Abstract
Morus alba L., a common traditional Chinese medicine (TCM) with a centuries-old medicinal history, owned various medicinal parts like Mori folium, Mori ramulus, Mori cortex and Mori fructus. Different medical parts exhibit distinct modern pharmacological effects. Mori folium exhibited analgesic, anti-inflammatory, hypoglycemic action and lipid-regulation effects. Mori ramulus owned anti-bacterial, anti-asthmatic and diuretic activities. Mori cortex showed counteraction action of pain, inflammatory, bacterial, and platelet aggregation. Mori fructus could decompose fat, lower blood lipids and prevent vascular sclerosis. The main chemical components in Morus alba L. covered flavonoids, phenolic compounds, alkaloids, and amino acids. This article comprehensively analyzed the recent literature related to chemical components and pharmacological actions of M. alba L., summarizing 198 of ingredients and described the modern activities of different extracts and the bioactive constituents in the four parts from M. alba L. These results fully demonstrated the medicinal value of M. alba L., provided valuable references for further comprehensive development, and layed the foundation for the utilization of M. alba L.
Keywords: Mori folium, Mori ramulus, Mori cortex, Mori fructus, chemical constituents, pharmacological activities
1 Introduction
Morus alba L., a deciduous tree species, belonging to the Moraceae family and Morus genus. China is the relatively early known country raising silkworms and growing M. alba L. The presence of M. alba L. could be traced back to thousands of years ago (Zeng et al., 2022). Besides, many medical classics such as Shennong Ben Cao, Tang Ben Cao and Ben Cao Gang Mu also recorded it (Wenmin Du, 2022). For the past few years, dozens of varieties of M. alba L. were widely planted in China, including cultivated and wild species (Ai et al., 2021). In TCM, M. alba L. is regarded as a treasure due to the rich active ingredients and modern activities of its different parts.
Mori cortex and Mori fructus taste slight cool and sweet. Mori cortex could purge and promoting water, relieve cough and asthma, reduce blood pressure, and against inflammatory (Batiha et al., 2023). Mori fructus could nourish blood and enhance immune function. Mori ramulus, which is mild and taste a litter bitter, owned functions of dispelling wind dampness and promoting blood circulation. Mori folium is a slight muted and possessed effects of dispelling wind, clearing heat, cooling blood, and improving eyesight. Mori fructus and Mori folium exhibit both medicinal and edible properties, making them widely used in medicine and food fields (Maqsood et al., 2022). In some Asian countries, Mori folium is used as a nutritional supplement (Liu et al., 2024). In South Korea, it is widely used as one ingredient of ice cream (Polumackanycz et al., 2021). In Japan, it is used as an anti-hyperglycemic supplement for the treatment of diabetes (Suthamwong et al., 2020). Recently, with the deepening awareness of M. alba L., its role in lowering blood sugar, alleviating depression, antioxidant and liver protection have been widely concerned.
The current pharmacological researches on M. alba L. mainly focus on Mori folium, Mori ramulus, Mori cortex and Mori fructus. With the rapid advance of science and technology, more bioactive substances covered flavonoids, alkaloids and phenols from M. alba L. were identified. In addition, there were several same biological active ingredients and some unique chemical components from different parts of M. alba L., the compositions were closely relevant to the pharmacological activities of each part. For example, 1-deoxynojirimycin, an alkaloid component only found in M. alba L., was the characteristic component with high-content from Mori folium, owns the intense inhibitory effect on α-glucosidase and exhibit obvious action in lowering blood glucose (Wang Shirui, 2023). Besides, on account of other affluent ingredients like proteins, carbohydrates, vitamins, trace elements and dietary fibre, Mori folium was also recognized as a high-quality food or mulberry tea (Polumackanycz et al., 2021). Thus it could be seen that due to the multifarious functional materials and particular pharmacological characteristics, different parts of M. alba L. maybe owned broad research prospects and were widely used in various scopes like medicine, food, and other fields (Maqsood et al., 2022).
On account of the favourable value of M. alba L., this review aimed to summarize the chemical components and the pharmacologic bioactivities of M. alba L., including Mori folium, Mori ramulus, Mori cortex and Mori fructus. The overall data in this present paper, could provide a helpful reference for further development and comprehensive utilization of M. alba L.
2 Chemical profiles of Morus alba L
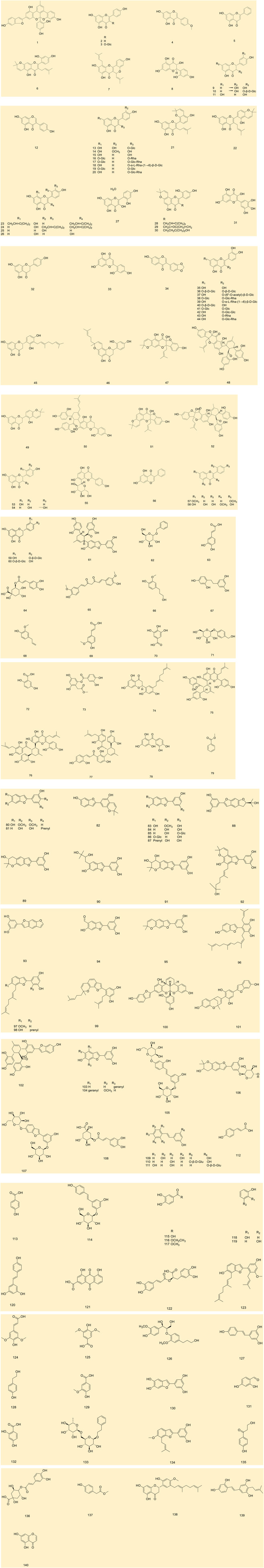
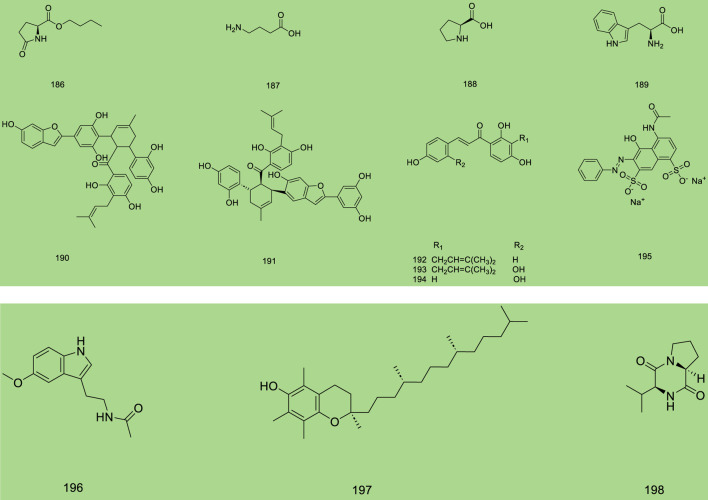
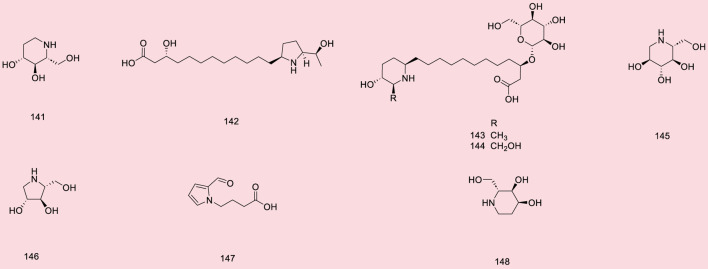
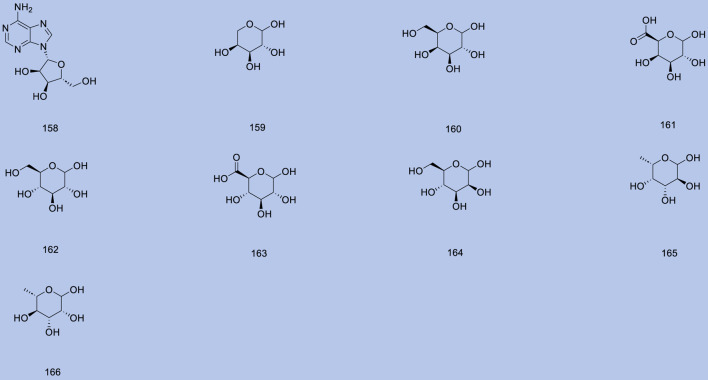
Up to 198 active compounds have been identified in the different parts of M. alba L. (Supplementary Table S1; Tables 1–7). Their structures are summarized in Figures 1–8.
TABLE 1.
Alkaloid in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological properties | Reference |
|---|---|---|---|---|---|
| 1 | 141 | fagomine | B | anti-obesity; anti-inflammatory | D'Urso et al. (2019) |
| Ramos-Romero et al. (2022) | |||||
| 2 | 142 | morusimic acid B | B | __ | D'Urso et al. (2019) |
| 3 | 143 | morusimic acid C | B | __ | D'Urso et al. (2019) |
| 4 | 144 | morusimic acid E | B | __ | D'Urso et al. (2019) |
| 5 | 145 | 1-deoxynojirimycin | A; B | antidiabetic | D'Urso et al. (2019) |
| Asai et al. (2011) | |||||
| 6 | 146 | 1,4-dideoxy-1,4-imino-D-arabinitol | D | hyperamnesia | Lei et al. (2022) |
| Gibbs (2016) | |||||
| 7 | 147 | 2-formyl-1H-pyrrole-1-butanoic acid | B | __ | D'Urso et al. (2019) |
| 8 | 148 | 3-epi-fagomine | A | anticancer; neuroprotection | Amezqueta et al. (2012) |
| Zabady et al. (2022) | |||||
| Bhuiyan et al. (2011) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
TABLE 7.
