Abstract
Fosfomycin (FOM) is an approved veterinary medicinal product for large animals in Japan, but Clinical breakpoint (CBP) for antimicrobial susceptibility test (AST) is not defined for animals. This study aimed at conducting a pharmacokinetics/pharmacodynamics (PK/PD) analysis to determine the PK/PD cutoff for the CBP in horses. Drug concentrations following single intravenous administration (IV) of 20 mg/kg body weight (BW) FOM in nine horses were measured using liquid chromatography/mass spectrometry. The data were modelled using a nonlinear mixed-effects model, followed by Monte Carlo simulations. A 90% probability of target attainment for a PK/PD target of the ratio of Area Under the free plasma concentration-time curve divided by the minimal inhibitory concentration (MIC) >24 hr was set as PK/PD cut-off. The PK/PD cutoff for FOM 20 mg/kg BW q12 hr IV was estimated with the MIC value of ≤16.0 mg/L, and this regimen was considered effective against E. coli (MIC90; 16.0 mg/L) in healthy horses based on the MIC90 values of the wild population. Owing to the relevance of FOM to human health, veterinarians should use q 12 hr FOM 20 mg /kg against E. coli infections with an MIC <16 µg/mL, as suggested by our PK/PD cutoff after AST.
Keywords: antimicrobial susceptibility testing, Escherichia coli, fosfomycin, horse, pharmacokinetics/pharmacodynamics (PK/PD) cutoff
Fosfomycin (FOM) is a bactericidal antimicrobial agent with broad antibacterial activity against both Gram-positive and Gram-negative pathogens [12]. Specifically, FOM is considered to be active against Gram-positive pathogens, including Staphylococcus aureus, and Staphylococcus spp. and Enterococcus spp. and against Gram-negative pathogens, including Salmonella spp., Escherichia coli, Klebsiella. [19]. In humans, FOM is prescribed mainly for urinary tract infections and for various other infections such as pneumonia, osteomyelitis, and septic arthritis [12].
Pharmacokinetic analysis has been reported in chickens, cattle, dogs, and horses, as well as in humans [2, 12, 14, 25, 28]. In Japan, FOM for intravenous (IV) administration was approved as veterinary medicinal products for cattle [28]. The recommended dosing regimen of FOM for horses and cattle based on pharmacokinetics studies is 20 mg/kg, q 8 to 12 hr [25, 28], and used for horses in Japan. European Committee on Antimicrobial Susceptibility Testing (EUCAST) indicated the Clinical breakpoint (CBP) for a susceptible minimum inhibitory concentrations (MIC) value <32.0 mg/L in Enterobacterales and Staphylococcus spp. in humans with the dose of 4 g/patient q 8 hr IV administration [8]. However, CBP is not established in horses by organizations such as EUCAST or the Clinical & Laboratory Standards Institute (CLSI) to interpret antimicrobial susceptibility test (AST) results. Since CBP has been established in horses, human CBP is currently used for AST in horses. Because CBPs are species-specific and depend on dosage regimens, CBP may be different in the current situation where the dosage is different between horses (20 mg/kg) and humans (60–80 mg/kg) [23]. In this case, the CBP in horses would be expected to be lower than that in humans, and FOM 20 mg/kg IV may be ineffective for horses according to the AST based on the human CBP.
The WHO ranked FOM as a critically important antimicrobial agent for human medicine [6]. Because of its importance for human health, the proper use of FOM in horses requires the implementation of an AST based on horse specific CBP. In this study, a pharmacokinetics/pharmacodynamics (PK/PD) analysis was conducted based on the pharmacokinetics of FOM and its MICs against bacteria isolated from horses to determine the PK/PD cutoff for CBP.
MATERIALS AND METHODS
Nine healthy 2–8 year-old Thoroughbred horses (four stallions and five mares) with body weights (BWs) of 416–557 kg were used. The horses were kept in individual stalls during the experiments and had ad libitum access to water and hay. The dose of FOM (20 mg/kg BW) was determined based on previous reports [28]. FOM (FOSMICIN® injection 2 g; Meiji Seika Pharma Co., Ltd., Tokyo, Japan) was dissolved in 50 mL sterile physiological saline and delivered into the right jugular vein by a short bolus infusion (<30 sec). This study was reviewed and approved by the Animal Care and Use Committee of the Equine Research Institute, Japan Racing Association, in accordance with ASPA (1986) legislation Protocol # 21-5.
Blood samples were collected at 0 (prior to administration), 5, 10, 20, 30, and 45 min and 1, 2, 3, 4, 6, 8, 12, and 21 hr after IV administration. All blood samples were collected from the left jugular vein using a 16G catheter (Becton Dickinson Co., Franklin Lakes, NJ, USA), which was inserted into the skin using local anesthesia and 1 mL lidocaine (Xylocaine Injection Polyamp 0.5%, Sandoz Pharma., Tokyo, Japan). Subsequently, 10 mL blood samples were collected in heparinized vacuum blood collection tubes (Venoject 2; Terumo Corporation, Tokyo, Japan). The samples were immediately centrifuged at 1,500 × g for 10 min, and the separated plasma samples were stored at −20°C until analysis.
