Abstract
Survey studies have played a significant role in understanding the gaps in the knowledge and practices of health practitioners. However, there have been no such survey studies on Ocular Allergy (OA). Thus, the purpose of this study was to develop and validate a survey on OA to better understand the gaps in the diagnostic, treatment, and collaborative care approaches of health practitioners in OA. The survey is titled “Survey on Ocular Allergy for Health Practitioners (SOAHP)”. SOAHP was developed in a five-stage process. First, item extraction via the use of a literature review, second, face and content validity, third, a pilot study, fourth, test–retest reliability, and fifth, finalisation of the survey. 65 items under 6 domains were initially generated in the item extraction phase. Content validity was conducted on 15 experts in the field. This was conducted twice to reach consensus whereby items and domains were added, edited, kept, or removed, resulting in 50 items under 7 domains. The pilot study was conducted on 15 participants from the five relevant health practitioner fields (Allergists/Immunologists, General Practitioners (GPs), Ophthalmologists, Optometrists and Pharmacists). This altered the survey further to 40 items under 7 domains. Test–retest reliability was conducted on 25 participants from the five health practitioner fields. Reliability was moderate to almost perfect for most (97%) investigated items. The finalised survey was 40 items under 7 domains. SOAHP is the first survey created to assess diagnostic, treatment and collaborative care approaches of Allergists/Immunologists, GPs, Ophthalmologists, Optometrists and Pharmacists on OA. SOAHP will be a useful tool in clinical research on OA.
Keywords: Ocular allergy, Health practitioners, Survey validation
Subject terms: Diagnosis, Disease prevention, Health services, Public health, Epidemiology, Outcomes research, Eye diseases, Immunological disorders, Eye manifestations
Introduction
Ocular Allergy (OA) refers to a spectrum of ocular allergic diseases of different severities and underlying pathophysiology1. These diseases lead to debilitating effects on the quality of life (QoL) of affected individuals2–6. OA may also lead to sequelae of other ocular diseases including dry eye7 and keratoconus8, which lead to further effects on QoL9,10 and severe visual impairment11. Further to this, the prevalence of allergy has been increasing worldwide12 and specifically, in Australia13. In fact, in 2016, the world’s most catastrophic thunderstorm asthma event occurred in Melbourne, Australia, due to the pollen of ryegrass (Lolium perenne). This resulted in 10 deaths and 3365 excess respiratory-related cases presenting to public hospital emergency departments, which was a 672% increase than the 3-year average of 50113. With known serious ocular effects and the increasing prevalence of allergy12,13, there needs to be a more effective, efficient and unified, diagnostic, treatment and collaborative care approach to OA.
OA is generally diagnosed and managed by Allergists/Immunologists, General Practitioners (GPs), Ophthalmologists, Optometrists, and Pharmacists in Australia14–16. However, the literature has shown disparities in these health practitioners’ diagnosis and management of the disease17–19. Yet the exact gaps in the knowledge and practices of that lead to these disparities are unknown. Thus, it is essential to understand current gaps in health practitioner diagnostic, treatment, and collaborative care approaches to OA. Through this, there will be less clogging of the healthcare system, a reduced economic burden, and less complications of OA20–22.
Previous literature has shown that survey studies have aided in understanding health practitioner gaps in knowledge and practices surrounding a health condition23–26. Through this, the disparities amongst health practitioners were alleviated and improved practices employed23–26. However, this has been an overlooked concept in OA. At present there are no surveys that have been validated to assess all aspects on OA. Therefore, the role of a health practitioner survey on OA is significant to address this health issue. Thus, the purpose of this paper is to develop such a survey to assess health practitioner diagnostic, treatment, and collaborative care approaches to OA.
Methods
The development and validation of the Survey on Ocular Allergy for Health Practitioners (SOAHP) followed a five-step method. This was established through consideration of the papers by Rodrigues et al.27, Hoffman et al.26, and Howell et al.28, the guidelines by Boateng et al.29, and Tsang et al.30, and the reliability and validity methods discussed by Mikhail et al.31. The five-step method involved; (1) item extraction, (2) face and content validity, (3) pilot study, (4) test–retest reliability, and (5) finalisation. The details of these methods are explained in detail below. Participants were recruited via publicly available email addresses. This study adhered to the tenets of the Declaration of Helsinki and human ethics was approved by Deakin University (reference number: SEBE-2020-68-MOD01). All methods were performed in accordance with the ethics approval and consent to participate was ensured through a plain language statement and consent form for participants to sign.
Step one: item extraction
Items and domains were created by the researchers in this study, via the use of a literature review. This is an established method in the current literature4–6,32–35. This covered the three aspects of diagnosis, treatment, and collaborative care27.
The terminology referring to OA used in the literature search included ‘ocular allergy,’ ‘allergic rhinoconjunctivitis,’ ‘allergic conjunctivitis,’ ‘allergic eye disease,’ ‘acute allergic conjunctivitis’, ‘seasonal allergic conjunctivitis,’ ‘perennial allergic conjunctivitis,’ ‘vernal keratoconjunctivitis,’ ‘atopic keratoconjunctivitis,’ ‘giant papillary conjunctivitis,’ and ‘contact blepharoconjunctivitis.’ A general search using these terms was made which then stemmed into further searches as detailed below.
Following the initial general search on OA, QoL appeared to be a significant topic in OA2–6,18,19,33–50. Therefore, a deeper search was conducted on this topic using the terms, ‘quality of life,’ ‘questionnaires,’ and ‘surveys.’ These keywords were used alongside the terms referring to OA mentioned above. 7 items were extracted, which included 2 items on awareness of QoL questionnaires, 3 items on implementation of QoL questionnaires, and 2 items covering reasons of use of QoL questionnaires. These formed the OA QoL Questionnaires domain.
Another topic of significance, in the initial search on OA, was patient history1,31,40,51–67. Thus, another literature search was conducted on this topic using the following terms; ‘history taking,’ ‘symptoms,’ and ‘signs.’ These keywords were used in multiple different arrangements alongside the terms referring to OA mentioned. 6 items were extracted, whereby 2 assessed awareness of types of OA, 1 assessed hallmark symptom of OA, 1 assessed all symptoms on OA, and 2 were targeted at eye rubbing. These formed the OA History Questions domain.
Likewise, in the initial search of OA, consideration of differential diagnosis appeared to be another significant topic58,68–81. Thus, a further literature search was conducted using the terms ‘red eye,’ and ‘differential diagnosis.’ These keywords were combined with the OA terms mentioned previously. 1 item was extracted, which covered all differential diagnosis on OA. These formed the Differential Diagnosis of OA domain.
Further, diagnostic tools in OA was another significant topic found in the initial literature search on OA56,68,82–99. Thus, an in-depth literature search included the terms ‘diagnostic methods,’ and ‘diagnosis.’ These keywords were arranged alongside the terms used for OA above. 2 items were extracted, which covered all diagnostic methods used in OA and referral pathways for diagnosis of OA. These formed the Diagnostic Methods in OA domain.
Moreover, management of OA was another significant topic found in the initial literature search8,9,52,54,72,82,100–113. Thus, a deeper literature search was conducted using terms including ‘management,’ and ‘treatment’. These keywords were combined with those referring to OA above. 45 items were extracted, which included 2 items covering all treatments in OA that was then broken down into 4 items on prevention strategies, 5 items on symptom and cosmetic remedies, 17 items on topical drops (including both allergy specific and anti-inflammatory eye drops), 6 items on systemic treatments, 8 items on referral pathways for management, and 3 items on knowledge of managements (which covered immunology of OA). These formed the Management Methods in OA domain.
Finally, collaborative care was another topic identified in the initial literature search on OA14–16,40,114–124. Thus, a further search was made using the terms ‘collaborative care,’ ‘interdisciplinary collaborations,’ and ‘health practitioners.’ 3 items were extracted. These formed the Collaborative Care in OA domain.
Additionally, it should be mentioned that 1 item allowed for additional information to be provided. Thus, the only headings in the literature review, which were not covered in SOAHP, were Epidemiology and Systemic Allergy, as these were found to be irrelevant to clinical practice, or difficult to be adapted to the five types of health practitioners, respectively.
The form of the items (i.e. single selection responses, multiple selection responses or open ended questions) was selected through researcher deliberation. Items were altered in the validation methods.
