Abstract
Allometric dose scaling aims to create isometric exposures between animals and humans and is often employed in preclinical pharmacokinetic/pharmacodynamic models. Bolus dose allometric scaling is the most simple and commonly used strategy in kidney injury studies; however, it is possible to humanize drug exposures. Currently, it is unknown if dose-matched bolus allometric scaling results in similar outcomes to humanized infusions in the vancomycin induced kidney injury model. We utilized a preclinical Sprague-Dawley rat model to compare traditional allometrically scaled dose-matched bolus administration of vancomycin to a computer-controlled and humanized infusion scheme to assess for differences in iohexol-measured kidney function and urinary kidney injury biomarkers.
Following 24 hours of vancomycin administration, rats in the humanized infusion group had equivalent area under the curve exposures to animals in the dose-matched bolus group (93.7 mg∙h/L [IQR 90.2 to 97.2] vs. 99.5 mg∙h/L [IQR 95.1 to 104.0], p=0.07). No significant differences in iohexol-measured kidney function nor the urinary kidney injury biomarkers, kidney injury molecule-1, clusterin, and osteopontin, were detected.
Administration of intravenous vancomycin as either a humanized infusion or dose-matched bolus resulted in similar vancomycin exposures, but no differences in iohexol-measured GFR and urinary kidney injury biomarkers among male Sprague-Dawley rats.
Keywords: vancomycin, allometric dosing, kidney injury
Introduction:
Vancomycin is a glycopeptide antibiotic which is known to be nephrotoxic [1]. To investigate this issue, preclinical studies have utilized animal models with allometric scaling of doses to approximate human exposures [2–4]. Allometric dose scaling is employed for a variety of purposes in drug development including dose finding for first-in-human clinical trials, dose extrapolation for veterinary applications, and dose extrapolation for experimental purposes [5–8]. It is particularly useful in bridging the gap from preclinical experiments to clinical applications when clinical evidence is either non-existent or difficult to obtain due to trial costs. Allometric dose scaling in preclinical models can be accomplished with a variety of methods ranging from allometrically-scaled bolus injections to humanized infusion schemes that mimic human pharmacokinetic and pharmacodynamic (PKPD) patterns [9, 10].
Although fully humanized preclinical infusion schemes for toxicity provide the theoretical advantage of being able to closely approximate human PKPD, these experiments often entail significant additional cost in terms of time, labor, and cost of materials. Thus, the sole purpose of this study was to compare kidney function between two preclinical models, i.e. a humanized infusion and a traditional dose-matched, allometrically scaled bolus dosing model for vancomycin in Sprague Dawley rats. We hypothesized that there would be no significant differences in kidney outcomes between the humanized infusion and dose-matched bolus.
Materials and Methods:
Experimental design and animal protocols:
Male Sprague-Dawley rats (n=19; age: 8 to 10 weeks; mean weight: 293.8 g) were utilized for this experiment. Animals were housed in a light- and temperature-controlled room for the duration of the study, with free access to water and food. All animals were placed in metabolic cages (Nalgene, Rochester, NY, USA) to allow for 24-hour urine collections, starting at baseline prior to dosing (day 0) with subsequent sampling over 2 days (Supplemental figure 1).
Rats were assigned to one of two treatment groups in which they received intravenous vancomycin, either as a humanized, variable-rate infusion [30.8 mg in 6.0 mL normal saline over 24 hours] or dose-matched bolus [15.4 mg in 0.31 mL normal saline over 2 minutes, every 12 hours for 2 doses] on day 1 of the experimental protocol (Supplemental figure 2, Supplemental Table 2) in order for the rat to recapitulate known human exposures. Pharmacokinetic parameters were taken from previous models [11, 12] Animals in the dose-matched bolus group were then volume-matched via administration of a normal saline infusion (i.e. 5.69 additional mL of saline given) according to the same infusion program as animals in the humanized vancomycin infusion group (Supplemental figure 3). Vancomycin doses were selected to approximate human doses allometrically scaled for rats (30.8 mg/300 g [100 mg/kg] in rats is equivalent to ~15 mg/kg in humans). All animals also received intravenous iohexol at 51.8 mg/day over 1 minute at baseline (day 0) and then on all subsequent study days (i.e., days 1 and 2). Following completion of the experimental protocol, all rats were euthanized and underwent nephrectomies.
