Sotorasib and other selected KRASG12C inhibitors leverage differential dependence on switch-II pocket binding at amino acid position 95 to target all RASG12C isoforms, which has critical implications for the treatment of NRASG12C- or HRASG12C-mutant tumors.
Abstract
KRASG12C inhibitors, like sotorasib and adagrasib, potently and selectively inhibit KRASG12C through a covalent interaction with the mutant cysteine, driving clinical efficacy in KRASG12C tumors. Because amino acid sequences of the three main RAS isoforms—KRAS, NRAS, and HRAS—are highly similar, we hypothesized that some KRASG12C inhibitors might also target NRASG12C and/or HRASG12C, which are less common but critical oncogenic driver mutations in some tumors. Although some inhibitors, like adagrasib, were highly selective for KRASG12C, others also potently inhibited NRASG12C and/or HRASG12C. Notably, sotorasib was five-fold more potent against NRASG12C compared with KRASG12C or HRASG12C. Structural and reciprocal mutagenesis studies suggested that differences in isoform-specific binding are mediated by a single amino acid: Histidine-95 in KRAS (Leucine-95 in NRAS). A patient with NRASG12C colorectal cancer treated with sotorasib and the anti-EGFR antibody panitumumab achieved a marked tumor response, demonstrating that sotorasib can be clinically effective in NRASG12C-mutated tumors.
Significance:
These studies demonstrate that certain KRASG12C inhibitors effectively target all RASG12C mutations and that sotorasib specifically is a potent NRASG12C inhibitor capable of driving clinical responses. These findings have important implications for the treatment of patients with NRASG12C or HRASG12C cancers and could guide design of NRAS or HRAS inhibitors.
See related commentary by Seale and Misale, p. 698.
This article is featured in Selected Articles from This Issue, p. 695
INTRODUCTION
The RAS family of protooncogenes, which includes KRAS, NRAS, and HRAS, represents the most commonly mutated family of oncogenes in human cancer (1). Although KRAS mutations represent the majority of RAS mutations present in approximately 18% of all cancers, NRAS mutations and HRAS mutations are found in approximately 3% and approximately 1% of all cancers, respectively. Despite the importance of mutant RAS as a potential therapeutic target in oncology, efforts to drug RAS directly were unsuccessful for almost 30 years. An important breakthrough arose through the development of KRASG12C inhibitors, which exploit a covalent interaction with the mutant cysteine in KRASG12C that is not present in wild-type KRAS to drive both binding affinity and selectivity (2). Clinical development of KRASG12C inhibitors in patients with KRASG12C cancers has demonstrated efficacy across a broad range of tumor types, leading to the FDA approval of the KRASG12C inhibitors sotorasib and adagrasib in KRASG12C non–small cell lung cancer (NSCLC; refs. 3–5). Together, the initial clinical experience with KRASG12C inhibitors has proven that direct targeting of mutant RAS can drive meaningful clinical antitumor responses.
On the basis of these initial observations, multiple classes of KRAS inhibitors are under development, including mutation-specific KRAS inhibitors selective for other mutations (e.g., G12D), pan-KRAS or pan-KRAS–selective inhibitors that target nearly all KRAS mutations but spare NRAS and HRAS, and pan-RAS inhibitors that target all RAS isoforms and mutations (6–9). However, there has so far been limited development and progress focused on agents that can directly target mutant NRAS or HRAS, which are present in a smaller but still meaningful population of patients with cancer. Farnesyl transferase inhibitors have demonstrated activity in HRAS-mutant tumors, due to a unique dependency of HRAS on this posttranslational modification, again demonstrating that targeting mutant RAS signaling can drive clinical efficacy (10). However, no inhibitors that bind directly and selectively to NRAS or HRAS have been reported or entered clinical trials. Given clinical proof of concept that targeting mutant RAS can drive clinical responses, direct NRAS or HRAS inhibitors could have substantial impact.
Here, based on the high amino acid sequence conservation between the RAS isoforms, we tested available KRASG12C inhibitors and observed that some demonstrate the ability to target G12C mutations in NRAS or HRAS. Notably, we find that sotorasib is a highly potent NRASG12C inhibitor and show proof of concept that sotorasib can drive clinical antitumor responses in a patient with NRASG12C cancer.
