Abstract
Expression of the NSP1 protein of Semliki Forest virus and Sindbis virus in cultured cells induced filopodia-like extensions containing NSP1 but not F actin. The actin stress fibers disappeared, whereas vimentin, keratin, and tubulin networks remained intact. The effects of NSP1 were dependent on its palmitoylation but not on its enzymatic activities and were also observed in virus-infected cells.
The RNA replication of alphaviruses such as Semliki Forest virus (SFV) and Sindbis virus (SIN) is directed by virus-specific nonstructural proteins (NSP1 to -4) and takes place in the cytoplasm of infected cells on modified endosomes and lysosomes (5, 17). The functions of the individual NSPs have been studied extensively (27). NSP1 (537 and 540 amino acids in SFV and SIN, respectively) is an mRNA capping enzyme with guanine-7-methyltransferase (MT) (11, 15) and guanylyltransferase (GT) activities (1, 2). Our recent study showed that SFV NSP1 was tightly associated with membranes due to the palmitoylation of cysteine residues 418 to 420. Interestingly, acylation also affected the subcellular distribution of NSP1, since only the palmitoyolated NSP1 was associated with the surface extensions of HeLa cells (10), which expressed NSP1 from the plasmid pTSF1 (18) by the aid of vaccinia virus vector vTF7-3 (6, 26).
Induction of filopodia by expression of SFV and SIN NSP1.
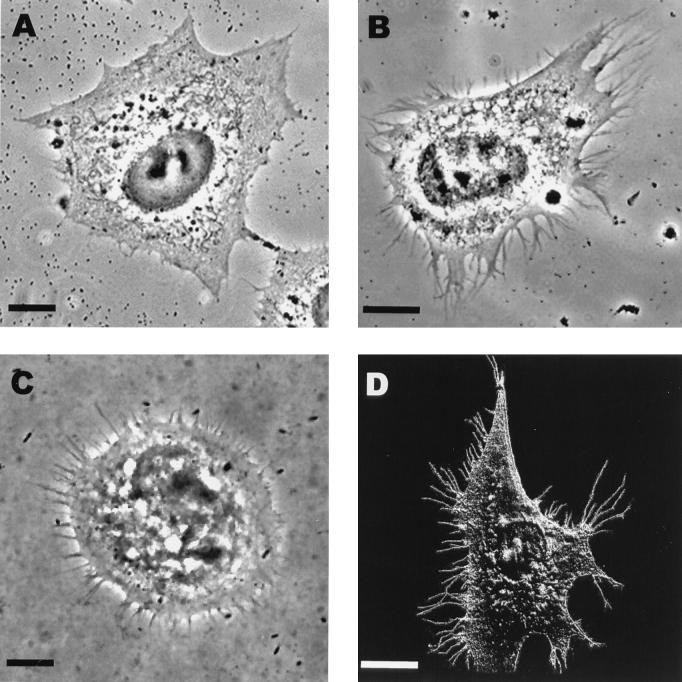
To study this phenomenon further, we expressed the acylated and unacylated forms of NSP1 of SIN. The gene coding for NSP1 was obtained from the infectious cDNA clone pToto1101 (19) by PCR, sequenced, and placed under the T7 promoter in pGEM3 to yield pTSIN1. HeLa cells were first infected with vTF7-3 for 45 min, followed by transfection with plasmid pTSF1 or pTSIN1 with the Lipofectin reagent (GIBCO, BRL) (10, 18) to express NSP1 of SFV or SIN, respectively. Hydroxyurea (10 mM) (Sigma) was applied to the cells simultaneously with Lipofectin to inhibit vaccinia virus DNA replication (3, 30). The cells were examined at 3 h posttransfection by using phase-contrast optics. Mock-infected (not shown) and vTF7-3-infected (Fig. 1A) cells served as controls. The cells expressing SFV (Fig. 1B) or SIN (Fig. 1C) NSP1 showed induction of a large number of filopodia-like structures, which hereafter are designated filopodia. These extensions often had a thick root and were branched at their distal parts to thin microspikes or filopodia. Similar structures were also observed in SFV- and SIN-infected cells, as demonstrated for SFV by scanning electron microscopy, which was stabilized as described elsewhere (24, 25) (Fig. 1D).