Other constituents in Morus alba L.
| No. | Sequential number | Name | Category | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|---|
| 1 | 186 | butyl pyroglutamate | amino acid derivatives | B | kidney protection | Lee et al. (2018) |
| 2 | 187 | γ-aminobutyric acid | amino acids | A | antifatigue | Chen et al. (2016) |
| 3 | 188 | L-proline | amino acids | B | anti-inflammatory; kidney protection | Lee et al. (2021b) |
| Li et al. (2019) | ||||||
| 4 | 189 | L-tryptophan | amino acids | A | antipyretic; mood improvement; sleep improvement | Qu et al. (2019) |
| Sutanto et al. (2022) | ||||||
| Kikuchi et al. (2021) | ||||||
| 5 | 190 | chalcomoracin | Diels–Alder adducts | A | anti-bacteria | Jeon and Choi (2019) |
| Kim et al. (2012) | ||||||
| 6 | 191 | guangsangon E | Diels–Alder adducts | A | anticancer | Shu et al. (2021) |
| 7 | 192 | isobavachalcone | chalcones | B | antidiabetic; antioxidant; anti-inflammatory; neuroprotection; antimicrobial | Wang et al. (2013) |
| Wang et al. (2021) | ||||||
| 8 | 193 | morachalcone A | chalcones | D | anti-melanogenesis | Zhang et al. (2016) |
| 9 | 194 | 2,4,2′,4′-tetrahydroxychalcone | chalcones | D | anti-melanogenesis | Zhang et al. (2016) |
| 10 | 195 | lignin | phenylpropanoids | A | anti-microbial | Chao et al. (2021) |
| Das et al. (2024) | ||||||
| 11 | 196 | melatonin | amines | A | antioxidant; anticancer; anti-aging | Panyatip et al. (2022) |
| Bhattacharya et al. (2019) | ||||||
| 12 | 197 | vitamin E | vitamins | C | antioxidant; anti-inflammatory | Kavitha and Geetha (2018) |
| 13 | 198 | cyclo (L-Pro-L-Val) | peptides | B | anti-inflammatory | Lee et al. (2021b) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
FIGURE 1.
Phenols.
FIGURE 8.
Other categories.
TABLE 2.
Coumarins in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|
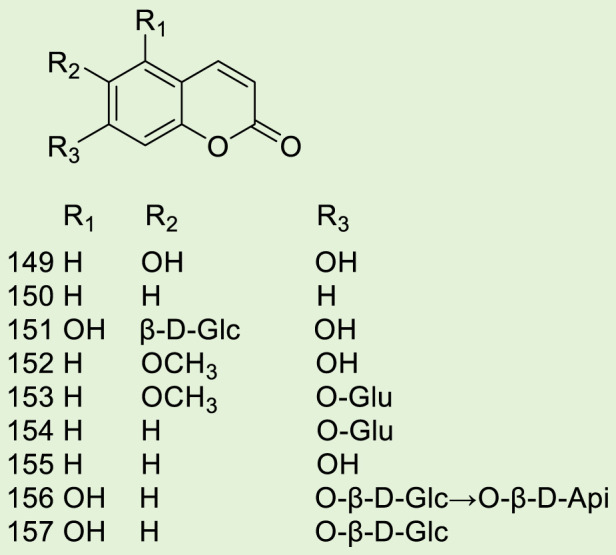
| 1 | 149 | aesculetin | A | anti-inflammatory | Li et al. (2020) |
| 2 | 150 | coumarin | C | anticancer; anti-inflammatory | Kavitha and Geetha (2018) |
| Bhattarai et al. (2021) | |||||
| 3 | 151 | mulberroside B | C | anti-obesity | Yang et al. (2011) |
| 4 | 152 | scopoletin | A | anti-inflammatory | Li et al. (2020) |
| 5 | 153 | scopolin | D | anti-inflammatory; anti-hyperuricemic | Yao et al. (2019) |
| Li et al. (2020) | |||||
| 6 | 154 | skimmin | A | cardioprotection | Doi et al. (2001) |
| Su et al. (2023) | |||||
| 7 | 155 | umbelliferone | C | antidiabetic nephropathy | Hyun et al. (2021) |
| Jin and Chen (2022) | |||||
| 8 | 156 | 5,7-dihydroxycoumarin 7-O-β-d-apiofuranosyl-(1→6)-O-β-d-glucopyranoside | C | anti-obesity | Yang et al. (2011) |
| 9 | 157 | 5,7-dihydroxycoumarin 7-O-β-D-glucopyranoside | C | anti-obesity | Yang et al. (2011) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
TABLE 3.
Carbohydrates in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|
| 1 | 158 | adenosine | C | anti-obesity | Yang et al. (2011) |
| 2 | 159 | arabinose | A | anti-obesity | Zhao et al. (2022) |
| 3 | 160 | D-galactose | A | anti-obesity | Zhao et al. (2022) |
| 4 | 161 | D-galacturonic acid | A | anti-obesity | Zhao et al. (2022) |
| 5 | 162 | D-glucose | A | anti-obesity | Zhao et al. (2022) |
| 6 | 163 | D-glucuronic acid | A | anti-obesity | Zhao et al. (2022) |
| 7 | 164 | D-mannose | A | anti-obesity | Zhao et al. (2022) |
| 8 | 165 | fucose | A | anti-obesity | Zhao et al. (2022) |
| 9 | 166 | L-rhamnose | A | anti-obesity | Zhao et al. (2022) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
TABLE 4.
Terpenoids in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|
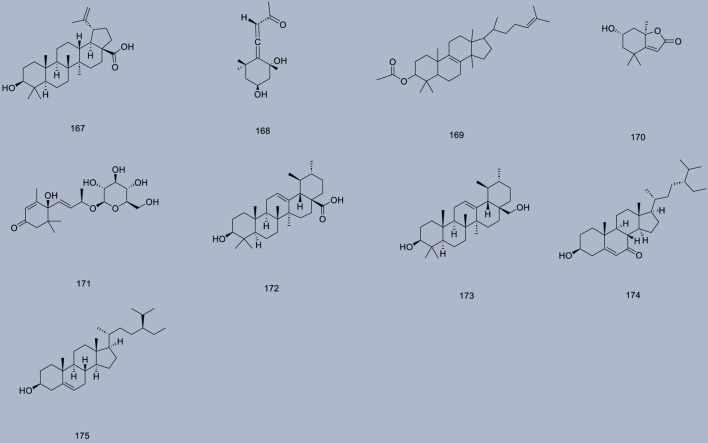
| 1 | 167 | betulinic acid | C | anticancer; anti-obesity | Yang et al. (2011) |
| Aswathy et al. (2022) | |||||
| 2 | 168 | grasshopper ketone | B | anti-inflammatory | Lee et al. (2021b) |
| 3 | 169 | lanosterol acetate | A | antigout | Oh et al. (2021) |
| 4 | 170 | loliolide | A | antidiabetic; anti-inflammatory; anti-aging | Hunyadi et al. (2013) |
| Park et al. (2019) | |||||
| 5 | 171 | roseoside | B | anti-inflammatory | D'Urso et al. (2019) |
| Wang et al. (2023b) | |||||
| 6 | 172 | ursolic acid | C; D | anti-inflammatory; antioxidant; antiviral | Yang et al. (2011) |
| Liu Ying (2023) | |||||
| Al-Kuraishy et al. (2022) | |||||
| Kornel et al. (2023) | |||||
| 7 | 173 | uvaol | C | anti-obesity | Yang et al. (2011) |
| anticancer | Bonel-Perez et al. (2020) | ||||
| 8 | 174 | 7-ketositosterol | B | kidney protection | Lee et al. (2021b) |
| 9 | 175 | β-sitosterol | C | anti-obesity; anticancer | Yang et al. (2011) |
| Khan et al. (2022) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
TABLE 5.
Organic acids in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|
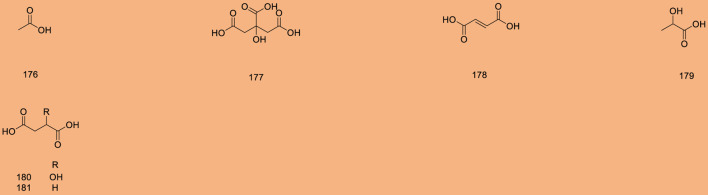
| 1 | 176 | Acetic acid | A | skin protectant | Chen et al. (2021) |
| 2 | 177 | citric acid | A | immuno-enhancement | Chen et al. (2021) |
| Hu et al. (2024) | |||||
| 3 | 178 | fumaric acid | A | anticancer | Chen et al. (2021) |
| Das et al. (2016) | |||||
| 4 | 179 | lactic acid | A | anti-inflammatory; anticancer | Chen et al. (2021) |
| Zhou et al. (2022a) | |||||
| 5 | 180 | malic acid | A | antioxidant; liver protection | Chen et al. (2021) |
| Koriem and Tharwat (2023) | |||||
| 6 | 181 | succinic acid | A | anticancer | Chen et al. (2021) |
| Kasarci et al. (2021) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
TABLE 6.
Anthocyanins in Morus alba L.
| No. | Sequential number | Name | Source | Pharmacological property | Reference |
|---|---|---|---|---|---|
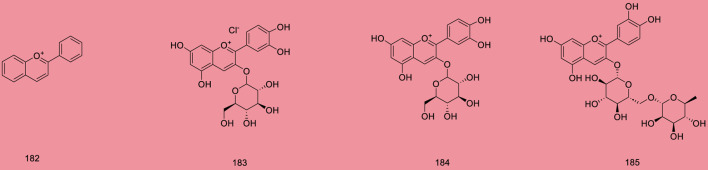
| 1 | 182 | anthocyanins | B | antioxidant; antibacterial | D'Urso et al. (2019) |
| Suriyaprom et al. (2021) | |||||
| 2 | 183 | cyanidin-3-glucoside | B | anticancer | Zabady et al. (2022) |
| 3 | 184 | cyanidin-3-O-glucoside | B | anticancer | Chen et al. (2022c) |
| Wei et al. (2021) | |||||
| 4 | 185 | cyanidin-3-O-rutinoside | B | antioxidant | Chen et al. (2022d) |
| Delazar et al. (2010) |
Note: A, Mori folium; B, Mori fructus; C, Mori cortex; D, Mori ramulus.
FIGURE 2.
Alkaloids.
FIGURE 3.
Coumarins.
FIGURE 4.
Carbohydrates.
FIGURE 5.
Terpenoids.
FIGURE 6.
Organic acids.
FIGURE 7.
Anthocyanin.
3 The pharmacological activities of components in Morus alba L
3.1 Hypoglycemic activity
1-deoxynojirimycin was the important active ingredient in M. alba L. Researchers have confirmed that 1-deoxynojirimycin exhibit an inhibitory effect on α-glucosidase, further reduced the postprandial blood glucose in pre-diabetic and mildly diabetic individuals (Asai et al., 2011). Current evidence showed that the same dose of Mori folium has similar biological activities like lowering blood sugar and protecting kidney in diabetic patient as the purified 1-deoxynojirimycin (Huang et al., 2014). In addition, Mori ramulus extract was reported effective hypoglycemic action and well inhibition of PTP1B and α-glucosidase, the main components were oxyresveratrol and kuwanon G (Kwon et al., 2022). Compared with Mori folium and Mori fructus, the hypoglycemic effects of Mori ramulus and Mori cortex were much more significant (Zhou Q. Y. et al., 2022). The various bioactive components of medicinal parts from M. alba L. expressed multiple antidiabetic targets and less adverse reactions. Thanks to the favourable hypoglycemic effect and the accessibility of M. alba L. resources, M. alba L. may exhibit a promising prospect in the preventing and treating of diabetes. The main hypoglycemic compounds in M. alba L. and their mechanisms are shown in Table 8.
