Abstract
This study has used the strategy of gene replacement to characterize the contribution of the adenovirus (Ad) capsid protein hexon to serotype definition. By replacing the Ad type 5 (Ad5) hexon gene with sequences from Ad2, we have changed the type specificity of the chimeric virus. The type-determining epitopes are primarily associated with loop 1 of hexon and, to a much lesser degree, with loop 2. In spite of the serotype distinctiveness of the chimeric hexon viruses, epitope similarity between the vectors resulted in a low level of cross-reactive neutralizing antibody, which in combination with activated cellular and innate arms of the immune system is sufficient to suppress gene transduction following readministration in vivo.
Efficient infection by adenovirus (Ad) or by a replication-defective (E1−) Ad vector of a cell or target tissue is mediated by the viral capsid proteins and their interaction with the host cell (6, 26, 27). The capsid proteins are also primary targets of the immune response to Ad infection, activating innate, cellular, and humoral arms of the immune cascade. The humoral immune response to Ad infection produces both neutralizing and nonneutralizing antibody. Neutralizing antibody protects the host by suppressing viral spread and reinfection, and this neutralizing immunity blocks efficient gene transduction by Ad vectors if repeat administration is attempted (5, 13, 14).
Neutralizing antibody generated against Ad has serotype specificity. An Ad serotype, as described by the International Committee on the Taxonomy of Viruses, is defined on the basis of immunological distinctiveness determined by quantitative in vitro neutralization mediated by animal antisera (20). A “type” either has no cross-reaction with others or shows a homologous-to-heterologous titer ratio of >16 in both directions (19). Applying the knowledge that there are 49 immunologically distinct Ad serotypes, we have previously shown that repeat administration can be accomplished if two vectors based on different serotypes are used sequentially (14, 17, 18). Based on these observations, if the type-determining epitopes of the Ad capsid proteins were known, they could be altered by genetic engineering to generate serologically distinct Ads. These modified Ads could then be used as effective vectors for repeat administration. However, since there is not an absolute correlation between in vitro neutralization and in vivo protection (some studies have correlated neutralization titers with protection [4, 21], but others have not [2, 7, 16, 29, 34] altering the type-determining epitopes of Ad may not be sufficient to functionally circumvent preexisting neutralizing antibodies.
The objective of this study is to identify the type determinants of serotypes 2 and 5 and to determine if replacing the identified Ad type determinants is sufficient to allow efficient gene transduction following repeat administration. The direct test for whether a capsid component of a virus particle contains epitopes involved in generation of protective immunity is to change the capsid components one at a time and assay for changes in immune recognition. The serotype chimeras described in this paper identify the serotype determinants of Ad type 5 (Ad5) and Ad2 and demonstrate the influence of the major type-determining epitopes when used in a readministration protocol for an immunocompetent host.
The three major components of the capsid, fiber, hexon, and penton base, are targets of neutralizing antibody in vitro, but the relative contribution of each to type determination and in vivo protection is not clear. Analysis of an Ad5-Ad7 chimera demonstrated that neutralizing epitopes on the fiber protein were not significant in vivo, since exchanging the Ad5 fiber protein with the Ad7 fiber did not prevent neutralization by anti-Ad5 antibodies in vivo (11). Additionally, antihexon antibody is considered to be the dominant neutralizing antibody in response to Ad infection, while infection is inhibited only 50% by anti-penton base antibody (12, 22, 30–33); thus, the hexon protein is the most likely candidate for containing the type determinants. Analysis of Ad hexon protein primary sequences identified two variable regions which correspond to the external loops L1 and L2, and antipeptide sera to these loops can neutralize Ad in a type-specific manner; thus, it has been proposed that L1 and L2 contain the Ad type determinants (1, 3, 8, 15, 23–25, 28).
Construction of Ad hexon serotype chimeras.