FIG. 1.
Phase-contrast images of HeLa cells after 3 h of incubation in the transfection medium. Shown are cells infected with vaccinia virus vTF7-3 for 45 min followed by incubation in transfection medium (A) and cells infected first with vTF7-3 for 45 min followed by transfection with plasmid pTSF1 encoding SFV NSP1 (B) or with pSIN1 encoding SIN NSP1 (C). Also shown is a scanning electron micrograph of an SFV-infected BHK cell at 4 h postinfection (D). Bars, 10 μm.
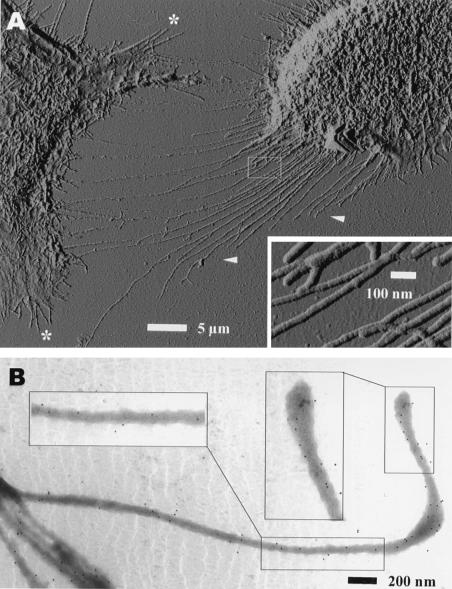
Tunneling microscopy revealed that the diameter of the filopodia was fairly uniform (about 50 nm [Fig. 2A]) and that many of them were branched (Fig. 2A and insert). Whole-mount immunotransmission electron microscopy of saponin (0.5%)-permeabilized cells carried out as described elsewhere (24, 25) revealed that NSP1 was present along the whole length of the filopodia (Fig. 2B).
FIG. 2.
Filopodia contain NSP1 along their whole length. HeLa cells infected with vTF7-3 and transfected with pTSF1 as described in the legend to Fig. 1 were processed at 3 h posttransfection for tunneling microscopy (A) or for whole-mount immunotransmission electron microscopy (B). For immunotransmission electron microscopy, treatment with anti-NSP1 was followed by the addition of protein A-gold particles. Arrowheads, retraction fibers; asterisks, filopodia.
Reorganization of F actin in cells expressing NSP1.
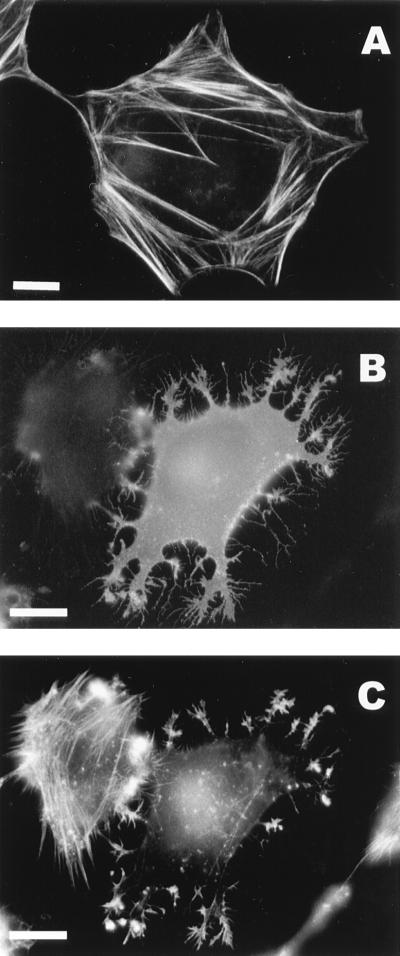
Since the formation of filopodia in normal cells is driven by the extension of F actin filaments, we labeled pTSF1-transfected cells with fluorescent phalloidin to visualize F actin. In mock-infected cells (Fig. 3A), phalloidin decorated the actin stress fibers, whereas no stress fibers were detectable in NSP1-expressing cells (Fig. 3C). Instead, F actin was brightly stained in the cell periphery and in the more thickly branched roots of the cellular extensions (Fig. 3C). However, double staining with anti-NSP1 and phalloidin revealed that the narrow filopodia-like branches did not contain detectable F actin filaments (compare Fig. 3B and C). Filopodia were also induced in BHK and PC12 cells and in the human fibrosarcoma cell line HT1080, all expressing SFV NSP1 (not shown).
