Key Points
-
•
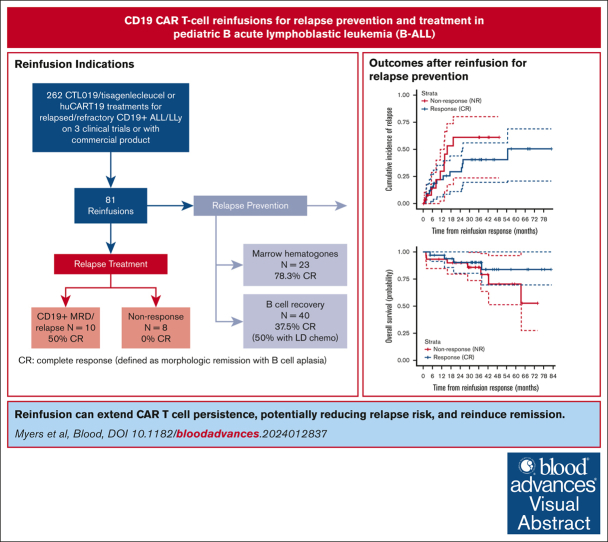
Reinfusion extended CAR T-cell persistence in 52% of patients reinfused for relapse prevention, thereby potentially reducing relapse risk.
-
•
Reinfusion induced remissions in 50% of patients with CD19+ relapses after initial CART, but durability was limited without further therapy.
Visual Abstract
Abstract
Relapse after CD19-directed chimeric antigen receptor (CAR)–modified T cells remains a substantial challenge. Short CAR T-cell persistence contributes to relapse risk, necessitating novel approaches to prolong durability. CAR T-cell reinfusion (CARTr) represents a potential strategy to reduce the risk of or treat relapsed disease after initial CAR T-cell infusion (CARTi). We conducted a retrospective review of reinfusion of murine (CTL019) or humanized (huCART19) anti–CD19/4-1BB CAR T cells across 3 clinical trials or commercial tisagenlecleucel for relapse prevention (peripheral B-cell recovery [BCR] or marrow hematogones ≤6 months after CARTi), minimal residual disease (MRD) or relapse, or nonresponse to CARTi. The primary endpoint was complete response (CR) at day 28 after CARTr, defined as complete remission with B-cell aplasia. Of 262 primary treatments, 81 were followed by ≥1 reinfusion (investigational CTL019, n = 44; huCART19, n = 26; tisagenlecleucel, n = 11), representing 79 patients. Of 63 reinfusions for relapse prevention, 52% achieved CR (BCR, 15/40 [38%]; hematogones, 18/23 [78%]). Lymphodepletion was associated with response to CARTr for BCR (odds ratio [OR], 33.57; P = .015) but not hematogones (OR, 0.30; P = .291). The cumulative incidence of relapse was 29% at 24 months for CR vs 61% for nonresponse to CARTr (P = .259). For MRD/relapse, CR rate to CARTr was 50% (5/10), but 0/8 for nonresponse to CARTi. Toxicity was generally mild, with the only grade ≥3 cytokine release syndrome (n = 6) or neurotoxicity (n = 1) observed in MRD/relapse treatment. Reinfusion of CTL019/tisagenlecleucel or huCART19 is safe, may reduce relapse risk in a subset of patients, and can reinduce remission in CD19+ relapse.
Introduction
Chimeric antigen receptor (CAR)–modified T cells targeting CD19 have produced remarkable responses in relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (R/R B-ALL). However, subsequent leukemia relapse occurs in at least 25% to 50% of pediatric patients.1, 2, 3, 4, 5, 6 One of the primary mechanisms of relapse after CAR T-cell therapy involves the loss of CAR T-cell surveillance due to short persistence allowing for relapse of residual leukemia. Indeed, CD19+ relapses, which account for 33% to 78% of relapses,2, 3, 4,7,8 are frequently associated with short persistence.3,4 B-cell recovery (BCR), a surrogate marker indicating loss of functional CD19 CAR T-cell persistence, within 6 months of infusion correlates with an increased risk of relapse.3,4,9 Thus, strategies to improve functional persistence to prevent and treat CD19+ relapses are crucial.
Reinfusion of the same CAR T-cell product (CARTr) represents a potential approach to mitigate the risk of or treat relapse after initial CAR T-cell infusion (CARTi). Published data on the efficacy and safety of reinfusion, however, are limited. Initial studies reported suboptimal outcomes after CARTr, but these reports focused on adult patients with lymphoma or pediatric patients treated with short-persisting anti-CD19/CD28 CAR T cells.4,10,11 Furthermore, prior studies have not evaluated the role of CARTr in relapse prevention for patients with early loss (<6 months after CARTi) of peripheral B-cell aplasia (BCA) or reappearance of CD19+ hematogones in the bone marrow.
Over the past decade, our center has administered reinfusions of murine and humanized CD19/4-1BB CAR T cells to children and young adults treated with investigational or commercial CD19 CAR T-cell products.1,3,12 Here, we report our experience administering CARTr to patients with demonstrated short persistence in an effort to prolong persistence to reduce relapse risk, treat CD19+ relapsed disease after CARTi, and produce responses after nonresponse to CARTi. We analyzed outcomes after CARTr, identified factors associated with response, established the incidence and severity of cytokine release syndrome (CRS) and neurotoxicity after CARTr, and described in vivo CAR T-cell expansion.
