Summary:
In Blood Cancer Discovery, Saygin and colleagues report that somatic variants that are recurrent in myeloid malignancies can also occur with high frequency (16%) in adult acute lymphoblastic leukemia (ALL) where they correlate with older age, diagnosis following genotoxic therapy for a prior malignancy and worse outcome to chemotherapy. Mutations in these “myeloid” genes can precede ALL diagnosis and arise in hematopoietic stem or progenitor cells that clonally expand and differentiate into both lymphoblasts and nonmalignant myeloid cells, supporting a role for clonal hematopoiesis as premalignant state outside the context of myeloid malignancies and providing implications for both ALL etiology and therapeutic intervention.
Clonal hematopoiesis refers to clonal outgrowth of hematopoietic stem and progenitor cells (HSPC) that carry somatic mutations in the absence of a diagnosed hematologic malignancy or cytopenia. Commonly mutated genes are epigenetic regulators such as DNMT3A, TET2, and ASXL1; DNA damage repair genes such as TP53 and PPM1D; JAK2; and splicing factor genes such as SF3B1, SRSF2, and U2AF1. Clonal hematopoiesis with mutations in these genes (myeloid clonal hematopoiesis/TP53 clonal hematopoiesis) is associated with an increased risk of developing myeloid malignancies, cardiovascular disease, and other nonmalignant disorders (1). Moreover, clonal hematopoiesis with mutations in genetic drivers of lymphoid malignancies (lymphoid clonal hematopoiesis) has been recently associated with an increased risk of developing lymphoid malignancies, such as chronic lymphocytic leukemia or lymphoma, and autoimmunity complications or immune deficiency (2). Nonetheless, the association between specific clonal hematopoiesis–related mutations and lineage (myeloid or lymphoid) of developed malignancies may not be unique. For example, mutations associated with myeloid clonal hematopoiesis (herein referred to as “myeloid mutations” or shortly “MyM”) such as DNMT3A, RUNX1, and ASXL1 were described in patients who developed ALL following genotoxic therapy for a prior malignancy. Patients with therapy-related ALL also had higher rates of poor-risk cytogenetic features (e.g., KMT2A-rearrangements) and inferior overall survival compared with patients with de novo ALL (3). These findings prompt the question of whether myeloid mutations may be recurrent in ALL and derive from clonal hematopoiesis, a mechanism well elucidated in the context of myeloid malignancies but mostly unexplored for lymphoblastic leukemogenesis. By using comprehensive bulk genetic profiling and cutting-edge single-cell immune genotyping and transcriptome sequencing technologies, Saygin and colleagues demonstrated that myeloid mutations are far more common in de novo adult ALL than previously appreciated and that these mutations can be preexisting to ALL diagnosis and derive from antecedent clonal hematopoiesis. Compared with ALL samples lacking these mutations, ALL patients with MyM exhibit distinct genetic and clinical features with adverse outcome after conventional chemotherapy (4) providing important implications for risk prognostication and therapy.
ALL encompasses over thirty different molecular subtypes of B- and T-lineage that are defined by distinct patterns of chromosomal abnormalities and genetic alterations, including chimeric gene fusions, cryptic rearrangements, and sequence mutations, that drive distinct gene expression profiles and display unique clinical features. The nature and frequency of subtype-defining genetic alterations vary according to age, suggesting a different etiology for genetic alterations across the age spectrum. For example, favorable-risk subtypes such as ETV6::RUNX1 and hyperdiploidy are common in children, whereas poor-risk subtypes such as BCR::ABL1 and BCR::ABL1-like are more frequent in adults (5). In addition to initiating genetic lesions, secondary alterations in lymphoid transcription factors, tumor suppressors, kinase/Ras signaling, transcriptional and epigenetic regulators are common and often used to refine risk stratification (6). Mutations in these genes contribute to a block of lymphoid differentiation and activation of multiple cellular pathways important for leukemic transformation and response to treatment. While many of these mutations are thought to originate in lymphoid progenitors, a full understanding of their origin, especially in adult patients, is lacking.