FIG. 3.
Expression of NSP1 causes destruction of actin stress fibers. (A) Oregon-green-conjugated phalloidin staining of stress fibers in a control HeLa cell. (B and C) Lack of stress fibers in a HeLa cell expressing NSP1 3 h after transfection with pTSF1; staining with anti-NSP1 antibodies (B) and Oregon-green phalloidin staining of the same cell (C). Note that stress fibers are present in a neighboring cell, which does not express NSP1. Bars, 10 μm.
We have previously identified critical amino acid residues in SFV NSP1, which are required for both MT and GT activities (e.g., D64 and D90), only for GT activity (H38) (2) or for palmitoylation (C418-420) (10). Moreover, removal of amino acid residues 121 to 148 destroyed both the MT and the GT activities and inhibited acylation and membrane association of this truncated NSP1 derivative (10). To study the effects of these mutations on the induction of filopodia and disappearance of stress fibers, the mutated NSP1 derivatives were expressed with the vaccinia virus vTF7-3 system (6). Table 1 shows that neither MT nor GT activity was required for the induction of filopodia and disassembly of actin stress fibers. However, the acylation of NSP1 of both SFV and SIN was necessary to cause these alterations.
TABLE 1.
Correlation of actin redistribution, palmitoylation, and enzymatic activities of NSP1 in HeLa cells expressing wild-type or mutant SFV or SIN NSP1 proteins
| Type of protein expressed | MT activity (11)a | GT activity (1)a | Palmitoyl-ationa | Stress fibers | Induction of filopodia |
|---|---|---|---|---|---|
| SFV NSP1 (wild type) | + | + | + | − | + |
| SFV C418-420A | + | + | − | + | − |
| SFV H38A | ++ | − | + | − | + |
| SFV D64A | − | − | + | − | + |
| SFV D90A | − | − | + | − | + |
| SFV Δ121-146 | − | − | − | + | − |
| SIN NSP1 (wild type) | + | + | + | − | + |
| SIN C420A | + | + | − | + | − |
NSP1-induced extensions differ from normal filopodia.
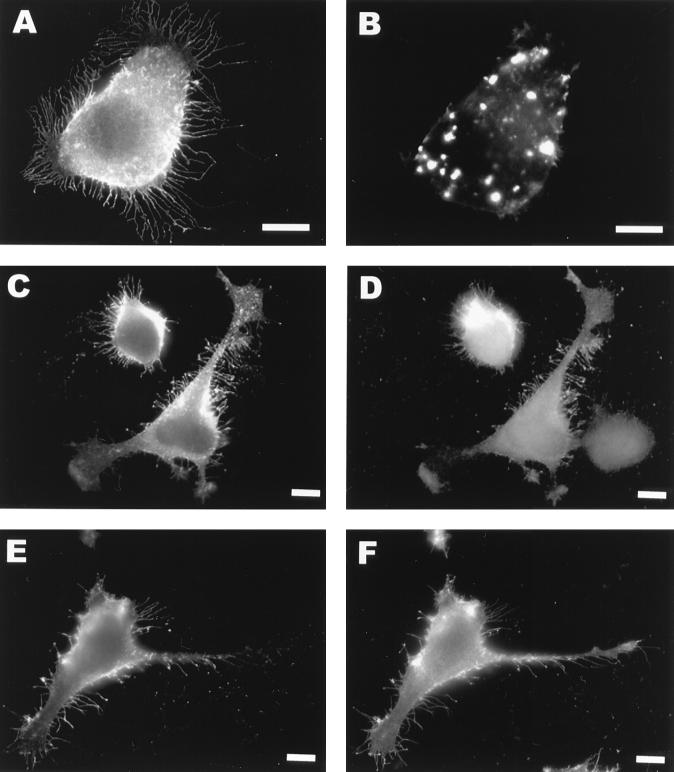
Cytochalasin D (CD) inhibits the polymerization of actin filaments, resulting in the disappearance of actin stress fibers and in the appearance of actin aggregates in the cytoplasm (Fig. 4B). When NSP1-expressing cells were treated with CD (0.1 μg/ml), numerous filopodia labeled with anti-nsP1 antiserum were observed around the cell periphery (Fig. 4A), indicating that F actin was not needed for their formation. The thicker surface extensions, which sometimes resembled neuronal growth cones (e.g., Fig. 3C), were never seen in CD-treated cells, suggesting that F actin had participated in their formation. In addition, no evidence for the presence of tubulin, vimentin, or keratin filaments in the filopodia of NSP1-expressing HeLa cells was obtained by staining with the respective antibodies (not shown). Addition of 0.1 or 0.5 mM nocodazole (12) or 50 μM vinblastine from 1 to 3 h posttransfection to disrupt microtubules did not inhibit the formation of filopodia, indicating that the integrity of microtubular and intermediate filament networks was not necessary for their formation (not shown).