Patients and methods
Patients and study design
We conducted a retrospective review of children and young adults with R/R B-ALL treated on 3 CD19 CAR clinical trials or with commercial tisagenlecleucel (Kymriah, Novartis) at Children’s Hospital of Philadelphia between 2012 and 2020 who received at least 1 reinfusion of the same product (CARTr) under the clinical trial, single-patient investigational new drug, or commercial release, due to 1 of the following indications: (1) clinical signs of poor persistence within 6 months of CARTi, defined as peripheral BCR (CD19+ cells ≥3% of lymphocytes) or CD19+ hematogones in the bone marrow (≥1% hematogones for patients treated after June 2015, detection of any hematogones for patients treated before then); (2) development of CD19+ minimal residual disease (MRD; defined as ≥0.01% bone marrow blasts by multiparameter flow cytometry) or morphologic relapse (defined as ≥5% bone marrow blasts); or (3) nonresponse to CARTi. Of note, for hematogones, the threshold for reinfusion changed in 2015 due to observations in clinical practice that <1% hematogones may not represent impending peripheral BCR. Patients treated on clinical trials received either the murine CD19/4-1BB CAR construct, CTL019 (Food and Drug Administration-approved as tisagenlecleucel; NCT01676495 and NCT02906371), or the humanized CD19/4-1BB CAR construct, huCART19 (NCT02374333). The primary results of each trial, including CAR manufacturing details, were previously published.1,3,12 Patients who were CAR naive were receiving their first CAR product at the time of CARTi. Patients who were exposed to CAR were those in the retreatment cohort of the huCART19 trial who had experienced early BCR or relapse after prior murine CD19 CAR. This retrospective study was approved by the Institutional Review Board of Children’s Hospital of Philadelphia.
With 1 exception, CARTr products were supplemental doses manufactured concurrently with the initial CD19 CAR T-cell manufacture. One patient had a new CARTr product manufactured after a second leukapheresis procedure. Lymphodepletion before CARTr was administered at the discretion of the treating physician. Before July 2016, most patients receiving CARTr for relapse prevention did not receive lymphodepletion. Beginning in July 2016, lymphodepletion was added for most patients receiving CARTr for peripheral BCR but not hematogones. CARTr doses ranged from 0.6 × 106 CAR T cells per kg to 13.2 × 106 CAR T cells per kg. Some patients received ≥1 reinfusion and/or the PD-1 inhibitor, pembrolizumab (Keytruda, Merck), after reinfusion.
Objectives
The primary objective was to evaluate the complete response (CR) rate at day 28 after first CARTr, defined as complete remission with establishment or maintenance of BCA (CD19+ cells <3% of peripheral blood lymphocytes). Secondary objectives were to identify factors associated with a CR to CARTr, describe patients’ clinical courses after initial response to CARTr, compare the cumulative incidence of relapse (CIR) and overall survival (OS) by response to CARTr, describe in vivo CAR T-cell expansion after CARTr, and assess the incidence of CRS and neurotoxicity.
Clinical response assessment
Initial responses to CARTr were evaluated at day 28 after reinfusion. All patients had peripheral blood samples tested for CD19+ lymphocytes to evaluate for BCA. Patients who received CARTr due to MRD/relapse or nonresponse to CARTi also had bone marrow evaluations completed at day 28 (as well as pre-CARTr). Complete remission was defined as M1 bone marrow (<5% blasts) with no evidence of extramedullary disease. MRD was assessed by multiparameter flow cytometry at the University of Washington. For patients with a CR to CARTr, peripheral CD19 levels were assessed every 2 weeks from day 28 to day 56, then monthly for at least 6 months provided ongoing CR. Bone marrow disease was assessed as clinically indicated after day 28.
Toxicity assessment
CRS was prospectively evaluated for all patients according to the Penn scale.13 Neurological toxicities were captured prospectively for study patients and retrospectively for nonstudy patients using the Common Terminology Criteria for Adverse Events (version 4.03; additional details in the supplemental Appendix).
Statistical analysis
Standard descriptive statistics were calculated for patient and disease characteristics, adverse events, and CR rates. To identify factors associated with response to CARTr for relapse prevention, univariate and multivariate logistic regression was used. To compare survival and relapse outcomes between patients with CR vs those with nonresponse to CARTr for relapse prevention, a landmark analysis was performed, in which time 0 was set as day 28 after CARTr, and only patients who were event-free and still under follow-up at day 28 were included. OS was calculated from day 28 after CARTr to time of any death and censored at last follow-up (with a data cutoff of 1 July 2021) if no death was observed. OS was evaluated using Kaplan-Meier methods and compared by response to CARTr using the log-rank test. Time to relapse/death was calculated from day 28 after CARTr to death or relapse, whichever occurred earlier, and censored at hematopoietic stem cell transplantation (HSCT), other anticancer therapy while in ALL remission, or last follow-up. CIR/death was estimated using 1 – Kaplan-Meier estimate and compared by response to CARTr using log-rank test. To evaluate CAR T-cell expansion after CARTr, peak expansion was compared between CR and NR using the Wilcoxon rank sum test. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC) and Stata, version 14.0 (StataCorp, College Station, TX).
Results
Patient and disease characteristics
Of 262 primary treatments with CD19 CAR T cells across the 3 clinical trials or with commercial tisagenlecleucel, 81 (30.9%) were subsequently followed by at least 1 reinfusion and were included in this analysis (Table 1). This represented 79 unique patients because 2 patients were first treated and reinfused with CTL019/tisagenlecleucel, then later treated and reinfused with huCART19. Sixty-eight patients were CAR-naïve at CARTi: 44 (65%) received investigational CTL019, 13 (19%) received investigational huCART19, and 11 (16%) received commercial tisagenlecleucel. Thirteen patients were CAR-exposed at CARTi, all of whom received huCART19. The majority were in second or greater relapse at CARTi (CAR-naïve, 56%; CAR-exposed, 77%). Before CARTi, 44% of patients who were CAR-naïve and 62% of patients who were CAR-exposed had undergone allogeneic HSCT. At the time of CARTi, 32% patients who were CAR-naïve and 31% patients who were CAR-exposed had ≥5% bone marrow blasts.
Table 1.