Notably, even within the same leukemia subtype the origin of specific genetic alterations may differ between children and adults with ALL. The best paradigm of this phenomenon is represented by low hypodiploid ALL (LH-ALL) which is defined by nonrandom losses of chromosomes leading to a karyotype with 32–39 chromosomes. In both children and adults with LH-ALL, biallelic TP53 mutations are present in almost all cases. Nevertheless, while in pediatric cases these mutations are also present in nontumor cells suggesting a germline origin, in adults with LH-ALL TP53 mutations are somatic and thought to originate from age-related clonal hematopoiesis as demonstrated by the persistence of these mutations in postremission samples (7). However, except for LH-ALL, the role of preleukemic clonal hematopoiesis for the development of ALL has been mostly unexplored. In this issue of Blood Cancer Discovery, Saygin and colleagues (4) profiled by comprehensive genetic analysis 400 adult ALL patients of B- (82%) and T-lineage (18%). Common mutations were detected in TP53 (18%) and in at least one of 16 myeloid clonal hematopoiesis–associated genes (16%) including, among the most frequently mutated, DNMT3A (5%), TET2 (4%), ASXL1 (3%), RUNX1 (3%), and IDH2 (2%). In contrast, mutations in genes that have been previously associated with lymphoid clonal hematopoiesis, such as KMT2D, KMT2C, MTOR, and ATM, were identified in a lower proportion of cases (7%). Consistent with previous studies in both pediatric and adult ALL, additional alterations were detected in signaling pathway genes (59%), transcription factors (37%), cell-cycle regulators (37%), and epigenetic regulators (19%). B-ALL patients with MyM mutations were often older adults, with therapy-related B-ALL and with BCR::ABL1/BCR::ABL-like ALL. As previously reported by an independent study (7), mutations in TP53 were common (>80%) in LH-ALL and near haploid ALL but they were also detected in other subtypes, including KMT2A-rearrenged ALL. On the basis of the mutational status of TP53/MyM, patients with ALL were stratified in three groups to explore clinical correlations: TP53-mutated-ALL, ALL with MyM, and ALL without MyM/TP53 mutations. In multivariable analysis, age, TP53 mutation, and MyM were independent predictors of poor overall survival to conventional chemotherapy. In patients with T-ALL, MyM or TP53 mutations were predominantly identified in the HOX-dysregulated, LMO2 and TAL1 subtypes. However, full genetic characterization and molecular annotation of different T-ALL subtypes were missing in this study and most cases (up to 50%) lacked information on the subtype-defining lesion and were grouped as “other.” Similar to B-ALL patients with MyM, T-ALL patients with MyM were prevalently older, with therapy-related T-ALL and showed worse outcome compared to patients without MyM/TP53 mutations.
An initial insight into unraveling the etiology of MyM derived from the analysis of bulk sequencing data which revealed a high variant allele frequency (VAF) of these mutations suggesting occurrence in most lymphoblasts and early acquisition from the founder clone. In contrast, mutations involving signaling pathway genes showed significantly lower VAFs, indicating subclonal pattern and late occurrence during leukemic evolution. However, although the temporal order and clonal architecture of mutations can be predicted by the analysis of VAF from bulk DNA sequencing, only single-cell DNA sequencing has the appropriate resolution to demonstrate mutational cooccurrence (mutations within the same cell) that can be used to infer clonal evolution (8). Thus, to investigate whether MyM were clonal and to infer their origin, Saygin and colleagues performed single cell–targeted DNA and cell surface protein sequencing (4). This approach has the power to measure both genotype and phenotype from the same cell across many cells by using oligo-conjugated antibodies. This analysis revealed that while most of the mature normal lymphoid cells were wild type, both lymphoblasts and nonmalignant myeloid cells shared myeloid or TP53 mutations, supporting a stem or progenitor cell origin that gives rise to both lymphoblasts and myeloid cells. This hypothesis was further enforced by the observation that MyM and TP53 mutations were detectable in patients with complete remission and negative measurable residual disease after treatment, confirming that a stem cell clone with these mutations contributed to reconstitute normal hematopoiesis after chemotherapy. Moreover, these mutations remained stable throughout the progression of the disease and persisted or expanded at the time of relapse, further supporting their stem cell origin.
These findings contrast with the notion that most B-ALL subtypes arise from progenitors already committed toward lymphoid lineage and thus raise the question of whether these mutations, similarly to their origin in myeloid malignancies, may be ancestral to ALL and derive from clonal hematopoiesis. The analysis of serial samples from patients with therapy-related B-ALL, which were obtained several years before the diagnosis of ALL, at the time of ALL diagnosis, and at the subsequent follow-up, revealed indeed the presence of MyM and TP53 mutations several years earlier the diagnosis of ALL, demonstrating that these mutations were antecedent to ALL. Interestingly, these mutations were often found to confer predisposition to more than one hematologic malignancy with distinct disease trajectories and treatment responses. For example, in a patient with therapy-related B-ALL after ALL-directing therapy, a clonal hematopoiesis TET2-mutated clone acquired SF3B1 mutation and expanded giving raise to myelodysplastic syndrome. These results highlight the importance of monitoring clonal hematopoiesis mutations for prevention and prognostic purposes.
