Abstract
Objective:
While evidence continues to emerge on the negative health effects of electronic cigarettes (e-cigarettes) on the lungs, little is known regarding their deleterious effects on the upper airway. The purpose of this review is to summarize the toxicological effects of e-cigarettes, and its components, on the upper airway.
Data Sources:
PubMed, SCOPUS, EMBASE databases
Review Methods:
Systematic searches were performed in accordance with PRISMA guidelines from 2003–2023. Studies were included if they investigated toxicological effects of e-cigarette exposure on human or animal upper airway tissue. Two authors independently screened, reviewed, and appraised all included articles.
Results:
822 unique articles were identified, of which 53 met inclusion criteria and spanned subsites including the oral cavity (22/53 studies), nasal cavity/nasopharynx (13/53), multiple sites (10/53), larynx (5/53), trachea (2/53), and oropharynx (1/53). The most commonly observed consequences of e-cigarette use on the upper airway included: pro-inflammatory (15/53 studies), histological (13/53), cytotoxicity (11/53), genotoxicity (11/53), and pro-carcinogenic (6/53). E-cigarette humectants independently induced toxicity at multiple upper airway subsites, however, effects were generally amplified when flavoring(s) and/or nicotine were added. Across almost all studies, exposure to cigarette smoke exhibited increased toxicity in the upper airway compared with exposure to e-cigarette vapor.
Conclusion:
Current data suggests that while e-cigarettes are generally less harmful than traditional cigarettes, they possess a distinct toxicological profile that is enhanced upon addition of flavoring(s) and/or nicotine. Future investigations into under-examined subsites such as the oropharynx and hypopharynx are needed to comprehensively understand the effects of e-cigarettes on the upper airway.
Keywords: E-cigarettes, E-cig, Vaping, Aerodigestive tract, Upper airway, PRISMA, Nicotine, Flavoring, Humectant
INTRODUCTION
Since their introduction into the United States market in the mid-2000s, electronic cigarettes, e-cigarettes or “vapes” have gained significant popularity. Much of the early wave of e-cigarette popularity was among the younger age groups. In 2018, 14.8% of all adults in the United States reported having used an e-cigarette, with the highest percentage (25.8%) in the 18 to 24 year-old range.1 In fact, in 2014, e-cigarettes surpassed conventional combustible cigarettes in popularity for this age range.2 This could be due to the fact that until 2016, e-cigarettes were left largely unregulated in the United States, and lacked the same advertisement and promotional restrictions placed on conventional cigarettes. E-cigarette companies benefited greatly from this delay, as made evident by the roughly $2.35 billion generated from e-cigarette sales in the United States in 2016.3
Among the many reasons that patients choose to use e-cigarettes, the one most commonly cited is smoking cessation and the resulting health benefits.4 In fact, the idea of utilizing e-cigarettes as a tool to stop smoking has been lauded among those in the harm reduction community and promoted by numerous governing health agencies, such as the British Royal College of General Practitioners.5 A 2021 Cochrane review comprising 61 separate studies found moderate evidence that nicotine-containing e-cigarettes increased quit rates of conventional cigarettes when compared to non-nicotine e-cigarettes and other nicotine replacement therapies.6 Nevertheless, despite the perceived benefits of e-cigarettes, their use is accompanied by their own unique risks.
Due to the heterogeneous composition of ingredients in these vaporized liquids and the mechanics of delivery, the overall health effects from using e-cigarettes are distinct from those associated with using conventional cigarettes and can be difficult to predict. Briefly, e-liquids (typically made of a combination of propylene glycol, glycerol, and flavoring with or without nicotine) are heated on a coiled wire, which creates a vapor/aerosol that can be inhaled through the mouthpiece. Compared to traditional tobacco smoke, particle size distribution in e-cigarette vapor is more wide ranging. In a recent study, aerosol particle size generated from e-cigarette use was shown to be tri-modal, with peaks at 40 nm, 200 nm, and 1 μm, and size distribution primarily impacted by the power of the vaping device being used.7 Based on particle deposition studies, it is estimated that smaller sized particles (40–200 nm) are more likely to deposit in the trachea and lower airway compared to large particles (1–2 μm) which primarily deposit in the oral cavity and oropharynx. The large distribution in particle sizes, primarily due to user device power levels, creates differential harmful effects of e-cigarette use at sites within the upper and lower airway. Additionally, e-cigarette users have been shown to practice exclusive nasal exhalation patterns at significantly higher rates than traditional cigarette users.8 These behavioral differences predispose the nasal cavity to toxicological impacts of e-cigarette use. However, while there has been significant research over the past 10 years regarding the effects of e-cigarette use on the lower airways, including in the context of Acute Respiratory Distress Syndrome (ARDS), diffuse alveolar hemorrhage, and lipid pneumonia, few studies have examined the effects on the upper airway.9 To fill this void, we examined the available evidence regarding the effect of e-cigarette use on the different subsites of the upper airway.
METHODS
Search Methodology, Inclusion Criteria, and Data Collection
A scoping review of the literature was performed following PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses)10 guidelines (Figure 1, Supplemental Data). The study did not require IRB approval from the authors’ institution. This scoping review was not registered online and a publically-available study protocol was not generated. A query of publications written in English between 2003, when the e-cigarette was invented, to November 2023 was performed in PubMed, SCOPUS, and EMBASE databases with the assistance of a research librarian. The complete search strategy for all three databases with included MeSH (Medical Subject Heading) terms is included (Supplemental Data). References from the retrieved articles were also analyzed for any missed studies that were relevant to and could be included in our review. Two authors independently screened all identified articles in Covidence (https://www.covidence.org/). A consensus was achieved based on pre-determined inclusion and exclusion criteria. Inclusion criteria encompassed studies that analyzed the toxicological effects of e-cigarette aerosol/vapor, condensate, or e-liquid exposure on human or animal upper airway tissue. Exclusion criteria included non-original research (e.g. review articles, editorials), case reports, inaccessible articles, Non-English studies, and articles investigating only dental subsites (e.g. dental enamel, periodontal ligament, gingiva) or saliva.
Figure 1.
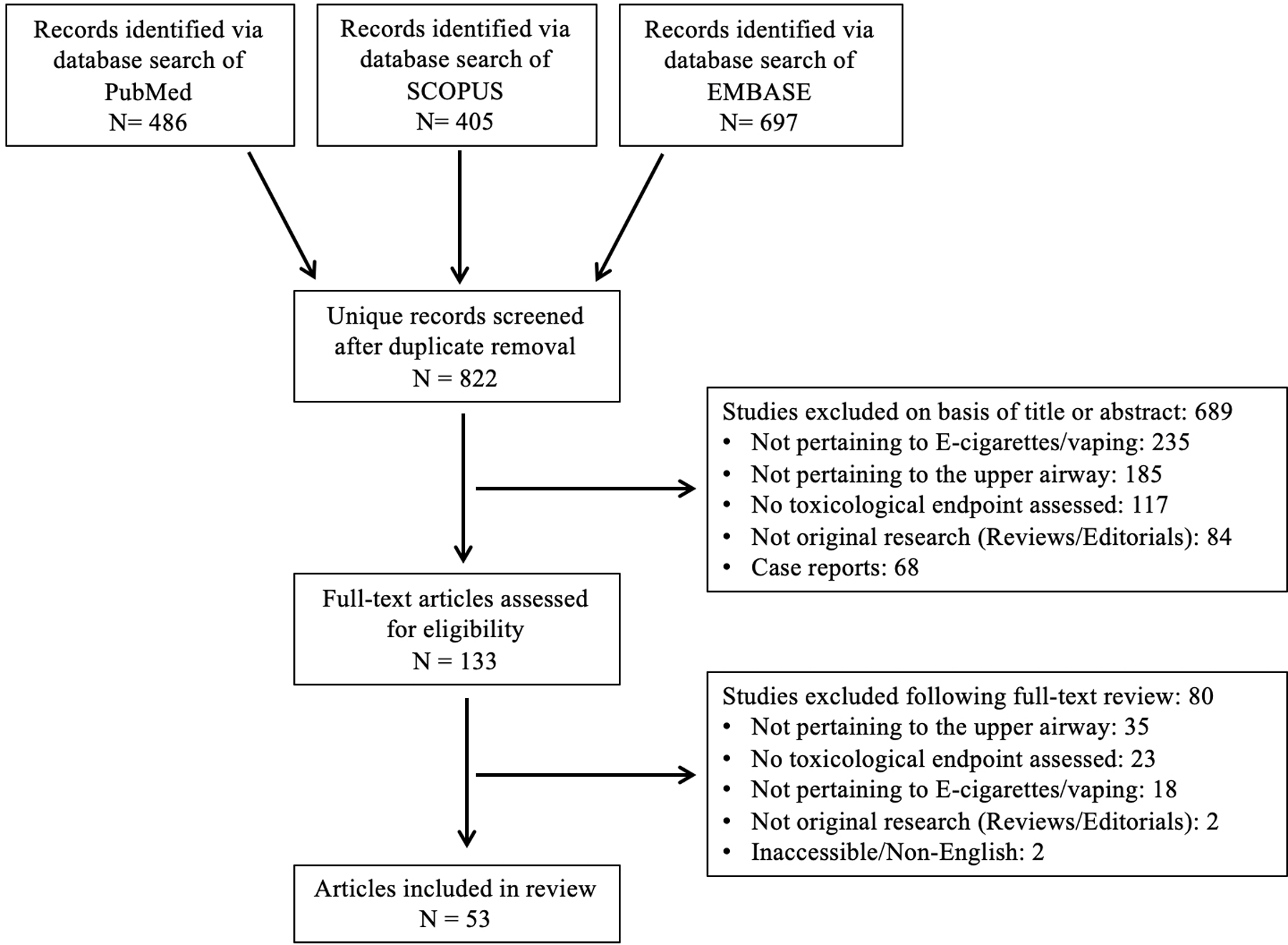
PRISMA Flow Diagram of Literature Search and Study Inclusion
Data Extraction, Analysis, and Quality Assessment
A schematic flow diagram highlighting the PRISMA search methodology is shown in Figure 1. Data were extracted from the included studies and reviewed independently by two investigators. A spreadsheet in Microsoft Excel (Redmond, WA, USA) was used to denote manuscript and author information, country, year of publication, study design, tissue/cell type, e-cigarette component or flavor studied (if applicable), research findings, and associated toxicological findings. Toxicological findings were classified as cytotoxicity, genotoxicity, pro-inflammatory, histological, pro-carcinogenic, immunosuppression/increased infectivity, ciliary dysfunction, and/or other according to pre-specific criteria outlined in Figure 2. Finally, each article was assigned a “level of evidence” for its study design based on the Oxford Centre for Evidence-based Medicine- 2011 Levels of Evidence guidelines11.
Figure 2.
