Abstract
A State of the Art lecture titled “Anticoagulation and Vascular Anomalies” was presented at the International Society on Thrombosis and Haemostasis (ISTH) Congress in 2023. Vascular anomalies have been classified by the International Society for the Study of Vascular Anomalies into vascular tumors and vascular malformations. Although some vascular tumors, such as tufted angioma and kaposiform hemangioendothelioma, and other vascular malformations can present with coagulation aberrancies, these are not generally managed with anticoagulation. A subclassification of vascular malformations includes slow-flow vascular malformations. It is this subgroup specifically that has a high risk of venous thromboembolism (VTE) and morbidity associated with coagulopathy that may be present. In these select cases, anticoagulation may be indicated to reduce the risk of VTE, treat VTE, or manage localized thrombosis in the malformation that causes significant pain and reduced quality of life. There are established risk factors for VTE in these patients that will be reviewed. Finally, we summarize relevant new data on this topic presented during the 2023 ISTH Congress.
Keywords: anticoagulant, coagulopathy, sclerotherapy, vascular malformation
Essentials
-
•
Vascular malformations can present with bleeding or clotting concerns that require a hematologist.
-
•
Slow blood flow through malformed vessels can cause abnormalities in clotting factors and platelets.
-
•
Additional risk factors or surgeries in these patients may lead to thrombosis or pulmonary embolism.
-
•
Blood thinners may play a role in reducing the risk of clots and pain in patients with higher-risk vascular malformations.
1. Classification of Vascular Anomalies
The International Society for the Study of Vascular Anomalies has proposed a complex classification system to better understand, diagnose, and manage vascular anomalies, last updated in 2018 [1] (Table). Appendix 4 of this classification includes a list of vascular anomalies possibly associated with platelet count/coagulation disorders. These include the better-described vascular tumors kaposiform endothelioma and tufted angioma, which can present with coagulation abnormalities known as Kassabach–Merritt phenomenon. This is typically defined by profound thrombocytopenia and hypofibrinogenemia and usually requires aggressive medical treatment with corticosteroids, vincristine, and/or sirolimus to treat the underlying vascular tumor. These patients are at exceedingly high risk of morbidity and mortality from bleeding complications [2].
Table.
Simplistic overview of classification schema of vascular anomalies.
| Vascular tumors | Vascular malformations |
||
|---|---|---|---|
| Slow flow | Fast flow | Other/unclassified | |
| Kaposiform hemangioendotheliomaa Tufted angiomaa Hemangioma Infantile Congenital Rapidly involutinga Noninvoluting |
Capillary malformation Venous malformationa Lymphatic malformation (LM) Macro- or microcystic LM Generalized lymphatic anomaly Kaposiform lymphangiomatosisa Combined malformationa CVMa CLMa CLVMa VLMa |
Arteriovenous malformation Arteriovenous fistula |
Multifocal lymphangiomatosis with thrombocytopeniaa |
CLM, capillary lymphatic malformation; CLVM, capillary lymphatic venous malformation; CVM, complex vertebral malformation; VLM, venous lymphatic malformation.
The conditions can be associated with various types of coagulation derangements.
Vascular malformations can also present with hematologic manifestations. Severe coagulopathy is a feature of a rare disorder known as kaposiform lymphangiomatosis. This is a complex lymphatic malformation involving multiple organs and bones. Patients frequently have thrombocytopenia, hypofibrinogenemia, and elevated D-dimer due to consumption of coagulation factors within the lymphatic channels [3]. More commonly, patients with venous malformations occurring in isolation or in combination with lymphatic and/or capillary malformations can have a similar picture with usually mild thrombocytopenia, hypofibrinogenemia, and/or elevated D-dimer. These malformations are collectively known as slow-flow vascular malformations (SFVM) to distinguish them from arterial malformations, which have high blood flow through the abnormal vascular channels. Most often, a good physical examination and imaging can help distinguish the various malformations. For the narrower topic of the use of anticoagulation in vascular anomalies, we will focus on SFVM for this review.
