Summary:
While the current approach to precursor hematologic conditions is to “watch and wait,” this may change with the development of therapies that are safe and extend survival or delay the onset of symptomatic disease. The goal of future therapies in precursor hematologic conditions is to improve survival and prevent or delay the development of symptomatic disease while maximizing safety. Clinical trial considerations in this field include identifying an appropriate at-risk population, safety assessments, dose selection, primary and secondary trial endpoints including surrogate endpoints, control arms, and quality-of-life metrics, all of which may enable more precise benefit–risk assessment.
INTRODUCTION
Precursor disease states require unique considerations for drug development. The natural history of precursor diseases requires efforts to minimize toxicity and balance safety and tolerability with therapeutic efficacy. These aspects of any therapeutic intervention in the space should be well characterized. Along with including a carefully defined, significantly at-risk patient population, we can make a more precise benefit–risk assessment. Trials should be designed to maximize our ability to interpret the results and confidently integrate findings into clinical practice. Finally, both early and late endpoints should be measured, recognizing the critical role of long-term outcomes in this setting. Early engagement with regulatory agencies can avoid delays due to the complexities of drug development in this space.
DEFINING AN “AT-RISK POPULATION” IN MULTIPLE MYELOMA PRECURSORS
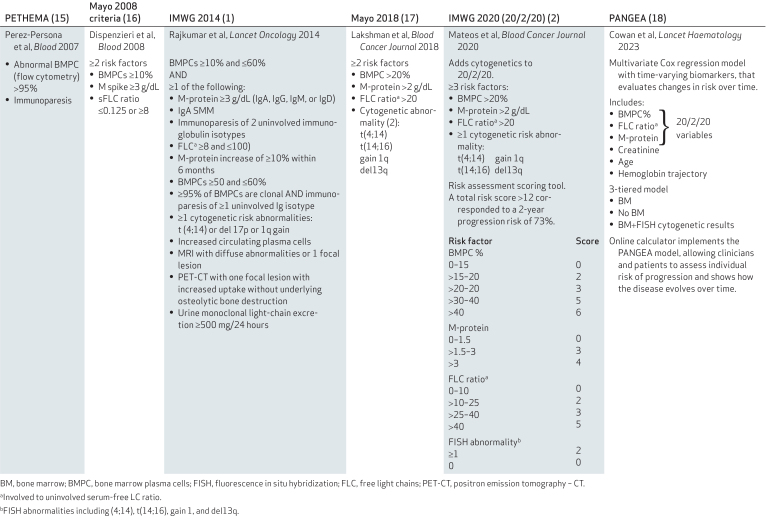
There are many ongoing studies in smoldering multiple myeloma (SMM), especially in high-risk SMM. However, the evolving definition of high-risk SMM and disparate inclusion criteria have made the results difficult to generalize. Multiple criteria can be used to define the risk of progression to multiple myeloma (Table 1). Several have demonstrated clinical utility but these distinct approaches have discordant results for the same patients. In 2014, the International Myeloma Working Group (IMWG) modified the hypercalcemia, renal failure, anemia, and bone disease (CRAB) definition of multiple myeloma by adding three criteria, ≥60% bone marrow plasma cells, free light chain (FLC) ratio ≥100, and >1 MRI-defined ≥5 mm focal lesion, called SLiM CRAB, to include cases previously defined as ultra-high-risk SMM and avoid undue harm that occurs during “watchful waiting” (1). In 2020, the 20/2/20 risk stratification model provided a new definition of high-risk SMM (2). However, patients with rapidly increasing M spike or serum-free light-chain levels or decreasing hemoglobin levels are also recognized as having a high risk of developing multiple myeloma. Studies have used this expanded definition of high-risk SMM based on dynamic monitoring of the disease rather than a one-time snapshot (3). To further improve risk assessment, new progression risk models are needed and will likely include factors such as circulating tumor cells and genomic aberrations (Myc, KRAS, etc.) that are indicative of high-risk SMM (4, 5). The development of additional markers or models to better stratify patients by progression risk and define an appropriate at-risk population should be encouraged and supported.
Table 1.