TABLE 8.
Hypoglycemic mechanisms of components from Morus alba L.
| Mechanism | Component | Reference |
|---|---|---|
| inhibition of α-glucosidase | chalcomoracin | Liu et al. (2022) |
| chlorogenic acid; rutin | Hunyadi et al. (2012) | |
| dihydromorin; kuwanon C; kuwanon G; moracin M; norartocarpetin | Kwon et al. (2022) | |
| kuwanon H | Zhou et al. (2022b) | |
| morin | Przeor (2022) | |
| morusin; morusinol | Zhou et al. (2022b) | |
| oxyresveratrol | Kwon et al. (2022) | |
| 1-deoxynojirimycin | Jan et al. (2022) | |
| Enhancement of glucose uptake via in-sulin signaling pathway/AMP-activated protein kinase | isoquercetin | Lim et al. (2021) |
| Increase insulin secretion of pancreatic β-cells | syringic acid | Jan et al. (2022) |
3.2 Antioxidant activity
Studies found that isoquercetin and 4-O-caffeoylquinic acid in Mori folium showed strong antioxidant activity, and the 50% radical-scavenging concentrations were 10.63 ± 0.96 μg/mL and 10.63 ± 0.96 μg/mL, respectively (Ganzon et al., 2018). The researchers comprehensively evaluated the antioxidant activities of bioactive components from M. alba L. in DPPH and ABTS radical scavenging assays, found that the acetone extract showed potential antioxidant activities with SC50 values of 242.33 ± 15.78 and 129.28 ± 10.53 μg/mL, respectively (Hsu et al., 2022). The antioxidant mechanisms of components from M. alba L. are summarized in Table 9.
TABLE 9.
Antioxidant mechanisms of components from Morus alba L.
| Mechanism | Component | Reference |
|---|---|---|
| inhibition of ROS production | astragalin; kaempferol; luteolin; quercetin; taxifolin | Yu et al. (2021) |
| inhibition of soluble epoxide hydrolase | aesculetin; moracin B; moracin J; moracin M; moracin M 3′-O-β-glucopyranoside; mulberroside F; scopoletin; scopoline | Li et al. (2020) |
| scavenging or inhibiting the production of free radicals | anthocyanins | Suriyaprom et al. (2021) |
| caffeic acid; chlorogenic acid; ferulic acid; gallic acid; myricetin; naringenin; p-coumaric acid; rosmarinic acid; rutin; sinapinic acid | Polumackanycz et al. (2021) | |
| mulberroside A; oxyresveratrol | Thomas et al. (2022) | |
| protocatechuic acid; isoquercetin | Leyva-Jimenez et al. (2020) | |
| 4-O-caffeoylquinic acid | Ganzon et al. (2018) |
3.3 Anti-inflammatory activity
Studies have demonstrated that M. alba L. and its active compounds could inhibit the inflammation by suppressing leukocyte chemotaxis, further data about the mechanism showed that oxyresveratrol in M. alba L. could inhibit the CXCR4-mediated leukocyte migration of the CXCR4 receptor by inactivating the MEK/ERK pathway (Chen et al., 2013). In addition, oxyresveratrol was alos reported favourable anti-inflammatory effect through the inhibitions of iNOS/NO production, synthesis of PGE2 and activation of NF-κB(Chung et al., 2003). The methanol extraction of mulberry bark showed that components named kuwanon T and sanggenon A in mulberry bark contribute to the anti-inflammatory activities on microglia (BV2) and macrophages (RAW264.7) by the inhibitions of productions of prostaglandin E2, interleukin-6 and tumour necrosis factor-α, and the stimulation of expression of cyclooxygenase-2 (Ko et al., 2021). The anti-inflammatory active ingredients in mulbery are displayed in Table 10.
TABLE 10.
Anti-inflammatory mechanisms of components from Morus alba L.
| Mechanism | Name | Reference |
|---|---|---|
| inhibition the release of pro-inflammatory cytokines | mulberroside A | Shi et al. (2023) |
| protocatechuic acid; isoquercetin | Leyva-Jimenez et al. (2020) | |
| inhibiting MEK/ERK signaling in leukocyte migration | oxyresveratrol | Chen et al. (2013) |
| inhibition of NF-κB pathway activity | morusin | Jia et al. (2020) |
| moracin O; moracin P | Hardianti et al. (2020) | |
| neochlorogenic acid | Gao et al. (2020a) | |
| kuwanon T | Ko et al. (2021) | |
| sanggenon A | ||
| downregulating INOS expression | astragalin; kaempferol; luteolin; quercetin; taxifolin | Yu et al. (2021) |
| regulating Nrf2 signaling pathways | neochlorogenic acid | Gao et al. (2020b) |
| kuwanon T | Ko et al. (2021) | |
| sanggenon A | ||
| removal of excess reactive oxygen/nitrogen species or interaction with their interacting enzymes | cudraflavone B; kuwanon E; 4′-O-methylkuwanon E | Kollar et al. (2013) |
| selective inhibition of COX-2 activity | kuwanon A | Baek et al. (2021) |
3.4 Anti-cancer activity
Moracin D was demonstrated that it could decrease cell proliferation and induce apoptosis in breast cancer cells by inhibiting the transduction pathway of Wnt3a/FOXM1/β-catenin signal and the activation of caspase and GSK3β(Hwang et al., 2018). Sanggenol L, another natural flavonoid compound in Mori cortex, could induce the apoptosis through inhibiting the PI3K/Akt/mTOR signaling pathway, and accelerate the cycle arrest of prostate cancer cells by activating the p53 protein (Won and Seo, 2020). In addition, sanggenol L could also reduce cytotoxicity and apoptosis in ovarian cancer cells through activating cysteine aspartase and inhibiting NF-κB (Ko et al., 2021). Moracin N was an active ingredient in Mori folium, which exhibit anti-lung cancer properties through apoptosis and autophagy (Gao C. et al., 2020). Morusin, which separate from Mori cortex, was demonstrated effective anticancer activity by inhibiting the vitality of prostate cancer cells with minimal impact on normal prostate epithelial cells, reducing STAT3 activity via the inhibition of phosphorylation, nuclear accumulation and DNA-binding activity. Moreover, morusin showed well downregulation effect on the expression of STAT1 target genes of Cyclin D2. Furthermore, morusin could also decrease the activity of STAT3 in inducing the apoptosis in prostate cancer cells (Lim et al., 2015). The anti-cancer components in M. alba L. and their mechanisms are summarized in Table 11.
TABLE 11.
Anticancer mechanisms of components from Morus alba L.
| Mechanism | Component | Reference |
|---|---|---|
| inhibition of the Akt/mTOR signalling pathway | morusin | Wu et al. (2023) |
| activating AMP-activated protein kinase | morusin | Park and Park (2020) |
| reducing STAT3 activity | morusin | Cho et al. (2017) |
| regulating bax and survivin expression | morusin | Kang et al. (2017) |
| induces autophagy | guangsangon E | Shu et al. (2021) |
| moracin N | Gao et al. (2020b) | |
| regulation of autophagy protein ATG3L16-related RNA molecule expression | cyanidin-3-glucoside | Zabady et al. (2022) |
| activating protein and inhibiting of signaling | sanggenol L | Won and Seo (2020) |
| moracin D | Hwang et al. (2018) | |
| inhibition of HIF-1α in tumours and DLL4 activity in the endothelium | steppogenin | Cha et al. (2023) |
| targeting the KDM4B-MYC axis | sanggenon C | Tang et al. (2023) |
| induces CHK1 degradation through the ubiquitin-proteasome pathway | morusinol | Guo et al. (2023) |
3.5 Other activities
Beside the aforementioned activities, the ingredients in the different parts from M. alba L. also exhibit other activities such as melanin inhibition effect, hair growth, etc. Up to now, multiple constituents including norluciferin, moracin B, moracin J, moracin M-3′-O-β-glucopyranoside and moracin M-6-O-β-D-glucopyranoside againsting melanin were separated from the ethanol extracts of M. alba L. These components have a significant dose-dependent inhibition of melanin production, effectively suppressed the activity of tyrosinase in B10-F1 cells induced by α-melanocyte stimulating hormone and exhibited inhibitory effects on the expression of associated proteins, such as microphthalmia-associated transcription factor, tyrosinase, and tyrosinase-associated protein-1 (Li Y. et al., 2018). Mulberroside F in Mori folium exhibit inhibitory effect on melanin and through the inhibition of tyrosinase and the formation of melanin in melanin-A cells (Lee et al., 2002). Moreover, little Mori cortex extract showed the stimulating on hair growth, enhance the secretion of growth factors, facilitating the transition of hair follicles from the resting phase to the growth phase, activating β-linker proteins, which is essential for inducing the growth phase (Hyun et al., 2021). Other pharmacological activities and the related mechanisms are summarized in Table 12.
TABLE 12.
Other pharmacological effects and their mechanisms of components from Morus alba L.
