Abstract
Preeclampsia is a syndrome that continues to be a major contributor to maternal and neonatal mortality, especially in low-income countries. Low-dose aspirin reduces the risk of preeclampsia, but the mechanism is still unknown. Risk factors to identify women at risk of preeclampsia are based on clinical characteristics. Women identified as high-risk would benefit from aspirin treatment initiated, preferably at the end of the first trimester. Current efforts have largely focused on developing screening algorithms that incorporate clinical risk factors, maternal biomarkers, and uterine artery Doppler evaluated in the first trimester. However, most studies on preeclampsia are conducted in high-income settings, raising uncertainties about whether the information gained can be totally applied in low-resource settings. In low- and middle-income countries, lack of adequate antenatal care and late commencement of antenatal care visits pose significant challenges for both screening for preeclampsia and initiating aspirin treatment. Furthermore, the preventive effect of first-trimester screening based on algorithms and subsequent aspirin treatment is primarily seen for preterm preeclampsia, and reviews indicate minimal or no impact on reducing the risk of term preeclampsia. The lack of evidence regarding the effectiveness of aspirin in preventing term preeclampsia is a crucial concern, as 75% of women will develop this subtype of the syndrome. Regarding adverse outcomes, low-dose aspirin has been linked to a possible higher risk of postpartum hemorrhage, a condition as deadly as preeclampsia in many low- and middle-income countries. The increased risk of postpartum hemorrhage among women in low-income settings should be taken into consideration when discussing which pregnant women would benefit from the use of aspirin and the ideal aspirin dosage for preventing preeclampsia. In addition, women's adherence to aspirin during pregnancy is crucial for determining its effectiveness and complications, an aspect often overlooked in trials. In this review, we analyze the knowledge gaps that must be addressed to safely increase low-dose aspirin use in low- and middle-income countries, and we propose directions for future research.
Key words: aspirin dosage, aspirin in preeclampsia, low- and middle-income countries, low-dose aspirin, low-resource settings, maternal and perinatal mortality, preeclampsia prevention, preeclampsia screening
Introduction
Preeclampsia is an obstetric complication that affects 3% to 8% of pregnant women. Together with hemorrhage, they are the leading cause of mortality for mothers and infants in low-resource settings.1,2 Every year, up to 70,000 women worldwide lose their lives, and 500,000 pregnancies result in stillbirths or neonatal deaths because of preeclampsia.3 Hemorrhage is responsible for another 70,000 maternal deaths each year.1 This is accompanied by an even greater excess of morbidity for affected women.
Meta-analyses have reported that the use of low-dose aspirin reduces the risk of preeclampsia by 18% to 62%.4,5 In addition, low-dose aspirin has also been associated with a reduction in stillbirths and neonatal deaths.5 Although some studies have found an association between low-dose aspirin and a decreased risk of a small-for-gestational-age infant,5 others have not found any such association.4,6,7 The latest Cochrane systematic review from 2019 showed that low-dose aspirin should ideally be initiated before 20 weeks of gestation for a protective effect against preeclampsia.5 Another review has suggested that even initiation before 16 weeks of gestation is preferable.8 This is a challenge in some low and middle-income countries (LMIC) in which women might have limited access to antenatal care.9 One of the most significant risk reductions for preterm preeclampsia was reported from the Aspirin for Evidence-based Preeclampsia Prevention (ASPRE) trial.8 This study was conducted in high-income settings, using a combined screening algorithm that included clinical risk factors, maternal biomarkers, and uterine artery Doppler. The intervention used 150 mg of aspirin, and quite importantly, the risk reduction was restricted to the 25% of women with preeclampsia who delivered preterm (<37 weeks). However, a secondary analysis of the ASPRE trial suggested that aspirin, in fact, delays the disease onset from preterm to term.10 Term preeclampsia is not benign and exerts long-lasting consequences on both mother and their children, in particular in LMIC.4 The interest in prescribing higher doses of aspirin is increasing.11,12 At the same time, a growing body of evidence suggests an association between low-dose aspirin use and postpartum hemorrhage.5 Higher aspirin dosages might increase the risk of bleeding complications, including neonatal hemorrhage. However, in low-resource settings, where hemorrhage poses the highest risk of morbidity and mortality, reliable information is extremely limited.
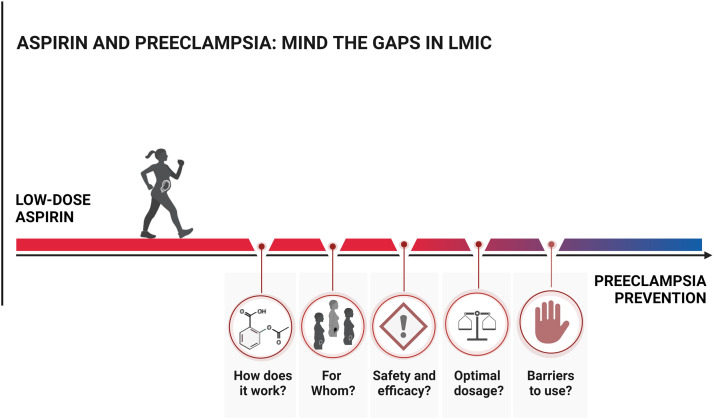
It is common to assume that information gained in studies in high-resource settings can be applied to low-resource settings. This might not be applicable regarding aspirin usage for preeclampsia. In this review, we analyze the existing gaps in knowledge concerning the use of low-dose aspirin in LMIC, including mechanisms of action, screening methods, safety, optimal dosage, adherence, and effectiveness. Addressing these gaps is essential to safely enhance aspirin therapy in LMIC and ultimately reduce maternal and perinatal mortality (Figure 1).
Figure 1.