Step two: face and content validity
It is generally recommended that 2 to 20 experts are involved in face and content validity125. The experts (n = 15) involved in this process included 1 Allergist/Immunologist, 1 Ophthalmologist, 1 General Paediatrician who is a researcher in Allergy, Asthma and Immunology, 3 Optometrists, 3 GPs, 3 Pharmacists, and 3 OA Researchers. Experts were selected based on the following guidelines: (a) involved in the care of OA patients, (b) is one of the health practitioners the final survey will be administered to, and/or (c) involved in allergy, asthma and immunology research.
Face validity was first conducted to assess if each item was suitable to the purpose of the topic of OA. Following this, content validity was conducted via the use of the modified Delphi technique126. This technique involves pre-meditated answers to each item in the survey whereby experts in the field assess the items and pre-meditated answers, until consensus is reached.
Experts assessed the relevance, essentiality, and clarity (according to a well-established scale) of each item in the domains27. The established Likert scales for relevance, essentiality, and clarity were selected from the Rodrigues et al.27 paper. The scale for relevance was a 4-point Likert scale, which was 1 = not relevant, 2 = somewhat relevant, 3 = quite relevant and 4 = very relevant, whereby 1 and 2 were considered content-not-relevant and 3 and 4 were considered content-relevant27. The scale for essentiality was also on a 3-point Likert scale whereby 1 = not essential, 2 = useful, but not essential and 3 = essential, whereby 3 was considered essential27. Finally, the scale for clarity was a 3-point Likert scale where 1 = not clear, 2 = item needs some revision and 3 = very clear27. This was conducted on Qualtrics, Provo, UT126. Finally, an open section for suggestions was provided to ensure nothing important surrounding the topic had been missed and to make appropriate amendments to the items.
Data was analysed through Content Validity Index (CVI) using item-CVI (I-CVI) for Relevance, Content Validity Ratio (CVR) for Essentiality, and Averaging Scores for Clarity27. I-CVI was calculated for each item as the number of experts rating the item as 3 or 4 (“quite relevant” and “very relevant”), divided by the total number of experts127. Values range from 0 to 1, where I-CVI > 0.79 means the item is relevant, between 0.70 and 0.79 means the item needs some revisions and below 0.70 means the item is removed27. CVR is calculated using the formula CVR = (Ne − N/2)/(N/2), where Ne is the number of panellist’s indicating the item as 3 (“essential”) and N is the total number of panellist’s27,127,128. Values range from 1 to − 1, and based on the numerical values in Lawshe’s table for n = 15 experts, CVR = 0.49 was the minimum value for an item to be considered essential128. For clarity, the scoring by each expert was averaged for each item and if comments were provided, the item was clarified. Finally, any comments regarding adding questions, editing questions, and removing questions were implemented, if deemed justifiable (e.g. if an item was suggested to be removed, but found to be important to the topic of OA and to be the only item covering this topic, then this was kept but edited as per comments provided).
Step three: pilot study
SOAHP was then administered to 15 participants129 (3 from each of the 5 specialities) who were not the same as those in the content validity phase, via Qualtrics, Provo, UT. This included 3 Allergists/Immunologists, 3 GPs, 3 Ophthalmologists, 3 Optometrists and 3 Pharmacists. The health practitioners needed to be Australian Health Practitioner Regulation Agency (AHPRA) registered and practicing in Australia. Health practitioners who were in the five health fields but were not fully qualified (i.e. students, interns, residents, registrars, and/or in training) were excluded and not allowed to participate in the project. Any other health practitioner not mentioned (e.g. Dermatologists) was also excluded. This was to ensure the pilot study simulated the purpose of SOAHP, which is to assess these five, fully qualified health practitioners’ knowledge and practices on OA.
The respondents were examined on how they ‘comprehended, interpreted and answered’ the survey questions27. This means the respondents were assessed to see if they all had the same understanding of the questions. Thus, the participants were asked if there were any difficulties following the wording of the questions or other problems that may lead to response error or bias. Additionally, data on time taken to complete the survey was also collected via Qualtrics, Provo, UT. It is important to note that data collected in the pilot study was not for the purpose of having a representative sample of health practitioner knowledge and practices but was collected to ensure the survey was piloted before wider administration and adjusted, if required.
Step four: test–retest reliability
SOAHP was administered via Qualtrics, Provo, UT to 25 participants, who were not the same as those from the content validity and pilot study phases130, to assess test–retest reliability. The five health practitioner groups were included: 2 Allergists/Immunologists, 5 GPs, 3 Ophthalmologists, 8 Optometrists and 3 Pharmacists. The same inclusion and exclusion criteria used in the pilot study was implemented in this step. An equal representation of each health practitioner was preferred but not necessary as the purpose was to assess reliability of the survey. These participants completed the survey twice at different times (in a 1–2 week time frame)28 to assess if the same responses were selected on both occasions.
Analysis was conducted on an item-by-item basis using percentage change, percentage agreement, intraclass correlation coefficient (ICC), and percent of agreement for dichotomised index. Percentage change was employed for questions with a high number of response selections. The average percentage change and its standard deviation were calculated for all the responses. An average of < 18 is considered almost perfect, 19–36 is strong, 37–65 is moderate, 66–85 is weak, 86–96 is minimal and > 96 is none131. Percentage agreement was used for questions with yes/no responses and correct/wrong responses. This was evaluated by assessing if the same item was chosen in the first and second completion of the survey. The expected score should be greater than 0.90 for almost perfect agreement, 0.80–0.90 for strong agreement, 0.60–0.79 for moderate agreement, 0.40–0.59 for weak agreement, 0.21–0.39 for minimal agreement and 0–0.20 for no agreement131. 95% confidence intervals were also calculated and reported. Furthermore, ICC was calculated for ordinal scales (e.g. never, rarely, sometimes, frequently and always). ICC > 0.9 is considered excellent, 0.75–0.9 is considered good, 0.5–0.75 is considered moderate and ICC < 0.5 is considered poor reliability132. For questions with nominal responses, agreement was measured by creating a dichotomised indicator that measured whether the same responses were selected the first and second time. This stratified the test–retest comparisons into full agreement (i.e. the same responses selected the first and second time), partial agreement (i.e. some of those selected the first time were also selected the second time) and complete disagreement (i.e. none of those selected the first time were selected the second time). The above cut-offs for percentage agreement was also used for the dichotomised index131.
Step five: finalisation of survey
SOAHP was then finalised ensuring the items and domains were clear, relevant, essential, accurate and consistent. It is essential to note that this survey was a smart survey, whereby certain items only appear if specific answers are selected. Not all items are assessed on each participant. This was a feature applied in Qualtrics, Provo, UT. The finalisation of SOAHP is purposed for wider administration to the five groups of health practitioners, who are AHPRA registered, fully qualified, and practicing in Australia.
Ethics approval and consent to participate
This study received Deakin University ethics approval (reference number: SEBE-2020-68-MOD01). Consent to participate was ensured through a plain language statement and consent form for participants to sign.
Results
Step one: item extraction
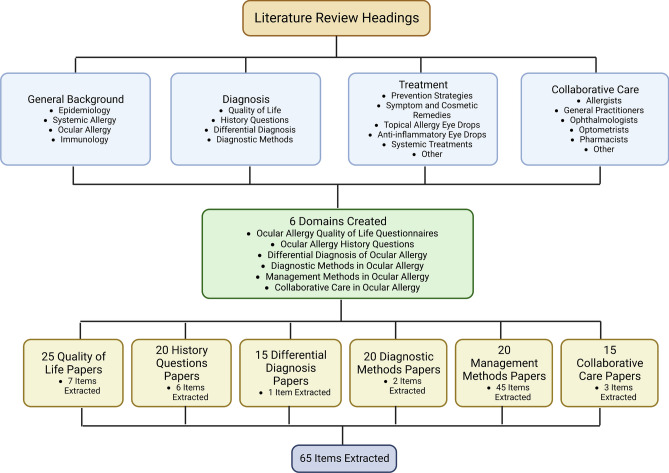
As aforementioned, the SOAHP items and domains were extracted through researcher deliberation via a literature review. The literature review covered the headings of general background, diagnosis, treatment and collaborative care, whereby appropriate subheadings were placed under these primary headings. Through the analysis of approximately 115 papers, 65 items were isolated, as they were considered significant to the topic of OA and placed under 6 domains including: OA QoL Questionnaires, OA History Questions, Differential Diagnosis of OA, Diagnostic Methods in OA, Management Methods in OA, and Collaborative Care in OA. This phase was conducted over a 6-month period. This process is demonstrated in Fig. 1.