2. Hematologic Complications of SFVM
2.1. Localized intravascular coagulopathy
In the vascular anomaly literature, the term localized intravascular coagulopathy (LIC) has been used to describe the frequently seen hematologic abnormalities of the patients. Although the precise pathophysiology is not well elucidated, it is thought to be similar to the consumptive coagulopathy seen in disseminated intravascular coagulopathy. In SFVM, the sluggish blood flow through the malformed, incompetent, valveless venous channels causes localized activation and consumption of platelets, fibrin deposition, localized thrombin generation, and subsequent consumption of multiple coagulation factors and natural anticoagulant factors [[4], [5], [6]]. This phenomenon may cause intralesional bleeding or localized venous thrombosis. This most frequently presents as localized hard, painful knots in the malformation or phleboliths. These superficial thrombi can cause significant pain and loss of function of the involved area. The term LIC is used to describe a distinct coagulopathy that occurs in approximately 40% to 60% of patients with SFVM and is characterized primarily by elevated D-dimer, but in more severe cases, patients may also have varying degrees of thrombocytopenia and hypofibrinogenemia, occasionally with prolongation of prothrombin time and partial thromboplastin time [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. This more severe form of LIC occurs in about 10% of patients, carries a high risk of bleeding, and is usually seen with larger venous malformations or following procedures or interventions that involve the abnormal vasculature (Figure 1) [5,12,14]. In addition to the hallmark LIC features, low levels of certain coagulation factors (factor [F]V, FVIII, prekallikrein, and von Willebrand factor) have been observed [9,15], but abnormalities in natural anticoagulant levels or activation of platelets have not been shown to occur [[6], [7], [8],16]. The presence of inherited thrombophilic conditions has been seen in patients with venous thromboembolism (VTE) and vascular malformations and could increase the risk of VTE [7,8,15,16].
Figure 1.
Patient with extensive multifocal venous malformation of his torso due to somatic TEK mutation. He has severe localized intravascular coagulopathy with hypofibrinogenemia, elevated D-dimer, and mild thrombocytopenia that has responded well to chronic anticoagulation with enoxaparin initially and, now, rivaroxaban.
3. VTE
3.1. Modifiable and nonmodifiable risk factors
Patients with SFVM are at an increased risk of VTE due to multiple factors. Abnormal venous channels lead to venous stasis, and patients may have altered mobility due to pain, procedures, or decreased range of motion from large malformation. No evidence supports an increased prevalence of thrombophilia among patients with SFVM compared with general population, but the presence of an acquired or inherited thrombophilia may certainly further increase the risk of VTE in these high-risk patients. Frequently, patients are advised to avoid estrogen-containing contraception for concern of increased risk of VTE; however, a recent large database study showed no association between hormonal contraception and hypercoagulation in a vascular malformation population [17]. However, given the high rate of VTE in patients with SFVM, caution to avoid any known modifiable thrombotic risk factor (eg, exogenous estrogen, immobility, smoking, and obesity) should be paramount.
3.2. Vascular malformation-related factors
The rate of VTE has been reported to be as high as 8% to 12.5% in patients with combined vascular malformations such as PIK3CA-related overgrowth syndrome (PROS), with a reported mortality of up to 20% to 50% [13,16,18,19]. Up to 90% of patients have evidence of superficial venous thrombosis in the malformation, which is referred to as phleboliths [13,16,18].