Aspirin and preeclampsia: mind the gaps LMIC
There are knowledge gaps concerning the use of low-dose aspirin in LMIC. They include aspirin's mechanism, how to identify women who benefit from aspirin, safety and efficacy, optimal aspirin dosage, and adherence to guidelines and treatment. Addressing and bridging these gaps is essential to safely enhance aspirin therapy in LMIC.
LMIC, low and middle-income countries.
Kupka. Aspirin and preeclampsia: mind the gaps. Am J Obstet Gynecol Glob Rep 2024.
How does aspirin reduce preeclampsia?
Aspirin has been proposed as a therapeutic intervention for preeclampsia since the early 1980s.13 The initial target effect was to reduce thromboxane without reducing prostacyclin. This would provide an antithrombotic effect. Nevertheless, even with the positive effects of low-dose aspirin therapy in restoring the placental tromboxane or prostacyclin balance,14 many women treated with aspirin will still develop preeclampsia. Other possible mechanisms include improvement of placentation15 and a reduction of endothelial cell dysfunction.16 However, the underlying course of action for aspirin-prevention of preeclampsia remains unclear. Therefore, it is not possible, other than empirically by outcome, to guide aspirin dosage in the setting of preeclampsia prevention.
Who should take aspirin?
Various screening methods for early pregnancy have been proposed to predict the maternal risk of developing preeclampsia and identify women who would benefit from aspirin use. Screening based on clinical risk factors is the most common approach. However, screening based on the National Institute for Health and Care Excellence (NICE) guideline has been reported to detect only 30% of all cases of preeclampsia and 41% of preterm preeclampsia cases.17 This has led to great debate on the implementation of multivariable prediction models that add maternal biomarkers and uterine artery Doppler to the traditional clinical risk factors.18
It has been demonstrated that combined screening algorithms demonstrate better test performance for preeclampsia requiring early delivery, typically before 37 weeks of gestation, compared with preeclampsia with later onset or all preeclampsia.18 However, as disease prevalence decreases, the positive predictive value declines. For rare conditions like preterm preeclampsia, the positive predictive value is notably low.19 International guidelines vary in their recommendations for screening methods. When accessible, the first-trimester combined test20 is recommended by the International Society for the Study of Hypertension and The International Federation of Gynecology and Obstetrics (FIGO) for universal screening.11,12 The latter acknowledges the challenges of implementing such a strategy in low- and middle-income countries but refers to prospective studies that have validated the effectiveness and predictive performance of the test, primarily in high-income countries (HIC).19,21, 22, 23, 24 In contrast, the American College of Obstetricians and Gynecologists (ACOG) and the NICE highlight limited evidence for the accuracy of predictive models, advocating for clinical risk factor-based screening (Table).12,18,25, 26, 27, 28, 29, 30, 31, 32, 33 A few guidelines on preeclampsia prevention are available from middle-income countries,34 and they are usually based on international recommendations (Table12,18,27, 28, 29, 30, 31, 32, 33). There is a lack of accessible guidelines from LMIC that are adapted to their local settings and population.
Table.
Preeclampsia guidelines
| ACOG27 | NICE28 | ISSHP29 | FIGO12 | SOMANZa.30 | FOGSI31 | SASOG32 | RBEHG33 | |
|---|---|---|---|---|---|---|---|---|
| Clinical risk factors | ||||||||
| Chronic hypertension | High | High | High | Included | High | 3 points | High | High |
| Type 1 or type 2 diabetes | High | High | High | Included | High | 3 points | High | High |
| Renal disease | High | High | High | Included | High | 3 points | High | High |
| Autoimmune disease (SLE, APLS) | High | High | High | Included | High | 3 points | High | High |
| History of preeclampsia | High | High | High | Included | High | 2 points | High | High |
| Multifetal gestation | High | Moderate | Moderate | Included | High | 2 points | Moderate | High |
| History of other pregnancy hypertensive disorder | Not included | High | Not included | Included | High | 2 points | High | Not included |
| Use of assisted reproductive technology | Moderate | Not included | High | Included | Moderate | Oocyte donation or surrogacy: 3 points IVF/ICSI: 1 point |
High | Moderate |
| Body mass index | >30 kg/m2: moderate | ≥35 kg/m2: moderate | >30 kg/m2: moderate | Included | ≥35 kg/m2: moderate | >30 kg/m2: 1 point; >35 kg/m2: 2 points Excessive weight gain during pregnancy: 1 point |
≥35 kg/m2: moderate | >30 kg/m2: high |
| Blood pressure | Not included | Not included | Not included | Not included | SBP >130 mmHg and/or DBP >80 mmHg: moderate |
Median artery pressure >85: 1 point | Not included | Not included |
| Nulliparity | Moderate | Moderate | Moderate | Included | Moderate | 1 point | Moderate | Moderate |
| Family history | Of preeclampsia (mother or sister): moderate | Of preeclampsia (mother or sister): moderate | Not included | Of preeclampsia (mother or sister): included | Of preeclampsia (mother or sister): moderate | Of cardiovascular disease: 1 point Woman born as SGA: 1 point |
Of preeclampsia (mother or sister): moderate | Of preeclampsia (mother or sister): moderate |
| Pregnancy interval | >10 y: moderate | >10 y: moderate | Not included | >10 y: included | >10 y: moderate | >7 y: 1 point | >10 y: moderate | >10 y: moderate |
| Short duration of sperm exposure | Not included | Not included | Not included | Not included | Not included | 1 point | Not included | Not included |
| Maternal age (age) | >35 y: moderate | ≥40 y: moderate | ≥40 y: moderate | Included | ≥40 y: moderate | >35 y or <19 y: 1 point | ≥40 y: moderate | >35 y: moderate |
| Maternal height | Not included | Not included | Not included | Included | Not included | Not included | Included | Not included |
| Obstetric history (LBW, SGA, or previous adverse pregnancy outcome) | Moderate | Not included | Moderate | Included | Not included | Not included | Included | Moderate |
| Other maternal conditions | Not included | Not included | Not included | Not included | Not included | Mental disorder: 3 points Thrombophilia: 3 points Gestational diabetes mellitus: 2 points Hypothyroidism: 2 points Anemia: 1 point Polycystic ovary syndrome: 1 point Chronic vascular disease (dyslipidemia): 1 point |
