Abstract
Vaccine-induced protection of chimpanzees against laboratory-adapted and syncytium-inducing, multiply passaged primary human immunodeficiency virus type 1 (HIV-1) isolates, but not against non-syncytium-inducing, minimally passaged ones, has been demonstrated. Following challenge with such an isolate, HIV-15016, we obtained complete protection in one of three chimpanzees previously protected against low- and high-dose HIV-1SF2 exposures after immunization with an adenovirus-HIV-1MN gp160 priming–HIV-1SF2 gp120 boosting regimen. At challenge, the protected chimpanzee exhibited broad humoral immunity, including neutralizing antibody activity. These results demonstrate the potential of this combination vaccine strategy and suggest that vaccine protection against an HIV isolate relevant to infection of people is feasible.
A combination vaccine approach consisting of intranasal priming with adenovirus (Ad)-human immunodeficiency virus type 1 strain MN (HIV-1MN) gp160 recombinants followed by intramuscular boosting with CHO cell-expressed HIV-1SF2 gp120 was previously shown to protect chimpanzees against low- and high-dose HIV-1SF2 intravenous (i.v.) challenges with only a minimal number of immunizations (24). High-dose protection was associated with long-lived antibodies which persisted for over a year following the last immunization and were capable of neutralizing both primary and laboratory-adapted HIV-1 isolates (24, 37). While all immunized chimpanzees developed cytotoxic T-lymphocyte (CTL) responses, one lacking neutralizing antibody nevertheless was completely protected against the low-dose challenge and exhibited a reduced viral burden following the high-dose challenge. Thus, a role for HIV-specific CTLs in vaccine-induced control of the viral load in chimpanzees was suggested. Mucosal immune responses in the form of antibodies in secretory fluids were also seen following immunization. Together with results of earlier immunogenicity studies in dogs and chimpanzees (23, 29, 30) and recently observed immune responses in rhesus macaques following Ad host range mutant simian immunodeficiency virus (SIV) env recombinant priming and SIV gp120 boosting (7), these findings suggest that the Ad recombinant-subunit boost approach provides a vaccine with the ability to stimulate production of a complete set of humoral, cellular, and mucosal immune responses.
To pursue these promising results, we decided to challenge the three previously protected chimpanzees a third time, with the heterologous primary isolate HIV-15016. Because of its non-syncytium-inducing (NSI) phenotype, established by lack of syncytial formation in MT2 cells (19), and its clade B V3 loop consensus sequence (10), the 5016 isolate is more representative of U.S. clinical isolates than the other available heterologous challenge isolate, the laboratory strain HIV-1IIIB. Moreover, the 5016 challenge stock, developed after only three passages in human peripheral blood mononuclear cells (PBMCs), gives a robust, persistent infection of chimpanzees. Two naive chimpanzees exhibited viral loads of >106 RNA copies/ml of plasma within 4 weeks of i.v. infection with 30,000 50% tissue culture infective doses (TCID50) (10). Vaccine-induced protection against such a minimally passaged NSI isolate has not previously been shown. A demonstration of protective efficacy against HIV-15016 would further validate our vaccine approach and establish the feasibility of preventing transmission of an isolate relevant to infection of people.
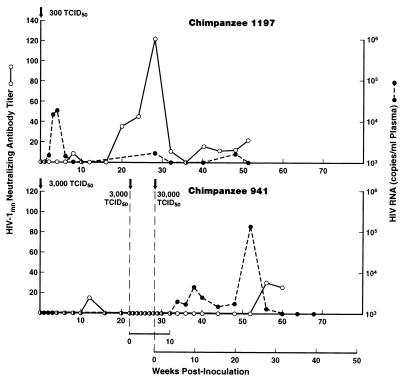
In vivo titration of HIV-15016 challenge stock.
Prior to the challenge experiment, the titer of HIV-15016 in vivo in two naive chimpanzees was determined. Infection was assessed by virus isolation and proviral DNA in PBMCs as previously described (24) and by determination of the level of viral RNA in plasma (33). Chimpanzee 1197, exposed i.v. to 300 TCID50, became infected and exhibited the expected viral burden of 104 RNA copies/ml of plasma within 4 weeks (Fig. 1). Persistent infection was demonstrated post-acute infection by occasional detection of viral RNA in plasma and the development of low-titer, long-lasting, neutralizing antibodies. Chimpanzee 941, initially exposed to 3,000 TCID50, exhibited no plasma viral RNA (Fig. 1). Attempts to isolate virus from or detect proviral DNA in PBMCs were also negative, and the animal did not seroconvert to gag antibodies (not shown). Exposure of chimpanzee 941 to the same dose 22 weeks later again failed to infect the chimpanzee. A dose of 30,000 TCID50 given at week 28 was shown necessary to infect this animal, which over time exhibited a viral burden of 105 RNA copies/ml of plasma (Fig. 1). Thus, to ensure infection of any naive control chimpanzee, the challenge dose was established at 30,000 TCID50. Four of four naive chimpanzees have been infected by this dose (reference 10; this report).