Patient characteristics
| Characteristic | CAR-naïve (n = 68) | CAR-exposed (n = 13) |
|---|---|---|
| CAR product | ||
| Investigational CTL019 | 44 (64.7) | |
| Investigational huCART19 | 13 (19.1) | 13 (100.0) |
| Commercial tisagenlecleucel | 11 (16.2) | |
| Median age at initial diagnosis (range), y | 6.0 (0.24-19.5) | 3.7 (1.3-15.0) |
| Median age at CARTi (range), y | 11.3 (1.7-24.5) | 9.7 (5.9-20.0) |
| Median age at CARTr (range), y | 11.9 (2.0-25.1) | 9.9 (6.2-20.4) |
| Female sex | 28 (41.2) | 3 (23.1) |
| Race | ||
| White | 53 (77.9) | 9 (69.2) |
| Black or African-American | 4 (5.9) | 0 (0.0) |
| Asian | 0 (0.0) | 1 (7.7) |
| Other | 11 (16.2) | 2 (15.4) |
| Unknown | 0 (0.0) | 1 (7.7) |
| Ethnicity | ||
| Non-Hispanic | 60 (88.2) | 10 (76.9) |
| Hispanic | 8 (11.8) | 3 (23.1) |
| Trisomy 21 | 2 (2.9) | 1 (7.7) |
| Prior HSCT | 30 (44.1) | 8 (61.5) |
| Prior blinatumomab | 6 (8.8) | 0 (0.0) |
| Prior inotuzumab | 3 (4.4) | 3 (23.1) |
| Disease status at CARTi | ||
| Primary refractory | 13 (19.1) | 1 (7.7) |
| First relapse | 17 (25.0) | 2 (15.4) |
| Second relapse | 29 (42.7) | 5 (38.5) |
| Third or greater relapse | 9 (13.2) | 5 (38.5) |
| BM blasts, pre-CARTi | ||
| MRD− | 28 (41.2) | 7 (53.9) |
| MRD+, < 5% | 18 (26.5) | 2 (15.4) |
| 5%-24.99% | 5 (7.4) | 1 (7.7) |
| ≥25% | 17 (25.0) | 3 (23.1) |
| CRS maximum grade after CARTi | ||
| None or mild (grade 1/2) CRS | 58 (85.3) | 11 (84.6) |
| Moderate (grade 3) CRS | 5 (7.4) | 2 (15.4) |
| Severe (grade 4) CRS | 5 (7.4) | 0 (0.0) |
Data are displayed as median (IQR) unless otherwise indicated.
BM, bone marrow; CNS, central nervous system; Cy, cyclophosphamide; Flu, fludarabine; LD, lymphodepleting.
Data are displayed as n (%) unless otherwise indicated.
BM, bone marrow.
CARTr indications and characteristics
Sixty-three patients received CARTr for relapse prevention, including 40 for peripheral BCR and 23 for emergence of CD19+ hematogones without peripheral BCR. Eighteen patients received CARTr to treat relapsed or residual disease; 10 for CD19+ MRD/relapse and 8 for nonresponse to CARTi.
Among patients who received CARTr for relapse prevention, median peripheral CD19+ cells or CD19+ bone marrow hematogones before CARTr was 7.3% (interquartile range [IQR], 5.5-19.7) or 2.0% (IQR, 0.1-6.5), respectively. Among patients who received CARTr for treatment of relapse, median bone marrow blasts before CARTr was 0.16% (IQR, 0.0-70.0) for MRD/relapse or 90.0% (IQR, 5.2-96.5) for nonresponse to CARTi. In addition, 4 patients with relapse had active extramedullary disease at CARTr.
Median time from CARTi to CARTr was 3.6 months (IQR, 3.2-5.2) for peripheral BCR, 3.8 months (IQR, 3.4-4.6) for hematogones, 9.5 months (IQR, 4.1-22.8) for MRD/relapse, and 1.2 months (IQR, 0.9-1.4) for nonresponse to CARTi. Lymphodepletion was administered before CARTr to 70% of patients with peripheral BCR, 22% with hematogones, 70% with MRD/relapse, and 50% with nonresponse to CARTi. Twenty-six patients received an additional reinfusion after the first reinfusion because of nonresponse to first CARTr (n = 6), peripheral BCR after first CARTr (n = 7), new CD19+ relapsed disease (n = 2), or as a preplanned second reinfusion to extend persistence further (n = 11; Table 2). Five patients who were exposed to CAR received PD-1 blockade after first reinfusion, and 6 patients who were CAR naive received PD-1 blockade after second reinfusion for relapse prevention. Four patients received PD-1 blockade after first (n = 3) or second reinfusion (n = 1) for relapse treatment and 3 after first reinfusion for nonresponse to CARTi (supplemental Table 1).14
Table 2.
CARTr characteristics by indication
| Peripheral BCR | Hematogones | CD19+ MRD or relapse | Nonresponse to CARTi | |
|---|---|---|---|---|
| n = 40 | n = 23 | n = 10 | n = 8 | |
| Peripheral CD19+ cells or CD19+ hematogones prereinfusion, % | 7.3 (5.5-19.7) Absolute CD19/μL, 86 (39-166) |
2.0 (0.1-6.5) | — | — |
| BM blasts prereinfusion, % | — | — | 0.16 (0.0-70.0)∗ | 90.0 (5.2-96.5)† |
| Extramedullary disease prereinfusion, n (site) | — | — | 4 (CNS, orbit, breast, mediastinum) | 0 |
| Months from initial infusion to reinfusion | 3.6 (3.2-5.2) | 3.8 (3.4-4.6) | 9.5 (4.1-22.8) | 1.2 (0.9-1.4) |
| CARTr dose, CAR T cells × 106/kg | 4.9 (2.3-6.5) | 4.1 (3.8-6.3) | 5.4 (2.4-6.7) | 5.6 (2.5-9.0) |
| Lymphodepletion prereinfusion | ||||
| Standard Flu/Cy | 24 (60.0) | 4 (17.4) | 6 (60.0) | 3 (37.5) |
| Lower dose Flu/Cy | 4 (10.0) | 1 (4.3) | 1 (10.0) | 0 (0.0) |
| Cy/Prednisone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| No lymphodepletion | 12 (30.0) | 18 (78.3) | 3 (30.0) | 4 (50.0) |
| Received pembrolizumab | 4 (10.0) | 1 (4.3) | 3 (30.0) | 3 (37.5) |
| CR to first reinfusion | 15 (37.5) | 18 (78.3) | 5 (50.0) | 0 (0.0) |
| With LD chemo | 14/28 (50.0) | 3/5 (60.0) | 4/7 (57.1) | 0/4 (0.0) |
| Without LD chemo | 1/12 (8.3) | 15/18 (83.3) | 1/3 (33.3) | 0/3 (0.0) |
| Received second reinfusion, n (%) | 13 (32.5) | 11 (47.8) | 2 (20.0) | 0 (0.0) |
Data are displayed as median (IQR) unless otherwise indicated.