RESULTS
Sotorasib Can Inhibit NRASG12C and HRASG12C
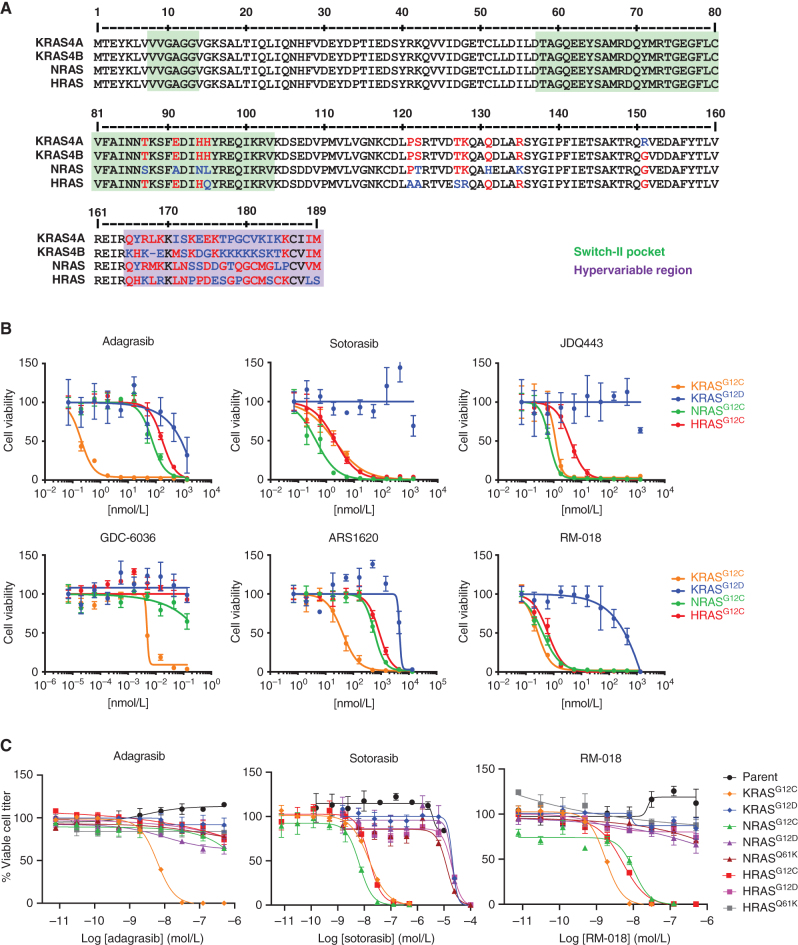
The three RAS family isoforms—KRAS, NRAS, and HRAS—have highly conserved amino acid sequences (Fig. 1A), with most differences occurring in the hypervariable domain represented by the last 25 amino acids of each isoform. Remarkably, there are no amino acid differences in the first 86 amino acids of each RAS isoform, and only four amino acid differences in the first 120 amino acids of each RAS isoform, which includes all residues contributing to the switch-II binding pocket to which most KRASG12C inhibitors bind. Thus, we hypothesized that some KRASG12C inhibitors might show similar potency against NRASG12C and/or HRASG12C mutations.
Figure 1.
Distinct RAS isoform selectivity profiles of KRASG12C inhibitors. A, Amino acid sequence aligments of the four RAS family isoforms, including KRAS4A and KRAS4B, NRAS, and HRAS. Amino acid differences are shown in red and blue. Regions involved in the switch-II binding pocket are shaded in green, and the hypervariable region is shaded in purple. B, Ba/F3 cells engineered to express either KRASG12C, NRASG12C, HRASG12C, or KRASG12D were treated for 72 hours in the absence of IL3 with various concentrations of the indicated inhibitors. Viable cell titer was measured using CellTiter Glo. C, Ba/F3 cells engineered to express the various mutant forms of KRAS, NRAS, and HRAS were treated for 72 hours with a range of concentrations of the indicated inhibitors, and viable cell titer was assessed as in B.
To test this hypothesis, we evaluated a range of commercially available KRASG12C inhibitors in Ba/F3 cells engineered to express either KRASG12C, NRASG12C, HRASG12C, or KRASG12D as a negative control (Fig. 1B; Supplementary Fig. S1). Ba/F3 cells depend on exogenous IL3 for survival unless engineered to express oncogenic RAS, thus providing a robust isogenic system to interrogate RAS inhibitor sensitivity. All compounds potently inhibited KRASG12C, as expected, and did not appreciably inhibit KRASG12D, a negative control that lacks the mutant cysteine required for covalent binding. However, striking molecule-specific differences in the ability to target NRASG12C and HRASG12C were noted. Adagrasib demonstrated little activity against NRASG12C and HRASG12C, with an IC50 similar to that observed with KRASG12D. Similarly, GDC-6036 and ARS-1620 showed markedly reduced IC50s for NRASG12C and HRASG12C inhibition relative to KRASG12C. Remarkably though, JDQ443 and sotorasib both demonstrated potent inhibition of NRASG12C and HRASG12C, comparable with that observed with KRASG12C. The IC50s of JDQ443 for NRASG12C and KRASG12C were equivalent, with only a slight decrease in potency noted for HRASG12C. Surprisingly, however, the IC50 of sotorasib was five-fold more potent against NRASG12C than for KRASG12C and HRASG12C, even though sotorasib was originally developed as a KRASG12C inhibitor. Finally, the tri-complex RASG12C inhibitor RM-018 (11, 12), which binds outside the switch-II pocket of RAS in a region where few amino acid differences exist between RAS isoforms, effectively inhibited NRASG12C, HRASG12C, and KRASG12C with equal potency, as expected.
To evaluate further the differences in isoform selectivity between KRASG12C inhibitors, we performed a detailed evaluation of adagrasib, sotorasib, and RM-018 against the G12C-, G12D-, and/or Q61K-mutated forms of KRAS, NRAS, and HRAS (Fig. 1C). As expected, none of these covalent inhibitors demonstrated inhibition of any RAS isoforms lacking the G12C mutation, confirming that their binding was still G12C-specific. Again, we observed that adagrasib only inhibited KRASG12C and did not show any appreciable inhibition of NRASG12C or HRASG12C. RM-018 again inhibited all G12C-altered RAS isoforms with similar potency. However, sotorasib inhibited KRASG12C and HRASG12C with similar IC50s, but again showed greater potency with NRASG12C.
Although NRASG12C and HRASG12C cancer cell models are limited, to further corroborate these findings, we evaluated the ability of sotorasib and adagrasib to inhibit NRASG12C signaling in two additional models: (i) an isogenic cell line system in which KRASG12C or NRASG12C were exogenously expressed in 293T cells and (ii) the MOLT-4 leukemia cell line, which harbors an endogenous NRASG12C mutation. In each model system, sotorasib, but not adagrasib, was able to inhibit NRASG12C signaling (Supplementary Figs. S2A, S2B, and S3). Furthermore, sotorasib, but not adagrasib, exhibited covalent modification of NRASG12C as evidenced by an upward shift in molecular weight upon Western blotting with an NRAS-specific antibody (Supplementary Fig. S2A and S2B).