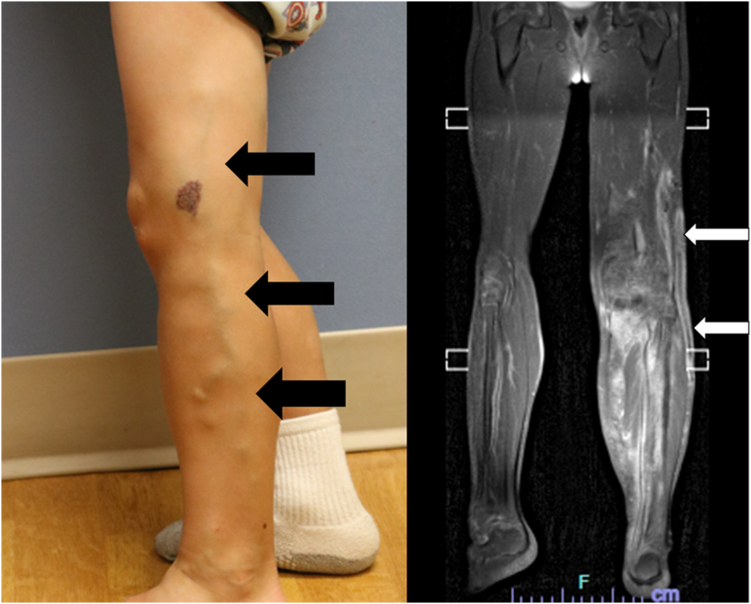
One of the most consistent risk factors for VTE in patients with complex vascular malformations such as PROS or Klippel-Trenaunay syndrome (KTS) is the presence of venous ectasia or persistence of embryonic veins (Figure 2). Venous ectasia is defined as a vein that is dilated 2 to 3 times the size of the normal surrounding veins or the normal vein size for age. Persistent embryonic veins are valveless veins that can drain straight into deeper veins, such as the femoral or iliac veins, and are associated with deep venous hypoplasia or aplasia. These usually occur as lateral medial/marginal veins or sciatic veins in PROS, and prevalence was 17.1% in a study of KTS screened with duplex ultrasonography [20]. Other reports have shown a higher prevalence of 60% to 80% [21,22]. In a nested case-control study of 97 patients within a larger cohort study of KTS patients showing a VTE incidence of 32.4%, 3 specific venous aberrations were shown to have an independent increased risk of thrombosis [23]. The presence of lateral marginal vein was associated with a statistically significant odds ratio (OR) of 2.71, insufficient vena perforans of upper leg OR of 4.08, and convoluted intramuscular veins OR of 2.67 for increased risk of VTE. These aberrant veins can be closed or removed to prevent VTE [20,[24], [25], [26], [27], [28]]. The risk of pulmonary embolism is highest in patients who have significant venous ectasia or persistent embryonic veins, which allow for a connection from the superficial vascular malformation to the deep veins [23]. A case-control study of 46 patients with SFVM revealed a statistically significantly increased OR of VTE if surface area of the malformation was ≥10 cm2 (OR, 6.18), if palpable phleboliths were present (OR, 20.17), or if D-dimer was >500 mg/mL (OR, 17.1) [18].
Figure 2.
Clinical photographs (black arrows) and magnetic resonance imaging (white arrows) of lateral marginal vein in a child with PIK3CA-related overgrowth syndrome. The patient has subsequently undergone several sclerotherapy procedures to ensure closure of the aberrant vein.
3.3. Procedural factors
The risk of VTE has been shown to be highest following surgical procedures. One study showed that 64% of pulmonary embolisms (14/22) occurred following surgery or sclerotherapy [19]. It is clear that different procedures have varied risks of VTE, with the more invasive procedures, especially those that involve manipulation of the abnormal vascular, conferring the highest risk. Examples of such a high-risk procedure would be glue embolization with subsequent resection of a venous malformation. Laser procedures and sclerotherapy procedures on venous malformations that are isolated or that drain into normal veins are likely less risky than those on malformations that drain into dysplastic veins or on venous ectasia [4,29]. The role of perioperative LIC in VTE risk is debated, especially if only D-dimer is elevated [4,15,24,[30], [31], [32]], but algorithms have been suggested to try to reduce perioperative risk [14,33].