Determination of plasma concentrations
Plasma concentrations of FOM were quantified via liquid chromatography/mass spectrometry as previously reported [27]. The FOM calibration curve and quality controls were prepared by spiking blank equine plasma with the reference standard at the concentration from 0.1 to 300 ng/mL. Quality control samples for the calibration of the plasma analysis were prepared by adding standard FOM (Sigma-Aldrich Co., St. Louis, MO, USA) to blank horse plasma. Then, 200 μL of acetonitrile and 20 μL of 1 μg/mL rac-fosfomycin-D5 (Toronto Research Chemicals Inc., Toronto, Canada) in methanol as an internal standard were added to 100 μL of plasma. The samples were incubated for 5 min at 25°C and centrifuged at 10,000 × g for 5 min. One microliter of each sample was injected into a liquid chromatography system (Nexera X2; Shimadzu Corporation, Kyoto, Japan) connected to a mass spectrometer (QTRAP4500; SCIEX Corp., Tokyo, Japan). Liquid chromatography was performed on the ZIC-HILIC Guard column (20 mm, 2.1 μm; Merck, Darmstadt, Germany) and ZIC-HILIC column (50 mm × 2.1 mm i.d., 3.5 μm; Merck, Darmstadt, Germany) with a mixture of 25 mmol/L ammonium formate (Fujifilm Wako, Osaka, Japan) and acetonitrile (Fujifilm Wako, Osaka, Japan) at a flow rate of 0.2 mL/min. The final calibration curve had a coefficient of correlation (R2) >0.995 over the concentration range of 0.1–300.0 µg/mL with a 1/y2 weighing factor. Accuracy and precision in quality control samples were determined at concentrations of 0.1, 0.3, 5, and 240 µg/mL (five replicates each). Accuracies were between 83.0% and 114.0%, and the precision of coefficient of variation (CV) were <15%. The lower limit of quantitation (LOQ) for FOM was 0.1 µg/mL.
Protein binding
The ultrafiltration method was used to separate free and bound drug for FOM; 200 µL samples were placed in a filter (Centrifree Ultrafiltration Device; Merck KGaA, Darmstadt, Germany) and centrifuged at 15,000 × g for 10 min at 25°C. The free drug concentration following ultrafiltration and the total drug concentration in samples not subjected to ultrafiltration were quantified using the same assay method, as previously described. Plasma samples for the assay were collected from nine horses 1, 3, and 5 hr after administration. The extent of protein binding and free fraction were calculated by comparing the free and total drug concentrations.
Pharmacokinetic data analysis
Estimation of the PK/PD cutoff requires the development of a population PK model to quantify typical PK parameters and their between-subject variability (BSV) [26]. Plasma pharmacokinetic analyses were conducted using a Nonlinear Mixed Effect (NLME) model on a commercially available software (Phoenix WinNonlin version 6.4; Certara, Princeton, NJ, USA). The ‘mixed effect’ of NLME refers to two types of effects; namely, fixed effects and random effects. Fixed effects correspond to typical pharmacokinetic parameters which characterize the structural model in all subjects in a population. Random effects like the BSV describe the variability around these fixed effects, making it possible to estimate individual values of the PK parameters. The distribution of PK parameters are assumed to be log-normal [18]. In a second step, the NLME model was used to generate a large sample of plasma disposition curves (typically 5000) via Monte Carlo simulations (MCS) based on typical PK parameters and the corresponding BSV to predict exposure for a large virtual population, allowing us to compute the Probability of Target Attainment (PTA). VetCAST recommended the use of this virtual population to estimate the PK/PD cutoffs for different possible MICs [26].
A three-compartment structural model was selected based on the likelihood ratio test and the Akaike information criterion. The model was parameterized in terms of clearance and distribution volume. The estimated parameters were the central (V1) and two peripheral (V2 and V3) volumes of distribution, plasma clearance (CL), and inter-compartmental distribution clearances (CL2 and CL3).
In a population model, the statistical model describing the BSV is added to the structural model. The BSV for a given parameter was described using an exponential model of the following form: θparameter_i = θtv_parameter (Eqn. 1)
where θparameter_i is the value of theta for a given parameter in the ith animal, θtv_parameter is the typical population value of parameters, and ηi (etai) is the deviation associated with the ith animal from the corresponding theta population value. The distribution of the etas was assumed normal with a mean of 0 and a variance ω2.
To report the BSV as a coefficient of variation, Equation 2 was used for conversion of the variance terms (ω2) into a coefficient of variation (CV%):
(Eqn. 2)
Shrinkage of the random effects (eta) toward the means was described as:
(Eqn. 3)
where var (ηr) is the variance of Empirical Bayes (“post hoc”) estimates (EBEs) of ηs. When the shrinkage of eta was >0.3, the data did not allow for a robust estimation of this random component. Estimates of the random effects for the IV model are given in Table 1, and all the eta shrinkage values were <0.3. A full OMEGA matrix, meaning that both variance and covariance terms were estimated, was used to determine the random components of the model, that is, the BSV associated with the fixed pharmacokinetic parameters.
