Abstract
There is evidence that CD46 (membrane cofactor protein) is a cellular receptor for vaccine and laboratory-passaged strains of measles virus (MV). Following infection with these MV strains, CD46 is downregulated from the cell surface, and consequent complement-mediated lysis has been shown to occur upon infection of a human monocytic cell line. The MV hemagglutinin (H) protein alone is capable of inducing this downregulation. Some wild-type strains of MV fail to downregulate CD46, despite infection being prevented by anti-CD46 antibodies. In this study we show that CD46 is also downregulated to the same extent by wild-type, vaccine, and laboratory-passaged strains of rinderpest virus (RPV), although CD46 did not appear to be the receptor for RPV. Expression of the RPV H protein by a nonreplicating adenovirus vector was also found to cause this downregulation. A vaccine strain of peste des petits ruminants virus caused slight downregulation of CD46 in infected Vero cells, while wild-type and vaccine strains of canine distemper virus and a wild-type strain of dolphin morbillivirus failed to downregulate CD46. Downregulation of CD46 can, therefore, be a function independent of the use of this protein as a virus receptor.
CD46 (membrane cofactor protein) is a widely distributed cell membrane protein, a member of the regulators of complement activation superfamily, that inhibits autologous complement activation on host cells (14, 18, 37). It binds the C3b and C4b components of the complement lysis pathway and facilitates their cleavage by factor 1 protease. This prevents further deposition of C3b and C4b on the cells and so protects them from lysis. There is now a great deal of evidence that CD46 acts as a receptor for vaccine strains and some wild-type strains of measles virus (MV) (9, 10, 22, 24, 26, 33). All nucleated human cells express CD46 on their surfaces, and there are four commonly occurring isoforms (13, 18, 28–30), all of which are used by MV as receptors (10, 22, 24, 26). There are numerous reports in the literature which show that MV infection causes downregulation of the expression of CD46 (all four isoforms) on the cell surface (3, 15, 27, 32, 34). Downregulation of CD46 from MV-infected cells is known to occur by internalization of the molecule due to interaction with the hemagglutinin (H) protein (15, 27), and correct glycosylation of the H protein is needed for this interaction (21, 22). Downregulation occurs following infection with any of the attenuated vaccine strains of MV but not following infection with some wild-type strains, while antibody against CD46 was shown to inhibit infection of cells by nearly all strains of the virus (3, 4, 10, 32, 34, 39). A recent review speculated that CD46 may be the main receptor only for vaccine-like strains of MV and that this may be a consequence of their adaptation to tissue culture (6). Recent reports have indicated the existence of other receptor molecules for nondownregulating wild-type strains, infection with which is not inhibited by anti-CD46 antibody, on human and marmoset B cells (4, 12). These results suggest that some MV wild-type strains use CD46 and an unknown molecule as receptors to different degrees, depending on their passage history. There are no previous reports of downregulation of CD46 by any of the other morbilliviruses. In this paper we report studies to determine if infections with other morbilliviruses result in the downregulation of CD46 expression on the cell surface and whether this is related to the use of CD46 as a receptor molecule.
All strains of RPV tested downregulate CD46 expression.
Analysis of nucleocapsid (N) protein gene sequences shows that rinderpest (cattle plague) virus (RPV) is the morbillivirus most closely related to MV (8); in fact, MV most likely originated from RPV (2). To date, no homologue of CD46 has been found in any bovine species, although homologues have been described in other species, e.g., the pig (38). Several strains of RPV, both vaccine and wild type, were investigated to determine if they could downregulate CD46 expression. The Kabete O and Saudi/81 wild-type strains of RPV were isolated on primary bovine skin fibroblasts, which were prepared from bovine skin biopsy tissues and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% (vol/vol) fetal calf serum (FCS), 3% amphotericin B, and 20 ng of epidermal growth factor/ml (20). The virus derived from the skin fibroblasts was passaged once on B95a cells (Epstein-Barr virus-transformed marmoset B lymphocytes) to raise the titer. These wild-type viruses were previously maintained by animal passage and were not adapted to tissue culture. The Saudi/81 laboratory-passaged strain of RPV was originally isolated from infected tissues, by using primary bovine kidney cells, and passaged several times on Vero cells and then on B95a cells. The RBOK vaccine strain of RPV, originally produced by passage of the virulent Kabete O strain in primary bovine kidney cells, was maintained on Vero cells. B95a and Vero cells were infected with RPV at a multiplicity of infection (MOI) of 1 and incubated for either 24 or 48 h. After staining, the intensity of the fluorescence was measured on a FACScan (Becton Dickinson), and the results were analyzed with the Lysis II software program.
