Abstract
Huntington's disease (HD) usually manifests in adulthood and is characterised by progressive neurodegeneration in the brain that causes worsening involuntary movements, mental health and cognition over many years. Depression, anxiety and apathy are common. HD is autosomal dominant and affects about 1 in 8,000 people in the UK. There are currently no disease-modifying treatments and so patient care centres on multidisciplinary therapy support and medical treatments to relieve distressing symptoms. Progression of HD is usually slow, and so acute deteriorations often indicate another problem, such as intercurrent infections, constipation, urinary retention, gastro-oesophageal reflux disease or poor dentition. In this review we outline common presentations in HD patients, both acute and chronic, consider therapeutic options and discuss specific considerations in advanced HD.
Introduction
Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder characterised clinically by progressive deterioration of movement control, mental health and cognition.1 Symptoms typically start in middle-age and progress relentlessly over the next 10–20 years, causing considerable morbidity. HD often leads to early loss of employment, reduced motivation and disordered behaviours. Over time, individuals lose the ability to function in daily life and become unable to care for themselves. HD reduces life-expectancy by an average of 13.3 years2 and there is currently no disease-modifying treatment. Current standard-of-care involves treatment of symptoms alongside multi-disciplinary support involving physicians, specialist nurses, therapists, clinical psychologists and dieticians to maintain independence and quality of life for as long as possible. Although HD is rare it has a prevalence of ∼1 in 8,000 in the UK3 making it likely that most physicians and general practitioners will need to manage HD patients during their careers, particularly given the high medical needs of this patient group. In this review we summarise aspects of the presentation, diagnosis and management of HD of most relevance to acute and general physicians.
Genetic testing
HD is caused by an expanded repeat of at least 36 CAGs in one copy of the Huntingtin (HTT) gene.4 In unaffected individuals the tract has ∼18–20 CAGs (range 6–355). Longer repeat lengths are associated with earlier onset and more severe disease, with CAG > 60 usually causing juvenile-onset HD before the age of 20.6 Genetic testing for HD, using a simple blood test to measure CAG length, is broadly carried out in two scenarios:
-
1)
Predictive testing in asymptomatic individuals at risk of developing HD
Asymptomatic adults with a family history of HD can request predictive testing to determine if they carry the HD repeat expansion. Each child of an affected individual has a 50% chance of inheriting the HD mutation. In the UK, predictive testing is managed by Clinical Genetics services and usually involves multiple consultations to explore an individual's understanding of the test, as well as their support networks and strategies to cope with a positive test result. It is important to be aware that a positive predictive test for an individual can have implications for other family members who might have chosen not to undertake predictive testing. At present under 20% of asymptomatic at-risk individuals request a predictive test, principally because of the lack of a disease-modifying treatment.7 In addition, insurance companies are permitted to ask for disclosure of predictive HD test results if more than £500,000 of cover is requested. Predictive testing is not recommended for those under 18.
-
2)
Diagnostic testing in individuals with symptoms/signs that could be HD
In individuals with symptoms and/or signs consistent with HD, most commonly chorea, a diagnostic HD genetic test might be offered. Mostly there will be a family history of HD, but ∼10% of positive diagnostic tests are apparently ‘de novo’. Often an absence of family history will be due to adoption or estrangement, or misdiagnosis in earlier generations. For example, for an individual presenting with chorea, a strong family history of severe mental illness, institutionalisation, alcohol or drug dependence, ‘dementia’ or ‘Parkinson's disease’ can be suspicious for HD. Occasionally patients’ relatives have been diagnosed with ‘HD’ prior to the era of genetic testing. In this scenario HD genetic testing is useful as there are rare phenocopies of HD caused by other repeat expansions in genes such as C9ORF72, TBP and ATN1. Diagnostic HD testing in symptomatic individuals requires appropriate consent for a genetic test but not the same degree of counselling as for predictive testing. It is usually carried out by neurologists, clinical geneticists or psychiatrists.
Presentation and investigation of HD
We will focus this primer on confirmed HD gene carriers. There are currently no NICE guidelines for investigation and management of HD and so recommendations are based on published expert consensus opinions.
Presentation
HD affects many aspects of movement control, mental health, behaviour and cognition over its course (Table 1). Involuntary, unsuppressible choreiform (‘dance-like’) movements are characteristic of, but not specific to, HD and found in almost all patients at some stage. They usually start insidiously, fluctuate diurnally (reducing at night) and day-to-day, and can affect all muscle groups including those of the face, trunk and limbs. Chorea tends to progress slowly and the patient is often less troubled by the movements than care-givers. Other motor problems that can develop include dystonia, rigidity and incoordination, and these contribute to a significant falls risk that increases as the disease progresses. Later in the disease course, eye movements, speech and swallowing can all be affected leading to problems with communication and nutrition, and risk of aspiration. Reduced food intake and the catabolic state seen in HD lead to weight loss which also contributes to frailty and impaired physical abilities.