Table 1. Estimates of the random effects (full variance/covariance matrix) and shrinkage with a 3 compartment model.
| Label | nV | nV2 | nV3 | nCl | nCl2 | nCl3 |
|---|---|---|---|---|---|---|
| Omega (variance/covariance) | ||||||
| nV | 0.0292 | |||||
| nV2 | 0.0049 | 0.0249 | ||||
| nV3 | −0.0139 | 0.0592 | 0.2708 | |||
| nCl | −0.0515 | 0.0222 | 0.1256 | 0.2206 | ||
| nCl2 | −0.0099 | 0.0089 | 0.042 | 0.0228 | 0.0103 | |
| nCl3 | −0.0159 | −0.0006 | −0.0257 | 0.0727 | −0.0035 | 0.052 |
| Correlation | ||||||
| nV | 1 | |||||
| nV2 | 0.1816 | 1 | ||||
| nV3 | −0.156 | 0.7201 | 1 | |||
| nCl | −0.6417 | 0.2999 | 0.5138 | 1 | ||
| nCl2 | −0.5678 | 0.5546 | 0.7973 | 0.4789 | 1 | |
| nCl3 | −0.4081 | −0.0174 | −0.2167 | 0.679 | −0.1521 | 1 |
The residual model was an additive plus a multiplicative (proportional) model of the form. C(t) = ƒ(θ,Time)×(1+Ɛ1)+Ɛ2 (Eqn. 4)
with ε1 is the multiplicative error term having a mean of 0 and a variance noted σ1 Ɛ1≈N(0,σ12)
and ε2 is the additive error term having a mean of 0 and a variance noted σ2 Ɛ2≈N(0,σ22)
The additive sigma was reported as its standard deviation noted with the same units as plasma concentration (µg/mL) and the multiplicative sigma was reported as coefficient of variation. Moreover, covariates were tested (age, body weight, and sex). The stepwise covariate search mode of Phoenix was used to define the statistically significant covariates for each of the structural parameters. The stepwise forward or backward addition or deletion of covariate effects (by adding one at a time) determines the improvement of the final model based on the Bayesian information criterion (BIC). A BIC value of 6.635 for adding a covariate and a value of 10.823 for deleting a covariate was used [5]. The Quasirandom Parametric Expectation Maximization (QRPEM) engine was used to maximize the likelihood.
Using the developed model and the free fraction, MCS were used to generate free plasma concentrations in a population of 5,000 horses using individual predictions or IPRED (eta was as estimated), corresponding to different dosage regimens. The simulation was performed for 20 mg/kg at four interval patterns for 24 hr from the first administration. We calculated for the 5,000 curves the ratio of the Area Under free plasma concentration-time Curve from 0–24 hr after administration divided by the MIC (fAUC0–24 hr/MIC) >24 [17, 22]. The highest MIC reaching the corresponding Probability of Target Attainment (PTA) of 90% after standard dosage regimen is considered PK/PD cutoff according to the VetCAST approach [26].
Minimum inhibitory concentrations
The MICs of FOM were obtained from unpublished data on the 138 strains of S. zooepidemicus, 65 strains of Staphylococcus aureus (without methicillin-resistant S. aureus), 87 strains of Escherichia coli and 58 strains of Pseudomonas aeruginosa isolated from infected Thoroughbred horses, including those with pneumonia and cellulitis. MICs were determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (M07-A9). Moreover, we compared MIC distributions and Epidemiology cut off (ECOFF) isolated from humans which were reported by EUCAST [10]. For bacteria for which ECOFF was not indicated by EUCAST, ECOFF was calculated from our MIC distributions using EcoFinder [9].
RESULTS
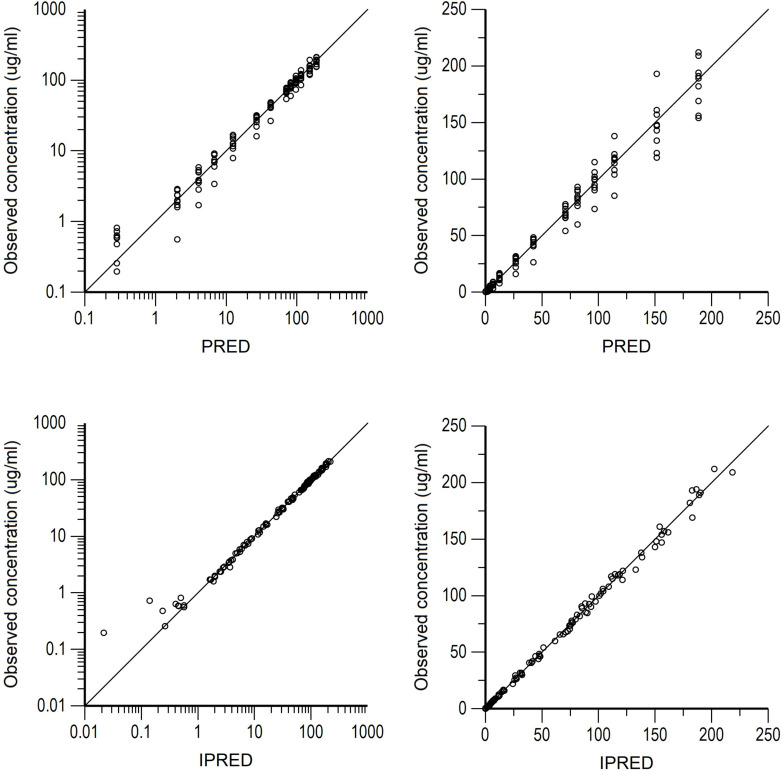
Semilogarithmic plots of the disposition curves of the FOM for each horse are shown in Fig. 1. The plasma concentrations of FOM were above the limit of quantification until 21 hr after administration at last sampling. Logarithmic plots of the observed drug plasma concentrations versus population prediction (PRED) and IPRED are shown in Fig. 2. Data were evenly distributed around the line of identity, indicating no major bias in the population components of the model. The plot of the conditional weighted residuals (CWRES) versus time indicated that the residuals were randomly scattered around zero with no systematic trend, supporting the selection of the residual error model (Fig. 3). None of the tested covariates (age, sex, or body weight) were significant in this model. Bootstrap estimates of the typical values of the primary structural parameters of the model (theta), secondary parameters, and their associated coefficients of variation as a measure of the precision of their estimation are given in Table 2. A Visual Predictive Check ensured that the simulated and observed data were consistent (Fig. 4).