Figure 1.
Flow chart of the item extraction process.
Step two: face and content validity
Fifteen experts participated in the face and content validity process, with a recruitment response rate of 40%. Table 1 describes the participant characteristics. There was an almost equal split between males and females, with a mean age of 44.5 ± 12.6 years and mean years of practice of 18.5 ± 13.1. Participants were heavily based in the Australian states of New South Wales and Victoria with 1 Participant from Queensland. Participants worked under different modalities and places of practice. Data was collected over a period of 11 weeks; 6 weeks for Round 1 and 5 weeks for Round 2. There was no participant dropout between Rounds 1 and 2.
Table 1.
Demographic characteristics of participants in content validity (n = 15), pilot study (n = 15), and test–retest reliability (n = 25).
| Characteristic | Content validity, n (%) | Pilot study, n (%) | Test–retest reliability, n (%) |
|---|---|---|---|
| Sex | |||
| Male | 7 (46.7) | 6 (40) | 13 (52) |
| Female | 8 (53.3) | 9 (60) | 12 (48) |
| Age (Years) | |||
| Range | 24–61 | 25–60 | 24–70 |
| Mean | 44.5 | 42.5 | 38.4 |
| Years of practice | |||
| Range | 2–38 | 1–26 | 2–48 |
| Mean | 18.5 | 13.7 | 12.4 |
| State | |||
| Australian Capital Territory | 0 (0) | 0 (0) | 0 (0) |
| New South Wales | 6 (40) | 10 (66.7) | 16 (64) |
| Northern Territory | 0 (0) | 1 (6.7) | 0 (0) |
| Queensland | 1 (6.7) | 0 (0) | 2 (8) |
| South Australia | 0 (0) | 0 (0) | 0 (0) |
| Tasmania | 0 (0) | 0 (0) | 0 (0) |
| Victoria | 8 (53.3) | 4 (26.6) | 3 (12) |
| Western Australia | 0 (0) | 0 (0) | 4 (16) |
| Modality of practice | |||
| Full time | 8 (53.3) | 9 (60) | 15 (60) |
| Part time | 4 (26.7) | 4 (26.7) | 6 (24) |
| Locum/Casual | 3 (20) | 2 (13.3) | 4 (16) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| Place of practice | |||
| Group practice | 7 (46.7) | 9 (60) | 12 (48) |
| Hospital | 5 (33.3) | 2 (13.3) | 7 (28) |
| Solo/Individual practice | 3 (20) | 3 (20) | 3 (12) |
| Community health centre | 0 (0) | 1 (6.7) | 1 (4) |
| Other | 0 (0) | 0 (0) | 2 (8) |
There were 65 total items in the first round of content validity. The results are summarised in Table 2. Face validity was deemed appropriate for all items. For content validity, 61 items were assessed, meaning 4 items were not assessed. Items not assessed were follow-up questions and thus, to reduce the burden of the survey on participants, they were not included in the content validity phases. This means that if the item relating to this follow-up question was removed then the follow-up question would also be removed. Based on relevance and/or essentiality, 17 items (27.9%) did not reach consensus and thus, were required to be re-run in the second round. 44 items reached consensus, whereby 26 items (42.6%) were kept in the survey and 18 items (29.5%) were removed from the survey. This process is demonstrated in Fig. 2. Overall, clarity of all items was 2.79 out of 3, revealing that the survey was clear.
Table 2.
Content validity round 1 and 2 results for relevance, essentiality, and clarity.
| Round 1 | Round 2 | |
|---|---|---|
| Number of items (%) | Number of items (%) | |
| Relevance | ||
| I-CVI 1 | 6/61 (9.8) | 1/23 (4.3) |
| I-CVI 0.93 | 8/61 (13.1) | 5/23 (21.8) |
| I-CVI 0.86 | 15/61 (24.6) | 8/23 (34.8) |
| I-CVI 0.8 | 16/61 (26.2) | 4/23 (17.4) |
| I-CVI 0.73 | 7/61 (11.5) | 3/23 (13.1) |
| I-CVI 0.66 | 5/61 (8.2) | 0/23 (0) |
| I-CVI 0.6 | 3/61 (4.9) | 1/23 (4.3) |
| I-CVI 0.53 | 1/61 (1.7) | 1/23 (4.3) |
| Essentiality | ||
| CVR 1 | 1/61 (1.7) | 0/35 (0) |
| CVR 0.86 | 3/61 (4.9) | 0/35 (0) |
| CVR 0.73 | 9/61 (14.8) | 1/35 (2.9) |
| CVR 0.6 | 17/61 (27.8) | 8/35 (22.8) |
| CVR 0.46 | 7/61 (11.5) | 8/35 (22.8) |
| CVR 0.3 | 8/61 (13.1) | 3/35 (8.6) |
| CVR 0.2 | 2/61 (3.3) | 3/35 (8.6) |
| CVR 0.06 | 3/61 (4.9) | 4/35 (11.4) |
| CVR − 0.06 | 6/61 (9.8) | 3/35 (8.6) |
| CVR − 0.2 | 3/61 (4.9) | 2/35 (5.7) |
| CVR − 0.3 | 2/61 (3.3) | 2/35 (5.7) |
| CVR − 0.46 | 0/61 (0) | 1/35 (2.9) |
| Clarity | ||
| Clarity 3 | 4/61 (6.5) | 3/19 (15.7) |
| Clarity 2.93 | 13/61 (21.3) | 3/19 (15.7) |
| Clarity 2.86 | 9/61 (14.8) | 4/19 (21.1) |
| Clarity 2.8 | 9/61 (14.8) | 1/19 (5.3) |
| Clarity 2.73 | 15/61 (24.6) | 6/19 (31.6) |
| Clarity 2.67 | 4/61 (6.5) | 1/19 (5.3) |
| Clarity 2.6 | 3/61 (4.9) | 0/19 (0) |
| Clarity 2.53 | 3/61 (4.9) | 1/19 (5.3) |
| Clarity 2.33 | 1/61 (1.7) | 0/19 (0) |
Figure 2.
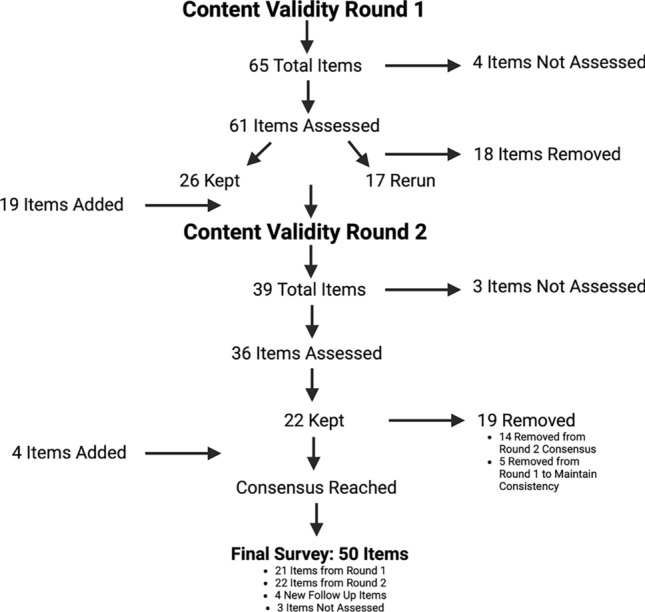
Flow chart of the content validity items that were kept, removed, rerun and added.
For the second round of content validity, there were 39 items. 3 items were those that were not assessed. Thus, 36 items were assessed; 17 were re-runs from the previous round and 19 were new questions suggested by experts. The results are summarised in Table 2. Consensus was reached whereby 22 items (61%) were kept and 14 (39%) were removed. This process is demonstrated in Fig. 2. However, it is significant to note that 5 items that were initially accepted in content validity round 1 were removed in content validity round 2, to maintain consistency of questions. These items were regarding prescribing patterns, however, as some items surrounding prescribing patterns were classed as not relevant and not essential, and thus, removed, then these items were also removed to ensure the survey was consistent. Furthermore, 4 new items were added, based on expert feedback and further deliberation. Overall, clarity for the 19 new items was 2.82 out of 3, again revealing the items were clear.