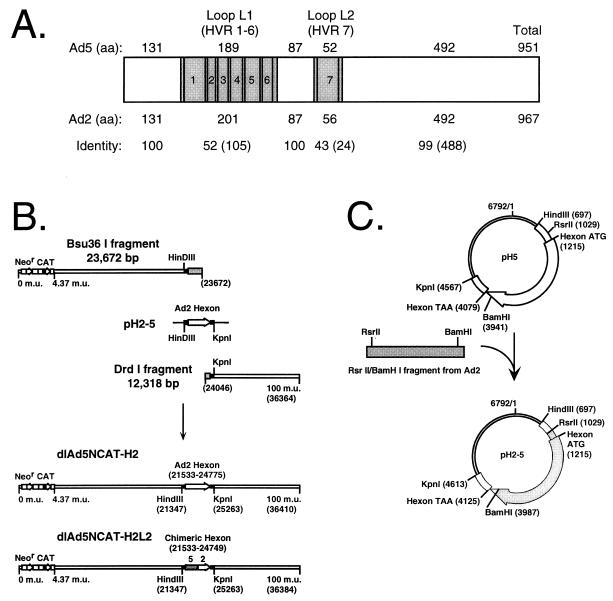
To define the type determinants present in hexon, Ad5-Ad2 hexon chimeras were constructed (Fig. 1). These two serotypes were chosen because their hexon proteins are essentially identical outside of loops L1 and L2; therefore, the serotype determinants must lie in L1 and/or L2, and exchanging their hexon proteins is equivalent to exchanging only L1 and L2 (Fig. 1A). Additionally, the hexon protein interacts with a large number of virion proteins in the capsid, and so there may be limited flexibility in the types of hexon protein changes that can be tolerated. An exchange of hexon proteins between closely related serotypes like Ad5 and Ad2 increases the likelihood of constructing viable serotype chimeras; however, it remains possible that a viable chimera can be constructed between two distantly related serotypes. Thus, in addition to Ad5-Ad2 chimeras, an attempt was made to engineer an Ad5-based, Ad7 hexon chimera. Recombinant Ads were constructed, purified, and propagated as in the work of Gall et al. (11). Our strategy for construction of a hexon serotype switch virus (Ad5 hexon > Ad2 hexon) (Fig. 1B) includes cotransfection of two viral DNAs due to the central position of the hexon gene at 51.9 map units: a left-hand end, a right-hand end, and a plasmid with the hexon replacement to bridge the gap between the left and right ends (Fig. 1C). Plasmid pH5 is a subclone of Ad5 viral DNA that extends 5′ and 3′ of coding sequence for hexon. pH2-5 was generated by direct-replacement subcloning of Ad2 hexon into pH5. The left- and right-hand-end viral fragments were generated by digestion of dlAd5NCAT with Bsu36I and DrdI, respectively (appropriate DNA fragments were isolated by sucrose gradient fractionation as in the work of Gall et al. [11]); the two subgenomic fragments cannot recombine because there is 374 bp of hexon gene sequence missing between the fragment termini. The Bsu36I fragment terminates 92 bp downstream of L2; thus, the Ad5 L1 and L2 are present in the transfections. The two subgenomic fragments and the plasmid pH2-5 (Fig. 1C) were cotransfected into HEK-293 cells, and the hexon chimera recombinant genomes of dlAd5NCAT-H2 (H2) and dlAd5NCAT-H2L2 (H2L2) were detected by modified HIRT DNA analysis and restriction enzyme digestion in a mixed lysate along with the parental dlAd5NCAT DNA (11). A combination of limiting dilution and multiple rounds of plaque purification was used to isolate the desired recombinant viruses from background. dlAd5NCAT-H2 represents the recombinant containing the entire hexon 2 region in place of Ad5. dlAd5NCAT-H2L2 (H2L2) encodes a chimeric hexon protein with the Ad5 L1 and the Ad2 L2 (Fig. 2A); the recombination junction is within a 120-bp region that straddles the border of conserved sequence and the start of L2 (data not shown). Southern blot analysis of genomic DNA from final-round plaque purification of both chimeras followed by a similar analysis of large-scale preparations was used to verify the homogeneity of the final viral stocks used in subsequent experiments (data not shown). To construct the Ad5-Ad7 hexon chimera, the two subgenomic fragments were cotransfected with pH7-5 (data not shown); however, despite multiple attempts, the recombinant virus could not be detected. That we could not identify an Ad5-Ad7 hexon recombinant could be due to limitations in our ability to generate hexon recombinants by our cotransfection strategy into 293 cells, or such a recombinant may not be viable.