Several criteria are used to define the risk of progression to overt multiple myeloma.
There is a need to define the population that is likely to experience the most favorable benefit-risk ratio with a given therapeutic. When considering the benefit-risk balance, we often refer to the uncertainty of efficacy and safety assessments. In the context of conditions like monoclonal gammopathy of undetermined significance (MGUS) and low-risk SMM with long natural histories and generally favorable outcomes (i.e., low progression rates) uncertainty must be minimized, emphasizing the need for robust and comprehensive clinical trials with precise assessments.
Uniform use of 20/2/20 to define high-risk SMM in clinical trials could help advance drug development in SMM. High variability in clinical trial inclusion criteria makes it difficult to perform meta-analyses or use data from past trials in new inquiries. It can also lead to challenges in clinical trial accrual in many countries or applying the inclusion criteria to real-world populations. Cross-trial comparisons and assessments of outcomes over time are challenging in the absence of standardized criteria. Dynamic changes in M spike or serum-free light-chain levels constitute a significant increase in the risk of progression, and clinicians use these factors to make risk assessments. Incorporating dynamic changes would be critical to define high-risk SMM cases that are likely to progress to multiple myeloma.
A major caveat of existing models is the lack of consideration for light-chain (LC) SMM. Patients with LC SMM may have a serum FLC ratio >100 and be diagnosed with overt multiple myeloma by SLiM-CRAB criteria, while they typically show no M spike. They also cannot be accurately stratified by 20/2/20. A recent publication from Mayo Clinic clarified that a patient with urinary free light chains of <200 mg/24 hours, even with a FLC ratio >100, is still at low risk of progression and can help differentiate overt multiple myeloma from SMM (6).
SAFETY ASSESSMENT AND APPROPRIATE DOSE SELECTION
Several questions must be addressed regarding safety. First, for a previously approved drug, are there different adverse reactions observed in the precursor patient population? What is the duration of treatment, and does it differ from the previously approved population? If this is a continuous therapy, the long-term safety would need to be adequately characterized and be deemed acceptable for this patient population. If it is a fixed duration, how do we decide on the length of treatment? What toxicity is acceptable in this patient population?
Patient-reported outcomes or patient preference assessments may be useful in this context. Regardless of the mechanism, toxicity, and tolerability data for both short and long-term assessments must be collected robustly.
Another important topic with both safety and efficacy implications that is often overlooked is the appropriate dosage in this patient population. There are several drugs in the oncology space whose development programs failed, largely due to unacceptable safety profiles, that may have benefited from additional dose optimization.
Recognizing the importance of dose optimization, the Oncology Center of Excellence initiated Project Optimus. The Project's objective is to ensure that cancer drug doses are optimized to maximize efficacy as well as safety and tolerability by reforming the dose selection and optimization process. As a field, we should emphasize the importance of dose optimization, especially for patient populations with precursor conditions.
Instead of identifying just one dose to move forward or accepting the dose identified in a relapsed or refractory patient population, a range of doses should be identified with multiple dosages moving forward ideally in a randomized trial. Both dose–response and exposure–response relationships for efficacy and toxicity should be characterized. The FDA recently published draft guidance on dose optimization, which may be helpful.
CLINICAL TRIAL ENDPOINTS
The International Conference and Harmonization (ICH) E9 guidance states that the primary endpoint should be a valid and reliable measure of a clinically relevant and important treatment benefit. Valid and reliable refers to the performance characteristics of the test. The endpoint should also be clinically relevant. When considering an appropriate endpoint, the natural history of the underlying disease and the desired outcomes for the patient population should be considered. The endpoint should capture the benefit conferred by the treatment. Specifically, the primary endpoint should evaluate the benefit in the particular aspect of disease targeted by the treatment.
Some challenges with endpoint selection in precursor diseases stem from the early disease setting and long natural history. It is challenging to assess long-term outcomes like overall survival, and endpoints that can be assessed earlier have either not been fully validated or only validated in late-stage disease. The clinical meaningfulness of delaying the onset of multiple myeloma should be assessed in the context of other long-term or subsequent outcomes.