All experiments were conducted at Midwestern University in Downers Grove, IL, in compliance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals [13], and were approved under the Institutional Animal Care and Use Committee protocol #3151.
Blood, urine, and kidney sampling:
Double jugular vein catheters with dual-channel vascular access buttons were surgically implanted 72 hours prior to protocol initiation (Instech, Plymouth Meeting, PA, USA). One channel was dedicated to blood draws, while drug dosing occurred via the other. Timed blood samples were obtained from the dedicated channel (0, 30, 60, and 240 minutes after iohexol dosing on each study day). For maintenance of euvolemia, each blood sample (0.2 mL/aliquot) was replaced with an equivalent volume of normal saline. Blood samples were prepared as plasma with EDTA (Sigma-Aldrich Chemical Company, Milwaukee WI, USA) and centrifuged at 3000 g for 10 minutes (Thermo Fisher Scientific, Waltham, MA, USA). Supernatants were collected and frozen at −80°C until time of batch analysis using LCMS. Urine samples were collected, and volume was measured every 24 hours, starting from day 0.
Infusion setup:
On experimental day 1, rats in the humanized infusion group were connected to the infusion pump syringe via a dual-channel tether that magnetically attached to the dual-channel vascular access button (Instech, Plymouth Meeting, PA, USA). The infusion pump was programmed to deliver a humanized vancomycin infusion scheme (Supplementary table 2). Animals in the dose-matched bolus group were also administered a normal saline infusion according to the same infusion program as animals in the humanized vancomycin infusion group (i.e., to volume match between the two treatment groups) (Supplemental figure 3).
GFR measurement:
GFR was assessed by iohexol clearance, similar to our previous study [14]. Iohexol was administered intravenously as an undiluted solution (240 mg/mL) on each experimental day, starting on day 0. Rats received once-daily doses of iohexol at 51.8 mg/0.22 mL, infused over 1 minute. On the day of vancomycin dosing (day 1), iohexol was administered 60 mins after the respective vancomycin treatment was administered. See Supplementary material for full methodology related to utilized LCMS and urinary biomarker assays.
Model building:
To describe iohexol clearance, a two-compartment model was created (Monolix 2023R1; Lixoft, Antony, France). Fixed estimates for peripheral compartment volume (V2) and intercompartmental transfer (Q) were derived from the base two-compartment model fitting. Random effects were estimated for clearance (CL) and central volume (V1). Evaluated covariates included log-transformed weight. To capture daily clearance changes, each experimental day was considered a separate occasion with clearance that could vary at the level of the individual animals Selection of the final model was based on the Akaike Information Criterion (AIC), between subject variability of the population estimates, goodness-of-fit plots for observed versus predicted, and the rule of parsimony [15]. Vancomycin model building was similarly performed without the occasions since only AUC0–24 hrs was desired. Individual Empirical Bayes estimates from the final two compartment model were then used to calculate 24-hour vancomycin AUC on day 1 (Simulx 2023R1; Lixoft, Antony, France).
For comparison of the allometrically scaled vancomycin infusion schemes (e.g. humanized infusion and dose-matched bolus) to exposures in human patients, we employed a published model of adult inpatients who received vancomycin and had two plasma levels drawn during the course of therapy [16]. Parameter estimates were utilized to simulate vancomycin exposures in a 70 kg human patient with normal kidney function (i.e., CrCL = 100 mL/min) receiving a vancomycin dose of 1000 mg every 12 hours (Simulx 2023R1; Lixoft, Antony, France).