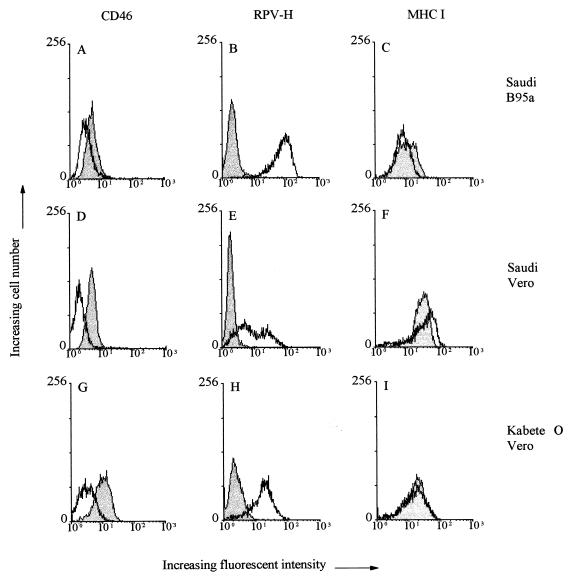
Cells infected with any of the four types of RPV showed high levels of H-antigen expression on their surfaces, as indicated by the peaks of fluorescence intensity for the infected and uninfected cell populations (Fig. 1B, E, and H). In contrast, while uninfected cells showed high expression of CD46 on their surfaces, the same cell types showed a much lower level of CD46 expression when infected with each strain of the virus (Fig. 1A, D, and G). The percentage of downregulation shown by the RBOK vaccine and the two different Saudi/81 viruses was similar to, or slightly greater than, that shown by the human 2 Edmonston vaccine strain (Table 1). The Kabete O wild-type virus had the greatest effect on downregulation (Table 1). Surface protein downregulation was not a general phenomenon observed following infection, since major histocompatibility complex (MHC) class I antigen expression remained at the same level in infected and uninfected cells (Fig. 1C, F, and I). This protein was used as a control because three different isolates of RPV, including Saudi/81 and RBOK, were reported not to affect MHC class I expression on the surfaces of infected bovine monocytes (31). This contrasts with certain strains of MV, which have been reported to augment MHC class I expression (7, 16, 33).
FIG. 1.
Flow-cytometric analysis of the surface expression of CD46, MHC class I, and RPV-H by RPV-infected Vero and B95a cells. Shaded peaks, uninfected cells; unshaded peaks, infected cells. Vero or B95a cells were infected with the laboratory-passaged Saudi/81 strain of RPV for 24 h at an MOI of 1, and Vero cells were infected with the wild-type Kabete O strain of RPV for 48 h at an MOI of 1. The cells were dissociated with trypsin EDTA in PBS for 10 min at 37°C and then washed with PBS supplemented with 1% FCS and 0.01% sodium azide (PBA). For single-color immunofluorescence, 2 × 105 cells were incubated for 30 min at 4°C with an anti-RPV-H specific monoclonal antibody (1:10) to detect infection (B, E, and H), an anti-CD46 polyclonal antibody (1:100) to detect downregulation (A, D, and G), or a monoclonal antibody against human MHC class I (1:100; Dako) as a control (C, F, and I). After being washed with PBA, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin (1:200; The Binding Site, Birmingham, United Kingdom) to detect binding of rabbit polyclonal antibodies or with phycoerythrin-conjugated rabbit anti-mouse immunoglobulin (1:200; The Binding Site, Birmingham, United Kingdom) to detect binding of mouse monoclonal antibodies for 30 min at 4°C. The cells were washed twice with PBA and then resuspended in 200 μl of PBA prior to analysis. At least 4 × 103 cells per sample were used for this assay. Where necessary, cell debris plus dead cells were excluded from the analysis by gating round the live cells.