The final survey following content validity was 50 items under 7 domains. These domains were Red Eye Case Scenario, QoL of OA Patients, OA History Questions, Diagnostic Methods in OA, Management Methods in OA, Knowledge on OA, and Collaborative Care in OA. The addition and removal of domains was based on the alteration of the items in the content validity process due to expert feedback. This was deliberated on by the researchers in this study.
Step three: pilot study
Fifteen participants were involved in the pilot study phase with a recruitment response rate of 25%. Participant characteristics are described in Table 1. There were 40% males and 60% females involved, with a mean age of 42.5 ± 11.6 years and mean years of practice being 13.7 ± 9.0. Participants were again heavily based in New South Wales, and Victoria with 1 participant from the Northern Territory. Like the content validity phase, participants worked under different modalities and places of practice.
The pilot study data was collected over a period of 4 weeks. The average completion time was 37.20 ± 17.4 min. There was an exclusion of one participant's time who was an outlier with an 87-min difference between this participant and the participant with the longest completion time. Participants provided comments, which resulted in the removal of 12 items and the addition of 2 items. The 12 items removed were regarding the management domain. They were all similar items assessing which management is applied to the different types of OA. However, it was found that these items required more context, as managements were applied depending on the severity of the OA, rather than the type, which was a notion expressed by most participants. Overall, SOAHP was clear to all participants. The pilot study reduced the survey to 40 items under the same 7 domains identified in the content validity phase. Thus, it was expected SOAHP would have a shorter completion time during the wider survey distribution.
Step four: test–retest reliability
SOAHP was administered to twenty-five participants for the test–retest reliability phase with a recruitment response rate of 57%. Participant characteristics are described in Table 1. There was almost an equal split between males and females, with a mean age of 38.4 ± 13.6 years, and mean years of practice being 12.4 ± 12.7. Participants were again heavily based in New South Wales with some participants from Victoria, Queensland, and Western Australia. Like the other phases, participants worked under different modalities and places of practice.
Data was collected over a period of 11 weeks and there was an average time of 10.92 ± 4.6 days between the first and second responses. The 40 items under the 7 domains identified in the pilot study were in the survey but only 39 items were analysed as the final item was an open-ended question regarding additional information. For the 39 items analysed, 14 were analysed by percentage change, 13 were analysed by percentage agreement, 4 were analysed using ICC, 6 were analysed using percent of agreement for dichotomised index and 2 were not analysed due to having only one participant response. A summary of the results are shown in Table 3. Overall, test–retest reliability was moderate to almost perfect for 97% of items assessed.
Table 3.
Test–retest reliability results for percentage change, percentage agreement, ICC, and dichotomised index.
| Number of items (%) | |
|---|---|
| Percentage change | |
| < 18 (Almost perfect) | 14/14 (100) |
| 19–36 (Strong) | 0/14 (0) |
| 37–65 (Moderate) | 0/14 (0) |
| 66–85 (Weak) | 0/14 (0) |
| 86–96 (Minimal) | 0/14 (0) |
| > 96 (None) | 0/14 (0) |
| Percentage agreement | |
| > 0.90 (Almost perfect) | 3/13 (23.1) |
| 0.80–0.90 (Strong) | 6/13 (46.2) |
| 0.60–0.79 (Moderate) | 3/13 (23.1) |
| 0.40–0.59 (Weak) | 0/13 (0) |
| 0.21–0.39 (Minimal) | 1/13 (7.6) |
| 0–0.20 (None) | 0/13 (0) |
| ICC | |
| > 0.9 (Excellent) | 3/4 (75) |
| 0.75–0.9 (Good) | 1/4 (25) |
| 0.5–0.75 (Moderate) | 0/4 (0) |
| < 0.5 (Poor) | 0/4 (0) |
| Dichotomised index | |
| > 90% (Almost perfect) | 1/6 (16.7) |
| 80–90% (Strong) | 3/6 (50) |
| 60–79% (Moderate) | 2/6 (33.3) |
| 40–59% (Weak) | 0/6 (0) |
| 21–39% (Minimal) | 0/6 (0) |
| 0–20% (None) | 0/6 (0) |
| Not assessed | 2/2 (100) |
Step five: finalisation of survey
The survey remained unchanged following the pilot study phase, which was an essential criterion to ensure test–retest reliability was accurate. The finalised survey included 40 items under the 7 domains, as described previously. This included 7 items under the Red Eye Case Scenario domain, which covered several topics including 1 item on history questions on OA, 1 item on differential diagnosis of OA, 1 item to diagnose the case scenario, 2 items on management methods in OA, and 2 items on referral pathways. Furthermore, the QoL of OA Patients domain had 3 items, which covered implementation and awareness of QoL in OA. The OA History Questions domain had 5 items, including 2 items on awareness of types of OA, 1 item on the hallmark symptom of OA, 1 item on all symptoms of OA, and 1 item on eye rubbing. Moreover, the Diagnostic Methods in OA domain included 2 items, on all diagnostic methods used in OA and referral pathways for diagnosis of OA. The Management Methods in OA domain had 9 items, with 2 items that broadly covered all types of management methods in OA, then 5 items which looked for the specific management methods in OA, and 2 on referral pathways for management of OA. Further, the Knowledge on OA domain had 9 items, with 3 items on immunology of OA, 3 items were on side effects and/or precautions of management methods, and 3 items on other considerations in the management of OA. Finally, the Collaborative Care in OA domain had 4 items to gauge on points of view and referral pathways in OA. An additional item was provided for comments.
The breakdown of results for Step 2–4 can be seen in Supplementary Material 1.
Discussion
SOAHP is the first validated survey, which assesses Allergists/Immunologists, GPs, Ophthalmologists, Optometrists and Pharmacists knowledge and practices on OA. SOAHP aimed to encompass all domains on OA to better understand these health practitioner diagnostic, treatment and collaborative care approaches to OA. There are 7 domains, excluding demographics: Red Eye Case Scenario, QoL of OA Patients, OA History Questions, Diagnostic Methods in OA, Management Methods in OA, Knowledge on OA, and Collaborative Care in OA. Although the survey was developed with the Australian healthcare model and scopes of practices in mind, this survey can be easily adapted and administered globally, but warrants validation in each country depending on the healthcare model of that country, and scope of practice of each health practitioner.
Although initially not included, the expert comments and further deliberation of researchers found that a case scenario domain was required. The implementation of a case scenario domain was further motivated by Ferreira133, to assess current approaches of health practitioners in real-life case scenarios. This domain, although helpful, can be omitted from the survey without affecting the validation, to allow for a shorter survey. However, the inclusion of a case scenario in SOAHP allows to gauge deeper understanding of gaps in knowledge and practices on OA, as it simulates a clinical environment133 and covers all domains on OA. Further to this, it was able to encapsulate other domains such as the initial differential diagnosis domain which only had 1 item. Thus, this domain was removed as it was covered in the case scenario.
The quality-of-life domain assesses awareness and implementation surrounding currently available questionnaires31, which has not been assessed previously120,134.
The history questions domain, for the first time, assesses the awareness of health practitioners of the different presentations of OA120. A prominent survey conducted in USA (2014) on OA for health practitioners (n = 500), was the AIRS survey120, which lacked specific history questions on OA. Instead, vague questions, (e.g. main symptom that resulted in patient attendance) were employed135. Practitioners stated that ‘itchy eyes’ (62%), being the hallmark symptom of OA, was the most common reason patients sought care135. However, no follow up questions were examined, such as eye rubbing, which is essential as this may lead to severe negative effects including ocular conditions such as keratoconus, which leads to progressive visual loss. The other survey study conducted in Italy (2015) on health practitioners (n = 200) was on allergic rhinitis, which revealed that GPs diagnosed the majority of allergic rhinitis cases134. However, ocular symptoms noted in the survey did not include ‘itchy eyes’ as one of the accompanying symptoms134. Instead, tearing, redness and conjunctivitis were noted. It is alarming that the hallmark symptom of OA (i.e. ‘itchy eyes’) was not queried. Therefore, targeted history taking items were implemented in SOAHP.