BM, bone marrow; CNS, central nervous system; Cy, cyclophosphamide; Flu, fludarabine; LD, lymphodepleting.
Data are displayed as n (%) unless otherwise indicated.
BM, bone marrow.
n = 9 (not available on 1 patient).
n = 7 (not available on 1 patient).
CRS and neurotoxicity
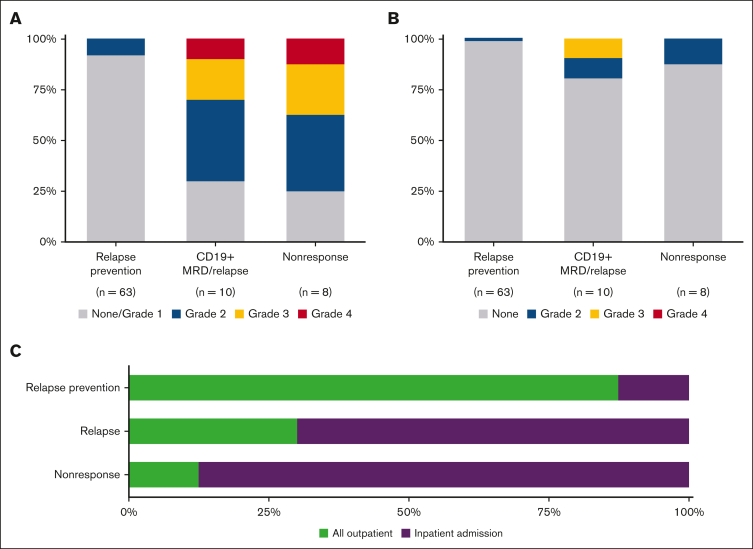
In the relapse prevention setting (n = 63), low rates of mild CRS and neurotoxicity were observed (Figure 1). Five patients (8%) developed grade 2 CRS, and 1 developed a grade 2 neurological serious adverse event (confusion). No grade ≥3 CRS or neurological events were observed. Furthermore, 55 patients (87%) remained in the outpatient setting throughout the 30-day post-CARTr period.
Figure 1.
Toxicity after CD19 CAR T-cell reinfusion. (A) CRS grade by indication for CARTr according to the Penn scale. (B) Neurotoxicity grade by indication for CARTr according to the CTCAE 4.03 grading scale. (C) Proportion of patients who required inpatient admission within 30 days after CARTr by indication for CARTr. CTCAE, Common Terminology Criteria for Adverse Events.
In the relapse treatment setting (n = 18), CRS was observed more frequently, but neurotoxicity was generally mild (Figure 1). Among 10 patients with MRD/relapse, 7 (70%) developed grade ≥2 CRS, of which 1 had grade 4 CRS. Two patients had neurological serious adverse events: encephalopathy grade 2 in 1 and grade 3 in 1. Among 8 patients with nonresponse to CARTi, 6 (75%) developed grade ≥2 CRS, of which 1 had grade 4 CRS. One patient experienced grade 2 encephalopathy. Most patients required at least 1 inpatient admission in the 30 days after CARTr (MRD/relapse, 70%; nonresponse to CARTi, 88%). All CRS and neurotoxicity events were reversible, and no CARTr-related deaths were observed.
CARTr for peripheral BCR
Fifteen (38%) of 40 patients reinfused for peripheral BCR had a CR to CARTr. The CR rate was higher among patients who received lymphodepletion prior to CARTr (14/28, 50%) compared to those who did not (1/12, 8%; OR, 11; P = .031). Other baseline factors, including time from CARTi to peripheral BCR, history of prior CAR exposure, history of prior HSCT, CAR product, or CARTr cell dose were not significantly associated with response to CARTr in univariate analysis (Table 3; supplemental Table 2). In a multivariate logistic regression model that included clinically relevant baseline factors, lymphodepletion remained highly associated with response (adjusted odds ratio [OR], 33.570; P = .013). There was a trend that did not reach statistical significance toward improved response rates among patients with a history of prior HSCT (adjusted OR, 5.175; P = .079). The adjusted OR for response among patients reinfused with commercial tisagenlecleucel compared with investigational CTL019 was 0.344, but this was not statistically significant (P = .334; Table 3).
Table 3.
Univariate and multivariate analysis of response to CARTr for peripheral BCR
| No. of participants = 40 | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Prior HSCT (n = 15) | 1.859 | 0.50-6.94 | .356 | 5.175 | 0.83-32.31 | .079 |
| BCR ≤3 mo after CARTi (n = 22) | 0.898 | 0.25-3.25 | .870 | 0.996 | 0.20-4.90 | .996 |
| CART product | ||||||
| Investigational CTL019 (n = 17) | REF | REF | ||||
| HuCART19 (n = 14) | 2.400 | 0.55-10.53 | .246 | 1.313 | 0.12-14.28 | .823 |
| Commercial tisagenlecleucel (n = 9) | 1.200 | 0.21-6.80 | .837 | 0.344 | 0.04-3.00 | .334 |
| Previously treated with a different CART product (n = 7) | 1.313 | 0.25-6.88 | .748 | 0.444 | 0.04-5.10 | .513 |
| Lymphodepletion prior to CARTr (n = 28) | 11.000 | 1.25-97.02 | .031 | 33.570 | 2.07-543.24 | .013 |
REF, reference.