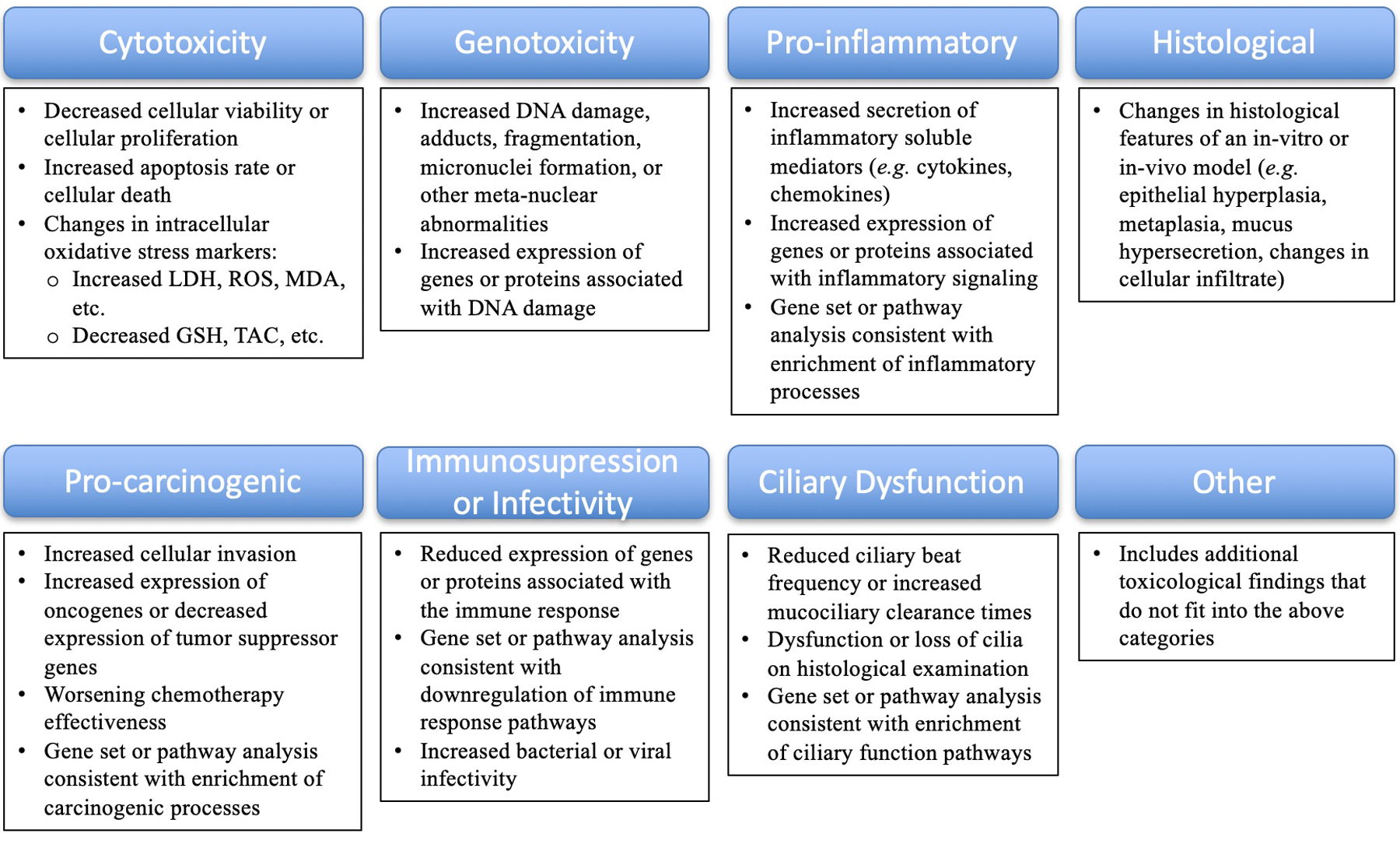
Toxicological Classification of Results
RESULTS
The literature search yielded 1588 total records. After removal of duplicates, 822 unique abstracts were screened, resulting in the inclusion of 53 studies (Figure 1, Table 1).12–64 Out of the 53 included articles, 34 (64 %) analyzed the effects of e-cigarette use on human subjects or cell lines, 16 (30%) utilized an animal model to investigate the in-vivo effects of e-cigarette aerosol exposure, and 3 (6%) studied both human and animal models. Human studies included: 1) in vitro assessments of e-cigarette aerosol, condensate, or e-liquid exposure on primary cultured cells or immortalized cell lines derived from upper airway tissue, or 2) clinical studies using samples obtained from current or former e-cigarette users. Using the Oxford Centre for Evidence-Based Medicine: Levels of Evidence guidelines, 36 of the included studies were graded as level 5 evidence, whereas 17 studies were graded as level 2b evidence (Table 1).
Table 1.
Major Characteristics of the 53 Included Studies
| Study | Year | City, Country |
Study Type | Tissue Type | Comparison to cigarette smoke | Sample, Participant, or Animal | Duration of Exposure | Methodology | Site | Flavors Examined (if stated) |
E-Cigarette Components (if stated) |
Oxford Level of Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral Cavity | |||||||||||||
| Ji EH, et al12 | 2016 | Multi-national (USA, China) | Basic Science | Human | No | Oral keratinocyte cell line | 24 hours of e-cigarette aerosol exposure | ATP assay, GSH assay, qPCR | Oral mucosa | Not stated | PG/VG + Nicotine + Flavor | 5 | |
| Franco T, et al13 | 2016 | Magna Graecia, Italy | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 23 cigarette smokers, 22 e-cigarette users, and 20 non-smokers | Not stated | Exfoliative cytology, Optical microscopy | Buccal mucosa | Not stated | Not stated | 2b | |
| Ganapathy V et al14 | 2017 | Multi-national (USA, India) | Basic Science | Human | Yes | Dysplastic oral keratinocyte cell line (POE9n), Oral SCCa cell line (UM-SCC-1) | 1, 10, or 100 puffs/5 L, 1 hour/day, every other day for 2 weeks of e-cigarette aerosol condensate exposure | q-PADDA assay, ELISA, MTT assay, TAC assay, DCFDA assay, RT-PCR, Western Blot | Oral mucosa | Traditional, Desert Sands | PG/VG +/− Nicotine + Flavor | 5 | |
| Tommasi S, et. al.15 | 2019 | Los Angeles, CA, USA | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 42 e-cigarette users, 24 cigarette smokers, and 27 non-smokers | Not stated | RNA-sequencing, qRT-PCR, IPA | Buccal mucosa | Not stated | Not stated | 2b | |
| Iskander AR, et al16 | 2019 | Multi-national (Switzerland, USA) | Basic Science | Human | Yes | Organotypic buccal epithelial cultures (MatTek) | 28 minutes of e-cigarette aerosol exposure | Histopathology, Multiplex immunoassay, qRT-PCR, IPA | Buccal mucosa | Not stated | PG/Glycerol +/− Nicotine +/− Flavor | 5 | |
| Ureña JF, et al17 | 2020 | University Park, PA, USA | Basic Science | Human | No | Oral SCCa cell line (SCC-25) | 45 total puffs of 4 seconds duration of e-cigarette aerosol exposure | MTT assay, Immunofluorescence | Oral mucosa, Gingiva | Lava Flow, Very Cool, Hawaiian Pog, American Patriots | PG/VG +/− Nicotine + Flavor | 5 | |
| Tsai KYF, et al18 | 2020 | Provo, UT, USA | Basic Science | Human | No | Oral SCCa cell line (Ca9-22), Tongue SCCa cell line (CAL-27) | 24 hours of e-liquid exposure | Real-Time Cell Invasion, Immunofluorescence, ELISA | Oral mucosa, Tongue | Red Hot, Green Apple | PG/VG +/− Nicotine + Flavor | 5 | |
| Pop AM, et. al.19 | 2021 | Tirgu Mures, Romania | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 25 cigarette smokers, 23 e-cigarette users and 20 non-smokers | ≥ 12 months of daily e-cigarette use | Exfoliative cytology, Optical microscopy | Buccal mucosa | Not stated | Not stated | 2b | |
| Guo J, et. al.20 | 2021 | Minneapolis, MN, USA | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 35 non-smokers, 30 cigarette smokers, and 30 e-cigarette users | ≥ 3 months of daily e-cigarette use; ≥ 12 months of daily cigarette use | LC-NSI-HRMS/MS | Buccal mucosa | Not stated | Not stated | 2b | |
| Muqawwi et al21 | 2021 | Bandung, Indonesia | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 20 non-smokers, 20 cigarette smokers, and 15 e-cigarette users | ≥ 12 months of e-cigarette use | Micronucleus assay | Buccal mucosa | Not stated | Not stated | 2b | |
| Tellez CS, et al22 | 2021 | Multi-national (USA, Japan) | Basic Science | Human | Yes | 3 immortalized oral epithelial cell lines | 20 minutes of e-cigarette aerosol exposure | Neutral red uptake assay, ROS-Glo assay, TBARS assay, Alkaline comet assay, Micronuclei assay | Oral mucosa | Arctic Blast, Blue Pucker, Jamestown, Love Potion, Mardi Gras, Midnight Splash, Port Royale, Tobacco Row, Tortuga, Uptown | PG/VG +/− Nicotine +/− Flavor | 5 | |
| Schwarzmeier LAT, et. al.23 | 2021 | Sao Jose dos Campos, Brazil | Clinical | Human | Yes | Oral cavity scrape biopsy from 20 e-cigarette users, 22 current cigarette smokers, 22 former cigarette smokers, and 27 non-smokers | ≥ 5 months of e-cigarette use | Exfoliative cytology, Optical microscopy | Lateral tongue, floor of mouth | Not stated | Not stated | 2b | |
| Hamad S, et. al.24 | 2021 | Chapel Hill, NC, USA | Clinical | Human | No | Buccal mucosa scrape biopsy from 3 healthy e-cigarette users | ≥ 2 months of daily e-cigarette use; Scripted vaping of 20 puffs over 20 minutes | qRT-PCR, IPA | Buccal mucosa | Not stated | PG/VG + Nicotine | 2b | |
| Cátala-Valentín AR, et al25 | 2022 | Orlando, FL, USA | Basic Science | Human | No | Oral epithelial cell line (OKF6) | 30 minutes of e-cigarette condensate exposure | EdU cell proliferation assay, Biofilm measurement, ELISA, qRT-PCR, Immunofluorescence | Oral mucosa | None | PG/VG +/− Nicotine | 5 | |
| Marinucci L et al26 | 2022 | Perugia, Italy | Basic Science | Human | Yes | Oral keratinocyte cell line (PSC-200-01) | 24 hours of e-cigarette aerosol condensate exposure | MTT assay, Scanning electron microscopy, Scratch assay, Flow cytometry, qRT-PCR | Oral mucosa | Tobacco | PG/VG + Nicotine + Flavor | 5 | |
| Cheng G et al27 | 2022 | Minneapolis, MN, USA | Clinical | Human | Yes | Buccal mucosa scrape biopsy from 8 cigarette smokers, 20 e-cigarette users and 20 non-smokers | ≥ 3 months of 4 days/week of e-cigarette use; ≥ 12 months of daily cigarette use | LC-NSI-HRMS/MS | Buccal mucosa | Not stated | Not stated | 2b | |
| Robin H et al28 | 2022 | South Jordan, UT, USA | Basic Science | Human | No | Oral SCCa cell line (Ca9-22), Tongue SCCa cell line (CAL-27) | 6 hours of e-cigarette aerosol condensate exposure | ELISA, Western Blot, Real time cell invasion | Oral mucosa | Red Hot, Green Apple | PG/VG +/− Nicotine + Flavor | 5 | |
| Reeve G et al29 | 2022 | New York, NY, USA | Clinical | Human | No | Non-smokers and e-cigarette users | Active e-cigarette users | Histopathology, Transcriptomics | Oral mucosa | Not stated | Not stated | 2b | |
| Mandour D et al30 | 2023 | Zagazig, Egypt | Basic Science | Murine | No | 30 adult male albino rats | 1 hour/day, 5 days/week, for 4 weeks of e-cigarette aerosol exposure | ELISA, Histopathology, Immunohistochemistry | Submandibular gland | Not stated | VG/PG + Nicotine + Flavor | 5 | |
| Elmahdi F et al31 | 2023 | Madinah, Saudi Arabia | Clinical | Human | No | Buccal mucosa scape biopsy from 250 non-smokers, 250 e-cigarette users | ≥ 6 months of daily e-cigarette use | Papanicolaou staining, Immunohistochemistry | Buccal mucosa | Not stated | Not stated | 2b | |
| Tommasi S et al32 | 2023 | Los Angeles, CA, USA | Clinical | Human | Yes | Oral mucosa scrape biopsy from 24 non-smokers, 24 e-cigarette users, 24 cigarette smokers | ≥ 3x week for 6 months of e-cigarette use; ≥ 3x week for 12 months of cigarette use | LA-QPCR | Oral epithelium | Fruit, Sweet, Mint/Menthol, Tobacco | VG/PG + Nicotine + Flavor | 2b | |
| de Lima JM, et al33 | 2023 | Multi-national (Brazil, Canada) | Basic Science | HumanMurine | No | Human oral mucosal keratinocyte and tongue epithelial cell lines, human tongue SCCa cell lines (CAL-27, HSC3), and mouse oral cancer cell line (AT84) | 7, 24, or 48 hours of e-liquid exposure | MTT assay, Colony formation assay, Wound healing migration assay, Cell invasion assay, Western Blot, qRT-PCR, Immunocytochemistry | Oral mucosa, Tongue | Not stated | PG/Glycerine + Nicotine + Flavor | 5 | |
| Oropharynx | |||||||||||||
| Welz C, et. al.34 | 2016 | Munich, Germany | Basic Science | Human | No | Primary oropharyngeal epithelial cells from healthy adult donors | 24 hours of e-liquid exposure | MTT assay, Alkaline microgel electrophoresis | Oropharynx mucosa | Apple. Cherry, Tobacco | PG/VG + Nicotine + Flavor | 5 | |
| Nasal Cavity/Nasopharynx | |||||||||||||
| Martin EM, et. al.35 | 2016 | Chapel Hill, NC, USA | Clinical | Human | Yes | Nasal biopsy and lavage fluid from 13 non-smokers, 14 cigarette smokers, 12 e-cigarette users | ≥ 6 months of e-cigarette use | Liquid chromatography tandem mass spectrometry, Nanostring based gene expression analysis, IPA, ELISA | Nasal mucosa, Nasal lavage fluid | Not stated | Not stated | 2b | |
| Kumral TL, et. al.36 | 2016 | Istanbul, Turkey | Clinical | Human | No | 72 current smokers in cessation clinic randomized to e-cigarette smoking group (n=42) or non e-cigarette group (n=30). | ≥ 5 years of daily cigarette use | Mucociliary clearance (via saccharin transit time), SNOT-22 | Nasal epithelium | Not stated | Not stated | 2b | |
| Carson JL, et al.37 | 2017 | Chapel Hill, NC, USA | Basic Science | Human | Yes | Primary nasal epithelial cells from healthy non-smoking adults | 20 consecutive e-cigarette “puffs” | Electron microscopy, Nitric oxide production, Ciliary beat frequency | Nasal epithelium | Tobacco | PG/VG + Nicotine + Flavor | 5 | |
