Abstract
Objective:
Patients with Alzheimer’s disease (AD) have diffuse brain atrophy, but some regions, such as the anterior cingulate cortex (ACC), are spared and may even show increase in size compared to controls. The extent, clinical significance, and mechanisms associated with increased cortical thickness in AD remain unknown. Recent work suggested neural facilitation of regions anticorrelated to atrophied regions in frontotemporal dementia. Here, we aim to determine whether increased thickness occurs in sporadic AD, whether it relates to clinical symptoms, and whether it occur in brain regions functionally connected to—but anticorrelated with—locations of atrophy.
Methods:
Cross-sectional clinical, neuropsychological, and neuroimaging data from the Alzheimer’s Disease Neuroimaging Initiative were analyzed to investigate cortical thickness in AD subjects vs. controls. Atrophy network mapping was used to identify brain regions functionally connected to locations of increased thickness and atrophy.
Results:
AD patients showed increased thickness in the ACC in a region-of-interest analysis and the visual cortex in an exploratory analysis. Increased thickness in the left ACC was associated with preserved cognitive function, while increased thickness in the left visual cortex was associated with hallucinations. Finally, we found that locations of increased thickness were functionally connected to, but anticorrelated with, locations of brain atrophy (r = −0.81, p < 0.05).
Interpretation:
Our results suggest that increased cortical thickness in Alzheimer’s disease is relevant to AD symptoms and preferentially occur in brain regions functionally connected to, but anticorrelated with, areas of brain atrophy. Implications for models of compensatory neuroplasticity in response to neurodegeneration are discussed.
Alzheimer’s disease (AD) involves widespread neurodegeneration of the cortex, which can be measured using MRI-based metrics of cortical thickness (1-7). However, some brain regions appear to be spared, and one recent study reported paradoxical increases in cortical thickness in the anterior cingulate cortex (ACC) (8). This provocative finding was in patients with rare genetic forms of AD, leaving it unknown whether increased thickness occurs in more common forms of AD. Answering this question could provide insight into how the brain responds to and compensates for neurodegeneration.
The relevance of increased cortical thickness for clinical symptoms remains unknown. One possibility is that increased cortical thickness is beneficial and associated with preserved cognitive function, a phenomenon sometimes referred to as cognitive reserve or resilience (9-12). Another possibility is that this increased thickness is problematic and associated with additional symptoms, such as increased cortical thickness in Parkinson’s disease patients with dyskinesias (13) and impulse control disorder (14,15).
It also remains unclear why some regions but not others would show increased thickness and if these locations are related to areas of brain atrophy. Structural and functional brain changes can occur as a direct effect of brain damage in remote but connected brain regions, a phenomenon referred to as diaschisis (16,17). One technique that appears helpful in understanding diaschisis in neurodegeneration (2,5,7,18-20), and brain damage in general (21-27), is resting-state functional connectivity. This technique uses MRI to measure spontaneous fluctuations in brain activity (28). Brain regions are considered functionally connected when the spontaneous activity in one region is correlated (or anticorrelated) with that of another. In neurodegenerative diseases, atrophy in one brain region predicts atrophy in other functionally connected brain regions (2,5,18). Prior studies have shown functional enhancement of the ACC and other salience network nodes that are anticorrelated to locations of atrophy in AD, with increased connectivity in the salience node correlating with neuropsychiatric symptoms (29-35). To our knowledge, prior studies have not investigated whether there is a similar reciprocal relationship between networks showing brain atrophy and increased cortical thickness in AD.
Here, we test whether increased thickness occurs in AD, is related to compensatory and maladaptive symptoms, and occurs in regions that are functionally connected, but anticorrelated, to locations of atrophy.
Methods
Subjects
Data used in this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). IRB approval was obtained from all sites, and informed consent obtained from all participants. The present study included subjects diagnosed with either AD or cognitively normal (CN) status from ADNI1 and ADNI2 with an available MP-RAGE or IR-SPGR MRI scan. Diagnosis was based on diagnostic criteria at the screening visit. We included subjects as healthy controls, even if they had AD-positive biomarkers or went on to develop cognitive impairment at subsequent visits. In ADNI1, we excluded one AD subject and one CN subject due to errors during FreeSurfer reconstruction. In ADNI2, three Alzheimer's disease subjects were excluded based on visual evaluation of segmentation quality. This resulted in 184 AD subjects and 227 age-matched CN subjects from ADNI-1, as well as 146 AD subjects and 201 age-matched CN subjects from ADNI-2.
Cerebrospinal fluid (CSF) Biomarkers
CSF biomarker data were retrieved from the ADNIMERGE dataset in ADNI, and each biomarker was used to derive AD-positive status based on previously determined thresholds (36). Further methodological details for processing of these biomarker values are provided in Methods S1.
MRI imaging and analysis
Magnetic resonance imaging (MRI) scans for ADNI1 were collected on a 1.5T scanner (MPRAGE protocol: sagittal plane, TR/TE/TI, 2400/3/1000 ms, flip angle 8°, 24 cm FOV, 192 × 192 in-plane matrix, 1.2 mm slice thickness (37)). MRI scans for ADNI2 were collected on a 3T scanner (IR-SPGR or MPRAGE protocol: sagittal plane, TR/TE/TI 2300/2.95/900 ms, flip angle 9°, 26 cm FOV, 256 × 256 in-plane matrix, 1.2 mm slice thickness). Quantitative morphometric analysis was performed using FreeSurfer version 6.0 (38). Cortical thickness was computed as the distance between the pial and white-matter surfaces.
Statistical and analysis software
Arithmetic computations and spatial statistical tests of neuroimaging data were conducted in FSL version 5.0 (39) and FreeSurfer version 6.0 (38). Visualizations of neuroimaging data on surfaces were generated using Surfice (https://www.nitrc.org/projects/surfice/). Statistical analyses were performed using the programming language R version 3.6.3 (40) equipped with the data.table package (41), and plots were generated by the ggplot2 package (42).