The diagnostic methods in OA domain aimed to encompass the variety of tools used by the five different health practitioners in diagnosing OA, which will likely deepen the understanding of the scopes of practice. This is significant as previous literature has revealed disparities in diagnostic methods of different health practitioners in allergic rhinitis120. The AIRS study found that most of those with allergic rhinoconjunctivitis were diagnosed by GPs (46% for age 18 + and 22% for < 18), rather than allergists/immunologists (17% for 18 + and 19% for < 18). However, only 37.4% of GPs employed an allergy test (i.e. skin prick or blood test), compared with 94.9% of allergists/immunologists120. Although, disparities were identified in practices, this did not aid in creating an appropriate model for scopes of practices to aid in a more efficient diagnosis, management, and collaborative care of OA patients. Since the AIRS study only included few diagnostic methods, SOAHP implemented an item that included all diagnostic methods of health practitioners involved in OA to better understand current gaps and therefore, scopes of practice. Through this, an appropriate model of care can be created.
The management methods in OA domain focused on practices of health practitioners in OA, whilst the knowledge on OA domain implemented items with correct and wrong answers to assess knowledge. This has not been gauged previously105,120. Treatment questions in the AIRS study were also not comprehensive for OA (i.e. studies only mentioned recommending immunotherapy referrals to aid in decreasing ocular symptoms). Although significant, other OA-specific treatments should have been questioned105. Likewise, in the Canonica et al. (2015) study, ocular treatments were not assessed as a targeted approach to these patients134. Thus, more targeted questions on ocular specific treatments were applied in SOAHP (e.g. topical treatments) in order to create a more effective treatment system surrounding OA, whereby scopes of practice can be further defined.
Finally, collaborative care in OA items were implemented throughout the survey, but also had a specific domain aimed at understanding the communication between health practitioners in OA, which is lacking in the current literature120,134. Current studies merely create collaborative care models without any profound evidence136. Thus, the need for an evidence based collaborative care model is required.
The survey had overall high content validity: I-CVI ranging from 0.73 to 1.00, average CVR 0.53, and average item clarity of 2.80. Moreover, the pilot study provided information on the participant’s understanding of the survey, thereby permitting necessary edits. Finally, the test–retest reliability phase allows researchers to ensure that the survey is reliable. One of the unforeseen effects of validation, which to our knowledge has not been mentioned in previous literature, is the power of the validation process (e.g. participant feedback), to result in the critical analysis of the items and domains in the survey. Through this, the addition, removal and/or re-establishing of items and domains is instigated. The final version of SOAHP is available as Supplementary Material 2.
Conclusions
SOAHP was formed as a response to current disparities in health practitioner approaches to OA. Additionally, with the aforementioned, effects of OA and the increasing prevalence, there needs to be a more unified approach. SOAHP was validated using appropriate methods to ensure it captures gaps in the knowledge and practitioners of relevant health care practitioners including Allergists/Immunologists, GPs, Ophthalmologists, Optometrists and Pharmacists. This will add fundamental knowledge to the current literature, whereby improved education can be implemented.
Supplementary Information
Acknowledgements
We would like to thank Dr Kyrillos Kaleeny for creating the heading of the survey. Figure created on Biorender.com by EM.
Author contributions
All authors were equally involved in the study design and discussion of each phase of the study. EM was involved in recruitment, data collection, data analysis and write up of manuscript. MM is the expert biostatistician in this study and was heavily involved in data analysis. SA was involved conception of the study and write up of the manuscript. MG was involved in overseeing each phase of the study design and write up of the manuscript. Finally, CS, was the lead supervisor of the project and was involved throughout the entire study including the write up of the manuscript.
Funding
The Deakin University COVID Accelerator Grant given by the School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, was used in this project to compensate participants. This grant had no role in the design or conduct of this research.
Data availability
Data breakdown and final survey available in Supplementary Materials 1 and 2, respectively.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60837-6.
References
- 1.Leonardi A, Bogacka E, Fauquert JL, Kowalski ML, Groblewska A, Jedrzejczak-Czechowicz M, et al. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–1337. doi: 10.1111/all.12009. [DOI] [PubMed] [Google Scholar]
- 2.Alexander M, Berger W, Buchholz P, Walt J, Burk C, Lee J, et al. The reliability, validity, and preliminary responsiveness of the Eye Allergy Patient Impact Questionnaire (EAPIQ) Health Qual. Life Outcomes. 2005;3:67. doi: 10.1186/1477-7525-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1991;21(1):77–83. doi: 10.1111/j.1365-2222.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 4.Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: Development and testing of a questionnaire for clinical trials. J. Allergy Clin. Immunol. 1994;93(2):413–423. doi: 10.1016/0091-6749(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Rohrbaugh T, Meltzer EO. A questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitis. J. Allergy Clin. Immunolol. 2003;111(3):484–490. doi: 10.1067/mai.2003.137. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti M, Baiardini I, Lambiase A, Aronni S, Fassio O, Gramiccioni C, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am. J. Ophthalmol. 2007;144(4):557–563. doi: 10.1016/j.ajo.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann. Allergy Asthma Immunol. 2012;108(3):163–166. doi: 10.1016/j.anai.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.McMonnies CW, Boneham GC. Keratoconus, allergy, itch, eye-rubbing and hand-dominance. Clin. Exp. Optometry. 2003;86(6):376–384. doi: 10.1111/j.1444-0938.2003.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 9.Aydin Kurna S, Altun A, Gencaga T, Akkaya S, Sengor T. Vision related quality of life in patients with keratoconus. J. Ophthalmol. 2014;2014:694542. doi: 10.1155/2014/694542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. JAMA Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Vazirani J, Basu S. Keratoconus: Current perspectives. Clin. Ophthalmol. 2013;7:2019–2030. doi: 10.2147/OPTH.S50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawankar R, Canonica GW, Holgate ST, Lockey RF. White Book on Allergy. World Allergy Organization (WAO); 2011. [Google Scholar]
- 13.Thien F, Beggs PJ, Csutoros D, Darvall J, Hew M, Davies JM, et al. The Melbourne epidemic thunderstorm asthma event 2016: An investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet. Health. 2018;2(6):e255–e263. doi: 10.1016/S2542-5196(18)30120-7. [DOI] [PubMed] [Google Scholar]
- 14.Services AGDoH. Medicare Item Reports 2018. http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp.
- 15.Health Do. Australia’s Future Health Workforce. Ophthalmology. (2018).
- 16.Katelaris CH. Ocular allergy in the Asia Pacific region. Asia Pac. Allergy. 2011;1(3):108–114. doi: 10.5415/apallergy.2011.1.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petricek I, Prost M, Popova A. The differential diagnosis of red eye: A survey of medical practitioners from Eastern Europe and the Middle East. Ophthalmologica. 2006;220(4):229–237. doi: 10.1159/000093076. [DOI] [PubMed] [Google Scholar]
- 18.Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62:17–25. doi: 10.1111/j.1398-9995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 19.Schatz M. A survey of the burden of allergic rhinitis in the USA. Allergy Eur. J. Allergy Clin. Immunol. 2007;62(SUPPL. 85):9–16. doi: 10.1111/j.1398-9995.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 20.Limited AEP. The Economic Impact of Allergic Disease in Australia. (2007).
- 21.ASCIA. Allergy in Australia in 2014: A submission for allergic diseases to be recognised as a National Health Priority Area. (2014).
- 22.ASCIA. Allergy and Immune Diseases in Australia (AIDA) Report. (2013).