FIG. 1.
In vivo titration of primary isolate HIV-15016. Chimpanzees 1197 and 941 were inoculated with the indicated doses of HIV-15016 at the times marked by the arrows. The viral burden is expressed as RNA copies/milliliter of plasma as assessed by the nucleic acid sequence-based amplification technique (33). Titers of neutralizing antibody to HIV-1MN, expressed as the reciprocal of the serum dilution at which 50% neutralization was observed, were assessed as previously described (31), with H9 cells as targets of infection.
The relative sensitivity of these chimpanzees to in vivo infection by HIV-15016 was not reflected by in vitro studies. Previously frozen PBMCs of chimpanzees 1197 and 941 obtained prior to 5016 exposure were infected in eight replicate microtiter wells with each of six 10-fold serial dilutions of the 5016 challenge stock following one cycle of freeze-thawing. Viral infectivity was determined by p24 antigen capture assay (National Cancer Institute-FCRDC, Frederick, Md.), and the TCID50 was calculated by the method of Spearman-Karber using a computer software program (28). The PBMCs of chimpanzees 941 and 1197 were shown to be equivalently infectable in vitro by the 5016 isolate (1.3 × 104 and 5.6 × 104 TCID50, respectively).
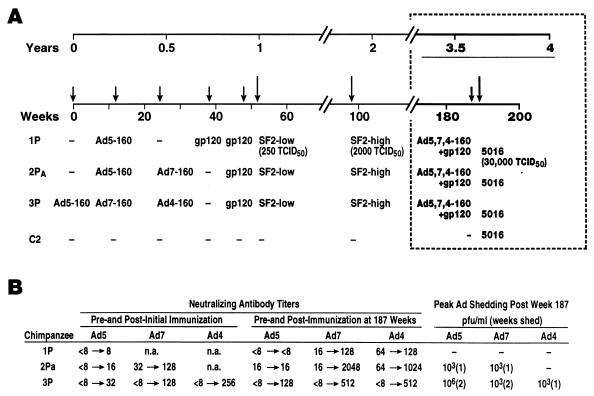
Reboost and challenge of chimpanzees.
Approximately 3.5 years had elapsed since initial immunization of the three chimpanzees, designated 1P, 2PA, and 3P, which were subsequently protected against low- and high-dose challenges with HIV-1SF2 (Fig. 2A; reference 24). Because of the length of time with no booster administrations, at week 187 following their initial immunization, the three chimpanzees were immunized intranasally with 107 PFU of each of the three HIV-1MN gp160 recombinants based in Ad4, Ad5, and Ad7 and intramuscularly with 50 μg of CHO-expressed SF2 gp160 in MF59 adjuvant. Replication of the recombinant Ad in nasal secretions and stool samples was then assessed by nested PCR as previously described (24), with primers and probes selected from the Ad fiber gene and specific for Ad4, Ad5, or Ad7. Ad titers were subsequently determined by serial 10-fold dilution of positive samples. The sensitivity of the nested PCR technique was equivalent to 0.1 PFU/10 μl of sample. Serum Ad neutralizing antibody titers were assessed by a type-specific microneutralization assay (22).
FIG. 2.
Immunization and challenge history of the three previously protected chimpanzees. (A) Prior administrations of one, two, or three HIV-1MN gp160 recombinants based in Ad4, Ad5, or Ad7 vectors and one or two boosts with CHO-expressed gp120 of the HIV-1SF2 strain are listed. The animals were protected against low- and high-dose HIV-1SF2 challenges at weeks 52 and 98, respectively, with no intervening immunizations (24). The timing of the boost and challenge described in this report is outlined in the boxed area. (B) Ad neutralizing antibody titers following initial and booster immunizations are listed. Postimmunization peak titers are listed. Duration of Ad shedding in nasal secretions, expressed in weeks, and peak viral titers of positive samples, expressed as PFU per milliliter, following the booster immunization at week 187 are also listed. Ad titers were adjusted for an ∼10-fold dilution of nasal secretions obtained by swabbing. n.a., not applicable.