Group-level comparisons of cortical thickness in AD patients vs. controls
We tested for increased cortical thickness in AD subjects in the anterior cingulate cortex (ACC) based on the findings from Benzinger et al. showing increased cortical thickness in the ACC in autosomal dominant AD patients (8). We defined our region of interest (ROI) using a recently published multimodal parcellation of the human cortex (43). By visual comparison, the parcel within this multimodal parcellation that most closely corresponded with the result by Benzinger et al. was area 33 prime (33pr), which corresponds approximately with Brodmann’s area 33 and lies on the ventral surface of the anterior cingulate cortex. We performed a group-level general linear model (GLM) to test for vertex-wise differences in cortical thickness between AD patients and control subjects within area 33 prime, controlling for age and gender as covariates and correcting for multiple comparisons using permutation testing (5mm FWHM smoothing kernel, 10,000 simulations, one-tailed cluster-forming threshold p < 0.05, cluster-wise FWE-corrected p < 0.05) (44,45). Because Benzinger et al. broadly identified the ACC and also appeared to observe increased thickness in the anterior insula (8), these analyses were also replicated for other ROIs within the anterior cingulate cortex and the anterior insula (see Methods S2 in Supplementary Materials). In an exploratory analysis, we also assessed for regions showing increased cortical thickness in AD subjects across the entire cortical surface for each hemisphere using an uncorrected vertex-wise threshold of p < 0.05.
Patient measures of clinical symptoms
Because our ROI and exploratory analyses found increased thickness in the ACC in AD vs. controls, we explored whether this finding could relate to cognitive functional status based on prior reports showing a potential association (12). Cognitive functional status was measured using the clinical dementia rating score sum of boxes (CDR-sob), a clinician-rating based on a semi-structured interview with the patient and caregiver. Additionally, because our exploratory analysis results found increased cortical thickness in the occipital cortex, we investigated whether this finding could relate to hallucinations based on prior reports showing a potential association (46). Hallucinations were identified using the neuropsychiatric inventory (NPI) or NPI-questionnaire (NPI-Q) (47) at the baseline or six-month visits. We also tested for symptoms corresponding with the other domains of the NPI and NPI-Q (Methods S3 in the Supplementary Materials).
Determining the relationship between increased cortical thickness and clinical symptoms
We performed a GLM to test for vertex-wise differences in cortical thickness associated with CDR-sob scores, controlling for age and gender as covariates and correcting for multiple comparisons using permutation testing, masked to area 33 prime. To demonstrate that significant results were due to increased cortical thickness, we tested for group-level differences in the average cortical thickness within significant clusters in the ACC in cognitively normal subjects, AD patients with higher cognitive impairment (CDR-sob ≤ 50th percentile among AD subjects), and AD patients with lower cognitive impairment (CDR-sob > 50th percentile among AD subjects) using a linear model with age and gender as covariates.
Given our finding of increased cortical thickness in this region in our initial exploratory analyses, we performed a similar GLM to test for vertex-wise differences in cortical thickness between AD patients with vs. without hallucinations, controlling for age and gender as covariates and correcting for multiple comparisons using permutation testing, masked to ROIs in the right and left visual cortex (V1). To demonstrate that significant results were due to increased cortical thickness, we tested for group-level differences in cortical thickness from significant clusters in cognitively normal controls subjects, AD patients with hallucinations, and AD patients without hallucinations using a linear model with age and gender as covariates.
To assess whether the results could be explained by motion or imaging quality, we also conducted similar control analyses using the Euler number-based count of holes/defects as a covariate. Further details are provided in Methods S4.
Atrophy Network Mapping and Growth Network Mapping
We generated atrophy and growth network maps using the same approach as in our prior work (7,19,48-51). First, we performed a vertex-wise GLM for cortical thickness for the cognitively normal subjects from ADNI using age and gender as covariates. Next, we used the beta term maps for age and gender, as well as the residuals from this normative model, to calculate a vertex-wise w-score for cortical thickness in each patient (a w-score is a z-score adjusted for age and gender) using the formula: w-score = (actual – expected)/RSD, where actual is the patient’s observed cortical thickness, expected is the predicted cortical thickness based on the control GLM, and RSD is the residual standard deviation from the control GLM (7,48-51). We defined single-subject “atrophy maps” as regions with a w-score < −2 and “growth maps” as regions with a w-score > 2, corresponding to cortical thickness two standard deviations below or above the mean of the population of healthy control subjects, respectively.
Surface-space atrophy maps (or growth maps) from each hemisphere were combined and converted to MNI volume space. Next, we derived an “atrophy network map,” defined as brain regions functionally connected to each patient’s single-subject atrophy map, and a “growth network map” for each patient, defined as brain regions functionally connected to each patient’s single-subject growth map (7,19). Functional connectivity was determined using a publicly available normative dataset of 1000 healthy subjects from the Brain Genomics Superstruct Project (GSP) (52,53).
Seeds corresponding to each patient’s atrophy or growth map were correlated with the blood-oxygen-level-dependent (BOLD) time-course at every other brain voxel. The resulting r-values were converted to a normal distribution using Fisher’s r-to-z transform and were used to compute a single-group, voxel-wise t-test across the 1,000 subjects in the normative connectome dataset to generate atrophy or growth network t-maps.
We specifically hypothesized that the increased cortical thickness in AD would occur in regions functionally connected to locations of atrophy. To generate a group-level AD atrophy network map, we performed a voxel-wise two-sample t-test of atrophy network maps between AD and cognitively normal subjects using the software Permutation Analysis of Linear Models (PALM). Similarly, to generate a group-level AD growth network map, we performed a voxel-wise two-sample t-test of growth network maps between AD and cognitively normal subjects. Finally, we generated an “atrophy-growth network spatial correlation” between the resulting AD atrophy and growth network maps.
