Abstract
Purpose of Review
Interfacial tissue exists throughout the body at cartilage-to-bone (osteochondral interface) and tendon-to-bone (enthesis) interfaces. Healing of interfacial tissues is a current challenge in regenerative approaches because the interface plays a critical role in stabilizing and distributing the mechanical stress between soft tissues (e.g., cartilage and tendon) and bone. The purpose of this review is to identify new directions in the field of interfacial tissue development and physiology that can guide future regenerative strategies for improving post-injury healing.
Recent Findings
Cues from interfacial tissue development may guide regeneration including biological cues such as cell phenotype and growth factor signaling; structural cues such as extracellular matrix (ECM) deposition, ECM, and cell alignment; and mechanical cues such as compression, tension, shear, and the stiffness of the cellular microenvironment.
Summary
In this review, we explore new discoveries in the field of interfacial biology related to ECM remodeling, cellular metabolism, and fate. Based on emergent findings across multiple disciplines, we lay out a framework for future innovations in the design of engineered strategies for interface regeneration. Many of the key mechanisms essential for interfacial tissue development and adaptation have high potential for improving outcomes in the clinic.
Keywords: Osteochondral interface, Enthesis, Extracellular matrix, Mechanical loading, Cellular microenvironment
Introduction
Musculoskeletal disorders such as tendinopathy and osteoarthritis are some of the most prevalent nonfatal diseases. For example, in 2019, over 500 million people are afflicted with osteoarthritis worldwide [1]. Connective tissues like tendons, ligaments, and cartilage rely on integration into bone for their form and function. Despite the significant burden associated with connective tissues, our ability to repair these tissues is limited by our ability to attach or repair them with bone. This is, in part, because of the major discrepancy in mechanical properties between bone and soft connective tissues. Additionally, repair strategies have primarily focused on individual tissues, overlooking the interface and its transitional and heterogeneous structure. Musculoskeletal interfaces (e.g., osteochondral interface and tendon/ligament-bone enthesis) are transitional tissues that play an essential role in dissipating localized stresses and deformations that accumulate at sites where connective tissues meet bone. A transitional gradient from bone to connective tissue, which forms in response to applied mechanical loads during growth, is not recreated following injury and is challenging to develop in engineered constructs. A major goal of the field in interfacial biology and mechanics is to understand how these musculoskeletal interfaces can be functionally repaired and regenerated to regain their mechanical function and biological homeostasis with diminished pain post-injury. Yet how these interfaces can regenerate is poorly understood, in part because of our limited understanding of how these tissues develop and heal following injury.
In this review, we highlight some of the current progress made in understanding the development of interfacial tissue and their adaptation during growth and following injury and share new innovations and approaches for interfacial tissue regeneration. We focus primarily on emerging challenges for studying the osteochondral interface and the enthesis revolving around the cellular (e.g., differentiation and metabolism) and extracellular matrix (ECM) processes (e.g., which contribute to its mechanical environment). We also discuss potential tools to advance knowledge in regenerative approaches for interfacial tissue regeneration, primarily through interfacial tissue development.
Cues from Interface Development to Guide Regenerative Approaches
A major challenge of interface regeneration is re-establishing the cellular and structural heterogeneity of healthy interfaces. This challenge arises from complexities in structure and function, as each interface has unique cellular and biomechanical demands [2]. Resident cells of tissue interfaces contribute to the establishment, remodeling, and maintenance of the interface, such as the ECM gradient that defines an osteochondral or tendon-bone interface. Interfacial tissues lack the innate ability to regenerate, in part because of dense ECM, low cellular density, and poor vascular supply. Guided regeneration will require novel engineered approaches to promote integration of one tissue with the other. For example, a cellular gradient exists at the tendon-bone enthesis (e.g., tenocytes, fibrochondrocytes, and osteocytes), and these cells remodel and deposit their local ECM (e.g., collagen types I, II, and X, and proteoglycans), which contributes to their local mechanical environment (e.g., stiffness). Platform-based engineered tissues have emerged to evaluate these dynamics by exploiting compressive boundary conditions in vitro [3•, 4]. Cells within interfacial tissues locally establish and remodel their surrounding environment by degrading or depositing new matrix. In the developing tendon-bone enthesis, cellular density is highest during its early growth phase, before which an organized, gradient ECM is established [5]. As the cells deposit ECM, a functional gradient forms at the tendon-to-bone insertion [6], and at the same time, cellular density decreases [5]. Interfacial tissue healing during enthesis development has recently shown that the tendon-bone enthesis also has innate regenerative properties which are not modeled in adult animals [7–9].
Post-injury in the neonatal enthesis, the injured tissue is hypocellular and avascular [9]. In tendon, Grinstein et al. demonstrated that tendon cells shift from rates of high to low proliferation during postnatal growth in mice, and expression of genes associated with tendon transcription factors and ECM decreases with age [10]. Additionally, neonatal tendon cells express markers of mesenchymal stem cells (MSC) but differ from bone-marrow MSCs as neonatal tendon cells demonstrate reduced differentiation potential toward chondrogenesis and osteogenesis compared to bone-marrow MSCs [11, 12]. Results from these studies reveal progenitor cells that have undergone differentiation toward tendon cells are terminal, introducing a potential challenge for interfacial tissue regeneration, as terminally differentiated cells post-injury may not have the high regeneration potential of progenitor cells typically involved during neonatal development. Thus, it is essential to understand the cellular mechanisms involved in interfacial tissue development that can be used to drive regeneration using native cell types.
Tools such as transgenic animals for lineage tracing, flow cytometry and cell sorting, and RNA sequencing have been foundational for understanding how the resident cell population establishes the interface. For example, it is known that cells rely on the expression of the transcription factor Scleraxis (Scx+) for enthesis development [13, 14]. Additionally, Scx+ cells that co-express SRY-box transcription factor 9 (Sox9+) are bi-fated and generate both Scx+ tendon fibroblasts and Sox9+ chondrocytes critical for enthesis formation (Fig. 2) [15, 16••]. Recent work by Best and Loiselle showed that Scx lineage cells are essential for generating an organized and bridging tissue following tendon injury, and ablation of Scx-lineage cells may improve tendon healing [17]. However, Scx + cells are required for adult tendon homeostasis [17–20], and expression of Scx is required to recruit mesenchymal progenitors during embryonic tendon elongation and regeneration in both mice and zebrafish [21–23]. Scx+ cells are also responsible for healing neonatal tendons [24, 25].