Care must be taken to ensure that early intervention does not lead to refractoriness to subsequent therapies, or decreased ability to receive or tolerate subsequent therapies with an overall worsening of long-term outcomes. In addition, the risk of refractoriness should be considered in balance with the opportunity to avoid the harm of more severe disease, as in multiple myeloma. It is well established that delayed treatment is associated with the expansion of more aggressive clones (7, 8). Therefore, trials should be continued for assessment of overall survival because this is the ultimate endpoint for early treatment. There is a need to evaluate endpoints that can be assessed at early and late times to provide definitive evidence of clinical benefit.
PROGRESSION-FREE SURVIVAL VERSUS END ORGAN DAMAGE AS AN ENDPOINT FOR SMM TRIALS
A major issue is whether a patient with high-risk SMM who received therapy for a fixed duration and then shows evidence of biochemical progression and meets the criteria of high-risk SMM anew should initiate therapy right away. An endpoint of many current clinical trials—including registration studies—is the time to develop SLiM CRAB. We consider several factors. First, PFS (defined as time to biochemical progression) has been used for all clinical trials of overt MM without waiting for patients to develop symptoms. Second, if a patient is in a clinical trial and their M-protein concentration is rising, they may not want to continue the treatment or continue observation until organ damage occurs. Third, it is hard to discern treatment-related renal function impairment and anemia from SLiM CRAB myeloma events. They can be intercurrent events and interfere with assessing the primary endpoint. In this instance, biochemical progression can be a clearer readout of the underlying premalignancy, malignancy development, or worsening of disease. Given these considerations, biochemical progression may be a reasonable endpoint. Importantly, from a patient standpoint, participation in the clinical trial was likely driven by the opportunity to avoid end-organ damage, and hence waiting for that to happen may not be acceptable.
RE-TREATING PATIENTS UPON BIOCHEMICAL PROGRESSION
Clonal selection posttherapy was identified as a critical issue. To date, we have no evidence that the underlying clones become more aggressive at progression in patients treated for 2 years or on a fixed duration therapeutic trial (9, 10). However, such patients are observed to progress again off therapy, with some progressing to high-risk SMM or SLiM CRAB multiple myeloma rapidly after completing therapy, indicating that the underlying disease is aggressive and requires further control.
Biochemical progression itself can be a clinical trial endpoint given that these patients not only biochemically progress but many also meet the criteria for what we consider high-risk SMM. If a patient's disease is evolving rapidly, they may consider going on therapy or subsequent clinical trials. There was a discussion of whether those patients should be included on the same trials as those for untreated high-risk SMM or at least identified as previously treated to define their response to subsequent therapy compared to untreated patients. We understand that they may have a very different response to therapy, especially if they are being re-treated with the same agents. Would a patient who responded to treatment with lenalidomide and dexamethasone but progressed 4 years later be treated again with lenalidomide and dexamethasone? Separate cohorts or arms on trials for these patients would be ideal for data interpretation.
Using biochemical progression as an endpoint, mirroring what is used in multiple myeloma, should be considered an option. We do not wait for patients with multiple myeloma who exhibit biochemical progression after treatment to have end-organ damage again before re-treatment, so we should investigate the risk–benefit balance of treating high-risk SMM similarly. In addition, we must consider whether biochemical progression allows us to re-treat these patients because they meet the criteria of high-risk disease once again.
It is important that criteria to define progression on a clinical trial are consistent with those used in clinical practice. This again highlights the benefit of randomized trials that properly address whether the effect of antimyeloma therapy used earlier is beneficial compared with waiting and using that therapy when patients develop MM.
Re-treatment population could be included as a separate cohort of an ongoing trial with strict and unified criteria as to when to re-treat. Further assessment of biochemical progression as an endpoint and a standardized approach to re-treatment in the precursor setting would significantly advance this field.
OVERALL SURVIVAL
The use of overall survival as a primary endpoint can be challenging in precursor disease states, and the FDA is amenable to considering other endpoints for specific trials. However, if early endpoints are used, late endpoints should also be measured to allow for a complete risk–benefit assessment. Even if accelerated approval is granted based upon the early endpoint, the trial is continued for assessment of late endpoints for regular approval. Overall survival is an important safety parameter. Although we may not use it as the primary endpoint, we need to make sure that we are assessing overall survival even in these early precursor trials. Despite the possibility of patients becoming refractory or precluded from the use of other agents, if overall survival ultimately is not negatively impacted and is potentially favorably impacted, a treatment can be deemed successful.