Isoform Selectivity of KRASG12C Inhibitors Is Modulated by the Amino Acid at Position 95
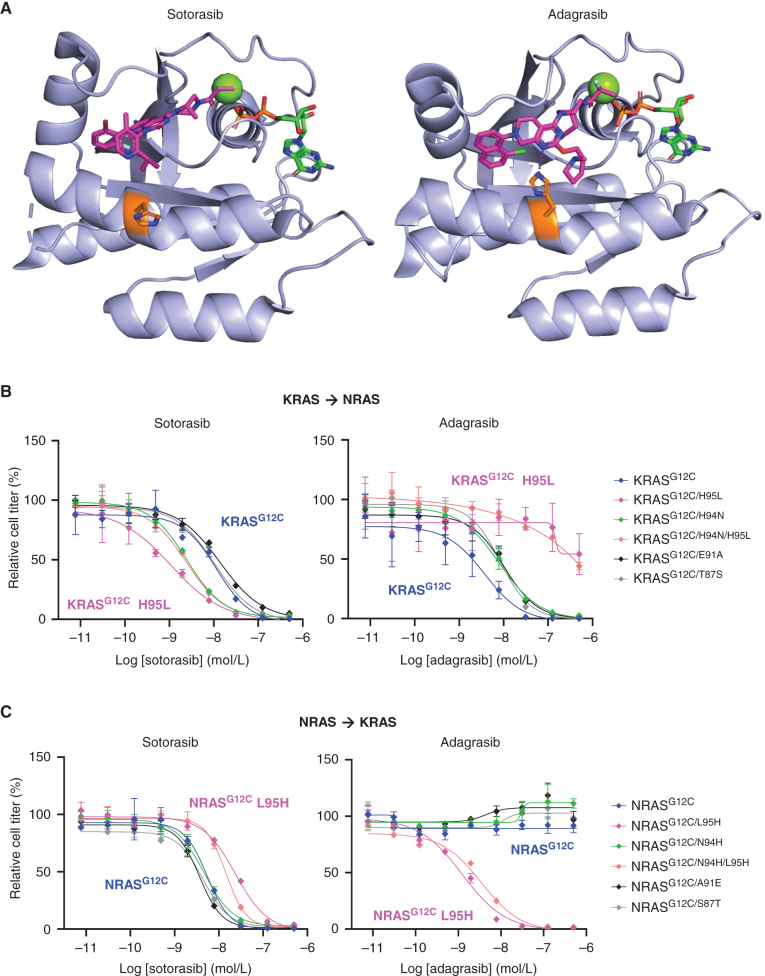
We assessed the structural basis for these differences in isoform-specific RASG12C inhibitory activity through comparative analyses of publicly available crystal structures (see Methods). As discussed above, the primary sequences of K-, N-, and HRAS are highly conserved; only four residues within 15Å of the switch-II binding pocket vary among the four family members (Fig. 1A; Supplementary Fig. S4). Three of these differences are only present in NRAS: position 87 (Ser in NRAS, Thr in KRAS/HRAS), 91 (Ala in NRAS, Glu in KRAS/HRAS), and 94 (Asn in NRAS, His in KRAS/HRAS), whereas position 95 is different in all three RAS family isoforms (Leu in NRAS, His in KRAS, and Gln in HRAS).
Of these four isoform-variable positions of interest, only residue 95 is observed to make direct contact with switch-II pocket ligands). Notably, adagrasib (KRASG12C-specific) and sotorasib (pan-RASG12C inhibitor) show markedly different interactions with His95 in KRASG12C. In binding KRASG12C, adagrasib forms a direct hydrogen bond with His95 that is predicted to be relatively strong given the favorable geometry of the interaction. Moreover, the strength of this hydrogen bond is likely further enhanced by the presence of Glu62 and Tyr64, which are situated such that they shield this key interaction from solvent (Fig. 2A; Supplementary Fig. S5A). In contrast, sotorasib binds to KRASG12C within the same cryptic groove but is not observed to interact with His95 and would therefore be predicted to be relatively insensitive to amino acid changes at that position. The importance of His95 in modulating isoform selectivity of KRASG12C inhibitors is further supported by the fact that JDQ443, which like sotorasib is a pan-RASG12C inhibitor, also does not form a direct hydrogen bond with His95 (Supplementary Fig. S5B). Conversely, ARS-1620 does form a direct hydrogen bond with His95 and exhibits a marked loss of potency for NRASG12C (Supplementary Fig. S5C). Notably, the three switch-II-pocket–binding molecules in this study showing the greatest selectivity for KRASG12C—adagrasib, GDC-6036, and ARS-1620—all share an unsubstituted core nitrogen that is well-positioned to form a hydrogen bond with His95. Furthermore, both adagrasib and GDC-6036 contain N-methyl-pyrrolidine moieties, which are likely key for orienting Glu62 and Tyr64 (Supplementary Fig. S6).
Figure 2.
Structural basis of RASG12C isoform selectivity. A, Crystal structures of sotorasib (6OIM) and adagrasib (6UT0) bound to KRASG12C are shown making distinct intractions with His95 (orange). Each inhibitor is colored magenta, GDP and a conserved Mg ion are shown in green, and the key hydrogen bond is shown in purple. B and C, Reciprocal mutagenesis studies were performed to explore the effects of substituting the four amino acid differences within the switch-II binding pocket, with NRAS amino acids substituted into KRASG12C (B) or KRAS amino acids substituted into NRASG12C. Constructs were expressed in Ba/F3 cells and treated for 72 hours in the absence of IL3 with various concentrations of the indicated inhibitors. Viable cell titer was measured using CellTiter Glo.