Fig. 1.
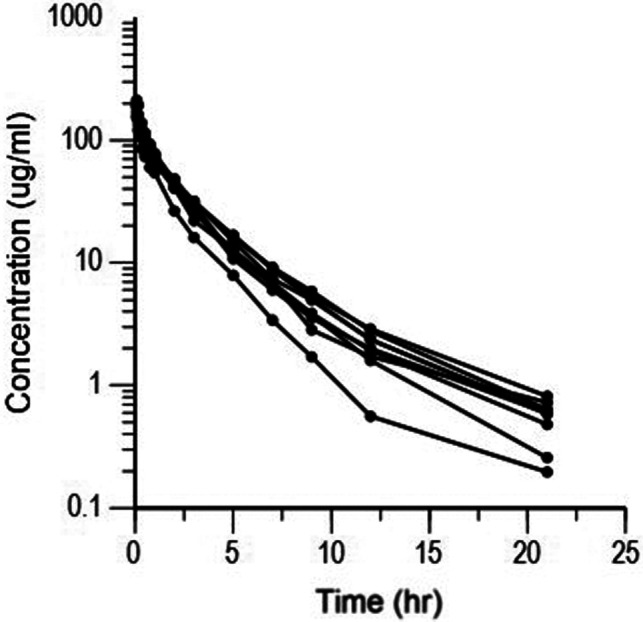
Semilogarithmic spaghetti plots of the disposition curves of fosfomycin after a single dose administration of 20 mg/kg BW fosfomycin in nine horses.
Fig. 2.
Arithmetic scale (left) and logarithmic scale (right) of observed fosfomycin plasma concentrations vs. population predictions (PRED) (top plots) and individual predictions (IPRED) (bottom plots).
Fig. 3.
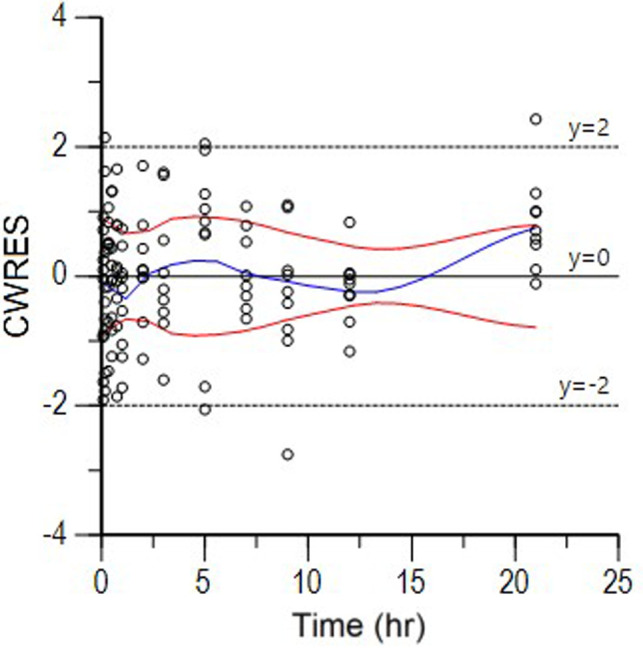
CWRES (conditional weighted residuals) vs time plot for fosfomycin. Values of CWRES should be approximately N (0, 1) and hence concentrated between y=−3 and y=+3. Inspection of the figure indicates that data were evenly distributed about zero and that the trends (as given by the blue line and the red line, its negative reflection) did not show any fanning, indicating no bias in the structural model.
Table 2. Population primary parameters of Fosfomycin in horses with a 3-compartment model (Legend: between-subject variability (BSV)%, CV%, and 2.5% and 97.5% percentiles give the precision of typical value estimates).
| Primary structural Parameters | Units | BSV% | Typical values (Median) | CV% | 2.50% | 97.50% |
|---|---|---|---|---|---|---|
| tvV | L/kg | 17.2 | 0.08 | 9 | 0.066 | 0.094 |
| tvV2 | L/kg | 10.2 | 0.055 | 9.8 | 0.044 | 0.066 |
| tvV3 | L/kg | 23.1 | 0.059 | 13.2 | 0.044 | 0.074 |
| tvCL | L/kg/hr | 15.9 | 0.07 | 7.4 | 0.06 | 0.08 |
| tvCL2 | L/kg/hr | 55.8 | 0.017 | 28.1 | 0.008 | 0.027 |
| tvCL3 | L/kg/hr | 49.7 | 0.22 | 29.6 | 0.09 | 0.35 |
| tvCMultStdev (residual, proportional) | Scalar | 0.048 | 18.5 | 0.03 | 0.066 | |
| stdev0 (residual, additive) | µg/L | 0.249 | 19.5 | 0.153 | 0.346 | |
| Secondary parameters | ||||||
| Half_life_alpha | hr | 0.099 | 26.3 | 0.047 | 0.151 | |
| Half_life_Beta | hr | 1.029 | 17.2 | 0.677 | 1.381 | |
| Half_life_Gamma | hr | 3.191 | 20.2 | 1.91 | 4.472 | |
| Vss (steady-state volume of distribution) | L/kg | 0.194 | 4.8 | 0.176 | 0.213 | |
| MRT (Mean residence time (IV)) | hr | 2.768 | 8.3 | 2.313 | 3.223 | |
| AUC | µg*hr/mL | 288.7 | 5.8 | 247.8 | 311.8 | |
| AUMC | µg*hr2/mL | 798.9 | 11.4 | 626 | 980.3 | |
The primary estimated parameters were the volume of distribution of the central compartment (V1), the volume of distribution of the peripheral compartments (V2, V3), the plasma clearance (CL) and the distribution clearances (CL2, CL3). CMultStdev corresponds to the proportional component of the residual error and stdev0 is the additive component of the residual. The estimated fixed parameters were reported as their typical values (tv) with their CV% and their confidence interval that is a measure of the precision of their estimation. Secondary parameters are the half-life of the different phases, the steady-state volume of distribution (Vss), the mean residence time (MRT), area under the curve (AUC) and area under the moment curve (AUMC).