USING SURROGATE EARLY TIME POINTS
Trials powered for endpoints like biochemical progression and long-term follow-up for a second progression to look at the effect of salvage therapy for patients who progress are critical, but an earlier or intermediate endpoint may be necessary. Intermediate endpoints include minimal residual disease (MRD) assessment and remission (complete remission).
CONSIDERATIONS FOR MRD
There are several considerations for an endpoint to be validated. One component is the analytic validity of the assay itself, which we have established in some disease states. Some MRD assays have been assessed and approved by the FDA. They have not, however, been approved for use as an endpoint. In addition to analytical validity, potential early endpoints need to be assessed for clinical validity. Previous efforts have included collaborative patient-level data gathering and submission for FDA review. The FDA examines whether the definitions of endpoints were consistent across studies and the associations between early surrogate endpoints and long-term endpoints (overall survival and progression) are proportional and consistent. While flexibility is needed, there must be confidence that the endpoint is a reliable marker in SMM. The field should consider sustained MRD as an early endpoint in these clinical trials.
COMPLETE REMISSION AS A SURROGATE ENDPOINT
Accelerated approval is often based on single-arm trials but randomized trials can also be used. Response rate data may be appropriate in such cases. A randomized study would help to better understand safety in this population and ultimately allow for truly effective therapies to get to patients sooner. The published myeloma response criteria can also be used in SMM (11).
Some agents being used in multiple myeloma are so effective that progression-free survival in the first-line setting is approaching 5–6 years, which would be a long time to wait for the primary endpoint. Ideally, SMM trials would use a proven surrogate endpoint around the time of response and continue follow-up for long-term outcomes.
Complete response (CR) or sustained CR can be considered as a primary initial surrogate endpoint while also collecting data on PFS and sensitivity or refractoriness to subsequent lines of therapy. An interception strategy does not need to be 100% effective, but rather needs to provide a meaningful risk reduction. The level of risk reduction that would be meaningful and benefit individual patients is a critical factor to consider.
COMPARATOR ARM IN A RANDOMIZED TRIAL: OBSERVATION VERSUS OTHER THERAPY
When assessing potential control arms, the FDA considers the current therapeutic context and benefit-risk assessment. Currently, observation is the standard of care for most hematologic precursors. Randomized controlled trials will be needed to understand and minimize the uncertainty regarding safety and efficacy of any proposed treatment in this patient population. It is challenging to compare to historical controls because of changes in management and outcomes over time, making selection of a comparably defined patient population difficult. Also, time-to-event endpoints can only be adequately assessed in randomized trials due to the potential for bias. Randomized controlled trials also provide a robust assessment of safety, which is important in this disease setting. However, we already have data from some phase three trials in patients with SMM treated with lenalidomide or lenalidomide and dexamethasone that have shown clear superiority over observation (12, 13). In this context, it is not clear that observation is still the ideal control arm. In the vast majority of precursor disease states observation is still the standard, but where there is evidence of benefit from other therapies, lenalidomide and dexamethasone may be used as a control.
DURATION OF THERAPY AND TREATMENT-FREE INTERVALS IN SMM
Most SMM clinical trials are designed with a fixed duration of therapy, but a treatment-free interval could allow for immune system recovery. Surrogate markers like T-cell types (response biomarker), less T-cell exhaustion, or actual reduction of T-cell exhaustion markers during a treatment-free interval could be considered recovery biomarkers. These could be used to design strategic treatment-free intervals to optimize patient outcomes.
SEQUENCING OF AGENTS
Using agents early should not prevent patients from receiving them later. Many agents are developed in later disease stages and demonstrate higher efficacy when subsequently used in earlier settings. We do not know how the reverse situation will play out, in which we develop an agent in early stages—how will this impact later use? The development of new resistance patterns may preclude the subsequent use of certain classes of agents. For clinical trials, randomization in this setting is critically important, keeping in mind the ultimate goal of prolonging life.