Following immunization, low-level shedding of Ad-HIV recombinants was detected in nasal secretions but not in stool samples of chimpanzees 2PA and 3P (Fig. 2B). Both animals exhibited up to sixfold increases in Ad neutralizing antibody titers, indicative of Ad replication in the upper respiratory tract. Previous administrations of Ad gp160 recombinants (Fig. 2A) did not necessarily preclude subsequent vector replication. However, variability in the response to Ad infection is shown by chimpanzee 1P, in which poor replication was reflected by low Ad neutralizing antibody titers and a lack of detectable Ad shedding (Fig. 2B).
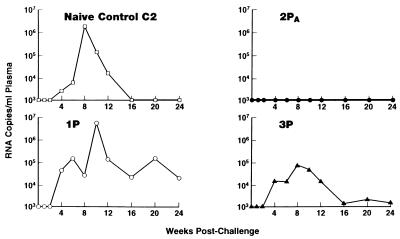
At 189 weeks, the three chimpanzees and naive control chimpanzee C2 were challenged i.v. with 30,000 TCID50 of HIV-15016. Chimpanzee 2PA was completely protected from 5016 infection. Viral RNA was not detected in the animal’s plasma for the entire monitoring period (Fig. 3). The animal remained negative for virus isolation and proviral DNA in PBMCs and did not exhibit HIV gag antibodies in Western blots (Table 1). In contrast, the other three chimpanzees became infected (Fig. 3; Table 1). Control C2 and chimpanzee 1P had viral burdens of >106 RNA copies/ml of plasma, while chimpanzee 3P exhibited a 10-fold reduction in peak plasma viral RNA copy number relative to the naive animal (Fig. 3).
FIG. 3.
Postchallenge assessment of plasma levels of HIV-15016 RNA.
TABLE 1.
Assessment of HIV-15016 infection of chimpanzees
| Chimpanzee | Virologic parameter postchallengea
|
||
|---|---|---|---|
| Virus isolation | Proviral DNA | Time of seroconversion to gag antibody (wk) | |
| 1P | + | + | 8 |
| 2PA | − | − | − |
| 3P | − | + | 4 |
| C2 | − | + | 6 |
Virologic parameters were monitored over 6 months as previously reported (24). +, positive result; −, negative result or no seroconversion.
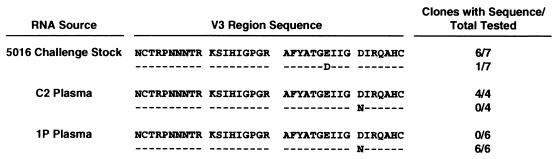
Identification of virus present in infected chimpanzees.
Because the immunized chimpanzees had been previously exposed to HIV-1SF2, viral RNA recovered from serum or plasma samples from the infected animals was analyzed to identify the virus being replicated. RNA was extracted by a guanidine thiocyanate–N-laurylsarcosine protocol (9). The envelope V3 region was amplified by using the GeneAmp RNA PCR kit (Perkin-Elmer Cetus, Norwalk, Conn.) and the primer 5′-CAUCAUCAUCAUCAAATTCTGGGTCCCCTCCTGA GG-3′ in the reverse transcriptase reaction and the primer 5′-CUACUACUACUAGCTAAAACCATAATAGTACAGC TG-3′ in the PCR master mix. PCR products were cloned in the pAMP vector plasmid of the CloneAMP System (Life Technologies, Inc., Gaithersburg, Md.) and sequenced. Four clones obtained from control chimp C2 exhibited a V3 region sequence identical to that of the predominant species present in the 5016 challenge stock (Fig. 4). In contrast, six clones obtained from chimp 1P had a variant V3 loop sequence, suggesting infection by a minor species present in the 5016 stock. Whether immune selection played a role in infection by this variant has not been determined. The V3 loop sequence of the variant differs from that of the SF2 isolate, indicating that the third viral challenge did not reactivate previously undetected virus in animal 1P. No clones could be obtained from the plasma of chimpanzee 3P.
FIG. 4.
Comparison of V3 region sequences of 5016 challenge stock with those present in infected chimpanzees.
Humoral immune responses.