Statistical analysis:
A mixed effects model was used to compare urine output, mean weight loss, GFR, and urinary injury biomarkers among the treatment groups, and by experimental day (StataCorp LLC, College Station, TX, USA). Log transformations were applied as needed to maintain parametric distributions. Margins were calculated for a full factorial of the variables, i.e., main effects for each variable and interactions. Day 0 served as a referent value for changes from baseline and between groups. Omnibus tests were first performed for the interaction, followed by a Bonferroni corrected treatment group by time analysis. P values are reported for the Bonferroni corrected and are only considered significant if the interaction was significant at the omnibus level. Mean vancomycin AUC for both treatment groups on day 1 was compared by t test. All tests conducted were two-tailed, with an a priori level of statistical significance set at α = 0.05.
Results:
A total of 19 male Sprague-Dawley rats were assigned to two different treatment groups to receive either the vancomycin humanized infusion (n=11) or dose-matched bolus (n=8). Three animals in the humanized infusion group did not contribute plasma data due to catheter occlusions; all other animals contributed complete plasma data. The mean baseline weight (SD) of the animals was 293.8 g (12.0), with no significant difference between the treatment groups.
Model characteristics and vancomycin exposure (AUC)
A two-compartment infusion model with first-order elimination fit the iohexol plasma data well. Mean population parameter values in the final iohexol model are listed in Supplemental table 3. The linear regression of the observed versus Bayesian posterior population predicted concentrations resulted in an intercept of −3.86 with a slope of 1.02 and R2 value of 0.94. The linear regression of the observed versus individual predicted concentrations resulted in an intercept of −4.71 with a slope of 1.03 and R2 value of 0.97 (Supplemental figure 4).
For vancomycin, a two-compartment infusion model with first-order elimination fit the plasma data well. In the final model, log-normalized weight was included as a covariate for central compartment volume (V1) and clearance (CL). V and CL were allometrically scaled to log-weight^1 and log-weight^0.75, respectively. Mean population parameter values in the final vancomycin model are listed in Supplemental table 4. The linear regression of the observed versus Bayesian posterior population predicted concentrations resulted in an intercept of −0.18 with a slope of 1.12 and R2 value of 0.87. The linear regression of the observed versus individual predicted concentrations resulted in an intercept of −0.13 with a slope of 1.14 and R2 value of 0.87 (Supplemental figure 5).
The mean vancomycin AUC [95% CI] on day 1 in the humanized infusion group was 93.7 mg∙h/L [90.5 to 96.9], while mean vancomycin AUC on day 1 in the dose-matched bolus group was 99.5 mg∙h/L [92.2 to 106.8]. Day 1 AUC was not significantly different between the treatment groups [p=0.07] (Figure 1).
Figure 1).

Comparison of representative plasma vancomycin elimination curves for one individual from each experimental dosing group (1 rat from VAN humanized infusion, 1 rat from VAN dose-matched bolus), and 1 simulated 70 kg human patient with normal kidney function (CrCL 100 mL/min) receiving an allometrically matched dose of VAN 1000 mg every 12 hours (for two doses).
Kidney function over time (iohexol-measured GFR)
Baseline iohexol-measured GFR (day 0) was not significantly different between the two treatment groups (marginal difference versus VAN humanized infusion group as referent [95% confidence interval]: 0.05 mL/min/100 g body weight [−0.01 to 0.09], p=0.16) (Table 1). Following administration of VAN on day 1 according to the assigned treatment groups, no significant differences in iohexol-measured GFR were detected between the two groups (0.04 mL/min/100 g body weight [−0.02 to 0.09], p=0.39. There were also no significant differences in iohexol-measured GFR on the day after VAN dosing (day 2).