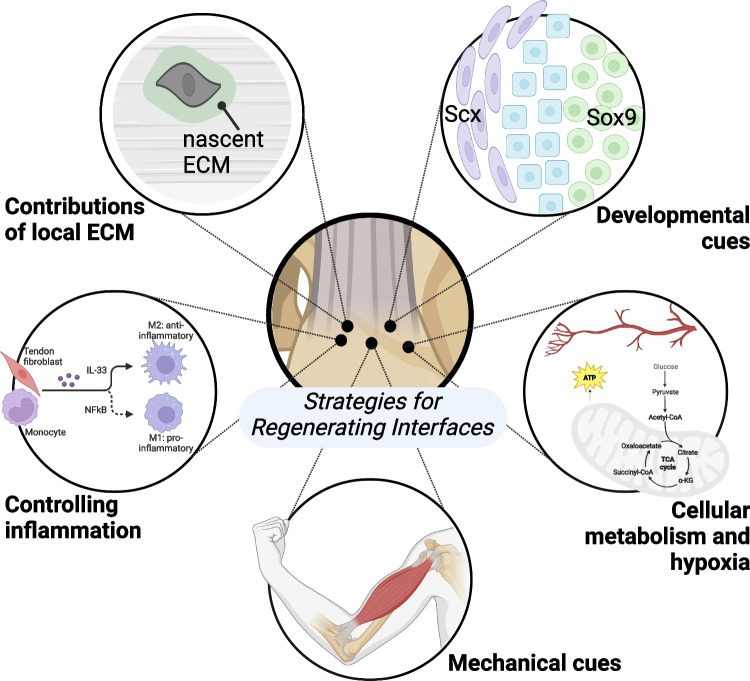
Fig. 2.
Strategies for regenerating interfacial tissues like the osteochondral interface and tendon-bone enthesis include: leveraging developmental cues to promote resident and progenitor cell remodeling of the interfacial tissues (e.g., Sox9 + /Scx + bi-fated cells, shown in blue, residing between Scx + cells in purple and Sox9 + cells in green); identifying factors that influence formation of nascent ECM in native tissues during remodeling and repair; promoting a regenerative, rather than destructive, inflammatory response; controlling the mechanical environment by increasing or decreasing applied loads (e.g., from skeletal muscle); and understanding and controlling interfacial cell metabolism and physiological response and sensitivity to their environment, such as hypoxia
The hedgehog (Hh) signaling pathway is critical for formation and maintenance of tissue interfaces [7, 26–29]. For example, Felsenthal et al. found that Sox9+ lineage cells are replaced with cells expressing Glioma-associated oncogene homolog 1 (Gli1+), a hedgehog-responsive transcription factor [30, 31]. These initial Sox9+ progenitor cells are necessary to establish the fibrocartilaginous template before cells are removed and replaced (during attachment migration) or further differentiate into Gli1 + cells [30] [32]. GLI family zinc finger 3 (Gli3) is also an essential regulator of patterning of attachment-site progenitors [33]. These findings during enthesis development support the balance Scx, Sox9, and hedgehog pathways for establishing the cell gradient necessary for enthesis function. Another emergent signaling pathway involved in post-natal interface development is fibroblast growth factor (FGF) signaling, in part because it plays a key role in mineralization at the tendon-to-bone enthesis and regulates cell fate in fibrocartilage tissues [5, 34–37]. Mechanobiological processes associated with cilia have recently been deemed critical for enthesis formation and healing and are mediated via Hh signaling and mechanical loading [28, 38–40]. The regulation of ECM deposition by mechanical loading and cellular pathways like Hh and FGF signaling suggests these are key targets and tools for regenerative approaches for the interface.
Contributions of the Local ECM on Interfacial Development and Healing
Cell-ECM interactions regulate mechanotransduction, particularly within key transitional tissues such as the osteochondral interface or the enthesis (Fig. 2) [41]. Mechanical and chemical cues are transduced from ECM to the cells to regulate production of nascent ECM. Remodeling of the ECM by matrix metalloproteases (MMPs) and tissue inhibitor of metalloprotease (TIMPs) and deposition of nascent ECM are critical to maintaining tissue homeostasis throughout changes in mechanical loading such as tension, compression, shear, and hydrostatic strains [42]. Within the enthesis, there is a complex ECM gradient from the tendon into the bone including a transition of primarily collagen types I and II, with collagen types III, V, VI, X, and XI contributing to the complex collagen and mineralized gradient [43–45]. Similarly, the osteochondral interface is also characterized by its unique cellular and ECM gradient [46, 47]. The mineralized and unmineralized regions of these interfaces are separated by a distinct tidemark, and the collagen organization and fibril size change, becoming smaller during the transition from soft to hard matrix (Fig. 1). Our ability to visualize and quantify specific spatial qualities of interfaces has been improved with refined techniques in fractionation, mass spectrometry, and proteomics in other interfacial tissues, like the myotendinous junction [48•]. The inability to remodel adult extracellular and pericellular matrix is a major obstacle to overcome in the context of interfacial tissue healing. The remodeling of ECM in mature interfaces is limited, in part, by increased collagen cross-linking due to advanced glycation end products (AGEs) or lysyl oxidase (LOX), increased ECM-to-cell ratio, and production, or lack thereof, of pericellular matrix [49–53]. Interfacial tissue ECM is also rich in proteoglycans, providing this tissue with osmotic properties to reduce compressive stress. Using hyperelastic characterization of strain-stiffening in cartilage, McCreery et al. found proteoglycans drive the strain-stiffening response in hyaline cartilage [54]. These data support that ECM components are key to mechanosensing and mechanical function of interfacial tissues.