Fig. 4.
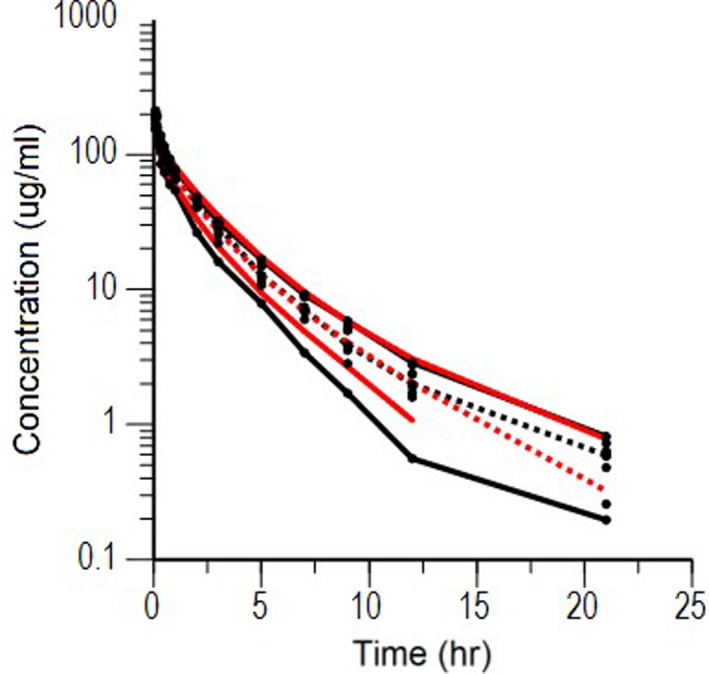
Visual Predictive Check of a single dose of 20 mg/kg BW fosfomycin. The observed and predicted 10th and 90th percentiles are shown in solid black and red lines, respectively. The observed and predicted 50th percentiles (median) are shown in black and red broken lines, respectively. Black dots are individual raw data.
The median plasma protein binding percentage of FOM was 1.5% (−3.9–4.6%), given the median and range, and the free fraction was determined as 1 to make the simulations of free plasma concentration. The PTA for the 5,000 free drug concentration profiles obtained by MCS for different possible MICs and FOM regimens are shown in Fig. 5. For an IV regimen of 20 mg/kg q12 hr IV a PTA of 90% was achieved for a MIC of ≤16.0 mg/L, and it was considered as PK/PD cutoff. The MIC90 of FOM against S. zooepidemicus, S. aureus, E. coli, and P. aeruginosa were 128.0, 2.0, 16.0, and >128.0 mg/L, respectively, isolated from horses. The ECOFF by EUCAST for S. aureus, E. coli and P. aeruginosa determined using EUCAST were 32, 4.0, and 256.0 mg/L, respectively. The ECOFF of S. zooepidemicus was calculated to be 256 mg/L using EcoFinder.
Fig. 5.
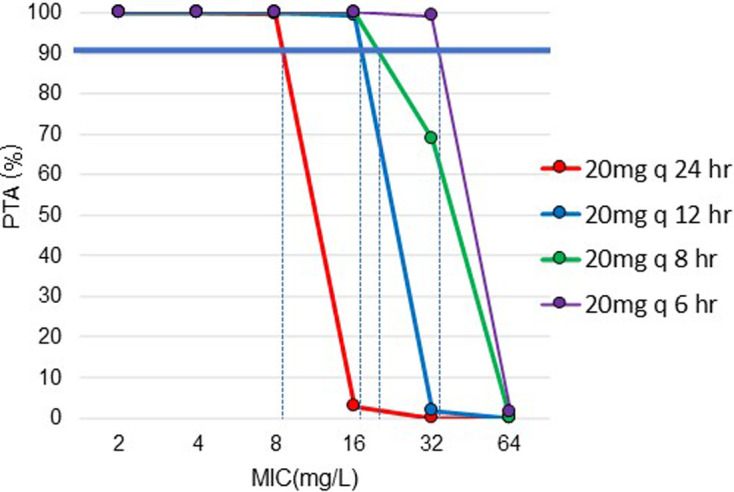
Probability of Target Attainment (PTA%) vs. minimal inhibitory concentration (MIC) (µg/mL) of fosfomycin for repeated administration of fosfomycin 20 mg/kg BW different dosing intervals ranging from 8 to 24 hr. Values were obtained from 5,000 simulated fosfomycin concentrations profiles generated from the population model by Monte Carlo simulations. PTA 90% is indicated by the solid blue line, which is considered as the target to achieve, and MIC that corresponds to PTA 90% is indicated by the dotted blue line.