| Activity | Mechanism | Component | Reference |
|---|---|---|---|
| antigout | blocking the RAS signaling pathway | lanosterol acetate | Oh et al. (2021) |
| antiviral | interference with cell damage caused by influenza virus infection | gallic acid | Kim and Chung (2018) |
| direct inhibition of influenza virus entry | morin hydrate | Hong et al. (2020) | |
| inhibition of viral neuraminidase | sanggenon C | Langeder et al. (2023a) | |
| inhibition of SARS-CoV-2 proteases | sanggenon C; sanggenon G; sanggenon O | Wasilewicz et al. (2023) | |
| antiulcer | inhibition the releasion of histamine | quercetin | Garg et al. (2022) |
| inhibition the formation of platelet-activating factor | rutin | Garg et al. (2022) | |
| antidepressant | interacts with the 5-hydroxytryptaminergic | sanggenon G | Lim et al. (2015) |
| antiplatelet | inhibition of thromboxane release | mulberroside C | Kwon et al. (2021) |
| inhibition of platelet aggregation | morusinol | Lee et al. (2012) | |
| anti-fatigue | increased glucose phosphatase activity | γ-aminobutyric | Chen et al. (2016) |
| anti-melanogenic | inhibition of tyrosinase activity | kuwanon G; mulberrofuran G | Koirala et al. (2018) |
| inhibition of S1P lyase activity | mulberroside A; oxyresveratrol | Zheng et al. (2023) | |
| anti-obesity | regulation of gut microbial communities and lipid indices | arabinose; D-galactose; D-galacturonic acid; D-glucose; D-glucuronic acid; D-mannose | Yang et al. (2022a) |
| fucose | |||
| L-rhamnose | |||
| anti-bacteria | blocking the binding of [1–14C]acetate to Staphylococcus aureus membrane lipids | chalcomoracin; moracin C | Kim et al. (2012) |
| neuroprotection | maintenance of mitochondrial membrane potential and mitochondrial function | cyanidin-3-glucoside | Bhuiyan et al. (2011) |
| promoted nuclear translocation of the mitophagy regulator TFEB and activated the AMPK-ULK1 pathway | morin | Wang et al. (2023c) | |
| cardioprotection | enhancement autophagy of hypoxia-induced | sanggenon C | Gu et al. (2017) |
| prevent hair loss | increased secretion of angiogenic paracrine factors | chlorogenic acid; umbelliferone | Hyun et al. (2021) |
| anti-alzheimer’s disease | reduction of intracellular amyloid-β oligomer-induced cytotoxicity | anthocyanins | Ochiishi et al. (2021) |
| against Benzo [a]pyrene in epidermal keratinocytes | activation of Nrf2-mediated signaling/inhibition of aryl hydrocarbon receptor signaling | maclurin | Kim et al. (2021) |
| relieve fever | inhibition of arachidonic acid metabolic pathway | tryptophan | Qu et al. (2019) |
| anti-hyperuricemia | inhibition of xanthine oxidase activity, and downregulation expression of mURAT1, mGLUT9, and mABCG2 | polydatin | Ge et al. (2023) |
4 Future perspective
In recent years, with the continuous advancement of modern science and technology, researchers conducted in-depth investigations of multifarious constituents and pharmacological activities of M. alba L., including Mori folium, Mori ramulus, Mori cortex and Mori fructus, making its high medicinal potential valuable in contemporary society. Until now, there were some reviews about the pharmacologic activities of M. alba L. (Hao et al., 2022), however, in view of the crucial connection between pharmacological actions and ingredients, the revelation of the overall constituents of M. alba L. was extremely important. When referred to constituents of M. alba L. concluded in this paper, the primary constituents were phenols, flavonoids, alkaloids, etc.. Summing up the pharmaceutical actions of M. alba L., hypoglycemic, antioxidant and anti-inflammatory were the common activities, and different constituents may owned similar effects. As is well konwn that, the connection between ingredients’s structure and pharmaceutical effects was extremely important. Take flavonoids ingredients, for example, the diversiform flavonoids in M. alba L. exhibited anti-inflammatory action. Popularly, the modifications could affect the mechanisms of inflammation, including glycosylation, hydroxylation, etc. (Chen et al., 2018). For example, both quercetin and its glycoside derivative quercetine-3-glucoside exhibit same anti-inflammatory activity with distinctive mechanisms of action. Quercetin downregulated the INOS expression (Leyva-Jimenez et al., 2020), however, isoquercetin inhibited the release of pro-inflammatory cytokines (Yu et al., 2021). Besides, different activities of constituents may owned the similar mechanisms. For example, AMP-activated protein kinase was related to both the anti-hpyerglycemic effect and anti-cancer action of M. alba L. Besides, when referred to the anti-oxidant and anti-inflammatory activities of M. alba L., the inhibition of soluble epoxide hydrolase was the same mechanism. These information indicated that one mechanism maybe related to diversified activities of M. alba L. based on the similar compounds. To sum up, the ingredients of M. alba L. were diverse, and the effect owned the characteristics of multiple approaches and multiple targets.
Nowadays, in order to extend the application of M. alba L. in TCM and food, the toxicity assessments of M. alba L. were evaluated by various experiments. When referred to Mori folium, the LD50 was higher than 15.0 g/kg bw in the acute toxicity test, indicating that Mori folium was deemed as safe and it may own a wide application as food or nutritional supplements (Li Y. et al., 2018). Besides, Mori fructus was a familiar edible food in daily life, and it was widely made into diverse foods such as fresh/dried fruit, fruit wine/juice, and other healthcare foods. From the sub-chronic oral toxicity test, the safe dose without observed adverse was up to 4200 mg/kg, meaning that Mori fructus was nontoxic under conventional edible dosage. Until now, there were none reports about the acute or chronic toxicity of the extracts of Mori cortex. However, the maximum tolerated dose of oral administration of the active ingredient named sanggenon C, an active ingredient derived from Mori cortex as well as identificated in Mori ramulus, was up to 100 mg/g (Langeder et al., 2023b). However, resveratrol, another active constituent in Mori cortex and Mori ramulus, was reported controversial toxicity, the metabolites of resveratrol may exhibit cytotoxic effects (Shaito et al., 2020), meaning that Mori cortex and Mori ramulus may owned a ralative reasonable safe space when applied. Hence, in order to improve the expansive value of M. alba L., the detailed illustrations of acute and long-term toxicity of Mori cortex and Mori ramulus were particularly vital in further studies for researchers.
5 Conclusion
This review summarized the chemical profiles and the pharmacological activities of M. alba L., as well as the safety and the structure-activity relationship. Totally 198 of constituents including phenols, alkaloids, coumarins, carbohydrates, terpenoids, organic acids, anthocyanins, and other constituents were concluded. Among the chemical ingredients, 140 of them were phenols, indicating that phenols may played a critical role in this plant. Modern pharmacological research showed that M. alba L. exhibited hypoglycemic, antioxidant, anti-inflammatory, anti-cancer and other activities, illustrating that M. alba L. has showed favourable applications in pharmaceutical and food fields. Furhter biological activities and the related mechanisms of the ingredients in M. alba L. were needed in order to promote the development of pharmaceutical industry. In addition, more nutritional valve analysis and toxicity research data were particularly important for the development of M. alba L. in food scope.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present study was supported by the Project from Qiqihar Academy of Medical Sciences (QMSI2023Z-16, 2021-ZDPY-011, QMSI2021M-12, and QMSI2021L16), Science and Technology Department of Heilongjiang Province (LH2023H100).
Author contributions
YW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Writing–original draft, Writing–review and editing. QA: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Supervision, Writing–original draft, Writing–review and editing. MG: Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Writing–review and editing. HG: Investigation, Resources, Supervision, Writing–review and editing. WY: Investigation, Resources, Supervision, Writing–review and editing. MZ: Data curation, Investigation, Supervision, Writing–review and editing. JM: Investigation, Methodology, Supervision, Writing–review and editing. ZL: Investigation, Resources, Writing–review and editing. QL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing–review and editing. JL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1364948/full#supplementary-material
References
- Agus H. H., Cetin A., Ozdemir N., Ozbay M. G., Caglar M. A., Sariyildiz M. A., et al. (2022). Resorcinol alleviates alpha-terpineol-induced cell death in Schizosaccharomyces pombe via increased activity of the antioxidant enzyme Sod2. FEMS Yeast Res. 22 (1), foac052. 10.1093/femsyr/foac052 [DOI] [PubMed] [Google Scholar]
- Ai J., Bao B., Battino M., Giampieri F., Chen C., You L., et al. (2021). Recent advances on bioactive polysaccharides from mulberry. Food Funct. 12 (12), 5219–5235. 10.1039/d1fo00682g [DOI] [PubMed] [Google Scholar]
- Alavi D. S., Farkhondeh T., Aschner M., Darroudi M., Samini H., Samarghandian S. (2023). Chrysin effect against gastric cancer: focus on its molecular mechanisms. Curr. Molec. Pharmacol. 16 (7), 707–711. 10.2174/1874467216666230103105725 [DOI] [PubMed] [Google Scholar]
- Alibakhshi A., Malekzadeh R., Hosseini S. A., Yaghoobi H. (2023). Investigation of the therapeutic role of native plant compounds against colorectal cancer based on system biology and virtual screening. Sci. Rep. 13 (1), 11451. 10.1038/s41598-023-38134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H. M., Al-Gareeb A. I., Negm W. A., Alexiou A., Batiha G. E. (2022). Ursolic acid and SARS-CoV-2 infection: a new horizon and perspective. Inflammopharmacology 30 (5), 1493–1501. 10.1007/s10787-022-01038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezqueta S., Galan E., Fuguet E., Carrascal M., Abian J., Torres J. L. (2012). Determination of D-fagomine in buckwheat and mulberry by cation exchange HPLC/ESI-Q-MS. Anal. Bioanal. Chem. 402 (5), 1953–1960. 10.1007/s00216-011-5639-2 [DOI] [PubMed] [Google Scholar]
- Asai A., Nakagawa K., Higuchi O., Kimura T., Kojima Y., Kariya J., et al. (2011). Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2 (4), 318–323. 10.1111/j.2040-1124.2011.00101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswathy M., Vijayan A., Daimary U. D., Girisa S., Radhakrishnan K. V., Kunnumakkara A. B. (2022). Betulinic acid: a natural promising anticancer drug, current situation, and future perspectives. J. Biochem. Mol. Toxicol. 36 (12), e23206. 10.1002/jbt.23206 [DOI] [PubMed] [Google Scholar]