FIG. 4.
CD (0.1 μg/ml) was added at 1 h posttransfection to HeLa cells infected with vTF7-3 and transfected with pTSF1 1 h after transfection. (A and B) HeLa cell stained with anti-NSP1 (A) and phalloidin (B) of the same cell. (C through F) Double-immunofluorescence staining of HeLa cells 3 h after transfection with pTSF1 stained with anti-NSP1 (C and E), anti-ezrin antibodies (D), and anti-CD44 antibodies (F). Bars, 10 μm.
Paxillin and vinculin, which are typical components of the focal adhesions for F actin bundles (4), were distributed similarly at the cell periphery both in mock- and pTSF1-transfected cells, suggesting that focal adhesions were not affected by NSP1 (not shown). Nor was β1-integrin, which normally mediates the contact of filopodia with the external matrix at the focal contact points (31), found in the filopodia (not shown). Instead, both the transmembrane protein CD44 (8) and the intracellularly located peripheral plasma membrane protein ezrin (29) colocalized with NSP1 in the filopodial extensions (Fig. 4C to F).
According to the present study, alphavirus NSP1 causes disassembly of the actin cytoskeleton when it is expressed in several different cell lines. In addition, it induces numerous surface extensions which stain positively with anti-NSP1 antibodies and morphologically resemble filopodia but lack, e.g., F actin. Only the acylated, tightly membrane-associated form of NSP1 causes these effects. The same phenomena take place in virus-infected cells, indicating that they are natural responses for alphavirus infection. Expression vectors which lack the genes for structural proteins have been developed both for SFV and SIN (13, 23). Since these vectors express the RNA replicase proteins, including NSP1, they also cause induction of filopodia and disruption of stress fibers, a fact which has to be taken into account when analyzing the effects of the expressed foreign proteins.
Analysis of interactions of viral proteins with host proteins has expanded our knowledge of many central cellular processes, such as DNA replication, transcriptional control, regulation of translation, nuclear transport, signal transduction, and apoptotic cell death, as well as folding, modification, transport, and targeting of exocytic membrane proteins (9). The present results for alphavirus NSP1 offer the possibility of studying its intervention with the delicately controlled and complex regulation of the actin cytoskeleton (7, 14, 16, 20–22, 28, 32). Understanding of the mechanism of action of NSP1 should reveal new aspects in this regulatory cascade, which controls the shape and movements of the cell.
Acknowledgments
We thank Marja Makarow for critical reading of the manuscript and Tarja Välimäki and Airi Sinkko for technical assistance. We thank Sirpa Jalkanen, Eero Lehtonen, Ismo Virtanen, and Jari Ylänne for antibodies. Charles M. Rice is acknowledged for the full-length SIN infectious cDNA clone pToto1101 and Tero Ahola for the SIN NSP1 constructs.
This work has been supported by Academy of Finland, Biocentrum Helsinki, and the Helsinki University Foundation.