TABLE 1.
Ability of different morbilliviruses to downregulate CD46
| Morbillivirus (type of strain) | Strain | % Downregulation (mean ± SD)a |
|---|---|---|
| MV (vaccine) | Human 2/Edmonston | 49 ± 9 |
| RPV (vaccine) | RBOK | 55 ± 4 |
| RPV (laboratory passaged) | Saudi | 67 ± 11 |
| RPV (wild type) | Kabete O | 80 ± 7 |
| Saudi/81 | 66 ± 11 | |
| CDV (vaccine) | Onderstepoort large plaque | 8 ± 6 |
| Onderstepoort small plaque | 6 ± 14 | |
| CDV (wild type) | Belfast dog isolate | 7 ± 19 |
| CDVA75-17 | 5 ± 13 | |
| PPRV (vaccine) | Nigeria 75/1 | 22 ± 6 |
| DMV (wild type) | MUC | 4 ± 8 |
| Ad-H recombinantb | RBOK | 54 ± 9 |
| Ad-N recombinantb | RBOK | 10 ± 14 |
Percentage of downregulation of CD46 = 100 − [(MFI of CD46 in infected cells × 100)/(MFI of CD46 in uninfected cells)] where MFI is mean fluorescence intensity. CD46 expression was detected by flow cytometry using a polyclonal antibody. Vero or B95a cells were infected for either 1 or 2 days at an MOI of 1. Results of at least three independent experiments per strain are given.
Vero or B95a cells were infected for either 1 or 2 days with the RPV Ad-H or RPV Ad-N recombinant at an MOI of 103.
It has been proposed that downregulation of CD46 by MV vaccine strains allows for rapid clearance of the virus from the body by facilitating complement lysis of the infected cells (35, 36) and greatly limits the pathology associated with infection (1). This effect is augmented by contact-mediated downregulation of CD46 in uninfected cells (15, 35). In agreement with this, MV strains that downregulate CD46 were found to be less virulent in experimental marmoset infections than MV strains that do not (1). However, highly virulent strains of RPV (such as Saudi/81 and Kabete O), which can cause 90 to 100% mortality in susceptible cattle herds, also downregulate CD46. Downregulation of CD46 from the surfaces of RPV-infected cells would be expected to allow complement-mediated lysis of these cells, and if this also occurs in vivo, it appears not to hinder the rapid spread of RPV through the infected animal. Pathological studies have shown that mild and virulent strains of RPV have identical tissue tropisms, the only difference being in the amount of viral antigen detected in the infected tissues (42). We have previously suggested that this is due to a more efficient growth of the virulent viruses in permissive cells in vivo, possibly due to differences in the polymerase proteins which alter their interaction with modulating factors (virus or cell), resulting in more extensive tissue destruction (42).
Downregulation by more distantly related morbilliviruses.
Other viruses in this genus include peste des petits ruminants virus (PPRV), which infects sheep, goats, and other small ruminants; canine distemper virus (CDV), which infects carnivores; and those which infect cetacean species, such as the dolphin morbillivirus (DMV). The highly laboratory-passaged vaccine strain of PPRV (Nigeria 75/1) was the only strain of this virus available for study. There was a high level of expression of the PPRV H antigen on the surfaces of infected cells and no expression of the H antigen on the surfaces of uninfected cells, as shown in Fig. 2D. There was a slight but significant (22%) downregulation of CD46 from the surfaces of cells infected with this virus (see Table 1), which was not clearly shown on the figure (Fig. 2A) printed from the FACScan data. MHC class I antigen expression again remained at approximately the same level on the surfaces of infected and uninfected cells (data not shown).