QUALITY OF LIFE
Quality-of-life (QOL) measures should be included in all clinical trials. For example, immunotherapies that potentially have high toxicity in patients with large tumor burden in the relapsed/refractory setting may have minimal toxicity and side effects in an earlier disease setting and potentially higher responses. Particularly, in the case of an observation control arm, we would be comparing a treatment intervention with toxicities to no treatment. In this case, the therapy would always appear worse in terms of safety, but the impact on delayed symptomatology would not be observed right away. Q-twist or other measures may help measure that trade-off between early treatment-related toxicity and the benefit of reduced or absent disease-related symptoms at a later timepoint. We do not necessarily capture all grade one or two adverse events that occur, but in this disease space, those become even more important. Because precursor conditions are largely asymptomatic, patient-reported outcomes, patient preference information, and other QOL measures have the potential to play a much greater role in the precursor disease space when evaluating the risk–benefit balance. However, correct metrics are critical to capturing complete information over long follow-up periods. It is imperative in the setting of precursor diseases to try to reduce toxicity and improve tolerability, emphasizing the importance of QOL measures in dose optimization and endpoint evaluation.
DIVERSITY AND INCLUSION
The FDA published draft guidance in April 2022 recommending submission of diversity plans with each registrational trial that outline strategies for enrollment of traditionally underrepresented populations based on race, ethnicity, sex, rural/urban status, economic status, etc. In December 2022, Congress passed the FDORA Omnibus bill, mandating the publication of final guidance on this issue, requiring sponsors for registrational trials to submit a diversity action plan (HR2617, Consolidated Appropriations Act 2023).
Some clinical trials in myeloma have shown somewhat disparate efficacy results based on race and ethnicity (14). Unfortunately, when those results were analyzed, the sample size was too small to draw any meaningful conclusions, revealing the need to address diversity in analysis and statistical plans. We need adequate representation within these trials so we can interpret the results and know if these products are beneficial for patients of all races, ethnicities, etc. Adequate diversity is important for both equitable access to cutting-edge interventions and understanding how the intervention performs in a variety of patient populations.
SUMMARY AND CONCLUSIONS
Advances in therapeutics that are safe and highly efficacious have enabled the development of clinical trials in early hematologic precursor conditions to change the status quo of “watch and wait” to early therapeutic interception and ultimately cure for those patients before symptoms even develop. To achieve this ultimate goal, clinical trials need to be designed with careful considerations including careful selection of the at-risk population to avoid over or under-treating asymptomatic patients, safety and appropriate dose selection, and primary endpoints and surrogate endpoints that are clinically meaningful and inform changing of practice in the future.
Authors’ Disclosures
Dr. Vassiliou reports personal fees from STRM.BIO and grants from AstraZeneca outside the submitted work. Dr. Munshi reports personal fees from Janssen, personal fees from BMS, and personal fees from Pfizer during the conduct of the study; in addition, Dr. Munshi has a patent for PVX-410 licensed to Oncopep. Dr. San-Miguel reports grants from CRUK-AECC-AIRC during the conduct of the study, other support from Abbvie, other support from Amgen, other support from BMS, other support from Celegene, other support from Janssen, other support from GSK, other support from Haemalogix, other support from Pfizer, other support from SecuraBio, other support from Roche, other support from Sanofi, other support from Regeneron, other support from Takeda, and other support from Kite outside the submitted work. Dr. Ghobrial reports personal fees from BMS, grants and personal fees from Janssen, personal fees from Abbvie, personal fees from Amgen, grants and personal fees from Takeda, personal fees from Novartis, grants and personal fees from Sanofi, personal fees from Pfizer, personal fees from Vor Biopharma, grants and personal fees from Menarini, personal fees from