| Miyashita L et al38 | 2018 | Multi-national (UK, South Africa) | Basic Science | Human, Murine | No | 11 adult vaping males; Primary nasal epithelial cells from a healthy never-smoking, never-vaping adult; Female CD1 mice | ≥ 1 week of e-cigarette use; 2x/day of e-cigarette aerosol condensate exposure | Immunostaining, Flow cytometry, Adhesion assay, Miles and Misra method | Nasal epithelium | Not stated | PG/VG +/− Nicotine + Flavor | 5 | |
| Jabba S et al39 | 2020 | Durham, NC, USA | Basic Science | Human | No | Primary nasal epithelial cells (PromoCell) | 24 hours of e-liquid flavor exposure | LIVE/DEAD Viability/Cytotoxicity assay | Nasal epithelium | Benzaldehyde, Vanillin | PG + Flavor | 5 | |
| Rouabhia M, et al40 | 2020 | Quebec, Canada | Basic Science | Human | Yes | Primary nasal epithelial cells from healthy non-smoking adults | 15 minutes, 2x/day, for 3 days of e-cigarette aerosol exposure | Tryptan blue exclusion assay, LDH assay, MTT assay, Ki67 immunostaining, ELISA assay | Nasal epithelium | Smooth Canadian Tobacco | PG/VG +/− Nicotine + Flavor | 5 | |
| Rebuli ME, et al41 | 2021 | Chapel Hill, NC, USA | Clinical | Human | Yes | Nasal lavage and epithelial lining fluid from 20 non-smokers, 14 cigarette smokers, and 15 e-cigarette users | Not stated | Viral load, NanoString nCounter, qRT-PCR, ELISA, Influenza-specific IgA quantification | Nasal epithelial-lining fluid, Nasal lavage fluid, Nasal mucosa | Not stated | Not stated | 2b | |
| Escobar YN et al42 | 2021 | Chapel Hill, NC, USA | Basic Science | Human | No | Primary nasal epithelial cells from adult smokers and nonsmokers |
20 consecutive e-cigarette “puffs” each of 4 secs duration | Western blot, ELISA | Nasal epithelium | None | PG/VG +/− Nicotine | 5 | |
| Kwak S et al43 | 2021 | Daegu, Republic of Korea | Basic Science | Human | No | Primary nasal epithelial cells (Epithelix) | 24 hours of Glyoxal or Methylglyoxal exposure | WST-1 assay, Western blot, ELISA | Nasal epithelium | None | Glyoxal or Methylglyoxal | 5 | |
| Hinds D et al44 | 2022 | Iowa City, IA, USA | Basic Science | Murine | No | Male golden Syrian Hamsters | 2 hours/day for 2 days of e-cigarette aerosol exposure | Histopathology, qRT-PCR | Nasal epithelium | None | PG/VG +/− Nicotine | 5 | |
| Karey E et al45 | 2022 | Chapel Hill, NC, USA | Clinical | Human | Yes | Nasal epithelial lining fluid from 37 never-smokers, 16 cigarette smokers, and 20 e-cigarette users | E-cigarette or cigarette use within last 7 days | ELISA | Nasal epithelial lining fluid | Not stated | Not stated | 2b | |
| Pozuelos et al46 | 2022 | Riverside, CA, USA | Clinical | Human | Yes | Nasal biopsy from 3 never-smokers, 3 cigarette smokers, and 3 e-cigarette users | Active e-cigarette or cigarette use | RNA sequencing, Gene Ontology, IPA | Nasal epithelium | Not stated | Not stated | 2b | |
| Nicholas BD, et. Al.47 | 2022 | Syracuse, NY, USA | Basic Science | Murine | No | Male and female BALB/cJ mice | 15 minutes, 3x/day for 5 days/week for 8 weeks of e-cigarette aerosol exposure | Histopathology | Eustachian tube mucosa | Tobacco | PG/VG + Flavor | 5 | |
| Larynx | |||||||||||||
| Salturk Z, et al.48 | 2015 | Istanbul, Turkey | Basic Science | Murine | No | Female Wistar albino rats | 1 hour/day for 4 weeks of e-cigarette aerosol exposure | Histopathology, Ki67 immunostaining | Vocal fold epithelium | None | PG/VG + Nicotine | 5 | |
| Ha TN, et. Al.49 | 2019 | Houston, TX, USA | Basic Science | Murine | Yes | C57BL/6 mice | 31 minutes/day, 5 days/week for 16 weeks of e-cigarette aerosol exposure | ELISA | Larynx | None | PG/VG +/− Nicotine | 5 | |
| Hassan NH, et al50 | 2022 | Zagazig, Egypt | Basic Science | Murine | No | Adult male Wistar albino rats | 1 hour/day, 5 days/week for 4 weeks of e-cigarette aerosol exposure | Histopathology, Ki67 and p53 immunostaining, MDA and TAC levels, Transmission electron microscopy | Larynx | Not stated | PG/VG + Nicotine + Flavor | 5 | |
| Martinez JD, et al.51 | 2022 | Palo Alto, CA, USA | Basic Science | Human | Yes | Immortalized vocal fold fibroblast cell line | 24 hours of e-cigarette condensate exposure | MTT assay, qRT-PCR, Immunofluorescence | Vocal fold epithelium | None | PG/VG +/− Nicotine | 5 | |
| Easwaran M et al52 | 2023 | Stanford, CA, USA | Basic Science | Murine | No | Male C57BL6/J mice | 2 hour/day for 1, 5, and 10 days of e-cigarette aerosol exposure | Histopathology, Immunohistochemistry, immunofluorescence, Scanning electron microscopy | Larynx | Mint, Mango | PG/VG + Nicotine + Flavor | 5 | |
| Trachea | |||||||||||||
| Wu Q et al53 | 2014 | Denver, CO, USA | Basic Science | Human | No | Primary tracheobronchial epithelial cells | 24 or 48 hours of e-liquid exposure | LDH assay, ELISA, qRT-PCR | Trachea | Tobacco | PG/VG +/− Nicotine | 5 | |
| El-Merhie et al54 | 2021 | Borstel, Germnay | Basic Science | Drosophilia melanogaster | No | L3 larvae | 2 min, every hour x 8 sessions of e-cigarette aerosol exposure | Stereomicroscope, NeuronJ | Trachea | None | PG/VG + Nicotine | 5 | |
| Multi-site | |||||||||||||
| Manyanga J, et al55 | 2021 | Oklahoma City, OK, USA | Basic Science | Human | Yes | Floor of mouth SCCa cell line (UM-SCC-1), Tongue SCCa cell line (WSU-HN6), and Pharyngeal SCCa cell line (WSU-HN30) | 48 hours of e-cigarette condensate exposure | Tryptan blue exclusion assay, MTT assay, qRT-PCR, Western Blot | Tongue, Floor of mouth, and Pharynx | Not stated | PG/VG +/− Nicotine | 5 | |
| Werley MS, et al56 | 2016 | Richmond, VA USA | Basic Science | Murine | No | Male and female Sprague-Dawley rats | 16, 48, or 160 minutes/day for 13 weeks of e-cigarette aerosol exposure | Histopathology | Nasal epithelium, Larynx | Not stated | PG/VG +/− Nicotine +/− Flavor | 5 | |
| Phillips B, et al57 | 2017 | Multi-national (Singapore, Germany, Switzerland) | Basic Science | Murine | No | Male and female Sprague-Dawley rats | 6 hours/day, 5 days/week for 13 weeks of e-cigarette aerosol exposure | Histopathology, DNA microarray, Proteomics | Nasal epithelium, Larynx | None | PG/VG +/−Nicotine | 5 | |
| Lee KM, et al58 | 2018 | Multi-national (Switzerland, USA) | Basic Science | Murine | Yes | Female C57BL/6 mice | 4 hours/day for 13 days of e-cigarette aerosol exposure | Histopathology | Nasal epithelium, Larynx, trachea | Not stated | PG/VG + Nicotine +/− Flavor | 5 | |
| Ho J, et al59 | 2020 | Multi-national (Singapore, Switzerland) | Basic Science | Murine | No | Male and female Sprague-Dawley rats | 6 hours/day, 5 days/week for 13 weeks of e-cigarette aerosol exposure | Histopathology, DNA microarray | Nasal epithelium, Larynx | Not stated | PG/VG +/− Nicotine +/− Flavor | 5 | |
| Ni F, et. al.60 | 2020 | Baltimore, MD, USA | Basic Science | Murine | No | Male and female C57BL/6 mice | 30 minutes of e-cigarette aerosol exposure | Immunohistochemistry, Event-related potential recordings | Nasal epithelium, Trachea | Vanilla, Menthol, Cinnamon | PG/VG + Nicotine + Flavor | 5 | |
| Wong ET et al61 | 2021 | Multi-national (Singapore, Switzerland, USA, Germany) | Basic Science | Murine | Yes | Female ApoE−/− mice | 3 hours/day, 5 days/week for 6 months of e-cigarette aerosol exposure | Histopathology, Transcriptomics | Nasal epithelium, Larynx, Trachea | Not stated | PG/VG +/− Nicotine +/− Flavor | 5 | |
| Kim M et al62 | 2022 | Kansas City, KS, USA | Clinical & Basic Science | Human, Sheep | No | Primary nasal epithelial cells and epithelial lining fluid from healthy non-smoking adults; Tracheal secretions from adult female sheep | Humans vaped 80 puffs/day for 8 days; Sheeps exposed to 80 puffs/day for 5 days of e-cigarette aerosols | Nasal ion transport assay, ELISA | Nasal epithelium, Nasal epithelial lining fluid, Tracheal secretions | None | VG +/− PG | 2b | |
| Wong ET, et al63 | 2022 | Multi-national (Singapore, Switzerland, USA) | Basic Science | Murine | Yes | Male and female A/J mice | 6 hours/day for 5 weeks of e-cigarette aerosol exposure | Histopathology | Nasal epithelium, Larynx, Trachea | Not stated |
PG/VG + Nicotine +/− Flavor | 5 | |
| Desai R et al64 | 2023 | Multi-national (USA, Greece) | Basic Science | Murine | Yes | Male and female Sprague-Dawley rats | 6 hours/day, 5 days/week for 90 days of e-cigarette aerosol exposure | Histopathology | Nasal epithelium, Larynx, Trachea | Virginia Tobacco, Menthol | PG/VG + Nicotine + Flavor | 5 | |
Abbreviations: ATP, Adenosine; GSH, Glutathione; qPCR, quantitative polymerase chain reaction, PG, propylene glycol; VG, vegetable glycerin; SCCa, squamous cell carcinoma; q-PADDA, quantitative primer-anchored DNA damage detection assay; ELISA, enzyme-linked immunosorbent assay; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; TAC, total antioxidant capacity; DCFDA, 2’,7’–dichlorofluorescin diacetate; qRT-PCR, reverse transcription quantitative polymerase chain reaction; IPA, Ingenuity Pathway Analysis; LC-NSI-HRMS/MS, Liquid Chromatography-Nanoelectrospray Ionization-High-Resolution Tandem Mass Spectrometry; ROS, reactive oxidative species; TBARS, Thiobarbituric Acid Reactive Substances; EdU, 5-ethynyl-2’-deoxyuridine; LA-QPCR, long-amplicon quantitative polymerase chain reaction; SNOT-22, Sino-nasal Outcome Test; LDH, lactatae dehydrogenase; WST-1, Water-soluble tetrazolium salt-1, MDA, malondialdehyde
Across included clinical studies, duration of e-cigarette use by study participants was highly variable, ranging from a minimum of seven days45 to over a year of daily use19 (Table 1). For human in vitro studies, exposure time to e-cigarette aerosols, condensate, or e-liquid also varied greatly, ranging from seconds37 to over two weeks14 (Table 1). Animal in-vivo studies were generally designed to simulate sub-chronic e-cigarette aerosol exposures and, as such, were typically multi-week in duration.