We performed a permutation test to assess the significance of the atrophy-growth network spatial correlation. We randomly permuted AD and cognitively normal group assignments for subjects, repeated the voxel-wise two-sample t-tests, and generated a pair of group-level AD atrophy and growth network maps. This process was repeated for 10,000 random permutations, and an atrophy-growth network spatial correlation was calculated between each corresponding pair of atrophy and growth network maps generated from these permutations. Significance was computed as the proportion of atrophy-growth network spatial correlations more anticorrelated than the non-permuted atrophy-growth network spatial correlation.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Results
Increased cortical thickness occurs in patients with Alzheimer’s disease
Based on the results from a previous study on rare genetic forms of AD (8), we hypothesized that AD subjects would have increased cortical thickness within area 33 prime, which is located on the ventral surface of the anterior cingulate cortex and is located similarly to Brodmann’s area 33 (43). We found a significant cluster of increased cortical thickness in AD patients within the left area 33 prime (FWE corrected p < 0.05; Fig. 1A). Because the previous study identified increased thickness as broadly falling within the ACC and also observed increased thickness in the anterior insula (8), we also looked at other parcels in the ACC and anterior insula, and the results are reported in Results S1 in Supplementary Materials.
Figure 1.
(A) A group-level linear model for cortical thickness, adjusted for age and gender, was conducted between AD patients and healthy control subjects. AD patients had a significant cluster of increased thickness in the left area 33 prime ROI. (B) An exploratory, whole-cortical analysis showed that AD patients also had regions of increased thickness in the left visual cortex.
In an exploratory analysis using a less stringent cutoff (uncorrected p < 0.05), we found increased cortical thickness in AD patients in the bilateral visual cortex (Fig. 1B; see Table S1 for other regions).
Increased cortical thickness in AD is associated with both compensatory and maladaptive symptoms
We next tested whether increased cortical thickness in AD is clinically significant to the disease phenotype. We hypothesized that increased cortical thickness in the ACC would relate to preserved cognitive functioning (12), whereas increased cortical thickness in the visual cortex would relate to hallucinations (46,54).
We found a significant cluster within the left area 33 prime ROI where increased cortical thickness was associated with better cognitive functioning (Fig. 2A). To further demonstrate that this effect was due to increased cortical thickness (rather than less atrophy), we found that AD patients with lower cognitive impairment (CDR-sob ≤ 50th percentile) had significantly greater thickness when compared to controls whereas there was no difference in cortical thickness between controls and AD patients with more cognitive impairment (CDR-sob > 50th percentile; Fig. 2B).
Figure 2.
(A) Among AD patients, cortical thickness in the left area 33 prime ROI was regressed against cognitive impairment (CDR-sob). This revealed a significant cluster of negative correlation in the left area 33 prime. (B) Within this significant cluster, AD patients with lower cognitive impairment had significantly higher average cortical thickness, adjusted for age and gender, than control subjects. (C) A group-level linear model of cortical thickness was conducted between AD patients with vs. without hallucinations, adjusted for age and gender. We observed a significant cluster where higher cortical thickness was associated with presence of hallucinations. (D) Within this significant cluster, we compared average cortical thickness, adjusted for age and gender, between control subjects vs. AD patients with or without hallucinations. AD patients with hallucinations had significantly greater average cortical thickness than healthy control subjects. AD patients without hallucinations had lower cortical thickness in this cluster. Horizontal bars indicate mean cortical thickness.
We also found a significant cluster within the left visual cortex where AD patients with hallucinations (n=26) had increased cortical thickness compared to AD patients without hallucinations (n=295, Fig. 2C). To further demonstrate that this effect was due to increased cortical thickness (rather than less atrophy), we found that AD patients with hallucinations had increased cortical thickness in this region compared to controls (p < 0.05). In contrast, AD patients without hallucinations had decreased cortical thickness in this region compared to controls (Fig. 2D). (Results for other neuropsychiatric symptoms are reported in Results S2 of the Supplementary Materials. Results for control analyses assessing whether the results could be explained by motion or image quality are given in Results S3 of the Supplementary Materials.)
Increased cortical thickness occurs in regions connected, but anticorrelated, to locations of atrophy in AD
Finally, we examined whether there was any relationship between the locations of increased cortical thickness and locations of cortical atrophy in AD subjects. We hypothesized that regions of increased cortical thickness would occur in brain regions connected to, and specifically anticorrelated with, regions of cortical atrophy.
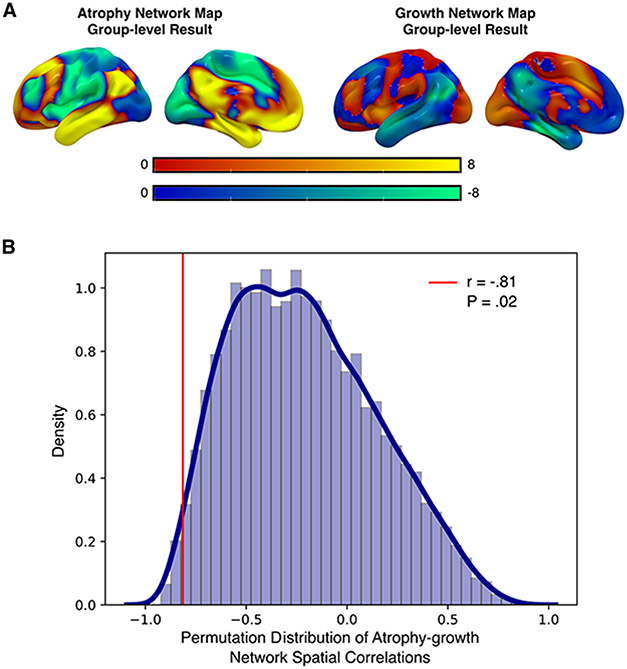
To test this hypothesis, we utilized recently developed methods to define the network of brain regions connected to each patient’s location of brain atrophy (“atrophy network map”) and increased cortical thickness (“growth network map”). Visualization indicated that the group-level AD atrophy and AD growth network maps were spatially anticorrelated (Fig. 3A). Spatial correlations between atrophy network maps and growth network maps in AD patients confirmed our hypothesis that these networks were anticorrelated (r = −0.81, p = 0.02, Fig. 3B).