GSK, personal fees from Regeneron, grants from 10X genomics, and other support from Disc Medicine outside the submitted work. Dr. Kumar reports grants from Abbvie, grants from BMS, grants from Janssen, grants from Roche-genentech, grants from Carsgen, grants from Oricell, grants from Pfizer, and grants from Sanofi during the conduct of the study. Dr. Mateos reports personal fees from Janssen, BMS, Amgen, GSK, Sanofi, Pfizer, Kite, Abbvie, and Astra Zeneca outside the submitted work. Dr. Bergsagel reports personal fees from CellCentrics, personal fees from Omeros, personal fees from Oncopeptides, personal fees from Salarius, and personal fees from AbbVie outside the submitted work; in addition, Dr. Bergsagel has a patent for GENETICALLY ENGINEERED MOUSE MODEL OF MULTIPLE MYELOMA VK*MYC and cell lines licensed to Opna Bio, Pi Therapeutics, Palleon Pharmaceuticals, Pfizer, and Abcuro, and a patent for Human CRBN transgenic mouse responsive to IMiDs licensed to Novartis. Dr. Chesi reports grants from Pfizer and personal fees from Novartis outside the submitted work; in addition, Dr. Chesi has a patent for Genetically Engineered, Clinically Predictive Mouse Model of Multiple Myeloma Responsive to IMiDs with royalties paid. Dr. Dhodapkar reports personal fees from BMS, Sanofi, and Lava Therapeutics outside the submitted work. Dr. Fonseca reports personal fees from Janssen, personal fees from BMS, personal fees from Sanofi, personal fees from Binding Site, other support from Antengene, other support from Adaptive Biotechnologies, personal fees from Karyopharm, personal fees from AbbVie, and other support from Caris Life Sciences outside the submitted work; in addition, Dr. Fonseca has a patent for FISH probes in MM issued and with royalties paid. Dr. Getz reports grants from Pharmacyclics, grants from IBM, grants from Bayer, grants from Ultima Genomics, grants from Genentech, personal fees and other support from Scorpion Therapeutics, and other support from PreDICTA Biosciences outside the submitted work; in addition, Dr. Getz has a patent for MinimuMM-Seq pending; and Dr. Getz is an inventor on patent applications filed by the Broad Institute related to MSMuTect, MSMutSig, POLYSOLVER, SignatureAnalyzer-GPU, and MSIDetect. Dr. Kastritis reports grants, personal fees, and nonfinancial support from Janssen, grants and personal fees from GSK, grants and personal fees from Pfizer, and nonfinancial support from Sanofi outside the submitted work. Dr. Martinez-Climent reports grants from Roche/Genentech, grants from Bristol Myers Squibb, grants from Janssen, grants from Regeneron, grants from AstraZeneca, grants from Priothera Pharmaceuticals, grants from Palleon Pharmaceuticals, and personal fees, nonfinancial support, and other support from MIMO Biosciences outside the submitted work; in addition, Dr. Martinez-Climent has a patent for GENETICALLY ENGINEERED ANIMAL MODELS FOR MULTIPLE MYELOMA (PT/EP2023/071025) licensed to MIMO Biosciences. Dr. Morgan reports personal fees from Jansen outside the submitted work. Dr. Davies reports other support from Abbvie, other support from BMS, other support from Janssen, other support from GSK, and other support from Regeneron outside the submitted work. Dr. Nadeem reports personal fees from BMS, personal fees and other support from Janssen, personal fees from GPCR therapeutics, personal fees from Takeda, personal fees from Sanofi, and personal fees from Pfizer outside the submitted work. Dr. Nuvolone reports grants and other support from Gate Bioscience, grants and personal fees from Pfizer, personal fees from Jannsen-Cilag, and grants from Oncopeptides outside the submitted work; in addition, Dr. Nuvolone has a patent for immunoglobulin gene sequencing pending. Dr. Paiva reports grants and other support from BMS, other support from Adaptive, other support from Janssen, grants and other support from GSK, grants and other support from Beigene, grants and other support from Roche, other support from Amgen, other support from Becton Dickinson, and grants and other support from Sanofi outside the submitted work. Dr. Shah reports grants and personal fees from Janssen, grants and personal fees from Celgene/BMS, personal fees from Sanofi, nonfinancial support from M and M labs, and nonfinancial support from Sabinsa Corporation outside the submitted work. Dr. Sklavenitis-Pistofidis reports other support from Pre-Seed Stage NewCo outside the submitted work. Dr. Sperling reports personal fees from Novartis and personal fees from Roche during the conduct of the study.