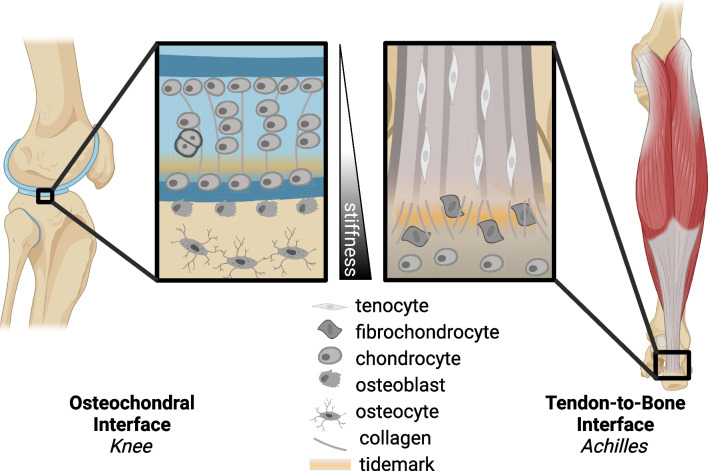
Fig. 1.
Schematics of the osteochondral interface (e.g., within the knee) and the tendon-to-bone interface (e.g., Achilles enthesis), which highlight the stiffness gradients (from bone to tendon), tidemark between mineralized and unmineralized fibrocartilage, variations in collagen alignment and fibril size, and cell type distribution inclusive of tendon fibroblast, fibrochondrocyte, and chondrocytes/osteoblasts
ECM composition may also play role in stimulating inflammation [55]. While inflammation is a key response to injury, chronic inflammation and accumulated fibrotic ECM can hinder the ability of interfacial tissue to regenerate. Clues from neonatal healing may provide insights of the regenerative potential of remodeling or maintenance of the native ECM by tissue resident cells, as a recent study by Vinestock et al. has shown that neonatal enthesis injury leads to generation of an acellular and low-inflammatory scar driven primarily by resident cells [9]. The intricate crosstalk between cells and their nascent ECM may influence the inflammatory response involved in tissue remodeling.
Controlling Inflammation to Influence Interfacial Tissue Healing
The inflammatory response contains a myriad of different cell types and functions making it challenging to decouple the beneficial or harmful mechanisms post-injury on interfacial tissues (Fig. 2) [56]. Inflammation may cause disruptions in homeostasis causing modulations in the native ECM architecture and mechanics that are challenging to reverse [42]. Regulatory T cells (Tregs) impact resident cells, and neonates have elevated levels of Tregs to help prevent autoimmune response [24, 57]. Tregs maintain the environment necessary for the switch from pro-inflammatory to anti-inflammatory macrophage phenotypes necessary to regulate tissue regeneration [55, 58]. Howell et al. found macrophages to be critical for neonatal tendon regeneration [24, 59]. Biomaterials can be designed to employ immunomodulatory effects to reduce the localized overactive immune response.
Cytokines, particularly IL-33, have been studied in the context of tendon and enthesis injury as well [60]. IL-33 expression is elevated in the human torn tendon and in early tendinopathy [60, 61]. Additional studies interrogating the inflammatory response post-injury or during disease progression (e.g., enthesitis) have revealed non-autonomous functions driving healing [18, 62], such as the protein complex known as nuclear factor kappa-light chain-enhancer of activated B cells (NF-kB) [63–65].
Leveraging Mechanical Cues to Understand Interfacial Tissue Development
The primary function of tissue interfaces is to transmit and dissipate forces between tissues with dissimilar mechanical properties (Fig. 2). In the absence of mechanical loading during periods of growth, these tissues do not form their hallmark gradient cellular and ECM morphology and are also remarkably weaker and less mechanically resilient [6, 13, 66]. Mechanical loading (e.g., from skeletal muscle contractions) can regulate cell-scale ciliary Hh signaling [26, 28, 29], interfacial matrix organization [6, 13, 67, 68], and accrual of mineral [6, 66]. Both the organization and deposition of mineral drive the mechanical toughness at the interface [66]. Furthermore, mechanical loading stimulates primary cilia assembly, which is required for Hh signaling [28]. These findings can be used in regenerative medicine approaches by mimicking loading using Hh activation via biomaterials or small molecules.
At the microscale, the ability of cells to respond to local substrate stiffness has been investigated for decades, yet our understanding of how cells interact within gradient materials is relatively new [69, 70]. Key studies have demonstrated the importance of loading in interface disorders such as rotator cuff disease and ligament repair [71–73]. At the cellular level, changes in stiffness elicit transcriptional changes which can lead to changes in cell fate [74–78]. Furthermore, tendon stromal compartments respond to mechanical unloading dependent on the vascular niche as well as reactive oxidative species (ROS) which can proteolytically break down functional collagen backbones [79]. Mechanical force has also been shown improve rotator cuff tendon-bone healing by activating the IL-4/JAK/STAT signaling pathway through mediation of macrophage polarization, indicating a feedback system involving both mechanosensing and the immune system [58, 80]. Therefore, in order to regenerate this tissue, strategies must consider mechanosensing of resident cells at tissue interfaces [81].
How Are Cellular Metabolism and Hypoxia Involved in Interface Healing?
Metabolism is a key driver of changes within the cell as it is typically the first approach for cells to adapt to changes in their environment, such as changes in oxygen availability and vasculature. Yet the metabolic profile of cells at tissue interfaces is poorly understood and a prime target for future studies. In cartilage, suppression of mitochondrial respiration is a key driver for chondrocyte survival under hypoxic conditions [82, 83]. In tendon, disorders such as tendinosis have been associated with changes in oxygen tension-dependent modulation of Rac1 activity [84]. Hypoxia inducible factor 1a (HIF-1α) is an oxygen-dependent transcription factor that regulates gene expression of genes affiliated with metabolism, angiogenesis, and matrix maturation. HIF-1α has recently gained more traction in the study of hypoxic, ECM-rich tissues such as the cartilage and tissue interfaces, and has potential to act as a therapeutic target for treating osteoarthritis [85]. HIF-1α metabolically regulates collagen synthesis and modification in chondrocytes [86]; thus, it may contribute to the establishment of the ECM gradient in the fibrochondrogenic enthesis (Fig. 2). In vitro, the deposition of osteochondrogenic matrix is mediated by HIF-1α in hypoxia [87]. However, prolonged HIF-1α signaling in chondrocytes via HIF prolyl hydroxylase 2 (PHD2) deactivation restricts cellular bioenergetics and biosynthesis, leading to skeletal dysplasia [86]. In addition to the metabolic response to changes in oxygen availability, increased matrix production is correlated with decreased mitochondrial gene expression as well as a lack of inflammatory signature [73]. With the close ties between oxygen availability and vascularity, studies focused on the effects of vascularity on cell fate within interfacial tissue are of particular interest. For instance, vascularity and lipid availability regulate skeletal progenitor cell fate while Sox9 suppresses fatty oxidation in chondrocytes [88]. More studies investigating vasculature in interfacial tissue are required to better inform regenerative approaches.