FIG. 2.
Flow-cytometric analysis of the surface expression of CD46 by PPRV-, CDV-, and DMV-infected Vero cells. Shaded peaks, uninfected cells; unshaded peaks, infected cells. Vero cells infected with the Nigeria 75/1 vaccine strain of PPRV for 48 h at an MOI of 1 were stained with an anti-PPRV-H specific monoclonal antibody (1:10) as a control for infection (D) and with an anti-CD46 polyclonal antibody (A). Vero cells infected with the wild-type Belfast dog strain of CDV for 24 h at an MOI of 1 were stained with anti-CDV polyclonal antibody (1:100) raised against the Onderstepoort strain of CDV in our laboratory as a control for infection (E) and with an anti-CD46 polyclonal antibody (B). Vero cells infected with DMV (MUC strain) for 24 h at an MOI of 1 were stained with rabbit hyperimmune anti-RPV antiserum (1:200), raised against the lapinized strain of the virus, to detect DMV infection (F) and with an anti-CD46 polyclonal antibody (C).
In the case of CDV, two wild-type isolates (CDV A75-17 and a dog isolate from Belfast) and two variants of the Onderstepoort vaccine strain (small- and large-plaque viruses) were tested, while only one DMV isolate (MUC strain, low passage number on Vero cells) was available for this study. Virus-specific antigens were expressed on the surfaces of Vero cells infected with either CDV or DMV (Fig. 2E and F). No significant downregulation of CD46 was observed with any strain of CDV used (Fig. 2B; Table 1). A previous paper reported that a vaccine strain of CDV did not downregulate CD46 (27), and our results provide further evidence that neither vaccine nor wild-type strains of CDV cause downregulation. Similarly, DMV did not downregulate CD46 from the surfaces of infected Vero cells (Fig. 2C; Table 1), and so it is clear that CD46 downregulation is not a property shared by all morbilliviruses.
Antibodies against CD46 do not inhibit RPV infection.
We tested to see if RPV also used CD46 as a receptor by using anti-CD46 antibodies to block attachment. Virus plaque titrations were prepared in cell monolayers (70 to 80% confluent) in six-well plates which were washed once with DMEM and incubated for 2 h at 37°C with dilutions of the RPV Saudi/81 laboratory-passaged strain or the MV vaccine strain (10−3 to 10−7). The virus inoculum was then removed, and the cells were washed twice with DMEM before being overlaid with 2 ml of Eagle’s medium containing 2% FCS and 0.8% low-melting-temperature agarose (Life Technologies). Cells were fixed 3 days (SF10 cells) or 4 days (Vero or MDBK cells) postinfection by overlaying the agarose with 2 ml of 4% formaldehyde in phosphate-buffered saline (PBS) for 18 to 20 h at 4°C. The agarose was removed from the cell monolayer, which was then washed three times with PBS without Ca2+ or Mg2+ (PBSB). Cells were permeabilized with 0.5% (vol/vol) Triton X-100 in PBSB for 5 min at room temperature, washed three times with PBSB, then incubated for 5 min in PBSB containing 0.2% (wt/vol) gelatin (type A from porcine skin; Sigma) (PBSB/G). Virus-specific antigen was labelled with rabbit hyperimmune anti-RPV antiserum (1/400 in PBSB/G) for 1 h at room temperature. The cells were washed three times for 5 min each time with PBSB/G and were incubated for 1 h with horseradish peroxidase-labelled goat anti-rabbit immunoglobulin G (Sigma or Dako). The cells were washed three more times for 5 min each time and then incubated for 5 to 30 s in 1 mg of diaminobenzidine/ml in PBS containing 0.16% (wt/vol) NiCl2 and 1 μl of 30% (wt/wt) H2O2/ml. The stained groups of RPV- or MV-infected cells were counted by using an inverted microscope. To examine whether CD46 was used as a receptor, cell monolayers (as above) were incubated for 1 h at 37°C with antibody diluted in 200 μl of DMEM. Virus (approximately 100 infectious units) was then added in 5 to 50 μl, and the number of infectious events was determined as described above.
