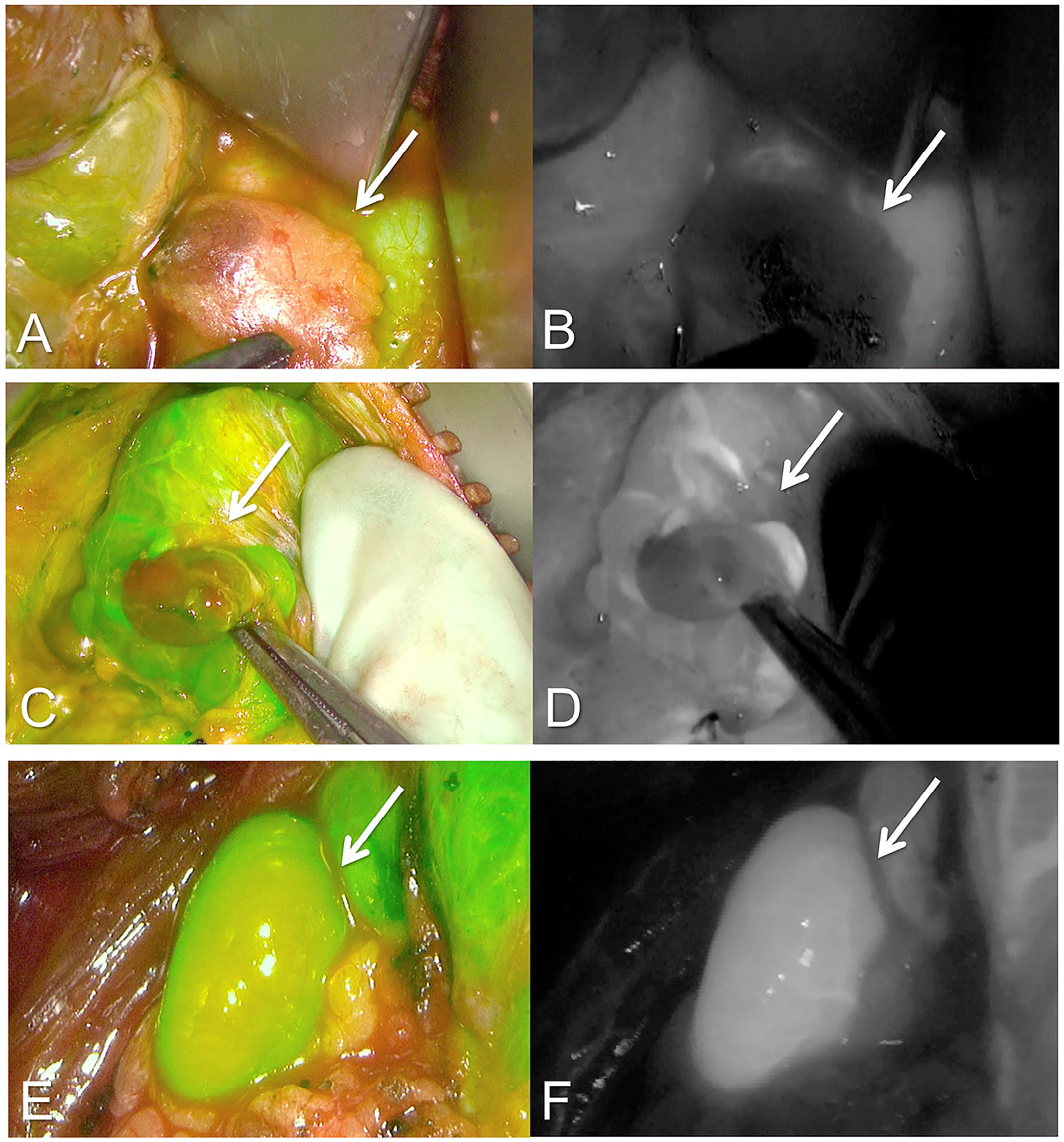
ICG fluorescence angiography modified grading scale for parathyroid adenomas. Computer-generated fluorescent overlay as well as the black and white modes of 3 separate patients. A and B have almost no fluorescent enhancement and receive a score of 0 on our modified scale. C and D demonstrate some uptake and receive a score of 1. E and F are greatly enhanced as evidenced by the strong green signal in E and the bright white area of demarcation in F and receive a score of 2.