Figure 3.
(A) Group-level AD atrophy and AD growth network maps were generated by comparing against controls. By visualization, these group-level maps appeared to be spatially anticorrelated. (B) The spatial anticorrelation between the group-level AD atrophy network map and the AD growth network map was significant by permutation testing.
Discussion
The main findings of the current study are that increased cortical thickness (a) occurs in AD patients compared to controls; (b) is associated with both preserved cognitive functioning and maladaptive symptoms like hallucinations; and (c) specifically occurs in regions connected, but functionally anticorrelated, to locations of brain atrophy.
AD patients have regions of increased cortical thickness compared to control subjects
Prior studies found increased cortical thickness in both symptomatic (8) and asymptomatic (55) autosomal dominant AD mutation carriers. Here, we show that regions of increased cortical thickness occur in sporadic AD, not just rare autosomal dominant AD cases. Further, we show that increased cortical thickness can be detected using several different neuroimaging approaches to study brain atrophy in neurodegenerative disorders, including group-level comparisons to normal subjects and methods to estimate increased cortical thickness and atrophy at the single-subject level.
Notably, we observed increased cortical thickness specifically in area 33 prime, which represents the ventral surface of the ACC. Our finding of increased cortical thickness in the 33 prime region of the ACC aligns visually with the results obtained by Benzinger et al., who found increased cortical thickness in this region in patients with autosomal dominant AD (8). We did not find increased thickness in other subregions within the ACC, which show different patterns of functional connectivity, cortical thickness, and functional activation during cognitive tasks, suggesting a biological plausibility for why this region, but not other regions within the ACC, would show increased cortical thickness in AD patients. In particular, area 33 prime has been found to be involved in both emotional regulation and complex motor function and is therefore particularly implicated in the ACC’s role as an intermediary for goal-directed behavior (56,57), which in turn also promotes social engagement and prosocial behavior (58). Prior studies have found dementia patients who engage in social activities tend to be more resilient against cognitive decline and other neurodegenerative symptoms (59,60). Hence, area 33 prime may be a neural substrate by which social engagement confers cognitive reserve to dementia patients.
Clinical symptoms associated with increased cortical thickness in neurodegenerative disorders
Our finding that increased cortical thickness within the ACC was associated with preserved cognitive functioning matches findings from prior work showing that glucose metabolism and cortical thickness in the ACC correlated with preserved cognitive functioning in older adults without cognitive impairment (12). Our study shows that increased cortical thickness in the ACC is associated with preserved cognitive performance in AD patients, suggesting that the ACC’s compensatory effects continue even after the onset of dementia. Second, we show that some AD patients have increased cortical thickness beyond what is expected for their age and gender in the ACC, and this observation may suggest cortical growth. This raises the possibility that brain growth might occur dynamically within resilience regions in response to cortical atrophy and/or cognitive dysfunction. However, it remains possible that baseline differences in the size of the ACC could account for this finding.
We also found increased cortical thickness in the visual cortex in AD patients with hallucinations. This observation matches one prior study showing a trend towards increased occipital lobe grey matter volume in a small number of AD patients with hallucinations (46). In contrast, another study found lower occipital lobe grey matter volume in AD patients with hallucinations (61). While the total number of subjects with hallucinations in our analysis (n=26) is larger than these prior studies, our finding of increased cortical thickness in the visual cortex in AD patients with hallucinations will need replication in other datasets and other dementia patient populations.
Early visual hallucinations are part of the diagnostic criteria for dementia with Lewy Bodies (DLB). DLB, compared to AD, is associated with less cortical atrophy (62-65), raising the possibility that co-morbid DLB pathology could contribute to the cortical-thickness differences in the occipital cortex between patients with vs. without hallucinations. However, the patient group with hallucinations had increased thickness in the occipital cortex compared to controls, suggesting that it is not just less cortical atrophy in patients with hallucinations compared to patients without. It is also possible that activity-dependent neuroplasticity in this region due to ongoing hallucinations could result in the increased cortical thickness found in patients with hallucinations.
Other studies in neurodegenerative patients have found increased cortical thickness associated with specific symptoms, including impulse control disorders (14,66,67), dyskinesias (13), and visual creativity (20,68). Our results demonstrate the value of including control subjects to help interpret whether these identified regions show increased cortical thickness or relatively less atrophy.
Locations of increased thickness were connected to, but functionally anticorrelated with, atrophy in AD patients
Prior studies have shown the clinical importance of regions functionally anticorrelated with locations of neuronal injury, including brain lesions causing hallucinations (22), delusions (23), criminal behavior (26), and alien limb (19).
A prior study found that patients with Alzheimer’s disease, who have atrophy in the default mode network, had increased functional connectivity within the salience network. In contrast, patients with FTD, who have atrophy in the salience network, had increased functional connectivity in the default mode network (29). Because the default mode network and salience network are functionally anticorrelated, this led to the hypothesis that atrophy may cause functional changes in reciprocal brain networks. A recent study demonstrated that anticorrelations between locations of brain atrophy and dorsal visual association areas relate to the emergence of visual creativity in some FTD patients (20), again suggesting that the remote effects of brain atrophy on functionally connected but anticorrelated brain regions can be clinically meaningful. Here, we extend support for this hypothesis by demonstrating increased cortical thickness in reciprocal brain networks related to both compensatory and maladaptive symptoms.