Table 1.
Common clinical manifestations of Huntington's disease.
| Domain | Clinical manifestation | Specific symptoms/signs commonly seen in HD | Medications that may be useful |
|---|---|---|---|
| Motor | Involuntary movements | Chorea | Tetrabenazine, 2nd generation anti-psychotics |
| Dystonia | Botulinum toxin | ||
| Bulbar dysfunction | Dysarthria | ||
| Dysphagia | |||
| Reduced balance and coordination | Eye movement problems | ||
| Rigidity | |||
| Ataxia | |||
| Psychiatric | Depression | Blunted affect | SSRI, SNRI |
| Suicidal ideation | SSRI, SNRI | ||
| Anxiety disorders | Constant worry, nervousness | SSRI | |
| Irritability (short fuse) | SSRI (higher doses), | ||
| Obsessive/compulsive phenomena | SSRI | ||
| Psychosis | Delusions, paranoia | 2nd generation anti-psychotics | |
| Hallucinations | 2nd generation anti-psychotics | ||
| Behavioural | Apathy | Reduced motivation | |
| Social withdrawal | |||
| Perseveration | Repetitive behaviours with little insight e.g. frequent toileting, hypersexual behaviours | SSRI, 2nd generation anti-psychotics | |
| Anger | Aggressive behaviours | 2nd generation anti-psychotics | |
| Cognitive | Inattention | Reduced concentration | |
| Executive dysfunction | Slowed thinking, impaired planning | ||
| Poor memory | Disorientation |
Symptoms and signs that are frequently encountered in individuals with HD are divided across four major domains. Most patients will experience symptoms from each domain across their disease course, although exact combinations vary by individual. Medications that can be helpful are indicated, although there is very little clinical trial evidence to base decisions on. Key: SSRI, Serotonin-Selective Reuptake Inhibitor (SSRI); SNRI, Serotonin-Noradrenaline Reuptake Inhibitor (SNRI).
HD also causes significant psychiatric, behavioural and cognitive problems that directly affect an individual's ability to hold down a job, manage their finances, maintain close relationships and, ultimately, care for themselves. In over 40% of gene carriers these symptoms will predate any motor symptoms of HD.5 A mixture of neuropsychiatric symptoms is commonly reported by care-givers, with apathy, loss of motivation and perseverative thoughts and actions prominent. Depression, anxiety, irritability, aggressive outbursts and obsessive/compulsive thoughts and behaviours are also regularly observed, all exacerbated by disrupted sleep patterns. Less frequently, hallucinations and delusional behaviours are reported. Rates of substance misuse, self-harm and suicide are higher in the HD population than in the general population and rates of addiction often increase with disease progression.8
Examination
Cranial nerve examination can highlight abnormal eye movements: jerky or incomplete ocular pursuit and/or slowed saccades. Dysarthria might be present as well as an inability to keep the tongue fully protruded. This ‘motor impersistence’ is a sign of chorea and may also be elicited through fluctuating grip strength when asked to grip the examiner's fingers (‘milkmaid sign’). Examination of the upper limbs can show incoordination of finger tapping and pronation/supination as well as a degree of rigidity. Observation of all body areas throughout a consultation can reveal chorea and dystonia, and these are often asymmetric, especially earlier in the disease course. Gait can be bradykinetic and stiff, and tandem walking difficult. As the disease progresses there is often loss of muscle mass, more widespread and unsuppressible involuntary movements, and progressive difficulty following commands in a standard examination. Cognitive testing often reveals impaired attention, executive function and memory.