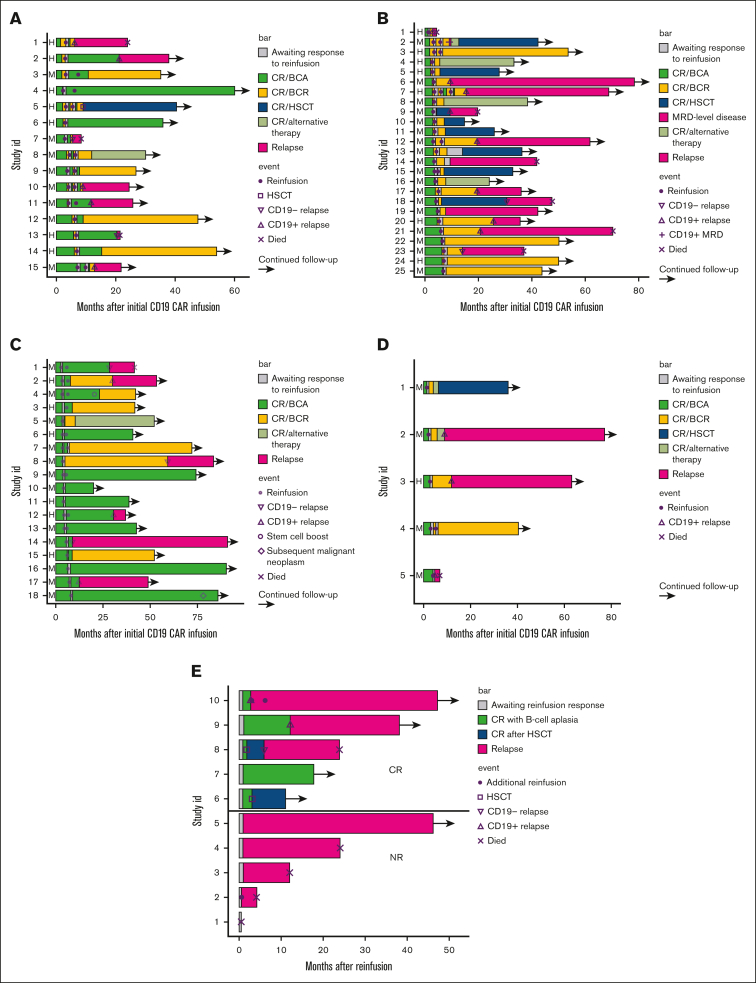
Of the 15 patients with a CR to CARTr, 3 remain in remission without further therapy, 3 remain in remission with an additional, preplanned reinfusion (each received a total of 2 CARTr), 2 remain in remission after receiving alternative anticancer therapy in remission (HSCT, n = 1; clinical trial of huCART19, n = 1), and 7 relapsed (CD19+, n = 5; CD19−, n = 2) (Figure 2A-B). Among the 7 patients with relapse, 5 successfully achieved another remission (remission induction therapies included: inotuzumab, n = 2; CD22 CAR, n = 1; blinatumomab, n = 1; cytotoxic chemotherapy and dasatinib, n = 1) and proceeded to HSCT, of which 4 remain alive in CR. Two patients with CD19− relapse died shortly after relapse without achieving another remission. The median duration of BCA after CARTr response assessment was 49 days (range, 15-1737). Among patients with BCR again within 42 days after CARTr (n = 4), 2 proceeded to alternative therapy in remission, and 2 experienced CD19+ relapse. Among patients with BCR 60 to 90 days after CARTr (n = 6), 3 remain in CR, and 3 relapsed. Among patients with BCR >90 days after CARTr (n = 5), 3 remain in CR, and 2 relapsed.
Figure 2.
Clinical courses of individual patients. Clinical courses are shown from the time of CARTi for patients who received CARTr for peripheral BCR followed by CR (A) or NR (B), and for patients who received CARTr for CD19+ hematogones followed by CR (C) or NR (D). (E) Clinical courses are shown from the time of CARTr for patients reinfused for CD19+ MRD/relapse. Data shown include duration of CR with BCA or BCR, duration of CR after HSCT or other alternative therapy, time to reinfusion(s), time to relapse denoted as CD19+ or CD19−, and time to death. BCA, B cell aplasia; BCR, B cell recovery; CR, complete response; HSCT, hematopoietic stem cell transplantation; NR, no response.
Of 25 patients with nonresponse to CARTr for peripheral BCR, 4 remain in remission without further therapy. Twelve proceeded to alternative therapy in remission (HSCT, n = 6; huCART19, n = 6), of which 9 remain in remission. Nine relapsed (CD19+, n = 8; CD19−, n = 1) at a median of 335 days (range, 22-593) after CARTr response assessment. Among the 9 patients with relapse, 7 were successfully bridged to HSCT, of which 5 are alive in CR, 1 died of progressive disease without achieving another remission, and 1 achieved another remission with CD22 CAR but then died of disease progression.
CARTr for CD19+ hematogones
Eighteen of 23 patients (78%) reinfused due to CD19+ hematogones maintained a CR to CARTr. The majority did not receive lymphodepletion. Lymphodepletion was not associated with response; 3 of 5 (60%) maintained CR with lymphodepletion, and 15 of 18 (83%) maintained CR without lymphodepletion (P = .291). Time from CARTi to detection of hematogones was associated with CR rates: 0 of 3 patients (0%) with hematogones at day 28 after CARTi maintained a CR at day 28 after CARTr compared with 13 of 15 (87%) with hematogones at month 3 and 5 of 5 (100%) with hematogones at month 6 or later (P = .009). Other baseline characteristics were not associated with response to CARTr by univariate analysis (supplemental Table 3). Multivariable models were not constructed due to small numbers.
Of 18 patients with CR to CARTr, 11 remain in remission without further anticancer therapy (4 received an additional reinfusion to extend persistence); 1 proceeded to huCART19 in remission; and 6 relapsed (CD19+, n = 3; CD19−, n = 3) (Figure 2C-D). Among the 6 patients with relapse, 5 remain alive with further therapies, and 1 died of progressive disease. The median duration of BCA was 765 days (range, 15-2528) after response to CARTr.
Of 5 patients with nonresponse to CARTr, 1 remains in remission without further therapy; 2 received huCART19, of whom 1 remains in remission after consolidative HSCT; and 2 relapsed before receiving other therapy, of whom 1 is alive and in remission after other therapies and 1 died of progressive disease.