Not included | Not included |
| Low socioeconomic status | Moderate | Not included | Not included | Included | Not included | Not included | Included | Moderate |
| Black race (as a proxy for underlying racism) | Moderate | Not included | Not included | Included | Not included | Not included | Included | Moderate |
| Recommendations for aspirin prophylaxis | ||||||||
| When to offer aspirin | The presence of any high-risk factor or the presence of any 2 moderate-risk factors | The presence of any high-risk factor or the presence of any 2 moderate-risk factors | Presence of any high-risk factor or presence of any 2 moderate-risk factors or high-risk on the Fetal Medicine Foundation first-trimester combined test | High-risk on the Fetal Medicine Foundation first-trimester combined test | The presence of any high-risk factor or the presence of any 2 moderate-risk factors | A total score of ≥3 | High-risk on the Fetal Medicine Foundation first-trimester combined test, or if not possible to use, presence of any high-risk factor or presence of any 2 moderate-risk factors | The presence of any high-risk factor or the presence of any 2 moderate-risk factors |
| Universal first trimester screening with multivariable prediction model | Does not recommend universal first-trimester screening | Does not recommend universal first-trimester screening | Supports universal first-trimester screening | Supports universal first-trimester screening | Conditionally supports the first trimester screening when accessible | Does not recommend universal first-trimester screening | Supports universal first-trimester screening | Does not recommend universal first-trimester screening |
| Recommended daily dose of aspirin | 81 mg initiated between 12 and 28 wk of gestation, ideally before 16 wk | 75–150 mg from 12 wk of gestation | Women screened with Fetal Medicine Foundation first-trimester combined test: 150 mg before 12 wk of gestation Women screened with risk factors: 100–162 mg before 16 wk of gestation |
150 mg initiated between 11 and 14 wk of (+6 days) gestation | 150 mg before 16 wk of gestation | 75–150 mg before 12 wk of gestation | Women screened with Fetal Medicine Foundation first-trimester combined test: 150 mg before 12 wk of gestation Women screened with risk factors: 75 mg before 12 wk of gestation |
100 mg from 12 wk of gestation |
| When to cease aspirin | Continue until delivery | Continue until delivery | Continue until 36 wk of gestation | Continue until 36 wk of gestation, delivery, or when preeclampsia is diagnosed | Cessation of aspirin between 34 wk of gestation and delivery | Continue until 2 days before delivery | 75 mg: until delivery 150 mg: until 36 wk of gestation |
Continue until 36 wk of gestation |
Adapted from Chappell et al,18 2021.
ACOG, American College of Obstetricians and Gynecologists; APLS, antiphospholipid syndrome; ASOG, The South African Society of Obstetricians and Gynaecologists; FIGO, The International Federation of Gynecology and Obstetrics; FOGSI, Federation of Obstetric and Gynecological Societies of India; ICSI, Intracytoplasmic sperm injection; ISSHP, International Society for the Study of Hypertension in Pregnancy; IVF, in vitro fertilization; LBW, low birthweight; NICE, National Institute for Health and Care Excellence; RBEHG, Brazilian Network For The Study Of Hypertension In Pregnancy; SGA, small-for-gestational-age; SLE, systemic lupus erythematosus; SOMANZ, Society of Obstetric Medicine of Australia and New Zealand.
Guideline still underrevision.
Kupka. Aspirin and preeclampsia: mind the gaps. Am J Obstet Gynecol Glob Rep 2024.
The use of multivariable prediction algorithms is a challenge in LMIC. There are important limitations regarding infrastructure, costs, and access to necessary equipment at antenatal healthcare facilities. FIGO recommends that in LMIC settings, prediction algorithms incorporating at least maternal characteristics, medical history, and blood pressure measurements should be employed. This can increase the detection rate compared with risk factor-based screening. However, many women fail to attend antenatal care in early pregnancy.9,35 Ultrasonographic assessment of gestational age was available in 38% of facilities, but in some countries, availability was as low as <10%. These factors pose a challenge to screening and initiating treatment with low-dose aspirin early in pregnancy in LMIC. In addition, it is unknown if risk factors for preeclampsia in HIC align with those in LMIC, considering potential unique factors such as poor healthcare access and high rates of diseases such as malaria and human immunodeficiency virus.36,37 For instance, there is an association between malaria infections in early pregnancy and defective placentation, potentially leading to hypertensive disorders, intrauterine growth restriction, and preterm birth.38 A recent substudy of the ASPIRIN trial, a randomized multicountry study in 6 LMICs, found that women with malaria in early pregnancy experienced higher rates of perinatal mortality than women without malaria when administered low-dose aspirin.39 Hence, the applicability of risk models developed in HIC to LMICs needs to be assessed carefully.
There has been a suggestion to implement universal aspirin prescription during pregnancy. However, there is no evidence to endorse such a recommendation. A clinical decision analysis study from 2019 evaluated the cost-effectiveness of various models of preeclampsia screening and aspirin prophylaxis strategies.23 The model assumed 100% adherence and that aspirin use was only associated with 2 possible side effects: gastrointestinal bleeding and aspirin-exacerbated respiratory disease. Universal aspirin administration was linked to a reduction in preeclampsia cases and lower overall costs than in scenarios in which no aspirin was administered or in which aspirin was given based on biomarkers and ultrasound measures or only clinical risk factors.23 However, it is unlikely that 100% compliance would be maintained with universal aspirin prescription. As a response, another analysis was performed under the assumption of greater compliance among women at high risk of preeclampsia.40 In this analysis, selectively prescribing aspirin was more efficient and also more cost-effective. These different results raise the controversial aspects of the universal use of low-dose aspirin. Importantly, none of these analyses considered the risk of postpartum hemorrhage.