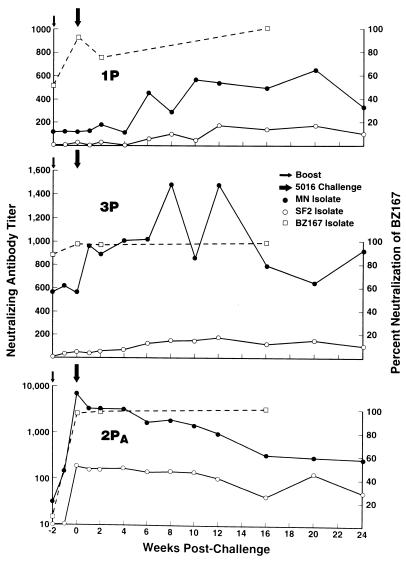
To investigate the basis for the complete protection of chimpanzee 2PA, numerous immunologic parameters were examined over time. Neutralizing antibodies in chimpanzee sera were assessed from week 187 through the postchallenge period. Chimpanzees 1P and 3P exhibited good neutralizing antibody titers against laboratory-adapted HIV-1MN at week 187 postimmunization, but these titers were not boosted until after challenge, reflecting infection (Fig. 5). In contrast, chimpanzee 2PA displayed a dramatic boost in HIV-specific neutralizing antibodies in response to the immunization, rising from a titer of 50 to >8,100 at the time of the boost and challenge, respectively, for the MN isolate and from a titer of <10 to nearly 300 for the SF2 isolate (Fig. 5; Table 2). All three chimpanzees were able to neutralize the primary isolate BZ167 at challenge, with chimpanzee 2PA again displaying an increased response after the booster immunization (Fig. 5; Table 2). Neutralization of HIV-15016 was seen at challenge only in the serum of chimpanzee 2PA (Table 2), in which antibody activity was at the threshold of detection as assessed by using phytohemagglutinin-stimulated human PBMC target cells (24). By using an infectivity reduction technique (26), small reductions in viral infectivity were observed for all three chimpanzees but did not reach statistical significance (Table 2). For the comparison of chimpanzee 2PA serum with that of the control, P was 0.1. The 5016 isolate appears particularly resistant to in vitro neutralization. Two naive chimpanzees persistently infected for over a year with 5016 have at present never developed homologous in vitro neutralizing antibody activity (10, 27).
FIG. 5.
Neutralizing antibody responses at boost and following challenge. Titers of neutralizing antibody to the laboratory isolates, HIV-1MN and HIV-1SF2, for the three immunized chimpanzees are expressed as reciprocal serum dilutions. Neutralization of the primary isolate, BZ167, is expressed as percent neutralization observed at a 1:20 serum dilution.
TABLE 2.
Immune responses at time of challenge
| Chimpanzee | Humoral immune responses
|
Cellular immune responses
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutralizing antibody
|
V3 loop binding antibodyd
|
CTL activity in PBMCse
|
CD8+ suppressor activityf
|
||||||||||
| MNa | SF2a | BZ167b | 5016a | 5016c | MN | SF2 | 5016 | MN | SF2 | 2:1 | 1:1 | 0.5:1 | |
| 2PA | >8,100 | 295 | 97 | 10 | 6 | 9,400 | 1,210 | 1,360 | 2 | 0 | 76 | 65 | 61 |
| 3P | 565 | 45 | 98 | − | 3.6 | 480 | − | − | 0 | 0 | 21 | 26 | 9 |
| 1P | 120 | 20 | 92 | − | 3.3 | 1,340 | − | − | 6 | 1 | 17 | 29 | 4 |
| C2 | − | − | 0 | − | 1 | − | − | − | 0 | 0 | 100 | 100 | 69 |
Mean titer expressed as the reciprocal of the serum dilution at which 50% neutralization was observed. The minus sign signifies a titer of <10.
Percent neutralization using a 1:20 dilution of serum (in the virus-serum incubation mixture).
Fold reduction in viral infectivity in the presence of test serum diluted 1:10 relative to infectivity in the presence of control serum.
Titer expressed as the reciprocal of the serum dilution at which absorbance of the test serum equaled absorbance of the control serum diluted 1:100. The minus sign signifies a titer of <100.
CTL activity expressed as percent cell lysis at an effector-to-target cell ratio of 100:1. Percent lysis of control target cells infected with vaccinia virus β-galactosidase has been subtracted.
Percent suppression of viral replication relative to control CD4+ cultures lacking CD8+ cells at the indicated effector-to-target cell ratios.