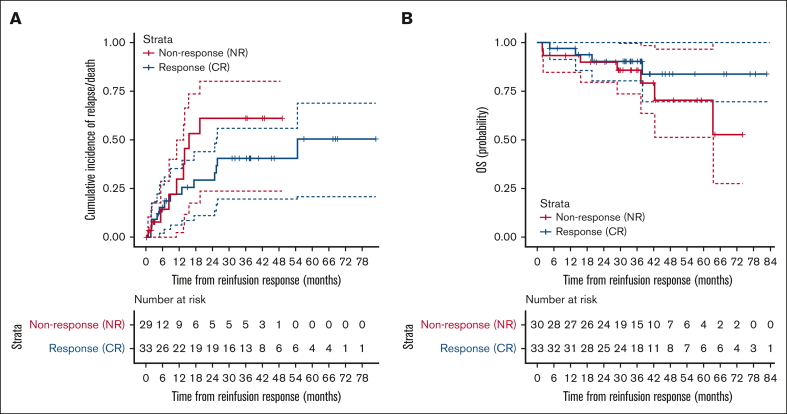
CIR/death and OS after CARTr for relapse prevention
To evaluate whether CARTr reduces relapse risk, the CIR or death (however, no nonrelapse mortality was observed) was compared between patients with CR vs nonresponse to CARTr (Figure 3). With a median potential follow-up of 38 months (IQR, 31-49) after CARTr response assessment, the 24-month CIR rates were 29% (95% confidence interval [CI], 11-44) for CR vs 61% (95% CI, 24-80) for nonresponse to CARTr, but the curves were not statistically significantly different (P = .259). OS was similar between the groups (P = .247), with 24-month OS rates of 90% (95% CI, 80-100) in both.
Figure 3.
Relapse and survival after CD19 CAR T-cell reinfusion for relapse prevention. (A) CIR or death from the time of assessment of response to CARTr among patients with a CR or NR to CARTr. Data were censored at the time of HSCT, other anticancer therapy while in remission, or last follow-up. (B) OS from the time of assessment of response to CARTr among patients with a CR or NR to CARTr. Data were censored at the time of last follow-up with a data cutoff of 1 July 2021. NR, nonresponse.
CARTr for MRD/relapse
Five of 10 patients (50%) reinfused for new CD19+ MRD or relapse achieved a CR to CARTr. CR rates were similar for patients with ≥5% bone marrow blasts (2/3 [67%]) or <5% bone marrow blasts (3/6 [50%]) before CARTr (P = 1.000); 1 patient did not have a pre-CARTr bone marrow evaluation.
Of the 5 patients with a CR to CARTr, 2 remain in remission; 1 after consolidative HSCT and the other without further therapy (Figure 2E). The other 3 patients experienced subsequent relapses; 2 had CD19+ relapses before additional therapy, and 1 had a CD19− relapse after consolidative HSCT. Of the 5 patients with nonresponse to CARTr, 4 died of progressive disease at a median of 247 days (range, 16-733) after CARTr. One was able to proceed to HSCT and remains in remission >3 years after CARTr.
CARTr for nonresponse to CARTi
Of the 8 patients reinfused for nonresponse to CARTi, 7 were evaluable for response to CARTr, but no CRs were observed. Progressive/residual disease was CD19+ in 4 patients, CD19− in 2 patients, and CD19-unknown in 1 patient. One patient was unevaluable due to progression of multisystem organ failure, likely related to sepsis and progressive disease that led to death 6 days after CARTr. All evaluable patients died of progressive disease at a median of 105 days (range, 27-669) after CARTr.
CARTr expansion
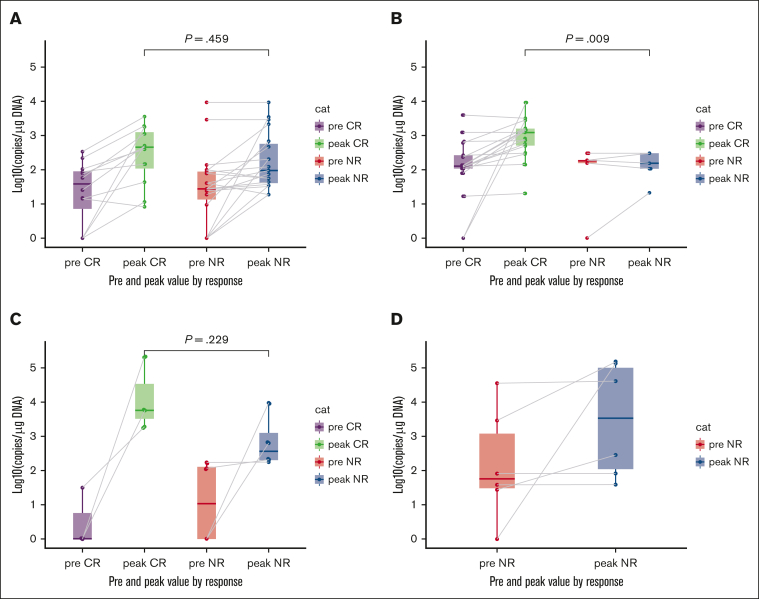
Pre-CARTr and post-CARTr measurements of CAR transgene in peripheral blood were quantified by quantitative real-time polymerase chain reaction as previously described (Figure 4).1,3 Median peak levels of CAR transgene were 2.66 log(10) copies per μg DNA for patients in CR to CARTr for peripheral BCR vs 1.98 log(10) copies per μg DNA for nonresponse (P = .459); 3.09 log(10) copies per μg DNA for patients in CR to CARTr for hematogones vs 2.20 log(10) copies per μg DNA for nonresponse (P = .009); 3.76 log(10) copies per μg DNA for patients in CR to CARTr for relapse vs 2.57 log(10) copies per μg DNA for nonresponse (P = .229); and 3.53 log(10) copies per μg DNA for patients with nonresponse to CARTi, all of whom had nonresponse to CARTr.
Figure 4.