One of the Editors-in-Chief is an author on this article. In keeping with the AACR's editorial policy, the peer review of this submission was managed by a member of Blood Cancer Discovery's Board of Scientific Editors, who rendered the final decision concerning acceptability. No other disclosures were reported.
Acknowledgments
Anna V. Justis, PhD, a medical writer employed by Dana-Farber Cancer Institute, edited this manuscript.
References
- 1. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. [DOI] [PubMed] [Google Scholar]
- 2. Mateos M-V, Kumar S, Dimopoulos MA, González-Calle V, Kastritis E, Hajek R, et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J 2020;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosiñol L, Bladé J, Esteve J, Aymerich M, Rozman M, Montoto S, et al. Smoldering multiple myeloma: natural history and recognition of an evolving type. Br J Haematol 2003;123:631–6. [DOI] [PubMed] [Google Scholar]
- 4. Bustoros M, Sklavenitis-Pistofidis R, Park J, Redd R, Zhitomirsky B, Dunford AJ, et al. Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J Clin Oncol 2020;38:2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Termini R, Žihala D, Terpos E, Perez-Montaña A, Jelínek T, Raab M, et al. Circulating tumor and immune cells for minimally invasive risk stratification of smoldering multiple myeloma. Clin Cancer Res 2022;28:4771–81. [DOI] [PubMed] [Google Scholar]
- 6. Visram A, Rajkumar SV, Kapoor P, Dispenzieri A, Lacy MQ, Gertz MA, et al. Monoclonal proteinuria predicts progression risk in asymptomatic multiple myeloma with a free light chain ratio ≥100. Leukemia 2022;36:1429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinhold N, Ashby C, Rasche L, Chavan SS, Stein C, Stephens OW, et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood 2016;128:1735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corre J, Cleynen A, Robiou du Pont S, Buisson L, Bolli N, Attal M, et al. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia 2018;32:2636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mateos M-V, Hernández M-T, Giraldo P, de la Rubia J, de Arriba F, Corral LL, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol 2016;17:1127–36. [DOI] [PubMed] [Google Scholar]
- 10. Sklavenitis-Pistofidis R, Aranha MP, Redd RA, Baginska J, Haradhvala NJ, Hallisey M, et al. Immune biomarkers of response to immunotherapy in patients with high-risk smoldering myeloma. Cancer Cell 2022;40:1358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328–46. [DOI] [PubMed] [Google Scholar]
- 12. Lonial S, Jacobus SJ, Weiss M, Kumar S, Orlowski RZ, Kaufman JL, et al. E3A06: Randomized phase III trial of lenalidomide versus observation alone in patients with asymptomatic high-risk smoldering multiple myeloma. J Clin Oncol 37, 2019. ( suppl 15; abstr 8001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mateos M-V, Hernández M-T, Salvador C, Rubia JDL, de Arriba F, López-Corral L, et al. Lenalidomide-dexamethasone versus observation in high-risk smoldering myeloma after 12 years of median follow-up time: a randomized, open-label study. Eur J Cancer 2022;174:243–50. [DOI] [PubMed] [Google Scholar]
- 14. Kanapuru B, Fernandes LL, Fashoyin-Aje LA, Baines AC, Bhatnagar V, Ershler R, et al. Analysis of racial and ethnic disparities in multiple myeloma US FDA drug approval trials. Blood Adv 2022;6:1684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez-Persona E, Vidriales M-B, Mateo G, García-Sanz R, Mateos M-V, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007;110:2586–92. [DOI] [PubMed] [Google Scholar]
- 16. Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008;111:785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lakshman A, Rajkumar SV, Buadi FK, Binder M, Gertz MA, Lacy MQ, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J 2018;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cowan A, Ferrari F, Freeman SS, Redd R, El-Khoury H, Perry J, et al. Personalised progression prediction in patients with monoclonal gammopathy of undetermined significance or smouldering multiple myeloma (PANGEA): a retrospective, multicohort study. Lancet Haematol 2023;10:e203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]