Potential mechanisms of increased cortical thickness in neurodegenerative disorders
Prior neuropathological studies have shown neuronal hypertrophy in the ACC and V1 in asymptomatic patients with AD neuropathology (69), providing a plausible biological substrate for increased cortical thickness in these regions in our analysis. In brain development and learning, increased cortical thickness is attributed to increased neuropil with increased dendritic spines, dendritic and axonal arborization, and glial presence (70,71). Additionally, cortical myelination could account for changes in cortical thickness measurements, since quantification of cortical thickness relies on the detection of the gray-white matter boundary, which can be affected by myelin distribution (72,73). Finally, prior research examining older adults with exceptionally preserved memory capacity found both increased cortical thickness in a region of the ACC as well as an overabundance of von Economo neurons (74). This overabundance of von Economo neurons may be related to increased thickness in area 33 prime among AD patients as well, especially those with lower cognitive impairment. However, the mechanisms leading to these cellular changes and increased cortical thickness remain unclear.
One possible interpretation is that increased cortical thickness occurs because of neuroplasticity. Neurological damage to one cortical location can cause functional and structural changes in remote undamaged brain regions, a phenomenon referred to as diaschisis. To our knowledge, no prior studies have demonstrated diaschisis causing increased cortical thickness in response to damage to a distal brain region. However, experience-induced increases in structural brain volume have been noted (75-77). It remains unclear whether neuroplasticity from network disinhibition, experience-induced changes related to compensatory efforts, or a combination of these effects could lead to the observed increased cortical thickness in the current study. However, our results suggest that neuroplasticity might be constrained to reciprocal brain networks from regions of cortical atrophy. While small, the overall magnitude of cortical thickness differences (~ 0.1 mm) is consistent with prior studies that have identified similar cortical thickness changes related to experience-induced neuroplasticity. For example, < 0.1 mm cortical thickness changes were noted after memory training compared with a control group, and the change in thickness after training correlated with improved memory performance (78).
A second possibility is that increased cortical thickness in AD patients correlates with differences in regional gene expression. Prior studies have found reciprocal genetic effects on structural brain size between networks, where genes associated with increased brain size in one network are also associated with decreased brain size in reciprocal networks (79,80). It, therefore, remains possible that patients at risk for AD also have baseline increased cortical thickness in reciprocal brain networks prior to the development of neuropathological changes.
Finally, a third interpretation is that increased cortical thickness is due to neuronal dysfunction and resulting toxic and/or inflammatory effects from AD pathology prior to cortical atrophy development, such as microglial activation (69,81,82). According to this model, patients with greater cortical thickness are those experiencing neuroinflammatory effects of AD pathology due to amyloid-beta deposition early in the disease course; this relative increase of cortical thickness would presumably dissipate over time and give way to cortical atrophy (83-85). We believe this explanation is less likely as one would expect area 33 prime thickness to relate to greater dysfunction, not cognitive resilience. Additionally, among AD patients, cortical thickness within area 33 prime was greater than or equal to that of controls, even in the subgroup with worse cognitive functioning. If the increase in cortical thickness in this area were due to the pathophysiology of AD, the expectation would be for that area to atrophy in more severe cases of dementia, not stay the same/increase compared to controls. Further studies investigating microstructural changes leading to increased cortical thickness in dementia patients or whether the increased thickness is associated with AD PET biomarkers in these regions could address this possible interpretation.
Limitations
We used cross-sectional neuroimaging comparing patients to controls rather than longitudinal neuroimaging to estimate increased cortical thickness. Cross-sectional estimates are by far the most common approach to studying cortical atrophy in neurodegenerative disorders due to technical challenges in optimizing longitudinal neuroimaging analyses (86-88). Longitudinal studies are also complicated by uncertainty regarding when increased cortical thickness would occur in the disease process and over what interval such changes might be detectable (8,69). Future studies investigating longitudinal changes in cortical thickness in asymptomatic biomarker-positive patients that progress to the dementia stage are needed to determine when and over what time period these cortical thickness increases occurs.
Supplementary Material
Table 1.