The majority of the assessed studies analyzed the effects of e-cigarette use on the oral cavity (22/53 studies), followed by the nasal cavity/nasopharynx (13/53), multiple sites (10/53), larynx (5/53), trachea (2/53), and oropharynx (1/53). The toxicological impact of e-cigarette use on the upper airway included: pro-inflammatory (15/53 studies), histological (13/53), cytotoxicity (11/53), genotoxicity (11/53), pro-carcinogenic (6/53), immunosuppression or infectivity (4/53), ciliary dysfunction (4/53), and other findings (3/53) (Table 2). In six studies, no toxicity was observed following exposure to e-cigarettes (Table 2).
Table 2.
Major Findings and Toxicities in the 53 Included Studies
| Upper Airway Site | Study | Major Findings | Toxicity |
|---|---|---|---|
| Oral Cavity | Ji EH, et al12 | Dose-dependent decrease in intracellular GSH, decreased cell viability, and increased gene expression of HO-1 in oral keratinocytes exposed to flavored e-cigarette aerosols (+ nicotine) vs air controls. | Cytotoxicity |
| Franco T, et al13 | Decreased micronuclei in oral epithelial cells of e-cigarette users compared to cigarette smokers; no difference between non-smoking controls and e-cigarette users. | None | |
| Ganapathy V et al14 | Dose-dependent increase in DNA damage, increased intracellular ROS, decreased intracellular TAC, and decreased gene expression of the DNA repair proteins ERCC1 and OGG1 in oral keratinocytes exposed to flavored e-cigarette aerosol condensate (+/− nicotine) vs vehicle controls. Cigarette smoke exerted increased DNA damage but similar intracellular oxidative stress and protein expression changes compared to e-cigarettes | Genotoxicity, Cytotoxicity | |
| Tommasi S, et al15 | Significantly increased numbers of differentially expressed genes in oral epithelial cells of cigarette smokers and e-cigarette users (1726 genes, 1152 genes; respectively) vs non-smokers. IPA showed that “Cancer” was the top disease pathway associated with differentially expressed genes in both e-cigarette users and cigarette smokers. The most enriched canonical pathway was “Wnt/Ca+” in e-cigarette users, and “integrin signaling” in cigarette smokers. Oral epithelial cells from e-cigarette users and cigarette smokers exhibited downregulation of tumor suppressors NOTCH1 and HERC2, and upregulation of BCL-2 and its binding heat-shock protein HSPA1B compared to non-smokers. | Pro-carcinogenic | |
| Iskander AR, et al16 | No changes in histopathology in buccal epithelial cells exposed to e-cigarette aerosols (+/− nicotine) compared to air controls. Increased secretion of IL-1α, decreased secretion of IL-13, and increased expression of genes associated with “inflammatory process” network family in buccal epithelial cells exposed to e-cigarette aerosols (+/− nicotine) vs air controls. Cigarette smoke exposure resulted in buccal epithelial cell morphological changes, increased secretion of IL-1β, and increased number of differentially expressed genes compared to e-cigarettes. | Pro-inflammatory | |
| Ureña JF, et al17 | Increased intracellular ROS and decreased cell viability in SCC-25 cells that were exposed to Lava Flow flavored e-cigarette aerosols (+/− nicotine) vs air controls. No cytotoxicity seen following exposure to other flavored e-cigarette aerosols. | Cytotoxicity | |
| Tsai KYF, et al18 | Increased Ca9-22 invasion and decreased Cal-27 invasion following exposure to Red Hot flavored e-liquid (+/− nicotine) vs media-only treated controls. Decreased Ca9-22 invasion following exposure to Green Apple flavored e-liquid (+/− nicotine) vs controls. Increased RAGE expression and differential expression of secreted cytokines IL-1α, IL-8, MMP-13 in both cell lines following exposure to Red Hot or Green Apple. Nicotine addition to both e-liquid flavors enhanced RAGE expression, however reduced secretion of IL-1α, IL-8. | Pro-carcinogenic, Pro-inflammatory | |
| Pop AM, et al19 | Increased micronuclei in oral epithelial cells of e-cigarette users and cigarette smokers vs non-smoking controls; no difference between e-cigarette users and cigarette smokers. | Genotoxicity | |
| Guo J, et al20 | Significantly lower apurinic/apyramidinic sites in e-cigarette users vs nonsmokers and cigarette smokers. | None | |
| Muqawwi et al21 | Decreased number of micronucleated cells and micronucleous in oral epithelial cells of e-cigarette users and non-smoking controls vs cigarette smokers (no difference between e-cigarette users and non-smoking controls). | None | |
| Tellez CS, et al22 | Significant heterogeneity across different flavors; however, oral epithelial cells exposed to flavored e-cigarette aerosols (+/− nicotine) generally displayed increased intracellular oxidative stress, lipid peroxidation, cellular toxicity, and micronuclei formation compared to cells exposed to unflavored e-cigarette aerosols. Cigarette smoke exposure induced dose-dependent cytotoxicity, intracellular oxidative stress, and DNA damage, which exceeded any flavored or unflavored e-cigarette aerosol. | Cytotoxicity, Genotoxicity | |
| Schwarzmeier LAT, et. al23 | Oral epithelial cells from e-cigarette users displayed increased metanuclear abnormities as evidenced by: increased karolysis (vs never-smokers and former cigarette smokers), increased binucleation (vs never-smokers and former smokers), increased “broken egg” (vs cigarette smokers, never-smokers, and former cigarette smokers), and increased “nuclear bud” (vs never-smokers and former smokers). | Genotoxicity | |
| Hamad S, et. al24 | Increased expression of genes related to DNA damage (TP53, FEN1, TREX1, XRCC2, AIFM1) in oral epithelial cells following scripted vaping session. Specifically, TP53 expression was puff volume and flow rate dependent. IPA showed that “cancer”, “cell cycle”, and “DNA repair” pathways were the most enriched pathways following e-cigarette exposure. | Genotoxicity, Pro-carcinogenic | |
| Cátala-Valentín AR, et al25 | Oral epithelial cells exposed to e-cigarette condensate (+/− nicotine) showed a dose-dependent decrease in cell viability, increased COX2 expression, increased pro-inflammatory pERK1/2 and NF-kB nuclear translocation and signaling, increased immunofluorescence of DNA damage marker pH2AX, and reduced secreted cytokines (IL-8, IL-6, IL-1β) vs controls who were treated with media exposed to air. | Cytotoxicity, Genotoxicity, Pro-inflammatory | |
| Marinucci L et al26 | Oral epithelial cells exposed to e-cigarette aerosol condensate (+nicotine) displayed no changes in cell viability, morphology, apoptosis rate, or gene expression compared to media-treated controls. Oral epithelial cells exposed to cigarette smoke condensate showed significant alterations in cellular morphology, increased apoptosis rate and cellular migration vs media-treated controls. | None | |
| Cheng G et al27 | Oral epithelial cells from e-cigarette users displayed higher levels of acrolein-DNA adducts compared to non-smokers, but less than cigarette smokers. | Genotoxicity | |
| Robin H et al28 | Human oral squamous cell carcinoma cells exposed to Green Apple flavored e-cigarette aerosol condensate (+ nicotine) displayed increased protein expression of NF-kB, TNF-α, ERK, JNK, MMP-13 and increased cell invasion vs media-exposed controls. Human tongue squamous cell carcinoma cells exposed to Green Apple or Red Hot flavored-cigarette aerosol condensate (+ nicotine) showed increased protein expression of TNF-α and JNK vs media-exposed controls. | Pro-inflammatory, Pro-carcinogenic | |
| Reeve G et al29 | There were no statistically significant histological or transcriptomic changes in the oral epithelium of e-cigarettes users vs non-smokers. | None | |
| Mandour D et al30 | Albino rats exposed to nicotine-containing e-cigarette aerosols demonstrated widening of submandibular acini and ducts along with epithelial degeneration, cytoplasm vacuolization, connective tissue septa thickening, and increased TNF-α immunostaining compared to submandibular glands from air controls. | Histological, Pro-inflammatory | |
| Elmahdi F et al31 | E-cigarette users displayed higher rates of oral epithelial cytological atypia (4.8 vs 0.4%) and HPV infection rates (3.2 vs 0.8%) vs non-smokers. Among e-cigarette users, higher atypia rates and HPV infection rates were seen in individuals with a longer duration of e-cigarette use (> 7 years). | Histological, Immunosupression or Infectivity | |
| Tommasi S et al32 | Oral epithelial cells from e-cigarette users displayed a dose-dependent increase in DNA damage vs non-smoking controls. There was no difference in DNA damage levels between e-cigarette users and smokers. Nicotine content was not associated with increased DNA damage. Among e-cigarette flavors, fruit, sweet, and mint/menthol showed the highest amount of DNA damage. | Genotoxicity | |
| Muniz de Lima J, et al33 | Increased cytotoxicity in normal oral epithelial cells treated with e-liquid (+ nicotine) vs untreated control. Human and murine oral SCCa cells treated with e-liquid (+ nicotine) showed increased cell proliferation, cell migration and invasion, as well as gene expression changes consistent with an epithelial to mesenchymal transition when compared to untreated controls. | Cytotoxicity, Pro-carcinogenic | |
| Oropharynx | Welz C, et al34 | Increased cytotoxicity and DNA fragmentation in oropharyngeal epithelial cells exposed to e-cigarette condensates (fruit flavors > tobacco) vs controls. | Cytotoxicity, Genotoxicity |
| Nasal Cavity/ Nasopharynx | Martin EM, et al35 | Decreased expression of immune-related genes (ZBTB16, PIGR, PTGS2, FKBP5) and transcription factors (NFKB1, ETS1, NOTCH1, BCL3, XBP1, BCL6, EGR1) in nasal epithelial cells from current e-cigarette users and cigarette smokers compared to non-smokers (> suppression in e-cigarette users vs. cigarette smokers). Top IPA canonical pathways in both e-cigarette users and cigarette smokers included: “cytokine-cytokine receptor interaction,” “apoptosis,” “Toll-like receptor signaling pathway,” and “NOD-like receptor signaling pathway.” | Immunosuppression or Infectivity, Pro-inflammatory |
| Kumral TL, et al36 | After 3 months of cigarette smoking cessation, both e-cigarette users and non-smokers displayed lower SNOT-22 scores vs baseline (SNOT-22 of non-smokers < e-cigarette users), whereas after 3 month of smoking cessation, only non-smokers had significantly lower mucociliary clearance times. | Ciliary dysfunction | |
| Carson JL, et al37 | Human nasal epithelial cells exposed to cigarette smoke or e-cigarette aerosols displayed similar declines in ciliary beat frequency with gradual return of function within 1 hour. Nitric oxide production was far greater in nasal epithelial cells exposed to cigarette smoke vs e-cigarette aerosol or unexposed controls. Following exposure to e-cigarette aerosols, nasal epithelial cells displayed secretory material overlying ciliary beds indicating mucous hypersecretion; however, this finding was much more prominent in nasal epithelial cells exposed to cigarette smoke. | Histological, Ciliary dysfunction | |