To more thoroughly explore the importance of the His95 interaction as well as other select amino acid differences in mediating distinctions in isoform selectivity, we performed reciprocal mutagenesis studies at the four amino acid positions within the switch-II binding pocket that differ between the RAS isoforms. When mutations corresponding to the NRAS amino acid sequence were introduced into KRASG12C, mutations at positions 87, 91, and 94 had minimal effects on the activities of adagrasib and sotorasib (Fig. 2B). However, the H95 L mutation in KRASG12C completely abrogated the activity of adagrasib. Conversely, the H95 L mutation actually increased the potency of sotorasib approximately five-fold, comparable with the greater potency observed for sotorasib in NRASG12C. These data suggest that the presence of a leucine at position 95 may lead to increased potency of sotorasib, potentially through an enhanced hydrophobic interaction between the isopropyl pyridine substituent of sotorasib and Leu95 (Supplementary Fig. S7). Reciprocal mutations in NRASG12C that introduced amino acids corresponding to the KRAS sequence further reinforced these observations, with minimal effects on potency observed with mutations at positions 87, 91, and 94 (Fig. 2C). However, mutations at position 95 again had striking effects, with a single L95H mutation in NRASG12C capable of conferring activity of adagrasib at similar potency to that observed in KRASG12C, despite complete lack of activity in parental NRASG12C. Similarly, in the reverse direction, the L95H mutation in NRASG12C led to an approximately five-fold loss of potency for sotorasib. Similar patterns for inhibition of RAS–MAPK signaling were observed in an isogenic system, in which various NRASG12C or KRASG12C constructs with reciprocal mutations at amino acid positions 94 and 95 were expressed in 293T cells (Supplementary Fig. S3). Taken together, these reciprocal mutagenesis data suggest that a single amino acid difference at position 95 is the primary driver of isoform-specific differences in activity of KRASG12C inhibitors.
Clinical Activity of Sotorasib in a Patient with NRASG12C Colorectal Cancer
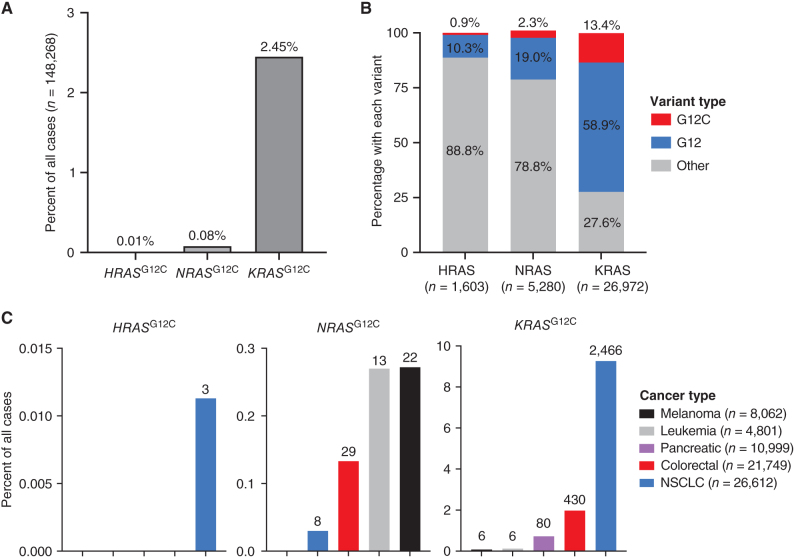
The observation that sotorasib can not only inhibit NRASG12C but is actually a more potent inhibitor of NRASG12C compared with KRASG12C raises the possibility that sotorasib and other inhibitors that do not strongly rely on their interaction with His95 could be potential therapeutic agents for NRASG12C cancers. Furthermore, the fact that sotorasib is an equipotent inhibitor of HRASG12C and KRASG12C further suggests that sotorasib could be used to target HRASG12C cancers. To assess the frequency of NRASG12C and HRASG12C mutations in various tumor types, we evaluated 148,268 cancers in the AACR GENIE database (13). We observed that although NRASG12C and HRASG12C mutations are rarer than KRASG12C mutations, these mutations are present in 0.08% and 0.01% of cancers, respectively, and are observed in specific cancer types, including melanoma, colorectal cancer, and leukemia (Fig. 3A and C).
Figure 3.
Frequencies of NRASG12C, HRASG12C, and KRASG12C mutations in cancer. A, Percentage of HRASG12C, NRASG12C, and KRASG12C mutations observed in 148,268 cancers from the AACR GENIE database. B, Of all cases with HRAS, NRAS, or KRAS mutations, the percentage with G12C mutations, other mutations of glycine 12 (G12), or other mutations not involving G12 are shown. C, Frequencies of HRASG12C, NRASG12C, and KRASG12C mutations observed in specific cancer types. Actual numbers of cases with each mutation in specific diseases are indicated above each column.