Impact in Discovery and Clinical Translation
Cues from interfacial tissue development and healing have the potential to inform how we treat and repair interfaces. One strategy for regeneration that has shown promise is biomaterial scaffolds; however, these strategies have been used for decades with limited translation to the clinic, and few of these are focused on complex tissue interfaces. To stimulate interfacial tissue regeneration, biomaterial design could promote a microenvironment like that of the developing interfacial tissue. To advance biomaterial design, continued study of interface development and healing is necessary, in addition to assessment of cell behavior in gradient-structured materials. To this end, biofabrication of interfaces highlights major advances in the field of tissue engineering, including development bi-zonal patterning of engineered constructs [89, 90] and preclinical translation of composite biomaterials for craniofacial osteochondral repair [91] and tendon-bone enthesis repair [92–94] have shown promise.
Biomaterials have proven to be a more popular method for interfacial tissue repair as they offer a more controllable system without the complexity of cell implantation (i.e., cells are challenging to scale-up production reproducibly for transplant) [95, 96]. There are several approaches to biomaterial design for interfacial tissue regeneration including synthetic vs. natural constructs, injectable vs. non-injectable materials, 3D-printed vs. electrospun, degradable vs. non-degradable, and multi-phasic vs. uni-phasic [97–104, 105••]. Currently, substantial progress has been made in osteochondral interfacial regeneration with bone using biomaterials, particularly in craniofacial bone defects [91, 97, 106–109]. These provide further motivation to investigate the use of biomaterials to heal the osteochondral interface or enthesis. As interfacial tissues with bone are graded, collagen-rich tissues, collagen scaffolds have been of particular interest to guide native cellular migration and spatial differentiation at the defect site. However, synthetic materials may provide a better scaffold for cells to interact with their microenvironment and generate the collagen or ECM required for regenerating the gradient. Overall, non-cellularized or acellularized multiphasic scaffolds generated to treat injured interfaces show promise in vitro, but require validation in vivo [110].
Interfacial tissue proves to be challenging to regenerate; however, increased studies in ECM remodeling, the immune system, mechanical loading, metabolism, hypoxia, and angiogenesis in development and healing will provide new insights into regenerative approaches. Cell functions within interfacial tissues contribute to the unique mechanical properties. ECM components derived from cells are key to mechanosensing and mechanical function of interfacial tissues. Furthermore, studies support that the tissue regeneration and healing can be regulated by mechanical force, the immune system, and metabolism as well. Knowing this, we can tune regenerative approaches using biomaterials or pharmaceuticals to promote interfacial tissue regeneration post-injury as we continue to study interfacial tissue development and healing.
Author Contribution
SSS and MLK drafted the manuscript. SSS, ACA, and MLK revised the submitted manuscript. All authors approved the final version.
Funding
MLK is supported by funding from the National Institutes of Health (R01AR077858 and R01AR079367) and the National Science Foundation (1944448).
Data Availability
Not applicable.
Compliance with Ethical Standards
Competing Interests
The authors have no competing interests of financial or personal nature. Authors’ contributions: SSS and MLK drafted the manuscript. SSS, ACA, and MLK revised the submitted manuscript. All authors approved the final version
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peniche Silva C, Müller S, Quirk N, De la Vega R, Coenen M, Evans C, Balmayor E, van Griensven M. Enthesis: Not the same in each localisation – A molecular, histological and biomechanical study. eCM. 2022;44:43–55. doi: 10.22203/eCM.v044a03. [DOI] [PubMed] [Google Scholar]
- 3.Brown ME, Puetzer JL. Driving native-like zonal enthesis formation in engineered ligaments using mechanical boundary conditions and β-tricalcium phosphate. Acta Biomater. 2022;140:700–716. doi: 10.1016/j.actbio.2021.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Puetzer JL, Ma T, Sallent I, Gelmi A, Stevens MM. Driving hierarchical collagen fiber formation for functional tendon, ligament, and meniscus replacement. Biomaterials. 2021;269:120527. doi: 10.1016/j.biomaterials.2020.120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganji E, Leek C, Duncan W, Patra D, Ornitz DM, Killian ML. Targeted deletion of Fgf9 in tendon disrupts mineralization of the developing enthesis. FASEB J. 2023;37:e22777. doi: 10.1096/fj.202201614R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz AG, Lipner JH, Pasteris JD, Genin GM, Thomopoulos S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013;55:44–51. doi: 10.1016/j.bone.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz AG, Galatz LM, Thomopoulos S. Enthesis regeneration: A role for Gli1+ progenitor cells. Development Dev. 2017;144(7):1159–64. [DOI] [PMC free article] [PubMed]