- 23.Gunn J, Lumley J, Young D. The role of the general practitioner in postnatal care: A survey from Australian general practice. Br. J. Gen. Pract. 1998;48(434):1570. [PMC free article] [PubMed] [Google Scholar]
- 24.Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner–recorded diagnosis of peanut allergy in England, 2001 to 2005. J. Allergy Clin. Immunol. 2011;127(3):623–30.e1. doi: 10.1016/j.jaci.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Roebroek YGM, Talib A, Muris JWM, van Dielen FMH, Bouvy ND, van Heurn LWE. Hurdles to take for adequate treatment of morbidly obese children and adolescents: Attitudes of general practitioners towards conservative and surgical treatment of paediatric morbid obesity. World J. Surg. 2019;43(4):1173–1181. doi: 10.1007/s00268-018-4874-5. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman SJ, Guindon GE, Lavis JN, Randhawa H, Becerra-Posada F, Dejman M, et al. Surveying the knowledge and practices of health professionals in China, India, Iran, and Mexico on treating tuberculosis. Am. J. Trop. Med. Hygiene. 2016;94(5):959–970. doi: 10.4269/ajtmh.15-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues IB, Adachi JD, Beattie KA, MacDermid JC. Development and validation of a new tool to measure the facilitators, barriers and preferences to exercise in people with osteoporosis. BMC Musculoskelet. Disord. 2017;18(1):540. doi: 10.1186/s12891-017-1914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell DR, Brilliant AN, Meehan WP., III Tandem gait test-retest reliability among healthy child and adolescent athletes. J. Athletic Train. 2019;54(12):1254–1259. doi: 10.4085/1062-6050-525-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: A primer. Front. Public Health. 2018;6:149. doi: 10.3389/fpubh.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J. Anaesth. 2017;11(Suppl 1):S80–S89. doi: 10.4103/sja.SJA_203_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikhail E, Azizoglu S, Gokhale M, Suphioglu C. Questionnaires assessing the quality of life of ocular allergy patients. J. Allergy Clin. Immunol. Pract. 2020 doi: 10.1016/j.jaip.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Sacchetti M, Baiardini I, Chini L, Moschese V, Bruscolini A, Lambiase A. Development and preliminary validation of a new screening questionnaire for identifying atopic children. Pediatr. Health Med. Ther. 2017;8:99–105. doi: 10.2147/PHMT.S142271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valero A. Development and validation of a new spanish instrument to measure health-related quality of life in patients with allergic rhinitis: The ESPRINT questionnaire. Value Health. 2007;10(6):466–477. doi: 10.1111/j.1524-4733.2007.00202.x. [DOI] [PubMed] [Google Scholar]
- 34.Valero A, Alonso J, Antepara I, Baro E, Colas C, del Cuvillo A, et al. Health-related quality of life in allergic rhinitis: Comparing the short form ESPRINT-15 and MiniRQLQ questionnaires. Allergy. 2007;62(12):1372–1378. doi: 10.1111/j.1398-9995.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 35.Ellwood P, Asher MI, Clayton TO, Beasley R, Stewart AW. The International Study of Asthma and Allergies in Childhood (ISAAC): Phase Three rationale and methods. Int. J. Tuberculosis Lung Dis. 2005;9(1):10–16. [PubMed] [Google Scholar]
- 36.Juniper, Thompson, Ferrie, Roberts, Juniper. Development and validation of the Mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin. Exp. Allergy 30(1):132–40 (2000). [DOI] [PubMed]
- 37.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J. Allergy Clin. Immunol. 1998;101(2 Pt 1):163–170. doi: 10.1016/S0091-6749(98)70380-X. [DOI] [PubMed] [Google Scholar]
- 39.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999;104(2):364–369. doi: 10.1016/S0091-6749(99)70380-5. [DOI] [PubMed] [Google Scholar]
- 40.Palmares J, Delgado L, Cidade M, Quadrado MJ, Filipe HP. Allergic conjunctivitis: A national cross-sectional study of clinical characteristics and quality of life. Eur. J. Ophthalmol. 2010;20(2):257–264. doi: 10.1177/112067211002000201. [DOI] [PubMed] [Google Scholar]
- 41.Virchow JC, Kay S, Demoly P, Mullol J, Canonica W, Higgins V. Impact of ocular symptoms on quality of life (QoL), work productivity and resource utilisation in allergic rhinitis patients—An observational, cross sectional study in four countries in Europe. J. Med. Econ. 2011;14(3):305–314. doi: 10.3111/13696998.2011.576039. [DOI] [PubMed] [Google Scholar]
- 42.Ait-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Allergy. 2009;64(1):123–148. doi: 10.1111/j.1398-9995.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 43.WHOQOL-100: World Health Organization. https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/index4.html. (1995).
- 44.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 45.Small M, Piercy J, Demoly P, Marsden H. Burden of illness and quality of life in patients being treated for seasonal allergic rhinitis: A cohort survey. Clin. Transl. Allergy. 2013;3(1):33. doi: 10.1186/2045-7022-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchholz P, Walt J, Wojcik A. Initial development and validation of the eye allergy patient impact questionnaire (EAPIQ) Value Health. 2002;5(6):558. doi: 10.1016/S1098-3015(10)61471-4. [DOI] [Google Scholar]
- 47.Valero A, Izquierdo I, Sastre J, Navarro AM, Baro E, Marti-Guadano E, et al. ESPRINT-15 questionnaire (Spanish version): Reference values according to disease severity using both the original and the modified ARIA classifications. J. Investig. Allergol. Clin. Immunol. 2013;23(1):14–19. [PubMed] [Google Scholar]
- 48.Okuda M, Ohkubo K, Goto M, Okamoto H, Konno A, Baba K, et al. Comparative study of two Japanese rhinoconjunctivitis quality-of-life questionnaires. Acta Oto-laryngologica. 2005;125(7):736–744. doi: 10.1080/00016480510026944. [DOI] [PubMed] [Google Scholar]
- 49.Pitt AD, Smith AF, Lindsell L, Voon LW, Rose PW, Bron AJ. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11(1):17–33. doi: 10.1076/opep.11.1.17.26437. [DOI] [PubMed] [Google Scholar]
- 50.Smith AF, Pitt AD, Rodruiguez AE, Alio JL, Marti N, Teus M, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a spanish setting. Ophthalmic Epidemiol. 2005;12(4):233–242. doi: 10.1080/09286580590967781. [DOI] [PubMed] [Google Scholar]
- 51.Uchio E, Kimura R, Migita H, Kozawa M, Kadonosono K. Demographic aspects of allergic ocular diseases and evaluation of new criteria for clinical assessment of ocular allergy. Graefe's Arch. Clin. Exp. Ophthalmol. 2008;246(2):291–6. doi: 10.1007/s00417-007-0697-z. [DOI] [PubMed] [Google Scholar]
- 52.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008*. Allergy. 2008;63(s86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 53.Leonardi A, Piliego F, Castegnaro A, Lazzarini D, La Gloria VA, Mattana P, et al. Allergic conjunctivitis: A cross-sectional study. Clin. Exp. Allergy. 2015;45(6):1118–1125. doi: 10.1111/cea.12536. [DOI] [PubMed] [Google Scholar]
- 54.Bielory L. Allergic and immunologic disorders of the eye. Part II: Ocular allergy. J. Allergy Clin. Immunol. 2000;106(6):1019–32. doi: 10.1067/mai.2000.111238. [DOI] [PubMed] [Google Scholar]
- 55.La Rosa M, Lionetti E, Leonardi S, Tomarchio S, Reibaldi M, Russo A, et al. Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 2013;39(1):18. doi: 10.1186/1824-7288-39-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonardi A, Doan S, Fauquert JL, Bozkurt B, Allegri P, Marmouz F, et al. Diagnostic tools in ocular allergy. Allergy. 2017;72(10):1485–1498. doi: 10.1111/all.13178. [DOI] [PubMed] [Google Scholar]
- 57.Dart JK, Buckley RJ, Monnickendan M, Prasad J. Perennial allergic conjunctivitis: Definition, clinical characteristics and prevalence. A comparison with seasonal allergic conjunctivitis. Trans. Ophthalmol. Soc. U. K. 1986;105(Pt 5):513–20. [PubMed] [Google Scholar]
- 58.Granet D. Allergic rhinoconjunctivitis and differential diagnosis of the red eye. Allergy Asthma Proc. 2008;29:565–574. doi: 10.2500/aap.2008.29.3170. [DOI] [PubMed] [Google Scholar]