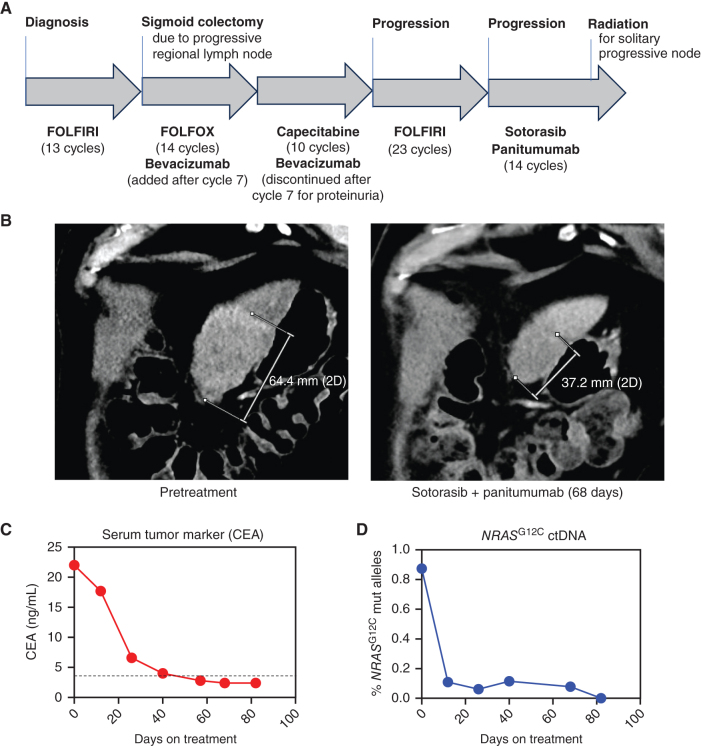
Given these findings, we predicted that sotorasib would have activity in NRASG12C cancers. We identified a patient with colorectal cancer who harbored an NRASG12C mutation and had exhausted all conventional therapy. Briefly, this 63-year-old man initially presented with vomiting, diarrhea, and marked anemia. A sigmoidoscopy demonstrated a mass with biopsy diagnostic of a microsatellite-stable, moderately differentiated adenocarcinoma. Staging evaluation with contrast-enhanced CT, MRI abdomen, and PET/CT showed for multiple hepatic metastases. A CEA was elevated at 84.7. Next-generation sequencing showed an NRASG12C alteration. The patient received extensive palliative chemotherapy consisting of 5-fluorouracil (5-FU), leucovorin, and irinotecan (FOLFIRI) and eventually underwent sigmoid colectomy for locoregional progression (Fig. 4A). He was subsequently treated with 5-fluorouracil (5-FU), leucovorin, oxaliplatin (FOLFOX) with bevacizumab, capecitabine/bevacizumab, capecitabine, and FOLFIRI retreatment. He ultimately progressed after 23 cycles of FOLFIRI with imaging showing new peritoneal nodularity and mild increase in hepatic metastatic disease.
Figure 4.
Clinical response in a patient with NRASG12C colorectal cancer treated with sotorasib and panitumumab. A, Graphical representation of the patient's treatment history. B, CT scans taken pretreatment and after 68 days of combined therapy with sotorasib and panitumumab with measurements of the dominant metastatic liver lesion shown. C, Serial serum CEA tumor marker measurements were obtained before and throughout treatment. The dotted horizontal line represents the upper limit of normal (3.5 ng/mL). D, The mutant allele fraction of NRASG12C was assessed in serial ctDNA speciments collected before and throughout therapy by ddPCR.
On the basis of the patient's NRASG12C mutation, the lack of other standard treatment options, and our data above, we explored whether sotorasib therapy might be clinically beneficial in this setting. Colorectal cancer is known to have robust adaptive feedback signaling that leads to reactivation of the RAS–MAPK pathway after treatment with BRAF or KRASG12C inhibitors (14–17). Preclinical and clinical studies have demonstrated that much of this feedback reactivation is mediated by EGFR. As a result, single-agent activity of BRAF and/or KRASG12C inhibitors has been lower in colorectal cancer compared with other cancers, but combination therapy with an anti-EGFR antibody has led to improved response rates and clinical benefit (18–20). Thus, we elected to treat this patient with NRASG12C colorectal cancer with the combination of sotorasib and the anti-EGFR antibody panitumumab based on established safety data from studies in patients with KRASG12C colorectal cancer (21). After 2 months of treatment, repeat imaging showed a dramatic reduction in the size of the patient's liver metastases (Fig. 4B). In addition, the patient's serum carcinoembryonic antigen (CEA) tumor markers, which were elevated prior to treatment, normalized after the first month of therapy (Fig. 4C). We also evaluated levels of NRASG12C in circulating tumor DNA (ctDNA) by droplet digital PCR (ddPCR), which similarly showed a dramatic reduction after initiation of treatment (Fig. 4D). Following cycle 12 of sotorasib/panitumumab, he received 40 Gy of radiation via MRI-guided stereotactic body radiotherapy to the solitary progressive mesenteric nodule. The patient remained on therapy with sotorasib and panitumumab and completed 14 treatment cycles. Therapy was well-tolerated with toxicities limited to grade 1 acneiform facial rash, grade 1 nausea, and grade 1 fatigue. Overall, these data provide clinical proof of concept that sotorasib may be effective in patients with NRASG12C cancers.
DISCUSSION
This study provides initial proof of concept that sotorasib, although originally developed as a KRASG12C inhibitor, is a potent inhibitor of NRASG12C that can potentially be used to treat patients with NRASG12C cancers. Furthermore, our preclinical data suggest that certain other KRASG12C inhibitors that do not strongly rely on their interaction with His95 (i.e., JDQ443) may also potently inhibit NRASG12C and could therefore also be evaluated in this population. Although we treated and observed a clinical response in only a single patient, when taken together with a similar recently published case report of a patient with NRASG12C colorectal cancer who also responded to treatment with sotorasib and panitumumab (22), these observations collectively provide support for the potential clinical efficacy of sotorasib in patients with NRASG12C cancers. Although NRASG12C mutations are rare (Fig. 3), these data suggest that sotorasib should be further evaluated as a treatment option for these patients when identified.