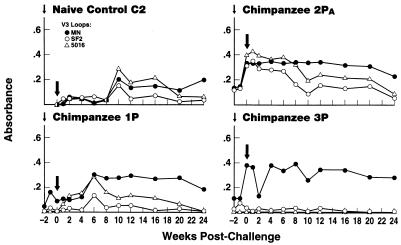
To examine the breadth of the antibody response, we assessed chimpanzee sera for binding to V3 loop peptides synthesized by Peptide Technologies, Inc., Gaithersburg, Md. Peptide sequences for the MN, SF2, and 5016 loops were YNKRKRIHIGPGRAFYTTKNIIGC, TRKSIYIGPGRAFHTT, and TRKSIHIGPGRAFY, respectively. Chimpanzee 2PA serum exhibited the broadest antibody responses, reacting with all three V3 loop peptides tested at the time of challenge (Fig. 6; Table 2). In contrast, sera of chimpanzees 1P and 3P exhibited good reactivity only with the MN peptide at challenge (Table 2). Broadening of the antibody response in chimpanzees 1P and C2 was only observed following infection (Fig. 6).
FIG. 6.
Binding antibodies to V3 loop peptides at boost and following challenge. Binding was assessed by peptide enzyme-linked immunosorbent assay (32) on sera diluted 1:100. Absorbance values of preimmune sera or prechallenge sera for chimpanzee C2 (ranging from 0.055 to 0.122 for the SF2 peptide, from 0.148 to 0.391 for the MN peptide, and from 0.039 to 0.102 for the 5016 peptide) have been subtracted from the values presented.
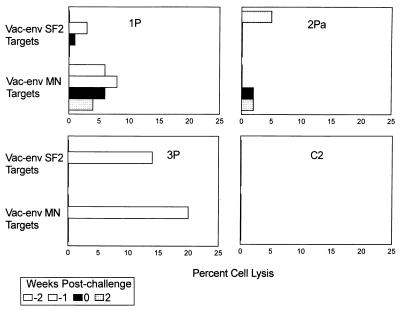
Cellular immune responses.
CTL activity in chimpanzee PBMCs was assessed as previously described (24) with, as target cells, Epstein-Barr virus-transformed autologous chimpanzee B cells infected with either a vaccinia virus-SF2 envelope recombinant (11), a vaccinia virus-MN gp160 recombinant (34), or a vaccinia virus β-galactosidase recombinant as control (8). After receiving the booster, only chimpanzee 3P PBMCs obtained 1 week postboost exhibited significant CTL activity (Fig. 7), which likely resulted from better replication of the Ad gp160 recombinants. Chimpanzee 3P shed Ad5 and Ad7 gp160 recombinants in nasal secretions for 2 weeks (compared to only 1 week for chimpanzee 2PA), exhibited a higher titer of the Ad5 gp160 recombinant shed, and replicated the Ad4 gp160 recombinant while chimpanzees 1P and 2PA did not (Fig. 2B). The CTL response may have contributed to the decreased viral burden observed in chimpanzee 3P following challenge (Fig. 3). Although PBMCs of this animal did not exhibit CTL activity at challenge (Table 2), we have previously seen CTL activity in tissue T lymphocytes when similar activity was not apparent in PBMCs (1, 24).
FIG. 7.
CTL activity observed in chimpanzee PBMCs at boost and following challenge. CTL activity is shown at the time of the booster immunization (−2 weeks postchallenge), 1 week later (−1 week), at the time of challenge (0 weeks), and 2 weeks postchallenge. Data were not recorded if the spontaneous lysis of target cells was greater than 30%. Values shown are for effector-to-target cell ratios of 100:1. No lysis of control targets infected with the vaccinia virus β-galactosidase construct over spontaneous levels was observed in these experiments.
We also considered that protection might have resulted from CD8+ T-cell suppressor activity (35). Suppressor activity of positively selected CD8+ T cells obtained from the four chimpanzees at the time of challenge was assessed in an acute infection system (21, 25) using elutriated human macrophages as targets of HIV-15016 infection. Viral infection was monitored by p24 antigen capture assay. Suppressor activity was high in chimpanzee 2PA and control C2 but low in chimpanzees 1P and 3P (Table 2). Therefore, the complete protection of chimpanzee 2PA was not strictly correlated with this nonlytic, non-major histocompatability complex-restricted, CD8+ antiviral activity.
In vitro HIV-15016 infection.