Of the 35 patients who had an ultrasonography, a parathyroid adenoma was identified in 25 patients, while of the 10 patients who had parathyroid disease that failed to localize, all 10 (100%) had an adenoma with fluorescence on ICG imaging, and specifically, 7 patients (70%) had strong fluorescence and 3 (30%) had intermediate fluorescence.
Of the 54 patients who had a preoperative sestamibi scan, a parathyroid adenoma was identified in 36 patients. Of the 18 patients whose disease failed to localize, all 18 (100%) had an adenoma that fluoresced on ICG imaging, and specifically, 15 patients (83%) had strong fluorescence and 3 (17%) had intermediate fluorescence.
Of the 25 patients who had a neck CT, a parathyroid adenoma was identified in 22 and of the 3 patients whose disease failed to localize, all 3 patients (100%) had an adenoma that fluoresced on ICG imaging and specifically, 2 patients had strong fluorescence and 1 had intermediate fluorescence.
Of the 4 patients who had a neck MRI, a parathyroid adenoma was identified in 2 and of the 2 patients whose disease failed to localize, 1 had an adenoma that strongly fluoresced on ICG imaging, and 1 had no fluorescence on ICG imaging.
Of the 60 patients, all had a decrease in their intraoperative PTH level of >50%. Of these patients, 19 had a documented cure at 6 months defined as serum calcium of ≤10.6 mg/dL; 31 had a documented cure at 3 months. The remaining patients had follow up at <3 months or did not receive follow-up through our institution. There were no patients with a documented failure of treatment, and there were no cases of recurrent hyperparathyroidism.
Discussion
Despite advances in preoperative techniques for localization of parathyroid glands, correct identification of the abnormal parathyroid glands can be challenging. Classic adjuncts, such as intraoperative changes in PTH levels and frozen sections, are helpful for confirmation but are time consuming and cannot be performed until after the specimen is removed. Quick and effective intraoperative localization modalities can improve the efficacy of the parathyroidectomy. In this report, we show that ICG fluorescence angiography can be a useful and safe technique to aid in the intraoperative localization of parathyroid adenomas during parathyroidectomy.
ICG is being used as a marker in the assessment of the perfusion of tissues and organs in many areas of medicine. The specific wavelength of light needed for excitation of the fluorescent agent is generated by a near infrared light source which is attached directly to a camera. A digital video camera allows the absorption of the fluorescence emitted by ICG to be recorded in real time, which means that perfusion can be assessed and documented immediately. Assessment of blood flow or tissue perfusion of parathyroid glands is an on-label use of ICG and the PINPOINT endoscopic fluorescence imaging system; however, the PINPOINT system and ICG are not specifically indicated for visualization/identification of parathyroid glands at the present time and would therefore, be considered off-label use by the Food & Drug Administration.
ICG fluorescence angiography is not the first intraoperative, image-guided modality to be used to identify the parathyroid glands. The use of aminolevulinic acid, an oral photosensitizer, has been described by Prosst et al. Aminolevulinic acid was administered preoperatively and enhanced the visualization of parathyroid glands in 48% of patients to the degree that it resulted in faster detection.13 For more than half of the patients, however, this technique enhanced inadequately the parenchyma of the parathyroid gland, thereby limiting its clinical utility. Intravenous administration of methylene blue also has been utilized to enhance and localize parathyroid glands during parathyroidectomy.14 Such as ICG, methylene blue also emits fluorescence in the NIR wavelength, but methylene blue is dosed once at the beginning of the case. In their series of 12 patients, Tummers et al14 were able to label parathyroid adenomas successfully in 10 of 12 cases with low dose administration (0.5 mg/kg). Success rates of up to 97% have been reported with this technique when high dose methylene blue (5–10 mg/kg) is given, but this dosing has been associated with cutaneous complications.15
In contrast to high dose methylene blue, ICG fluorescence angiography is a safe and effective method for intraoperative localization of the parathyroid glands. The risk of serious adverse events with ICG injection has been reported previously to be about 0.05%.16 At our institution we have not experienced any adverse events. Of note, patients with iodine or shellfish allergies did not undergo ICG fluorescence angiography in our center. Per the drug information sheet of the manufacturer, IC-Green (Akorn Pharmaceuticals, Lake Forest, IL) contains sodium iodide and therefore, should be used with caution in patients with allergies to iodides.