To understand the role that MyM play into lymphoid leukemogenesis and resistance to therapy, single-cell RNA sequencing (scRNA-seq) was performed on representative samples of B-ALL with MyM, B-ALL without MyM and in samples with myeloid clonal hematopoiesis. If single-cell DNA sequencing can dissect mutational cooccurrence and reconstruct clonal evolution, scRNA-seq provides information on intra- and intertumor transcriptomic heterogeneity which is relevant to understand mechanisms driving both leukemogenesis and therapeutic response (8). Compared with ALL without MyM and individuals with clonal hematopoiesis, BCR::ABL1–negative ALL with MyM showed distinct genetic features with upregulation of genes promoting cell survival, invasion, and tumor growth and upregulation of genes (e.g., GNLY, GZMB, and GZMH), which define activated T cells. These expression patterns could explain the results obtained by the in vitro drug screening which revealed poor sensitivity of B-ALL cells with MyM to chemotherapeutic agents but not to the bispecific T-cell engager blinatumomab. Consistently, when patient outcome data were analyzed, complete remission rates to cytotoxic chemotherapy were lower in B-ALL patients with MyM or TP53 mutations and higher in those without MyM/TP53. However, complete remission rates were independent on MyM status when patients were treated with antibody-based therapies (blinatumomab or inozutumab). Collectively, these findings have substantial clinical implications for ALL treatment and prediction of relapse because they suggest that lymphoblasts with MyM are intrinsically more resistant to cytotoxic chemotherapy compared with B-ALL without MyM/TP53. However, the presence of activated T cells may favor responses to antibody-based therapies providing a strong rational for their clinical use.
In conclusion, the identification by Saygin and colleagues (4) of myeloid/TP53-mutant clonal hematopoiesis in the context of ALL challenges the traditional notion of ALL etiology and provides valuable insights into diagnosis, prognosis, and treatment approaches for adult patients with ALL (Fig. 1). The demonstration that TP53 and myeloid clonal hematopoiesis mutations precede ALL and arise from multipotent stem cells raises important biological and clinical considerations. First, myeloid mutations were found associated with specific ALL subtypes such as BCR::ABL1/BCR::ABL1-like and mutually exclusive with others, such as TCF3::PBX1, ETV6::RUNX1, ZNF384, PAX5-alt, PAX5 P80R, and iAMP21. Although these associations may partially reflect the distribution of subtype-defining lesions across age, the exact molecular mechanisms and consequences of these associations were not investigated. Most myeloid mutations are in epigenetic regulators and transcription factors, thus they may impair chromatin status, transcriptome signatures and shape leukemia cell developmental state composition that can predispose or cooperate with ALL genetic drivers. Recently, a multipotent cell of origin has been described for a subset of BCR::ABL1 ALL patients with multilineage features (9, 10). Through functional experimental assays and genetic analysis, in these cases the BCR::ABL1 rearrangement was hypothesized to arise in a multipotent stem cell that can differentiate in myeloid cells and transform in ALL when cooperating secondary alterations are acquired in a downstream B-cell progenitor. Notably, RUNX1 mutations are common in these BCR::ABL1 patients in line with findings from Saygin and colleagues who identified a similar association. These findings highlight the importance of enhancing our comprehension of myeloid clonal hematopoiesis in the context of B-ALL cell of origin with the final aim to understand disease etiology and guide therapeutic intervention. Second, the high frequency of “myeloid” mutations in ALL and other lymphoid malignancies, as well as in solid cancers, underscores the need to revisit the conventional “myeloid” versus “lymphoid” clonal hematopoiesis terminology. The term “myeloid genes” originated from their initial association with myeloid cancers through clonal hematopoiesis. However, with increasing evidence of mutations in lymphoid cancers, including the article by Saygin and colleagues, this terminology is becoming obsolete and potentially misleading, especially because it does not involve a direct biological role of most of these “myeloid” mutations in the myeloid program. From a clinical perspective, the involvement of clonal hematopoiesis as preleukemic state of lymphoid leukemogenesis highlights the need for future studies investigating ALL clonal evolution and prevention (in addition to myeloid neoplasms) in individuals with clonal hematopoiesis. Finally, the intrinsic resistance of ALL with MyM to cytotoxic chemotherapy but not to immunotherapeutic approaches highlights the importance of screening and detecting these mutations to inform and refine treatment strategies. However, follow-up studies in larger and subtype-unbiased cohorts of ALL patients, are warranted to understand long-term responses and mechanisms of disease progression.