| Miyashita L et al38 | Nasal epithelial PAFR expression increased following vaping session in e-cigarette users. Pneumococcal adhesion increased in human nasal epithelial cells following exposure to e-cigarette aerosol condensate (+/− nicotine) compared to vehicle controls. In CD1 mice, e-cigarette aerosol condensate (+ nicotine) exposure increased nasal PAFR expression and nasopharyngeal pneumococcal colonization vs vehicle controls. | Immunosupression or infectivity | |
| Jabba S et al39 | Human nasal epithelial cells exposed to the benzaldehyde-propylene glycol adduct (berry/fruit e-cigarette flavoring) showed increased cell death vs benzaldehyde exposure alone. | Cytotoxicity | |
| Rouabhia M, et al40 | Nasal epithelial cells exposed to e-cigarette aerosol (+/− nicotine) or cigarette smoke showed increased LDH levels, fewer Ki67+ cells, reduced cellular proliferation rate, and increased secretion of IL-6, IL-8, TNF-α, and MCP-1 compared to controls (Magnitude of effects: cigarette smoke > nicotine-rich e-cigarette aerosols > nicotine-free e-cigarette aerosols). | Pro-inflammatory, Cytotoxicity | |
| Rebuli ME, et al41 | Compared to non-smokers, e-cigarette users and cigarette smokers showed suppression of live attenuated influenza vaccine (LAIV)-induced nasal immune response as evidenced by decreased gene expression, decreased cytokine/chemokine secretion, and decreased LAIV-specific IgA levels (Magnitude of effects: e-cigarette users > cigarette smokers > non-smokers) | Immunosuppression or infectivity | |
| Escobar YN et al42 | Human nasal epithelial cells from non-smokers exposed to e-cigarette aerosols (+/− nicotine) demonstrated increased protein expression of MUC5AC vs air controls. Human nasal epithelial cells from non-smokers exposed to e-cigarette aerosol (+ nicotine) showed elevated MUC5AC and MUC5B protein levels vs air controls. Human nasal epithelial cells from smokers exposed to e-cigarette aerosols (+/− nicotine) showed elevated pro-inflammatory cytokine secretion. | Pro-inflammatory | |
| Kwak S et al43 | Human nasal epithelial cells exposed to the e-cigarette compounds (glyoxal or methylglyoxal) demonstrated increased secretion of IL-1β, IL-6, MUC5AC, MUC5B, and increased activation of nuclear factors (ERK1/2, p38, and NF-κB) compared to culture-exposed controls. | Pro-inflammatory | |
| Hinds D et al44 | Golden Syrian mice exposed to 2 days of e-cigarette aerosols displayed nicotine-dependent increases in nasal epithelial gene expression of CCL-5, CXCL-10, TNF-α, IL-1β, TGF-β, and nicotine-independent increases in gene expression of SOD-2 and decreases in gene expression of VEGF vs air controls. There were no changes in the histology of the nasal epithelium of e-cigarette aerosol exposed mice compared to air controls. | Pro-inflammatory | |
| Karey E et al45 | Pro-inflammatory cytokine levels (IL-2, IL-4, IL-8, IL-10, IL-12p70, IL-13, TNF-α, IL-1β, IFN-γ) were significantly increased in the nasal fluid of e-cigarette users compared to cigarette users and non-smoking controls. | Pro-inflammatory | |
| Pozuelos et al46 | Significantly increased number of differentially expressed genes in primary human nasal epithelial cells of cigarette smokers and e-cigarette users (407 genes, 1817 genes; respectively) vs non-smokers. Enriched gene ontology terms for the downregulated genes between e-cigarette users and non-smokers included “cilium assembly and function” and for the upregulated genes included “immune response”, “neutrophil activation”, “leukocyte degranulation”, and “granulocyte activation”. | Pro-inflammatory, Ciliary dysfunction, Immunosupression or Infectivity | |
| Nicholas BD, et al47 | BALB/cJ mice exposed to 8 weeks of e-cigarette aerosols displayed decreased goblet cells in Eustachian tube mucosa vs controls (no significant differences in cilia, mucin, and squamous metaplasia). In BALB/cJ mice exposed to e-cigarette aerosol, trans-tympanic application of anti-IL-13 or AG1478 led to increased goblet cells in Eustachian tube mucosa. | Histological | |
| Larynx | Salturk Z, et al48 | Wistar albino rats exposed to 4 weeks of e-cigarette aerosols (+ nicotine) displayed increased vocal fold hyperplasia and metaplasia, however results were not statistically significant. | None |
| Ha TN, et al49 | C57BL/6 mice exposed to 16 weeks of e-cigarette aerosols (+ nicotine) or cigarette smoke displayed significantly elevated levels of IL-4 in larynx homogenates compared to control mice exposed to air or e-cigarette aerosols without nicotine. | Pro-inflammatory | |
| Hassan NH, et al50 | Wistar albino rats exposed to 4 weeks of e-cigarette aerosols (+ nicotine) displayed increased MDA levels and decreased TAC levels in laryngeal tissue homogenates vs control rats exposed to air. Histologically in the larynx, e-cigarette-exposed rats showed increased cilia loss, increased epithelial hyperplasia, increased vascular dilation of the lamina propria, increased p53 immunostaining, and decreased Ki67 immunostaining compared to control rats exposed to air. | Cytotoxicity, Histological, Ciliary dysfunction | |
| Martinez JD, et al51 | Human vocal fold fibroblasts (hVFFs) exposed to e-cigarette condensate (+/− nicotine) showed decreased cell viability vs untreated controls. Compared to e-cigarette condensate, hVFFs exposed to cigarette smoke condensate showed a greater reduction in cell viability and increased DNA damage. Gene expression was not significantly altered in hVFFs following either e-cigarette or cigarette smoke condensate exposure. | Cytotoxicity, Genotoxicity | |
| Easwaran M et al52 | There were no differences in vocal fold epithelial thickness, cellular proliferation, glandular surface area, surface topography between C57BL6/J rats exposed to flavored e-cigarette aerosol (+ nicotine) or air controls. There was a slight increase in acidic mucus content in the subglottis and increase in macrophage and CD3+ T cell infiltration in the vocal cords of e-cigarette exposed mice vs air controls. | Histological | |
| Trachea | Wu Q et al53 | Tracheobronchial epithelial cells exposed to tobacco flavored e-liquid (+/− nicotine) showed increased IL-6 secretion, enhanced human rhinovirus infection, and decreased SPLUNC1 expression. There was no change in cytotoxicity of tracheobronchial epithelial cells exposed to flavored e-liquid (+/− nicotine) at 24 or 48 hours post exposure. | Pro-inflammatory, Immunosupression or Infectivity |
| El-Merhie et al54 | Maternal Drosophilia Melanogaster flies exposed to nicotine-containing e-cigarette aerosols prior to mating produced offspring with abnormalities in tracheal length compared to sham controls. | Other | |
| Multisite | Manyanga J, et al55 | E-cigarette (+/− nicotine) or cigarette smoke condensate exposure in various oral cavity and oropharyngeal cancer cell lines reduced cisplatin-induced cytotoxicity, reduced expression of DNA repair genes MMS19 and ERCC1, decreased expression of drug influx protein CTR1, and increased expression of drug efflux proteins ABCG2 and ATP7A compared to cells exposed to saline treated controls. | Pro-carcinogenic, Genotoxicity |
| Werley MS, et al56 | Sprague-Dawley rats chronically exposed to 13 weeks of e-cigarette aerosols (+/− nicotine or flavor) displayed increased nasal secretions, nasal mucous cell hyperplasia and epithelial vacuolization, and intraluminal mucin exudate in the larynx; histological changes did not resolve following 42-day recovery period. | Histological | |
| Phillips B, et al57 | Sprague-Dawley rats chronically exposed to 13 weeks of e-cigarette aerosols (+ nicotine) displayed mild adaptive squamous cell metaplasia and basal cell hyperplasia in the larynx vs air controls. No statistically significant histological changes in nasal epithelium. There were no changes in nasal epithelial gene or protein expression between rats exposed to e-cigarettes or filtered air. | Histological | |
| Lee KM, et al58 | C57BL/6 exposed to 13 days of e-cigarette aerosols had a minimal increase in microscopic squamous metaplasia of the nasal turbinates and epiglottis compared to sham controls. All histological findings were significantly more severe in cigarette smoke-exposed group. | Histological | |
| Ho J, et al59 | Sprague-Dawley rats exposed to 13 weeks of e-cigarette aerosols (+ nicotine) displayed mild adaptive squamous cell metaplasia and lamina propria infiltration in the larynx. No statistically significant histological changes in nasal epithelium. No biologically significant gene expression differences in nasal epithelium of rats exposed to e-cigarettes aerosols vs PBS vehicle controls. | Histological | |
| Ni F, et al60 | C57BL/6 mice exposed to flavored e-cigarette aerosols (+/− nicotine) displayed increased numbers of stimulated nociceptive neurons (nasal cavity > trachea) vs air controls. | Other | |
| Wong ET et al61 | ApoE−/− mice exposed to 6 months of e-cigarette aerosols (+/− nicotine) displayed no differences in nasal or tracheal histology and only minimal squamous metaplasia at the epiglottis compare to the sham control group. All histological findings were significantly more severe in cigarette smoke-exposed group. E-cigarette exposed mice showed no significant changes in gene expression in the nasal epithelium or trachea following exposure. | Histological | |
| Kim M, et al62 | Reduced nasal CFTR function was seen in human subjects after 7 days of vaping vegetable glycerol (VG)-containing e-cigarettes vs baseline. Primary human nasal epithelial cells exposed to VG-containing e-liquid displayed increased secretions of pro-inflammatory mediators (IL-6, IL-8, MMP-2, MMP-9, TGF-β) vs baseline. Tracheal secretions from sheep exposed to 5 days of VG-containing e-cigarette aerosols showed increased mucus concentrations and MMP-9 activity vs baseline | Pro-inflammatory, Other | |
| Wong ET, et al63 | A/J mice exposed to 5 weeks of flavored e-cigarette aerosols (+ nicotine) displayed a mild adaptive increase in nasal and laryngeal epithelial hyperplasia and laryngeal squamous metaplasia. No significant histological changes in trachea. All histological findings were significantly more severe in cigarette smoke-exposed group. | Histological | |
| Desai R, et al64 | Sprague-Dawley rats exposed to 13 weeks of e-cigarette aerosols (+ nicotine) displayed mild squamous cell metaplasia in the larynx vs air controls. No statistically significant histological changes were seen in the nasal epithelium. All histological findings were significantly more severe in cigarette smoke-exposed group | Histological |
Abbreviations: GSH, glutathione; ROS, reactive oxidative species, TAC, total antioxidant capacity; IPA, Ingenuity pathway analysis; RAGE, receptor for advanced glycation end-products; HPV, human papilloma virus; SNOT-22, Sino-nasal Outcome Test; MDA, malondialdehyde