- 59.Leonardi A. Allergy and allergic mediators in tears. Exp. Eye Res. 2013;117:106–117. doi: 10.1016/j.exer.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 60.Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, et al. Japanese guidelines for allergic conjunctival diseases. Allergol. Int. 2017;2017:220–229. doi: 10.1016/j.alit.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: A case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157–1163. doi: 10.1016/S0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 62.Bremond-Gignac D, Donadieu J, Leonardi A, Pouliquen P, Doan S, Chiambarretta F, et al. Prevalence of vernal keratoconjunctivitis: A rare disease? Br. J. Ophthalmol. 2008;92(8):1097–1102. doi: 10.1136/bjo.2007.117812. [DOI] [PubMed] [Google Scholar]
- 63.De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: An update. Br. J. Ophthalmol. 2013;97(1):9–14. doi: 10.1136/bjophthalmol-2011-301376. [DOI] [PubMed] [Google Scholar]
- 64.Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2010;10(5):478–485. doi: 10.1097/ACI.0b013e32833e16e4. [DOI] [PubMed] [Google Scholar]
- 65.Leonardi A, Busca F, Motterle L, Cavarzeran F, Fregona IA, Plebani M, et al. Case series of 406 vernal keratoconjunctivitis patients: A demographic and epidemiological study. Acta Ophthalmol. Scand. 2006;84(3):406–410. doi: 10.1111/j.1600-0420.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 66.Patel N, Venkateswaran N, Wang Z, Galor A. Ocular involvement in atopic disease: A review. Curr. Opin. Ophthalmol. 2018;29(6):576–581. doi: 10.1097/ICU.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 67.Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: A severe allergic eye disease with remodeling changes. Pediatr. Allergy Immunol. 2014;25(4):314–322. doi: 10.1111/pai.12197. [DOI] [PubMed] [Google Scholar]
- 68.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II report executive summary. Ocular Surf. 2017;15(4):802–812. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 70.Jackson WB. Differentiating conjunctivitis of diverse origins. Surv. Ophthalmol. 1993;38(Suppl):91–104. doi: 10.1016/0039-6257(93)90034-5. [DOI] [PubMed] [Google Scholar]
- 71.Mohamed-Noriega K, Mohamed-Noriega J, Valdés-Navarro MA, Cuervo-Lozano EE, Fernández-Espinosa MC, Mohamed-Hamsho J. Conjunctival infection with Chlamydia trachomatis in sexual partners of patients with adult inclusion conjunctivitis. Int. Ophthalmol. 2015;35(2):179–185. doi: 10.1007/s10792-014-9930-z. [DOI] [PubMed] [Google Scholar]
- 72.Schmid KL, Schmid LM. Ocular allergy: Causes and therapeutic options. Clin. Exp. Optometry. 2000;83(5):257–270. doi: 10.1111/j.1444-0938.2000.tb05014.x. [DOI] [PubMed] [Google Scholar]
- 73.Amerasinghe N, Aung T. Angle-closure: Risk factors, diagnosis and treatment. In: Nucci C, Cerulli L, Osborne NN, Bagetta G, editors. Progress in Brain Research. Elsevier; 2008. pp. 31–45. [DOI] [PubMed] [Google Scholar]
- 74.Cheung CSY, Noordeh N, Gottlieb CC. A national survey of Canadian ophthalmologists to determine awareness of published guidelines for the management of uveitis. J. Ophthal. Inflamm. Infect. 2016;6(1):38. doi: 10.1186/s12348-016-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choong YF, Irfan S, Menage MJ. Acute angle closure glaucoma: An evaluation of a protocol for acute treatment. Eye. 1999;13(5):613–616. doi: 10.1038/eye.1999.168. [DOI] [PubMed] [Google Scholar]
- 76.Gutteridge IF, Hall AJ. Acute anterior uveitis in primary care. Clin. Exp. Optometry. 2007;90(2):70–82. doi: 10.1111/j.1444-0938.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 77.Mabey DCW, Solomon AW, Foster A. Trachoma. Lancet. 2003;362(9379):223–229. doi: 10.1016/S0140-6736(03)13914-1. [DOI] [PubMed] [Google Scholar]
- 78.Roscoe M, Landis T. How to diagnose the acute red eye with confidence. JAAPA. 2006;19(3):24–30. doi: 10.1097/01720610-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Tarlan B, Kiratli H. Subconjunctival hemorrhage: Risk factors and potential indicators. Clin. Ophthalmol. 2013;7:1163–1170. doi: 10.2147/OPTH.S35062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watson PG, Hayreh SS. Scleritis and episcleritis. Br. J. Ophthalmol. 1976;60(3):163. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carnt NA, Evans VE, Naduvilath TJ, Willcox MDP, Papas EB, Frick KD, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch. Ophthalmol. 2009;127(12):1616–1623. doi: 10.1001/archophthalmol.2009.313. [DOI] [PubMed] [Google Scholar]
- 82.2007 Report of the International Dry Eye WorkShop (DEWS). 2007.
- 83.Papillary Versus Follicular Conjunctivitis: American Academy of Ophthalmology. https://www.aao.org/bcscsnippetdetail.aspx?id=9d2ac3f7-43cb-4096-9c26-3c7b6d052e20.
- 84.Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge: A clinical approach to studying allergic conjunctivitis. JAMA Ophthalmol. 1990;108(1):84–88. doi: 10.1001/archopht.1990.01070030090035. [DOI] [PubMed] [Google Scholar]
- 85.Ackerman S, D'Ambrosio F, Jr, Greiner JV, Villanueva L, Ciolino JB, Hollander DA. A multicenter evaluation of the efficacy and duration of action of alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J. Asthma Allergy. 2013;6:43–52. doi: 10.2147/JAA.S38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol. Scand. Suppl. 2000;230:52–5. doi: 10.1034/j.1600-0420.2000.078s230052.x. [DOI] [PubMed] [Google Scholar]
- 87.Bielory L, Dinowitz M, Rescigno R. Ocular allergic diseases: Differential diagnosis, examination techniques, and testing. J. Toxicol. Cutaneous Ocular Toxicol. 2002;21(4):329–351. doi: 10.1081/CUS-120016394. [DOI] [PubMed] [Google Scholar]
- 88.Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol. Allergy Clin. N. Am. 2008;28(1):43–58. doi: 10.1016/j.iac.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Hazarika AK, Singh PK. Efficacy of topical application of 0.03% tacrolimus eye ointment in the management of allergic conjunctivitis. J. Nat. Sci. Biol. Med. 2015;6(Suppl 1):S10–S2. doi: 10.4103/0976-9668.166051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moller C, Bjorksten B, Nilsson G, Dreborg S. The precision of the conjunctival provocation test. Allergy. 1984;39(1):37–41. doi: 10.1111/j.1398-9995.1984.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 91.Secchi A, Leonardi A, Discepola M, Deschenes J, Abelson MB. An efficacy and tolerance comparison of emedastine difumarate 0.05% and levocabastine hydrochloride 0.05%: Reducing chemosis and eyelid swelling in subjects with seasonal allergic conjunctivitis. Emadine Study Group. Acta Ophthalmol. Scand. Suppl. 2000;78(230):48–51. doi: 10.1034/j.1600-0420.2000.078s230048.x. [DOI] [PubMed] [Google Scholar]
- 92.Avunduk AM, Avunduk MC, Kapıcıoğlu Z, Akyol N, Tavlı L. Mechanisms and comparison of anti-allergic efficacy of topical lodoxamide and cromolyn sodium treatment in vernal keratoconjunctivitis. Ophthalmology. 2000;107(7):1333–1337. doi: 10.1016/S0161-6420(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 93.Bartlett JD, Howes JF, Ghormley NR, Amos JF, Laibovitz R, Horwitz B. Safety and efficacy of loteprednol etabonate for treatment of papillae in contact lens-associated giant papillary conjunctivitis. Curr. Eye Res. 1993;12(4):313–321. doi: 10.3109/02713689308999455. [DOI] [PubMed] [Google Scholar]
- 94.Eperon S, Berguiga M, Ballabeni P, Guex-Crosier C, Guex-Crosier Y. Total IgE and eotaxin (CCL11) contents in tears of patients suffering from seasonal allergic conjunctivitis. Graefe's Arch. Clin. Exp. Ophthalmol. 2014;252(9):1359–1367. doi: 10.1007/s00417-014-2683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fauquert JL, Jedrzejczak-Czechowicz M, Rondon C, Calder V, Silva D, Kvenshagen BK, et al. Conjunctival allergen provocation test: Guidelines for daily practice. Allergy. 2017;1:43. doi: 10.1111/all.12986. [DOI] [PubMed] [Google Scholar]
- 96.Mashige KP. Ocular allergy. Health SA Gesondheid. 2017;1:112. doi: 10.1016/j.hsag.2016.07.001. [DOI] [Google Scholar]