Zaidi et al have demonstrated the feasibility of localizing parathyroid glands intraoperatively with ICG fluorescence angiography in a case series of 33 patients where the technique was successful in 93% of parathyroid glands that were detected by the naked eye.11 They concluded that ICG can localize parathyroid glands reliably during parathyroidectomy and in addition, allow for assessment of parathyroid perfusion in patients undergoing subtotal resection.
In the present report, we compared intraoperative ICG localization with the subset of patients who did not localize parathyroid adenomas on preoperative imaging studies. We found that ICG fluorescence was detected in all patients who failed to localize on preoperative ultrasonography and sestamibi scanning, indicating that this technique can be a useful adjunct in patients with nonlocalizing preoperative imaging studies. In the one patient who demonstrated a benign parathyroid cyst on pathology, this technique identified the parathyroid gland and was scored 2 (strong vascular enhancement). There were no cases where the ICG gave a false positive.
A potential drawback of ICG fluorescence angiography during parathyroidectomy is that currently, ICG fluorescence angiography can be used only as a confirmatory mechanism once a candidate gland is identified and brought into the field. We have found it helpful in reoperative parathyroidectomies when scar tissue is present. As the surgeon dissects in the area of the suspected adenoma, the fluorescence of the parathyroid adenoma can be detected which can guide the surgeon to find the adenoma. The initial cost of a fluorescence imaging device is another potential drawback; however, this technology is becoming standard on many of the latest platforms of laparoscopic and robotic surgery. Furthermore, ICG is a relatively inexpensive medical dye.
In conclusion, ICG fluorescence angiography has the potential to assist surgeons in identifying parathyroid adenomas rapidly with minimal risk. With the high safety profile of the dye and wide availability of NIR fluorescence imaging devices available currently, ICG fluorescence angiography should be considered as an adjunctive localization method during parathyroid surgery.
Footnotes
Presented at the Academic Surgical Congress on February 7–9, 2017, Las Vegas, NV.
References
- 1.Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005;132:359–72. [DOI] [PubMed] [Google Scholar]
- 2.Madkhali T, Alhefdhi A, Chen H, Elfenbein D. Primary hyperparathyroidism. Ulus Cerrahi Derg 2016;32:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren Frunzac R, Richards M. Computed tomography and magnetic resonance imaging of the thyroid and parathyroid glands. Front Horm Res 2016;45:16–23. [DOI] [PubMed] [Google Scholar]
- 4.DeLong JC, Hoffman RM, Bouvet M. Current status and future perspectives of fluorescence-guided surgery for cancer. Expert Rev Anticancer Ther 2016;16:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh YJ, Choi JY, Chai YJ, et al. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc 2015;29:2811–7. [DOI] [PubMed] [Google Scholar]
- 6.McCarty P, Metildi C, Kelly K, Maser C, Bouvet M. Parathyroidectomy using indocyanine green fluorescence imaging. J Video Endocrinol 2015;2. [Google Scholar]
- 7.Chakedis JM, Maser C, Brumund KT, Bouvet M. Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep 2015;2015:pii: bcr2015211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sound S, Okoh A, Yigitbas H, Yazici P, Berber E. Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg Innov 2015;doi: 10.1177/1553350615613450. [DOI] [PubMed] [Google Scholar]
- 9.Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal Fortuny J, Karenovics W, Triponez F, Sadowski SM. Intra-operative indocyanine green angiography of the parathyroid gland. World J Surg 2016;40:2378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidi N, Bucak E, Okoh A, Yazici P, Yigitbas H, Berber E. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 2016;113:771–4. [DOI] [PubMed] [Google Scholar]
- 12.Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775–8. [DOI] [PubMed] [Google Scholar]
- 13.Prosst RL, Weiss J, Hupp L, Willeke F, Post S. Fluorescence-guided minimally invasive parathyroidectomy: clinical experience with a novel intraoperative detection technique for parathyroid glands. World J Surg 2010;34:2217–22. [DOI] [PubMed] [Google Scholar]
- 14.Tummers QR, Schepers A, Hamming JF, et al. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose Methylene Blue. Surgery 2015;158:1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman ED, Thambi R, Pytynia KB. Methylene blue and parathyroid adenoma localization: three new cases of a rare cutaneous complication. Ear Nose Throat J 2016;95:70–2. [PubMed] [Google Scholar]
- 16.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529–33. [DOI] [PubMed] [Google Scholar]