Cytotoxicity was seen in all upper airway subsites, except the trachea, following e-cigarette exposure and was most commonly measured via changes in markers of cellular apoptosis rate, cellular metabolic activity, and/or intracellular oxidative species or antioxidant levels.12,14,17,22,25,33,34,39,40,50,51 Pro-inflammatory effects were seen in all upper airway subsites, except for the oropharynx. These included elevated levels of secreted soluble inflammatory mediators (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, IFN-γ, CCL-5, CXCL-10, MCP-1, MMP-2, MMP-9, MMP-13, MUC5AC, MUC5B), increased expression of genes or proteins involved in inflammatory signaling (ERK1/2, p38, COX-2, JNK, and NF-kB), and enrichment of gene sets or pathways associated with inflammatory processes.16,18,25,28,30,35,40,42–46,49,53,62 Genotoxicity was seen primarily in the oral cavity, but also in the oropharynx and larynx, and was detected by increased DNA fragmentation, adducts, double- or single-strand breaks, appearance of micronuclei or metanuclear abnormalities, or increased expression of genes or proteins involved with DNA damage or repair pathways.14,19,22–25,27,32,51
Histological changes associated with acute and sub-chronic e-cigarette aerosol exposure in animal models were seen in the oral cavity, submandibular gland, nasal cavity, larynx, and trachea; however, the majority of these changes were considered adaptive and resolved following a recovery period.30,31,37,47,50,52,56–59,61,64 Persistent histological changes in the nasal cavity and larynx included: mild epithelial hyperplasia and squamous metaplasia, and mucus hypersecretion. In the submandibular gland, ductal dilation, connective tissue thickening, and epithelial degeneration with cytoplasmic vacuolization was observed.30 Pro-carcinogenic features such as downregulation of tumor suppression genes were seen in normal oral cavity epithelial cells following exposure to e-cigarette aerosols or e-liquid.15,33 Additionally, in both human oral and oropharyngeal squamous cell carcinoma cell lines, exposure to e-cigarette aerosols and flavorings led to increased cellular proliferation/survival, migration, invasion, and resistance to cytotoxic chemotherapeutic agents.18,28,55 Various mechanisms for e-cigarette-induced immunosuppression were seen in the nasal cavity, including suppression of an influenza-induced immune response and decreased expression of genes related to immune cell function.35,41,46 Additionally, enhanced bacterial and viral infectivity following e-cigarette exposure were observed and included increased nasopharyngeal pneumococcal colonization38, and increased human papillomavirus and rhinovirus infection rates in oral and tracheobronchial epithelial cells, respectively31,53. Ciliary dysfunction was also seen in the nasal cavity of e-cigarette users and in primary nasal epithelial cells following exposure to e-cigarette aerosols, and was measured by increased mucociliary clearance times36, decreased ciliary beat frequency37, and downregulation of genes associated with cilium assembly and function45. Other toxicological effects of e-cigarettes that were identified included: embryological abnormalities in tracheal development in Drosophilia Melanogaster flies54, enhanced stimulation of nociceptive neurons in the nasal cavity and trachea of mice60, and reduced nasal cystic fibrosis transmembrane conductance regulator protein function in human subjects62.
A variety of e-cigarette components were analyzed. In clinical studies, the brand of e-cigarettes used by subjects was generally not provided, and no information was included regarding whether devices contained added flavorings and/or nicotine. Thirteen human in-vitro studies14,16,17,18,22,25,28,38,40,42,51,53,55 analyzed the effects of e-cigarette aerosol/condensate exposure with and without nicotine and showed mixed toxicological effects: eight studies14,16,17,22,25,51,53,55 demonstrated similar effects with and without nicotine; whereas five studies18,28,38,40,42 showed aerosol/condensate exposure from nicotine-containing e-cigarettes had enhanced toxicity. In these five studies, the addition of nicotine to e-cigarettes enhanced cytotoxicity, increased secretion of inflammatory mediators, increased intracellular oxidative stress, increased bacterial infectivity, and enhanced cellular invasion and RAGE expression. In sub-chronic exposure studies in animal models49,56,57,59, histological findings of squamous cell metaplasia in the larynx was predominately seen in those exposed to nicotine-containing e-cigarette aerosols as opposed to nicotine-free e-cigarette aerosols.
Twenty-six studies investigated the effects of flavored e-cigarettes.12,14,16–18,22,26,28,30,32–34,37–40,47,50,52,56,58,59,61,63,64 The most commonly studied flavors were tobacco and mint/menthol, which appeared in 9/26 and 4/26 studies, respectively. One study22 compared the effects of ten different flavored e-liquids vs. unflavored e-liquid of the same brand on oral epithelial cells and showed that, in general, flavored e-liquids (+/− nicotine) were associated with increased oxidative stress, lipid peroxidation, cytotoxicity, and micronuclei formation compared to unflavored e-liquid. Eleven studies14,17,18,22,28,32,34,39,52,60,64 directly compared various e-cigarette flavors to one another; however, the flavors varied between studies limiting the generalizability of results.
Twenty-five studies13–16,19–23,26,27,32,35,37,40,41,45,46,49,51,55,58,61,63,64 directly compared the effects of exposure to e-cigarette vapor vs. traditional cigarette smoke. In general, exposure to traditional cigarette smoke/condensate resulted in increased cytotoxicity, intracellular oxidative stress, DNA damage, and upregulated pro-inflammatory response compared with exposure to e-cigarette aerosol/condensate (+/− nicotine). Additionally, in sub-chronic animal exposure studies, cigarette smoke led to more severe and permanent histological changes in both the nasal cavity and larynx compared to e-cigarette aerosols (+/− nicotine).58,61,63,64
DISCUSSION
This scoping review collates the current literature and highlights the effects of e-cigarettes on the upper airway, a topic that is becoming a greater public health concern. The studies we analyzed are heterogeneous and include: human clinical studies using samples from current or former e-cigarette users; in-vitro experiments utilizing primary cell cultures or immortalized cell lines derived from human or animal upper airway tissue; or in-vivo studies utilizing animal models to characterize sub-chronic e-cigarette aerosol exposure. A majority of studies primarily analyzed tissue from the oral cavity and were spearheaded by dental or basic science researchers. Few studies analyzed other subsites in the upper aerodigestive tract and even less were led by Otolaryngologist.
E-liquids require solvents/humectants such as propylene glycol (PG) and glycerol (vegetable glycerin) to maintain moisture and the ability to vaporize. Studies in this review demonstrated toxicity from exposure to e-cigarette aerosols or e-liquids in the absence of nicotine or flavoring, which highlights the potential harmful effects of these solvents/humectants on the upper airway.16,22,25,42,44,49,51,53,55,56,57,59,61,62 Across human in-vitro studies, exposure to e-cigarette humectants alone increased the secretion of soluble inflammatory mediators in the oral cavity22,25, nasal cavity42,44, and trachea62; induced cytotoxicity in oral epithelial cells22 and vocal fold fibroblasts51; promoted genotoxicity in oral epithelial cells22,25; and enhanced human rhinovirus infection in tracheobronchial epithelial cells62. Additionally, exposing oral cavity and oropharyngeal cancer cell lines to humectant condensate reduced cisplatin-induced cytotoxicity.55 This finding supports the idea that exposure to e-cigarette vapor promotes resistance to chemotherapy and may worsen the efficacy of oral cavity and oropharyngeal cancer treatment, similar to the effects of tobacco smoke.
Studies investigating histological changes in the upper airway following sub-chronic humectant aerosol exposure in animal models were mixed. Increased nasal secretions, nasal mucous cell hyperplasia and epithelial vacuolization, and increased intraluminal mucin exudate was observed in the larynx of Sprague-Dawley rats following a 90-day exposure56; however, in two other murine exposure studies57,59, histological changes were only seen when nicotine was added to humectants. The toxicological effects of humectants have been corroborated in lower airway studies as humectant aerosol exposure increased the secretion of inflammatory cytokines compared to air controls in human bronchial epithelial cells.65 Further, humectant aerosol exposure disrupted lipid homeostasis in murine alveolar macrophages and airway epithelial cells resulting in decreased surfactant production and impaired immunological response to viral infections.66
While the above studies demonstrate that e-cigarettes can cause damage to tissues of the upper airway, even if they only including humectants such as glycerol and propylene glycol, it is probable that the addition of flavorings that make e-cigarettes so popular contribute additional deleterious effects. In lower airway studies65,67,68, e-cigarette flavorings directly influence cytotoxicity. However, only one study22 included in this review directly compared flavored vs. unflavored e-cigarettes of the same brand. In this study, out of the ten flavors tested only one, Arctic Blue, demonstrated lower combined toxicity scores compared to the unflavored e-liquid. While data from this singular study strongly suggest that the addition of flavorings to e-liquids has toxicological impact, additional future studies are needed to better characterize the specific effect in the upper airway.
One challenge of investigating the toxicological impact of flavorings is the heterogeneity of flavors between studies, which limits the generalizability of results. Additionally, flavoring compounds associated with common flavor names (e.g. fruit) often vary across brands, which precludes accurate comparisons of their toxicological impact. Our review found eleven studies14,17,18,22,28,32,34,39,52,60,64 that directly compared flavors against one another; however, only menthol and tobacco were included in more than two studies. Notably, tobacco flavoring appeared to be generally less harmful than the other flavors studied. Tobacco flavored e-cigarettes were less cytotoxic and genotoxic to oropharyngeal cells than fruit flavors34 and were less cytotoxic to oral epithelial cells compared with other e-cigarette flavorings (sweet, mint/menthol, fruit)32. Given the wide variety of e-cigarette flavorings available on the market–from tobacco to chocolate to strawberry–and the different chemicals used to achieve those flavors, there is much to glean from specifically assessing the interactions of these additives with upper aerodigestive tract cells. Testing the effects of available flavorings should be prioritized in future studies given the allure that flavors hold in the public perception of these products.