CAR T-cell expansion after CD19 CAR T-cell reinfusion. Measurements of CAR-modified T cells in peripheral blood by quantitative real-time PCR assay before CARTr and peak measurements in the 30 days after CARTr among patients with CR vs no response (NR) to CARTr for peripheral BCR (A), CD19+ hematogones (B), CD19+ MRD/relapse (C), and nonresponse to CARTi (D). P values compare peak measurements between CR and NR for each indication by the Wilcoxon rank sum test. Box-and-whisker plots show the median (horizontal line), IQR (box), and 1.5× the IQR (whiskers). Each observation is overlaid as a dot. Gray lines connect individual patient’s pre-CARTr measurement with his/her peak measurement. Data are only available for patients treated with CARTr on a clinical trial. PCR, polymerase chain reaction.
Discussion
CD19-directed CAR T cells can produce durable remissions in children and young adults with R/R B-ALL; however, up to 50% of patients relapse. A key contributor to relapse risk is short persistence of CAR-modified T cells.3,4,9 We hypothesized that reinfusion of supplemental doses of CAR T cells could extend persistence and reduce the risk of relapse. We performed a retrospective analysis of CTL019/tisagenlecleucel or huCART19 reinfusion for prevention or treatment of relapse in patients treated on 3 clinical trials or with commercial tisagenlecleucel. Response to CARTr for relapse prevention was observed in 52% and 50% for CD19+ MRD or relapse. There were no responses for those with nonresponse to CARTi.
Short persistence of CD19-targeted CAR T cells is a substantial problem in B-ALL, observed in 15% to nearly 100%, with variations by product and trial.1, 2, 3, 4, 5,15 Although several studies have shown an increased risk of relapse without consolidative therapy, the best choice of consolidation is not clear and may be both patient dependent and product dependent. In a trial of CD19 CAR T cells with the CD28 costimulatory domain at the National Cancer Institute (NCI), 75% of patients received consolidative HSCT, with a 24-month CIR of 9.5% (95% CI, 1.5-26.8). All those who did not receive consolidative HSCT relapsed.16 In a trial of CD19 CAR T cells containing the 4-1BB costimulatory domain, Seattle Children’s showed a benefit to HSCT in those with early BCR by day 63 but not in patients with a history of prior HSCT.17 Furthermore, HSCT carries substantial risks of treatment-related morbidity and mortality. In the NCI study, one-third of patients who received consolidative HSCT died of transplant-related complications, infection, or subsequent malignancy. In adults receiving a CD19/CD28 CAR for R/R B-ALL, Memorial Sloan Kettering Cancer Center demonstrated no difference in event-free survival or OS with HSCT, in part due to transplant-related mortality.18 In early studies, most patients had already undergone HSCT before CD19 CAR T-cell therapy, making the benefit of consolidative HSCT after CAR less clear. As an alternative approach to reduce relapse risk, we studied reinfusion in patients who experienced short persistence after the 4-1BB–containing CD19 CARs CTL019/tisagenlecleucel or huCART19. In this study, reinfusion resulted in 24-month CIR of 29% (95% CI, 11-44) for patients with a CR compared with 61% (95% CI, 24-80) for patients with nonresponse. These estimates lack precision, however, due to patients being censored for HSCT and other therapy after experiencing nonresponse. Although this difference was not statistically significant, the effect size suggests a clinically meaningful difference.
A leading concern with the reinfusion approach is losing disease control if reinfusion proves ineffective. For reinfusion to represent a favorable alternative to consolidative HSCT, not only are safety and efficacy paramount; the ability to subsequently proceed to HSCT is also critical. In this study, 14 patients with nonresponse to reinfusion maintained remission to consolidative HSCT or other therapy. Of the 16 with nonresponse who elected no further therapy, 5 remain in remission, and 11 relapsed at 0.8 to 21 months after reinfusion. There were no reinfusion-related toxicities that precluded subsequent therapy, no related deaths, and no cases of grade ≥3 CRS or neurotoxicity in the relapse prevention setting. The ability to safely proceed to further therapy is also reflected in the similarly excellent OS in patients with or without a CR to reinfusion (24-month OS, 90% in both groups). In summary, reinfusion did not preclude further consolidative therapy, including HSCT, supporting the clinical practice of offering reinfusion as an option for relapse prevention and then using response to guide further management.
Mixed results have been observed with reinfusion, with some small studies reporting similar response rates, whereas others noted little to no response. Several potential reasons for this discrepancy exist, including indication for reinfusion, CAR product and costimulatory domain, and lymphodepletion. The NCI reported no responses to reinfusion for R/R disease after initial infusion on their CD19.CD28 study but observed responses to reinfusion with their CD22.4-1BB and CD19/CD22.4-1BB products (7/18 [38.9%]).11 Gauthier et al reported a CR rate of 21% in 14 adults with B-ALL reinfused with a CD19.4-1BB CAR for relapse or nonresponse and found that increased CAR dose and incorporation of fludarabine into lymphodepletion were associated with improved CR rates.10 With the same product, Seattle Children’s reported a response to reinfusion in 1 of 4 patients with CD19+ relapse and 1 of 6 patients with early BCR.4 Finally, Memorial Sloan Kettering Cancer Center observed a response to reinfusion in 4 of 10 patients with progression after CD19.28 CAR.15,18 Although we observed no responses to reinfusion in patients with nonresponse to CARTi in our study, the NCI and Seattle showed responses in this setting and suggested that increased CAR dose and intensified lymphodepletion contributed. Most of the prior studies focused on relapse after CAR and reported no durable remissions without further therapy. We similarly observed only 1 durable remission without further therapy in the relapse setting. This study differed in the focus on relapse prevention, suggesting that reinfusion may be effective in reducing relapse risk. There may also be product-specific differences, as suggested by these studies. The reasons for different results by product are unclear, but it has been suggested that immunogenicity plays a role in reinfusion nonresponse.10
The retrospective design led to some limitations. Changes in practice, such as the inclusion of lymphodepletion once it was observed that lymphodepletion was needed to produce responses to CARTr for peripheral BCR, led to differences in treatment. Our clinical observation that emergence of bone marrow hematogones signaled impending peripheral BCR in many patients led to our practice of offering CARTr for hematogones; however, it is possible that some patients with hematogones would have maintained CAR persistence even without reinfusion. PD-1 blockade in combination with reinfusion may have contributed to response or persistence; however, small subsets precluded conclusions. In addition, small subgroup sizes led to some analyses being underpowered to detect differences. For example, subgroup sizes precluded an appropriately powered analysis of the contribution of CAR product to CARTr response. The nonrandomized design could have introduced bias and made teasing out the contribution of reinfusion infeasible. Nevertheless, the large sample size of this study and long follow-up enabled analyses of factors associated with response to reinfusion and the description of long-term outcomes.