4. Management Strategies For VTE Risk
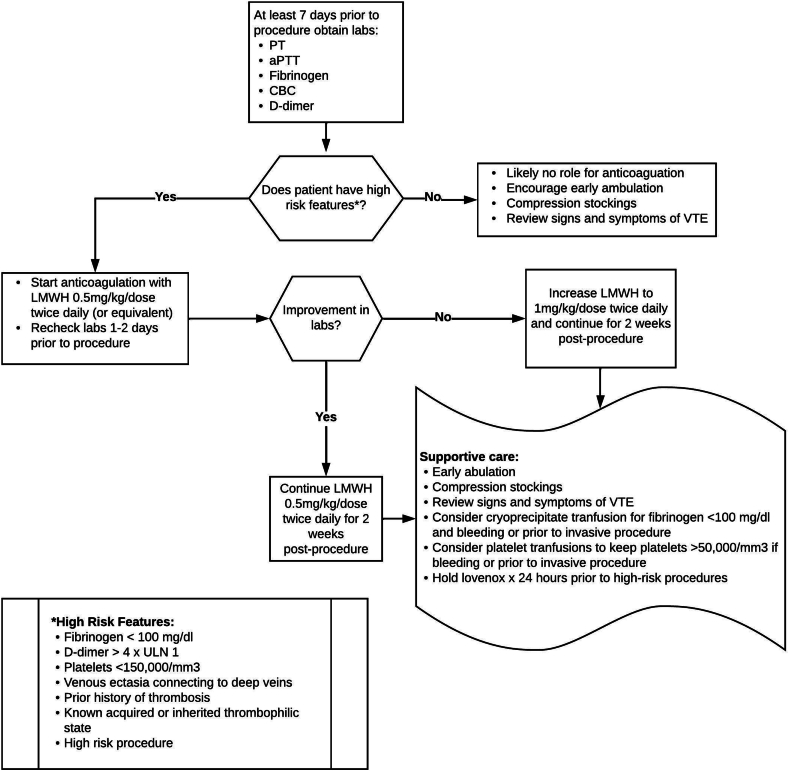
Mitigating periprocedural risk of VTE is an area of interest, although no universal guideline exists. It is important to assess an individual’s risk in combination with the procedure-related risk of thrombosis. The American Society for Pediatric Hematology/Oncology Vascular Anomalies Special Interest Group adopted an institutional protocol as a consensus document in periprocedural management, which was adapted by our institution (Figure 3). Treatment guidelines may differ in D-dimer cutoffs and length of anticoagulation before and after procedure [14]. Some experts argue that D-dimer alone, regardless of level of elevation, is not sufficient to guide management of LIC. High-risk features, including fibrinogen level of <100 mg/dL, platelet count of <150,000 mm3, venous ectasia connecting to deep veins, and a history of thrombosis or known thrombophilia in combination with an elevated D-dimer could provide more evidence of potential risk of complications and need for anticoagulation and supportive care.
Figure 3.
Proposed periprocedural management of localized intravascular coagulopathy to reduce risk of venous thromboembolism (VTE). aPTT, activated partial thromboplastin time; CBC, complete blood count; LMWH, low-molecular-weight heparin; PT, prothrombin time.
Generally, laboratory evaluation should be performed 1 to 2 weeks prior to an invasive procedure and include prothrombin time, activated partial thromboplastin time, fibrinogen, complete blood count, and D-dimer to determine if patient has high-risk features subsequently needing anticoagulation. Obtaining repeat laboratory results 24 to 48 hours prior to the procedure after starting anticoagulation will help assess response and determine if cryoprecipitate, platelets, or increase in dose of anticoagulation are needed or should result in postponement of elective procedure. If high-risk features are not present, supportive treatment will usually suffice. This may also be true for low-risk procedures such as sclerotherapy or laser therapy [30].
Conservative treatment with compression garments can reduce risk of VTE, pain, and swelling if fitted accurately and used regularly [34,35]. Closure of the embryonic veins that are connected to the deep venous system may reduce the risk of VTE and other sequelae of an aging, valveless, compliant vessel [36]. Postprocedural anticoagulation is desirable in cases of venous ectasia or thrombophilia without evidence of LIC before procedure. Direct oral anticoagulants (DOACs) are becoming increasingly utilized instead of low-molecular-weight heparin (LMWH) due to ease of administration; however, they may not be as effective as LMWH in correcting severe LIC before or after procedure [37]. Holding anticoagulation is usually not needed for minor procedures but may need to be held 24 hours prior to high-risk procedures. As seen in some patients with extensive venous malformations and severe LIC, holding anticoagulation for an extended time may actually worsen postprocedural LIC, leading to increased risk of bleeding.
5. Management of Pain in SFVM
Pain in patients with SFVM is frequently multifactorial, including chronic venous insufficiency, inflammation, cellulitis, arthritis, hemarthropathy, and/or neuropathic pain [38]. Pain is often associated with hard, painful knots in superficial malformations (phleboliths) usually in the acute setting but can also present in the chronic setting with ongoing LIC.