- 8.Vervaecke AJ, Carbone AD, Abraham A, Bernstein Z, Laudier D, Verborgt O, Galatz LM, Huang AH. Tendon progenitor cells as biological augmentation improve functional gait and reduce scar formation after rotator cuff repair. J Shoulder Elbow Surg. 2022;31:2366–2380. doi: 10.1016/j.jse.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinestock RC, Felsenthal N, Assaraf E, et al. Neonatal enthesis healing involves noninflammatory acellular scar formation through extracellular matrix secretion by resident cells. Am J Pathol. 2022;192:1122–1135. doi: 10.1016/j.ajpath.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL. A distinct transition from cell growth to physiological homeostasis in the tendon. eLife. 2019;8:e48689. doi: 10.7554/eLife.48689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walia B, Li TM, Crosio G, Montero AM, Huang AH. Axin2-lineage cells contribute to neonatal tendon regeneration. Connect Tissue Res. 2022;63:530–543. doi: 10.1080/03008207.2022.2036732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walia B, Huang AH. Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res. 2019;37:1270–1280. doi: 10.1002/jor.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB j. 2016;30:301–311. doi: 10.1096/fj.14-258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Xu J, Lan Y, Lim H-W, Jiang R. The Scleraxis transcription factor directly regulates multiple distinct molecular and cellular processes during early tendon cell differentiation. Front Cell Dev Biol. 2021;9:654397. doi: 10.3389/fcell.2021.654397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx - and Sox9 -positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- 16.•• Kult S, Olender T, Osterwalder M, et al. Bi-fated tendon-to-bone attachment cells are regulated by shared enhancers and KLF transcription factors. eLife. 2021;10:e55361. Our understanding of the cell fate at musculoskeletal interfaces was rapidly advanced by Kult et al., whose work describes multi-omic approaches at the single cell level for describing the epigenetic and transcriptomic profile of attachment resident cells. These attachment-resident cells, which are uniquely identified during development as both Sox9+ and Scx+, have unique and overlapping features of cells in adjacent tissues, and these insights can be leveraged for improving interface regeneration using epigenetic modifiers for controlling cell fate. [DOI] [PMC free article] [PubMed]
- 17.Best KT, Loiselle AE. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB j. 2019;33:8578–8587. doi: 10.1096/fj.201900130RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackerman JE, Best KT, Muscat SN, Pritchett EM, Nichols AEC, Wu C-L, Loiselle AE. Defining the spatial-molecular map of fibrotic tendon healing and the drivers of Scleraxis-lineage cell fate and function. Cell Rep. 2022;41:111706. doi: 10.1016/j.celrep.2022.111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best KT, Korcari A, Mora KE, Nichols AE, Muscat SN, Knapp E, Buckley MR, Loiselle AE. Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. eLife. 2021;10:e62203. doi: 10.7554/eLife.62203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korcari A, Nichols AE, Buckley MR, Loiselle AE. Scleraxis-lineage cells are required for tendon homeostasis and their depletion induces an accelerated extracellular matrix aging phenotype. eLife. 2023;12:e84194. doi: 10.7554/eLife.84194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang AH, Watson SS, Wang L, Baker B, Akiyama H, Brigande JV, et al. Requirement for Scleraxis in the recruitment of mesenchymal progenitors during embryonic tendon elongation. Development Dev. 2019;146(20):dev182782. [DOI] [PMC free article] [PubMed]
- 22.Ideo K, Tokunaga T, Shukunami C, Takimoto A, Yoshimoto Y, Yonemitsu R, Karasugi T, Mizuta H, Hiraki Y, Miyamoto T. Role of Scx+/Sox9+ cells as potential progenitor cells for postnatal supraspinatus enthesis formation and healing after injury in mice. PLoS One. 2020;15:e0242286. doi: 10.1371/journal.pone.0242286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu X, Subramanian A, Hwang TH, Schilling TF, Galloway JL. Tendon cell regeneration is mediated by attachment site-resident progenitors and BMP signaling. Curr Biol. 2020;30:3277–3292.e5. doi: 10.1016/j.cub.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, Huang AH. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep. 2017;7:45238. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser HL, Abraham AC, Howell K, Laudier D, Zumstein MA, Galatz LM, Huang AH. Cell lineage tracing and functional assessment of supraspinatus tendon healing in an acute repair murine model. J Orthop Res. 2021;39:1789–1799. doi: 10.1002/jor.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breidenbach AP, Aschbacher-Smith L, Lu Y, et al. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res. 2015;33:1142–1151. doi: 10.1002/jor.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyment NA, Breidenbach AP, Schwartz AG, et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. 2015;405:96–107. doi: 10.1016/j.ydbio.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang F, Schwartz AG, Moore ER, Sup ME, Thomopoulos S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci Adv. 2020;6:eabc1799. doi: 10.1126/sciadv.abc1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz AG, Long F, Thomopoulos S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development. 2015;142:196–206. doi: 10.1242/dev.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felsenthal N, Rubin S, Stern T, Krief S, Pal D, Pryce BA, et al. Development of migrating tendon-bone attachments involves replacement of progenitor populations. Development Dev. 2018;145(24):dev165381. [DOI] [PMC free article] [PubMed]
- 31.Jing D, Li C, Yao K, Xie X, Wang P, Zhao H, Feng JQ, Zhao Z, Wu Y, Wang J. The vital role of Gli1+ mesenchymal stem cells in tissue development and homeostasis. J Cell Physiol. 2021;236:6077–6089. doi: 10.1002/jcp.30310. [DOI] [PubMed] [Google Scholar]
- 32.Fang F, Xiao Y, Zelzer E, Leong KW, Thomopoulos S. A unique mineralizing pool of Gli1+ stem cells builds the tendon enthesis and demonstrates therapeutic potential. 2022. 10.1101/2022.02.17.480929. [DOI] [PMC free article] [PubMed]
- 33.Eyal S, Kult S, Rubin S, et al. Bone morphology is regulated modularly by global and regional genetic programs. Development Dev. 2019;146(14):dev167882. [DOI] [PMC free article] [PubMed]
- 34.Duboc V, Sulaiman F, Feneck E, Kucharska A, Bell D, Holder-Espinasse M, et al. Tbx4 function during hindlimb development reveals a novel mechanism to explain the origins of proximal limb defects. Development Dev. 2021;148(19):dev199580. [DOI] [PMC free article] [PubMed]
- 35.Roberts RR, Bobzin L, Teng CS, Pal D, Tuzon CT, Schweitzer R, et al. FGF signaling patterns cell fate at the interface between tendon and bone. Development Dev. 2019;146(15):dev170241. [DOI] [PMC free article] [PubMed]