However, one of the greatest difficulties in many LMICs is limited access to antenatal care. Without access to antenatal care in the first trimester, neither universal aspirin prescription nor universal screening based on complex algorithms is feasible. Improving access to antenatal care is an immense challenge but would have many benefits apart from making first-trimester screening and aspirin initiation possible.
Is aspirin safe in pregnancy?
For decades, the use of low-dose aspirin in pregnancy has been considered safe.41 However, previous research on low-dose aspirin and bleeding events has been scarce and controversial. Bleeding events, and in particular postpartum hemorrhage, have been difficult to study in meta-analyses because of uncertainties in the assessment of blood loss, bleeding often being an exploratory endpoint, different definitions of postpartum hemorrhage, and different national or organizational guidelines for the approach to postpartum hemorrhage. Importantly, some studies have suggested that low-dose aspirin use is associated with an increased risk of bleeding complications during pregnancy and, in particular, postpartum.5,42,43 However, there is no randomized controlled trial that has investigated bleeding events as a primary outcome after low-dose aspirin use in pregnancy.
The 2019 Cochrane Systematic Review of antiplatelet agents for the prevention of preeclampsia included 77 trials and 40,249 women using aspirin at a dosage of 50–150 mg.5 Three large trials included women from upper-middle-income countries, whereas no large trials were conducted in low-income countries. The occurrence of postpartum hemorrhage >500 mL was slightly increased (23,769 women, 19 trials; relative relative risk [RR], 1.06 [95% CI, 1.00–1.12]). Nonetheless, the quality of evidence for this outcome was downgraded to moderate because of differences in measurements of blood loss. In addition, the authors suggested that antiplatelet agents probably marginally increase placental abruption.5
Following the 2019 Cochrane meta-analysis, a more recent meta-analysis from 2023 was published that focused on postpartum hemorrhage in women using low-dose aspirin. The authors reported an increased risk of postpartum hemorrhage among women using low-dose aspirin (odds ratio [OR], 1.20 [95%, CI, 1.07–1.34]).42 It contained 21 studies, including 17 randomized controlled trials. The increased risk remained when only randomized controlled trials were retained (OR, 1.12 [95% CI, 1.00–1.25]), but the estimate was unsure, ranging between 0% to 25% increased risk.42 Five studies originated from LMIC, 14 studies were from HIC, and 3 studies involved multiple-country collaboration. The average dosage of aspirin was 81 mg, with a range from 60 to 150 mg, and the definition of postpartum hemorrhage and time for cessation of aspirin differed across studies. The association between aspirin use and postpartum hemorrhage was stronger in HIC. The cause for this is unknown but might be attributed to reduced adherence to aspirin and challenges in measuring blood loss in LMIC.
A systematic review from 2014, including 27,032 neonates to women using 60–150 mg aspirin, found no association between low-dose aspirin use in pregnancy and neonatal intracranial hemorrhage. In most of these trials, aspirin treatment was continued until delivery, and the predominant aspirin dosages were 60 or 100 mg.44 The Cochrane Systematic Review reported that low-dose aspirin use was associated with no risk of intraventricular hemorrhage (RR, 0.99 [95% CI, 0.72–1.36]) and neonatal bleeding (RR, 0.90 [95% CI, 0.75–1.08]) among 26,184 neonates in 10 trials.5
Hence, robust data suggest a higher risk of postpartum hemorrhage with aspirin use during pregnancy, whereas the link with neonatal intracranial hemorrhage is unclear. Low-dose aspirin use has been associated with an increased risk of placental abruption and intrapartum bleeding.5,45 However, there are conflicting data, with some studies reporting no association.46
Most of these studies were conducted in HIC, and it remains uncertain whether these results apply to low-resource settings. The ASPIRIN trial reported no difference in postpartum hemorrhage >1000 mL between women using aspirin 81 mg (0.9%) and placebo (0.7%). However, the study population consisted of healthy nulliparous women, and the reported incidence of postpartum hemorrhage was unexpectedly low, questioning the accuracy in reporting bleeding outcomes.6 It is possible that the resulting morbidity and mortality would be worse in LMIC without rapid access to emergency obstetric care. Complications like anemia further increase the risk of postpartum hemorrhage and death in LMICs.47 In HIC, maternal deaths because of hemorrhage and preeclampsia are comparable; however, in LMIC, this is not always true. Although hemorrhage and hypertension equally contribute to maternal mortality in Latin America and the Caribbean, hemorrhage accounts for more than twice as many direct maternal deaths as preeclampsia in Asia and Northern Africa.1 However, it is important to note that many women who died because of hemorrhage also had preeclampsia.
Thus, there is an urgent need for large, randomized trials in LMIC that investigate bleeding complications after aspirin use in pregnancy as a primary outcome alongside the protective effects against preeclampsia and its related maternal complications to determine whether the benefits outweigh the harms in these settings.
What is the optimal aspirin dosage?