Table 1:
Marginal differences versus VAN humanized infusion group as referent in models that demonstrated a significant omnibus interaction term.
| Dosing day | Treatment groups |
|---|---|
| VAN dose-matched bolus | |
| GFR difference (mL/min/100 g body weight), mean (95% CI), p-value | |
| Baseline (day 0) | 0.05 (−0.01 to 0.11), 0.16 |
| Day 1 | 0.04 (−0.02 to 0.09), 0.39 |
| Day 2 | 0.04 (−0.02 to 0.10), 0.30 |
| Urinary clusterin difference (ng), mean (95% CI), p-value | |
| Baseline (day 0) | 879 (−3831 to 5589), p>0.99 |
| Day 1 | 1932 (−2778 to 6641), p=0.98 |
| Day 2 | −3103 (−7813 to 1607), p=0.34 |
| Urinary OPN difference (ng), mean (95% CI), p-value | |
| Baseline (day 0) | −0.43 (−4.41 to 3.55), p>0.99 |
| Day 1 | −1.76 (−5.73 to 2.22), p=0.87 |
| Day 2 | 0.52 (−3.46 to 4.50), p>0.99 |
| Urine output difference (mL), mean (95% CI), p-value | |
| Baseline (day 0) | 0.58 (−2.83 to 3.99), p>0.99 |
| Day 1 | 2.10 (−1.31 to 5.51), p=0.42 |
| Day 2 | 0.07 (−3.34 to 3.48), p>0.99 |
| KIM-1 difference (ng), mean (95% CI), p-value | |
| Baseline (day 0) | 2.33 (−6.30 to 10.95), p>0.99 |
| Day 1 | 9.80 (1.17 to 18.4), p=0.02 |
| Day 2 | −2.27 (−10.90 to 6.35), P>0.99 |
Urine output and kidney injury biomarkers
Baseline urine output (day 0) was not significantly different between treatment groups (marginal difference versus VAN humanized infusion group as referent [95% CI]: 0.58 mL [−2.83 to 3.99], p>0.99) (Table 1, Figure 1). No significant differences in urine output were identified on subsequent experimental days 1 and 2.
Baseline urinary kidney injury biomarker levels were similar between treatment groups and few significant differences were identified (Table 1). For urinary KIM-1, a significant interaction between time and group was identified in the vancomycin dosed-matched bolus group, resulting in a 9.8 ng/24 hours (p=0.02) increase compared to the humanized dosing group. For urinary clusterin, no significant differences were identified on day 1 (marginal difference versus VAN humanized infusion group as referent [95% CI]: 1932 ng/24 hours [−2778 to 6641], p=0.98) or day 2 (−3103 [−7813 to 1607], p=0.34). For urinary osteopontin (OPN), there were also no significant differences identified on day 1 (−1.76 [−5.73 to 2.22], p=0.87) or day 2 (0.52 [−3.46 to 4.50], p=1.00).
Discussion:
In this investigation, we did not find significant differences for the outcomes of iohexol-measured kidney function and kidney injury between animals that either received vancomycin administered as a humanized infusion or dose-matched bolus. This outcome was expected based on our initial hypothesis because the humanized infusion scheme employed mimics standard vancomycin administration methods in humans (i.e., infusion over 1 hour for each gram of administered vancomycin). For vancomycin, clinical data has shown that only extended infusion schemes such as continuous infusion (not employed in this study) are associated with decreased nephrotoxicity [17, 18].
It should be mentioned that urinary KIM-1 was statistically higher in the dose-matched bolus group on the day of vancomycin (day 1). However, this difference of 10 ng/24 hours between the groups is not expected to reflect significantly more kidney injury. As an example, our recent work showed that flucloxacillin added to vancomycin increased urinary KIM-1 58 to 140 ng/24 hours (increased nephrotoxic effect) when compared to vancomycin whereas adding imipenem cilastatin resulted in 61 to 107 ng/24 hours less KIM-1 (nephroprotective) [19]. Thus, changes in magnitude of 50 ng/24 hours is expected to be more scientifically relevant. Overall, animals in this experiment had lower levels of urinary kidney injury biomarkers compared to other studies that have employed larger vancomycin doses and/or longer dosing protocols [14, 20]. Furthermore, the administration of a large volume of normal saline (6 mL/24 hours) in both treatment groups may have ameliorated overall vancomycin-induced kidney injury among this cohort of animals.