E-cigarettes are promoted as a tool to stop smoking conventional cigarettes and are perceived as relatively safe; therefore, it is critically important to assess the effects of exposure to e-cigarettes on tissues of the upper aerodigestive tract compared to the effects of exposure to conventional cigarette smoke. A majority of studies in this review found that upper airway tissue that was exposed to cigarette smoke exhibited more severe toxicological effects compared to tissue exposed to e-cigarettes vapors. In particular, exposure to conventional cigarette smoke induced dose-dependent cytotoxicity, intracellular oxidative stress, and DNA damage in oral epithelial cells at levels that far exceeded the effects of any flavored or unflavored e-cigarette aerosol either with or without the addition of nicotine.22 Similar effects were shown across other upper airway subsites. Human vocal fold fibroblasts that were exposed to media treated with cigarette smoke extract demonstrated a greater reduction in cell viability and increased DNA damage compared to cells exposed to media treated with e-cigarette extract.51 In sub-chronic animal exposure studies, cigarette smoke consistently led to more severe and permanent histological changes in the nasal cavity and larynx compared to e-cigarette vapor either with or without the addition of nicotine.58,61,63,64 In the nasal cavity, nasal epithelial cells exposed to e-cigarette aerosol (+/− nicotine) or cigarette smoke showed increased intracellular oxidative stress, reduced cellular proliferation rate, and increased secretion of inflammatory mediators compared to air controls, although the magnitude of effects were larger in the group exposed to cigarette smoke.40 Of note, e-cigarettes seem to exert a comparable or possibly greater immunosuppressive effect in the nasal cavity compared to conventional tobacco cigarettes. In particular, nasal epithelial cells from current e-cigarette users and cigarette smokers exhibited decreased expression of immune-related genes and transcription factors compared to non-smokers, with greater suppression observed in the e-cigarette cohort.35 More recent work has shown that following inoculation with the live-attenuated influenza virus, current e-cigarette users and conventional cigarette smokers demonstrated similar impairment in viral nasal immune responses, as evidenced by decreased gene expression, secretion of inflammatory cytokines and chemokines, and influenza-specific IgA as compared with non-smokers.41
This scoping review had several limitations. Firstly, clinical studies had varying inclusion criteria for the duration of e-cigarette use and many studies had limited information regarding the types of e-cigarettes used by participants. Future clinical studies should include information regarding the brand of e-cigarette used by subjects as well as whether the device contains nicotine and/or flavoring additives. An additional concern was the limited number of studies investigating the toxicological effects of e-cigarettes at upper airway sites outside of the oral and nasal cavities. There was only one study that investigated effects in the oropharynx and no studies examined effects in the hypopharynx. Future studies investigating these other subsites are needed to fully characterize the toxicological profile of e-cigarettes on the upper airway. Finally, across the in-vitro studies there was significant heterogeneity in the methods used to expose cells to e-cigarettes. These included exposing upper airway cells: 1) directly to e-liquid, 2) to e-cigarette aerosol condensate in culture media, and 3) to e-cigarette aerosols utilizing an exposure chamber. It is possible that there are differential toxicological effects following exposure to heated e-cigarette aerosols vs. e-liquid vs. e-cigarette condensate. As in-vivo exposure to e-cigarettes in the upper airway physiological occurs in aerosol form, priority should be place on using this method of exposure in future in vitro studies.
CONCLUSIONS
E-cigarette use has become increasingly mainstream in the last two decades, especially in younger populations; however, there is a lack of research investigating its deleterious effects, particularly in the upper airway. This scoping review compiled the current literature reporting the effects of e-cigarettes in the upper aerodigestive tract to better understand their harms and to identify avenues for future research. The consensus from our analysis is that exposure to e-cigarette aerosols, condensate, or e-liquids can increase cytotoxicity, pro-inflammatory effects, genotoxicity, histological changes, pro-carcinogenic effects, immunosuppression, healing impairment, ciliary dysfunction, microbiome changes, and neurotoxicity at multiple sites within the upper airway. While future investigations are needed to fully characterize the effects of e-cigarettes on less studied sites such as the oropharynx and hypopharynx, the otolaryngologist should counsel patients that emerging evidence indicates that vaping is toxic to upper airway tissue and should ideally be avoided. Patients should be advised that while e-cigarettes are likely less harmful to the upper airway compared to traditional cigarettes, they have an independent toxicological profile that is enhanced with the addition of nicotine or flavorings that often vary considerably between brands. If e-cigarettes are to being considered as a bridge for smoking cessation, the clinician should have a detailed discussion with patients about the potential risks and benefits of this approach and a shared decision should be made. Finally, patients should be made aware that due to their recency of introduction, data regarding the health effects of long-term e-cigarette use are not currently known. As the primary caregivers for diseases affecting the upper airway, otolaryngologists should take initiative in forwarding research to help identify the possible injurious effects of e-cigarettes so that they can appropriately counsel patients.
Supplementary Material
Financial Disclosure:
Research in this publication was supported by the NIDCD branch of the NIH under award number 5T32DC005360 (C.P.W., Z.F). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
No authors have any financial conflicts of interest.
The authors have no financial disclosures or conflicts of interest.
Presented as an oral presentation at the Triological Society Meeting at Combined Otolaryngology Sections Meeting in April 2022 in Dallas, Texas.
Level of Evidence: 2
REFERENCES
- 1.Villarroel MA, Cha AEVA. Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief, no 365. [PubMed]
- 2.Centers for Disease Control and Prevention. Frequency of tobacco use among middle and high school students—United States, 2014. Morbidity and Mortality Weekly Report 2015b;64(38):1061–5. [DOI] [PubMed] [Google Scholar]
- 3.Electronic Cigarettes (E-Cigarettes) Dollar Sales in the United States from 2014 to 2018 (in Billion U.S. Dollars)* Statista Research Department. 2015. https://www.statista.com/statistics/285143/us-e-cigarettes-dollar-sales/.
- 4.Romijnders KAGJ, Osch L van, Vries H de, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: A narrative literature review. Int J Environ Res Public Health 2018;15(6):11–22. doi: 10.3390/ijerph15061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of Physicians (2016) Nicotine without smoke: Tobacco harm reduction London: Royal College of Physicians. [Google Scholar]
- 6.Hartmann-Boyce J, McRobbie H, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2020. Oct 14;10(10):CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floyd EL, Queimado L, Wang J, Regens JL, Johnson DL. Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS One 2018;13(12):1–15. doi: 10.1371/journal.pone.0210147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karey E, Reed T, Katsigeorgis M, Farrell K, Hess J, Gibbon G, Weitzman M, Gordon T. Exhalation of alternative tobacco product aerosols differs from cigarette smoke-and may lead to alternative health risks. Tob Use Insights 2022. Feb 28;15:1179173X221078200. doi: 10.1177/1179173X221078200. PMID: 35250322; PMCID: PMC8891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherian SV, Kumar A, Estrada-Y-Martin RM. E-Cigarette or Vaping Product-Associated Lung Injury: A Review. Am J Med 2020;133(6):657–663. doi: 10.1016/j.amjmed.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6(7). doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.OCEBM Levels of Evidence Working Group. “The Oxford 2011 Levels of Evidence” Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653 [Google Scholar]
- 12.Ji EH, Sun B, Zhao T, et al. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLoS One 2016. May 25;11(5):e0154447. doi: 10.1371/journal.pone.0154447. Erratum in: PLoS One. 2016 Dec 29;11(12 ):e0169380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco T, Trapasso S, Puzzo L, Allegra E. Electronic Cigarette: Role in the Primary Prevention of Oral Cavity Cancer. Clin Med Insights Ear Nose Throat 2016. Oct 17;9:7–12. doi: 10.4137/CMENT.S40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, Queimado L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One 2017. May 18;12(5):e0177780. doi: 10.1371/journal.pone.0177780. PMID: 28542301; PMCID: PMC5436899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tommasi S, Caliri AW, Caceres A, et al. Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int J Mol Sci 2019;20(3):1–18. doi: 10.3390/ijms20030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iskandar AR, Zanetti F, Kondylis A, et al. A lower impact of an acute exposure to electronic cigarette aerosols than to cigarette smoke in human organotypic buccal and small airway cultures was demonstrated using systems toxicology assessment. Intern Emerg Med 2019. Sep;14(6):863–883. doi: 10.1007/s11739-019-02055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ureña JF, Ebersol LA, Silakov A, et al. Impact of Atomizer Age and Flavor on In Vitro Toxicity of Aerosols from a Third-Generation Electronic Cigarette against Human Oral Cells. Chem Res Toxicol 2020. Oct 19;33(10):2527–2537. doi: 10.1021/acs.chemrestox.0c00028. [DOI] [PubMed] [Google Scholar]
- 18.Tsai KYF, Hirschi Budge KM, Lepre AP, et al. Cell invasion, RAGE expression, and inflammation in oral squamous cell carcinoma (OSCC) cells exposed to e-cigarette flavoring. Clin Exp Dent Res 2020; 6: 618–625. doi: 10.1002/cre2.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pop AM, Coroș R, Stoica AM, Monea M. Early diagnosis of oral mucosal alterations in smokers and e-cigarette users based on micronuclei count: A cross-sectional study among dental students. Int J Environ Res Public Health 2021;18(24). doi: 10.3390/ijerph182413246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Ikuemonisan J, Hatsukami DK, Hecht SS. Liquid Chromatography-Nanoelectrospray Ionization-High-Resolution Tandem Mass Spectrometry Analysis of Apurinic/Apyrimidinic Sites in Oral Cell DNA of Cigarette Smokers, e-Cigarette Users, and Nonsmokers. Chem Res Toxicol 2021;34(12):2540–2548. doi: 10.1021/acs.chemrestox.1c00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muqawwi ASY. The Genotoxic Potential of Electronic Cigarettes on Micronucleus Count: A Preliminary Study. International Journal of Clinical Dentistry 2021;14(2):133–140. [Google Scholar]
- 22.Tellez CS, Juri DE, Phillips LM, Do K, Yingling CM, Thomas CL, Dye WW, Wu G, Kishida S, Kiyono T, Belinsky SA. Cytotoxicity and Genotoxicity of E-Cigarette Generated Aerosols Containing Diverse Flavoring Products and Nicotine in Oral Epithelial Cell Lines. Toxicol Sci 2021. Jan 28;179(2):220–228. doi: 10.1093/toxsci/kfaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzmeier LÂT, da Cruz BS, Ferreira CCP, et al. E-cig might cause cell damage of oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131(4):435–443. doi: 10.1016/j.oooo.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Hamad SH, Brinkman MC, Tsai YH, et al. Pilot study to detect genes involved in DNA damage and cancer in humans: Potential biomarkers of exposure to e-cigarette aerosols. Genes (Basel) 2021;12(3). doi: 10.3390/genes12030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cátala-Valentín AR, Almeda J, Bernard JN, Cole AM, Cole AL, Moore SD, Andl CD. E-Cigarette Aerosols Promote Oral S. aureus Colonization by Delaying an Immune Response and Bacterial Clearing. Cells 2022. Feb 23;11(5):773. doi: 10.3390/cells11050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinucci L, Coniglio M, Valenti C, et al. In Vitro effects of alternative smoking devices on oral cells: Electronic cigarette and heated tobacco product versus tobacco smoke. Arch Oral Biol 2022;144:105550. doi: 10.1016/j.archoralbio.2022.105550 [DOI] [PubMed] [Google Scholar]