- 97.Mimura T, Mori M, Usui T, Amano S, Funatsu H, Noma H. Specific tear IgE in patients with moderate-to-severe autumnal allergic conjunctivitis. Int. Arch. Allergy Immunol. 2011;156(4):381–386. doi: 10.1159/000323908. [DOI] [PubMed] [Google Scholar]
- 98.Mimura T, Usui T, Mori M, Funatsu H, Noma H, Yamamoto H, et al. Relationship between total tear and serum IgE in allergic conjunctivitis. Cornea. 2011;154:349–352. doi: 10.1159/000321828. [DOI] [PubMed] [Google Scholar]
- 99.Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy. 2017;72(8):1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 100.Sharma N, Rao K, Maharana PK, Vajpayee RB. Ocular allergy and keratoconus. Indian J. Ophthalmol. 2013;61(8):407–409. doi: 10.4103/0301-4738.116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bielory L. Update on ocular allergy treatment. Expert Opin. Pharmacother. 2002;3(5):541–553. doi: 10.1517/14656566.3.5.541. [DOI] [PubMed] [Google Scholar]
- 102.Donshik PC. Giant papillary conjunctivitis. Trans. Am. Ophthalmol. Soc. 1994;92:687–744. [PMC free article] [PubMed] [Google Scholar]
- 103.Pas-Wyroślak A, Wiszniewska M, Kręcisz B, Świerczyńska-Machura D, Pałczyński C, Walusiak-Skorupa J. Contact blepharoconjunctivitis due to black henna—A case report. Int. J. Occup. Med. Environ. Health. 2012;25(2):196–199. doi: 10.2478/s13382-012-0019-5. [DOI] [PubMed] [Google Scholar]
- 104.Wilkinson CP. Why Should I Wait Between Putting in Eye Drops? American Academy of Ophthalmology; 2016. [Google Scholar]
- 105.Bielory L, Dykewicz M, Craig T, Blaiss M, Leatherman B, Skoner D, et al. Ophthalmology/Optometry Allergic Rhinoconjunctivitis Patient Treatment; The Allergies, Immunotherapy & RhinoconjunctivitiS (AIRS) Provider Survey. Investig. Ophthalmol. Vis. Sci. 2013;54(15):888. [Google Scholar]
- 106.Kamegasawa A, Chaoul MM, El Dib R. Oral antihistamines for seasonal allergic conjunctivitis. Cochrane Database Syst. Rev. 2017;2017(4):CD011172. [Google Scholar]
- 107.Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, et al. Management of ocular allergy. Allergy. 2019;74(9):1611–1630. doi: 10.1111/all.13786. [DOI] [PubMed] [Google Scholar]
- 108.Nabe M, Miyagawa H, Agrawal DK, Sugiyama H, Townley RG. The effect of ketotifen on eosinophils as measured at LTC4 release and by chemotaxis. Allergy Proc. 1991;12(4):267–271. doi: 10.2500/108854191778879313. [DOI] [PubMed] [Google Scholar]
- 109.Shoja MR, Behsharaty MR. Comparison of efficacy and safety of topical Ketotifen (Zaditen) and cromolyn sodium in vernal keratoconjunctivitis. Med. J. Islamic World Acad. Sci. 2006;16(1):35–40. [Google Scholar]
- 110.Sokol KC, Amar NK, Starkey J, Grant JA. Ketotifen in the management of chronic urticaria: Resurrection of an old drug. Ann. Allergy Asthma Immunol. 2013;111(6):433–436. doi: 10.1016/j.anai.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008;122(2 Suppl):S1–84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 112.AMH. Australian Medicines Handbook https://amhonline.amh.net.au/auth.
- 113.MIMS. Monthly Index of Medical Specialities https://www.mims.com.au/index.php/products/mims-online.
- 114.WH Organization . Framework for Action on Interprofessional Education and Collaborative Practice. World Health Organization; 2010. [PubMed] [Google Scholar]
- 115.Orchard C, Bainbridge L, Bassendowski S, Stevenson K, Wagner SJ, Weinberg L, et al. A National Interprofessional Competency Framework. (2010).
- 116.Koff E, Pearce S, Peiris DP. Collaborative Commissioning: Regional funding models to support value-based care in New South Wales. Med. J. Aust. 2021;215(7):297–301.e1. doi: 10.5694/mja2.51243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baker L, Egan-Lee E, Martimianakis MA, Reeves S. Relationships of power: Implications for interprofessional education. J. Interprof. Care. 2011;25(2):98–104. doi: 10.3109/13561820.2010.505350. [DOI] [PubMed] [Google Scholar]
- 118.Hall P. Interprofessional teamwork: Professional cultures as barriers. J. Interprof. Care. 2005;19(sup1):188–196. doi: 10.1080/13561820500081745. [DOI] [PubMed] [Google Scholar]
- 119.World Health O . Interprofessional Collaborative Practice in Primary Health Care: Nursing and Midwifery Perspectives. World Health Organization; 2013. p. 2013. [Google Scholar]
- 120.Blaiss MS, Dykewicz MS, Skoner DP, Smith N, Leatherman B, Craig TJ, et al. Diagnosis and treatment of nasal and ocular allergies: The Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Ann. Allergy Asthma Immunol. 2014;112(4):322–8.e1. doi: 10.1016/j.anai.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 121.Schmid KL, Schmid LM, Swann PG, Hartley L. A survey of ocular therapeutic pharmaceutical agents in optometric practice. Clin. Exp. Optometry. 2000;83(1):16–31. doi: 10.1111/j.1444-0938.2000.tb05071.x. [DOI] [PubMed] [Google Scholar]
- 122.Bilkhu P, Wolffsohn JS, Taylor D, Gibson E, Hirani B, Naroo SA. The management of ocular allergy in community pharmacies in the United Kingdom. Int. J. Clin. Pharmacy. 2013;35(2):190–194. doi: 10.1007/s11096-012-9742-z. [DOI] [PubMed] [Google Scholar]
- 123.Marquez GE, Torres VE, Sanchez VM, Gramajo AL, Zelaya N, Peña FY, et al. Self-medication in ophthalmology: A questionnaire-based study in an Argentinean population. Ophthal. Epidemiol. 2012;19(4):236–241. doi: 10.3109/09286586.2012.689076. [DOI] [PubMed] [Google Scholar]
- 124.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armstrong TS, Cohen MZ, Eriksen L, Cleeland C. Content validity of self-report measurement instruments: An illustration from the development of the Brain Tumor Module of the M.D. Anderson Symptom Inventory. Oncol. Nurs. Forum. 2005;32(3):669–76. doi: 10.1188/05.ONF.669-676. [DOI] [PubMed] [Google Scholar]
- 126.Fernández-Domínguez JC, Sesé-Abad A, Morales-Asencio JM, Sastre-Fullana P, Pol-Castañeda S, de Pedro-Gómez JE. Content validity of a health science evidence-based practice questionnaire (HS-EBP) with a web-based modified Delphi approach. Int. J. Qual. Health Care. 2016;28(6):764–773. doi: 10.1093/intqhc/mzw106. [DOI] [PubMed] [Google Scholar]
- 127.Zamanzadeh V, Ghahramanian A, Rassouli M, Abbaszadeh A, Alavi-Majd H, Nikanfar AR. Design and implementation content validity study: Development of an instrument for measuring patient-centered communication. J. Car. Sci. 2015;4(2):165–178. doi: 10.15171/jcs.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lawshe CH. A quantitative approach to content validity. Pers. Psychol. 1975 doi: 10.1111/j.1744-6570.1975.tb01393.x. [DOI] [Google Scholar]
- 129.Johanson GA, Brooks GP. Initial scale development: Sample size for pilot studies. Educ. Psychol. Meas. 2009;70(3):394–400. doi: 10.1177/0013164409355692. [DOI] [Google Scholar]
- 130.Bujang MA, Baharum N. A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: A review. Arch. Orofacial Sci. 2017;12(1):1–11. [Google Scholar]
- 131.McHugh ML. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 2012;22(3):276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferreira MB. Differences between general practitioners and allergists in treating moderate to severe persistent rhinitis. J. Investig. Allergol. Clin. Immunol. 2012;22(2):136–138. [PubMed] [Google Scholar]
- 134.Canonica GW, Triggiani M, Senna G. 360 degree perspective on allergic rhinitis management in Italy: A survey of GPs, pharmacists and patients. Clin. Mol. Allergy CMA. 2015;13:25. doi: 10.1186/s12948-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bielory L, Skoner DP, Blaiss MS, Leatherman B, Dykewicz MS, Smith N, et al. Ocular and nasal allergy symptom burden in America: The Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Allergy Asthma Proc. 2014;35(3):211–218. doi: 10.2500/aap.2014.35.3750. [DOI] [PubMed] [Google Scholar]
- 136.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020;16(1):5. doi: 10.1186/s13223-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data breakdown and final survey available in Supplementary Materials 1 and 2, respectively.