Preincubation of Vero cells with rabbit anti-CD46 antibody strongly reduced infection by MV Edmonston vaccine strain. The number of plaques seen was 7% ± 2.2% (mean ± standard deviation [SD]) (n = 3) of the number seen in cells treated with nonimmune rabbit serum. Treatment of Vero cells, MDBK cells, or primary bovine fibroblasts with the same anti-CD46 antiserum had no apparent effect on infection with the RPV laboratory-passaged strain Saudi/81; plaque numbers were 99.4% ± 9.1% (n = 5), 100.4% ± 4.3% (n = 4), and 94.6% ± 10.4% (n = 4), respectively. Similarly, monoclonal anti-CD46 at 25 μg/ml inhibited MV infection of Vero cells (plaque numbers, 47.9% [n = 2] compared with the control) but not RPV infection (95.9% ± 6.1% [n = 4]) of Vero cells. RPV does not, therefore, use CD46 to infect Vero cells. In addition, RPV does not appear to use a bovine homologue to attach to bovine cells. RPV is capable of infecting a wide range of cell types, including bovine, monkey, pig, rabbit, lamb, human, canine, and hamster cells (20), and the receptor must be a widely distributed and conserved cellular protein. The virus could use a common receptor initially and then broaden its receptor specificity with passage to utilize CD46. However, there is no evidence from our results to suggest that RPV infection is blocked by anti-CD46 antibody, although this antibody stained 25% of bovine cells, suggesting that there is a structural homologue on bovine cells.
Our results have shown that CD46 downregulation by RPV is entirely separate from utilization of CD46 as a receptor. It has been suggested that downregulation of CD46 from the surfaces of MV-infected cells relates to usage of this molecule as a virus receptor (3, 27, 34–36). However, some wild-type strains of MV do not downregulate CD46, despite the fact that they appear to use it as a receptor (3, 4, 34). Furthermore, recent reports show that infection with a nondownregulating wild-type strain of MV, grown on B95a cells, is not inhibited by anti-CD46 antibody (and so this strain must use an alternative receptor), while the related Vero cell-adapted strain does appear to use CD46 as a receptor (4).
Expression of RPV-H alone downregulates CD46.
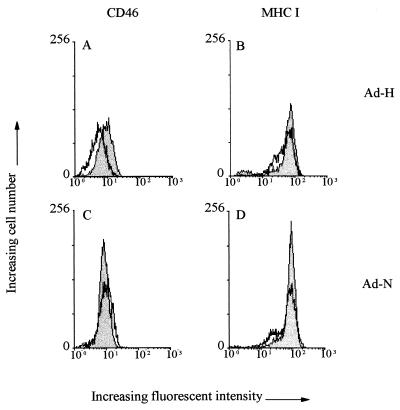
Replication-defective adenovirus recombinants expressing either the H or the N protein of RPV (Ad-H and Ad-N) were constructed with the E1A-deleted adenovirus expression vector pJM17 by conventional techniques (25, 41). Monoclonal antibodies (40) against the H and N proteins of RPV were used to confirm authentic expression of these proteins (data not shown). In Vero and B95a cells infected with the Ad-H recombinant at an MOI of 103, CD46 expression on the cell surface was decreased by an amount similar to that seen in cells infected with the RBOK virus, the strain from which the H-protein gene was cloned (Fig. 3A; Table 1). Downregulation of CD46 on B95a cells occurred after infection with the Ad-H recombinant at MOIs ranging from 50 to 103. However, H-gene expression was detected only on the surfaces of cells infected at MOIs between 500 and 103 (20). This indicates that even low levels of the H protein are capable of effecting downregulation. MHC class I expression remained unchanged in Ad-H-infected cells (Fig. 3B), while infection with Ad-N had no effect on either protein (Fig. 3C and D).