Similar to conservative treatment for decreasing VTE risk, compression therapy may help with decreasing pain. Antiplatelet therapy has shown equivocal benefit but is frequently prescribed [39]. With more aggressive or aspirin-refractory pain, LMWH and DOACs are commonly prescribed [37]. Recommended dosing at our institution starts with LMWH 0.5 mg/kg/dose every 12 hours with the option to increase to 1 mg/kg/dose every 12 hours or rivaroxaban 10 mg by mouth daily, which can be increased to 15 to 20 mg daily at 2 weeks after initiation if little to no benefit is seen. Other DOACs, such as apixaban, can be utilized, especially in the setting of bleeding complications like menorrhagia due to rivaroxaban [40]. Treatment may be long-term for chronic pain/severe LIC or for 2- to 12-week intervals if treating acute pain and phleboliths.
Anticoagulation may help with improving the quality of life in patients with SFVM, and this question is currently being studied through a multi-institutional clinical trial by the Consortium of Investigators of Vascular Anomalies. Consortium of Investigators of Vascular Anomalies is a group of pediatric hematologists in the United States representing 22 institutions and 6 patient advocacy groups who are all committed to improving the lives of patients with vascular anomalies.
6. Future Directions
As the involvement of hematologists in the field of vascular anomalies is relatively new, most of the recommendations included are based on expert opinion or small case series and retrospective reviews. Very few clinical trials of anticoagulation in vascular malformations have been completed. However, given the complexities of the coagulopathies that occur in this population as well as the significant risk of VTE, it is very important that multidisciplinary teams, consisting of a trained hematologist in addition to the surgical specialties, are involved in managing these patients. High-quality clinical trials to establish evidence-based guidelines for prevention and treatment of VTE and coagulopathy are of utmost importance to this growing field.
Acknowledgments
Funding
None.
Author contributions
S.E.C. and J.M.M. both wrote and edited the manuscript.
Relationship Disclosure
J.M.M. has no conflicts to disclose. S.E.C. received honoraria for participation in advisory boards from Pfizer, Medexus, and Sanofi, and consultancy for data safety monitoring boards from ASC Therapeutics and Novartis.
Footnotes
Handling Editor: Michael Makris
References
- 1.International Society for the Study of Vascular Anomalies ISSVA Classification of Vascular Anomalies. 2018. http://www.issva.org/classification
- 2.Ji Y., Yang K., Peng S., Chen S., Xiang B., Xu Z., et al. Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol. 2018;179:457–463. doi: 10.1111/bjd.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croteau S.E., Kozakewich H.P., Perez-Atayde A.R., Fishman S.J., Alomari A.I., Chaudry G., et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164:383–388. doi: 10.1016/j.jpeds.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci K.W., Chute C., Hammill A.M., Dasgupta R., Patel M. Retrospective study of hematologic complications in patients with slow-flow vascular malformations undergoing sclerotherapy. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28277. [DOI] [PubMed] [Google Scholar]
- 5.Mack J.M., Verkamp B., Richter G.T., Nicholas R., Stewart K., Crary S.E. Effect of sirolimus on coagulopathy of slow-flow vascular malformations. Pediatr Blood Cancer. 2019;66 doi: 10.1002/pbc.27896. [DOI] [PubMed] [Google Scholar]
- 6.Mack J.M., Pierce C.D., Richter G.T., Spray B.J., Nicholas R., Lewis P.S., et al. Analyzing coagulation dynamics during treatment of vascular malformations with thromboelastography. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28824. [DOI] [PubMed] [Google Scholar]