Investigations
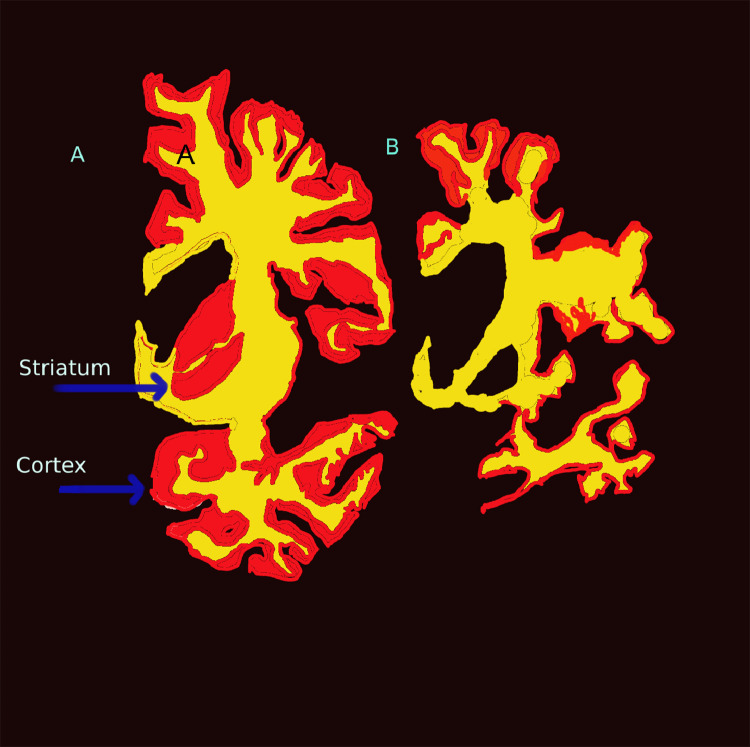
Beyond genetic testing, there are no specific investigations required for a diagnosis of HD. MRI or CT brain scanning can show caudate and then putaminal atrophy early in the disease course,9 with more widespread brain atrophy later on (Fig. 1).
Fig. 1.
Progression of Huntington's disease from early motor stage (A) to late stage (B). The coronal sections through the left hemisphere demonstrate whole brain atrophy that is disproportionately focussed on the grey matter of the striatum and cortex (in red). The white matter (in yellow) is relatively preserved. There is almost complete loss of the striatum in later stages of the illness (B). Figure designed after Vonsattel, 1998.10
Acute presentations in individuals with HD
Progression of HD is usually slow, occurring over years. Therefore, acute deteriorations in HD-related symptoms often indicate another problem. For example, intercurrent urinary tract, chest or other infections can exacerbate HD symptoms as can pain, constipation and urinary retention. In our experience, gastro-oesophageal reflux disease and poor dentition can also often cause symptomatic exacerbation in HD. Changes in work, home, social or financial circumstances, and drug or alcohol use or abuse can all contribute to an acute deterioration in mental or physical health. Importantly, individuals with HD often lack insight into their condition and may also not be able to communicate their symptoms clearly, particularly in advanced disease. They can perseverate on particular symptoms and so careful assessment is needed to prioritise those requiring treatment.
As well as exacerbation of existing symptoms, individuals with HD can develop new problems unrelated to HD. These can be difficult to identify if they affect the nervous system: for example, a TIA or stroke might cause acute neurological symptoms that are mistaken for HD. Seizures are not usually part of adult HD and so their occurrence should prompt a full work-up.
Management of HD-related symptoms
There are currently no treatments that can slow down or prevent onset or progression of HD. Medical and non-medical interventions are aimed at symptom management and maintenance of quality of life.11 Ideally, HD patients are managed by a specialist multi-disciplinary team including doctors, specialist nurses and a range of therapists.
Medical therapy (Table 1)
If chorea is intrusive, tetrabenazine three-times daily can be used to reduce its severity.12 This depletes central monoamines and should be used with caution in those with depression or parkinsonism. Alternative, off-licence, medications for chorea include second generation antipsychotics such as olanzapine or quetiapine. These are favoured in those with co-existing behavioural or psychotic symptoms, and in those where once-daily dosing is easier, but should be used cautiously in those with rigidity and bradykinesia. Anti-choreic drugs should ideally be started and managed by a specialist.
Depression and anxiety are common in HD. First-line treatments include selective serotonin reuptake inhibitors (SSRIs), such as citalopram or sertraline, and serotonin-noradrenaline reuptake inhibitors (SNRIs), such as venlafaxine or duloxetine. Mirtazapine can also be useful, particularly if sleep is problematic. Irritability can respond to SSRIs, but often requires higher doses. Agitation and aggressive outbursts, as well as delusions and hallucinations, can be treated with antipsychotics. Short-term benzodiazepines can be used in acute situations.11
Non-medical therapies
Therapies are central to enabling individuals with HD to live independently and well for as long as possible. Physiotherapy can help with balance, coordination and mobility.13 Speech and language therapists are essential for monitoring and improving communication and swallowing. Dietitians and nutritionists can advise on suitable soft but calorific diets to help patients maintain body mass. In individual cases where dysphagia is particularly problematic in advance of other aspects of HD, percutaneous endoscopic gastrostomy (PEG) tube placement and feeding may be considered.14 Occupational therapists can help adapt a patient's home to enable them to live there longer; for example, various assistance aids are available to assist mobility and communication in those with involuntary movements and impaired cognition. Psychological therapies are very useful for managing behavioural and psychiatric symptoms but are unevenly available through the UK.