FIG. 1.
Construction of the hexon chimeras dlAd5NCAT-H2 and dlAd5NCAT-H2L2 by recombination. (A) Schematic and amino acid alignment of the Ad5 and Ad2 hexon proteins showing the locations of conserved regions (open bars) and hypervariable regions (HVR; gray bars) and the numbers of amino acid residues (aa) per region. Identity is shown as percent with the number of identical residues shown in parentheses. (B) Virus construction. dlAd5NCAT DNA was digested with either Bsu36I or DrdI to generate left- and right-hand-end subgenomic fragments, respectively. The fragments were cotransfected with linearized pH2-5. Shaded boxes, Ad5 hexon sequence; black boxes, regions of homology between the plasmid and the subgenomic fragments; thin lines, plasmid sequence. (C) Plasmid constructs for introducing mutations into the Ad5 hexon gene. Plasmid pH5 contains the HindIII-KpnI fragment (map units [m.u.] 51.03 to 61.81) of Ad5.
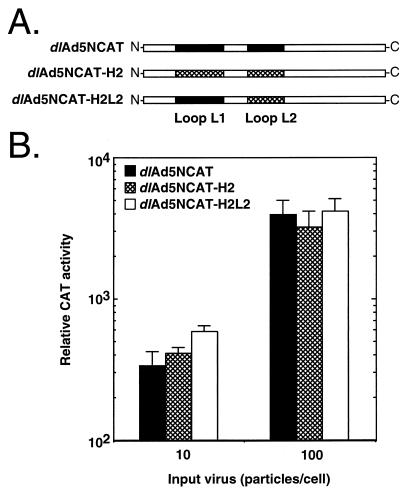
FIG. 2.
Transduction of A549 lung cells by Ad hexon chimeras. (A) Schematics of the hexon proteins encoded by dlAd5NCAT, dlAd5NCAT-H2, and dlAd5NCAT-H2L2. Open bars, conserved sequence; black bars, Ad5 unique sequence; crosshatched bars, Ad2 unique sequence. (B) CAT activity in extracts of A549 cells infected with 10 or 100 particles of dlAd5NCAT, dlAd5NCAT-H2, or dlAd5NCAT-H2L2 per cell. CAT activity is expressed as the calculated total activity in the total extract. Values are means ± standard deviations of triplicates.
The hexon chimeras H2 and H2L2 replicate as well as the parental virus dlAd5NCAT in HEK-293 cells (data not shown). The yield of virus from 4 × 108 cells is typically 2.5 × 1013 to 3.0 × 1013 particles. A relative measure of particle number to infectious units is a chloramphenicol acetyltransferase (CAT) transduction assay, in which A549 cells are infected with 10 or 100 particles of H2, H2L2, or dlAd5NCAT per cell. Under identical infection conditions, the three viruses transduce A549 cells with the CAT gene (11) with the same efficiency (Fig. 2B), despite the differing amounts of Ad2 sequences in the major capsid protein (Fig. 2A).
Hexon loops 1 and 2 contain the Ad2 and Ad5 determinants as defined by in vitro neutralization assays.