- 27.Cheng G, Guo J, Carmella SG, et al. Increased acrolein-DNA adducts in buccal brushings of e-cigarette users. Carcinogenesis 2022;43(5):437–444. doi: 10.1093/carcin/bgac026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robin HP, Trudeau CN, Robbins AJ, et al. Inflammation and Invasion in Oral Squamous Cell Carcinoma Cells Exposed to Electronic Cigarette Vapor Extract. Front Oncol 2022;12:917862. Published 2022 Jul 22. doi: 10.3389/fonc.2022.917862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeve GS, Rostami MR, Reich RF, et al. Oral epithelium response of electronic cigarette users to electronic cigarette. J Oral Pathol Med 2023;52(5):431–439. doi: 10.1111/jop.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandour DA; Abdelfattah MT; Saber SM; Soliman RHM. Vapor of Electronic Cigarettes Induces Histopathological Changes in the Rat Submandibular Gland. Egyptian Journal of Histology, 46, 1, 2023, 150–162. doi: 10.21608/ejh.2021.90525.1555 [DOI] [Google Scholar]
- 31.Elmahdi FM, Aljohani RS, Alharbi NA, Yousef SE, Alharbi NM, Afasha RB, Aljohani RB, Alhejaili YK, Almuzaini NO. A Cytological Study of Oral Human Papillomavirus (HPV) Infection Among Electronic Cigarette Smokers in Al-Madinah Al-Munawara. Cureus 2023. Jun 14;15(6):e40421. doi: 10.7759/cureus.40421. PMID: 37456376; PMCID: PMC10348396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tommasi S, Blumenfeld H, Besaratinia A. Vaping Dose, Device Type, and E-Liquid Flavor are Determinants of DNA Damage in Electronic Cigarette Users. Nicotine Tob Res 2023;25(6):1145–1154. doi: 10.1093/ntr/ntad003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lima JM, Macedo CCS, Barbosa GV, Castellano LRC, Hier MP, Alaoui-Jamali MA, da Silva SD. E-liquid alters oral epithelial cell function to promote epithelial to mesenchymal transition and invasiveness in preclinical oral squamous cell carcinoma. Sci Rep 2023. Feb 27;13(1):3330. doi: 10.1038/s41598-023-30016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welz C, Canis M, Schwenk-Zieger S, Becker S, Stucke V, Ihler F BP. Cytotoxic and Genotoxic Effects of Electronic Cigarette Liquids on Human Mucosal Tissue Cultures of the Oropharynx. J Env Pathol Toxicol Oncol 2016;35(4):343–354. [DOI] [PubMed] [Google Scholar]
- 35.Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol - Lung Cell Mol Physiol 2016;311(1):L135–L144. doi: 10.1152/ajplung.00170.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumral TL, Saltürk Z, Yildirim G, et al. How does electronic cigarette smoking affect sinonasal symptoms and nasal mucociliary clearance? B-ENT 2016;12(1):17–21. [PubMed] [Google Scholar]
- 37.Carson JL, Zhou L, Brighton L, Mills KH, Zhou H, Jaspers I, Hazucha M. Temporal structure/function variation in cultured differentiated human nasal epithelium associated with acute single exposure to tobacco smoke or E-cigarette vapor. Inhal Toxicol 2017. Feb;29(3):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyashita L, Suri R, Dearing E, et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J 2018;51(2):1701592. Published 2018 Feb 7. doi: 10.1183/13993003.01592-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabba SV, Diaz AN, Erythropel HC, Zimmerman JB, Jordt SE. Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells. Nicotine Tob Res 2020;22(Suppl 1):S25–S34. doi: 10.1093/ntr/ntaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouabhia M, Piché M, Corriveau MN, Chakir J. Effect of e-cigarettes on nasal epithelial cell growth, Ki67 expression, and pro-inflammatory cytokine secretion. Am J Otolaryngol 2020. Nov-Dec;41(6):102686. doi: 10.1016/j.amjoto.2020.102686. [DOI] [PubMed] [Google Scholar]
- 41.Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, Pawlak EA, Dhingra R, Noah TL, Jaspers I. Electronic-Cigarette Use Alters Nasal Mucosal Immune Response to Live-attenuated Influenza Virus. A Clinical Trial. Am J Respir Cell Mol Biol 2021. Jan;64(1):126–137. doi: 10.1165/rcmb.2020-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escobar YH, Morrison CB, Chen Y, et al. Differential responses to e-cig generated aerosols from humectants and different forms of nicotine in epithelial cells from nonsmokers and smokers. Am J Physiol Lung Cell Mol Physiol 2021;320(6):L1064–L1073. doi: 10.1152/ajplung.00525.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwak S, Choi YS, Na HG, Bae CH, Song SY, Kim YD. Glyoxal and Methylglyoxal as E-cigarette Vapor Ingredients-Induced Pro-Inflammatory Cytokine and Mucins Expression in Human Nasal Epithelial Cells. Am J Rhinol Allergy 2021;35(2):213–220. doi: 10.1177/1945892420946968 [DOI] [PubMed] [Google Scholar]
- 44.Hinds DM, Nick HJ, Vallin TM, et al. Acute vaping in a golden Syrian hamster causes inflammatory response transcriptomic changes. Am J Physiol Lung Cell Mol Physiol 2022;323(5):L525–L535. doi: 10.1152/ajplung.00162.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karey E, Hess J, Farrell K, et al. The Nose Knows: Sniffing out the Unique Immunological Risk of Alternative Tobacco Products. Am J Respir Cell Mol Biol 2022;66(4):461–464. doi: 10.1165/rcmb.2021-0368LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozuelos GL, Kagda M, Rubin MA, Goniewicz ML, Girke T, Talbot P. Transcriptomic Evidence That Switching from Tobacco to Electronic Cigarettes Does Not Reverse Damage to the Respiratory Epithelium. Toxics 2022;10(7):370. Published 2022 Jul 4. doi: 10.3390/toxics10070370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholas BD, Kiprovski A, Perez D, et al. Changes in Eustachian Tube Mucosa in Mice After Short-Term Tobacco and E-cigarette Smoke Exposure. Laryngoscope 2022;132(3):648–654. doi: 10.1002/lary.29887 [DOI] [PubMed] [Google Scholar]
- 48.Salturk Z, Çakir Ç, Sünnetçi G, et al. Effects of Electronic Nicotine Delivery System on Larynx: Experimental Study. J Voice 2015;29(5):560–563. doi: 10.1016/j.jvoice.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 49.Ha TAN, Madison MC, Kheradmand F, Altman KW. Laryngeal inflammatory response to smoke and vape in a murine model. Am J Otolaryngol - Head Neck Med Surg 2019;40(1):89–92. doi: 10.1016/j.amjoto.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 50.Hassan NH, El-Wafaey DI. Histopathological scoring system role in evaluation of electronic cigarette’s impact on respiratory pathway in albino rat: Biochemical, histo-morphometric and ultrastructural study. Tissue Cell 2022. Dec;79:101945. doi: 10.1016/j.tice.2022.101945. [DOI] [PubMed] [Google Scholar]
- 51.Martinez JD, Easwaran M, Ramirez D, Erickson-DiRenzo E. Effects of Electronic (E)-cigarette Vapor and Cigarette Smoke in Cultured Vocal Fold Fibroblasts. Laryngoscope 2022:1–8. doi: 10.1002/lary.30073 [DOI] [PubMed]
- 52.Easwaran M, Maria CS, Martinez JD, et al. Effects of Short-term Electronic(e)-Cigarette Aerosol Exposure in the Mouse Larynx. Laryngoscope Published online September 12, 2023. doi: 10.1002/lary.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 2014;9(9):e108342. Published 2014 Sep 22. doi: 10.1371/journal.pone.0108342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Merhie N, Krüger A, Uliczka K et al. Sex dependent effect of maternal e-nicotine on F1 Drosophila development and airways. Sci Rep 11, 4441 (2021). 10.1038/s41598-021-81607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manyanga J, Ganapathy V, Bouharati C, Mehta T, Sadhasivam B, Acharya P, Zhao D, Queimado L. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci Rep 2021. Jan 19;11(1):1821. doi: 10.1038/s41598-021-81148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werley MS, Kirkpatrick DJ, Oldham MJ, Jerome AM, Langston TB, Lilly PD, Smith DC, Mckinney WJ Jr. Toxicological assessment of a prototype e-cigaret device and three flavor formulations: a 90-day inhalation study in rats. Inhal Toxicol 2016;28(1):22–38. doi: 10.3109/08958378.2015.1130758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips B, Titz B, Kogel U, Sharma D, Leroy P, Xiang Y, Vuillaume G, Lebrun S, Sciuscio D, Ho J, Nury C, Guedj E, Elamin A, Esposito M, Krishnan S, Schlage WK, Veljkovic E, Ivanov NV, Martin F, Peitsch MC, Hoeng J, Vanscheeuwijck P. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food Chem Toxicol 2017. Nov;109(Pt 1):315–332. doi: 10.1016/j.fct.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Lee KM, Hoeng J, Harbo S, Kogel U, Gardner W, Oldham M, Benson E, Talikka M, Kondylis A, Martin F, Titz B, Ansari S, Trivedi K, Guedj E, Elamin A, Ivanov NV, Vanscheeuwijck P, Peitsch MC, McKinney WJ Jr. Biological changes in C57BL/6 mice following 3 weeks of inhalation exposure to cigarette smoke or e-vapor aerosols. Inhal Toxicol 2018. Nov-Dec;30(13–14):553–567. doi: 10.1080/08958378.2019.1576807. [DOI] [PubMed] [Google Scholar]
- 59.Ho J, Sciuscio D, Kogel U, Titz B, Leroy P, Vuillaume G, Talikka M, Martin E, Pospisil P, Lebrun S, Xia W, Lee T, Chng YX, Phillips BW, Veljkovic E, Guedj E, Xiang Y, Ivanov NV, Peitsch MC, Hoeng J, Vanscheeuwijck P. Evaluation of toxicity of aerosols from flavored e-liquids in Sprague-Dawley rats in a 90-day OECD inhalation study, complemented by transcriptomics analysis. Arch Toxicol 2020. Jun;94(6):2179–2206. doi: 10.1007/s00204-020-02759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni F, Ogura T, Lin W. Electronic Cigarette Liquid Constituents Induce Nasal and Tracheal Sensory Irritation in Mice in Regionally Dependent Fashion. Nicotine Tob Res 2020;22:S35–S44. doi: 10.1093/ntr/ntaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong ET, Szostak J, Titz B, et al. A 6-month inhalation toxicology study in Apoe−/− mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract. Arch Toxicol 2021;95(5):1805–1829. doi: 10.1007/s00204-021-03020-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MD, Chung S, Dennis JS, et al. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front Pharmacol 2022;13:1012723. Published 2022 Sep 26. doi: 10.3389/fphar.2022.1012723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong ET, Luettich K, Cammack L, Chua CS, Sciuscio D, Merg C, Corciulo M, Piault R, Ashutosh K, Smith C, Leroy P, Moine F, Glabasnia A, Diana P, Chia C, Tung CK, Ivanov N, Hoeng J, Peitsch M, Lee KM, Vanscheeuwijck P. Assessment of inhalation toxicity of cigarette smoke and aerosols from flavor mixtures: 5-week study in A/J mice. J Appl Toxicol 2022. Oct;42(10):1701–1722. doi: 10.1002/jat.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desai RW, Demir K, Tsolakos N, et al. Comparison of the toxicological potential of two JUUL ENDS products to reference cigarette 3R4F and filtered air in a 90-day nose-only inhalation toxicity study. Food Chem Toxicol 2023;179:113917. doi: 10.1016/j.fct.2023.113917 [DOI] [PubMed] [Google Scholar]
- 65.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control 2016. Nov;25(Suppl 2):ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. Epub 2016 Sep 15. PMID: 27633767; PMCID: PMC5784427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 2019;129(10):4290–4304. doi: 10.1172/JCI128531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bengalli R, Ferri E, Labra M, Mantecca P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int J Environ Res Public Health 2017. Oct 20;14(10):1254. doi: 10.3390/ijerph14101254. PMID: 29053606; PMCID: PMC5664755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P. High-Nicotine Electronic Cigarette Products: Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chem Res Toxicol 2019. Jun 17;32(6):1058–1069. doi: 10.1021/acs.chemrestox.8b00381. Epub 2019 Apr 17. PMID: 30896936; PMCID: PMC6579667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