Considerations in advanced HD
HD inevitably progresses after symptom onset, usually over 10–20 years. As such, early assistance with applying for benefits, liaising with the DVLA about driving, and considering lasting powers of attorney, for both health and financial matters, can be invaluable for patients. Advanced care plans can be useful, including the patient's wishes around refusing treatment including resuscitation, and appropriate documentation put in place in both hospital and community settings. As the disease becomes more advanced, cognitive impairment becomes increasingly problematic, alongside neuropsychiatric symptoms, lack of insight and severely impaired mobility. Eventually patients are unable to manage daily tasks. Living at home might become impossible and patients often have to move to care settings. Palliative care input can be invaluable in the final stages of life to ensure comfort and dignity.15 The leading causes of death in HD are pneumonia and suicide.2
Conclusions
Individuals with HD require considerable medical and psychiatric support over the course of their lives. Careful assessment is needed to discriminate between symptoms directly caused by HD and those of intercurrent illnesses. Although HD is progressive and there are no disease-modifying treatments, many acute deteriorations are reversible, particularly early in the disease course, with a combination of medications and therapist support. Pioneering treatments that lower mutant huntingtin protein levels or prevent CAG repeat expansions in the brain are currently in development, and there is optimism that a drug that slows or prevents onset of HD will be available in the next 5–10 years.16
Take-home messages
-
•
Acute deterioration in HD patients usually indicates acute illness.
-
•
Gastro-oesophageal reflux disease and impaired dentition are common mimics of HD ‘progression’.
-
•
Chorea is often not troublesome for patients, and may not need medication.
-
•
Neuropsychiatric symptoms such as depression and irritability cause severe distress to patients and carers, but can respond well to SSRIs
-
•
End-of-life care planning should begin early, with clear documentation linked to patient records
Declaration of competing interest
T.H.M. is an associate member of the scientific advisory board of LoQus23 Therapeutics Ltd. D.J.M. reports no conflicts of interest.
References
- 1.Bates G.P., et al. Huntington disease. Nat Rev Dis Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 2.Solberg O.K., Filkukovà P., Frich J.C., Feragen K.J.B. Age at death and causes of death in patients with huntington disease in Norway in 1986-2015. J Huntingtons Dis. 2018;7:77–86. doi: 10.3233/JHD-170270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans S.J.W., et al. Prevalence of adult Huntington's disease in the UK based on diagnoses recorded in general practice records. J Neurol Neurosurg Psychiatry. 2013;84:1156–1160. doi: 10.1136/jnnp-2012-304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's disease collaborative research group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 5.McAllister B., et al. Timing and impact of psychiatric, cognitive, and motor abnormalities in huntington disease. Neurology. 2021;96:e2395–e2406. doi: 10.1212/WNL.0000000000011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronin T., Rosser A., Massey T. Clinical presentation and features of juvenile-onset Huntington's disease: a systematic review. J Huntingtons Dis. 2019;8:171–179. doi: 10.3233/JHD-180339. [DOI] [PubMed] [Google Scholar]
- 7.Baig S.S., et al. 22 Years of predictive testing for Huntington's disease: the experience of the UK Huntington's Prediction Consortium. Eur J Hum Genet. 2016;24:1515. doi: 10.1038/ejhg.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symonds A.L., Macerollo A., Foy K., Alusi S.H., Davies R. Genetic and environmental contributors to neurodegeneration: an exploration of the effects of alcohol on clinical features of Huntington's disease using the enroll-HD global platform. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18105113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabrizi S.J., et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 10.Vonsattel J.P.G., DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bachoud-Lévi A.-C., et al. International guidelines for the treatment of Huntington's disease. Front Neurol. 2019;10:710. doi: 10.3389/fneur.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huntington Study Group Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366–372. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- 13.Quinn L., et al. Physical activity and exercise outcomes in Huntington's disease (PACE-HD): results of a 12-month trial-within-cohort feasibility study of a physical activity intervention in people with Huntington's disease. Parkinsonism Relat Disord. 2022;101:75–89. doi: 10.1016/j.parkreldis.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Frank E., Dyke A., MacKenzie S., Maskwa E., Frank S. Effects of percutaneous endoscopic gastrostomy in patients with huntington disease. Neurol Clin Pract. 2021;11:517–520. doi: 10.1212/CPJ.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiblin L. An introduction to neuropalliative care: a growing need. Clin Med. 2024;24:100038. doi: 10.1016/j.clinme.2024.100038. [DOI] [PubMed] [Google Scholar]
- 16.Tabrizi S.J., et al. Potential disease-modifying therapies for Huntington's disease: lessons learned and future opportunities. Lancet Neurol. 2022;21:645–658. doi: 10.1016/S1474-4422(22)00121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]