Ad serotypes have immunological distinctiveness, as determined by an in vitro assay with animal (nonhuman) antiserum. The antiserum used to define type is both highly avid and highly specific, as it is generated by multiple booster injections of the virus antigens. Sera were obtained from the American Type Culture Collection (ATCC) (Manassas, Va.), were produced by the National Institute of Allergy and Infectious Diseases, and were rated to be specific for Ad2 (VR-1079 AS/Rab) or Ad5 (VR-1082 AS/Rab). The results compiled from multiple in vitro neutralization assays with the hyperimmune sera confirmed the specificity of the sera and validated the neutralization assay employed here (11), as the experimentally determined titers for Ad2 and Ad5 matched the declared titers and specificity for their respective sera (Table 1). The two hexon chimeras, H2 and H2L2, reacted differently in the neutralization assays. Anti-Ad2 serum neutralized H2 with a titer that was within two- to fourfold of the neutralization titer of Ad2 but neutralized H2L2 very weakly, with a titer difference of 128. The results from the anti-Ad5 neutralization assays complement the anti-Ad2 results. The Ad5-specific serum neutralized the H2L2 chimera as well as Ad5, but the neutralization titer against H2 was 16- to 32-fold less than the Ad5 titer. Thus, based on the neutralization profile with the ATCC sera, H2 is distinct from Ad5 but not from Ad2, and H2L2 is distinct from Ad2 but not from Ad5.
TABLE 1.
Neutralizing antibody titersa of hyperimmune sera
| Virus | Titer of serumb
|
|||
|---|---|---|---|---|
| Anti-Ad2 | Anti-Ad5 | Anti-dlAd5NCAT-H2 | Anti-dlAd5NCAT-H2L2 | |
| Ad2 | >5,120 | 0c | 640 | 160–320 |
| Ad5 | 0c | 5,120 | 40–80 | 2,560–5,120 |
| dlAd5NCAT-H2 | 1,280–2,560 | 160–320 | 640 | 2,560 |
| dlAd5NCAT-H2L2 | 40 | 5,120 | 160–320 | 5,120 |
| dlAd5NCAT-F7 | NDd | 5,120 | 40–80 | 5,120 |
Inverse of dilution giving 50% neutralization.
Serum sources: anti-Ad2 and anti-Ad5, ATCC (National Institute for Allergy and Infectious Diseases); anti-dlAd5NCAT-H2 and anti-dlAd5NCAT-H2L2, rat (without adjuvant).
No detectable neutralization.
Nd, not determined.
The same pattern of neutralization is seen with serum generated against the chimeras. Adult Sprague-Dawley rats received three administrations of 1012 particles of cesium-chloride-gradient-purified H2 or H2L2 intraperitoneally at 2-week intervals (two animals per virus). Neither serum was specific, as they cross-reacted with all viruses tested (Table 1); however, there are meaningful differences in the titers. The homologous anti-H2 titer was surprisingly low in both sera generated, eightfold less than the anti-H2L2 homologous titer. The anti-H2 serum neutralization titer against Ad2 matched the homologous titer; likewise, anti-H2L2 serum neutralized Ad5 to the same extent as it did H2L2. Anti-H2 sera neutralized Ad5 weakly, but the low homologous titer prevents the assignment of distinctiveness, since the homologous-to-heterologous ratio is 8/16. The anti-H2L2 sera distinguished Ad5 and Ad2, with a titer difference of 16 to 32 (H2L2/Ad2 ratio). When the sera generated against the chimeras were tested against the heterologous chimera, there was a greater degree of cross-neutralization than that seen with Ad2 and Ad5. In both cases, anti-H2 versus anti-H2L2 and anti-H2L2 versus anti-H2, the titers were only about twofold less than the homologous titers. Thus, it would appear that the hexon serotype chimeras are presenting neutralizing epitopes that are not on Ad2 or Ad5 or that different epitopes are immunodominant in the chimeras. It is clear that L1 is more important in neutralization than L2, since the serotype source of L1 determined type (Table 1, dlAd5NCAT-H2 and dlAd5NCAT-H2L2 versus anti-Ad2 and anti-Ad5). However, since loop L2 conferred cross-neutralization, it also contains significant neutralization epitopes. The cross-neutralization of Ad5 by anti-H2 serum raised the possibility that the Ad5 fiber protein was the target of neutralizing antibody in hyperimmune serum. However, the neutralization titer of this serum for dlAd5NCAT-F7 was identical to that for Ad5, even though the fiber proteins are different, lending support to the conclusion that the fiber protein does not contain type-determining epitopes.