- Baek S., Hwang S., Park T., Kwon Y., Cho M., Park D. (2021). Evaluation of selective COX-2 inhibition and in silico study of kuwanon derivatives isolated from Morus alba . Int. J. Mol. Sci. 22 (7), 3659. 10.3390/ijms22073659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha G. E., Al-Snafi A. E., Thuwaini M. M., Teibo J. O., Shaheen H. M., Akomolafe A. P., et al. (2023). Morus alba: a comprehensive phytochemical and pharmacological review. Schmiedeb. Arch. Pharmacol. 396 (7), 1399–1413. 10.1007/s00210-023-02434-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Patel K. K., Dehari D., Agrawal A. K., Singh S. (2019). Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 462 (1-2), 133–155. 10.1007/s11010-019-03617-5 [DOI] [PubMed] [Google Scholar]
- Bhattarai N., Kumbhar A. A., Pokharel Y. R., Yadav P. N. (2021). Anticancer potential of coumarin and its derivatives. Mini-Rev. Med. Chem. 21 (19), 2996–3029. 10.2174/1389557521666210405160323 [DOI] [PubMed] [Google Scholar]
- Bhuiyan M. I. H., Kim H., Kim S. Y., Cho K. (2011). The neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J. Physiology Pharmacol. 15 (6), 353–361. 10.4196/kjpp.2011.15.6.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonel-Perez G. C., Perez-Jimenez A., Gris-Cardenas I., Parra-Perez A. M., Lupianez J. A., Reyes-Zurita F. J., et al. (2020). Antiproliferative and pro-apoptotic effect of uvaol in human hepatocarcinoma HepG2 cells by affecting G0/G1 cell cycle arrest, ROS production and AKT/PI3K signaling pathway. Molecules 25 (18), 4254. 10.3390/molecules25184254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S., Kim H., Jang H., Lee J., Chao T., Baek N., et al. (2023). Steppogenin suppresses tumor growth and sprouting angiogenesis through inhibition of HIF-1α in tumors and DLL4 activity in the endothelium. Phytomedicine 108, 154513. 10.1016/j.phymed.2022.154513 [DOI] [PubMed] [Google Scholar]
- Chaita E., Lambrinidis G., Cheimonidi C., Agalou A., Beis D., Trougakos I., et al. (2017). Anti-melanogenic properties of Greek plants. A novel depigmenting agent from Morus alba wood. Mol Basel, Switz. 22 (4), 514. 10.3390/molecules22040514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao N., Yu T., Hou C., Liu L., Zhang L. (2021). Genome-wide analysis of the lignin toolbox for morus and the roles of lignin related genes in response to zinc stress. PeerJ (San Francisco, CA) 9, e11964. e11964-e11964. 10.7717/peerj.11964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Mohamad Razali U. H., Saikim F. H., Mahyudin A., Mohd Noor N. Q. I. (2021). Morus alba L. Plant: bioactive compounds and potential as a functional food ingredient. Foods 10 (3), 689. 10.3390/foods10030689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Mokhtar R. A. M., Sani M. S. A., Noor N. Q. I. M. (2022a). The effect of maturity and extraction solvents on bioactive compounds and antioxidant activity of mulberry (Morus alba) fruits and leaves. Molecules 27 (8), 2406. 10.3390/molecules27082406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., He X., Liu Y., Li J., He Q., Zhang C., et al. (2016). Extraction, purification and anti-fatigue activity of gamma-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci. Rep. 6, 18933. 10.1038/srep18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li G., Sun C., Peng F., Yu L., Chen Y., et al. (2022b). Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 36 (4), 1545–1575. 10.1002/ptr.7397 [DOI] [PubMed] [Google Scholar]
- Chen L., Teng H., Xie Z., Cao H., Cheang W. S., Skalicka-Woniak K., et al. (2018). Modifications of dietary flavonoids towards improved bioactivity: an update on structure-activity relationship. Crit. Rev. Food Sci. Nutr. 58 (4), 513–527. 10.1080/10408398.2016.1196334 [DOI] [PubMed] [Google Scholar]
- Chen T., Shuang F., Fu Q., Ju Y., Zong C., Zhao W., et al. (2022c). Evaluation of the chemical composition and antioxidant activity of mulberry (Morus alba L.) fruits from different varieties in China. Molecules 27 (9), 2688. 10.3390/molecules27092688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Han Y., Chen L., Tian Q. L., Yin Y. L., Zhou Q., et al. (2022d). Discovery and characterization of the flavonoids in Cortex Mori Radicis as naturally occurring inhibitors against intestinal nitroreductases. Chem.-Biol. Interact. 368, 110222. 10.1016/j.cbi.2022.110222 [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Tien Y. J., Chen C. H., Beltran F. N., Amor E. C., Wang R. J., et al. (2013). Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 13, 45. 10.1186/1472-6882-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. W., Na W., Choi M., Kang S. J., Lee S., Choi C. Y. (2017). Autophagy inhibits cell death induced by the anti-cancer drug morusin. Am. J. Cancer Res. 7 (3), 518–530. [PMC free article] [PubMed] [Google Scholar]
- Choi S. W., Jang Y. J., Lee Y. J., Leem H. H., Kim E. O. (2013). Analysis of functional constituents in mulberry (Morus alba L.) twigs by different cultivars, producing areas, and heat processings. Prev. Nutr. Food Sci. 18 (4), 256–262. 10.3746/pnf.2013.18.4.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. K., Mitra K., Conte A. J., Sarker A., Chowdhury A., Ragauskas A. J. (2024). Lignin - a green material for antibacterial application - a review. Int. J. Biol. Macromol. 261 (Pt 2), 129753. 10.1016/j.ijbiomac.2024.129753 [DOI] [PubMed] [Google Scholar]
- Das R. K., Brar S. K., Verma M. (2016). Recent advances in the biomedical applications of fumaric acid and its ester derivatives: the multifaceted alternative therapeutics. Pharmacol. Rep. 68 (2), 404–414. 10.1016/j.pharep.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Delazar A., Khodaie L., Afshar J., Nahar L., Sarker S. (2010). Isolation and free-radical-scavenging properties of cyanidin 3-O-glycosides from the fruits of Ribes biebersteinii Berl. Acta Pharm. Zagreb. Croat. 60 (1), 1–11. 10.2478/v10007-010-0007-x [DOI] [PubMed] [Google Scholar]
- Doi K., Kojima T., Makino M., Kimura Y., Fujimoto Y. (2001). Studies on the constituents of the leaves of Morus alba L. Chem. Pharm. Bull. 49 (2), 151–153. 10.1248/cpb.49.151 [DOI] [PubMed] [Google Scholar]
- Dong H., Yu P., Long B., Peng T., He Y., Xu B., et al. (2023). Total synthesis of kuwanons A and B and discovery of their antibacterial mechanism. J. Nat. Prod. 86 (8), 2022–2030. 10.1021/acs.jnatprod.3c00466 [DOI] [PubMed] [Google Scholar]
- D'Urso G., Mes J. J., Montoro P., Hall R. D., de Vos R. C. H. (2019). Identification of bioactive phytochemicals in mulberries. Metabolites 10 (1), 7. 10.3390/metabo10010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi-Tago M., Nonaka Y., Tago K., Takeda M., Ishihara Y., Sakai A., et al. (2020). Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2. Sci. Rep. 10 (1), 2584. 10.1038/s41598-020-59380-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzon J. G., Chen L. G., Wang C. C. (2018). 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 26 (3), 985–993. 10.1016/j.jfda.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Sun X., Wu Z., Yuan H., Han H., Huang H., et al. (2020a). A novel benzofuran derivative moracin N induces autophagy and apoptosis through ROS generation in lung cancer. Front. Pharmacol. 11, 391. 10.3389/fphar.2020.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. H., Zhang S. D., Wang L. T., Yu L., Zhao X. L., Ni H. Y., et al. (2020b). Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules 25 (6), 1385. 10.3390/molecules25061385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Singla R. K., Rahman M. M., Sharma R., Mittal V. (2022). Evaluation of ulcer protective activity of Morus alba L. Extract-loaded chitosan microspheres in ethanol-induced ulcer in rat model. Evid.-based Complement. Altern. Med. 2022, 4907585–4907617. 10.1155/2022/4907585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Su Z., Wang Y., Zhao X., Hou K., Zheng S., et al. (2023). Identifying the intervention mechanisms of polydatin in hyperuricemia model rats by using UHPLC-Q-Exactive Orbitrap mass spectroscopy metabonomic approach. Front. Nutr. 10, 1117460. 10.3389/fnut.2023.1117460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M. E. (2016). Role of glycogenolysis in memory and learning: regulation by noradrenaline, serotonin and ATP. Neurosci 9, 70. 10.3389/fnint.2015.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienke U., Richter M., Walther E., Hoffmann A., Kirchmair J., Makarov V., et al. (2016). Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumoniae. Sci. Rep. 6 (1), 27156. 10.1038/srep27156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Gao L., Chen Y., Xu Z., Yu K., Zhang D., et al. (2017). Sanggenon C protects against cardiomyocyte hypoxia injury by increasing autophagy. Mol. Med. Rep. 16 (6), 8130–8136. 10.3892/mmr.2017.7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Dong Z., Zhang X., Yang Y., Hu X., Ji Y., et al. (2023). Morusinol extracted from Morus alba induces cell cycle arrest and apoptosis via inhibition of DNA damage response in melanoma by CHK1 degradation through the ubiquitin-proteasome pathway. Phytomedicine 114, 154765. 10.1016/j.phymed.2023.154765 [DOI] [PubMed] [Google Scholar]
- Guzman L., Balada C., Flores G., Alvarez R., Knox M., Vinet R., et al. (2018). t-Resveratrol protects against acute high glucose damage in endothelial cells. Plant Food Hum. Nutr. 73 (3), 235–240. 10.1007/s11130-018-0683-0 [DOI] [PubMed] [Google Scholar]
- Hao J., Gao Y., Xue J., Yang Y., Yin J., Wu T., et al. (2022). Phytochemicals, pharmacological effects and molecular mechanisms of mulberry. Foods 11 (8), 1170. 10.3390/foods11081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardianti B., Umeyama L., Li F., Yokoyama S., Hayakawa Y. (2020). Anti-inflammatory compounds moracin O and P from Morus alba Linn. (Sohakuhi) target the NF-kappaB pathway. Mol. Med. Rep. 22 (6), 5385–5391. 10.3892/mmr.2020.11615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E., Song J., Kim S., Cho J., Jeong B., Yang H., et al. (2020). Morin hydrate inhibits influenza virus entry into host cells and has anti-inflammatory effect in influenza-infected mice. Immune Netw. 20 (4), e32. 10.4110/in.2020.20.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. H., Yang C. S., Chen J. J. (2022). Antioxidant, anti-alpha-glucosidase, antityrosinase, and anti-inflammatory activities of bioactive components from Morus alba . Antioxidants 11 (11), 2222. 10.3390/antiox11112222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Yuan M., Guo B., Lin J., Yan S., Huang H., et al. (2024). Citric acid promotes immune function by modulating the intestinal barrier. Int. J. Mol. Sci. 25 (2), 1239. 10.3390/ijms25021239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Yu M., Yan G., Wang H., Hou A., Lei C. (2018). Isoprenylated phenolic compounds with tyrosinase inhibition from Morus nigra . J. Asian Nat. Prod. Res. 20 (5), 488–493. 10.1080/10286020.2017.1350653 [DOI] [PubMed] [Google Scholar]