- 7.Keppler-Noreuil K.M., Lozier J.N., Sapp J.C., Biesecker L.G. Characterization of thrombosis in patients with Proteus syndrome. Am J Med Genet A. 2017;173:2359–2365. doi: 10.1002/ajmg.a.38311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen I., Hedelund L., Larsen J., Hvas A. Thrombophilia in patients with venous malformations. Thromb Res. 2022;212:1–3. doi: 10.1016/j.thromres.2022.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Kapp F.G., Schneider C., Holm A., Glonnegger H., Niemeyer C.M., Rößler J., et al. Comprehensive analyses of coagulation parameters in patients with vascular anomalies. Biomolecules. 2022;12:1840. doi: 10.3390/biom12121840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel E.R., Hammill A., Adams D., Phillips R.J., Jeng M., Tollefson M.M., et al. Response to sirolimus in capillary lymphatic venous malformations and associated syndromes: impact on symptomatology, quality of life, and radiographic response. Pediatric Blood Cancer. 2023;70 doi: 10.1002/pbc.30215. [DOI] [PubMed] [Google Scholar]
- 11.Mazoyer E., Enjolras O., Bisdorff A., Perdu J., Wassef M., Drouet L. Coagulation disorders in patients with venous malformation of the limbs and trunk: a case series of 118 patients. Arch Dermatol. 2008;144:861–867. doi: 10.1001/archderm.144.7.861. [DOI] [PubMed] [Google Scholar]
- 12.Dompmartin A., Acher A., Thibon P., Tourbach S., Hermans C., Deneys V., et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144:873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Es J., Kappelhof N.A., Douma R.A., Meijers J.C.M., Gerdes V.E.A., van der Horst C. Venous thrombosis and coagulation parameters in patients with pure venous malformations. Neth J Med. 2017;75:328–334. [PubMed] [Google Scholar]
- 14.Ricci K.W., Brandao L.R. Coagulation issues in vascular anomalies. Semin Pediatr Surg. 2020;29 doi: 10.1016/j.sempedsurg.2020.150966. [DOI] [PubMed] [Google Scholar]
- 15.Adams D.M. Special considerations in vascular anomalies: hematologic management. Clin Plast Surg. 2011;38:153–160. doi: 10.1016/j.cps.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Oduber C.E., van Beers E.J., Bresser P., van der Horst C.M., Meijers J.C., Gerdes V.E. Venous thromboembolism and prothrombotic parameters in Klippel-Trenaunay syndrome. Neth J Med. 2013;71:246–252. [PubMed] [Google Scholar]
- 17.Wang J., Qian M.F., Jeng M.R., Teng J.M.C. Assessment of hormonal contraceptive utilization and associated odds of hypercoagulopathy in patients with venous malformations using a national claims database. Clin Drug Investig. 2023;43:141–145. doi: 10.1007/s40261-022-01228-5. [DOI] [PubMed] [Google Scholar]
- 18.Sepulveda P., Zavala A., Zuniga P. Factors associated with thrombotic complications in pediatric patients with vascular malformations. J Pediatr Surg. 2017;52:400–404. doi: 10.1016/j.jpedsurg.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Reis J. 3.r.d., Alomari A.I., Trenor C.C., 3rd, Adams D.M., Fishman S.J., Spencer S.A., et al. Pulmonary thromboembolic events in patients with congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and spinal/skeletal abnormalities and Klippel-Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2018;6:511–516. doi: 10.1016/j.jvsv.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Oduber C.E., Young-Afat D.A., van der Wal A.C., van Steensel M.A., Hennekam R.C., van der Horst C.M. The persistent embryonic vein in Klippel-Trenaunay syndrome. Vasc Med. 2013;18:185–191. doi: 10.1177/1358863X13498463. [DOI] [PubMed] [Google Scholar]
- 21.Bastarrika G., Redondo P., Sierra A., Cano D., Martínez-Cuesta A., López-Gutiérrez J.C., et al. New techniques for the evaluation and therapeutic planning of patients with Klippel-Trénaunay syndrome. J Am Acad Dermatol. 2007;56:242–249. doi: 10.1016/j.jaad.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 22.Abdel Razek A.A.K. Imaging findings of Klippel-Trenaunay syndrome. J Comput Assist Tomogr. 2019;43:786–792. doi: 10.1097/RCT.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 23.Zwerink L.G.J.M., Te Loo D.M.W.M., Praster R., Verhoeven B.H., van der Vleuten C.J.M. Aberrant venous anatomy as a risk factor for thromboembolic events in patients with Klippel-Trénaunay syndrome: case-control study within a cohort study. J Am Acad Dermatol. 2021;84:1470–1472. doi: 10.1016/j.jaad.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Dharmarajan H., McCoy J.L., Jabbour N., McCormick A., Xavier F., Correa D., et al. Peri-procedural anticoagulation in patients with head and neck versus extremity venous malformations. Laryngoscope. 2021;131:1163–1167. doi: 10.1002/lary.29123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noel A.A., Gloviczki P., Cherry K.J., Rooke T.W., Stanson A.W., Driscoll D.J. Surgical treatment of venous malformations in Klippel-Trénaunay syndrome. J Vasc Surg. 2000;32:840–847. doi: 10.1067/mva.2000.110343. [DOI] [PubMed] [Google Scholar]