Our data also demonstrate that sotorasib can inhibit HRASG12C with similar potency to KRASG12C. Although HRASG12C mutations are rare and no patients with HRASG12C cancer have yet been treated with sotorasib, these preclinical data suggest that sotorasib and/or other KRASG12C inhibitors that are not isoform-restricted might also be effective in these patients. These findings also have implications for other current or future mutation-selective KRAS inhibitors, such as KRASG12D inhibitors (9), which are currently in clinical trials. Our data, as well as other recent reports (23, 24), suggest that it is possible that some mutation-selective inhibitors may retain the ability to inhibit a specific RAS mutation across all RAS isoforms, thereby expanding the population of patients that may benefit. This could certainly have broader impact for mutations, such as G12D, that represent more common NRAS and HRAS mutations, and thus novel mutation-selective inhibitors should be evaluated for their ability to inhibit a specific mutation in the context of each RAS isoform going forward.
Our study has several limitations that affect the generalizability of the conclusions. First, it involves clinical evaluation of only a single patient. Although this patient did respond to therapy, supporting a potential role for sotorasib in this patient population, further study in a larger patient cohort will be needed to assess its utility as a possible treatment strategy for NRASG12C cancers. Second, our patient was treated with the combination of sotorasib and the anti-EGFR antibody panitumumab rather than single-agent sotorasib based on historical experience in colorectal cancer that suggests that coinhibition of EGFR can block adaptive feedback signaling and improve efficacy specifically in patients with colorectal cancer. Because there is extensive evidence that NRAS-mutant colorectal cancer does not respond to anti-EGFR antibodies alone, it is unlikely that the efficacy observed was due uniquely to panitumumab. Still, future evaluation of sotorasib monotherapy in NRASG12C tumor types that are more likely to respond to single-agent inhibition (e.g., melanoma or NSCLC, based on experience with KRASG12C inhibitors) would provide a more conclusive demonstration of clinical potential and would be warranted. Finally, as NRASG12C and HRASG12C models are limited, our analysis was restricted to a limited number of available or engineered models, and more extensive evaluation of patient-derived models and more detailed biochemical studies would be warranted.
Importantly, our study has potential implications for the design of inhibitors of KRAS and/or other RAS isoforms. Structural and reciprocal mutagenesis data suggest a mechanistic basis for RAS isoform selectivity of switch-II pocket inhibitors and the important role for interactions with the amino acid at position 95. Indeed, recent studies have demonstrated the potential for KRAS isoform–specific inhibitors (also called pan-KRAS or pan-KRAS–selective inhibitors), which have been shown to utilize a key interaction with His95 (8). The purpose of such inhibitors would be to effectively target the full spectrum of KRAS mutations while leaving NRAS and HRAS signaling in normal cells unaffected to provide for a tolerable therapeutic index. This is also supported by prior data that H95 mutations can drive acquired resistance to adagrasib (which our data confirm is highly selective for KRASG12C vs. NRASG12C or HRASG12C), but not other less isoform-selective inhibitors like sotorasib (25). Understanding these key structure activity relationships can help drive the design of desired inhibitor profiles.
This study is also one of the first demonstrations that direct targeting of mutant NRAS can lead to clinical response. These data provide strong support for the development and clinical evaluation of direct NRAS inhibitors as a potential novel therapeutic class. The increased potency seen with sotorasib in the presence of Leu95, either native in NRAS or when substituted into KRAS, suggests a potential avenue for NRAS isoform selectivity that could be further exploited to generate NRAS isoform–selective inhibitors similar to the KRAS isoform–selective inhibitors discussed above and capable of inhibiting the full spectrum of more common NRAS mutations. Enhancing the potential hydrophobic interaction with Leu95 could be one avenue to achieve this desired selectivity profile to spare inhibition of signaling through all RAS isoforms in normal cells as a potential means of achieving favorable therapeutic index. Together, our data highlight the potential for clinical efficacy in patients with NRASG12C cancers with currently available KRASG12C inhibitors like sotorasib, and potentially also agents in clinical trials such as JDQ443 and RMC-6291 (the investigational KRASG12C-selective tri-complex inhibitor, for which RM-018 is a representative preclinical tool; ref. 12), and point to a potential path for designing direct NRAS inhibitors capable of benefitting a broader patient population.
METHODS
Patient Care and Specimen Collection
All patient data and peripheral blood draws for plasma isolation were collected in accordance with Institutional Review Board–approved protocols, to which patients provided written informed consent, and all studies were conducted in accordance with the Declaration of Helsinki. The patient was treated with sotorasib and panitumumab, both approved by the FDA, off-label with informed consent, and the patient's insurance company covered the cost of this therapy. Sotorasib was administered as 960 mg once daily. Panitumumab was administered at a dose of 6 mg/kg intravenously once every 2 weeks. Imaging studies, including CT scans as well as serum CEA tumor marker measurements, were obtained as part of routine clinical care.
Cell Lines and Reagents
Parental Ba/F3 cells were maintained in DMEM supplemented with 10% FBS and 10 ng/mL IL3. Oncogene-addicted Ba/F3 cells and HEK-293T cells were maintained in DMEM supplemented with 10% FBS. MOLT-4 cells were cultured in RPMI1640 medium (ATCC, #30–2001) with 10% FBS. All cell lines were grown in the presence of penicillin–streptomycin at 37°C and 5% CO2. All cell lines were obtained from ATCC or from the MGH Center for Molecular Therapeutics cell line collection, which performs routine short tandem repeat genotyping and Mycoplasma testing, and all cell lines were maintained in culture for less that 6 months from receipt. Sotorasib, JDQ443 (opnurasib), and GDC-6036 (divarasib) were purchased from MedChemExpress. Adagrasib and ARS-1620 were purchased from Selleck Chemicals. RM-018 was kindly provided by Revolution Medicines.