- Hua F., Li J. Y., Zhang M., Zhou P., Wang L., Ling T. J., et al. (2022). Kaempferol-3-O-rutinoside exerts cardioprotective effects through NF-κB/NLRP3/Caspase-1 pathway in ventricular remodeling after acute myocardial infarction. J. Food Biochem. 46 (10), e14305. 10.1111/jfbc.14305 [DOI] [PubMed] [Google Scholar]
- Huang S. S., Yan Y. H., Ko C. H., Chen K. M., Lee S. C., Liu C. T. (2014). A comparison of food-grade folium mori (sang ye) extract and 1-deoxynojirimycin for glycemic control and renal function in streptozotocin-induced diabetic rats. J. Tradit. Complement. Med. 4 (3), 162–170. 10.4103/2225-4110.131639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyadi A., Liktor-Busa E., Márki Á., Martins A., Jedlinszki N., Hsieh T. J., et al. (2013). Metabolic effects of mulberry leaves: exploring potential benefits in type 2 diabetes and hyperuricemia. Evid.-based Complement. Altern. Med. 2013, 948627–948710. 10.1155/2013/948627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyadi A., Martins A., Hsieh T. J., Seres A., Zupko I. (2012). Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One 7 (11), e50619. 10.1371/journal.pone.0050619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. M., Lee H. J., Jung J. H., Sim D. Y., Hwang J., Park J. E., et al. (2018). Inhibition of wnt3a/FOXM1/β-catenin Axis and activation of GSK3β and caspases are critically involved in apoptotic effect of moracin D in breast cancers. Int. J. Mol. Sci. 19 (9), 2681. 10.3390/ijms19092681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J., Im J., Kim S., Kim H. Y., Seo I., Bhang S. H. (2021). Morus alba root extract induces the anagen phase in the human hair follicle dermal papilla cells. Pharmaceutics 13 (8), 1155. 10.3390/pharmaceutics13081155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A., Islam M. S., Rahman M. K., Uddin M. N., Akanda M. R. (2020). The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 43 (6), 582–592. 10.1007/s12272-020-01243-0 [DOI] [PubMed] [Google Scholar]
- Jan B., Zahiruddin S., Basist P., Irfan M., Abass S., Ahmad S. (2022). Metabolomic profiling and identification of antioxidant and antidiabetic compounds from leaves of different varieties of Morus alba linn grown in kashmir. ACS Omega 7 (28), 24317–24328. 10.1021/acsomega.2c01623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y., Choi S. (2019). Isolation, identification, and quantification of tyrosinase and α-glucosidase inhibitors from UVC-irradiated mulberry (Morus alba L.) leaves. Prev. Nutr. Food Sci. 24 (1), 84–94. 10.3746/pnf.2019.24.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. Y., Liu Q., Kim S. B., Jo Y. H., Mo E. J., Yang H. H., et al. (2015). Characterization of melanogenesis inhibitory constituents of Morus alba leaves and optimization of extraction conditions using response surface methodology. Molecules 20 (5), 8730–8741. 10.3390/molecules20058730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., He W., Zhang H., He L., Wang Y., Zhang T., et al. (2020). Morusin ameliorates IL-1β-induced chondrocyte inflammation and osteoarthritis via NF-κB signal pathway. Drug Des. Devel Ther. 14, 1227–1240. 10.2147/DDDT.S244462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Chen C. (2022). Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem. Toxicol. 163, 112892. 10.1016/j.fct.2022.112892 [DOI] [PubMed] [Google Scholar]
- Jongkon N., Seaho B., Tayana N., Prateeptongkum S., Duangdee N., Jaiyong P. (2022). Computational analysis and biological activities of oxyresveratrol analogues, the putative cyclooxygenase-2 inhibitors. Mol Basel, Switz. 27 (7), 2346. 10.3390/molecules27072346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W. T., Kwon O. C., Kim H. B., Sung G. B., Kim H. W., Kim Y. S. (2018). Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol.-Mysore. 55 (5), 1789–1796. 10.1007/s13197-018-3093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Kim E., Kim S., Lee J., Ahn K. S., Yun M., et al. (2017). Morusin induces apoptosis by regulating expression of Bax and Survivin in human breast cancer cells. Oncol. Lett. 13 (6), 4558–4562. 10.3892/ol.2017.6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasarci G., Ertugrul B., Iplik E. S., Cakmakoglu B. (2021). The apoptotic efficacy of succinic acid on renal cancer cell lines. Med. Oncol. 38 (12), 144. 10.1007/s12032-021-01577-9 [DOI] [PubMed] [Google Scholar]
- Kavitha Y., Geetha A. (2018). Anti-inflammatory and preventive activity of white mulberry root bark extract in an experimental model of pancreatitis. J. Tradit. Complement. Med. 8 (4), 497–505. 10.1016/j.jtcme.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Nath N., Rauf A., Emran T. B., Mitra S., Islam F., et al. (2022). Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications. Chem.-Biol. Interact. 365, 110117. 10.1016/j.cbi.2022.110117 [DOI] [PubMed] [Google Scholar]
- Kikuchi A. M., Tanabe A., Iwahori Y. (2021). A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 18 (3), 316–333. 10.1080/19390211.2020.1746725 [DOI] [PubMed] [Google Scholar]
- Kim D., Lee K., Kwon J., Lee H. J., Lee D., Mar W. (2015). Neuroprotection against 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells by 5,7-Dihydroxychromone: activation of the Nrf2/ARE pathway. Life Sci. 130, 25–30. 10.1016/j.lfs.2015.02.026 [DOI] [PubMed] [Google Scholar]
- Kim D. S., Irfan M., Sung Y. Y., Kim S. H., Park S. H., Choi Y. H., et al. (2017). Schisandra chinensis and Morus alba synergistically inhibit in vivo thrombus formation and platelet aggregation by impairing the glycoprotein VI pathway. Evid.-based Complement. Altern. Med. 2017, 7839658. 10.1155/2017/7839658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. S., Kang Y. M., Jin W. Y., Sung Y. Y., Choi G., Kim H. K. (2014). Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Biomed. Rep. 2 (5), 675–680. 10.3892/br.2014.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Chung M. S. (2018). Antiviral activities of mulberry (Morus alba) juice and seed against influenza viruses. Evid.-based Complement. Altern. Med. 2018, 2606583. 10.1155/2018/2606583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Park S., Yang S., Oh S. W., Kwon K., Park S. J., et al. (2021). Protective effects of maclurin against benzo[a]pyrene via aryl hydrocarbon receptor and nuclear factor erythroid 2-related factor 2 targeting. Antioxidants 10 (8), 1189. 10.3390/antiox10081189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Doh E. J., Lee G. (2020a). Quantitative comparison of the marker compounds in different medicinal parts of Morus alba L. Using high-performance liquid chromatography-diode array detector with chemometric analysis. Molecules 25 (23), 5592. 10.3390/molecules25235592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Nam D., Ju W., Choe J., Choi A. (2020b). Response surface methodology for optimization of process parameters and antioxidant properties of mulberry (Morus alba L.) leaves by extrusion. Molecules 25 (22), 5231. 10.3390/molecules25225231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J., Sohn M., Kim W., Korea R. I. O. B. (2012). Chalcomoracin and moracin C, new inhibitors of Staphylococcus aureus enoyl-acyl carrier protein reductase from Morus alba . Biol. Pharm. Bull. 35 (5), 791–795. 10.1248/bpb.35.791 [DOI] [PubMed] [Google Scholar]
- Ko W., Liu Z., Kim K., Dong L., Lee H., Kim N. Y., et al. (2021). Kuwanon T and sanggenon a isolated from Morus alba exert anti-inflammatory effects by regulating NF-κB and HO-1/Nrf2 signaling pathways in BV2 and RAW264.7 cells. Molecules 26 (24), 7642. 10.3390/molecules26247642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala P., Seong S. H., Zhou Y., Shrestha S., Jung H. A., Choi J. S. (2018). Structure(-)Activity relationship of the tyrosinase inhibitors kuwanon G, mulberrofuran G, and albanol B from morus species: a kinetics and molecular docking study. Molecules 23 (6), 1413. 10.3390/molecules23061413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar P., Barta T., Hosek J., Soucek K., Zavalova V. M., Artinian S., et al. (2013). Prenylated flavonoids from Morus alba L. Cause inhibition of G1/S transition in THP-1 human leukemia cells and prevent the lipopolysaccharide-induced inflammatory response. Evid.-based Complement. Altern. Med. 2013, 350519. 10.1155/2013/350519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriem K. M. M., Tharwat H. A. K. (2023). Malic acid improves behavioral, biochemical, and molecular disturbances in the hypothalamus of stressed rats. J. Integr. Neurosci. 22 (4), 98. 10.31083/j.jin2204098 [DOI] [PubMed] [Google Scholar]
- Kornel A., Nadile M., Retsidou M. I., Sakellakis M., Gioti K., Beloukas A., et al. (2023). Ursolic acid against prostate and urogenital cancers: a review of in vitro and in vivo studies. Int. J. Mol. Sci. 24 (8), 7414. 10.3390/ijms24087414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. W., Lee D. H., Rhee M. H., Shin J. H. (2021). In vitro antiplatelet activity of mulberroside C through the up-regulation of cyclic nucleotide signaling pathways and down-regulation of phosphoproteins. Genes 12 (7), 1024. 10.3390/genes12071024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon R. H., Thaku N., Timalsina B., Park S. E., Choi J. S., Jung H. A. (2022). Inhibition mechanism of components isolated from Morus alba branches on diabetes and diabetic complications via experimental and molecular docking analyses. Antioxidants 11 (2), 383. 10.3390/antiox11020383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeder J., Döring K., Schmietendorf H., Grienke U., Schmidtke M., Rollinger J. M. (2023a). 1H NMR-based biochemometric analysis of Morus alba extracts toward a multipotent herbal anti-infective. J. Nat. Prod. 86 (1), 8–17. 10.1021/acs.jnatprod.2c00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeder J., Koch M., Schmietendorf H., Tahir A., Grienke U., Rollinger J. M., et al. (2023b). Correlation of bioactive marker compounds of an orally applied Morus alba root bark extract with toxicity and efficacy in BALB/c mice. Front. Pharmacol. 14, 1193118. 10.3389/fphar.2023.1193118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Lee S. R., Kang K. S., Kim K. H. (2021a). Bioactive phytochemicals from mulberry: potential anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Sci. 22 (15), 8120. 10.3390/ijms22158120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Lee S. R., Park B. J., Song J. H., Kim J. K., Ko Y., et al. (2021b). Identification of renoprotective phytosterols from mulberry (Morus alba) fruit against cisplatin-induced cytotoxicity in LLC-PK1 kidney cells. Plants 10 (11), 2481. 10.3390/plants10112481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Yu J., Lee S., Hwang G., Kang K., Park J., et al. (2018). Beneficial effects of bioactive compounds in mulberry fruits against cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 19 (4), 1117. 10.3390/ijms19041117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Yang H., Yoo Y. M., Hong S. S., Lee D., Lee H. J., et al. (2012). Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease. J. Atheroscler. Thromb. 19 (6), 516–522. 10.5551/jat.10058 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Choi S. Y., Kim H., Hwang J. S., Lee B. G., Gao J. J., et al. (2002). Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol. Pharm. Bull. 25 (8), 1045–1048. 10.1248/bpb.25.1045 [DOI] [PubMed] [Google Scholar]