- 26.Bittles M., Jodeh D.S., Mayer J.L.R., Gallant M., Rottgers S.A. Laser ablation of embryonic veins in children. Pediatr Int. 2019;61:358–363. doi: 10.1111/ped.13804. [DOI] [PubMed] [Google Scholar]
- 27.Nassiri N., Crystal D., Huntress L.A., Murphy S. Transcatheter embolization of persistent embryonic veins in venous malformation syndromes. J Vasc Surg Venous Lymphat Disord. 2017;5:749–755. doi: 10.1016/j.jvsv.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Dahal S., Karmacharya R.M., Vaidya S., Gautam K., Bhatt S., Bhandari N. A rare case of persistent lateral marginal vein of Servelle in Klippel Trenaunay Syndrome: a successful surgical management. Int J Surg Case Rep. 2022;94 doi: 10.1016/j.ijscr.2022.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puig S., Aref H., Chigot V., Bonin B., Brunelle F. Classification of venous malformations in children and implications for sclerotherapy. Pediatr Radiol. 2003;33:99–103. doi: 10.1007/s00247-002-0838-9. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlin R.F., Briones M.A., Gill A.E., Hawkins C.M. Coagulopathy and related complications following sclerotherapy of congenital venous malformations. Pediatr Blood Cancer. 2022;69 doi: 10.1002/pbc.29610. [DOI] [PubMed] [Google Scholar]
- 31.Zhuo K.Y., Russell S., Wargon O., Adams S. Localised intravascular coagulation complicating venous malformations in children: associations and therapeutic options. J Paediatr Child Health. 2017;53:737–741. doi: 10.1111/jpc.13461. [DOI] [PubMed] [Google Scholar]
- 32.Leung Y.C., Leung M.W., Yam S.D., Hung J.W., Liu C.S., Chung L.Y., et al. D-dimer level correlation with treatment response in children with venous malformations. J Pediatr Surg. 2018;53:289–292. doi: 10.1016/j.jpedsurg.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Mack J.M., Crary S.E. How we approach coagulopathy with vascular anomalies. Pediatr Blood Cancer. 2022;69 doi: 10.1002/pbc.29353. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta R., Patel M. Venous malformations. Semin Pediatr Surg. 2014;23:198–202. doi: 10.1053/j.sempedsurg.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Cooke-Barber J., Kreimer S., Patel M., Dasgupta R., Jeng M. Venous malformations. Semin Pediatr Surg. 2020;29 doi: 10.1016/j.sempedsurg.2020.150976. [DOI] [PubMed] [Google Scholar]
- 36.Uller W., Hammer S., Wildgruber M., Muller-Wille R., Goessmann H., Wohlgemuth W.A. Radiofrequency ablation of the marginal venous system in patients with venous malformations. Cardiovasc Interv Radiol. 2019;42:213–219. doi: 10.1007/s00270-018-2099-5. [DOI] [PubMed] [Google Scholar]
- 37.Mack J.M., Richter G.T., Crary S.E. Effectiveness and safety of treatment with direct oral anticoagulant rivaroxaban in patients with slow-flow vascular malformations: a case series. Lymphat Res Biol. 2018;16:278–281. doi: 10.1089/lrb.2017.0029. [DOI] [PubMed] [Google Scholar]
- 38.Lee A., Driscoll D., Gloviczki P., Clay R., Shaughnessy W., Stans A. Evaluation and management of pain in patients with Klippel-Trenaunay syndrome: a review. Pediatrics. 2005;115:744–749. doi: 10.1542/peds.2004-0446. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen J.T., Koerper M.A., Hess C.P., Dowd C.F., Hoffman W.Y., Dickman M., et al. Aspirin therapy in venous malformation: a retrospective cohort study of benefits, side effects, and patient experiences. Pediatr Dermatol. 2014;31:556–560. doi: 10.1111/pde.12373. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson-Kelly A.E., Samuelson Bannow B.T. Abnormal uterine bleeding in users of rivaroxaban and apixaban. Hematology Am Soc Hematol Educ Program. 2020;2020:538–541. doi: 10.1182/hematology.2020000166. [DOI] [PMC free article] [PubMed] [Google Scholar]