Chimpanzee 2PA might have remained uninfected as a result of a natural resistance to HIV-15016 infection. The ability of chimpanzee PBMCs to support infection of the 5016 isolate in vitro was determined with previously frozen cells obtained prior to any immunization or challenge. PBMCs were thawed, cultured overnight, and stimulated for 3 days with phytohemagglutinin prior to infection with serial dilutions of a fourth-passage PBMC stock of HIV-15016. TCID50 were calculated as described above for animals 941 and 1197. The results indicated that all prebleed chimpanzee PBMCs were equivalently infectable. The TCID50 on cells from protected chimpanzee 2PA was 1.2 × 102. Values on cells of the other chimpanzees were 6.5 × 102, 7.6 × 102, and 1 × 102 for chimpanzees 1P, 3P, and C2, respectively. Thus, complete protection of chimpanzee 2PA was not correlated with natural resistance to 5016 infection.
Discussion.
In this study, three previously protected chimpanzees were challenged a third time to assess the breadth of protective immunity established by a combination vaccine strategy. The results, first demonstrating vaccine-induced protection against a minimally passaged, heterologous NSI primary HIV isolate, support further development of Ad-HIV recombinants in a bimodal approach for an AIDS vaccine. Only one of three immunized chimpanzees was protected against HIV-15016 infection, but the protection was achieved by using a vaccine regimen based only on the HIV envelope. It is clear from the work of other groups that vaccines incorporating additional HIV genes are more effective (18). We expect that future experiments using multicomponent Ad-HIV recombinants under development will result in greater protective efficacy.
It is not possible to determine whether the two prior HIV-1SF2 challenges contributed to the protection seen in chimpanzee 2PA. The previous exposures may have served as boosts to the immune system, resulting in greater resistance to infection than would have been achieved solely by the immunization regimen. We can speculate that in general such natural boosting, initiated by a level of vaccine-induced immunity able to protect against a first virus exposure, might ultimately provide the basis for long-lasting vaccine protection. In any case, a new study will be required to assess protective efficacy of our combination vaccine approach against an initial HIV-15016 challenge. It is noteworthy, however, that the immunization regimen used here based on envelopes of syncytium-inducing laboratory strains, with or without an additional boosting effect by subinfectious HIV-1SF2 exposure, resulted in protection against an NSI, primary isolate. The result suggests that some common protective mechanisms against syncytium-inducing and NSI viruses may exist.
With regard to the basis for complete protection of chimpanzee 2PA, the striking rise in antibody titer at the time of challenge, the broad antibody reactivity, and the degree of neutralizing activity against the 5016 isolate are notable. Whether the latter, magnified in the undiluted in vivo environment, was sufficient to confer protection is not known, and we cannot conclude that antibody alone provided the protective mechanism. While neutralizing antibodies have been correlated with protection of chimpanzees against i.v. challenge (3, 6, 13, 14, 16, 17) and probably exert their greatest influence against this route of transmission, it is likely that other factors play a role as well. CTL activity, while not observed in PBMCs of chimpanzee 2PA at challenge, may have been sequestered in tissues. In addition, while not correlated here with a lack of infection, a high level of CD8+ suppressor activity was exhibited by chimpanzee 2PA at challenge and could have contributed to its protection. This nonlytic non-major histocompatability complex-restricted inhibitory activity has been associated with protection in SIV macaque models (20, 21, 36).
Other immunization strategies have shown some success in protecting chimpanzees from i.v. challenge with cell-free or cell-associated virus. These include use of subunit immunogens and inactivated virus (2–4, 6, 12, 15–17) and more recent experiments with DNA vaccines (5). At present, the combination Ad recombinant priming-subunit boosting approach has been most promising in terms of duration of protection following few immunizations, the development of antibodies able to neutralize both primary and laboratory-adapted isolates, and most importantly as shown here, protection against a representative U.S. clinical isolate of the NSI type believed responsible for the majority of HIV transmissions between people. Whether this vaccine regimen will also protect against transmission via the highly relevant mucosal route can only be determined following development of a mucosal challenge stock for chimpanzees.
Acknowledgments
We thank Michael Lubeck, John Eldridge, and Stephen Udem for helpful discussion; Ali Javadian and Patrice Frost for carrying out all aspects of the study directly involving the chimpanzees; John Bisbing, Michael Justice, and Lina Cuadra for excellent technical assistance; and Larry Arthur and Genoveffa Franchini for critical reviews of the manuscript.