Demographic characteristicsa
| ADNI1 | ADNI2 | |||
|---|---|---|---|---|
| AD | CN | AD | CN | |
| N | 184 | 227 | 146 | 201 |
| Age, mean (SD), years | 75.3 (7.6) | 76.0 (5.0) | 74.6 (8.2) | 73.3 (6.4) |
| Gender, No. (%) | ||||
| Female | 89 (48) | 109 (48) | 65 (45) | 106 (53) |
| Male | 95 (52) | 118 (52) | 81 (55) | 95 (47) |
| Hallucinations, No. (%) | ||||
| No | 171 (93) | --- | 124 (91) | --- |
| Yes | 13 (7) | --- | 13 (9) | --- |
| CDR-SOB, mean (SD)b | 4.28 (1.6) | --- | 4.49 (1.7) | --- |
| APOE4 status, No. (%) | ||||
| APOE4− | 63 (34) | 166 (73) | 42 (31) | 133 (72) |
| APOE4+ | 121 (66) | 61 (27) | 92 (69) | 53 (28) |
| Aβ42, mean (SD), pg/mLc | 638. (304.) | 1136. (453.) | 680. (315.) | 1232. (448.) |
| t-tau, mean (SD), pg/mLc | 355. (133.) | 236. (87.) | 383. (158.) | 238. (92.) |
| p-tau, mean (SD), pg/mLc | 35.9 (16.) | 21.9 (9.0) | 37.9 (16.) | 21.8 (9.3) |
| t-tau/Aβ42 ratio, mean (SD)c | 0.637 (0.29) | 0.260 (0.21) | 0.643 (0.34) | 0.232 (0.16) |
| Aβ42 status, No. (%)c | ||||
| Aβ42− | 9 (9) | 60 (54) | 11 (9) | 96 (60) |
| Aβ42+ | 87 (91) | 51 (46) | 108 (91) | 64 (40) |
| p-tau status, No. (%)c | ||||
| p-tau− | 15 (16) | 64 (58) | 13 (11) | 95 (59) |
| p-tau+ | 81 (84) | 47 (42) | 106 (89) | 65 (41) |
| t-tau status, No. (%)c | ||||
| t-tau− | 13 (14) | 52 (47) | 11 (9) | 82 (51) |
| t-tau+ | 83 (86) | 59 (53) | 108 (91) | 78 (49) |
| t-tau/Aβ42 status, No. (%)c | ||||
| t-tau/Aβ42− | 9 (9) | 78 (70) | 10 (8) | 116 (72) |
| t-tau/Aβ42+ | 87 (91) | 33 (30) | 109 (92) | 44 (28) |
| Total triglycerides, mean (SD), mmol/ld | 1.27 (0.50) | 1.24 (0.57) | 1.04 (0.39) | 1.17 (0.55) |
| Total cholesterol, mean (SD), mmol/ld | 5.09 (1.0) | 4.88 (1.0) | 4.88 (0.99) | 4.81 (0.89) |
| LDL cholesterol, mean (SD), mmol/ld | 2.03 (0.48) | 1.95 (0.46) | 1.87 (0.43) | 1.85 (0.42) |
| HDL cholesterol, mean (SD), mmol/ld | 1.47 (0.35) | 1.45 (0.39) | 1.57 (0.39) | 1.54 (0.35) |
| Systolic blood pressure, mean (SD), mmHge | 135. (17.) | 133. (16.) | 132. (17.) | 133. (16.) |
| Diastolic blood pressure, mean (SD), mmHge | 73.2 (9.5) | 73.9 (9.9) | 74.3 (9.3) | 73.6 (9.7) |
AD = Alzheimer’s disease, CN = cognitively normal/healthy control, Aβ42 = amyloid-beta peptide 1-42
CDR-SOB scores not used for CN subjects (n=184 in ADNI1 AD, n=146 in ADNI2 AD)
Amyloid-beta and tau data available for 96 subjects in ADNI1 AD, 111 subjects in ADNI1 CN, 119 subjects in ADNI2 AD, 160 subjects in ADNI2 CN. Patients were designated positive (+) biomarker status based on the following criteria: Aβ42+ status if Aβ42 levels ≤ 1054 pg/mL, t-tau+ if t-tau ≥ 213 pg/mL, p-tau+ if p-tau ≥ 21.3 pg/mL, and t-tau/Aβ42+ if t-tau/Aβ42 ≥ 0.258.
Metabolomic data available for 173 subjects in ADNI1 AD, 215 subjects in ADNI1 CN, 125 subjects in ADNI2 AD, 182 subjects in ADNI2 CN
Blood-pressure data available for 184 subjects in ADNI1 AD, 227 subjects in ADNI1 CN, 134 subjects in ADNI2 AD, 183 subjects in ADNI2 CN
Summary for Social Media If Published.
What is the current knowledge on the topic?:
Patients with Alzheimer’s disease (AD) have brain atrophy but some regions are spared and may even show increase in size.
What question did this study address?:
Does increased cortical thickness occur in Alzheimer’s disease, and if so, what is the clinical significance, and how does it relate to locations of brain atrophy?
What does this study add to our knowledge?:
Increased cortical thickness relative to controls occurs in AD, is associated with both adaptive and maladaptive symptoms, and occurs in brain regions that are functionally anticorrelated to regions of brain atrophy.
How might this potentially impact on the practice of neurology?:
Increased cortical thickness, not just atrophy, may be relevant to the clinical symptoms in AD and relates to the brain’s intrinsic organization into reciprocal, functionally anticorrelated brain networks.
Acknowledgments
This work was funded by grants from the Alzheimer's Association, BrightFocus Foundation, National Institutes of Health (1K23AG070320-01A1, R56AG069086), Vanderbilt Institute for Clinical and Translational Research (VICTR), Vanderbilt University, Sidney Baer Foundation, McKnight Brain Foundation, American Brain Foundation, and American Academy of Neurology.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Data used in preparation of this article were generated by the Alzheimer's Disease Metabolomics Consortium (ADMC). As such, the investigators within the ADMC provided data but did not participate in analysis or writing of this report. A complete listing of ADMC investigators can be found at: https://sites.duke.edu/adnimetab/team/
Potential Conflicts of Interest
The authors declared no conflicts of interest.
Data Availability Statement
The data underlying this article may be shared on reasonable request to the corresponding author.