DISCUSSION
FOM is an old antimicrobial; however, its importance has increased in recent years because of the worldwide emergence of resistant bacteria [12]. In Europe, the Antimicrobial Advice Ad Hoc Expert Group (AMEG) of the European Medicine Agency categorized FOM as a category A antimicrobial; it may be administered to horses under exceptional circumstances [11]. However, FOM has been approved as veterinary product in Japan. This motivated the present study to delimit its use based on an evaluation of the susceptibility of pathogens involved in horse infections. Currently, there is no horse CBP available for interpreting AST results; only human CBP is available. Given that CBPs are species-specific and depend on dosage regimens [26], there is no indication that human CBPs can be used for horses, particularly because of the difference in the recommended dosages in horses (20 mg/kg) and in men (60–80 mg/kg) [23]. The present study aimed to determine the PK/PD cutoff of the FOM, i.e. the highest value of the MIC that can be reached in 90% of horses with the recommended FOM dosage according to VetCAST approach [26]. This PK/PD cutoff, in the absence of CBP, can be used to interpret AST and consider ECOFFs for the involved pathogen, and allows for the prudent use of FOM in horses.
PK/PD cutoff of FOM was estimated using NLME model and free fraction according to VetCAST approach [26]. The extent of protein binding was important to establish a PK/PD cutoff because only the free drug concentration is microbiologically active [1]. It was reported that FOM was not to bind to human plasma proteins and good diffusion into tissues and body fluids in humans [15]. In this study the protein binding rate of FOM in horses was also almost 0 at all-time points and considered not bind to equine plasma protein same as humans. The selection of a PK/PD index is necessary to compute a PK/PD cutoff as a surrogate of antimicrobial efficacy based on an in vivo or in vitro infection model [26]. Antimicrobials are classified as time-dependent or concentration-dependent, with the former index being the time for which the free drug concentration exceeds the MIC (fT >MIC), and the latter being fAUC/MIC [7]. Previous reports on mouse infection models have indicated that FOM has bacteriostatic effects against the Enterobacteriaceae group, with an AUC/MIC (equivalently an fAUC/MIC with f=1) ratio of 24 [17].
In this study, PK/PD cutoff of FOM with 20 mg/kg q12 hr IV administration were calculated as MIC of ≤16.0 mg/L. CLSI indicated the breakpoint for a susceptible MIC value <64.0 mg/L in Enterobacterales and Enterococcus spp. in humans (M100 Ed32E). The EUCAST indicated a breakpoint for a susceptible MIC value of <32.0 mg/L in Enterobacterales and Staphylococcus spp. in humans at a dose of 4 g/patient q 8 hr IV administration (Version 12.0). Compared with these human breakpoints, the PK/PD cutoff was estimated to be lower in horses. These differences are related to the difference between the dosage in humans (60–80 mg/kg q 8 hr) and in horses (20 mg/kg q 12 hr) and the plasma clearance in horses (1.17 mL/kg/min) vs humans (approximately 2 mL/kg/min) [13]. Since there is a difference between the human breakpoints and result of the present paper, the dose of 20 mg/kg q 12 hr administration may be ineffective for ‘susceptible’ bacteria by AST based on human breakpoints with the MIC located between human CBP (32–64 mg/L) and PK/PD cutoff in this study (16 mg/L) in horses. We recommend discarding human CBP and considering the PK/PD cut-off estimated in this study for horses.
The determination of a PK/PD cut-off is first required to develop a population model to subsequently simulate a large meta-population using MCS. In the present study, we used only nine horses, which is a limited number. That said, for the IV route, the only pharmacokinetic determinant of the fAUC/MIC index for FOM was plasma clearance (0.070 L/kg/hr) and its BSV (15.9%). If the BSV of plasma clearance is increased for a given typical PK value, the PK/PD cut off to reach 90% PTA is expected to be lower than our results. Since this study only included a limited number of healthy young thoroughbred horses and none of the tested covariates (age, sex, and body weight) were significant, it is difficult to predict what the PK/PD cutoff would be in other populations. In quarter horses aged 5 to 15 years, the clearance was shown to be lower and AUC to be higher than in the present study. It is therefore likely that the threshold value that we proposed is also valid in that population, since the PK/PD cutoff is inversely proportional to plasma clearance. [28]. To explore the robustness of PTA for AUC/MIC, meta-analysis including various horses and conditions using multiple previously published data based on NLME model with covariate model may be efficient and have recently reported on marbofloxacin and penicillin in horses [4, 16].
The pharmacokinetics of FOM have also been reported in cattle, chicken, pig, dog, and humans [2, 13, 14, 20, 24, 25]. The plasma clearance was higher in horses than in chickens, cattle, and dogs, lower than in pigs, and similar to humans’ clearance [13, 20]. Since AUC is controlled by the administered dose and the plasma clearance, the PK/PD cutoff is expected to decrease with increased clearance and/or lower doses. Dosing regimens are also different, so different PK/PD cutoffs are expected in different animal species. FOM breakpoints have not been indicated in other animals from any organizations such as CLSI and EUCAST. Since FOM is considered a critically important antimicrobial agent for human medicine [10], its use in animal medicine is limited. It’s use has been reported in chickens and pigs in Central and South America, and in cattle in Japan [20]. Whatever the treated species, an appropriate use of FOM after AST based on scientific PK/PD cutoffs is recommended to prevent antimicrobial resistance.