Several limitations of this study should be noted. First, animals in this study only received vancomycin over a 24-hour period. It is possible that repeated dosing over several days with either humanized infusion or dose-matched bolus could result in different outcomes in terms of kidney function and kidney injury. However, our previous experiments have shown that differences in KIM-1 are detectable on day 1 in our translational rat model [20]. Future studies may consider administration of repeated vancomycin doses over a longer time, as this would have greater translational applicability to repeated dosing in human patients. It is notable that continuous infusion studies in rodents is a much more technically difficult model, however. Second, this study employed only male Sprague-Dawley rats since this was an initial investigation of the humanized dosing protocol for vancomycin. While we have not observed sex differences in the outcomes of kidney function and kidney injury during our previous investigations of vancomycin nephrotoxicity using this Sprague-Dawley rat model, future studies should utilize female animals as well, in accordance with the latest guidelines from the U.S. National Institutes of Health [21]. Finally, the sole purpose of this study was to compare two vancomycin-induced, pre-clinical models of kidney damage. The rationale for the comparison is that the humanized model is more technically complex in conduct and cost, and it was previously unclear if there was a benefit to the more complex humanized model. Our findings do not apply to efficacy models where other approaches are primarily used.
Conclusion:
In conclusion, administration of intravenous vancomycin as either a humanized infusion scheme or dose-matched bolus did not result in meaningful differences in iohexol-measured GFR and urinary kidney injury biomarkers in a male Sprague-Dawley rat toxicity model.
Supplementary Material
Figure 2).
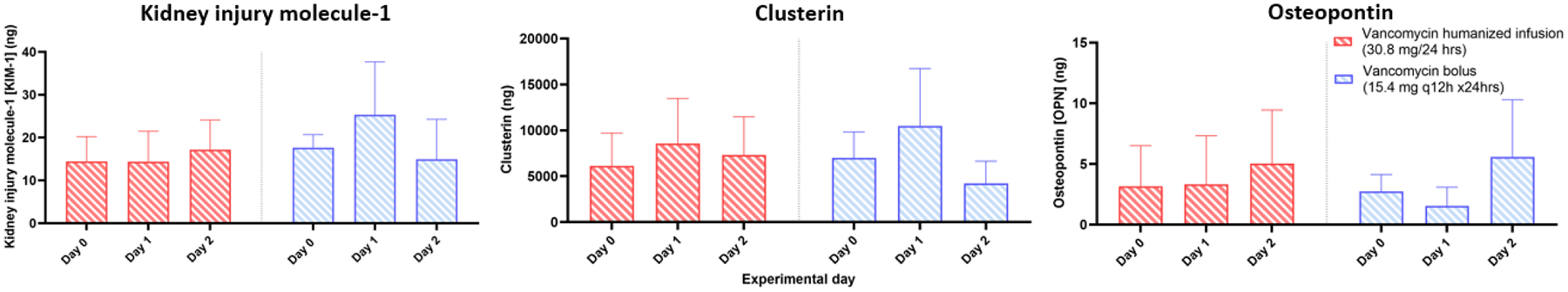
Comparison of urinary kidney injury biomarkers between rats that received a humanized vancomycin infusion vs. dose matched bolus. No significant differences were detected between the groups on any experimental day.
Highlights.
Translational allometric scaled rat models have previously demonstrated the pharmacokinetic/pharmacodynamic relationship between vancomycin and kidney injury, but previous models have used bolus dosing that results in high peak concentrations not observed in humans.
In studies that focus on the pharmacodynamic outcome of kidney injury, utilization of kidney injury to prolong exposure is not a possible approach.
Computerized pump schemes were employed in this study to mimic vancomycin parameters and create humanized vancomycin exposures (i.e. maximum concentrations and area under the concentration curve).