This study was supported in part by a Cooperative Research and Development Agreement with Lederle-Praxis Biologicals Division, American Cyanamid Company.
REFERENCES
- 1.Abimiku A G, Franchini G, Aldrich K, Myagkikh M, Markham P, Gard E, Gallo R C, Robert-Guroff M. Humoral and cellular immune responses in rhesus macaques infected with human immunodeficiency virus type 2. AIDS Res Hum Retroviruses. 1995;11:383–393. doi: 10.1089/aid.1995.11.383. [DOI] [PubMed] [Google Scholar]
- 2.Barrett N, Eder G, Dorner F. –1991. Characterization of a vaccinia-derived recombinant HIV-1 gp160 candidate vaccine and its immunogenicity in chimpanzees. Biotechnol Ther. 1990;2:91–106. [PubMed] [Google Scholar]
- 3.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant gp120 but not gp160 glycoproteins. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 4.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, Bussiere J, Francis D P, Matthews T, Gregory T J, Obijeski J F. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 5.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 6.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Opstal O M, Culp J, Rosenberg M, De Wilde M, Heidt P, Heeney J. HIV-1 envelope-elicited neutralizing antibody titers correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 7.Buge S L, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio-Castro M, Koening S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conley A J, Kessler II J A, Boots J, McKenna P M, Schleif W A, Emini E A, Mark III G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doe B, Steimer K S, Walker C M. Induction of HIV-1 envelope (gp120)-specific cytotoxic T lymphocyte responses in mice by recombinant CHO cell-derived gp120 is enhanced by enzymatic removal of N-linked glycans. Eur J Immunol. 1994;24:2369–2376. doi: 10.1002/eji.1830241017. [DOI] [PubMed] [Google Scholar]
- 12.El-Amad Z, Murthy K K, Higgins K, Cobb E K, Haigwood N L, Levy J A, Steimer K S. Resistance of chimpanzees immunized with recombinant gp120SF2 to challenge by HIV-1SF2. AIDS. 1995;9:1313–1322. doi: 10.1097/00002030-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Emini E A, Nara P L, Schleif W A, Lewis J A, Davide J P, Lee D R, Kessler J, Conley S, Matsushita S, Putney S D, Gerety R J, Eichberg J W. Antibody-mediated in vitro neutralization of human immunodeficiency virus type 1 abolishes infectivity for chimpanzees. J Virol. 1990;64:3674–3678. doi: 10.1128/jvi.64.8.3674-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 15.Fultz P N, Nara P, Barre-Sinoussi F, Chaput A, Greenberg M L, Muchmore E, Kieny M P, Girard M. Vaccine protection of chimpanzees against challenge with HIV-1-infected peripheral blood mononuclear cells. Science. 1992;256:1687–1690. doi: 10.1126/science.256.5064.1687. [DOI] [PubMed] [Google Scholar]
- 16.Girard M, Kieny M P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore M, Ronco J, Kaczorek M, Gomard E, Gluckman J-C. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard M, Meignier B, Barre-Sinoussi F, Kieny M P, Matthews T, Muchmore E, Nara P, Wei Q, Rimsky L, Weinhold K, Fultz P N. Vaccine-induced protection of chimpanzees against infection by a heterologous human immunodeficiency virus type 1. J Virol. 1995;69:6239–6248. doi: 10.1128/jvi.69.10.6239-6248.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S L, Polacino P, Stallard V, Klaniecki J, Pennarthu S, Travis B M, Misher L, Kornas H, Langlois A J, Morton W R, Benveniste R E. Recombinant subunit vaccine as an approach to study correlates of protection against primate lentivirus infection. Immunol Lett. 1996;51:115–119. doi: 10.1016/0165-2478(96)02564-3. [DOI] [PubMed] [Google Scholar]
- 19.Koot M, Vos A H, Keet R P, de Goede R E, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. HIV-1 biological phenotype in long-term infected individuals evaluated with an MT-2 cocultivation assay. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Kavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 21.Leno, M., L. Carter, D. J. Venzon, J. Romano, P. D. Markham, K. Limbach, J. Tartaglia, E. Paoletti, J. Benson, G. Franchini, and M. Robert-Guroff. CD8+ lumphocyte antiviral activity in monkeys immunized with SIV recombinant poxvirus vaccines: potential role in vaccine efficacy. Submitted for publication. [DOI] [PubMed]