Furthermore, ECOFF and MIC distributions are important for CBP along with PK/PD cutoff and clinical cutoff [26]. The clinical cutoff is an MIC cutoff related to clinical outcomes, but the data required for this value are limited in veterinary medicine. ECOFF is defined as the upper end of the wild-type MIC distribution and is a biological parameter that is not affected by the source (human or animal). If the PK/PD cut-off is below the ECOFF, the current dosage regimen is too low to treat the wild-type population. In this case, VetCAST does not establish a CBP dividing the wild-type MIC distributions to prevent the wild-type strain from becoming resistant [3, 26]. Compared to PK/PD cutoff (16.0 mg/L), ECOFF (4.0 mg/L) and MIC distribution in horses (MIC90; 16.0 mg/L) for E. coli, 16 mg/L may be set as possible CBP for this pathogen. E. coli is sometimes isolated from the lower respiratory tract in horses and is associated with death and severe pleuropneumonia [21], and FOM 20 mg/kg q 12 hr administration was expected to be controlled from this study. Because the PK/PD cutoff (16 mg/L) in this study was below the ECOFF of S. zooepidemicus (256 mg/L), P. aeruginosa (256 mg/L), and S. aureus (32 mg/L), the PK/PD cutoff cannot be set as the CBP for these pathogens. In particular, the ECOFF and MIC distributions in horses of S. zooepidemicus and P. aeruginosa were extremely high; FOM is ineffective and should not be used for these infections. For S. aureus, the PK/PD cutoff was higher than the MIC90 in horses (2.0 mg/L), but lower than the ECOFF (32.0 mg/L). Currently, 20 mg/kg q 12 hr administration is expected to be effective against the MIC90 value of S. aureus isolated from horses, however, q 6h administration is required to cover all wild-type strains. The results of this study will help clarify which pathogens can be targeted in horses by FOM and should promote more responsible prescription trends by reducing empirical use, thus preventing the development of resistant pathogens.
Our study indicated that the PK/PD cutoff in 20 mg/kg BW q12 hr IV were MIC value ≤16.0 mg/L and attained therapeutic concentrations to control E. coli in healthy horses up to the MIC90 values of the wild population. Owing to its importance in human health, veterinarians should use FOM 20 mg /kg q 12 hr mainly against E. coli infections with a MIC <16 µg/mL, as suggested by our PK/PD cutoff after AST.
CONFLICT OF INTEREST
The authors disclose no conflict of interest.
Acknowledgments
This study was supported by a grant from the Japan Racing Association.
REFERENCES
- 1.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 44: 79–86. doi: 10.1086/510079 [DOI] [PubMed] [Google Scholar]
- 2.Aramayona JJ, Bregante MA, Solans C, Rueda S, Fraile LJ, Garcia MA. 1997. Pharmacokinetics of fosfomycin in chickens after a single intravenous dose and tissue levels following chronic oral administration. Vet Res 28: 581–588. [PubMed] [Google Scholar]
- 3.Arendrup MC, Kahlmeter G, Rodriguez-Tudela JL, Donnelly JP. 2009. Breakpoints for susceptibility testing should not divide wild-type distributions of important target species. Antimicrob Agents Chemother 53: 1628–1629. doi: 10.1128/AAC.01624-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet-Mélou A, Schneider M, El Garch F, Broussou DC, Ferran AA, Lallemand EA, Triboulloy C, Damborg P, Toutain PL. 2021. Determination of the pharmacokinetic-pharmacodynamic cut-off values of marbofloxacin in horses to support the establishment of a clinical breakpoint for antimicrobial susceptibility testing. Equine Vet J 53: 1047–1055. doi: 10.1111/evj.13385 [DOI] [PubMed] [Google Scholar]
- 5.Cagnardi P, Di Cesare F, Toutain PL, Bousquet-Mélou A, Ravasio G, Villa R. 2018. Population pharmacokinetic study of cefazolin used prophylactically in canine surgery for susceptibility testing breakpoint determination. Front Pharmacol 9: 1137. doi: 10.3389/fphar.2018.01137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collignon PJ, Conly JM, Andremont A, McEwen SA, Aidara-Kane A, Agerso Y, Andremont A, Collignon P, Conly J, Dang Ninh T, Donado-Godoy P, Fedorka-Cray P, Fernandez H, Galas M, Irwin R, Karp B, Matar G, McDermott P, McEwen S, Mitema E, Reid-Smith R, Scott HM, Singh R, DeWaal CS, Stelling J, Toleman M, Watanabe H, Woo GJ. World Health Organization Advisory Group, Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR).2016. World health organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis 63: 1087–1093. doi: 10.1093/cid/ciw475 [DOI] [PubMed] [Google Scholar]
- 7.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22: 89–96. doi: 10.1016/0732-8893(95)00053-D [DOI] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing (EUCAST).2023. European Committee on Antimicrobial Susceptibility Testing, Breakpoint tables for interpretation of MICs and zone diameters Version 13.1. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf [accessed on November 24, 2023].
- 9.European Committee on Antimicrobial Susceptibility Testing (EUCAST).2023. MIC and zone diameter distributions and ECOFFs. https://www.eucast.org/mic_and_zone_distributions_and_ecoffs [accessed on November 24, 2023].