REFERENCES
- 1.Ahola T, Kääriäinen L. Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci USA. 1995;92:507–511. doi: 10.1073/pnas.92.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahola T, Laakkonen P, Vihinen H, Kääriäinen L. Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities. J Virol. 1997;71:392–397. doi: 10.1128/jvi.71.1.392-397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucci C, Parton R G, Mather I H, Stunnenberg H G, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 4.Burridge K, Chrzanowska-Wodnicka M, Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- 5.Froshauer S, Kartenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988;107:2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 8.Jalkanen S T, Bargatze R F, Herron L R, Butcher E C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986;16:1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- 9.Knipe D M. Virus-host cell interactions. In: Fields B N, Knipe D M, Howley P M, et al., editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 273–299. [Google Scholar]
- 10.Laakkonen P, Ahola T, Kääriäinen L. The effects of palmitoylation on membrane association of Semliki Forest virus RNA capping enzyme. J Biol Chem. 1996;271:28567–28571. doi: 10.1074/jbc.271.45.28567. [DOI] [PubMed] [Google Scholar]
- 11.Laakkonen P, Hyvönen M, Peränen J, Kääriäinen L. Expression of Semliki Forest virus nsP1-specific methyltransferase in insect cells and in Escherichia coli. J Virol. 1994;68:7418–7425. doi: 10.1128/jvi.68.11.7418-7425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao G, Nagasaki T, Gundersen G G. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- 13.Liljeström P, Lusa S, Huylebroeck D, Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991;65:4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machesky L M, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- 15.Mi S, Stollar V. Expression of Sindbis virus NSP1 and methyltransferase activity in Escherichia coli. Virology. 1991;184:423–427. doi: 10.1016/0042-6822(91)90862-6. [DOI] [PubMed] [Google Scholar]
- 16.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 17.Peränen J, Kääriäinen L. Biogenesis of type I cytopathic vacuoles in Semliki Forest virus-infected BHK cells. J Virol. 1991;65:1623–1627. doi: 10.1128/jvi.65.3.1623-1627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peränen J, Laakkonen P, Hyvönen M, Kääriäinen L. The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles. Virology. 1995;208:610–620. doi: 10.1006/viro.1995.1192. [DOI] [PubMed] [Google Scholar]
- 19.Rice C M, Levis R, Strauss J H, Huang H V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridley A J, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 22.Schafer D A, Cooper J A. Control of actin assembly at filament ends. Annu Rev Cell Dev Biol. 1995;11:497–518. doi: 10.1146/annurev.cb.11.110195.002433. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger S. Alphaviruses—vectors for the expression of heterologous genes. Trends Biotechnol. 1993;11:18–22. doi: 10.1016/0167-7799(93)90070-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer R H, Langevin G L, Lawrence J B. Ultrastructural visualization of cytoskeletal mRNAs and their associated proteins using double-label in situ hybridization. J Cell Biol. 1989;108:2342–2353. doi: 10.1083/jcb.108.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sormunen R. α-Spectin in detergent-extracted whole-mount cytoskeletons of chicken embryo heart fibroblasts. Histochem J. 1997;25:678–686. doi: 10.1007/BF00157882. [DOI] [PubMed] [Google Scholar]
- 26.Stenmark H, Bucci C, Zerial M. Expression of Rab GTPases using recombinant vaccinia viruses. Methods Enzymol. 1995;257:155–164. doi: 10.1016/s0076-6879(95)57021-7. [DOI] [PubMed] [Google Scholar]
- 27.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaheri A, Carpén O, Heiska L, Helander T S, Jääskeläinen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O. The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Opin Cell Biol. 1997;9:659–666. doi: 10.1016/s0955-0674(97)80119-6. [DOI] [PubMed] [Google Scholar]
- 30.Vos J C, Stunnenberg H G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988;7:3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ylänne J, Cheresh D A, Virtanen I. Localization of beta 1, beta 3, alpha 5, alpha v, and alpha lib subunits of the integrin family in spreading human erythroleukemia cell. Blood. 1990;76:570–577. [PubMed] [Google Scholar]
- 32.Zigmond S H. Signal transduction and actin filament organization. Curr Opin Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]