FIG. 3.
Flow-cytometric analysis of the surface expression of CD46 and MHC class I on Vero cells infected by adenovirus recombinants expressing either the H (Ad-H) or the N (Ad-N) protein of RPV. Shaded peaks, uninfected cells; unshaded peaks, infected cells. Stocks were grown and titered in 293 cells, which were maintained on DMEM containing 5% FCS, 100 U of benzylpenicillin/ml, and 100 U of streptomycin/ml. Vero cells were infected with the Ad-H or Ad-N recombinant for 24 h at an MOI of 103 and were stained with anti-CD46 polyclonal antibody (A and C) and with anti-MHC class I monoclonal antibody (B and D). The adenovirus expression of the RPV-H protein has been submitted for publication (20).
The H protein of MV has been shown to bind to CD46 short consensus repeat regions (SCR) I (amino acids 11 to 56) and II (amino acids 70 to 104) (5, 11, 23), and amino acids at positions 451 and 481 are crucial for determining the downregulation phenotype of the virus (3, 17). Changing the wild-type N451 and E481 to vaccine-like V451 and Y481 changes a nondownregulating H protein to a downregulating one. These residues are not present in RPV and are in areas which are poorly conserved between morbilliviruses. They are probably involved in receptor binding and may, therefore, account for differences in receptor specificity between viruses. These small changes in the MV H protein probably change the nature of the H-CD46 interaction, possibly by increasing binding affinity for CD46 or inducing conformational changes in CD46 that lead to its downregulation. Although our data indicate that CD46 is not the receptor for RPV, we cannot completely eliminate the possibility that RPV-H can interact directly with CD46 and thereby induce its downregulation. It is also possible that the RPV H protein could downregulate CD46 by an indirect mechanism, secondary to interaction of RPV-H with another cellular membrane protein, perhaps the real receptor for the virus, which in turn affects CD46 transport to, or reinsertion into, the cell membrane. CDV, on the other hand, neither downregulates CD46 nor uses it as a receptor (19, 27).
This raises the intriguing possibility that infections with RPV, MV, or other morbilliviruses have a variety of effects on the surface proteins of infected cells, even those not used as receptors. Such effects might be immunomodulatory and could be very important determinants of virus pathogenicity. Even where viruses act on the same protein, the overall pathogenic effect may be different: in this case, MV downregulation of CD46 appears to be associated with limitation of viral virulence, while highly virulent strains of RPV are very active in inducing downregulation, irrespective of their passage histories. In the case of MV, downregulation of CD46 on infected cells, and contact-mediated downregulation of the molecule on adjacent uninfected cells (35), appears to limit infection by resulting in the death of infected and neighboring cells. If we assume that the bovine homologue of CD46 serves a similar complement-regulating function, it could be that the resultant complement-mediated lysis of RPV-infected cells, instead of limiting the infection, is a necessary means of releasing the virus to spread and infect other cells.
Acknowledgments
We thank the following colleagues for cells and reagents: Fumio Kobune, National Institute of Health, Tokyo, Japan, for providing the B95a cells and Keith Leppard, University of Leicester, Leicester, United Kingdom, for providing the 293 cells; Fabian Wild (Institut Pasteur de Lyon, Lyon, France, for providing the rabbit anti-CD46 polyclonal antibody; Jürgen Schneider-Schaulies, University of Würzburg, Würzburg, Germany, for providing the monoclonal antibody against the H protein of MV and monoclonal antibody against CD46 (13/42); Albert Osterhaus, Erasmus University, Rotterdam, The Netherlands, for providing the DMV; and Frank Graham, McMaster University, Hamilton, Ontario, Canada, for providing the pJM17 adenovirus expression vector. We also thank Bert Rima, The Queen’s University of Belfast, for many helpful discussions and critical reading of the manuscript.