References
- 1.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex (New York, NY : 1991). 2009. Mar;19(3):497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron. 2009. Apr;62(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. NeuroImage. 2011. Jan 15;54(2):1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 5.Darby RR, Joutsa J, Fox MD. Network localization of heterogeneous neuroimaging findings. Brain : a journal of neurology. 2019. Jan 1;142(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson MA, Lim C, Cooke D, Darby RR, Wu O, Rost NS, et al. A human memory circuit derived from brain lesions causing amnesia. Nature Communications. 2019;10(1):3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tetreault AM, Phan T, Orlando D, Lyu I, Kang H, Landman B, et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2020. Apr 1;143(4):1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benzinger TLS, Blazey T, Jack CR, Koeppe RA, Su Y, Xiong C, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2013. Nov 19;110(47):E4502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet neurology. 2012. Nov;11(11):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Vol. 17, Trends in Cognitive Sciences. 2013. p. 502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darby RR, Brickhouse M, Wolk DA, Dickerson BC, Alzheimer’s Disease Neuroimaging Initiative. Effects of cognitive reserve depend on executive and semantic demands of the task. Journal of neurology, neurosurgery, and psychiatry. 2017. Jun 19;jnnp-2017-315719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, Graff-Radford J, Machulda MM, Knopman DS, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain : a journal of neurology. 2019. Mar; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, et al. Prefrontal thickening in PD with levodopa-induced dyskinesias: New evidence from cortical thickness measurement. Parkinsonism and Related Disorders. 2013;19(1):123–5. [DOI] [PubMed] [Google Scholar]
- 14.Tessitore A, Santangelo G, De Micco R, Vitale C, Giordano A, Raimo S, et al. Cortical thickness changes in patients with Parkinson’s disease and impulse control disorders. Parkinsonism & Related Disorders. 2016;24:119–25. [DOI] [PubMed] [Google Scholar]
- 15.Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, et al. Patterns of cortical thickness associated with impulse control disorders in Parkinson’s disease. Movement Disorders. 2015;30(5):688–95. [DOI] [PubMed] [Google Scholar]
- 16.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014. Sep;137(9):2408–22. [DOI] [PubMed] [Google Scholar]
- 17.Monakow C. Die Lokalisation im Grosshirn : und der Abbau der Funktion durch kortikale Herde. Wiesbaden: Verlag von J.F. Bergmann; 1914. [Google Scholar]
- 18.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting Regional Neurodegeneration from the Healthy Brain Functional Connectome. Neuron. 2012;73(6):1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetreault AM, Phan T, Petersen KJ, Claassen DO, Neth BJ, Graff-Radford J, et al. Network Localization of Alien Limb in Patients with Corticobasal Syndrome. Annals of neurology. 2020. Sep 15;ana.25901. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg A, Pasquini L, Diggs R, Glaubitz EA, Lopez L, Illán-Gala I, et al. Prevalence, Timing, and Network Localization of Emergent Visual Creativity in Frontotemporal Dementia. JAMA Neurology. 2023. Feb 27;94158:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD. Localizing symptoms to brain networks using the human connectome. New England Journal of Medicine. 2018;2237–45. [DOI] [PubMed] [Google Scholar]
- 22.Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015. Aug 10;138(10):3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain. 2017. Feb 12;140(2):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darby RR, Fox MD. Reply: Capgras syndrome: neuroanatomical assessment of brain MRI findings in an adolescent patient. Brain : a journal of neurology. 2017. Jul 1;140(7):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darby RR, Joutsa J, Fox MD. Lesion network localization of free will. Proceedings of the National Academy of Sciences. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proceedings of the National Academy of Sciences of the United States of America. 2018. Dec 16;115(3):601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016. Dec 6;87(23):2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–11. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(5):1352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiology of Aging. 2012;33(8):1564–78. [DOI] [PubMed] [Google Scholar]
- 31.Bai F, Shi Y, Yuan Y, Wang Y, Yue C, Teng Y, et al. Altered self-referential network in resting-state amnestic type mild cognitive impairment. Cortex. 2012;48(5):604–13. [DOI] [PubMed] [Google Scholar]
- 32.Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Archives of Neurology. 2011;68(9):1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balthazar MLF, Pereira FRS, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Human Brain Mapping. 2014;35(4):1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow TE, Veziris CR, La Joie R, Lee AJ, Brown JA, Yokoyama JS, et al. Increasing empathic concern relates to salience network hyperconnectivity in cognitively healthy older adults with elevated amyloid-β burden. NeuroImage: Clinical. 2023;37(December 2022):103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredericks CA, Sturm VE, Brown JA, Hua AY, Bilgel M, Wong DF, et al. Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2018;10:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doecke JD, Ward L, Burnham SC, Villemagne VL, Li QX, Collins S, et al. Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid-PET imaging. Alzheimers Res Ther. 2020. Mar 31;12(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Vol. 27, Journal of Magnetic Resonance Imaging. 2008. p. 685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999. Feb;9(2):179–94. [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012. Aug 15;62(2):782–90. [DOI] [PubMed] [Google Scholar]
- 40.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/ [Google Scholar]
- 41.Dowle M, Srinivasan A. data.table: Extension of `data.framè [Internet]. 2021. Available from: https://CRAN.R-project.org/package=data.table [Google Scholar]
- 42.Wickham H. ggplot2: Elegant Graphics for Data Analysis [Internet]. Springer-Verlag; New York; 2016. Available from: https://ggplot2.tidyverse.org [Google Scholar]
- 43.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greve DN, Fischl B. False positive rates in surface-based anatomical analysis. NeuroImage. 2018;171(July 2017):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middelkoop HAM, Van Der Flier WM, Burton EJ, Lloyd AJ, Paling S, Barber R, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57(11):2117–20. [DOI] [PubMed] [Google Scholar]
- 47.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(2):233–9. [DOI] [PubMed] [Google Scholar]