- 36.Vieira WA, Wells KM, Raymond MJ, De Souza L, Garcia E, McCusker CD. FGF, BMP, and RA signaling are sufficient for the induction of complete limb regeneration from non-regenerating wounds on Ambystoma mexicanum limbs. Dev Biol. 2019;451:146–157. doi: 10.1016/j.ydbio.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wernlé KK, Sonnenfelt MA, Leek CC, Ganji E, Sullivan AL, Offutt C, et al. Loss of Fgfr1 and Fgfr2 in Scleraxis-lineage cells leads to enlarged bone eminences and attachment cell death. Dev Dyn. 2023;252(9):1180–8. [DOI] [PMC free article] [PubMed]
- 38.Kamalitdinov TB, Fujino K, Keith Lang S, Jiang X, Madi R, Evans MK, Zgonis MH, Kuntz AF, Dyment NA. Targeting the hedgehog signaling pathway to improve tendon-to-bone integration. Osteoarthritis Cartilage. 2023;31:1202–1213. doi: 10.1016/j.joca.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopinke D, Norris AM, Mukhopadhyay S. Developmental and regenerative paradigms of cilia regulated hedgehog signaling. Semin Cell Dev Biol. 2021;110:89–103. doi: 10.1016/j.semcdb.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao H, Zhang T, Li C, et al. Mechanical stimulation promotes enthesis injury repair by mobilizing Prrx1+ cells via ciliary TGF-β signaling. eLife. 2022;11:e73614. doi: 10.7554/eLife.73614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development. 2015;142:4191–4204. doi: 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo V, El Khatib M, Prencipe G, et al. Tendon immune regeneration: Insights on the synergetic role of stem and immune cells during tendon regeneration. Cells. 2022;11:434. doi: 10.3390/cells11030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acuna A, Drakopoulos MA, Leng Y, Goergen CJ, Calve S. Three-dimensional visualization of extracellular matrix networks during murine development. Dev Biol. 2018;435:122–129. doi: 10.1016/j.ydbio.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed S, Nowlan N. Initiation and emerging complexity of the collagen network during prenatal skeletal development. eCM. 2020;39:136–155. doi: 10.22203/eCM.v039a09. [DOI] [PubMed] [Google Scholar]
- 45.Deymier AC, An Y, Boyle JJ, Schwartz AG, Birman V, Genin GM, Thomopoulos S, Barber AH. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017;56:25–35. doi: 10.1016/j.actbio.2017.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansari S, Khorshidi S, Karkhaneh A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019;87:41–54. doi: 10.1016/j.actbio.2019.01.071. [DOI] [PubMed] [Google Scholar]
- 47.Niu X, Li N, Du Z, Li X. Integrated gradient tissue-engineered osteochondral scaffolds: Challenges, current efforts and future perspectives. Bioactive Materials. 2023;20:574–597. doi: 10.1016/j.bioactmat.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobson KR, Lipp S, Acuna A, Leng Y, Bu Y, Calve S. Comparative analysis of the extracellular matrix proteome across the myotendinous junction. J Proteome Res. 2020;19:3955–3967. doi: 10.1021/acs.jproteome.0c00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolger MW, Romanowicz GE, Kohn DH. Advancements in composition and structural characterization of bone to inform mechanical outcomes and modeling. Current Opinion in Biomedical Engineering. 2019;11:76–84. doi: 10.1016/j.cobme.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Depalle B, Duarte AG, Fiedler IAK, Pujo-Menjouet L, Buehler MJ, Berteau J-P. The different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen. Bone. 2018;110:107–114. doi: 10.1016/j.bone.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Loebel C, Saleh AM, Jacobson KR, Daniels R, Mauck RL, Calve S, Burdick JA. Metabolic labeling of secreted matrix to investigate cell–material interactions in tissue engineering and mechanobiology. Nat Protoc. 2022;17:618–648. doi: 10.1038/s41596-021-00652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNerny EM, Gong B, Morris MD, Kohn DH. Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J Bone Miner Res. 2015;30:455–464. doi: 10.1002/jbmr.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stammers M, Ivanova IM, Niewczas IS, Segonds-Pichon A, Streeter M, Spiegel DA, Clark J. Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon. J Biol Chem. 2020;295:10562–10571. doi: 10.1074/jbc.RA119.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCreery KP, Luetkemeyer CM, Calve S, Neu CP. Hyperelastic characterization reveals proteoglycans drive the nanoscale strain-stiffening response in hyaline cartilage. J Biomech. 2023;146:111397. doi: 10.1016/j.jbiomech.2022.111397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 56.Crosio G, Huang A. Innate and adaptive immune system cells implicated in tendon healing and disease. eCM. 2022;43:39–52. doi: 10.22203/eCM.v043a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell C, Rudensky A. Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab. 2020;31:18–25. doi: 10.1016/j.cmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong Y, Mi B-B, Lin Z, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil Med Res. 2022;9:65. doi: 10.1186/s40779-022-00426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howell KL, Kaji DA, Li TM, Montero A, Yeoh K, Nasser P, Huang AH. Macrophage depletion impairs neonatal tendon regeneration. FASEB J. 2021 doi: 10.1096/fj.202100049R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GAC, Liew FY, Kurowska-Stolarska M, McInnes IB. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6:6774. doi: 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA. Tendinopathy Nat Rev Dis Primers. 2021;7:1. doi: 10.1038/s41572-020-00234-1. [DOI] [PubMed] [Google Scholar]
- 62.Ackerman JE, Nichols AE, Studentsova V, Best KT, Knapp E, Loiselle AE. Cell non-autonomous functions of S100a4 drive fibrotic tendon healing. eLife. 2019;8:e45342. doi: 10.7554/eLife.45342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abraham AC, Shah SA, Golman M, et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Sci Trans Med. 2019;11:eaav4319. doi: 10.1126/scitranslmed.aav4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Best KT, Nichols AEC, Knapp E, Hammert WC, Ketonis C, Jonason JH, Awad HA, Loiselle AE. NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Sci Signal. 2020;13:eabb7209. doi: 10.1126/scisignal.abb7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Best KT, Lee FK, Knapp E, Awad HA, Loiselle AE. Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing. Sci Rep. 2019;9:10926. doi: 10.1038/s41598-019-47461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golman M, Abraham AC, Kurtaliaj I, et al. Toughening mechanisms for the attachment of architectured materials: The mechanics of the tendon enthesis. Science Advances. 2021;7:eabi5584. doi: 10.1126/sciadv.abi5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganji E, Lamia SN, Stepanovich M, Whyte N, Goulet RW, Abraham AC, Killian ML. Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice. Sci Adv. 2023;9:eadf4683. doi: 10.1126/sciadv.adf4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsinman TK, Huang Y, Ahmed S, Levillain AL, Evans MK, Jiang X, et al. Lack of skeletal muscle contraction disrupts fibrous tissue morphogenesis in the developing murine knee. J Orthop Res. 2023;41(10):2305–14. [DOI] [PMC free article] [PubMed]