S. Galbraith was the recipient of a Department of Education for Northern Ireland studentship under the Co-operative Awards in Science and Technology scheme. A. Tiwari was the recipient of a Department for International Development scholarship from the British Government.
REFERENCES
- 1.Albrecht P, Lorenz D, Klutch M J, Vickers J H, Ennis F A. Fatal measles infection in marmosets: pathogenesis and prophylaxis. Infect Immun. 1980;27:969–978. doi: 10.1128/iai.27.3.969-978.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett T. Rinderpest and distemper viruses. In: Webster R G, Granoff A, editors. Encyclopedia of virology. Vol. 3. London, United Kingdom: Academic Press; 1994. pp. 1260–1268. [Google Scholar]
- 3.Bartz R, Brinckmann U, Dunster L M, Rima B, ter Meulen V, Schneider-Schaulies J. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]
- 4.Bartz R, Firsching R, Rima B K, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 6.Buckland R, Wild T F. Is CD46 the cellular receptor for measles virus? Virus Res. 1996;48:1–9. doi: 10.1016/s0168-1702(96)01421-9. [DOI] [PubMed] [Google Scholar]
- 7.Dhib-Jalbut S S, Xia Q, Drew P D, Swoveland P T. Differential up-regulation of HLA class I molecules on neuronal and glial cell lines by virus infection correlates with differential induction of IFN-β. J Immunol. 1995;155:2096–2108. [PubMed] [Google Scholar]
- 8.Diallo A, Barrett T, Barbron M, Meyer G, Lefevre P C. Cloning of the nucleocapsid protein gene of peste des petits ruminants virus: relationship to other morbilliviruses. J Gen Virol. 1994;75:233–237. doi: 10.1099/0022-1317-75-1-233. [DOI] [PubMed] [Google Scholar]
- 9.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 10.Gerlier D, Loveland B, Varior-Krishnan G, Thorley B, McKenzie I F C, Rabourdin-Combe C. Measles virus receptor properties are shared by several different CD46 isoforms differing in extracellular regions and cytoplasmic tails. J Gen Virol. 1994;75:2163–2171. doi: 10.1099/0022-1317-75-9-2163. [DOI] [PubMed] [Google Scholar]
- 11.Hsu E C, Dorig R E, Sarangi F, Marcil A, Iorio C, Richardson C D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston R W, Russel S M, Loveland B E, McKenzie I F C. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T. Biology of complement: the overture. Immunol Today. 1991;12:291–295. doi: 10.1016/0167-5699(91)90001-A. [DOI] [PubMed] [Google Scholar]
- 15.Krantic K, Gimenez C, Rabourdin-Combe C. Cell-to-cell contact via measles virus haemagglutinin-CD46 interaction triggers CD46 downregulation. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 16.Kraus E, Schneider-Schaulies S, Miyasaka M, Tamatani T, Sedgwick J. Augmentation of major histocompatibility complex class I and ICAM-1 expression on glial cells following measles virus infection: evidence for the role of type-1 interferon. Eur J Immunol. 1992;22:175–182. doi: 10.1002/eji.1830220126. [DOI] [PubMed] [Google Scholar]
- 17.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of the complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 19.Loffler S, Lottspeich F, Lanza F, Azersa D O, terMeulen V, Schneider-Schaulies J. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J Virol. 1997;71:42–49. doi: 10.1128/jvi.71.1.42-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund, B. T., A. Tiwari, S. Galbraith, W. I. Morrison, and T. Barrett. Submitted for publication.