- 48.La Joie R, Perrotin A, Barré L, Hommet C, Mézenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (Aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012. Nov 14;32(46):16265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ossenkoppele R, Pijnenburg YAL, Perry DC, Cohn-Sheehy BI, Scheltens NME, Vogel JW, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138(9):2732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Möller C, Lehmann M, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Human Brain Mapping. 2015;36(11):4421–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011. Sep;106(3):1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Scientific Data. 2015;2:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter R, ffytche DH. On visual hallucinations and cortical networks: a trans-diagnostic review. Journal of Neurology. 2015;262(7):1780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortea J, Sala-Llonch R, Bartrés-Faz D, Bosch B, Lladó A, Bargalló N, et al. Increased cortical thickness and caudate volume precede atrophy in psen1 mutation carriers. Journal of Alzheimer’s Disease. 2010;22(3):909–22. [DOI] [PubMed] [Google Scholar]
- 56.Oane I, Barborica A, Chetan F, Donos C, Maliia MD, Arbune AA, et al. Cingulate cortex function and multi-modal connectivity mapped using intracranial stimulation. NeuroImage. 2020. Oct;220:117059. [DOI] [PubMed] [Google Scholar]
- 57.Baker CM, Burks JD, Briggs RG, Stafford J, Conner AK, Glenn CA, et al. A Connectomic Atlas of the Human Cerebrum—Chapter 4: The Medial Frontal Lobe, Anterior Cingulate Gyrus, and Orbitofrontal Cortex. Operative Surg. 2018. Dec;15(suppl_1):S122–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gęsiarz F, Crockett MJ. Goal-directed, habitual and Pavlovian prosocial behavior. Front Behav Neurosci. 2015;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002. Jun 15;155(12):1081–7. [DOI] [PubMed] [Google Scholar]
- 60.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001. Dec 26;57(12):2236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holroyd S, Shepherd ML, Downs JH, Ph D. Associated With Visual Hallucinations in Alzheimer ’ s Disease. Journal Of Neuropsychiatry. 2000;25–8. [DOI] [PubMed] [Google Scholar]
- 62.Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: A distinct pattern from Alzheimer’s disease. Brain. 2007;130(3):708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Constant AB, Basavaraju R, France J, Honig LS, Marder KS, Provenzano FA. Longitudinal Patterns of Cortical Atrophy on MRI in Patients With Alzheimer Disease With and Without Lewy Body Pathology. Neurology. 2022;99(17):E1843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiology of Aging. 2015;36(1):452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mak E, Su L, Williams GB, Watson R, Firbank M, Blamire AM, et al. Longitudinal assessment of global and regional atrophy rates in Alzheimer’s disease and dementia with Lewy bodies. NeuroImage: Clinical. 2015;7:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellicano C, Niccolini F, Wu K, O’Sullivan SS, Lawrence AD, Lees AJ, et al. Morphometric changes in the reward system of Parkinson’s disease patients with impulse control disorders. Journal of Neurology. 2015;262(12):2653–61. [DOI] [PubMed] [Google Scholar]
- 67.Hammes J, Theis ÃH, Giehl ÃK, Hoenig MC, Greuel A, Tittgemeyer M, et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. 2019;1–11. [DOI] [PubMed] [Google Scholar]
- 68.Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, et al. Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131(1):39–49. [DOI] [PubMed] [Google Scholar]
- 69.Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, et al. Neuronal hypertrophy in asymptomatic Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2008;67(6):578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental Trajectories of the Human Cerebral Cortex. J Neurosci. 2008. Apr 2;28(14):3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mechelli A, Price C, Friston K, Ashburner J. Voxel-Based Morphometry of the Human Brain: Methods and Applications. CMIR. 2005. Jun 1;1(2):105–13. [Google Scholar]
- 72.Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci USA. 2019. Oct 8;116(41):20750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parent O, Olafson E, Bussy A, Tullo S, Blostein N, Dai A, et al. High spatial overlap but diverging age-related trajectories of cortical magnetic resonance imaging markers aiming to represent intracortical myelin and microstructure. Human Brain Mapping. 2023. Jun;44(8):3023–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, et al. Morphometric and Histologic Substrates of Cingulate Integrity in Elders with Exceptional Memory Capacity. J Neurosci. 2015. Jan 28;35(4):1781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000. Apr 11;97(8):4398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey matter induced by training. Nature. 2004. Jan;427(6972):311–2. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt S, Gull S, Herrmann KH, Boehme M, Irintchev A, Urbach A, et al. Experience-dependent structural plasticity in the adult brain: How the learning brain grows. NeuroImage. 2021. Jan;225:117502. [DOI] [PubMed] [Google Scholar]
- 78.Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, et al. Effects of memory training on cortical thickness in the elderly. NeuroImage. 2010;52(4):1667–76. [DOI] [PubMed] [Google Scholar]
- 79.Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, et al. Hierarchical Genetic Organization of Human Cortical Surface Area (SUPLEMENTARY MATERIAL). Science. 2012;335(6076):1634–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen CH, Panizzon MS, Eyler LT, Jernigan TL, Thompson W, Fennema-Notestine C, et al. Genetic Influences on Cortical Regionalization in the Human Brain. Neuron. 2011;72(4):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. Journal of Cell Biology. 2018. Feb 5;217(2):459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montal V, Vilaplana E, Alcolea D, Pegueroles J, Pasternak O, González-Ortiz S, et al. Cortical microstructural changes along the Alzheimer’s disease continuum. Alzheimer’s & Dementia. 2018. Mar;14(3):340–51. [DOI] [PubMed] [Google Scholar]
- 83.Fortea J, Vilaplana E, Alcolea D, Carmona-Iragui M, Sánchez-Saudinos MB, Sala I, et al. Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease: CSF β-Amyloid and p-Tau in AD. Ann Neurol. 2014. Aug;76(2):223–30. [DOI] [PubMed] [Google Scholar]
- 84.Salvadó G, Shekari M, Falcon C, Operto G, Milà-Alomà M, Sánchez-Benavides G, et al. Brain alterations in the early Alzheimer’s continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Communications. 2022. May 2;4(3):fcac134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams ME, Elman JA, Bell TR, Dale AM, Eyler LT, Fennema-Notestine C, et al. Higher cortical thickness/volume in Alzheimer’s-related regions: protective factor or risk factor? Neurobiology of Aging. 2023. Sep;129:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011. Jul;57(1):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012. Jul;61(4):1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beare R, Ball G, Yang JYM, Moran C, Srikanth V, Seal M. Participant followup rate can bias structural imaging measures in longitudinal studies. Neuroimage: Reports. 2021. Dec;1(4):100066. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
The data underlying this article may be shared on reasonable request to the corresponding author.