It is unknown if humanized infusion schemes result in similar outcomes to traditional allometric scaled models.
We found that the more complicated humanized infusion schemes in rats resulted in similar kidney outcomes to the more standard allometric bolus methodologies.
Funding:
This research was supported in part by NIAID award number R21AI149026 (MS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Approval: Institutional Animal Care and Use Committee protocol #3151.
Declaration of Competing Interests:
MS reports previous research contracts with Nevakar and SuperTrans Medical, ongoing consultancy with DoseMe, and has filed patent US10688195B2. All other authors have no other related conflicts of interest to declare.
References
- [1].Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin Area Under the Curve and Acute Kidney Injury: A Meta-analysis. Clin Infect Dis. 2019;69:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, et al. 24-Hour Pharmacokinetic Relationships for Vancomycin and Novel Urinary Biomarkers of Acute Kidney Injury. Antimicrob Agents Chemother. 2017;61:e00416–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Avedissian SN, Pais GM, O’Donnell JN, Lodise TP, Liu J, Prozialeck WC, et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother. 2019;74:2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pais GM, Liu J, Zepcan S, Avedissian SN, Rhodes NJ, Downes KJ, et al. Vancomycin-Induced Kidney Injury: Animal Models of Toxicodynamics, Mechanisms of Injury, Human Translation, and Potential Strategies for Prevention. Pharmacotherapy. 2020;40:438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79:373–82. [DOI] [PubMed] [Google Scholar]
- [7].Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005.
- [8].Ings RM. Interspecies scaling and comparisons in drug development and toxicokinetics. Xenobiotica. 1990;20:1201–31. [DOI] [PubMed] [Google Scholar]
- [9].Gad SC. Animal models in toxicology: CRC press; 2006. [Google Scholar]
- [10].Mahmood I Application of allometric principles for the prediction of pharmacokinetics in human and veterinary drug development. Adv Drug Deliv Rev. 2007;59:1177–92. [DOI] [PubMed] [Google Scholar]
- [11].Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–14. [DOI] [PubMed] [Google Scholar]
- [12].O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, et al. 24-Hour Pharmacokinetic Relationships for Vancomycin and Novel Urinary Biomarkers of Acute Kidney Injury. Antimicrob Agents Chemother. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guide for the care and use of laboratory animals, 8th edition. 8th ed. Washington DC, US: National Academies Press; 2011. [PubMed] [Google Scholar]
- [14].Chang J, Pais GM, Engel PL, Klimek P, Marianski S, Valdez K, et al. Impact of Vancomycin Loading Doses and Dose Escalation on Glomerular Function and Kidney Injury Biomarkers in a Translational Rat Model. Antimicrob Agents Chemother. 2023;67:e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mould DR, Upton RN. Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development—Part 2: Introduction to Pharmacokinetic Modeling Methods. CPT: Pharmacometrics & Systems Pharmacology. 2013;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang J, Patel D, Vega A, Claeys KC, Heil EL, Scheetz MH. Does calculation method matter for targeting vancomycin area under the curve? J Antimicrob Chemother. 2022;77:2245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Flannery AH, Bissell BD, Bastin MT, Morris PE, Neyra JA. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis. Crit Care Med. 2020;48:912–8. [DOI] [PubMed] [Google Scholar]
- [18].Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, et al. Continuous versus Intermittent Infusion of Vancomycin in Severe Staphylococcal Infections: Prospective Multicenter Randomized Study. Antimicrob Agents Chemother. 2001;45:2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pais GM, Marianski S, Valdez K, Melicor RP, Liu J, Rohani R, et al. Flucloxacillin worsens while imipenem-cilastatin protects against vancomycin-induced kidney injury in a translational rat model. Br J Pharmacol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pais GM, Avedissian SN, O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, et al. Comparative Performance of Urinary Biomarkers for Vancomycin-Induced Kidney Injury According to Timeline of Injury. Antimicrob Agents Chemother. 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