Plasmid Construction and Establishment of Ba/F3 Line Series
Plasmids for KRASG12C, NRASG12C, and HRASG12C were purchased from Addgene. pDONR223_KRAS_p.G12C and pDONR223_NRAS_p.G12C were a gift from Jesse Boehm, William Hahn, and David Root (pDONR223_KRAS_p.G12C: Addgene plasmid # 81667; http://n2t.net/addgene:81667; RRID:Addgene_81667, pDONR223_NRAS_p.G12C: Addgene plasmid # 81660; http://n2t.net/addgene:81660; RRID:Addgene_81660; ref. 26). Hs.HRAS G12C was a gift from Dominic Esposito (Addgene plasmid # 83182; http://n2t.net/addgene:83182; RRID:Addgene_83182). The insert genes were subcloned into pMXs-Puro Retrovrial Expression Vector, and the mutagenesis was performed for constructing the appropriate plasmids used in the study. Series of Ba/F3 lines were established as previously described (11).
Cell Viability Assay
Cells were seeded in 96-well plates at 5–10 × 103 cells/well and incubated for 24 hours. Each cells were treated with a serial dilution of drugs for 72 hours, and then the cell viabilities were measured CellTiter-Glo (Promega) following manufacturer's instructions.
Structural Modeling
Publicly available crystal structures showing the interaction between the drugs and protein were downloaded from the RCSB Protein Data Bank, and the computational structural modelings were performed using PyMol (The PyMOL Molecular Graphics System) and MOE (Chemical Computing Group). Models used in this study were: 6UT0 (adagrasib), 6OIM (sotorasib), 7R0M (JDQ443), and 5V9U (ARS-1620).
Analysis of the Frequency of RASG12C Variants
Mutational data were obtained from the AACR GENIE database.
Cell-free DNA Isolation and Droplet Digital PCR
Cell-free DNA was isolated from plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen). For droplet digital PCR (ddPCR) analysis, DNA template (up to 10 μL for a total of 20 ng) was added to 10 μL of ddPCR Supermix for Probes (Bio-Rad) and 2 μL of the custom primer/probe mixture. This reaction mix was added to a DG8 cartridge together with 60 μL of Droplet Generation Oil for Probes (Bio-Rad) and used for droplet generation. Droplets were then transferred to a 96-well plate (Eppendorf) and then thermal cycled with the following conditions: 10 minutes at 95°C, 40 cycles of 94°C for 30 seconds, and 55°C (with a few grades of difference among assays) for 1 minute followed by 98°C for 10 minutes (ramp rate 2°C/second). Droplets were analyzed with the QX200 Droplet Reader (Bio-Rad) for fluorescent measurement of FAM and HEX probes. Gating was performed on the basis of positive and negative controls, and mutant populations were identified. The ddPCR data were analyzed with QuantaSoft analysis software (Bio-Rad) to obtain fractional abundance of the mutant DNA alleles in the WT/normal background. The quantification of the target molecule was presented as the number of total copies (mutant plus WT) per sample in each reaction. Fractional abundance is calculated as follows: F.A. % = [Nmut/(Nmut + Nwt)] × 100, in which Nmut is the number of mutant events and Nwt is the number of WT events per reaction. ddPCR analysis of normal control plasma DNA (from cell lines) and no-DNA template controls were always included. Probe and primer sequences are available upon request.
Data Availability
Accession numbers for publicly available crystal structures used in this study are provided in the respective figure legends. Raw data are available upon reasonable request from the corresponding authors.
Supplementary Material
Supplementary Figure S1 shows IC50 values of KRASG12C inhibitors for KRASG12C, NRASG12C, HRASG12C.
Supplementary Figure S2 shows inhibition of NRASG12C by sotorasib in 293T and MOLT-4 cells.
Supplementary Figure S3 shows effect of amino acid substitutions in NRASG12C or KRASG12C on signaling in response to sotorasib and adagrasib.
Supplementary Figure S4 who's location of isoform-variable residues relative to Switch-II binders.
Supplementary Figure S5 shows crystal structures of KRASG12C inhibitors and interactions with His95.
Supplementary Figure S6 shows chemical structures of switch-II pocket-binding KRASG12C inhibitors.
Supplementary Figure S7 shows structural model of sotorasib interaction with Leu95.