- Lee Y. J., Lee S. Y. (2021). Maclurin exerts anti-cancer effects in human osteosarcoma cells via prooxidative activity and modulations of PARP, p38, and ERK signaling. IUBMB Life 73 (8), 1060–1072. 10.1002/iub.2506 [DOI] [PubMed] [Google Scholar]
- Lei L., Huan Y., Liu Q., Li C., Cao H., Ji W., et al. (2022). Morus alba L. (Sangzhi) alkaloids promote insulin secretion, restore diabetic β-cell function by preventing dedifferentiation and apoptosis. Front. Pharmacol. 13, 841981. 10.3389/fphar.2022.841981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Jimenez F. J., Ruiz-Malagon A. J., Molina-Tijeras J. A., Diez-Echave P., Vezza T., Hidalgo-Garcia L., et al. (2020). Comparative study of the antioxidant and anti-inflammatory effects of leaf extracts from four different Morus alba genotypes in high fat diet-induced obesity in mice. Antioxidants 9 (8), 733. 10.3390/antiox9080733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li S., Yang H., Wang Y., Wang J., Zheng N. (2019). l-Proline alleviates kidney injury caused by AFB1 and AFM1 through regulating excessive apoptosis of kidney cells. Toxins 11 (4), 226. 10.3390/toxins11040226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. X., Heo M., Go Y., Kim Y. S., Kim Y. H., Yang S. Y., et al. (2020). Coumarin and moracin derivatives from mulberry leaves (Morus alba L.) with soluble epoxide hydrolase inhibitory activity. Molecules 25 (17), 3967. 10.3390/molecules25173967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. X., Park J. U., Su X. D., Kim K. T., Kang J. S., Kim Y. R., et al. (2018a). Identification of anti-melanogenesis constituents from Morus alba L. Leaves. Mol Basel, Switz. 23 (10), 2559. 10.3390/molecules23102559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dou L., Chen S., Zhou H., Mou F. (2021a). Neochlorogenic acid: an anti-HIV active compound identified by screening of Cortex Mori [Morus Alba L. (Moraceae)]. Pharm. Biol. 59 (1), 1517–1527. 10.1080/13880209.2021.1995005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang X., Liang C., Hu J., Yu Z. (2018b). Safety evaluation of mulberry leaf extract: acute, subacute toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol. 95, 220–226. 10.1016/j.yrtph.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Li Z., Chen X., Liu G., Li J., Zhang J., Cao Y., et al. (2021b). Antioxidant activity and mechanism of resveratrol and polydatin isolated from mulberry (Morus alba L.). Molecules 26 (24), 7574. 10.3390/molecules26247574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D. W., Jung J., Park J., Baek N., Kim Y. T., Kim I., et al. (2015). Antidepressant-like effects of sanggenon G, isolated from the root bark of Morus alba, in rats: involvement of the serotonergic system. Biol. Pharm. Bull. 38 (11), 1772–1778. 10.1248/bpb.b15-00471 [DOI] [PubMed] [Google Scholar]
- Lim S. H., Yu J. S., Lee H. S., Choi C. I., Kim K. H. (2021). Antidiabetic flavonoids from fruits of Morus alba promoting insulin-stimulated glucose uptake via akt and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Pharmaceutics 13 (4), 526. 10.3390/pharmaceutics13040526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Peng Y., Chen C., Ren H., Zhu J., Deng Y., et al. (2024). Flavonoids from mulberry leaves inhibit fat production and improve fatty acid distribution in adipose tissue in finishing pigs. Anim. Nutr. 16, 147–157. 10.1016/j.aninu.2023.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou X., Zhou D., Jian Y., Jia J., Ge F. (2022). Isolation of chalcomoracin as a potential α-glycosidase inhibitor from mulberry leaves and its binding mechanism. Molecules 27 (18), 5742. 10.3390/molecules27185742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ying G. M. G. J. (2023). Identification of chemical constituents and optimization of total flavonoids extraction process for Mori Ramulus. Chin. Tradit. Pat. Med. 45 (06), 1892–1901. [Google Scholar]
- Maqsood M., Anam S. R., Sahar A., Khan M. I. (2022). Mulberry plant as a source of functional food with therapeutic and nutritional applications: a review. J. Food Biochem. 46 (11), e14263. 10.1111/jfbc.14263 [DOI] [PubMed] [Google Scholar]
- Martins B. D. A., Sande D., Solares M. D., Takahashi J. A. (2021). Antioxidant role of morusin and mulberrofuran B in ethanol extract of Morus alba roots. Nat. Prod. Res. 35 (24), 5993–5996. 10.1080/14786419.2020.1810036 [DOI] [PubMed] [Google Scholar]
- Miklasinska M., Kepa M., Wojtyczka R. D., Idzik D., Zdebik A., Orlewska K., et al. (2015). Antibacterial activity of protocatechuic acid ethyl ester on Staphylococcus aureus clinical strains alone and in combination with antistaphylococcal drugs. Molecules 20 (8), 13536–13549. 10.3390/molecules200813536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K. M., Yang J., Lee M., Kwon E., Baek J., Hwang T., et al. (2022). Maclurin exhibits antioxidant and anti-tyrosinase activities, suppressing melanogenesis. Antioxidants 11 (6), 1164. 10.3390/antiox11061164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Hano Y., Fukai T. (2009). Chemistry and biosynthesis of isoprenylated flavonoids from Japanese mulberry tree. Proc. Jpn. Acad. Ser. B 85 (9), 391–408. 10.2183/pjab.85.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiishi T., Kaku M., Kajsongkram T., Thisayakorn K. (2021). Mulberry fruit extract alleviates the intracellular amyloid-β oligomer-induced cognitive disturbance and oxidative stress in Alzheimer's disease model mice. Genes cells. 26 (11), 861–873. 10.1111/gtc.12889 [DOI] [PubMed] [Google Scholar]
- Oh K. K., Adnan M., Cho D. H. (2021). Network Pharmacology study on Morus alba L. Leaves: pivotal functions of bioactives on RAS signaling pathway and its associated target proteins against gout. Int. J. Mol. Sci. 22 (17), 9372. 10.3390/ijms22179372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyatip P., Padumanonda T., Yongram C., Kasikorn T., Sungthong B., Puthongking P. (2022). Impact of tea processing on tryptophan, melatonin, phenolic and flavonoid contents in mulberry (Morus alba L.) leaves: quantitative analysis by LC-MS/MS. Molecules 27 (15), 4979. 10.3390/molecules27154979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. J., Park S. H. (2020). Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells. Nutr. Res. Pract. 14 (5), 478–489. 10.4162/nrp.2020.14.5.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H., Kim D. S., Kim S., Lorz L. R., Choi E., Lim H. Y., et al. (2019). Loliolide presents antiapoptosis and antiscratching effects in human keratinocytes. Int. J. Mol. Sci. 20 (3), 651. 10.3390/ijms20030651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. J., Seong S. H., Kim M. S., Seo S. W., Kim M. R., Kim H. S. (2017). High-throughput detection of antioxidants in mulberry fruit using correlations between high-resolution mass and activity profiles of chromatographic fractions. Plant Methods 13, 108. 10.1186/s13007-017-0258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel P., Park S. E., Seong S. H., Jung H. A., Choi J. S. (2019). Novel diels-alder type adducts from Morus alba root bark targeting human monoamine oxidase and dopaminergic receptors for the management of neurodegenerative diseases. Int. J. Mol. Sci. 20 (24), 6232. 10.3390/ijms20246232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Gong L., Si K., Bai X., Du G. (2011). Fluorescence resonance energy transfer assay for high-throughput screening of ADAMTS1 inhibitors. Molecules 16 (12), 10709–10721. 10.3390/molecules161210709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T. N., Kim O., Ha M. T., Hwangbo C., Min B. S., Lee J. H. (2020). Albanol B from mulberries exerts anti-cancer effect through mitochondria ROS production in lung cancer cells and suppresses in vivo tumor growth. Int. J. Mol. Sci. 21 (24), 9502. 10.3390/ijms21249502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov M. B., Plotnikova T. M. (2021). Tyrosol as a neuroprotector: strong effects of a "weak" antioxidant. Curr. Neuropharmacol. 19 (4), 434–448. 10.2174/1570159X18666200507082311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polumackanycz M., Wesolowski M., Viapiana A. (2021). Morus alba L. And Morus nigra L. Leaves as a promising food source of phenolic compounds with antioxidant activity. Plant Food Hum. Nutr. 76 (4), 458–465. 10.1007/s11130-021-00922-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przeor M. (2022). Some common medicinal plants with antidiabetic activity, known and available in europe (A mini-review). Pharmaceuticals 15 (1), 65. 10.3390/ph15010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Wang L., Guo W. (2019). Screening and identification of antipyretic components in the postfrost leaves of Morus alba based on multivariable and continuous-index spectrum-effect correlation. J. Anal. Methods Chem. 2019, 8796276. 10.1155/2019/8796276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Romero S., Ponomarenko J., Amezqueta S., Hereu M., Miralles-Perez B., Romeu M., et al. (2022). Fiber-like action of d-fagomine on the gut microbiota and body weight of healthy rats. Nutrients 14 (21), 4656. 10.3390/nu14214656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Gupta S., Panda G., Chatterjee P., Das A., Patawri P., et al. (2023). Identification of pseudobaptigenin as a novel polyphenol-based multi-target antagonist of different hormone receptors for breast cancer therapeutics. J. Biomol. Struct. Dyn., 1–13. 10.1080/07391102.2023.2226750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razliqi R. N., Ahangarpour A., Mard S. A., Khorsandi L. (2023). Gentisic acid ameliorates type 2 diabetes induced by Nicotinamide-Streptozotocin in male mice by attenuating pancreatic oxidative stress and inflammation through modulation of Nrf2 and NF-кB pathways. Life Sci. 325, 121770. 10.1016/j.lfs.2023.121770 [DOI] [PubMed] [Google Scholar]
- Ren B., Kwah M. X., Liu C., Ma Z., Shanmugam M. K., Ding L., et al. (2021). Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 515, 63–72. 10.1016/j.canlet.2021.05.001 [DOI] [PubMed] [Google Scholar]