- 10.European Committee on Antimicrobial Susceptibility Testing (EUCAST).2023. MIC distributions for Fosfomycin. https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=100&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 [accessed on November 24, 2023].
- 11.European Medicine Agency.2023. Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals −Categorisation of antimicrobials. https://www.ema.europa.eu/en/documents/other/answer-request-european-commission-updating-scientific-advice-impact-public-health-animal-health-use_en.pdf [accessed on November 24, 2023].
- 12.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29: 321–347. doi: 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20: 393–397. doi: 10.1128/AAC.20.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez OL, Ocampo CL, Aguilera JR, Luna J, Sumano LH. 2008. Pharmacokinetics of disodium-fosfomycin in mongrel dogs. Res Vet Sci 85: 156–161. doi: 10.1016/j.rvsc.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 15.Kirby WM. 1977. Pharmacokinetics of fosfomycin. Chemotherapy 23Suppl 1: 141–151. doi: 10.1159/000222040 [DOI] [PubMed] [Google Scholar]
- 16.Lallemand EA, Bousquet-Mélou A, Chapuis L, Davis J, Ferran AA, Kukanich B, Kuroda T, Lacroix MZ, Minamijima Y, Olsén L, Pelligand L, Portugal FR, Roques BB, Santschi EM, Wilson KE, Toutain PL. 2023. Pharmacokinetic-pharmacodynamic cutoff values for benzylpenicillin in horses to support the establishment of clinical breakpoints for benzylpenicillin antimicrobial susceptibility testing in horses. Front Microbiol 14: 1282949. doi: 10.3389/fmicb.2023.1282949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (Fosfomycin for Injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61: e00476–e00417. doi: 10.1128/AAC.00476-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2: e38. doi: 10.1038/psp.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SS, Balfour JA, Bryson HM. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53: 637–656. doi: 10.2165/00003495-199753040-00007 [DOI] [PubMed] [Google Scholar]
- 20.Pérez DS, Tapia MO, Soraci AL. 2014. Fosfomycin: uses and potentialities in veterinary medicine. Open Vet J 4: 26–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Racklyeft DJ, Love DN. 2000. Bacterial infection of the lower respiratory tract in 34 horses. Aust Vet J 78: 549–559. doi: 10.1111/j.1751-0813.2000.tb11901.x [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Gascón A, Canut-Blasco A. 2019. Deciphering pharmacokinetics and pharmacodynamics of fosfomycin. Rev Esp Quimioter 32Suppl 1: 19–24. [PMC free article] [PubMed] [Google Scholar]
- 23.Sojo-Dorado J, López-Hernández I, Rosso-Fernandez C, Morales IM, Palacios-Baena ZR, Hernández-Torres A, Merino de Lucas E, Escolà-Vergé L, Bereciartua E, García-Vázquez E, Pintado V, Boix-Palop L, Natera-Kindelán C, Sorlí L, Borrell N, Giner-Oncina L, Amador-Prous C, Shaw E, Jover-Saenz A, Molina J, Martínez-Alvarez RM, Dueñas CJ, Calvo-Montes J, Silva JT, Cárdenes MA, Lecuona M, Pomar V, Valiente de Santis L, Yagüe-Guirao G, Lobo-Acosta MA, Merino-Bohórquez V, Pascual A, Rodríguez-Baño J. REIPI-GEIRAS-FOREST group.2022. Effectiveness of fosfomycin for the treatment of multidrug-resistant escherichia coli bacteremic urinary tract infections: a randomized clinical trial. JAMA Netw Open 5: e2137277. doi: 10.1001/jamanetworkopen.2021.37277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soraci AL, Perez DS, Martinez G, Dieguez S, Tapia MO, Amanto F, Harkes R, Romano O. 2011. Disodium-fosfomycin pharmacokinetics and bioavailability in post weaning piglets. Res Vet Sci 90: 498–502. doi: 10.1016/j.rvsc.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 25.Sumano LH, Ocampo CL, Gutierrez OL. 2007. Intravenous and intramuscular pharmacokinetics of a single-daily dose of disodium-fosfomycin in cattle, administered for 3 days. J Vet Pharmacol Ther 30: 49–54. doi: 10.1111/j.1365-2885.2007.00812.x [DOI] [PubMed] [Google Scholar]
- 26.Toutain PL, Bousquet-Mélou A, Damborg P, Ferran AA, Mevius D, Pelligand L, Veldman KT, Lees P. 2017. En route towards european clinical breakpoints for veterinary antimicrobial susceptibility testing: a position paper explaining the VetCAST approach. Front Microbiol 8: 2344. doi: 10.3389/fmicb.2017.02344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijma RA, Bahmany S, Wilms EB, van Gelder T, Mouton JW, Koch BCP. 2017. A fast and sensitive LC-MS/MS method for the quantification of fosfomycin in human urine and plasma using one sample preparation method and HILIC chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 1061-1062: 263–269. doi: 10.1016/j.jchromb.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 28.Zozaya DH, Gutiérrez OL, Ocampo CL, Sumano LH. 2008. Pharmacokinetics of a single bolus intravenous, intramuscular and subcutaneous dose of disodium fosfomycin in horses. J Vet Pharmacol Ther 31: 321–327. doi: 10.1111/j.1365-2885.2008.00970.x [DOI] [PubMed] [Google Scholar]