The question about the optimal aspirin dosage for preeclampsia prevention is still unresolved. International guidelines recommend 75–150 mg per day and vary between countries and regions (Table).12,18,25, 26, 27, 28, 29, 30, 31, 32, 33,48
In the 2019 Cochrane Systematic Review, 80% of women received aspirin 50–75 mg, and 20% received 100–150 mg.5 Although not statistically significant, there was an indication that a higher dosage (≥75 mg) may be more effective than doses <75 mg. In trials using individual participant data, the RR of preeclampsia was 0.92 for women using aspirin <75 mg, although the CI included the possibility of no effect (22,618 women, 11 trials; RR, 0.92 [95% CI, 0.85–1.00]). For women using aspirin ≥75 mg, the RR of preeclampsia was 0.78 (9107 women, 16 trials; 95% CI, 0.66–0.92) in trials using individual participant data.5 A systematic review and meta-analysis from 2017 reported a dose-response effect for the prevention of preeclampsia when aspirin treatment (50–150 mg) was initiated at ≤16 weeks. Higher dosages of aspirin were associated with greater risk reduction. However, out of 21 included studies, only one study from 1985 used 150 mg of aspirin.49 A 2018 study analyzing individual participant data found decreasing rates of preterm preeclampsia with increasing dosage of aspirin but with overlapping confidence intervals.50 In this study, the use of 100 mg aspirin resulted in the lowest predicted rate of preterm preeclampsia of 1.6%.50 Nevertheless, a recent meta-analysis favored 150–162 mg over 75–80 mg for preventing preterm preeclampsia,51 although limited by few studies and methodological issues.52 If a higher dose proves to be more effective, the impact would be greater for women in LMIC than in HIC. Preventing preeclampsia and eclampsia is even more urgent in LMICs, where antenatal care is not always available, access to emergency obstetric services might be limited, and magnesium sulfate is often unavailable or underused.53 In addition, survival rates for preterm infants are significantly lower in LMIC than in HIC.54
The benefit of preeclampsia prevention with increasing dosage must be weighed against the potential bleeding risk. Although higher doses might be more effective, there is concern about whether higher doses increase bleeding complications. This is a very important consideration in LMIC, in which aspirin-associated hemorrhage is likely to be more consequential. In a worst-case scenario, low-dose aspirin would increase maternal mortality because of increased bleeding risk in low-income settings.
Trials assessing the association between low-dose aspirin use and bleeding complications have mainly utilized aspirin dosages below 100 mg,5 and it is essential to confirm the safety of doses exceeding 100 mg in low-resource settings. The ASPRE study did not include postpartum hemorrhage as a primary or secondary outcome, limiting data for assessing bleeding risks with higher dosages (150 mg).4
To resolve the question about the optimal aspirin dosage for preeclampsia prevention, large trials comparing dosages in LMIC assessing both efficacy in preventing preeclampsia and risk of bleeding complications are necessary.
Is there adherence to current guidelines regarding screening and treatment?
To prevent preeclampsia in the clinical setting, 2 conditions must be met. First, the obstetric care provider must adhere to the guidelines for preeclampsia prevention and advise women at risk to use low-dose aspirin. Second, the woman needs to be compliant with the therapy.
Although low-dose aspirin to prevent preeclampsia has been indicated mainly on the basis of the presence of high-risk conditions, the prescription of low-dose aspirin has been described as underused. Retrospective cohort studies from the United States and England, in which risk factors-based screening was employed, found that only 30% of women identified as high-risk for preeclampsia received recommendations for aspirin use.48,55
In addition, women's adherence to aspirin treatment during pregnancy is essential for defining both its effectiveness and potential complications. Despite this importance, most trials have not addressed this topic appropriately, and there is a gap in research regarding aspirin adherence among pregnant women in LMIC. A 2017 review of randomized trials from HIC and LMIC in cardiology and obstetrics found that only 37% (25 out of 68) of obstetric trials and 32% (20 out of 62) of cardiology trials referred to aspirin adherence. It is important to keep in mind that poor adherence not only compromises the success of trials but also can mask adverse effects. The reported aspirin adherence differs between studies. In the ASPRE trial, 80% of participants exhibited an adherence rate of ≥85%, correlating with a decreased risk of preterm preeclampsia.4 However, medical adherence is likely greater in a randomized controlled trial compared with real-world settings. A 2020 Australian prospective cohort study found that only 56% of high-risk women were adherent, defined as having a higher than 90% adherence rate.56 Adherent women experienced lower rates of preeclampsia, intrauterine growth restriction, and preterm birth. A prospective multicenter study in England reported that women screened with the NICE guidelines risk factor-based screening had a compliance rate of only 23%.17 Postpartum hemorrhage was not assessed in either study.
In the nonobstetric literature, adherence to chronic medication is generally lower in LMIC than in HIC.57 Numerous factors serve as barriers to medication adherence in LMICs. They include reliance on traditional medicine, religious beliefs, limited communication with healthcare providers, financial constraints, limited healthcare access, and challenges related to medication availability.58 Interventions such as mobile health services and facility-based interventions have the potential to increase adherence to antenatal healthcare.59 However, this intervention may be difficult to implement in low-resource settings. To increase the utilization of low-dose aspirin among women at risk of preeclampsia, it is crucial to assess the perspectives and beliefs of both pregnant women and obstetric healthcare providers in these settings.