- 69.Wilmoth RL, Ferguson VL, Bryant SJ. A 3D, dynamically loaded hydrogel model of the osteochondral unit to study osteocyte mechanobiology. Adv Healthcare Mater. 2020;9:2001226. doi: 10.1002/adhm.202001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burdis R, Chariyev-Prinz F, Browe DC, Freeman FE, Nulty J, McDonnell EE, Eichholz KF, Wang B, Brama P, Kelly DJ. Spatial patterning of phenotypically distinct microtissues to engineer osteochondral grafts for biological joint resurfacing. Biomaterials. 2022;289:121750. doi: 10.1016/j.biomaterials.2022.121750. [DOI] [PubMed] [Google Scholar]
- 71.Abraham AC, Fang F, Golman M, Oikonomou P, Thomopoulos S. The role of loading in murine models of rotator cuff disease. J Orthop Res. 2022;40:977–986. doi: 10.1002/jor.25113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Locke RC, Peloquin JM, Lemmon EA, Szostek A, Elliott DM, Killian ML. Strain distribution of intact rat rotator cuff tendon-to-bone attachments and attachments with defects. J Biomech Eng. 2017;139:111007. doi: 10.1115/1.4038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steffen D, Mienaltowski MJ, Baar K. Scleraxis and collagen I expression increase following pilot isometric loading experiments in a rodent model of patellar tendinopathy. Matrix Biol. 2022;109:34–48. doi: 10.1016/j.matbio.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Darnell M, Gu L, Mooney D. RNA-seq reveals diverse effects of substrate stiffness on mesenchymal stem cells. Biomaterials. 2018;181:182–188. doi: 10.1016/j.biomaterials.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konar S, Bolam SM, Coleman B, Dalbeth N, McGlashan SR, Leung S, Cornish J, Naot D, Musson DS. Changes in physiological tendon substrate stiffness have moderate effects on tendon-derived cell growth and immune cell activation. Front Bioeng Biotechnol. 2022;10:800748. doi: 10.3389/fbioe.2022.800748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loebel C, Mauck RL, Burdick JA. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat Mater. 2019;18:883–891. doi: 10.1038/s41563-019-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vermeulen S, Roumans N, Honig F, Carlier A, Hebels DGAJ, Eren AD, ten Dijke P, Vasilevich A, de Boer J. Mechanotransduction is a context-dependent activator of TGF-β signaling in mesenchymal stem cells. Biomaterials. 2020;259:120331. doi: 10.1016/j.biomaterials.2020.120331. [DOI] [PubMed] [Google Scholar]
- 78.Hussien AA, Niederoest B, Bollhalder M, Goedecke N, Snedeker JG. The stiffness-sensitive transcriptome of human tendon stromal cells. Adv Healthcare Materials. 2023;12:2101216. doi: 10.1002/adhm.202101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wunderli SL, Blache U, Beretta Piccoli A, Niederöst B, Holenstein CN, Passini FS, Silván U, Bundgaard L, auf dem Keller U, Snedeker JG. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biology. 2020;89:11–26. doi: 10.1016/j.matbio.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Wang L, Li S, Zhang T, Chen C, Hu J, Sun D, Lu H. Mechanical stimulation improves rotator cuff tendon-bone healing via activating IL-4/JAK/STAT signaling pathway mediated macrophage M2 polarization. J Orthop Trans. 2022;37:78–88. doi: 10.1016/j.jot.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selig M, Lauer JC, Hart ML, Rolauffs B. Mechanotransduction and stiffness-sensing: Mechanisms and opportunities to control multiple molecular aspects of cell phenotype as a design cornerstone of cell-instructive biomaterials for articular cartilage repair. IJMS. 2020;21:5399. doi: 10.3390/ijms21155399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao Q, Parvez-Khan M, Schipani E. In vivo survival strategies for cellular adaptation to hypoxia: HIF1α-dependent suppression of mitochondrial oxygen consumption and decrease of intracellular hypoxia are critical for survival of hypoxic chondrocytes. Bone. 2020;140:115572. doi: 10.1016/j.bone.2020.115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao Q, Khan MP, Merceron C, et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell. 2019;49:748–763.e7. doi: 10.1016/j.devcel.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McBeath R, Edwards RW, O’Hara BJ, Maltenfort MG, Parks SM, Steplewski A, Osterman AL, Shapiro IM. Tendinosis develops from age- and oxygen tension-dependent modulation of Rac1 activity. Aging Cell. 2019;18:e12934. doi: 10.1111/acel.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H, Wang L, Cui J, et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci Adv. 2023;9:eabo7868. doi: 10.1126/sciadv.abo7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stegen S, Laperre K, Eelen G, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Calejo I, Costa-Almeida R, Reis RL, Gomes ME. In vitro temporal HIF-mediated deposition of osteochondrogenic matrix governed by hypoxia and osteogenic factors synergy. J Cell Physiol. 2021;236:3991–4007. doi: 10.1002/jcp.30138. [DOI] [PubMed] [Google Scholar]
- 88.van Gastel N, Stegen S, Eelen G, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579:111–117. doi: 10.1038/s41586-020-2050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kilian D, Cometta S, Bernhardt A, Taymour R, Golde J, Ahlfeld T, Emmermacher J, Gelinsky M, Lode A. Core–shell bioprinting as a strategy to apply differentiation factors in a spatially defined manner inside osteochondral tissue substitutes. Biofabrication. 2022;14:014108. doi: 10.1088/1758-5090/ac457b. [DOI] [PubMed] [Google Scholar]