In conclusion, reinfusion of CTL019/tisagenlecleucel or huCART19 can extend persistence and reinduce remission in antigen-positive relapse. In the relapse treatment setting, successful reinfusions should be followed by additional consolidative therapy given limited remission durability with reinfusion alone. Reinfusion may reduce the risk of relapse in a subset of patients with short persistence and, importantly, does not preclude HSCT if ineffective; therefore, reinfusion may represent a reasonable alternative to consolidative HSCT in selected patients.
Conflict-of-interest disclosure: S.K. has received research support from Miltenyi Biotec and Lonza. J.A.F. has received research funding and personal fees from Cartography; research funding from Tmunity Therapeutics; and personal fees from Retro Bio and Shennan Bio. S.P.H. has received consulting fees from Novartis; honoraria from Amgen, Jazz Pharmaceuticals, and Servier; and is a current equity holder in Amgen. S.R.R. has received consulting fees from Abbvie and Pfizer. C.H.J. has received royalties from Kite Pharma and Novartis; has served on advisory committees for Kite Pharma, AC Immune, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Celldex, Decheng, Poseida, Verismo, WIRB-Copernicus, and Danaher; and has received research funding from Kite Pharma. S.A.G. has received consulting/speaking fees and has served on advisory committees for Jazz Pharmaceuticals, Vertex, Novartis, Adaptive, Adaptimmune, Allogene, Eureka, Kyttaro, and Cabaletta; and has received research funding from Cellectis, Jazz Pharmaceuticals, Kite Pharma, Vertex, Novartis, and Servier. S.L.M. has received clinical trial support from Novartis and Wugen; has served on advisory and study steering committees for Novartis and Wugen; and has a patent pending and licensed to Novartis Pharmaceuticals without royalty for PCT/US2017/044425: Combination Therapies of CAR and PD-1 inhibitors. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by clinical trial awards funded by a research alliance between the University of Pennsylvania and Novartis Pharmaceuticals, the Children’s Hospital of Philadelphia Frontier Program, and the V Foundation Pediatric Cancer Research Award (S.L.M.) as well as 5-K08-CA-277013 (R.M.M.) and grants from the American Society of Hematology and Alex’s Lemonade Stand Foundation (R.M.M.). S.P.H. is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at The Children's Hospital of Philadelphia. S.L.M. is a Scholar in Clinical Research of The Leukemia & Lymphoma Society.
Authorship
Contribution: R.M.M., K.D., L.W., and S.L.M. conceptualized, designed, and planned the study; R.M.M., K.J.D., S.L.M., and R.M. collected the data; R.M.M., Y.L., and H.L. performed the statistical analysis; and all authors reviewed the analyses, contributed to interpretation of results and writing of the manuscript, and approved the final version of the submitted report.
Footnotes
L.W. and S.L.M. contributed equally to this study.
Initial analyses from this manuscript were previously presented in oral abstract form at the 2021 American Society of Hematology annual meeting.
Deidentified, individual participant data may be shared with investigators. To access data, investigators will be required to provide a methodologically sound proposal with approved aims. Data will only be shared if they do not compromise an ongoing trial or study, if there is strong scientific rationale for the data to be used for the requested purpose and if the investigators who have invested time and efforts into development these trials have a period of exclusivity to pursue their own aims with the data. Proposals should be directed to the corresponding author, Shannon L. Maude (maude@chop.edu). To gain access, data requestors will need to sign a data access agreement.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. 2021;39(27):3044–3055. doi: 10.1200/JCO.20.03458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361–2368. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamble A, Myers RM, Taraseviciute A, et al. Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv. 2023;7(4):575–585. doi: 10.1182/bloodadvances.2022007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz LM, Baggott C, Prabhu S, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J Clin Oncol. 2022;40(9):945–955. doi: 10.1200/JCO.20.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulsipher MA, Han X, Maude SL, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3(1):66–81. doi: 10.1158/2643-3230.BCD-21-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier J, Bezerra ED, Hirayama AV, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137(3):323–335. doi: 10.1182/blood.2020006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland EM, Molina JC, Dede K, et al. Efficacy of second CAR-T (CART2) infusion limited by poor CART expansion and antigen modulation. J Immunother Cancer. 2022;10(5) doi: 10.1136/jitc-2021-004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadauke S, Myers RM, Li Y, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39(8):920–930. doi: 10.1200/JCO.20.02477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li AM, Hucks GE, Dinofia AM, et al. Checkpoint inhibitors augment CD19-directed chimeric antigen receptor (CAR) T cell therapy in relapsed B-cell acute lymphoblastic leukemia. Blood. 2018;132(suppl 1):556. [Google Scholar]
- 15.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NN, Lee DW, Yates B, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 2021;39(15):1650–1659. doi: 10.1200/JCO.20.02262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summers C, Wu QV, Annesley C, et al. Hematopoietic cell transplantation after CD19 chimeric antigen receptor T cell-induced acute lymphoblastic lymphoma remission confers a leukemia-free survival advantage. Transplant Cell Ther. 2022;28(1):21–29. doi: 10.1016/j.jtct.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Wudhikarn K, Flynn JR, Rivière I, et al. Interventions and outcomes of adult patients with B-ALL progressing after CD19 chimeric antigen receptor T-cell therapy. Blood. 2021;138(7):531–543. doi: 10.1182/blood.2020009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.