Acknowledgments
This work was supported by NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003, R01 CA262805, and a Stand Up To Cancer Colorectal KRAS Catalyst Grant (SU2C Grant #6331, to R.B. Corcoran). R.B. Corcoran is also supported by the Mark J. Kusek Endowed Chair in Colorectal Cancer. M.H. Rosenthal receives support from NIH/NCI U01 CA210171 and R01 CA255184.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Authors’ Disclosures
D.A. Rubinson reports personal fees from Boston Scientific, Instylla, and Axial Therapeutics and personal fees from Sirtex outside the submitted work. M.H. Rosenthal reports personal fees from Merck outside the submitted work. A.J. Aguirre reports grants and personal fees from Boehringer Ingelheim, Mirati Therapeutics, Revolution Medicines, and Syros Pharmaceuticals; personal fees from Anji Pharmaceuticals, Affini-T Therapeutics, Arrakis Therapeutics, AstraZeneca, Kestrel Therapeutics, Merck & Co., Inc., Nimbus Therapeutics, Oncorus, Inc., Plexium, Quanta Therapeutics, Reactive Biosciences, Riva Therapeutics, Servier Pharmaceuticals, T-knife Therapeutics, Third Rock Ventures, and Ventus Therapeutics; grants from Bristol Myers Squibb, Deerfield, Inc., Eli Lilly, and Novartis; and grants from Novo Ventures outside the submitted work. R.B. Corcoran reports other support from Erasca and Avidity Biosciences; personal fees and other support from Alterome Therapeutics and Sidewinder Therapeutics, C4 Therapeutics, Cogent Biosciences, Kinnate Biopharma, Interline Therapeutics, Nested Therapeutics, nRichDx, Remix Therapeutics, and Revolution Medicines; personal fees from Abbvie, Amgen, Pfizer, Asana Biosciences, Astellas, Daiichi-Sankyo, Elicio, FOG Pharma, Roche, Guardant Health, Mirati Therapeutics, Natera, Qiagen, Syndax, Tango Therapeutics, and Taiho; grants from Novartis, Pfizer, Lilly, and Invitae; and grants from Asana Biosciences outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
D.A. Rubinson: Conceptualization, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. N. Tanaka: Conceptualization, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. F. Fece de la Cruz: Conceptualization, formal analysis, validation, investigation, writing–original draft, writing–review and editing. K.S. Kapner: Formal analysis, investigation, methodology, writing–original draft, writing–review and editing. M.H. Rosenthal: Formal analysis, investigation, writing–review and editing. B.L. Norden: Formal analysis, investigation, writing–review and editing. H. Barnes: Formal analysis, investigation, writing–review and editing. S. Ehnstrom: Formal analysis, investigation, writing–review and editing. A.A. Morales-Giron: Formal analysis, validation, investigation, writing–review and editing. L.K. Brais: Investigation, writing–review and editing. C.T. Lemke: Conceptualization, formal analysis, validation, investigation, writing–review and editing. A.J. Aguirre: Conceptualization, resources, formal analysis, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, writing–review and editing. R.B. Corcoran: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol 2018;15:709–20. [DOI] [PubMed] [Google Scholar]
- 2. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503:548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021;384:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in Non-small-cell lung cancer harboring a KRAS(G12C) mutation. N Engl J Med 2022;387:120–31. [DOI] [PubMed] [Google Scholar]
- 5. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020;383:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov 2022;12:924–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corcoran RB. A single inhibitor for all KRAS mutations. Nat Cancer 2023;4:1060–2. [DOI] [PubMed] [Google Scholar]
- 8. Kim D, Herdeis L, Rudolph D, Zhao Y, Bottcher J, Vides A, et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 2023;619:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J Med Chem 2022;65:3123–33. [DOI] [PubMed] [Google Scholar]
- 10. Ho AL, Brana I, Haddad R, Bauman J, Bible K, Oosting S, et al. Tipifarnib in head and neck squamous cell carcinoma with HRAS mutations. J Clin Oncol 2021;39:1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J, Kiedrowski LA, et al. Clinical acquired resistance to KRAS(G12C) inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov 2021;11:1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulze CJ, Seamon KJ, Zhao Y, Yang YC, Cregg J, Kim D, et al. Chemical remodeling of a cellular chaperone to target the active state of mutant KRAS. Science 2023;381:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consortium APG. AACR Project GENIE: powering precision medicine through an International Consortium. Cancer Discov 2017;7:818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Cancer Res 2020;26:1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100–3. [DOI] [PubMed] [Google Scholar]
- 17. Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov 2020;10:1129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov 2018;8:428–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 2019;381:1632–43. [DOI] [PubMed] [Google Scholar]
- 20. Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med 2023;388:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuboki Y, Yaeger R, Fakih M, Strickler JH, Masuishi T, Kim EJH, et al. 45MO Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: Safety and efficacy for phase Ib full expansion cohort. Ann Oncol 2022;33:S1445–S6. [Google Scholar]
- 22. Mohrmann L, Cuberi A, Bruckmann S, Stasik S, Heukamp LC, Bornhauser M, et al. Response to (K)RAS(G12C) and EGFR Inhibition in a patient with NRAS(G12C)-mutated rectal cancer. JCO Precis Oncol 2023;7:e2200666. [DOI] [PubMed] [Google Scholar]
- 23. Qian J, Li Z, Pei K, Li Z, Li C, Yan M, et al. Effects of NRAS mutations on leukemogenesis and targeting of children with acute lymphoblastic leukemia. Front Cell Dev Biol 2022;10:712484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saiki AY, Mohn D, Li Y, Osgood T, Rex K, Wang HL, et al. In vitro characterization of sotorasib and other RAS ‘His95-groove’ binders and investigation of resistance mechanisms [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2021; 2021 Apr 10–15 and May 17–21. Philadelphia (PA): AACR; 2021. Abstract nr 1285. [Google Scholar]
- 25. Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med 2021;384:2382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim E, Ilic N, Shrestha Y, Zou L, Kamburov A, Zhu C, et al. Systematic functional interrogation of rare cancer variants identifies oncogenic alleles. Cancer Discov 2016;6:714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 shows IC50 values of KRASG12C inhibitors for KRASG12C, NRASG12C, HRASG12C.
Supplementary Figure S2 shows inhibition of NRASG12C by sotorasib in 293T and MOLT-4 cells.
Supplementary Figure S3 shows effect of amino acid substitutions in NRASG12C or KRASG12C on signaling in response to sotorasib and adagrasib.
Supplementary Figure S4 who's location of isoform-variable residues relative to Switch-II binders.
Supplementary Figure S5 shows crystal structures of KRASG12C inhibitors and interactions with His95.
Supplementary Figure S6 shows chemical structures of switch-II pocket-binding KRASG12C inhibitors.
Supplementary Figure S7 shows structural model of sotorasib interaction with Leu95.
Data Availability Statement
Accession numbers for publicly available crystal structures used in this study are provided in the respective figure legends. Raw data are available upon reasonable request from the corresponding authors.