- 90.Zlotnick HM, Locke RC, Hemdev S, et al. Gravity-based patterning of osteogenic factors to preserve bone structure after osteochondral injury in a large animal model. Biofabrication. 2022;14:044101. doi: 10.1088/1758-5090/ac79cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dewey MJ, Milner DJ, Weisgerber D, et al. Repair of critical-size porcine craniofacial bone defects using a collagen–polycaprolactone composite biomaterial. Biofabrication. 2022;14:014102. doi: 10.1088/1758-5090/ac30d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarafder S, Brito JA, Minhas S, Effiong L, Thomopoulos S, Lee CH. In situ tissue engineering of the tendon-to-bone interface by endogenous stem/progenitor cells. Biofabrication. 2019;12:015008. doi: 10.1088/1758-5090/ab48ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han J, Han SC, Kim YK, Tarafder S, Jeong HJ, Jeong HJ, Chung JY, Lee CH, Oh JH. Bioactive scaffold with spatially embedded growth factors promotes bone-to-tendon interface healing of chronic rotator cuff tear in rabbit model. Am J Sports Med. 2023;51:2431–2442. doi: 10.1177/03635465231180289. [DOI] [PubMed] [Google Scholar]
- 94.Ma H, Yang C, Ma Z, et al. Multiscale hierarchical architecture-based bioactive scaffolds for versatile tissue engineering. Adv Healthcare Materials. 2022;11:2102837. doi: 10.1002/adhm.202102837. [DOI] [PubMed] [Google Scholar]
- 95.Diederichs S, Shine K, Tuan R. The promise and challenges of stem cell-based therapies for skeletal diseases: Stem cell applications in skeletal medicine: Potential, cell sources and characteristics, and challenges of clinical translation. BioEssays. 2013;35:220–230. doi: 10.1002/bies.201200068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loebel C, Burdick JA. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. 2018;22:325–339. doi: 10.1016/j.stem.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amann E, Amirall A, Franco AR, Poh PSP, Sola Dueñas FJ, Fuentes Estévez G, Leonor IB, Reis RL, Griensven M, Balmayor ER. A graded, porous composite of natural biopolymers and octacalcium phosphate guides osteochondral differentiation of stem cells. Adv Healthcare Mater. 2021;10:2001692. doi: 10.1002/adhm.202001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chae S, Yong U, Park W, Choi Y, Jeon I-H, Kang H, Jang J, Choi HS, Cho D-W. 3D cell-printing of gradient multi-tissue interfaces for rotator cuff regeneration. Bioactive Materials. 2023;19:611–625. doi: 10.1016/j.bioactmat.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C, Shi Q, Li M, et al. Engineering an enthesis-like graft for rotator cuff repair: An approach to fabricate highly biomimetic scaffold capable of zone-specifically releasing stem cell differentiation inducers. Bioactive Materials. 2022;16:451–471. doi: 10.1016/j.bioactmat.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen P, Wang A, Haynes W, Landao-Bassonga E, Lee C, Ruan R, Breidahl W, Shiroud Heidari B, Mitchell CA, Zheng M. A bio-inductive collagen scaffold that supports human primary tendon-derived cell growth for rotator cuff repair. J Orthop Trans. 2021;31:91–101. doi: 10.1016/j.jot.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottardi R, Moeller K, Di Gesù R, Tuan RS, van Griensven M, Balmayor ER. Application of a hyperelastic 3D printed scaffold for mesenchymal stem cell-based fabrication of a bizonal tendon enthesis-like construct. Front Mater. 2021;8:613212. doi: 10.3389/fmats.2021.613212. [DOI] [Google Scholar]
- 102.Kent RN, Said M, Busch ME, et al. Physical and soluble cues enhance tendon progenitor cell invasion into injectable synthetic hydrogels. Adv Funct Materials. 2022;32:2207556. doi: 10.1002/adfm.202207556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Owida HA, Yang R, Cen L, Kuiper NJ, Yang Y. Induction of zonal-specific cellular morphology and matrix synthesis for biomimetic cartilage regeneration using hybrid scaffolds. J R Soc Interface. 2018;15:20180310. doi: 10.1098/rsif.2018.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peniche Silva CJ, Müller SA, Quirk N, et al. Enthesis healing is dependent on scaffold interphase morphology—Results from a rodent patellar model. Cells. 2022;11:1752. doi: 10.3390/cells11111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.•• Sun Han Chang RA, Shanley JF, Kersh ME, Harley BAC. Tough and tunable scaffold-hydrogel composite biomaterial for soft-to-hard musculoskeletal tissue interfaces. Science Advances. 2020;6:eabb6763. This work highlights the importance of inclusion of interfacial hydrogels in biofabrication for increasing toughness and improving tunability of enthesis-mimetic materials. By controlling gelation rate and diffusive mixing, this work details ways to improve manufacturing of gradient biomaterial structure and improved mechanical function. [DOI] [PMC free article] [PubMed]
- 106.Dewey MJ, Nosatov AV, Subedi K, Shah R, Jakus A, Harley BAC. Inclusion of a 3D-printed hyperelastic bone mesh improves mechanical and osteogenic performance of a mineralized collagen scaffold. Acta Biomater. 2021;121:224–236. doi: 10.1016/j.actbio.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dewey MJ, Johnson EM, Slater ST, Milner DJ, Wheeler MB, Harley BAC. Mineralized collagen scaffolds fabricated with amniotic membrane matrix increase osteogenesis under inflammatory conditions. Regenerative Biomaterials. 2020;7:247–258. doi: 10.1093/rb/rbaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dewey MJ, Johnson EM, Weisgerber DW, Wheeler MB, Harley BAC. Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells. J Mech Behav Biomed Mater. 2019;95:21–33. doi: 10.1016/j.jmbbm.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wong SA, Hu DP, Slocum J, Lam C, Nguyen M, Miclau T, Marcucio RS, Bahney CS. Chondrocyte-to-osteoblast transformation in mandibular fracture repair. J Orthop Res. 2021;39:1622–1632. doi: 10.1002/jor.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Locke RC, Ford EM, Silbernagel KG, Kloxin AM, Killian ML. Success criteria and preclinical testing of multifunctional hydrogels for tendon regeneration. Tissue Eng Part C Methods. 2020;26:506–518. doi: 10.1089/ten.tec.2020.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.