In vitro neutralization of Ad hexon protein chimeras correlates with in vivo protection.
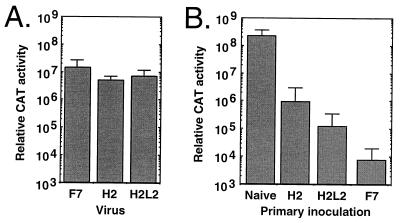
The practical significance of changing the type determinants of a vector would be to allow readministration of a gene to a host with circulating neutralizing antibodies. To determine the contribution of hexon loops to in vivo protection, a sequential administration protocol was performed with adult Sprague-Dawley rats. Groups of rats received either dlAd5NCAT-H2, dlAd5NCAT-H2L2, or dlAd5NCAT-F7 (1012 particles per animal) via the jugular vein (9). At 3 days postinfection, the CAT activities in liver extracts from rats infected with the three viruses were identical, indicating that the injections were effective at delivering consistent doses of Ad vectors systemically to rats (Fig. 3A). Groups of rats (n = 5/group) that received either H2, H2L2, or F7 were reinjected 28 days postinfection with 1012 particles of dlAd5NCAT via the jugular vein and sacrificed 24 h later, and liver extracts were prepared for CAT activity assays. Based on the in vitro neutralization results (Table 1), we would predict the level of CAT expressed from dlAd5NCAT in the animals immunized with the three viruses to be dlAd5NCAT-H2 >> dlAd5NCAT-H2L2 > dlAd5NCAT-F7. The apparent experimental results (Fig. 3B) agree with the prediction, but more importantly, the level of CAT gene expression was significantly reduced in all groups immunized with the Ad variants compared with the naive group (Fig. 3B) (P < 0.05). The analysis of CAT expression levels indicates that the ability to readminister in this strategy and generate high levels of CAT activity is severely compromised even with the dlAd5NCAT-H2 chimera, which by definition is a distinct serotype. Serum taken from the animals used in Fig. 3B before administration of dlAd5NCAT was examined for the presence of circulating neutralizing antibodies by the in vitro neutralization assay. Anti-Ad5 neutralization titers of all the tested sera were low (dlAd5NCAT-H2 = 110 ± 114, dlAd5NCAT H2L2 = 400 ± 113, and dlAd5NCAT-F7 = 293 ± 167), since the antisera were generated in response to a single Ad vector exposure.
FIG. 3.
Effect of immunization with Ad hexon or fiber chimeras on the in vivo transduction efficiency of dlAd5NCAT. (A) Evaluation of the efficacy of intravenous administration. Adult female Sprague-Dawley rats were administered 1012 particles of dlAd5NCAT-H2, dlAd5NCAT-H2L2, or dlAd5NCAT-F7 via the jugular vein, and the livers were assayed for CAT activity 3 days later. Data are the means of triplicates ± standard deviations. (B) Readministration. Adult female Sprague-Dawley rats were administered 1012 particles of dlAd5NCAT-H2, dlAd5NCAT-H2L2, or dlAd5NCAT-F7 via the jugular vein. At 28 days postinfection, the same animals received 1012 particles of dlAd5NCAT via the jugular vein and were sacrificed 24 h later, and their livers were processed for CAT activity assays. Values shown are a compilation of two independent experiments and are the means ± 1 standard deviation. Abbreviations: F7, dlAd5NCAT-F7; H2, dlAd5NCAT-H2; H2L2, dlAd5NCAT-H2L2.
Exchanging the Ad5 hexon protein with the Ad2 hexon protein did not allow readministration despite the determination in vitro that Ad5 and the chimera are immunologically distinct. The cross-neutralization of Ad5 (dlAd5NCAT) by the anti-chimera H2L2 sera was predictive of in vivo protection, but more surprisingly, the low level of cross-neutralization of Ad5 by dlAd5NCAT-H2 was also effective in significantly blocking efficient CAT expression in the repeat administration protocol: weak anti-Ad5 neutralization titers in rats immunized with 1012 particles of either H2 or H2L2 were sufficient to compromise efficient readministration. Thus, under the present experimental conditions, the definition of type by the quantitative neutralization assay does not correlate with immunological distinctiveness in vivo. The neutralizing epitopes that are responsible for cross-reactivity between H2 and Ad5 are not known at this time. There are at least two possibilities, however: there could be epitopes on another capsid protein (although not the fiber protein), or epitopes may become more antigenic or immunodominant in the context of the Ad2-Ad5 chimeric capsid.