- Shaito A., Posadino A. M., Younes N., Hasan H., Halabi S., Alhababi D., et al. (2020). Potential adverse effects of resveratrol: a literature review. Int. J. Mol. Sci. 21 (6), 2084. 10.3390/ijms21062084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Sharma A., Bhatia G., Landi M., Brestic M., Singh B., et al. (2019). Isolation of phytochemicals from Bauhinia variegata L. Bark and their in vitro antioxidant and cytotoxic potential. Antioxidants 8 (10), 492. 10.3390/antiox8100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Qian J., Miao H., Zhang S., Hu Y., Liu P., et al. (2023). Mulberroside A ameliorates CCl4-induced liver fibrosis in mice via inhibiting pro-inflammatory response. Food Sci. Nutr. 11 (6), 3433–3441. 10.1002/fsn3.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Seong S. H., Park S. G., Min B. S., Jung H. A., Choi J. S. (2019). Insight into the PTP1B inhibitory activity of arylbenzofurans: an in vitro and in silico study. Molecules 24 (16), 2893. 10.3390/molecules24162893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Yuan H., Xu M., Hong Y., Gao C., Wu Z., et al. (2021). A novel Diels-Alder adduct of mulberry leaves exerts anticancer effect through autophagy-mediated cell death. Acta Pharmacol. Sin. 42 (5), 780–790. 10.1038/s41401-020-0492-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilioti E., Jaakkola M., Tolonen T., Lipponen M., Virtanen V., Chinou I., et al. (2014). Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS One 9 (4), e94860. 10.1371/journal.pone.0094860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Cui J., Zhao J., Fu X. (2023). Skimmin ameliorates cardiac function via the regulation of M2 macrophages in a myocardial infarction mouse model. Perfusion-UK 38 (6), 1298–1307. 10.1177/02676591221100742 [DOI] [PubMed] [Google Scholar]
- Suriyaprom S., Kaewkod T., Promputtha I., Desvaux M., Tragoolpua Y. (2021). Evaluation of antioxidant and antibacterial activities of white mulberry (Morus alba L.) fruit extracts. Plants 10 (12), 2736. 10.3390/plants10122736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutanto C. N., Loh W. W., Kim J. E. (2022). The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutr. Rev. 80 (2), 306–316. 10.1093/nutrit/nuab027 [DOI] [PubMed] [Google Scholar]
- Suthamwong P., Minami M., Okada T., Shiwaku N., Uesugi M., Yokode M., et al. (2020). Administration of mulberry leaves maintains pancreatic β-cell mass in obese/type 2 diabetes mellitus mouse model. BMC Complement. Med. Ther. 20 (1), 136. 10.1186/s12906-020-02933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Zhang Z. N., Cai H. R., Sun W., Yang H., Zhao E. H., et al. (2023). Effect of Morus alba extract sanggenon C on growth and proliferation of glioblastoma cells. Zhongguo Zhong Yao Za Zhi 48 (1), 211–219. 10.19540/j.cnki.cjcmm.20220905.701 [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Eilstein J., Prasad A., Ekhar P., Shetty S., Peng Z., et al. (2022). Comprehensive characterization of naturally occurring antioxidants from the twigs of mulberry (Morus alba) using on-line high-performance liquid chromatography coupled with chemical detection and high-resolution mass spectrometry. Phytochem. Anal. 33 (1), 105–114. 10.1002/pca.3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tianqiao Yong D. L. C. X., Liang D., Xiao C., Huang L., Chen S., Xie Y., et al. (2022). Hypouricemic effect of 2,4-dihydroxybenzoic acid methyl ester in hyperuricemic mice through inhibiting XOD and down-regulating URAT1. Biomed. Pharmacother. 153, 113303. 10.1016/j.biopha.2022.113303 [DOI] [PubMed] [Google Scholar]
- Wang M., Lin L., Lu J., Chen X. (2021). Pharmacological review of isobavachalcone, a naturally occurring chalcone. Pharmacol. Res. 165, 105483. 10.1016/j.phrs.2021.105483 [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang L., Pang P. (2023a). Dihydrokaempferol attenuates LPS-induced inflammation and apoptosis in WI-38 cells. Allergol. Immunopath. 51 (6), 23–29. 10.15586/aei.v51i6.971 [DOI] [PubMed] [Google Scholar]
- Wang W., Mei L., Yue H., Tao Y., Liu Z. (2023b). Targeted isolation of cyclooxygenase-2 inhibitors from Saussurea obvallata using affinity ultrafiltration combined with preparative liquid chromatography. J. Chromatogr. B 1217, 123620. 10.1016/j.jchromb.2023.123620 [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiang L., Wang C., Tang C., He X. (2013). Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One 8 (7), e71144. 10.1371/journal.pone.0071144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cui J., Li D., Ran S., Huang J., Chen G. (2023c). Morin exhibits a neuroprotective effect in MPTP-induced Parkinson's disease model via TFEB/AMPK-mediated mitophagy. Phytomedicine 116, 154866. 10.1016/j.phymed.2023.154866 [DOI] [PubMed] [Google Scholar]
- Wasilewicz A., Kirchweger B., Bojkova D., Abi Saad M. J., Langeder J., Bütikofer M., et al. (2023). Identification of natural products inhibiting SARS-CoV-2 by targeting viral proteases: a combined in silico and in vitro approach. J. Nat. Prod. 86 (2), 264–275. 10.1021/acs.jnatprod.2c00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Ji X., Xue J., Gao Y., Zhu X., Xiao G. (2021). Cyanidin-3-O-glucoside represses tumor growth and invasion in vivo by suppressing autophagy via inhibition of the JNK signaling pathways. Food Funct. 12 (1), 387–396. 10.1039/d0fo02107e [DOI] [PubMed] [Google Scholar]
- Wenmin Du Z. Z. J. W. (2022). Herbal textual research on mori in famous classical formulas. Chin. J. Exp. Traditional Med. Formulae 28 (10), 11–21. 10.13422/j.cnki.syfjx.20212149 [DOI] [Google Scholar]
- Won Y., Seo K. (2020). Sanggenol L induces apoptosis and cell cycle arrest via activation of p53 and suppression of PI3K/Akt/mTOR signaling in human prostate cancer cells. Nutrients 12 (2), 488. 10.3390/nu12020488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. E., Su C. C., Wang S. C., Liu P. L., Cheng W. C., Yeh H. C., et al. (2023). Anticancer effects of morusin in prostate cancer via inhibition of akt/mTOR signaling pathway. Am. J. Chin. Med. 51 (4), 1019–1039. 10.1142/S0192415X23500477 [DOI] [PubMed] [Google Scholar]
- Xu L., Yu M., Niu L., Huang C., Wang Y., Wu C., et al. (2020). Phenolic compounds isolated from Morus nigra and their alpha-glucosidase inhibitory activities. Nat. Prod. Res. 34 (5), 605–612. 10.1080/14786419.2018.1491041 [DOI] [PubMed] [Google Scholar]
- Yang J., Chen X., Rao S., Li Y., Zang Y., Zhu B. (2022a). Identification and quantification of flavonoids in okra (abelmoschus esculentus L. Moench) and antiproliferative activity in vitro of four main components identified. Metabolites 12 (6), 483. 10.3390/metabo12060483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Kang Z., Zhang J., Qu F., Song B. (2021). Neuroprotective effects of isoquercetin: an in vitro and in vivo study. Cell J. (Yakhteh) 23 (3), 355–365. 10.22074/cellj.2021.7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. Y., Wu Y. L., Yu M. H., Hung T. W., Chan K. C., Wang C. J. (2022b). Mulberry leaf and neochlorogenic acid alleviates glucolipotoxicity-induced oxidative stress and inhibits proliferation/migration via downregulating ras and FAK signaling pathway in vascular smooth muscle cell. Nutrients 14 (15), 3006. 10.3390/nu14153006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gong T., Liu C., Chen R., Chinese A. O. M. S., Key L. O. B. S., et al. (2010). Four new 2-arylbenzofuran derivatives from leaves of Morus alba L. Chem. Pharm. Bull. 58 (2), 257–260. 10.1248/cpb.58.257 [DOI] [PubMed] [Google Scholar]
- Yang Z., Matsuzaki K., Takamatsu S., Kitanaka S. (2011). Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells. Molecules 16 (7), 6010–6022. 10.3390/molecules16076010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., He H., Xue J., Wang J., Jin H., Wu J., et al. (2019). Mori ramulus (Chin.Ph.)-the dried twigs of Morus alba L./Part 1: discovery of two novel coumarin glycosides from the anti-hyperuricemic ethanol extract. Molecules 24 (3), 629. 10.3390/molecules24030629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. S., Lim S. H., Lee S. R., Choi C., Kim K. H. (2021). Antioxidant and anti-inflammatory effects of white mulberry (Morus alba L.) fruits on lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules 26 (4), 920. 10.3390/molecules26040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabady S., Mahran N., Soltan M. A., Alaa E. M., Eid R. A., Albogami S., et al. (2022). Cyanidin-3-Glucoside modulates hsa_circ_0001345/miRNA106b/atg16l1 Axis expression as a potential protective mechanism against hepatocellular carcinoma. Curr. Issues Mol. Biol. 44 (4), 1677–1687. 10.3390/cimb44040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Chen M., Wang S., Xu X., Li T., Xiang Z., et al. (2022). Comparative and phylogenetic analyses of the chloroplast genome reveal the taxonomy of the Morus genus. Front. Plant Sci. 13, 1047592. 10.3389/fpls.2022.1047592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Tao G., Chen J., Zheng Z. (2016). Characterization of a new flavone and tyrosinase inhibition constituents from the twigs of Morus alba L. Molecules 21 (9), 1130. 10.3390/molecules21091130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Shi Y., Petrova V., Yue G. G. L., Negrin A., Wu S., et al. (2019). Jaboticabin and related polyphenols from jaboticaba (myrciaria cauliflora) with anti-inflammatory activity for chronic obstructive pulmonary disease. J. Agric. Food Chem. 67 (5), 1513–1520. 10.1021/acs.jafc.8b05814 [DOI] [PubMed] [Google Scholar]
- Zhao X., Fu Z., Yao M., Cao Y., Zhu T., Mao R., et al. (2022). Mulberry (Morus alba L.) leaf polysaccharide ameliorates insulin resistance- and adipose deposition-associated gut microbiota and lipid metabolites in high-fat diet-induced obese mice. Food Sci. Nutr. 10 (2), 617–630. 10.1002/fsn3.2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Lee E. H., Lee S. Y., Lee Y., Shin K. O., Park K., et al. (2023). Morus alba L. root decreases melanin synthesis via sphingosine-1-phosphate signaling in B16F10 cells. J. Ethnopharmacol. 301, 115848. 10.1016/j.jep.2022.115848 [DOI] [PubMed] [Google Scholar]
- Zhou H., Yan X., Yu W., Liang X., Du X., Liu Z., et al. (2022a). Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Int. Rev. Immunol. 41 (1), 4–18. 10.1080/08830185.2021.1955876 [DOI] [PubMed] [Google Scholar]
- Zhou J., Li S. X., Wang W., Guo X. Y., Lu X. Y., Yan X. P., et al. (2013). Variations in the levels of mulberroside A, oxyresveratrol, and resveratrol in mulberries in different seasons and during growth. Sci. World J. 2013, 380692. 10.1155/2013/380692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Y., Liao X., Kuang H., Li J., Zhang S. (2022b). LC-MS metabolite profiling and the hypoglycemic activity of Morus alba L. Extracts. Molecules 27 (17), 5360. 10.3390/molecules27175360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Tang X., Zhu Z., Gong Z., Tang W., Hu Y., et al. (2023). STING activation in macrophages by vanillic acid exhibits antineoplastic potential. Biochem. Pharmacol. 213, 115618. 10.1016/j.bcp.2023.115618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.