Concluding remarks and perspective
Reducing the incidence of preeclampsia is a crucial step toward decreasing maternal and neonatal mortality in LMICs. However, this cannot come at the expense of an increased risk of bleeding complications. The implementation of aspirin prophylaxis in LMIC is challenging because of economic constraints, lack of necessary equipment, and impairments in providing or attending to antenatal care. The ideal aspirin dosage for preeclampsia prevention remains uncertain. Although higher doses of aspirin might be more effective, there are some concerns that they could raise the likelihood of bleeding events. Another question concerns the timing of aspirin use during pregnancy. Although there is a prevailing belief that it must be started before the 16th week of pregnancy, not all meta-analyses support this view.60
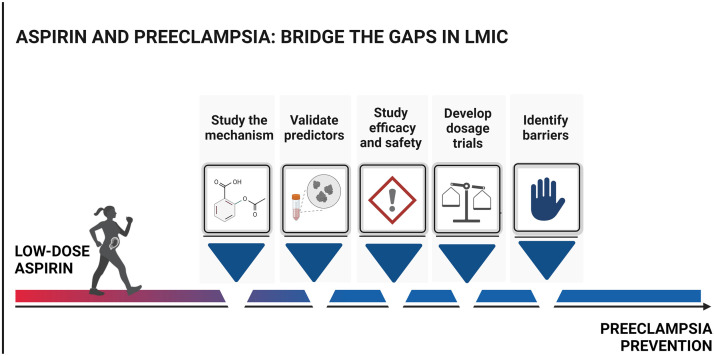
In addition, the adherence of both healthcare providers and pregnant women to aspirin therapy is essential. To safely increase aspirin therapy in LMIC and reduce the burden of preeclampsia, we recommend the investigation of the following research areas for appropriate decisions (Figure 2):
-
•
Incorporate research on aspirin's mechanism of action in clinical trials.
-
•
Identify and validate predictors that are pertinent and feasible in LMIC contexts to use as screening tools.
-
•
Study aspirin's efficacy and safety as primary outcomes in LMIC.
-
•
Trial different dosages of aspirin and measure bleeding as a main outcome in aspirin trials in LMIC.
-
•
Identify barriers and facilitators to antenatal care in LMIC and work with remote solutions to increase adherence and the number of visits in antenatal care.
-
•
Evaluate women's views regarding compliance and what outcomes matter to women with a specific focus on LMIC.
Figure 2.
Aspirin and preeclampsia: bridge the gaps in LMIC
To bridge the knowledge gaps concerning the use of low-dose aspirin in LMIC, we suggest further studies on aspirin's mechanism of action, predictors for screening in LMIC, safety and efficacy, the effects of different dosages of aspirin in randomized trials, and barriers to aspirin use.
LMIC, low and middle-income countries.
Kupka. Aspirin and preeclampsia: mind the gaps. Am J Obstet Gynecol Glob Rep 2024.
The importance of building research capacity in LMICs has long been recognized. However, research challenges in LMICs persist. There is often limited access to scientific infrastructure, including adequate access to clinical data and scientifically trained personnel, and funding constraints.61 The use of mobile phone applications has shown potential to facilitate data collection in LMIC.53 These applications could serve as valuable tools for self-reporting pregnancy outcomes and complications, such as bleeding during aspirin therapy, and for collecting data on predictors of preeclampsia. In addition, they can play a significant role in educating women about the importance of antenatal care, improving communication with healthcare providers, and collecting data on barriers and facilitators to attending antenatal care.
CRediT authorship contribution statement
Ellen Kupka: Writing – review & editing, Writing – original draft. James M. Roberts: Writing – review & editing, Writing – original draft. Zaleha A. Mahdy: Writing – review & editing, Writing – original draft. Carlos Escudero: Writing – review & editing, Writing – original draft. Lina Bergman: Writing – review & editing, Writing – original draft. Leandro De Oliveira: Writing – review & editing, Writing – original draft.
Acknowledgments
Special thanks to the speakers and panelists of the Global Pregnancy Collaboration - CoLab Workshop “Pre- and Peri-conceptional physiology and pathophysiology: Target for innovative therapy,” September 17–19, 2023, whose comments and presentations informed the objectives of this manuscript: Ashley Moffett, PhD (University of Cambridge), Kirk Conrad, PhD (University of Florida College of Medicine), Barbara Luke, PhD (Michigan State University), Mellissa Mann, PhD (University of Pittsburgh), McKenzie Jancsura, PhD (The Ohio State University), Keith Godfrey, PhD (University of Southampton), Ira Bernstein, PhD (University of Vermont), Stephen Lye, PhD (University of Toronto), Graham Burton, PhD (University of Cambridge), Stefan Hansson, PhD (Lund University), David Cantonwine, PhD (Brigham and Women's Hospital, Boston), Swati Shree, MD (University of Washington), Judith Stephenson, PhD (University College London), Shane Norris, PhD (University of the Witwatersrand, Johannesburg), Régine Steegers-Theunissen, PhD (Erasmus University Rotterdam), Dominik Heider, PhD (University of Marburg), Mike Gravett, PhD (University of Washington), Bob Davis, PhD (University of Tennessee), Jenny Myers, PhD (University of Manchester), Manu Vatish, PhD (University of Oxford), Chileshe Mabula-Bwalya, MD (University Teaching Hospital - Women and Newborn Hospital Zambia), Leslie Myatt, PhD (Oregon Health Sciences University), Tom McElrath, PhD (Harvard University). The figures were created by the first author using Biorender.
Footnotes
The authors report no conflict of interest.
E.K. has received funding from the Center for Clinical Research Center Dalarna (grant number CKFUU-974487), C.E. received funding from Fondecyt Regular (identification number 1240295, 1200250), and L.B. received funding from the Wallenberg Center for Molecular and Translational Research.