We have used the strategy of capsid gene replacement combined with the CAT reporter gene in replication-defective viruses as a method to better understand the biology of the viral capsid in virus entry as well as the host response to the major capsid proteins. Using our strategy of complete gene replacement, we have found that complete complementation of fiber can occur across subgroup divisions. We have found that hexon may be more restricted in a replacement strategy. The construction of H2 demonstrates the functional conservation of the Ad5 and Ad2 hexons (subgroup C viruses), since the chimera grows to the same titer and transduces cells with the same efficiency as Ad5. However, our inability to isolate an Ad7 hexon chimera indicates less efficient complementation when we are expanding the evolutionary distance between viruses used in chimera constructions.
The possibility exists that changing the hexon protein of Ad5 to that of a more divergent serotype than Ad2 may result in a greater degree of immunological distinctiveness than was observed with Ad5 and Ad2. Since attempts to construct an Ad5-Ad7 hexon chimera were unsuccessful, perhaps due to incompatibilities of the Ad7a hexon protein with Ad5 proteins such as the 100K protein or any of the four capsid proteins with which hexon interacts, an alternative strategy to exchanging the whole hexon protein is to replace only the external loops. This would conserve the essential intercapsomere interactions required of the hexon trimer and yet introduce variable regions that have already been selected for having distinct epitopes. Since L1 and L2 make contacts with loop L4 to form the tower region of the hexon trimer (35), it may be necessary to change all three loops (even though L4 is relatively conserved) (3). However, the results presented here imply that another capsid protein, perhaps penton base, may have significant neutralization epitopes that are targets for protective antibody in vivo. The use of highly specific hyperimmune serum like the anti-Ad5 and anti-Ad2 sera from the ATCC in in vitro neutralization assays should be adequate to predict in vivo protection with the model system used here, with the caveat that any cross-neutralization can be indicative of cross-protection in vivo regardless of the homologous-to-heterologous titer ratios.
The in vivo model system that we have chosen in this study, systemic administration of the vector by injection into the vasculature and analysis of reporter gene expression in the liver, may not be predictive for other model systems. It is likely, for example, that mucosal immunity to Ad vectors administered to the respiratory tract is different from the immune response to vectors administered via the vasculature, and so one must be cautious in extrapolating from the animal model system to not only human gene therapy but also other animal models. An additional complication of the in vivo model is the implication that limited expression of the CAT transgene can be influenced only by the humoral arm of the immune system. Prior stimulation of the innate immune system or the activation of cellular immunity against a dlAd5NCAT-based vector may also be contributing to the repression of CAT gene expression in these studies. The route of administration and the target organ must be considered as important determinants of transduction efficiency and the nature of the immune response (13). For example, a recent study of human sera from patients who received an intratumoral injection of an Ad reporter vector implicated penton base and fiber as targets of the humoral immune response (10).
Acknowledgments
This work was supported by a sponsored research grant from GenVec Inc. to E.F.-P. and by grant PO1 HL51746 to E.F.-P. and R.G.C. R.G.C. receives sponsored research support from GenVec Inc.