- 22.Lubeck M D, Davis A R, Chengalvala M, Natuk R J, Morin J E, Molnar-Kimber K, Mason B B, Bhat B M, Mizutani S, Hung P P, Purcell R H. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci USA. 1989;86:6763–6767. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubeck M D, Natuk R J, Chengalvala M, Chanda P K, Murthy K K, Murthy S, Mizutani S, Lee S-G, Wade M S, Bhat B M, Bhat R, Dheer S K, Eichberg J W, Davis A R, Hung P P. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res Hum Retroviruses. 1995;10:1443–1449. doi: 10.1089/aid.1994.10.1443. . (Erratum, 11:189.) [DOI] [PubMed] [Google Scholar]
- 24.Lubeck M D, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy S C S, Chanda P K, Nigida S M, Jr, Markham P, Zolla-Pazner S, Steimer K, Wade M, Reitz M, Jr, Arthur L, Mizutani S, Davis A, Hung P P, Gallo R C, Eichberg J, Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 25.Mackewicz C, Levy J A. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res Hum Retroviruses. 1992;8:1039–1050. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 26.Mascola J R, Louder M K, Surman S R, Vancott T C, Yu X F, Bradac J, Porter K R, Nelson K E, Girard M, McNeil J G, McCutchan F E, Birx D L, Burke D S. Human immunodeficiency virus type 1 neutralizing antibody serotyping using serum pools and an infectivity reduction assay. AIDS Res Hum Retroviruses. 1996;12:1319–1328. doi: 10.1089/aid.1996.12.1319. [DOI] [PubMed] [Google Scholar]
- 27.Murthy, K. K., and A. J. Conley. Unpublished data.
- 28.National Center for Biotechnology Information. Spouge ID-50 software, version 2.0. Bethesda, Md: National Library of Medicine, National Institutes of Health; 1993. [Google Scholar]
- 29.Natuk R J, Chanda P K, Lubeck M D, Davis A R, Wilhelm J, Hjorth R, Wade M S, Bhat B M, Mizutani S, Lee S, Eichberg J, Gallo R C, Hung P P, Robert-Guroff M. Adenovirus-human immunodeficiency virus (HIV) envelope recombinant vaccines elicit high-titered HIV-neutralizing antibodies in the dog model. Proc Natl Acad Sci USA. 1992;89:7777–7781. doi: 10.1073/pnas.89.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natuk R J, Lubeck M D, Chanda P K, Chengalvala M, Wade M S, Murthy S C S, Wilhelm J, Vernon S K, Dheer S K, Mizutani S, Lee S-G, Murthy K K, Eichberg J W, Davis A R, Hung P P. Immunogenicity of recombinant human adenovirus-human immunodeficiency virus vaccines in chimpanzees. AIDS Res Hum Retroviruses. 1993;9:395–404. doi: 10.1089/aid.1993.9.395. [DOI] [PubMed] [Google Scholar]
- 31.Robert-Guroff M. Neutralizing antibodies. In: Aldovini A, Walker B, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 179–185. [Google Scholar]
- 32.Robert-Guroff M, Aldrich K, Muldoon R, Stern T L, Bansal G P, Matthews T J, Markham P D, Gallo R C, Franchini G. Cross-neutralization of human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus isolates. J Virol. 1992;66:3602–3608. doi: 10.1128/jvi.66.6.3602-3608.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano J W, van Gemen B, Kievits T. A novel, isothermal detection technology for qualitative and quantitative HIV-1 RNA measurements. Clin Lab Med. 1996;16:89–103. [PubMed] [Google Scholar]
- 34.Takahashi H, Merli S, Putney S D, Houghten R, Moss B, Germain R N, Berzofsky J A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989;246:118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- 35.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Tao L, Mitchell E, Bogers W M J M, Doyle C, Bravery C A, Bergmeier L A, Kelly C G, Heeney J L, Lehner T. Generation of CD8 suppressor factor and β chemokines, induced by xenogenic immunization, in the prevention of simian immunodeficiency virus infection in macaques. Proc Natl Acad Sci USA. 1998;95:5223–5228. doi: 10.1073/pnas.95.9.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zolla-Pazner S, Lubeck M, Xu S, Burda S, Natuk R J, Sinangil F, Steimer K, Gallo R C, Eichberg J W, Matthews T, Robert-Guroff M. Induction of neutralizing antibodies to T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J Virol. 1998;72:1052–1059. doi: 10.1128/jvi.72.2.1052-1059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]