References
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Global data . 2020. Fragile States Index.https://fragilestatesindex.org/data/ Available at: Accessed October 1, 2023. [Google Scholar]
- 3.World Health Organization; 2018. Recommendations: policy of Interventionist versus expectant management of severe pre-eclampsia before term.http://www.ncbi.nlm.nih.gov/books/NBK535829/ Available at: Accessed October 1, 2023. [PubMed] [Google Scholar]
- 4.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for preterm Preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 5.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019 doi: 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:285–293. doi: 10.1016/S0140-6736(19)32973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastie R, Tong S, Wikström AK, et al. Low-dose aspirin for preventing birth of a small-for-gestational age neonate in a subsequent pregnancy. Obstet Gynecol. 2022;139:529–535. doi: 10.1097/AOG.0000000000004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 9.Beyuo TK, Lawrence ER, Oppong SA, et al. Impact of antenatal care on severe maternal and neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia in Ghana. Pregnancy Hypertens. 2023;33:46–51. doi: 10.1016/j.preghy.2023.07.177. [DOI] [PubMed] [Google Scholar]
- 10.Wright D, Nicolaides KH. Aspirin delays the development of preeclampsia. Am J Obstet Gynecol. 2019;220:580.e1–580.e6. doi: 10.1016/j.ajog.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl1):1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh SW, Strauss JF. The road to low-dose aspirin therapy for the prevention of preeclampsia began with the placenta. Int J Mol Sci. 2021;22:6985. doi: 10.3390/ijms22136985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perneby C, Vahter M, Akesson A, Bremme K, Hjemdahl P. Thromboxane metabolite excretion during pregnancy—influence of preeclampsia and aspirin treatment. Thromb Res. 2011;127:605–606. doi: 10.1016/j.thromres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Su MT, Wang CY, Tsai PY, Chen TY, Tsai HL, Kuo PL. Aspirin enhances trophoblast invasion and represses soluble fms-like tyrosine kinase 1 production: a putative mechanism for preventing preeclampsia. J Hypertens. 2019;37:2461–2469. doi: 10.1097/HJH.0000000000002185. [DOI] [PubMed] [Google Scholar]
- 16.Dzeshka MS, Shantsila A, Lip GYH. Effects of aspirin on endothelial function and hypertension. Curr Hypertens Rep. 2016;18:83. doi: 10.1007/s11906-016-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51:743–750. doi: 10.1002/uog.19039. [DOI] [PubMed] [Google Scholar]
- 18.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 19.O'Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49:751–755. doi: 10.1002/uog.17399. [DOI] [PubMed] [Google Scholar]
- 20.Risk for preeclampsia. The Fetal Medicine Foundation. Available at: https://fetalmedicine.org/research/assess/preeclampsia/first-trimester. Accessed September 7, 2023.
- 21.Poon LC, Rolnik DL, Tan MY, et al. ASPRE trial: incidence of preterm pre-eclampsia in patients fulfilling ACOG and NICE criteria according to risk by FMF algorithm. Ultrasound Obstet Gynecol. 2018;51:738–742. doi: 10.1002/uog.19019. [DOI] [PubMed] [Google Scholar]
- 22.Lobo GAR, Nowak PM, Panigassi AP, et al. Validation of Fetal Medicine Foundation algorithm for prediction of pre-eclampsia in the first trimester in an unselected Brazilian population. J Matern Fetal Neonatal Med. 2019;32:286–292. doi: 10.1080/14767058.2017.1378332. [DOI] [PubMed] [Google Scholar]
- 23.Mallampati D, Grobman W, Rouse DJ, Werner EF. Strategies for prescribing aspirin to prevent preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2019;134:537–544. doi: 10.1097/AOG.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 24.Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126:1242–1250. doi: 10.1097/AOG.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 25.Guidance: hypertension in pregnancy: diagnosis and management. National Institute for Health and Care Excellence. Available at:https://www.nice.org.uk/guidance/ng133. Accessed May 2, 2021. [PubMed]
- 26.ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–e52. doi: 10.1097/AOG.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 27.Low-dose aspirin use for the prevention of preeclampsia and related morbidity and mortality. American College of Obstetricians and Gynecologists. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/12/low-dose-aspirin-use-for-the-prevention-of-preeclampsia-and-related-morbidity-and-mortality. Accessed September 27, 2023.
- 28.National Institute for Health and Care Excellence; 2019. Recommendations: hypertension in pregnancy: diagnosis and management.https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#reducing-the-risk-of-hypertensive-disorders-in-pregnancy Available at: Accessed September 27, 2023. [PubMed] [Google Scholar]
- 29.Magee LA, Brown MA, Hall DR, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148–169. doi: 10.1016/j.preghy.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 30.SOMANZ; 2023. SOMANZ hypertension in pregnancy guideline.https://www.somanz.org/content/uploads/2023/06/SOMANZ_Hypertension_in_Pregnancy_Guideline_2023_Updated_30.6.23.pdf Available at: Accessed September 27, 2023. [Google Scholar]
- 31.FOGSI; 2020. FOGSI's GCPR on hypertensive disorders in pregnancy (HDP) 2019.https://www.fogsi.org/fogsi-hdp-gcpr-2019/ Available at: Accessed September 27, 2023. [Google Scholar]
- 32.Pre-eclampsia: first trimester risk screening and prevention. South African Society of Obstetricians and Gynaecologists. Available at: https://sasog.co.za/wp-content/uploads/2021/10/PREECLAMPSIA-FIRST-TRIMESTER-RISK- SCREENING-AND-PREVENTION.pdf. Accessed September 28, 2023.