REFERENCES
- 1.Athappilly F K, Murali R, Rux J J, Cai Z, Burnett R M. The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution. J Mol Biol. 1994;242:430–455. doi: 10.1006/jmbi.1994.1593. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 3.Crawford-Miksza L, Schnurr D P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cybinski D H, Walker P J, Byrne K A, Zakrzewski H. Mapping of antigenic sites on the bovine ephemeral fever virus glycoprotein using monoclonal antibodies. J Gen Virol. 1990;71:2065–2072. doi: 10.1099/0022-1317-71-9-2065. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX and vector antigens allows for long term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defer C, Belin M-T, Caillet-Boudin M-L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietzschold B, Kao M, Zheng Y M, Chen Z Y, Maul G, Fu Z F, Rupprecht C E, Koprowski H. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci USA. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiz B, Adrian T, Pring-Akerblom P. Immunological adenovirus variant strains of subgenus D: comparison of the hexon and fiber sequences. Virology. 1995;213:313–320. doi: 10.1006/viro.1995.0004. [DOI] [PubMed] [Google Scholar]
- 9.Elkon K B, Liu C-C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Song X, Old L J, Crystal R G, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahery-Segard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet J-G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase A T, Pereira H G. The purification of adenovirus neutralizing antibody: adenovirus type 5 hexon immunoadsorbent. J Immunol. 1972;108:633–636. [PubMed] [Google Scholar]
- 13.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 14.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 15.Kinloch R, Mackay N, Mautner V. Adenovirus hexon. Sequence comparison of subgroup C serotypes 2 and 5. J Biol Chem. 1984;259:6431–6436. [PubMed] [Google Scholar]
- 16.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of Alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 17.Mack C A, Song W-R, Carpenter H, Wickham T J, Kovesdi I, Harvey B-G, Magovern C J, Isom O W, Rosengart T, Falck-Pedersen E, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 18.Mastrangeli A, Harvey B-G, Yao J, Wolff G, Kovesdi I, Crystal R G, Falck-Pedersen E. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of antiadenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 19.Matthews R E F. Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17:1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- 20.Summers M D, editor. Virus taxonomy. Sixth report of the International Committee on Taxonomy of Viruses. Arch Virol Suppl. 1995;10:1–586. [PubMed] [Google Scholar]
- 21.Pay T W, Hingley P J. Correlation of a 140S antigen dose with the serum neutralising antibody response and level of protection induced in cattle by FMD vaccines. Vaccine. 1987;5:60–70. doi: 10.1016/0264-410x(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 22.Pettersson U. Structural proteins of adenoviruses. VI. On the antigenic determinants of the hexon. Virology. 1971;43:123–136. doi: 10.1016/0042-6822(71)90230-3. [DOI] [PubMed] [Google Scholar]
- 23.Pring-Akerblom P, Adrian T. The hexon genes of adenoviruses of subgenus C: comparison of the variable regions. Res Virol. 1993;144:117–127. doi: 10.1016/s0923-2516(06)80020-8. [DOI] [PubMed] [Google Scholar]
- 24.Pring-Akerblom P, Trijssenaar F E, Adrian T. Sequence characterization and comparison of human adenovirus subgenus B and E hexons. Virology. 1995;212:232–236. doi: 10.1006/viro.1995.1474. [DOI] [PubMed] [Google Scholar]
- 25.Roberts M M, White J L, Grutter M G, Burnett R M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986;232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- 26.Svensson U, Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984;51:687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svensson U, Persson R, Eviritt E. Virus-receptor interaction in the adenovirus system. I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981;38:70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toogood C I, Crompton J, Hay R T. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73:1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 29.Tyler K L, Mann M A, Fields B N, Virgin H W., IV Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J Virol. 1993;67:3446–3453. doi: 10.1128/jvi.67.6.3446-3453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox N, Mautner V. Antigenic determinants of adenovirus capsids. II. Homogeneity of hexons, and accessibility of their determinants, in the virion. J Immunol. 1976;116:25–29. [PubMed] [Google Scholar]
- 32.Wilcox W C, Ginsberg H S. Production of specific neutralizing antibody with soluble antigens of type 5 adenovirus. Proc Soc Exp Biol Med. 1963;114:37–42. doi: 10.3181/00379727-114-28579. [DOI] [PubMed] [Google Scholar]
- 33.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright K W, Buchmeier M J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991;65:3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia D, Henry L J, Gerard R D, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994;2:1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]