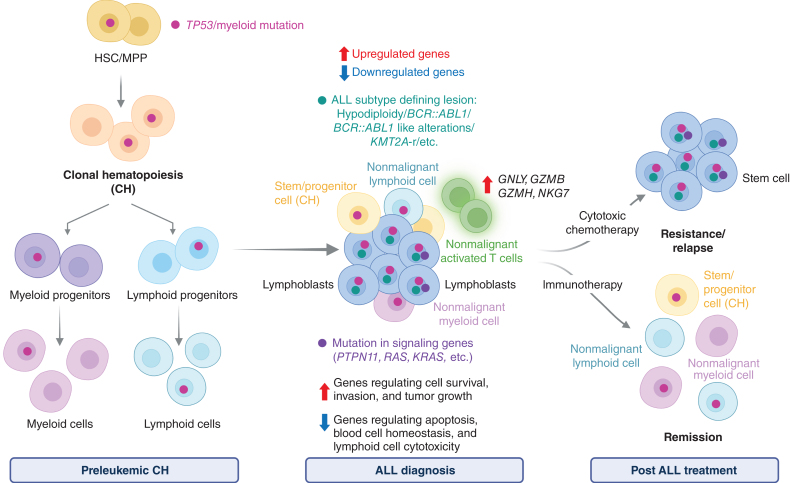
Figure 1.
ALL with TP53/myeloid mutations arising from clonal hematopoiesis. Simplified cartoon depicting the evolution of acute lymphoblastic leukemia from antecedent clonal hematopoiesis with mutations in “myeloid” genes. Mutations in myeloid genes (e.g., DNMT3A, TET2, ASXL1, RUNX1, and IDH2) or TP53 are acquired in hematopoietic stem cells (HSC)/multipotent progenitors (MPP) and promote the expansion of a clone that can differentiate in both nonmalignant mature myeloid and lymphoid cells and evolve in lymphoblasts. Lymphoblasts with TP53/myeloid mutations are characterized by upregulation of prosurvival genes and downregulation of proapoptotic genes that confer resistance to conventional chemotherapy: (as represented in the cartoon by the expansion of leukemic cells). Moreover, activated T cells are present in these leukemic samples and thought to confer sensitivity to immunotherapy or chemo-immunotherapy combination approaches. Abbreviation: KMT2A-r, KMT2A-rearrangments. This figure was created with BioRender.com.
Authors’ Disclosures
I. Iacobucci reports reimbursement for travel and accommodation costs from Mission Bio and consultation fee from Arima Genomics.
References
- 1. Weeks LD, Ebert BL. Causes and consequences of clonal hematopoiesis. Blood 2023;142:2235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niroula A, Sekar A, Murakami MA, Trinder M, Agrawal M, Wong WJ, et al. Distinction of lymphoid and myeloid clonal hematopoiesis. Nat Med 2021;27:1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saygin C, Kishtagari A, Cassaday RD, Reizine N, Yurkiewicz I, Liedtke M, et al. Therapy-related acute lymphoblastic leukemia is a distinct entity with adverse genetic features and clinical outcomes. Blood Adv 2019;3:4228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saygin C, Zhang P, Stauber J, Aldoss I, Sperling AS, Weeks LD, et al. Acute lymphoblastic leukemia with myeloid mutations is a high-risk disease associated with clonal hematopoiesis. Blood Cancer Discov 2024;5:164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iacobucci I, Kimura S, Mullighan CG. Biologic and therapeutic implications of genomic alterations in acute lymphoblastic leukemia. J Clin Med 2021;10:3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brady SW, Roberts KG, Gu Z, Shi L, Pounds S, Pei D, et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat Genet 2022;54:1376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim R, Bergugnat H, Larcher L, Duchmann M, Passet M, Gachet S, et al. Adult low-hypodiploid acute lymphoblastic leukemia emerges from preleukemic TP53-mutant clonal hematopoiesis. Blood Cancer Discov 2023;4:134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iacobucci I, Witkowski MT, Mullighan CG. Single-cell analysis of acute lymphoblastic and lineage-ambiguous leukemia: approaches and molecular insights. Blood 2023;141:356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim JC, Chan-Seng-Yue M, Ge S, Zeng AGX, Ng K, Gan OI, et al. Transcriptomic classes of BCR-ABL1 lymphoblastic leukemia. Nat Genet 2023;55:1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bastian L, Beder T, Barz MJ, Bendig S, Bartsch L, Walter W, et al. Developmental trajectories and cooperating genomic events define molecular subtypes of BCR::ABL1-positive ALL. Blood 2023Dec 28 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]