- 21.Maisner A, Alvarez J, Liszewski M K, Atkinson D J, Atkinson J P, Herrler G. The N-glycan of the SCR 2 region is essential for membrane cofactor protein (CD46) to function as a measles virus receptor. J Virol. 1996;70:4973–4977. doi: 10.1128/jvi.70.8.4973-4977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisner A, Schneider-Schaulies J, Liszewski M K, Atkinson J P, Herrler G. Binding of measles virus to membrane cofactor protein (CD46): importance of disulfide bonds and N-glycans for the receptor function. J Virol. 1994;68:6299–6304. doi: 10.1128/jvi.68.10.6299-6304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchester M, Gairin J E, Patterson J E, Alvarez J, Liszewski M K, Eto D S, Atkinson J P, Oldstone M B A. Measles virus recognises its receptor, CD46, via two distinct binding domains within SCR1-2. Virology. 1997;233:174–184. doi: 10.1006/viro.1997.8581. [DOI] [PubMed] [Google Scholar]
- 24.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptor for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrory W J, Bautista D S, Graham F L. A simple technique for the rescue of early region mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 26.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naniche D, Wild T F, Rabourdin-Combe C, Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993;74:1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- 28.Post T W, Liszewski M K, Adams E M, Tedja I, Miller E A, Atkinson J P. Membrane cofactor protein of the complement system: alternative splicing of the serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991;174:93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell D F J, Johnstone R W, McKenzie I F C. Identification of four different CD46 (MCP) molecules with anti-peptide antibodies. Biochem Biophys Res Commun. 1991;180:1091–1097. doi: 10.1016/s0006-291x(05)81178-7. [DOI] [PubMed] [Google Scholar]
- 30.Purcell D F J, Russel S M, Deacon N J, Brown M A, Hooker D J, McKenzie I F C. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics. 1991;33:335–344. doi: 10.1007/BF00216692. [DOI] [PubMed] [Google Scholar]
- 31.Rey Nores J E, McCullough K C. Rinderpest virus isolates of different virulence vary in their capacity to infect bovine monocytes and macrophages. J Gen Virol. 1997;78:1875–1884. doi: 10.1099/0022-1317-78-8-1875. [DOI] [PubMed] [Google Scholar]
- 32.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B, ter Meulen V. Differential downregulation of CD46 by measles virus strains. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider-Schaulies J, Dunster L M, Schwartz-Albiez R, Krohne G, ter Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider-Schaulies J, Schnorr J-J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnorr J-J, Dunster L M, Nanan R, Schneider-Schaulies J, Schneider-Schaulies S, ter Meulen V. Measles virus-induced downregulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur J Immunol. 1995;25:976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 37.Seya T, Atkinson J P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264:581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.vanden Berg C W, dela Lastra J M P, Llanes D, Morgan B P. Purification and characterization of the pig analogue of human membrane cofactor protein (CD46/MCP) J Immunol. 1997;158:1703–1709. [PubMed] [Google Scholar]
- 39.Varior-Krishnan G, Trescol-Biemont M-C, Naniche D, Rabourdin-Combe C, Gerlier D. Glycosylphosphatidylinositol-anchored and transmembrane forms of CD46 display similar measles virus receptor properties: virus binding, fusion, and replication; down-regulation by hemagglutinin; and virus uptake and endocytosis for antigen presentation by major histocompatibility complex class II molecules. J Virol. 1994;68:7891–7899. doi: 10.1128/jvi.68.12.7891-7899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wamwayi H M, Fleming M, Barrett T. Characterisation of African isolates of rinderpest virus. Vet Microbiol. 1995;44:151–163. doi: 10.1016/0378-1135(95)00008-x. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson G W G, Akrigg A. Constitutive and enhanced expression from the CMV major IE promotor in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlsein P, Wamwayi H M, Trautwein G, Pohlenz J, Liess B, Barrett T. Pathomorphological and immunohistological findings in cattle experimentally infected with rinderpest virus isolates of different pathogenicity. Vet Microbiol. 1995;44:141–147. doi: 10.1016/0378-1135(95)00007-w. [DOI] [PubMed] [Google Scholar]