- 33.RBEHG; 2023. Brazilian Network for the Study of Hypertension in Pregnancy.https://rbehg.com.br/ Available at: Accessed September 28, 2023. [Google Scholar]
- 34.Scott G, Gillon TE, Pels A, von Dadelszen P, Magee LA. Guidelines-similarities and dissimilarities: a systematic review of international clinical practice guidelines for pregnancy hypertension. Am J Obstet Gynecol. 2022;226:S1222–S1236. doi: 10.1016/j.ajog.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Madaj B, Gopalakrishnan S, Quach A, et al. Where is the “C” in antenatal care and postnatal care: a multi-country survey of availability of antenatal and postnatal care in low- and middle-income settings. BJOG. 2022;129:1546–1557. doi: 10.1111/1471-0528.17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol. 2009;170:847–853. doi: 10.1093/aje/kwp207. [DOI] [PubMed] [Google Scholar]
- 37.Sansone M, Sarno L, Saccone G, et al. Risk of preeclampsia in human immunodeficiency virus-infected pregnant women. Obstet Gynecol. 2016;127:1027–1032. doi: 10.1097/AOG.0000000000001424. [DOI] [PubMed] [Google Scholar]
- 38.Bauserman M, Conroy AL, North K, Patterson J, Bose C, Meshnick S. An overview of malaria in pregnancy. Semin Perinatol. 2019;43:282–290. doi: 10.1053/j.semperi.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauserman M, Leuba SI, Hemingway-Foday J, et al. The efficacy of low-dose aspirin in pregnancy among women in malaria-endemic countries. BMC Pregnancy Childbirth. 2022;22:303. doi: 10.1186/s12884-022-04652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuckle H. Strategies for prescribing aspirin to prevent preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2020;135:217. doi: 10.1097/AOG.0000000000003631. [DOI] [PubMed] [Google Scholar]
- 41.CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-Dose Aspirin Study in Pregnancy) Collaborative Group. Lancet (Lond Engl) 1994;343:619–629. [PubMed] [Google Scholar]
- 42.Jiang Y, Chen Z, Chen Y, et al. Low-dose asprin use during pregnancy may be a potential risk for postpartum hemorrhage and increased blood loss: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5 doi: 10.1016/j.ajogmf.2023.100878. [DOI] [PubMed] [Google Scholar]
- 43.Hastie R, Tong S, Wikström AK, Sandström A, Hesselman S, Bergman L. Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population-based cohort study. Am J Obstet Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.07.023. 95.e1–12. [DOI] [PubMed] [Google Scholar]
- 44.Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 45.Souter V, Painter I, Sitcov K, Khalil A. Propensity score analysis of low dose aspirin and bleeding complications in pregnancy. Ultrasound Obstet Gynecol. 2024;63:81–87. doi: 10.1002/uog.27472. [DOI] [PubMed] [Google Scholar]
- 46.Roberge S, Bujold E, Nicolaides KH. Meta-analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol. 2018;218:483–489. doi: 10.1016/j.ajog.2017.12.238. [DOI] [PubMed] [Google Scholar]
- 47.WOMAN-2 Trial Collaborators Maternal anaemia and the risk of postpartum haemorrhage: a cohort analysis of data from the WOMAN-2 trial. Lancet Glob Health. 2023;11:e1249–e1259. doi: 10.1016/S2214-109X(23)00245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Preventive Services Task Force. Davidson KW, Barry MJ, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:1186–1191. doi: 10.1001/jama.2021.14781. [DOI] [PubMed] [Google Scholar]
- 49.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:110–120.e6. doi: 10.1016/j.ajog.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 50.Seidler AL, Askie L, Ray JG. Optimal aspirin dosing for preeclampsia prevention. Am J Obstet Gynecol. 2018;219:117–118. doi: 10.1016/j.ajog.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Ghesquiere L, Guerby P, Marchant I, et al. Comparing Aspirin 75 to 81 mg vs 150 to 162 mg for prevention of preterm preeclampsia: systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5 doi: 10.1016/j.ajogmf.2023.101000. [DOI] [PubMed] [Google Scholar]
- 52.Cluver C, Kupka E, Hesselman S, Tong S, Hastie R, Bergman L. Comparing Aspirin 75 to 81 mg vs 150 to 162 mg for prevention of preterm preeclampsia: systematic review and meta-analysis: questionable quality and small study effects? Am J Obstet Gynecol MFM. 2023;5 doi: 10.1016/j.ajogmf.2023.101098. [DOI] [PubMed] [Google Scholar]
- 53.Mayrink J, Reis ZSN. Pre-eclampsia in low and middle-income settings: what are the barriers to improving perinatal outcomes and evidence-based recommendations? Int J Gynaecol Obstet. 2024;164:33–39. doi: 10.1002/ijgo.14913. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg RL, McClure EM. Improving birth outcomes in low- and middle-income countries. N Engl J Med. 2017;377:2387–2388. doi: 10.1056/NEJMe1713831. [DOI] [PubMed] [Google Scholar]
- 55.Guy GP, Leslie K, Diaz Gomez D, et al. Implementation of routine first trimester combined screening for pre-eclampsia: a clinical effectiveness study. BJOG. 2021;128:149–156. doi: 10.1111/1471-0528.16361. [DOI] [PubMed] [Google Scholar]
- 56.Shanmugalingam R, Wang X, Motum P, et al. Clinical influence of nonadherence with prophylactic aspirin in preventing preeclampsia in high-risk pregnancies: a multicenter, prospective, observational cohort study. Hypertension. 2020;75:1125–1132. doi: 10.1161/HYPERTENSIONAHA.119.14107. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen JØ, Shrestha AD, Neupane D, Kallestrup P. Non-adherence to anti-hypertensive medication in low- and middle-income countries: a systematic review and meta-analysis of 92443 subjects. J Hum Hypertens. 2017;31:14–21. doi: 10.1038/jhh.2016.31. [DOI] [PubMed] [Google Scholar]
- 58.Khoiry QA, Alfian SD, van Boven JFM, Abdulah R. Self-reported medication adherence instruments and their applicability in low-middle income countries: a scoping review. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mzembe T, Chikwapulo V, Kamninga TM, et al. Interventions to enhance healthcare utilisation among pregnant women to reduce maternal mortality in low- and middle-income countries: a review of systematic reviews. BMC Public Health. 2023;23:1734. doi: 10.1186/s12889-023-16558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative Group Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 61.Hasan BS, Rasheed MA, Wahid A, Kumar RK, Zuhlke L. Generating evidence from contextual clinical research in low- to middle income countries: a roadmap based on theory of change. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.764239. [DOI] [PMC free article] [PubMed] [Google Scholar]